Abstract
Annual surveys of Irish soil samples identified three isolates, CBS 16921 (UCD88), CBS 18246 (UCD443), and CBS 18247 (UCD483), of an apiculate yeast species within the Hanseniaspora genus. The internal transcribed spacer (ITS) and D1/D2 region of the large subunit (LSU) rRNA sequences showed that these are isolates of the recently described species Hanseniaspora menglaensis, first isolated from Southwest China. No genome sequence for H. menglaensis is currently available. The genome sequences of the three Irish isolates were determined using short-read (Illumina) sequencing, and the sequence of one isolate (CBS 16921) was assembled to chromosome level using long-read sequencing (Oxford Nanopore Technologies). Phylogenomic analysis shows that H. menglaensis belongs to the fast-evolving lineage (FEL) of Hanseniaspora. Only one MAT idiomorph (encoding MATα1) was identified in all three sequenced H. menglaensis isolates, consistent with one mating type of a heterothallic species. Genome comparisons showed that there has been a rearrangement near MATα of FEL species compared to isolates from the slowly evolving lineage (SEL).
Keywords: heterothallic, MAT locus, sporulation, yeast mitochondrion, phylogenomics, nanopore sequencing, Illumina sequencing, genome assembly, chromosomes, fast-evolving lineage
1. Introduction
Hanseniaspora species are apiculate yeasts found abundantly on a variety of ripening fruits, flowers and barks [1]. They are particularly prevalent and diverse within grape musts [2,3]. Hanseniaspora species have long since been associated with wine fermentation, commonly as pests, and more recently as potential bio-flavouring agents [3,4]. Most species are not prolific fermenters with low ethanol tolerance between 3 and 5% and are quickly outcompeted by Saccharomyces species in early fermentation [5,6]. Those that do exceed this threshold, such as Hanseniaspora osmophila, often produce “off flavour” compounds such as acetic acid, acetaldehyde, and ethyl acetates, which are considered detrimental to the flavour profile of the wine [7,8]. Hanseniaspora species may function as bio-flavouring agents and potential co-fermenters with Saccharomyces cerevisiae because they can metabolise cellobiose [9,10].
Hanseniaspora species fall into two subclades commonly known as the fast (FEL) and slow (SEL) evolving lineages within the Saccharomycodaceae [11]. The clades are distinguished by the loss of genes involved in DNA repair, cell cycle repair, and mitotic checkpoints [11], with more extensive loss observed within the FEL. The loss of repair genes enabled the rapid accumulation of mutations, resulting in significant protein divergence. The FEL is separated from other lineages by a distinctly long branch length, similar to many hyper-mutator fungal lineages [11]. Members of the SEL are more proficient fermenters, yielding higher concentrations of ethanol [12].
Currently, 24 species of Hanseniaspora are known, consisting of 18 species within the FEL and 6 within the SEL [13]. Three yeast strains, CBS 16921 (UCD88), CBS 18246 (UCD443), and CBS 18247 (UCD483), were collected from soil samples as part of undergraduate projects at University College Dublin, Ireland, from 2017 to 2020 [14,15,16]. Strains were isolated from Ballawley Park, Co. Dublin (53.2799, −6.23317) (CBS 16921) and wooded roadsides in Kilcommon, Co. Tipperary (52.692835, −8.146708) (CBS 18247) and Aughnagarnon, Co. Longford (53.788433, −7.423251) (CBS 18246). Internal transcribed spacer (ITS) sequence analysis suggests they represent new isolates of the recently described species Hanseniaspora menglaensis [13] (Table A1). Because there is currently no genome sequence of H. menglaensis available, we sequenced the genomes of the three Irish isolates in order to contribute to our understanding of the phylogeny and evolution of the Hanseniaspora genus.
2. Materials and Methods
Yeast isolation and identification: Yeasts were isolated from soil samples as described in Sylvester et al. [17] and Bergin et al. [16]. In brief, ~2.5 g of soil was incubated at room temperature for 5 days in 9 mL yeast–peptone–dextrose (YPD) (1% yeast extract, 2% peptone, 2% glucose) broth containing chloramphenicol (30 μg/mL) and ampicillin (100 μg/mL). A total of 10 µL of homogenised cultures was inoculated into fresh media for a further 2-day incubation. Next, 100 µL of both 1:100 and 1:10,000 diluted cultures was plated onto YPD agar (1% yeast extract, 2% peptone, 2% agar, 2% glucose) and incubated for 5 days. Single colonies were obtained and potential yeast isolates were chosen for further investigation. The ITS regions of selected isolates were amplified by colony PCR using universal primers ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′) [18] using 35 cycles of 95° for 15 s, 48 °C for 15 s, and 72 °C for 30 s. PCR products were sequenced by the Eurofins Genomics Mix2Seq platform using ITS1 as a primer. Three isolates with ITS sequences identical to the ITS sequence of Hanseniaspora menglaensis (CICC 33364/NYNU 181083) were deposited to the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands, as CBS 16921, CBS 18246 and CBS 18247 (Table A1) [13]. Additional deposits have been made to the Portuguese Yeast Culture Collection, Portugal, as PYCC 9756 (CBS 16921), PYCC 9757 (CBS 18246) and PYCC 9758 (CBS 18247) and at University College Dublin as UCD88 (CBS 16921), UCD443 (CBS 18246) and UCD483 (CBS 18247).
Genome sequencing: We sequenced the genome of one isolate (CBS 16921) to chromosome level using a combination of long-read (Oxford Nanopore) and short-read (Illumina) technologies, and we used short-read sequencing to survey the genomes of the other two Irish isolates. For Illumina sequencing, total genomic DNA was prepared from all three Irish H. menglaensis isolates using phenol–chloroform–isoamyl alcohol (Sigma-Aldrich P3803, Gillingham, Dorset, UK. All three genomes were sequenced by BGI Tech Solutions Co. (Hong Kong, China) from 1 µg of genomic DNA using an Illumina HiSeq 4000 instrument for CBS 16921 and an Illumina HiSeq X for CBS 18246 and CBS 18247. A total of 6.4 million, 6.7 million, and 4 million paired-end reads (2 × 150 bp) were obtained for CBS 16921, CBS 18246 and CBS 18247, respectively. Reads were trimmed using Skewer v. 0.2.2 [19] to minimum mean qualities of 30 and minimum lengths of 35. To increase the proportion of high-molecular-weight DNA for long-read sequencing which facilitates chromosomal-level assembly, genomic DNA was also extracted from CBS 16921 using a Qiagen Genomic Tip 100/G kit according to the manufacturer’s instructions. Genomic DNA (400 ng) was sequenced with MinION technology using the rapid barcoding kit (SQK-RBK004) from Oxford Nanopore Technologies (ONT, Oxford, UK), following the manufacturer’s instructions. Sequencing was performed on a MinION MK1B device (MinKNOW v. 19.05.0) (ONT) using an r9.4.1 chemistry flowcell (FLO-MIN109). Basecalling was performed using guppy v. 16.04.5 (ONT) and reads were demultiplexed using qcat v. 1.1.0 (ONT). This generated 213,571 raw reads, which were reduced to 182,436 reads by filtering with NanoFilt v. 2.8.0 (ONT) to remove reads with quality scores < 7 or lengths of <1 kb. The filtered reads were assembled into 13 contigs using Canu v. 2.2 [20]. The raw assembly was polished with the trimmed Illumina reads using five rounds of correction with NextPolish v. 1.4.1 [21]. Two contigs containing partial arrays of the rDNA locus at one end each were manually joined. Four short, overlapping contigs derived from the mitochondrial genome were removed. The mitochondrial genome was annotated using MITOS2 [22] and trimmed to one copy using bedtools [23]. The VAR1 gene was identified by BLAST analysis of an unannotated open reading frame. Contig-level assemblies for samples CBS 18246 and CBS 18247 were generated using SPAdes v. 3.14.0 [24]. Only contigs larger than 500 bases with an average coverage greater than 10 were retained.
