Abstract
Interaction of retrovirus vectors and endogenous retroviruses present in packaging cell lines and target cells may result in unwanted events, such as the formation of recombinant viruses and the mobilization of therapeutic vectors. Using sensitive reverse transcriptase PCR assays, we investigated human and murine gene therapy packaging cell lines for incorporation of endogenous retrovirus transcripts into murine leukemia virus (MLV) vector particles and, conversely, whether vector genomes are incorporated into human endogenous retrovirus (HERV) particles. VL30 endogenous retrovirus sequences were efficiently packaged in particles produced by the murine AM12 packaging system. For every seven MLV-derived β-galactosidase (β-Gal) vector genomes present in the particles, one copy of VL30 was also packaged. Although human FLY packaging cells expressed several classes of HERV transcripts (HERV-K, HuRT, type C, and RTVL-H), none was detectable in the MLV vector particles released from the cells. Nonspecific packaging of the MLV Gag-Pol expression vector transcripts was detected in the FLY virions at a low level (1 in 17,000 sequences). These findings indicate that human packaging cells produce retrovirus particles far less contaminated by endogenous viral sequences than murine packaging cells. Human teratocarcinoma cells (GH cells), which produce HERV-K particles, were transduced with an MLV-derived β-Gal vector. Although both HERV-K and RTVL-H sequences were found in association with the particles, β-Gal transcripts were not detected, indicating that HERV Gag proteins do not efficiently package MLV-based vectors.
Although retroviral gene therapy holds promise for the treatment of human disease, the technique also poses a number of important safety questions. Ideally, a packaging system will produce high-titer virus containing vector sequences only; once transduced into target cells, these sequences should not replicate further. Viewed as a significant safety concern of packaging systems is the possible generation of new replication-competent retroviruses (RCR) due to the recombination of expression plasmids (13). Split packaging cell lines, such as GP+E (11) and FLY (6), which separate the coding regions of virus structural proteins onto two separate plasmids, have been developed decreasing the probability of RCR generation. However, packaging of RNAs coding for packaging functions and cross-packaging of endogenous retrovirus (ERV) genomes expressed in packaging cells may result in the transfer of unwanted genetic information and its recombination with therapeutic vectors. Such recombination may give rise to RCR. The recent isolation of an RCR from a third-generation murine packaging cell line serves as a further safety warning (3).
ERVs belong to a diverse family of genetic elements related to infectious retroviruses, which form part of the genome of all vertebrates, including humans (27). ERV genomes have the same basic organization as infectious retrovirus genomes and possess sequence similarity to gag, pol, and env genes. Human ERVs (HERVs) constitute up to 0.1% of the human genome and possess diverse biological activities, including transcription, protein synthesis, and in some cases particle production, although an infectious HERV has yet to be identified (17, 27). Infectious ERVs have been identified in chickens, mice, cats, pigs, and baboons. Although not all ERVs are infectious for the cells of the host species, many can infect and replicate in cells of other species, a phenomenon called xenotropism (10). HERVs which show sequence similarity to several retrovirus families, including the A-type particles (e.g., murine intracisternal A-type particles [IAP]), type B viruses (e.g., mouse mammary tumor virus), type C viruses (e.g., murine leukemia viruses [MLV]), type D viruses (e.g., langur and squirrel monkey viruses), and foamy viruses, have been identified (4, 17, 27). Classification based on particle morphology is inappropriate for HERVs because particle formation has been detected for only a few HERVs. Instead, a system based on the identity of the primer binding site is most commonly used, e.g., with HERV-K possessing a primer binding site utilizing a lysine tRNA. Two families of HERVs studied in particular depth are HERV-K and HERV-H (RTVL-H). The HERV-K family has been studied for its possession of open reading frames for gag, pol, and env, while the RTVL-H family has particularly high transcriptional activity and copy numbers (26).
If ERV RNAs are cross-packaged into vector particles, they will be more likely than other cellular sequences to recombine with vector genomes (1). The transfer of two ERV sequences, VL30 and IAP, has been described for murine packaging cell lines (2, 8, 19), and the recombination of VL30 and MLV sequences has been reported (9). Some murine packaging cell lines have been shown to readily give rise to RCR which can be transferred to primates (18) and can be pathogenic (7). It is therefore appropriate to screen human packaging cell lines for the packaging of HERV sequences.