Genome annotation: Protein-coding sequences in all Hanseniaspora genomes were identified using BRAKER3 v. 3.0.2 [25]. Genomes were first soft-masked using both RepeatModeler v.2.0.4 [26] and RepeatMasker v. 4.1.2-p1 [27]. The training set for protein annotation was derived from the OrthoDB Fungi clade partition [28]. Annotation was performed with a lambda parameter of 1 for intron downsampling [25]. For CBS 16921, InterProScan v. 5.61-93.0 [29] was used to further annotate putative protein function using PANTHER, TIGRFAM, PFAM and SUPERFAMILY databases. tRNA-scan v. 2.0.5 [30] was used to annotate tRNAs, and barrnap v. 0.9 [31] was used to annotate rRNAs. In total, 4260 genomic protein-coding genes, 162 tRNAs, and a three-copy array of the rDNA locus were annotated in the CBS 16921 reference assembly.
Trimmed reads for CBS 16921, CBS 18246 and CBS 18247 were mapped to the CBS 16921 reference genome using BWA v. 0.7.17-r1188 [32]. Alignments were sorted and indexed using SAMtools v. 1.10 [33]. Duplicate reads were marked using Picard tools [34]. Variants were called and filtered using BCFtools v. 1.10.2 [35] and VCFtools v. 0.1.16 [36], respectively. Sites missing in any sample, sites with quality < 30, and sites with depth < 15 or >200 were removed. Only single-nucleotide polymorphisms (SNPs) were analysed. This identified 122 variant sites, all of which were manually verified in IGV [37] using BAM alignment to ensure that the sequencing depth matched the surrounding regions. SNP, protein-coding, tRNA, and rRNA annotations were visualised in R using the Circlize v. 0.4.15 [38] package.
Phylogenomic analysis: Phylogenomic analysis was performed using single-copy orthologs from the H. menglaensis CBS 16921 chromosome-level assembly, short-read assemblies for CBS 18246 and CBS 18247, and 20 scaffold-level assemblies for other Hanseniaspora species. Four outgroup species were included (Saccharomyces cerevisiae S288C, Kluyveromyces marxianus DMKU3-1042, Wickerhamomyces anomalus NRRL Y-366-8 and Cyberlindnera jadinii NRRL Y-1542). All included Hanseniaspora genomes were annotated as described above. Average nucleotide identity (ANI) was determined pairwise between CBS 16921 and all other assemblies using OrthoAni [39]. Single-copy orthologs were identified as described in Steenwyk et al. [11] with some modifications. In brief, putative orthologs were clustered using OrthoMCL [40] with pairing evidence from BLASTP v.2.10.0 [41] searches. BLASTP hits were filtered for E-values ≤ 1 × 10−10, percent identities ≥ 30%, and percent match length ≥ 70%. An inflation parameter of 4 was used to cluster putative orthologs with MCL v.14-137 [40]. Unlike Steenwyk et al. [11], only single-copy orthologs were used. In total, 548 single-copy orthologs were identified. Orthologs were aligned using MAFFT v.7.520 [42] with the following parameters: “--op 1.0 --maxiterate 1000 --genafpair”. Alignments were trimmed using trimAl v.1.4.rev15 (http://trimal.cgenomics.org/ (accessed on 14 August 2023)) [43] using the “automated 1” parameter. Trees were calculated for each ortholog alignment using RAxML v.8.2.12 [11,44] with the model of substitution set to PROTGAMMAAUTO and the seed set to “12345”. Ortholog trees where all four outgroup species were not found to be earliest diverging were discarded. Alignments of the remaining 522 orthologs were concatenated, and trees were inferred using RAxML with 100 bootstraps and the PROTGAMMALG model of substitution and a seed of “12345” [44]. The corresponding tree file was visualised in iTOL [45].
Mating-type locus annotation: The mating-type locus and neighboring genes in H. menglaensis isolates CBS 16921, CBS 18246 and CBS 18247 were identified using BLASTN and TBLASTN [41] against a dataset of Saccharomyces cerevisiae reference proteins. Similar sequences were identified in other Hanseniaspora species using BLAST [11,41]. Pairwise identity was calculated using the H. menglaensis sequences of SLA2, SUI1, MATα1, VPS75, YNL247W, GNEAS1 and CWC25 and MATa2 from H. valbyensis.
Physiological characterization: Morphology, nutritional growth and additional phenotypic profiles were characterised using standard protocols as described in Kurtzman et al. [46]. Most growth tests were performed at 25 °C, except for fermentation, which was assayed at 20 °C. Growth at 30 °C was assessed on (GYPA-2% glucose, 1% peptone, 0.5% yeast extract, 1.5% agar, pH 6.8). Ascus and ascospore formation were investigated by growing CBS 16921, CBS 18246 and CBS 18247 separately and as mixed cultures on 2% Difco malt extract agar (MEA) (pH 5.5) at 25 °C. Cells were examined daily for up to 7 days.
3. Results
3.1. Genome Sequence of H. menglaensis
An isolate of the new species Hanseniaspora menglaensis was recently identified from rotting wood in Southwest China [13]. We identified three isolates from soil in Ireland: CBS 16921, CBS 18246 and CBS 18247. The ITS regions of CBS 16921, CBS 18246, CBS 18247 are 99% identical to H. menglaensis CICC 33364/NYNU 181083, with lower similarity (96.7%) to the next closest sequence (Hanseniaspora lindneri) (Table A1). Extraction of the D1/D2 regions shows that CBS 16921, CBS 18246, CBS 18247 and H. menglaensis CICC 33364/NYNU 181083 share >99% identity (Table A1). Current guidelines (ITS sequence divergence of <2% and D1/D2 divergence < 1%) indicate that CBS 16921, CBS 18246, CBS 18247 and CICC 33364/NYNU 181083 are isolates of the same species, H. menglaensis, separate to H. lindneri [47,48,49].
We sequenced the genome of one isolate (H. menglaensis CBS 16921) using a combination of long read (Oxford Nanopore, Oxford, UK) and short read (Illumina, Cambridge, UK) technologies, and we used short-read sequencing to survey the genomes of the other two isolates. The final assembly of H. menglaensis CBS 16921 consists of 8 contigs (7 chromosome-level contigs, named from 1 to 7 in order of size, and 1 mitochondrial contig) (Figure 1). This assembly is 9,558,052 bp with an N50 of 1,490,982 bp and G+C content of 30.34%. This assembly is likely chromosome-level, but no telomere repeats were identified. The mitochondrial genome consists of a circular contig of 19.63 kb. G+C content is lower than that of the nuclear genome (23.61%). All core mitochondrial components (rrnL, rrnS, cob, cox1, cox2, cox3, atp6, atp8 and atp9) are present, as well as 27 tRNA genes. The NADH ubiquinone oxidoreductase genes (nad1, nad2, nad3, nad4, nad4L, nad5, nad6) are not present, similar to species in the Saccharomycetaceae [50]. The ribosomal protein gene VAR1 is missing from Hanseniaspora uvarum [50,51]. However, it is present in the H. menglaensis mitochondrial genome. Approximately 43% of the mitochondrial genome consists of intergenic regions.