The consequences to the recipient of exposure to an RCR will depend ultimately upon the precise genetic structure of the virus. Although some primates exposed to RCR have not developed disease (5), others have (7). In vivo, RCR can give rise to chronic viremia and in the long term to neoplasia due to insertional mutagenesis next to host oncogenes (14). This risk has been highlighted by the development of lymphomas in monkeys exposed to an RCR. Tumors developed in 3 of 10 animals (7) and murine ERV (VL30) sequences were also detected in the tissues of these animals (25).
While there has been much concern about the safety of retrovirus vector packaging cell lines, particularly over the generation of RCR by recombination events between vector genomes and packaging plasmids, less attention has been paid to mobilization by ERV elements and to the possible generation of RCR by recombination of vector genomes with ERV sequences. In this study, we tested whether various families of murine and human ERV sequences are packaged into particles and, if so, whether they are transferred to and expressed in target cells. We quantified the packaged ERV sequences in comparison to the vector genomes designed to be packaged.
The mobilization of vector sequences by HERV proteins in human cells transduced with a retrovirus vector also has safety consequences. It is desirable that, after transduction of a target cell, no further vector replication occurs. If expressed vector sequences were to be repackaged by endogenous Gag proteins in target cells, further replicative cycles might occur, resulting in an increased risk of mobilization, insertional mutagenesis, and generation of RCR. Therefore, the packaging of MLV-based vector genomes by HERV core proteins was also tested.
MATERIALS AND METHODS
Cells.
Human and murine packaging cell lines FLY, FLYA4, and FLYA4L3 (previously named FLYA4LacZ3) (6) and AM12Lac25 (previously named GP+EAM12LacZ25) (11) have been described previously. FLYA4L3 and AM12Lac25 produce amphotropic retrovirus vectors encoding MFGnlsLacZ vector genomes. GH cells were kindly provided by Roswitha Löwer (Paul-Ehrlich Institute, Langen, Germany). GHLacZ and RDLacZ cells were produced by high-multiplicity transduction of the parental cell lines, GH and RD/TE671, respectively, with FLYA4L3 supernatant. RDLacZ-MPMV cells were obtained by infection of RDLacZ cells with cell-free Mason-Pfizer monkey virus. Other cells are as described previously (16, 22). β-Galactosidase (β-Gal) expression from the MFGnlsLacZ vector was determined by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining techniques (23). DNA and RNA were prepared from mink lung cells (Mv-1-Lu) 72 h after transduction at a high multiplicity of infection with a filtered (0.45-μm-pore-size filter) packaging cell line supernatant in the presence of 8 μg of Polybrene per ml. Replication-competent virus was not detected in the supernatant of the packaging cell lines, as determined by the inability of the supernatant to rescue an MLV vector from TELacZ cells (6).
Sucrose density gradients.
The overnight culture supernatant or cell lysate from 3 × 106 to 4 × 106 cells (at confluency) was fractionated by centrifugation through 40-ml linear sucrose density gradients (20 to 65%) at 100,000 × g and 4°C for 16 h in an SW28 rotor (Beckman). Cell preparations were lysed by three rapid freeze-thaw cycles in 1 ml of virus preparation buffer (50 mM Tris-HCl [pH 7.9], 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 20% glycerol), and cell debris was removed by centrifugation at 17,500 × g and 4°C for 20 min prior to loading. Supernatants were filtered (0.45-μm-pore-size filter). The gradients were collected in 1-ml serial fractions and analyzed for reverse transcriptase (RT) activity and viral genomes by RT PCR.
RT assays.
The RT activity of gradient fractions was determined with a PCR-based RT assay as previously described (15).
RT-PCR.