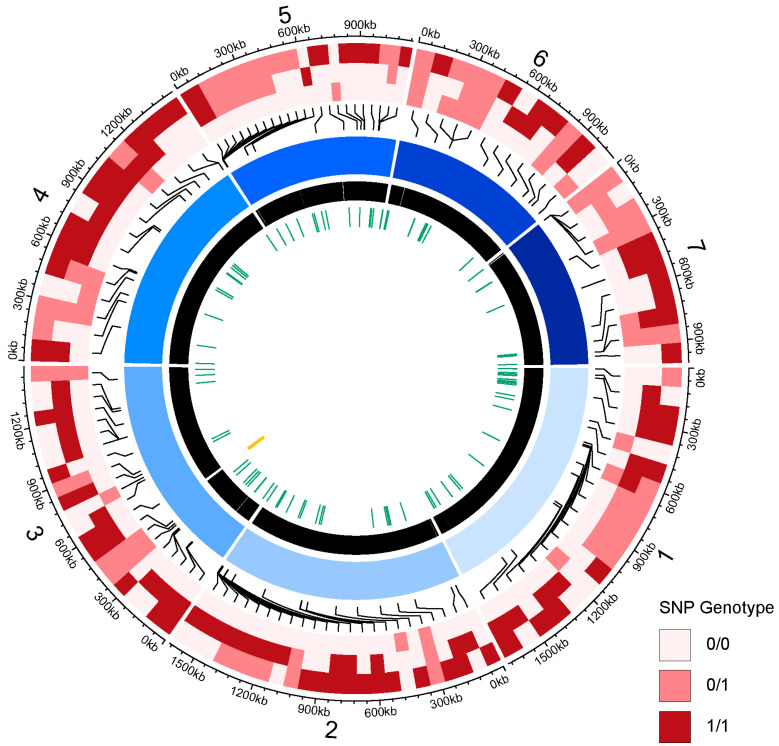
Figure 1.
Chromosome circle diagram of H. menglaensis CBS 16921 genome assembly. The central circle (blue) shows each chromosome, labelled by number 1 through 7 on the outermost ring. Chromosome sizes are shown in 300 kb intervals. The pink-to-red heatmap rings show the genotypes of well-supported SNPs in comparison to the reference genome, in the order CBS 16921, CBS 18246 and CBS 18247 (from inner to outer). “0/0” represents sites called as homozygous for the reference allele, “1/1” represents sites called as homozygous for an alternative allele, and “0/1” represents sites called as heterozygous. The black ring shows protein-coding sequences, the green ring shows tRNA genes, and the gold ring shows the rRNA array on chromosome 3.
Mapping the individual reads from H. menglaensis CBS 16921, CBS 18246 and CBS 18247 to the haploid genome assembly identified 122 variant sites (Figure 1). In each isolate, a small number of sites were called as heterozygous with high confidence: there are 24 such sites in CBS 16921, 29 in CBS 18246, and 40 in CBS 18247. In addition, 53 sites in CBS 18246 and 55 sites in CBS 18247 were called as homozygous for an allele different from the reference (the CBS 16921 haploid assembly). The genomes of the three Irish isolates are therefore very similar (~99.987%), but they are not identical (Figure 1). No high-confidence SNPs were identified between the mitochondrial genomes.
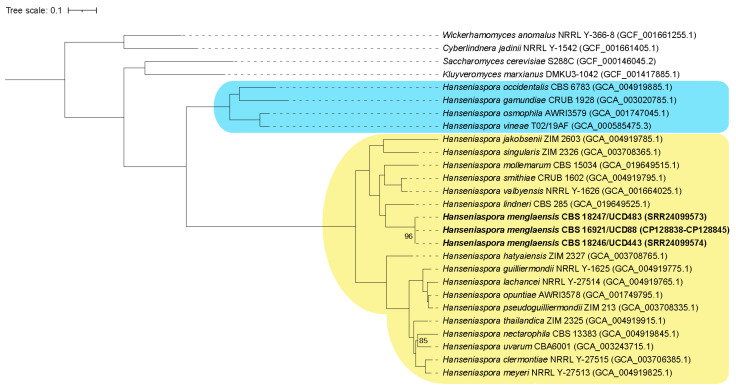
Phylogenomic analysis (Figure 2) shows that H. menglaensis CBS 16921, CBS 18246 and CBS 18247 belong to the fast-evolving lineage (FEL) of Hanseniaspora and form a subclade with Hanseniaspora lindneri, Hanseniaspora valbyensis, Hanseniaspora smithiae, Hanseniaspora mollemarum, Hanseniaspora singularis and Hanseniaspora jakobsenii. They are most closely related to H. lindneri CBS 285 but are separated with bootstrap support of 100%. This tree provides strong support for placing H. menglaensis within the FEL as a close relative of H. lindneri, similar to a previous analysis which used only the ITS region, D1/D2 domain of the LSU and ACT1 [13] (bootstrap support of 78%). In addition, comparing the ANI over the entire genome sequences showed that H. menglaensis CBS 16921 and H. lindneri have an ANI of 75.5% (determined using OrthoAni [39]) (Table A2), supporting the designation of H. menglaensis as a separate species.
Figure 2.
Phylogenomic tree generated from 522 single-copy orthologs from 23 Hanseniaspora isolates and 4 outgroup species, S. cerevisiae, K. marxianus, W. anomalus and C. jadinii. Bootstraps lower than 100% are shown. The accession of the reference assembly or protein set used is shown in parentheses. The slow (SEL) and fast (FEL) evolving lineages are shown with blue and yellow boxes, respectively. The new species H. menglaensis is marked in bold text.
3.2. Characterization of the Mating-Type Locus
Yeast mating occurs as a fusion of haploid cells of opposing mating types [52]. Mating types are determined by idiomorphs of the mating-type (MAT) locus, and species may be either heterothallic or homothallic [52,53,54]. Heterothallic species encode one of two MAT idiomorphs, MATa or MATα. Mating occurs between haploid cells of opposite mating type, generating a MATa/MATα diploid [52,54]. Homothallic species encode mating identity genes from both MATa and MATα idiomorphs [54,55]. Such isolates can mate with any other related cell [52,53,54,55].
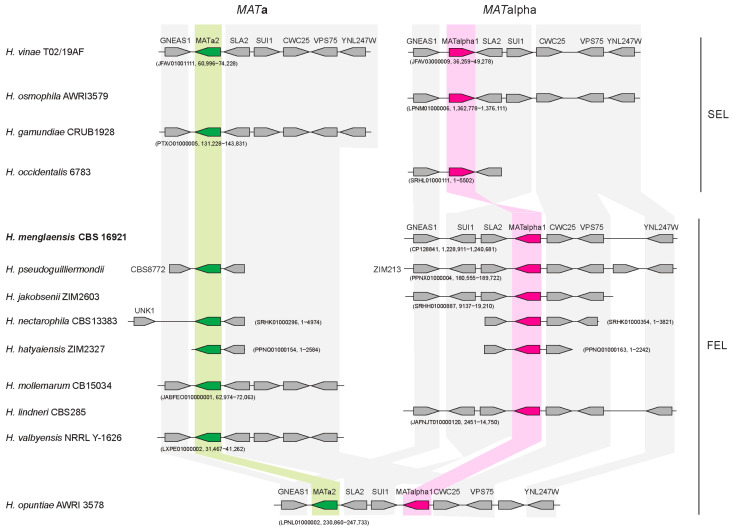
Heterothallic isolates of Hanseniaspora species have previously been identified from both haploid and diploid genome assemblies [55]. The MATa and MATα idiomorphs encode only one gene each: MATa2 and MATα1, respectively [53,54,55,56]. Both idiomorphs are found adjacent to SLA2, SUI1, CWC25 and GNEAS1 [56]. However, previous analysis has shown that that there is an inversion between SUI1 and MATα1 in H. pseudoguillermondii, H. opuntiae, H. uvarum and H. guilliermondii (Figure 3) [56].