RNA was purified from gradient fractions as previously described (21). DNA-free cellular mRNA was purified with RNAsolB (Biogenesis) and a PolyAtract kit (Promega) following the manufacturers’ instructions. The purity of the RNA was confirmed by PCR analysis of RNA which had not been subjected to reverse transcription, and the quality of the RNA was confirmed by amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) sequences. Oligonucleotide primer pairs designed to amplify conserved Pol regions of retroviruses and control sequences were as follows. The pan-retrovirus (20) 5′ primer was TGGAAAGTGYTRCCMCARGG, and the 3′ primer was GGMGGCCAGSAKGTCATCCAYGTA. The type B or D virus (12) 5′ primer was TCCCCTTGGAATACTCCTGTTTTYGT, and the 3′ primer was CATTCCTTGTGGTAAAACTTTCCAYTG. The type C virus 5′ primer was ACCAAIGAYTAYMGRCCWGTICARGA, and the 3′ primer was TTGAAMCCYTGKGGIARYCKIGTCCA. The RTVL-H (26) 5′ primer was CCTCACCCTGATCACRYTTG, and the 3′ primer was GAATTATGTCTGACAGAAGGG. The VL30 5′ primer was GTTGACATCTGCAGAGAAAGACC, and the 3′ primer was TCTGAGGTCTGTACACACAATGG. The β-Gal 5′ primer was CTCTGGCTCACAGTACGCGTAG, and the 3′ primer was CCATCAATCCGGTAGGTTTTCCG. The GAPDH 5′ primer was TGGATATTGTTGCCATCAATGACC, and the 3′ primer was GATGGCATGGACTGTGGTCATG. The type C virus-specific primers were designed to conserve motifs of type C retrovirus pol genes.
Following reverse transcription of RNA with the 3′ primers, PCR amplification was performed on a Omnigene thermal cycler (Hybaid) with the following conditions for all reactions except for RTVL-H for which a 1-min extension was used: 92°C for 4 min, 1 cycle; 94°C for 30 s, annealing for 45 s, and 72°C for 30 s, 30 cycles; and 72°C for 5 min, 1 cycle. Annealing temperatures were as follows: pan-retrovirus, 45°C; type B or D virus, 50°C; type C virus, 50°C; RTVL-H, 52°C; VL30, 62°C; β-Gal, 63°C; and GAPDH, 63°C. PCR products were visualized by ethidium bromide-agarose gel electrophoresis. Following T-tailed cloning into the pBluescript KS− vector EcoRV site, automated sequencing was performed on several clones and products were analyzed by a FASTA search of the GenBank and EMBL databases.
RT PCR sensitivity.
Clones of KS− containing ERV sequences were linearized with NotI or SalI to prevent runoff transcripts, and DNA-free RNA was produced by T3/T7 in vitro transcription following the manufacturer’s instructions (Riboprobe kit; Promega). The quality of the RNA was confirmed by gel electrophoresis and quantification against brome mosaic virus RNA (Promega) by ethidium bromide-ammonium acetate gel spotting. Known amounts of the transcribed RNA were titrated into the RT PCR mixtures, enabling the sensitivity of the assays to be determined. The combination of these data, along with the dilution to which test samples could be taken and still provide a positive RT PCR signal, allowed calculation of the concentration of RNA in the test samples.
RESULTS
ERV expression in murine and human cell lines.
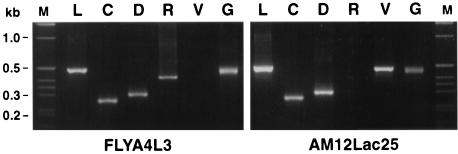
Human cells not only contain several classes of ERV sequence but also express several as RNA transcripts. Table 1 lists the types of ERV that we found to be transcribed by RT PCR in a number of human cell lines as well as murine NIH 3T3 cells. We examined in more detail FLYA4L3 packaging cells (derived from HT1080 cells) and AM12Lac25 packaging cells (derived from murine NIH 3T3 cells), which express the MFGnlsLacZ vector and produce amphotropic LacZ particles. ERV and MFGnlsLacZ transcripts were detected in both AM12Lac25 and FLYA4L3 cells at levels comparable to those of the GAPDH housekeeping gene (Fig. 1). RT PCR products were cloned and sequenced. Sequences were identified by nucleotide sequence analysis and comparison with the GenBank and EMBL data bases (Table 2). The results indicated that FLYA4L3 cells, like AM12Lac25 cells, express significant levels of ERV RNA and confirm the need to test for packaging of HERV sequences in viral vector particles released by these cells.
TABLE 1.
ERV RNA expression in human and murine cells
| Cells | ERV RNA expressiona:
|
|||
|---|---|---|---|---|
| Type B or D virus | Type C virus | RTVL-H | VL30 | |
| RD | + | + | + | − |
| HT1080 | + | + | + | − |
| HeLa | + | + | + | − |
| GH | + | + | + | − |
| T47D | + | + | + | − |
| PBMC | + | + | + | − |
| NIH 3T3 | + | + | − | + |
+, expression; −, no expression. PCR analysis with pan-retrovirus primers was not applicable due to nonspecific amplification of ribosomal sequences and therefore is not presented.
FIG. 1.