Figure 3.
Identification of mating-type (MAT) loci. Mating loci were identified using BLAST analysis. Orthologous genes are indicated with shading in the background. MATa2 genes are colored in green and orthologs are indicated with green shading, whereas MATα1 genes are colored in pink and orthologs are indicated with pink shading. The accession numbers and locations of the MAT loci are indicated. MATa and MATα loci were identified in different assemblies of H. vinae TO2/19AF [56]. Some MAT loci are assembled into short contigs (e.g., H. nectarophila and H. hatyaiensis). The MATa loci of H. pseudoguilliermondii CBS8772 and H. opuntiae AWRI 357 are described in Saubin et al. [56]. There is a rearrangement around SUI1/MATα1 in FEL isolates, as previously described by Saubin et al. [56]. The three H. menglaensis isolates have identical MATα loci. Pairwise similarity with the reference H. menglaensis sequence varies for each gene: CWC25 (34–61.5%), GNEAS1 (35.4–67.4%), MATα1 (34.6–52.9%), SLA2 (10.8–76%), SUI1 (10.1–89.7%), VPS75 (41.1–77.3%) and YNL247W (63.5–80.7%). MATa2 was compared to H. valbyensis and ranges from 37.6 to 65.7% identity.
We used BLASTN and TBLASTN [41] to extend the analysis of the MAT locus across 13 Hanseniaspora species, including species from both the FEL and SEL (Figure 3). Only one MAT idiomorph (encoding MATα1) was identified in all three Irish H. menglaensis isolates (Figure 3). The structure of the region resembles the MATα locus in other FEL isolates, with MATα1 lying between SLA2 and CWC25 (Figure 3). We find that the inversion of SUI1-SLA2-MATα1 previously described [56] is restricted to FEL isolates (Figure 3). The MATa locus has the same structure in both SEL and FEL isolates, with a single MAT gene (MATa2) between SLA2 and GNEAS1 (Figure 3).
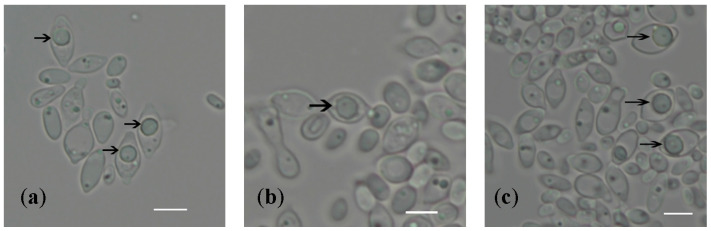
Some isolates of the Hanseniaspora species are diploid, and contain both MATa and MATα loci (e.g., H. vinae, H. nectarophila and H. hatyaenisis) (Figure 3). These isolates are likely to be heterothallic, with one MAT locus originating from one parent and the other from a second parent. In other isolates, only one MAT locus has been identified: only MATα in H. osmophila, H. occidentalis, H. menglaensis, H. jakobsenii, and H. lindneri, and only MATa in H. gamundiae, H. mollemarum and H. valbyensis (Figure 3). These are likely to be haploid and heterothallic species, and the missing MAT locus may be present in other isolates of the same species. For example, MATa and MATα have been identified in different isolates of H. pseudoguilliermondii (Figure 3; [56]). However, it is also possible that the isolates are diploid, and the second MAT locus has not been identified in the genome assemblies. For example, the genome of H. vinae TO2/19AF was assembled twice (from the same data), and in one iteration, the MATa locus was assembled, and in the second, the MATα locus was assembled (Figure 3). In addition, although Chen et al. [13] did not observe ascospores in the Chinese isolate of H. menglaensis, ascospores are formed by the Irish isolates (Figure 4). The species is likely to be homothallic as asci with warty ascospores were observed routinely after 7 days for each of the studied strains, CBS 16921, CBS 18246 and CBS 18247, when grown as separate cultures on sporulation medium. The sexual cycle of H. menglaensis therefore requires further exploration.
Figure 4.
Formation of ascospores of Hanseniaspora menglaensis strains. (a) CBS 16921 ascospores (indicated with arrows) in asci; (b) CBS 18246 ascospore (indicated with arrow) in ascus; (c) CBS 18247 ascospores (indicated with arrows) in asci. Scale bar = 5 µm.
It is notable that the MAT locus in H. opuntiae appears to have arisen from a recombination between MATa and MATα, and contains both MATa2 and MATα1 (Figure 3) (previously described in Saubin et al. [56]). This may be a homothallic species. However, some H. opuntiae isolates appear to encode only MATa2, and hybrids between H. opuntiae and H. pseudoguilliermondii have been identified [56].
3.3. Physiological Analysis
All described H. menglaensis isolates have a narrow range of carbon utilisation. Glucose, cellobiose, arbutin, salicin and glucono D-lactone are assimilated (Table 1). Unlike H. menglaensis CICC 33364/NYNU 181083, galactose and inulin are assimilated by the three Irish isolates, albeit weakly, with delayed assimilation for inulin (Table 1) [13]. We did not observe growth of the Irish isolates on D-gluconate, which has been reported for H. menglaenisis CICC 33364/NYNU 181083 (Table 1) [13]. There are some differences in nitrogen utilisation between the isolates. All isolates assimilate L-lysine, but only H. menglaenisis CICC 33364/NYNU 181083 grows on tryptophan (Table 1) [13]. All Irish isolates also use cadaverine and creatine, unlike CICC 33364/NYNU 181083 (Table 1) [13]. Unlike H. menglaenisis CICC 33364/NYNU 181083, the Irish isolates do not grow at 30 °C (Table 1) [13].
Table 1.
Biochemical characteristics of Hansenisaspora menglaensis and Hansenisaspora linderi. Characteristics of H. menglaensis CICC 33364/NYNU 181083 and H. linderi are taken from previous studies [1,13,57].
| Species | H. menglaensis CBS 16921 | H. menglaensis CBS 18246 | H. menglaensis CBS 18247 | H. menglaensis CICC 33364/NYNU 181083 | H. lindneri |
|---|---|---|---|---|---|
| Glucose | + | + | + | + | + |
| Cellobiose | + | + | + | + | d |
| Arbutin | + | + | + | + | d |
| Salicin | + | + | + | + | d |
| Glucono D-lactone | + | + | + | + | d |
| D-Galactose | w | w | w | - | - |
| Inulin | dw | dw | dw | - | - |
| Soluble Starch | dw | dw | dw | n | n |
| D-Gluconate | - | - | - | + | - |
| Lysine | + | + | + | + | + |
| Ethylamine | - | - | - | - | + |
| Cadaverine | + | + | + | - | + |
| Creatine | + | + | + | - | n |
| Tryptophan | - | - | - | + | n |
| 30 °C | - | - | - | + | + |
+, positive; -, negative; w, weak; d, delayed; dw, delayed and weak; n, not available.
4. Discussion
As of September 2023, 60 Hanseniaspora assemblies are publicly available from NCBI GenBank [58]. These include 1 complete, chromosome-level assembly (H. meyeri, GCA_030370665.1), 9 contig-level assemblies, and 50 scaffold-level assemblies. We have added another complete chromosome-level assembly for a newly discovered species (H. menglaensis), which will facilitate future comparative analysis.
Yeast mitochondrial genomes vary greatly in size, ranging from 18 to more than 105 kb [50]. H. menglaensis contains a small mitochondrial genome, similar in size to that of its close relative H. uvarum (~19.6 kb and ~18.5 kb, respectively). The H. uvarum mitochondrial genome is linear and has identical repeat regions of 3543 bp at each end [51], similar to the mitochondrial genome of H. meyeri [59], whereas the H. menglaensis mitochondrial genome is circular. H. meyeri and H. uvarum belong to a different branch than H. menglaensis within the FEL (Figure 2), suggesting that there may be a difference in mitochondrial organization between sub-lineages of the FEL clade. However, mitochondrial genome assemblies of other FEL species are needed to confirm this. The mitochondrial genomes from H. uvarum and H. menglaensis differ in their G+C content (~30% and 24%, respectively). The gene content of Hanseniaspora mitochondrial genomes are similar to other Saccharomycetaceae species, containing all core components except for NADH ubiquinone oxidoreductase genes [50]. The mitochondrial genes are also short, similar to those in H. uvarum [51]. The RNaseP subunit (rpm1) is absent from the H. menglaensis assembly; however, this element is consistently poorly annotated among yeast species [50]. The ribosomal protein VAR1 gene is present in the SEL Hanseniaspora clade and in the FEL subclade that includes H. menglaensis, H. singularis, H. mollemarum, H. smithiae, H. valbyensis, and H. lindneri (Figure 2). However, VAR1 is missing from the FEL subclade containing H. uvarum [50,51] (Figure 2). VAR1 is also missing from species in the CTG-Ser1 clade but is present in most other Saccharomycetaceae [50,60]. The functional consequence of this gene loss is not clear.
H. menglaensis was identified from rotting wood in China [13] and from soil in Ireland, suggesting that it may be a soil saprobic yeast. The genomes of the Irish isolates are highly similar, with a sequence divergence of ~0.0013%. In wild and domestic S. cerevisiae populations, sequence divergence between 0.001 and 1.1% has been observed, with an average of 0.5% [61]. Isolates of the human pathogen Candida albicans have a divergence of ~0.5% (between isolates of the same clade) and 1.1% (between isolates of different clades) [62]. The sequence divergence in the Irish H. menglaensis is surprisingly low, considering that they originated from locations up to 180 km apart and that they belong to a fast-evolving lineage (Figure 2). It is possible that there was a recent genetic bottleneck or a founder effect in the evolutionary history of the Irish population. Comparisons of the whole-genome sequences of the Irish and Chinese isolates (which have not yet been sequenced) may help to address this in the future.
Heterothallic isolates from both the FEL and SEL lineages of Hanseniaspora have been described previously [55,56]. MATa and MATα idiomorphs have been identified in haploid and diploid isolates [55,56] (Figure 3). Each idiomorph encodes only one protein (Mata2 or Matα1, respectively), unlike many other yeast species where the MATa locus encodes Mata1 and Mata2, and MATα encodes Matα1 and Matα2 [52,56]. In S. cerevisiae, Mata1 and Matα2 are homeodomain proteins that repress the expression of cell-type specific genes in diploid isolates [52,53,54,63,64]. This dimeric repressor appears to be absent in Hanseniaspora. Matα1 regulates the expression of α-specific genes, and (outside the immediate neighbors of S. cerevisiae) Mata2 plays a similar role in activating the expression of a-specific genes [53,54,55,65,66].
The synteny of the MAT locus is generally well conserved in yeast, and it is often adjacent to the SLA2 gene [52,53,54,55,56]. This pattern is also observed in Hanseniaspora species (Figure 3) [56]. Saubin et al. [56] previously identified an inversion around SUI1-SLA2 in MATα idiomorphs in some Hanseniaspora isolates. Our analyses show that the rearrangement occurs exclusively in members of the FEL and likely occurred in an ancestor of this lineage (Figure 3). The locus in H. opuntiae AWRI 3578, which is probably homothallic, likely arose from a recombination between a MATa and a rearranged MATα locus (Figure 3).
All three sequenced H. menglaensis isolates contain only a MATα locus, consistent with a haploid and heterothallic structure. Chen et al. [13] did not observe ascospore formation in the Chinese isolate (CICC 33364/NYNU 181083), which also suggests that they are haploid [13]. However, ascospores are formed by the Irish isolates (Figure 4). In addition, 24–40 heterozygous sites were identified in the three isolates. It therefore remains possible that the genomes are highly homozygous diploids and that MATa is present but was not assembled. As the mechanisms of mating and sporulation in Hanseniaspora are poorly understood, further investigation is required to underline the processes at work.
The physiology of all five H. menglaensis isolates is similar (Table 1) [13], but there are some differences. For example, all isolates assimilate nitrogen from lysine, but only the Chinese isolate uses tryptophan and only the Irish isolates use cadaverine (Table 1). All are signatures of association with plant material, where these nitrogen sources are commonly found. Other differences include the ability of the Chinese isolate to grow at temperatures up to 30 °C, which may indicate an adaptation to different locations. Chen et al. [13] suggest that the ability to assimilate D-gluconate is a distinguishing factor between H. menglaensis and H. lindneri. However, the Irish isolates cannot assimilate D-gluconate (Table 1). We do note that an inability to metabolise ethylamine distinguishes all five H. menglaensis isolates from H. lindneri [13,57].
Acknowledgments
Many thanks to the undergraduate and postgraduate students who helped in the initial isolation, sequencing and analysis of these samples, including Elijah Bahate, Jade Norton, Caoimhe O’Brien, Eoin O Cinnéide and Ísla O’Connor. We are particularly grateful to the students of Saint Bernard’s Mixed National School, Abbeylara, County Longford, and Kilcommon National School, Thurles, County Tipperary, who provided soil samples that led to the identification of CBS 18246 and CBS 18247.
Appendix A
Table A1.
Comparison of ITS and D1/D2 regions to CBS 16921.
| ITS | D1/D2 | |||
|---|---|---|---|---|
| Isolate | % Identity | Accession | % Identity | Accession |
| CBS 16921 * | 100 | OR939358 | 100 | SRR24099575 |
| CBS 18247 * | 99.3 | OR939360 | 99.8 | SRR24099573 |
| CBS 18246 * | 99.3 | OR939359 | 99.8 | SRR24099574 |
| CICC33364 | 99 | MK682803 | 99.3 | MK682799 |
| NYNU 181083 | 98.9 | OQ168353 | 99.3 | OQ168352 |
| H. lindneri * | 96.7 | GCA_019649525 | 98.4 | GCA_019649525 |
| H. valbyensis | 95.8 | KY103578 | 97.9 | KY107858 |
* sequences derived from whole-genome assembly.
Table A2.