ERV expression in FLYA4L3 and AM12Lac25 cells. Lanes: M, molecular size markers; L, β-Gal; C, type C virus; D, type B or D virus; R, RTVL-H; V, VL30; G, GAPDH.
TABLE 2.
Identity of packaging cell line RT PCR products
| Cell line | RT PCR for: | Product size (bp) | Database sequencea | % Similarity to database |
|---|---|---|---|---|
| FLYA4L3 | Type B or D virus | 295 | HERV-K10 | 90 |
| Type B or D virusb | 298 | HERV-K10 | 72 | |
| Type B or D virusb | 297 | HERV-K10 | 75 | |
| Type C virusc | 263 | HERV | 97 | |
| RTVL-H | 411 | RTVL-H2 | 94 | |
| Pan-retrovirus | 135 | Human RT | 66 | |
| AM12Lac25 | VL30 | 484 | VL30 | 99 |
| Type B or D virus | 298 | Murine IAP | 95 |
GenBank database accession numbers: HERV-K10, gb_pr:m14123; HERV, gb_pr:m74509; RTVL-H2, gb_pr:m18048; human RT, gb_pr:m25767; VL30, gb_ro:m21123; and murine IAP, gb_ro:m17551.
Sequences correspond to the 70A and 70B HERV-K10 families (21).
RNA transcripts from the CeB plasmid were also detected at approximately the same frequency as HERV transcripts.
RT PCR sensitivities.
ERV and β-Gal RNAs were produced by in vitro transcription and quantified. Known amounts of RNA were diluted into RT PCR mixtures, and the sensitivity of the reactions was determined (Table 3). The possibility that template DNA present in the transcription reactions contaminated the RT PCRs was excluded, as amplifications performed in the absence of RT did not yield any products.
TABLE 3.
Presence and quantification of retroviral sequences in gradient-purified virus particlesa
| Sequence | Sensitivity (fg)b | AM12Lac25 cell line
|
FLYA4L3 cell line
|
||
|---|---|---|---|---|---|
| Peak RNA level (fg) | LacZ/ERV ratio | Peak RNA level (fg) | LacZ/ERV ratio | ||
| LacZ | 527 | 6.3 × 103 | NA | 171 × 103 | NA |
| VL30 | 212 | 848 | 7.2:1 | NA | NA |
| Type B or D virus (murine) | 61 | <61 | >62:1 | NA | NA |
| Pan-retrovirus | 1.59 | <1.59 | >1,100:1 | <1.59 | >29,000:1 |
| Type C virus | 4.76 | <4.76 | >700:1 | 4.76 | 19,000:1 |
| RTVL-H | 160 | NA | NA | <160 | >880:1 |
| HERV-K10 family | |||||
| 90 | 175 | NA | NA | <175 | >580:1 |
| 70A | 87 | NA | NA | <87 | >1,200:1 |
| 70B | 160 | NA | NA | <160 | >650:1 |
NA, not applicable.
Minimum input of standard RNA which resulted in a positive RT PCR signal.
Packaging of ERV by packaging cell lines.
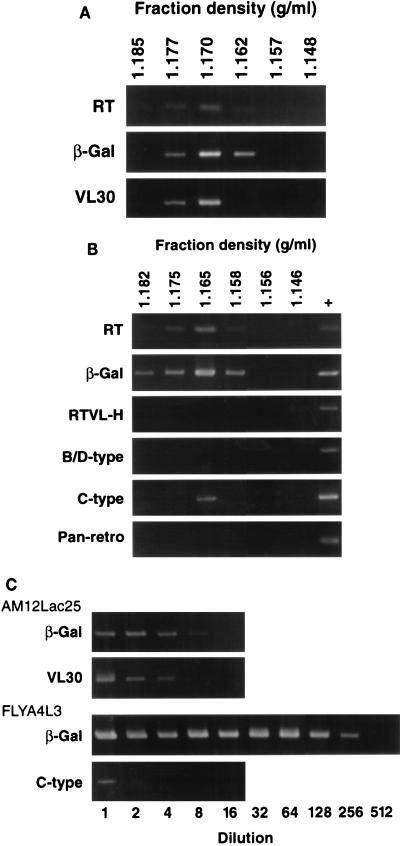
Virus produced by AM12Lac25 cells was purified on sucrose gradients. As shown in Fig. 2A, RT activity and β-Gal vector RNA banded at a density of 1.170 g/ml, appropriate for retrovirus particles. In addition, significant levels of packaged ERV (VL30) RNA were also detected in these fractions. RT PCRs with pan-retrovirus, type C virus, and type B or D virus primers were negative (data not shown).