Comparison of average nucleotide identity (ANI) of CBS 16921 to available Hanseniaspora genomes.
| Isolate | OrthoANI Value | Average Aligned Length | Query Coverage |
Subject Coverage |
Subject Length |
|---|---|---|---|---|---|
| H. menglaensis CBS 16921 | 100 | 9,523,740 | 1 | 1 | 9,553,320 |
| H. menglaensis CBS 18246 | 99.96 | 7,358,704 | 0.77 | 0.78 | 9,432,960 |
| H. menglaensis CBS 18247 | 99.96 | 6,908,493 | 0.72 | 0.74 | 9,348,300 |
| H. valbyensis NRRL Y-1626 | 76 | 2,853,907 | 0.3 | 0.3 | 9,664,500 |
| H. smithiae CRUB 1602 | 75.87 | 3,063,016 | 0.32 | 0.33 | 9,319,740 |
| H. lindneri CBS 285 | 75.5 | 2,340,075 | 0.24 | 0.22 | 10,647,780 |
| H. singularis ZIM 2326 | 74.87 | 2,343,548 | 0.25 | 0.27 | 8,784,240 |
| H. mollemarum CBS 15034 | 74.59 | 2,715,924 | 0.28 | 0.3 | 8,941,320 |
| H. hatyaiensis ZIM 2327 | 74.29 | 1,621,195 | 0.17 | 0.17 | 9,581,880 |
| H. uvarum CBA6001 | 74.14 | 1,723,829 | 0.18 | 0.19 | 8,958,660 |
| H. thailandica ZIM 2325 | 74.14 | 1,655,772 | 0.17 | 0.18 | 9,229,980 |
| H. opuntiae AWRI3578 | 74.04 | 1,434,013 | 0.15 | 0.16 | 8,820,960 |
| H. jakobsenii ZIM 2603 | 73.96 | 1,648,240 | 0.17 | 0.12 | 13,340,580 |
| H. guilliermondii NRRL Y-1625 | 73.94 | 1,582,303 | 0.17 | 0.18 | 8,974,980 |
| H. meyeri NRRL Y-27513 | 73.94 | 1,462,711 | 0.15 | 0.15 | 9,746,100 |
| H. nectarophila CBS 13383 | 73.79 | 1,636,636 | 0.17 | 0.18 | 9,218,760 |
| H. clermontiae NRRL Y-27515 | 73.77 | 1,487,723 | 0.16 | 0.17 | 8,667,960 |
| H. pseudoguilliermondii ZIM 213 | 73.42 | 1,509,933 | 0.16 | 0.17 | 8,747,520 |
| H. lachancei NRRL Y-27514 | 73.39 | 1,449,502 | 0.15 | 0.16 | 8,821,980 |
| H. occidentalis CBS 6783 | 72.26 | 712,638 | 0.07 | 0.06 | 11,567,820 |
| H. osmophila AWRI3579 | 71.96 | 647,824 | 0.07 | 0.06 | 11,449,500 |
| H. gamundiae CRUB 1928 | 71.73 | 597,327 | 0.06 | 0.06 | 10,006,200 |
| H. vineae T02/19AF | 71.58 | 689,535 | 0.07 | 0.06 | 11,293,440 |
Author Contributions
Conceptualization, G.B. and K.H.W.; methodology, A.P.R.; software, formal analysis, A.P.R., K.H.W. and G.B.; investigation, A.P.R., M.T.S., M.G. and T.B.; resources, C.H.; data curation, A.P.R.; writing—original draft preparation, A.P.R. and G.B.; writing—review and editing, A.P.R., M.G., T.B., K.H.W. and G.B.; visualization, A.P.R., K.H.W. and G.B.; supervision, G.B., M.G., T.B. and K.H.W.; project administration, G.B.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Whole-genome sequencing data for strains CBS 18246 (SRR24099574), CBS 18247 (SRR24099573), and CBS 16921 (SRR24099575/SRR24099572), and the genome assembly for CBS 16921 (CP128838-CP128845) are available at NCBI GenBank under BioProject PRJNA950348.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This work was funded by a UCD programme for undergraduate research and was supported by Science Foundation Ireland (grant numbers 19/FFP/6668 to G.B. and 20/FFP-A/8795 to K.H.W.), European Research Council (789341) to K.H.W, and the Irish Research Council (A.R.). T.B acknowledges funds from the Distinguished Scientist Fellow Programme of King Saud University, Riyadh, Saudi Arabia.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Čadež N., Smith M.T. The Yeasts. Elsevier; Amsterdam, The Netherlands: 2011. Hanseniaspora Zikes (1912) pp. 421–434. [Google Scholar]
- 2.Čadež N., Bellora N., Ulloa R., Hittinger C.T., Libkind D. Genomic Content of a Novel Yeast Species Hanseniaspora Gamundiae Sp. Nov. from Fungal Stromata (Cyttaria) Associated with a Unique Fermented Beverage in Andean Patagonia, Argentina. PLoS ONE. 2019;14:e0210792. doi: 10.1371/journal.pone.0210792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Wyk N., Badura J., von Wallbrunn C., Pretorius I.S. Exploring Future Applications of the Apiculate Yeast Hanseniaspora. Crit. Rev. Biotechnol. 2023;44:100–119. doi: 10.1080/07388551.2022.2136565. [DOI] [PubMed] [Google Scholar]
- 4.Bourbon-Melo N., Palma M., Rocha M.P., Ferreira A., Bronze M.R., Elias H., Sá-Correia I. Use of Hanseniaspora guilliermondii and Hanseniaspora opuntiae to Enhance the Aromatic Profile of Beer in Mixed-Culture Fermentation with Saccharomyces Cerevisiae. Food Microbiol. 2021;95:103678. doi: 10.1016/j.fm.2020.103678. [DOI] [PubMed] [Google Scholar]
- 5.Borren E., Tian B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods. 2020;10:13. doi: 10.3390/foods10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Celis M., Ruiz J., Vicente J., Acedo A., Marquina D., Santos A., Belda I. Expectable Diversity Patterns in Wine Yeast Communities. FEMS Yeast Res. 2022;22:foac034. doi: 10.1093/femsyr/foac034. [DOI] [PubMed] [Google Scholar]
- 7.Dzialo M.C., Park R., Steensels J., Lievens B., Verstrepen K.J. Physiology, Ecology and Industrial Applications of Aroma Formation in Yeast. FEMS Microbiol. Rev. 2017;41:S95–S128. doi: 10.1093/femsre/fux031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escott C., Loira I., Morata A., Bañuelos M.A., Suárez-Lepe J.A. Wine Spoilage Yeasts: Control Strategy. In: Morata A., Loira I., editors. Yeast—Industrial Applications. InTech; London, UK: 2017. [Google Scholar]
- 9.Medina K., Boido E., Fariña L., Gioia O., Gomez M.E., Barquet M., Gaggero C., Dellacassa E., Carrau F. Increased Flavour Diversity of Chardonnay Wines by Spontaneous Fermentation and Co-Fermentation with Hanseniaspora vineae. Food Chem. 2013;141:2513–2521. doi: 10.1016/j.foodchem.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 10.Zhang P., Zhang R., Sirisena S., Gan R., Fang Z. Beta-Glucosidase Activity of Wine Yeasts and Its Impacts on Wine Volatiles and Phenolics: A Mini-Review. Food Microbiol. 2021;100:103859. doi: 10.1016/j.fm.2021.103859. [DOI] [PubMed] [Google Scholar]
- 11.Steenwyk J.L., Opulente D.A., Kominek J., Shen X.-X., Zhou X., Labella A.L., Bradley N.P., Eichman B.F., Čadež N., Libkind D., et al. Extensive Loss of Cell-Cycle and DNA Repair Genes in an Ancient Lineage of Bipolar Budding Yeasts. PLoS Biol. 2019;17:e3000255. doi: 10.1371/journal.pbio.3000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granchi L., Ganucci D., Messini A., Vincenzini M. Oenological Properties of and from Wines Produced by Spontaneous Fermentations of Normal and Dried Grapes. FEMS Yeast Res. 2002;2:403–407. doi: 10.1016/S1567-1356(02)00089-2. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Qiao Y.-Z., Hui F.-L. Hanseniaspora Menglaensis f.a., Sp. Nov., a Novel Apiculate Yeast Species Isolated from Rotting Wood. Int. J. Syst. Evol. Microbiol. 2023;73:005970. doi: 10.1099/ijsem.0.005970. [DOI] [PubMed] [Google Scholar]