FIG. 2.
RT PCR for RT activity and MFGnlsLacZ (β-Gal) or ERV RNA in sucrose gradient fractions from AM12Lac25 and FLYA4L3 cells. Positive controls (+) were 10−4 U of Moloney MLV RT (Gibco) for RT activity; FLYA4L3 total cell RNA for detection of β-Gal, RTVL-H, type B or D virus, or type C virus RNAs; and in vitro-transcribed human RT for pan-retrovirus (Pan-retro) RNAs (see Table 1). (A) AM12Lac25 supernatants. (B) FLYA4L3 supernatants. (C) Titration of peak fractions identified in panels A and B.
Analysis of FLYA4L3 supernatants revealed higher levels of packaged RT activity and β-Gal RNA than in AM12Lac25 supernatants (Fig. 2B and C). This result was expected, as the infectious titer of FLYA4L3 supernatants (4.2 × 106 IU/ml) was found to be approximately 30-fold higher than that of AM12Lac25 supernatants (1.5 × 105 IU/ml). Despite the increased virus titer, we were unable to detect the presence of viral sequences in any FLYA4L3 gradient fractions using pan-retrovirus, type B or D virus, or RTVL-H primers. In addition, supernatants from parental “empty” FLYA4 cells, which produce virus particles with no vector genome, did not contain detectable levels of HERV sequences (data not shown), ruling out the possibility that packaging of the lacZ genome at a high titer was competing HERV RNA from virus particles. However, RT PCR with primers for type C virus sequences gave a weak positive signal. Following sequencing, the product was identified as MLV and represents packaging RNA transcribed from an MLV Gag-Pol expression plasmid. This result is consistent with the observation that LacZ particles produced by human packaging cells TELCeb6/AF7 (6) and FLYA4L3 (23a) can transfer Gag-Pol function to target cells. Packaging of endogenous human type C virus sequences was not detected. The RT level in FLYA4 cells was very similar to that in FLYA4L3 cells, indicating that similar levels of virus particles were produced (results not shown). These results indicate that the packaging of HERV sequences by FLY cell lines, if it occurred at all, occurred at a frequency much lower than that observed for VL30 sequences in murine cells.
The concentrations of β-Gal and ERV RNAs in the peak gradient fractions of FLYA4L3 and AM12Lac25 cells were estimated by titration (Fig. 2C). Table 3 shows the estimated RNA concentrations and the vector/ERV molar ratios, indicating a much improved specificity of packaging of FLYA4L3 cells over AM12Lac25 cells.
Transfer of ERV to target cells.
DNA and RNA extracted from mink Mv-1-Lu cells which had been exposed to vector supernatants were analyzed in order to confirm previous reports that VL30 can be transferred from murine packaging cells to target cells (2, 8, 19) and to test if any transfer of HERV from human packaging cells to target cells can be detected. Mv-1-Lu cells, which had been transduced with AM12Lac25 supernatant and were more than 90% LacZ positive, contained and expressed VL30 sequences, as demonstrated by PCR analyses with the same primers and conditions as those described above. This observation confirmed that at least some types of ERV can be mobilized by vector virions and expressed in target cells. In contrast, exposure of Mv-1-Lu cells to supernatants from either FLYA4L3 or empty FLYA4 cells resulted in no detectable transfer of RTVL-H or type B or D virus sequences, consistent with the absence of detectable packaging of these sequences.
Interaction of MLV vectors with HERV Gag proteins.
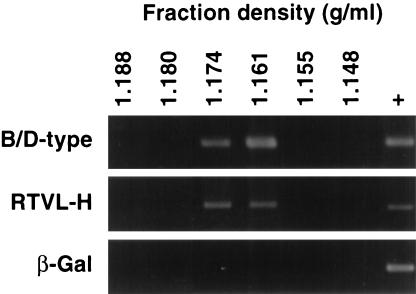
GH cells were transduced with the MFGnlsLacZ retroviral vector. β-Gal expression in >90% of the cells was confirmed by X-Gal staining (data not shown). RT PCR of RNA fractionated on sucrose gradients identified the presence of cell-associated virus particles containing type B or D virus (HERV-K-related) and RTVL-H sequences. However, no packaging of β-Gal vector transcripts into these virus particles was detected, despite their expression in the cells (Fig. 3). Estimation of the concentration of ERV RNA indicated that the β-Gal/HERV ratios were <1:2 for RTVL-H sequences and <1:7 for HERV-K10-related sequences.