- 14.Ryan A., Ó Cinnéide E., Bergin S.A., Alhajeri G., Almotawaa H., Daly I., Heneghan S., Horan K., Kavanagh R., Keane C., et al. Draft Genome Sequence of a Diploid and Hybrid Candida Strain, Candida sanyaensis UCD423, Isolated from Compost in Ireland. Microbiol Resour. Announc. 2021;10:e00761-21. doi: 10.1128/MRA.00761-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ó Cinnéide E., Jones M., Bahate E., Boyd E., Clavero R., Doherty H., Drozdz I., Dumana M., Gonzales C., Kennedy J., et al. Draft Genome Sequence of the Yeast Ogataea degrootiae Strain UCD465, Isolated from Soil in Ireland. Microbiol. Resour. Announc. 2021;10:e00736-21. doi: 10.1128/MRA.00736-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergin S.A., Allen S., Hession C., Ó Cinnéide E., Ryan A., Byrne K.P., Ó Cróinín T., Wolfe K.H., Butler G. Identification of European Isolates of the Lager Yeast Parent Saccharomyces eubayanus. FEMS Yeast Res. 2022;22:foac053. doi: 10.1093/femsyr/foac053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sylvester K., Wang Q.-M., James B., Mendez R., Hulfachor A.B., Hittinger C.T. Temperature and Host Preferences Drive the Diversification of Saccharomyces and Other Yeasts: A Survey and the Discovery of Eight New Yeast Species. FEMS Yeast Res. 2015;15:fov002. doi: 10.1093/femsyr/fov002. [DOI] [PubMed] [Google Scholar]
- 18.Xie J., Fu Y., Jiang D., Li G., Huang J., Li B., Hsiang T., Peng Y. Intergeneric Transfer of Ribosomal Genes between Two Fungi. BMC Evol. Biol. 2008;8:87. doi: 10.1186/1471-2148-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang H., Lei R., Ding S.-W., Zhu S. Skewer: A Fast and Accurate Adapter Trimmer for next-Generation Sequencing Paired-End Reads. BMC Bioinform. 2014;15:182. doi: 10.1186/1471-2105-15-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koren S., Walenz B.P., Berlin K., Miller J.R., Bergman N.H., Phillippy A.M. Canu: Scalable and Accurate Long-Read Assembly via Adaptive k-Mer Weighting and Repeat Separation. Genome Res. 2017;27:722–736. doi: 10.1101/gr.215087.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Z., Erickson D.L., Meng J. Polishing the Oxford Nanopore Long-Read Assemblies of Bacterial Pathogens with Illumina Short Reads to Improve Genomic Analyses. Genomics. 2021;113:1366–1377. doi: 10.1016/j.ygeno.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Donath A., Jühling F., Al-Arab M., Bernhart S.H., Reinhardt F., Stadler P.F., Middendorf M., Bernt M. Improved Annotation of Protein-Coding Genes Boundaries in Metazoan Mitochondrial Genomes. Nucleic Acids Res. 2019;47:10543–10552. doi: 10.1093/nar/gkz833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quinlan A.R., Hall I.M. BEDTools: A Flexible Suite of Utilities for Comparing Genomic Features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prjibelski A., Antipov D., Meleshko D., Lapidus A., Korobeynikov A. Using SPAdes De Novo Assembler. Curr. Protoc. Bioinform. 2020;70:e102. doi: 10.1002/cpbi.102. [DOI] [PubMed] [Google Scholar]
- 25.Brůna T., Hoff K.J., Lomsadze A., Stanke M., Borodovsky M. BRAKER2: Automatic Eukaryotic Genome Annotation with GeneMark-EP+ and AUGUSTUS Supported by a Protein Database. NAR Genom. Bioinform. 2021;3:lqaa108. doi: 10.1093/nargab/lqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flynn J.M., Hubley R., Goubert C., Rosen J., Clark A.G., Feschotte C., Smit A.F. RepeatModeler2 for Automated Genomic Discovery of Transposable Element Families. Proc. Natl. Acad. Sci. USA. 2020;117:9451–9457. doi: 10.1073/pnas.1921046117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarailo-Graovac M., Chen N. Using RepeatMasker to Identify Repetitive Elements in Genomic Sequences. CP Bioinform. 2009;25:4.10.1–4.10.14. doi: 10.1002/0471250953.bi0410s25. [DOI] [PubMed] [Google Scholar]
- 28.Zdobnov E.M., Kuznetsov D., Tegenfeldt F., Manni M., Berkeley M., Kriventseva E.V. OrthoDB in 2020: Evolutionary and Functional Annotations of Orthologs. Nucleic Acids Res. 2021;49:D389–D393. doi: 10.1093/nar/gkaa1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones P., Binns D., Chang H.-Y., Fraser M., Li W., McAnulla C., McWilliam H., Maslen J., Mitchell A., Nuka G., et al. InterProScan 5: Genome-Scale Protein Function Classification. Bioinformatics. 2014;30:1236–1240. doi: 10.1093/bioinformatics/btu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan P.P., Lowe T.M. tRNAscan-SE: Searching for tRNA Genes in Genomic Sequences. In: Kollmar M., editor. Gene Prediction. Volume 1962. Springer New York; New York, NY, USA: 2019. pp. 1–14. Methods in Molecular Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seemann T. Prokka: Rapid Prokaryotic Genome Annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 32.Li H. Aligning Sequence Reads, Clone Sequences and Assembly Contigs with BWA-MEM. arXiv. 2013 doi: 10.48550/ARXIV.1303.3997.1303.3997 [DOI] [Google Scholar]
- 33.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map Format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Auwera G.A.V., O’Connor B.D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra. 1st ed. O’Reilly; Beijing, China: Boston, MA, USA: Farnham, UK: Sebastopol, CA, USA: Tokyo, Japan: 2020. [Google Scholar]
- 35.Danecek P., Bonfield J.K., Liddle J., Marshall J., Ohan V., Pollard M.O., Whitwham A., Keane T., McCarthy S.A., Davies R.M., et al. Twelve Years of SAMtools and BCFtools. GigaScience. 2021;10:giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., et al. The Variant Call Format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative Genomics Viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gu Z., Gu L., Eils R., Schlesner M., Brors B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- 39.Lee I., Ouk Kim Y., Park S.-C., Chun J. OrthoANI: An Improved Algorithm and Software for Calculating Average Nucleotide Identity. Int. J. Syst. Evol. Microbiol. 2016;66:1100–1103. doi: 10.1099/ijsem.0.000760. [DOI] [PubMed] [Google Scholar]