FIG. 3.
RT PCR for β-Gal and ERV RNAs in sucrose gradient fractions from GH cell lysates. Positive controls (+) represent amplifications from GH total cell RNA.
DISCUSSION
This study addresses two important issues of safety for retroviral gene therapy. First, we compared a human packaging cell line (FLYA4L3) with a murine cell line (AM12Lac25) for packaging of ERV sequences into virions. The delivery of ERV sequences and their subsequent integration are themselves unwanted and potentially pathogenic events (25). Copackaging of ERV and vector sequences could lead to recombination events between the transcripts and the possible generation of RCR. Second, we investigated the interaction of HERV with retroviral vectors after transduction of target cells. If retroviral vectors could be repackaged by HERV Gag proteins in human target cells, mobilization of the vectors might occur, increasing the risk of less specific cell targeting, insertional mutagenesis of host genes, and generation of RCR.
The human packaging cell line FLYA4L3 expresses high levels of MLV Gag-Pol proteins, producing virions at titers 10- to 100-fold higher than those obtained with murine packaging cell lines, such as AM12LacZ25. Packaging of murine VL30 sequences was readily detected in AM12Lac25 cells. In comparison, although RT PCR confirmed the presence of many classes of HERV mRNA in FLYA4L3 cells, we were unable to detect any evidence of packaging of these sequences into virions produced by these cells. The detection of VL30 packaging has been reported (2, 8, 19); the packaging sequence of VL30 elements, although divergent from the MLV packaging sequence, is known to be compatible with MLV Gag proteins (24). Although little is known of the packaging specificity of the various HERV sequences, the RNA packaging signals of the HERVs investigated in this study may be more divergent from MLV than that of murine ERV. This possibility could result in a reduced efficiency of the interaction of HERV RNA transcripts with the MLV Gag proteins expressed in the packaging cells and hence in the absence of HERV transcripts in the virions. In a more stringent test of HERV packaging, we also tested the empty FLYA4 packaging cell line, as FLYA4L3 cells express high levels of MFGnlsLacZ retroviral vector RNA, which will efficiently compete with HERV transcripts for packaging into MLV virions. Significantly, even in the absence of such competing sequences, HERV packaging was undetectable.
The concentration of RNA packaged into vector particles was estimated by RT PCR titration with RNA templates produced by in vitro transcription. We calculated that the peak AM12Lac25 fraction contained 6.3 pg of β-Gal RNA and 850 fg of VL30 RNA (Table 3). Converted into molar terms, these values represent a β-Gal/VL30 ratio of approximately 7:1. The detection of significant VL30 packaging by murine packaging cells and the transfer of these sequences to target cells are in agreement with previously published results (2, 8, 18, 19). The peak FLYA4L3 fraction contained 171 pg of β-Gal RNA and undetectable levels of HERV transcripts. Thus, according to the sensitivity of the assays, for every virion containing β-Gal RNA, fewer than 1 in 30,000 virions carried sequences identified with pan-retrovirus primers, fewer than 1 in 600 to 1 in 1,200 carried type B or D virus (HERV-K10-related) sequences, and fewer than 1 in 900 carried RTVL-H sequences (Table 3). HERV sequences were not detected in cells exposed at a high multiplicity to FLYA4L3 or empty FLYA4 supernatants. These results indicate that human packaging cell lines produce retroviral vectors less contaminated with ERV sequences and therefore less likely to give rise to unwanted ERV mobilization and generation of RCR due to recombination between ERV and vector genomes than murine packaging cell lines. The possibility exists that other, as-yet-unknown HERV families not detected by our RT PCR methods interact with MLV Gag proteins and are packaged into virions. It will be important to determine whether such HERV families exist.
MLV sequences were detected with type C virus primers in the supernatants of FLYA4L3 cells. As the supernatants of these cells did not contain detectable levels of RCR, the sequences were most likely derived from the CeB Gag-Pol expression plasmid. It is possible that cells transduced by contaminated particles can express MLV proteins (6). The 1:19,000 ratio of packaging is consistent with that observed for the transfer of Gag-Pol function to target cells by FLYA4L3 supernatants (6, 23a).
Once target cells are transduced with a retroviral vector, it is desirable that its replication be limited to a single integration event, as all cycles of retroviral replication carry a risk of recombination events and of insertional mutagenesis. The second safety aspect investigated, therefore, was that of vector mobilization by HERV Gag proteins. Associated with HERV Gag expression is the presence of intracellular virus particles. In vivo, the presence of high levels of HERV particles is a rare phenomenon. A notable exception is the placenta trophoblast, which expresses high levels of type B or D virus particles packaging HERV-K10-related sequences (21). We therefore examined GH teratocarcinoma cells which, like the trophoblast produce HERV-K10-derived particles, for MLV vector packaging. Despite the presence of virus particles carrying HERV-K and RTVL-H sequences, we did not detect the packaging of MLV vector transcripts into virions, presumably due to an inability of the Gag proteins to interact efficiently with MLV RNA. These results suggest that vector mobilization by HERV Gag proteins is unlikely to present a significant safety problem. Furthermore, RDLacZ-MPMV cells producing type D virions did not cross-package MLV vector sequences into supernatant particles (data not shown).
In summary, human packaging cell lines such as FLY appear to be a promising packaging system possessing some significant advantages over murine-based systems. Not only are serum-resistant particles produced (6), but also they are less contaminated by ERV than particles from murine packaging cells. In addition, MLV-derived vectors packaged by FLY or GP+EAM12 cells and transduced into target cells do not interact with HERV Gag proteins, such as those expressed in GH cells, and are not recovered by replication-competent type D virus.
ACKNOWLEDGMENTS
We thank Mark Boyd, David Wilkinson, and Mary Collins for helpful discussions and David Griffiths for the design of the type C virus-specific primers.
This work was supported by the Health and Safety Executive and the Medical Research Council.
REFERENCES
- 1.Anderson R A, Kato S, Camerini-Otero R D. A pattern of partially homologous recombination in mouse L cells. Proc Natl Acad Sci USA. 1984;81:206–210. doi: 10.1073/pnas.81.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty A K, Zink M A, Hodgson C P. Transmission of endogenous VL30 retrotransposons by helper cells used in gene therapy. Cancer Gene Ther. 1994;1:113–118. [PubMed] [Google Scholar]
- 3.Chong H, Vile R G. Replication-competent retrovirus produced by a ‘split-function’ third generation amphotropic packaging cell line. Gene Ther. 1996;3:624–629. [PubMed] [Google Scholar]
- 4.Cordonnier A, Casella J F, Heidmann T. Isolation of novel human endogenous retrovirus-like elements with foamy virus-related pol sequence. J Virol. 1995;69:5890–5897. doi: 10.1128/jvi.69.9.5890-5897.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornetta K, Moen R C, Culver K, Morgan R A, McLachlin J R, Sturm S, Selegue J, London W, Blaese R M, Anderson W F. Amphotropic murine leukemia retrovirus is not an acute pathogen for primates. Hum Gene Ther. 1990;1:15–30. doi: 10.1089/hum.1990.1.1-15. [DOI] [PubMed] [Google Scholar]
- 6.Cosset F-L, Takeuchi Y, Battini J-L, Weiss R A, Collins M K L. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J Virol. 1995;69:7430–7436. doi: 10.1128/jvi.69.12.7430-7436.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donahue R E, Kessler S W, Bodine D, McDonagh K, Dunbar C, Goodman S, Agricola B, Byrne E, Raffeld M, Moen R, Bacher J, Zsebo K M, Nienhuis A W. Helper virus induced T cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J Exp Med. 1992;176:1125–1135. doi: 10.1084/jem.176.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzoglou M, Hodgson C P, Mularo F, Hanson R W. Efficient packaging of a specific VL30 retroelement by psi 2 cells which produce MoMLV recombinant retroviruses. Hum Gene Ther. 1990;1:385–397. doi: 10.1089/hum.1990.1.4-385. [DOI] [PubMed] [Google Scholar]