- 40.Li L., Stoeckert C.J., Roos D.S. OrthoMCL: Identification of Ortholog Groups for Eukaryotic Genomes. Genome Res. 2003;13:2178–2189. doi: 10.1101/gr.1224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altschul S. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katoh K. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Capella-Gutiérrez S., Silla-Martínez J.M., Gabaldón T. trimAl: A Tool for Automated Alignment Trimming in Large-Scale Phylogenetic Analyses. Bioinformatics. 2009;25:1972–1973. doi: 10.1093/bioinformatics/btp348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stamatakis A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Letunic I., Bork P. Interactive Tree Of Life (iTOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurtzman C.P., Fell J.W., Boekhout T., Robert V. The Yeasts. Elsevier; Amsterdam, The Netherlands: 2011. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts; pp. 87–110. [Google Scholar]
- 47.Kurtzman C.P., Robnett C.J. Identification and Phylogeny of Ascomycetous Yeasts from Analysis of Nuclear Large Subunit (26S) Ribosomal DNA Partial Sequences. Antonie Van Leeuwenhoek. 1998;73:331–371. doi: 10.1023/A:1001761008817. [DOI] [PubMed] [Google Scholar]
- 48.Vu D., Groenewald M., Szöke S., Cardinali G., Eberhardt U., Stielow B., De Vries M., Verkleij G.J.M., Crous P.W., Boekhout T., et al. DNA Barcoding Analysis of More than 9 000 Yeast Isolates Contributes to Quantitative Thresholds for Yeast Species and Genera Delimitation. Stud. Mycol. 2016;85:91–105. doi: 10.1016/j.simyco.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boekhout T., Aime M.C., Begerow D., Gabaldón T., Heitman J., Kemler M., Khayhan K., Lachance M.-A., Louis E.J., Sun S., et al. The Evolving Species Concepts Used for Yeasts: From Phenotypes and Genomes to Speciation Networks. Fungal Divers. 2021;109:27–55. doi: 10.1007/s13225-021-00475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freel K.C., Friedrich A., Schacherer J. Mitochondrial Genome Evolution in Yeasts: An All-Encompassing View. FEMS Yeast Res. 2015;15:fov023. doi: 10.1093/femsyr/fov023. [DOI] [PubMed] [Google Scholar]
- 51.Pramateftaki P.V., Kouvelis V.N., Lanaridis P., Typas M.A. The Mitochondrial Genome of the Wine Yeast Hanseniaspora uvarum: A Unique Genome Organization among Yeast/Fungal Counterparts. FEMS Yeast Res. 2006;6:77–90. doi: 10.1111/j.1567-1364.2005.00018.x. [DOI] [PubMed] [Google Scholar]
- 52.Butler G., Kenny C., Fagan A., Kurischko C., Gaillardin C., Wolfe K.H. Evolution of the MAT Locus and Its Ho Endonuclease in Yeast Species. Proc. Natl. Acad. Sci. USA. 2004;101:1632–1637. doi: 10.1073/pnas.0304170101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee S.C., Ni M., Li W., Shertz C., Heitman J. The Evolution of Sex: A Perspective from the Fungal Kingdom. Microbiol. Mol. Biol. Rev. 2010;74:298–340. doi: 10.1128/MMBR.00005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni M., Feretzaki M., Sun S., Wang X., Heitman J. Sex in Fungi. Annu. Rev. Genet. 2011;45:405–430. doi: 10.1146/annurev-genet-110410-132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krassowski T., Kominek J., Shen X.-X., Opulente D.A., Zhou X., Rokas A., Hittinger C.T., Wolfe K.H. Multiple Reinventions of Mating-Type Switching during Budding Yeast Evolution. Curr. Biol. 2019;29:2555–2562.e8. doi: 10.1016/j.cub.2019.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saubin M., Devillers H., Proust L., Brier C., Grondin C., Pradal M., Legras J.-L., Neuvéglise C. Investigation of Genetic Relationships Between Hanseniaspora Species Found in Grape Musts Revealed Interspecific Hybrids With Dynamic Genome Structures. Front. Microbiol. 2020;10:2960. doi: 10.3389/fmicb.2019.02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ouoba L.I.I., Nielsen D.S., Anyogu A., Kando C., Diawara B., Jespersen L., Sutherland J.P. Hanseniaspora jakobsenii Sp. Nov., a Yeast Isolated from Bandji, a Traditional Palm Wine of Borassus Akeassii. Int. J. Syst. Evol. Microbiol. 2015;65:3576–3579. doi: 10.1099/ijsem.0.000461. [DOI] [PubMed] [Google Scholar]
- 58.Opulente D.A., LaBella A.L., Harrison M.-C., Wolters J.F., Liu C., Li Y., Kominek J., Steenwyk J.L., Stoneman H.R., VanDenAvond J., et al. Genomic and Ecological Factors Shaping Specialism and Generalism across an Entire Subphylum. bioRxiv. 2023 doi: 10.1101/2023.06.19.545611. [DOI] [Google Scholar]
- 59.Rueda-Mejia M.P., Bühlmann A., Ortiz-Merino R.A., Lutz S., Ahrens C.H., Künzler M., Freimoser F.M. Pantothenate Auxotrophy in a Naturally Occurring Biocontrol Yeast. Appl. Environ. Microbiol. 2023;89:e00884-23. doi: 10.1128/aem.00884-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dujon B.A., Louis E.J. Genome Diversity and Evolution in the Budding Yeasts (Saccharomycotina) Genetics. 2017;206:717–750. doi: 10.1534/genetics.116.199216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peter J., De Chiara M., Friedrich A., Yue J.-X., Pflieger D., Bergström A., Sigwalt A., Barre B., Freel K., Llored A., et al. Genome Evolution across 1,011 Saccharomyces cerevisiae Isolates. Nature. 2018;556:339–344. doi: 10.1038/s41586-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J.M., Bennett R.J., Anderson M.Z. The Genome of the Human Pathogen Candida Albicans Is Shaped by Mutation and Cryptic Sexual Recombination. mBio. 2018;9:e01205-18. doi: 10.1128/mBio.01205-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson A.D. Molecular Mechanisms of Cell-Type Determination in Budding Yeast. Curr. Opin. Genet. Dev. 1995;5:552–558. doi: 10.1016/0959-437X(95)80022-0. [DOI] [PubMed] [Google Scholar]
- 64.Hull C.M., Johnson A.D. Identification of a Mating Type-Like Locus in the Asexual Pathogenic Yeast Candida albicans. Science. 1999;285:1271–1275. doi: 10.1126/science.285.5431.1271. [DOI] [PubMed] [Google Scholar]
- 65.Sengupta P., Cochran B.H. MAT Alpha 1 Can Mediate Gene Activation by A-Mating Factor. Genes Dev. 1991;5:1924–1934. doi: 10.1101/gad.5.10.1924. [DOI] [PubMed] [Google Scholar]
- 66.Coughlan A.Y., Lombardi L., Braun-Galleani S., Martos A.A., Galeote V., Bigey F., Dequin S., Byrne K.P., Wolfe K.H. The Yeast Mating-Type Switching Endonuclease HO Is a Domesticated Member of an Unorthodox Homing Genetic Element Family. eLife. 2020;9:e55336. doi: 10.7554/eLife.55336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Whole-genome sequencing data for strains CBS 18246 (SRR24099574), CBS 18247 (SRR24099573), and CBS 16921 (SRR24099575/SRR24099572), and the genome assembly for CBS 16921 (CP128838-CP128845) are available at NCBI GenBank under BioProject PRJNA950348.