- 9.Itin A, Keshet E. Apparent recombinants between virus-like (VL30) and murine leukemia virus-related sequences in mouse DNA. J Virol. 1983;47:178–184. doi: 10.1128/jvi.47.1.178-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy J A. Xenotropic viruses: murine leukemia viruses associated with NIH Swiss, NZB and other mouse strains. Science. 1973;182:1151–1153. doi: 10.1126/science.182.4117.1151. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz D, Goff S, Bank A. Construction and use of a safe and efficient amphotropic packaging cell line. Virology. 1988;167:400–406. [PubMed] [Google Scholar]
- 12.Medstrand P, Lindeskog M, Blomberg J. Expression of human endogenous retroviral sequences in peripheral blood mononuclear cells of healthy individuals. J Gen Virol. 1992;73:2463–2466. doi: 10.1099/0022-1317-73-9-2463. [DOI] [PubMed] [Google Scholar]
- 13.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moolten F L, Cupples L A. A model for predicting the risk of cancer consequent to retroviral gene therapy. Hum Gene Ther. 1992;3:479–486. doi: 10.1089/hum.1992.3.5-479. [DOI] [PubMed] [Google Scholar]
- 15.Patience C, Simpson G R, Colletta A A, Welch H M, Weiss R A, Boyd M T. Human endogenous retrovirus expression and reverse transcriptase activity in the T47D mammary carcinoma cell line. J Virol. 1996;70:2654–2657. doi: 10.1128/jvi.70.4.2654-2657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patience C, Takeuchi Y, Weiss R A. Infection of human cells by an endogenous retrovirus of pigs. Nat Med. 1997;3:282–286. doi: 10.1038/nm0397-282. [DOI] [PubMed] [Google Scholar]
- 17.Patience C, Wilkinson D A, Weiss R A. Our retroviral heritage. Trends Genet. 1997;13:116–120. doi: 10.1016/s0168-9525(97)01057-3. [DOI] [PubMed] [Google Scholar]
- 18.Purcell D F, Broscius C M, Vanin E F, Buckler C E, Nienhuis A W, Martin M A. An array of murine leukemia virus-related elements is transmitted and expressed in a primate recipient of retroviral gene transfer. J Virol. 1996;70:887–897. doi: 10.1128/jvi.70.2.887-897.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scadden D T, Fuller B, Cunningham J M. Human cells infected with retrovirus vectors acquire an endogenous murine provirus. J Virol. 1990;64:424–427. doi: 10.1128/jvi.64.1.424-427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shih A, Misra R, Rush M G. Detection of multiple, novel reverse transcriptase coding sequences in human nucleic acids: relation to primate retroviruses. J Virol. 1989;63:64–75. doi: 10.1128/jvi.63.1.64-75.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson G R, Patience C, Löwer R, Tönjes R R, Moore H D, Weiss R A, Boyd M T. Endogenous D-type (HERV-K) related sequences are packaged into retroviral particles in the placenta and possess open reading frames for reverse transcriptase. Virology. 1996;222:451–456. doi: 10.1006/viro.1996.0443. [DOI] [PubMed] [Google Scholar]
- 22.Sommerfelt M A, Weiss R A. Receptor interference groups of 20 retroviruses plating on human cells. Virology. 1990;176:58–69. doi: 10.1016/0042-6822(90)90230-o. [DOI] [PubMed] [Google Scholar]
- 23.Tailor C S, Takeuchi Y, O’Hara B, Johann S V, Weiss R A, Collins M K L. Mutation of amino acids within the gibbon ape leukemia virus (GALV) receptor differentially affects feline leukemia virus subgroup B, simian sarcoma-associated virus, and GALV infections. J Virol. 1993;67:6737–6741. doi: 10.1128/jvi.67.11.6737-6741.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Takeuchi, Y. Unpublished data.
- 24.Torrent C, Gabus C, Darlix J L. A small and efficient dimerization/packaging signal of rat VL30 RNA and its use in murine leukemia virus-VL30-derived vectors for gene transfer. J Virol. 1994;68:661–667. doi: 10.1128/jvi.68.2.661-667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanin E F, Kaloss M, Broscius C, Nienhuis A W. Characterization of replication-competent retroviruses from nonhuman primates with virus-induced T-cell lymphomas and observations regarding the mechanism of oncogenesis. J Virol. 1994;68:4241–4250. doi: 10.1128/jvi.68.7.4241-4250.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilkinson D A, Goodchild N L, Saxton T M, Wood S, Mager D L. Evidence for a functional subclass of the RTVL-H family of human endogenous retrovirus-like sequences. J Virol. 1993;67:2981–2989. doi: 10.1128/jvi.67.6.2981-2989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson D A, Mager D L, Leong J-A C. Human endogenous retroviruses. In: Levy J A, editor. The Retroviridae. Vol. 3. New York, N.Y: Plenum Press; 1994. pp. 465–535. [Google Scholar]