Abstract
BACKGROUND
Hemangiopericytoma (HPC) is a rare malignancy accounting for 0.4% of intracranial tumors. HPCs are characterized by local aggressiveness, high rates of recurrence, and a tendency to metastasize to extracranial sites. These features make management of HPCs challenging, often requiring a combination of radical resection and radiation. Given their rarity, optimal treatment algorithms remain undefined.
OBSERVATIONS
The authors report a series of four patients who underwent resection of intracranial HPC. Mean age at presentation was 49.3 years. Three patients had reoperation for progression of residual tumor, and one patient was surgically retreated for recurrence. One patient received adjuvant radiotherapy following initial resection, and three patients received adjuvant radiotherapy following resection of recurrent or residual disease. There was one death in the series. Average progression-free survival and overall survival following the index procedure were 32.8 and 82 months, respectively. Progression occurred locally in all patients, with metastatic recurrence in one patient.
LESSONS
The current gold-standard treatment for intracranial HPC consists of gross-total resection followed by radiation therapy. This approach allows satisfactory local control; however, given the tendency for these tumors to recur either locally or distally within or outside of the central nervous system, there is a need for salvage therapies to improve long-term outcomes for patients.
Keywords: hemangiopericytoma, solitary fibrous tumor, recurrence, radiotherapy
ABBREVIATIONS: AP = anteroposterior, CC = craniocaudal, CNS = central nervous system, CT = computed tomography, EEA = endoscopic endonasal approach, GTR = gross-total resection, HPC = hemangiopericytoma, ML = mediolateral, MRI = magnetic resonance imaging, NTR = near-total resection, PET = positron emission tomography, STR = subtotal resection, WHO = World Health Organization
Intracranial hemangiopericytoma (HPC)/solitary fibrous tumor is a rare malignancy of the mesenchymal lineage that accounts for an estimated 0.4% of tumors affecting the central nervous system (CNS).1 HPC is believed to originate from Zimmerman pericytes, which function in forming and supporting capillary walls.2 These tumors are characterized by local aggressiveness, a high rate of both local and distant recurrence within the CNS, and a tendency to metastasize to extracranial sites.2,3 This propensity toward progression makes the management of HPCs challenging. Recurrence rates up to 30% have been reported following gross-total resection (GTR) and adjuvant radiotherapy.4 Although they arise in arachnoid locations similar to meningiomas and present with similarities both clinically and radiographically, HPCs are notably more aggressive and can only be definitively diagnosed using histopathological or genomic testing.5
Management of HPC typically involves aggressive resection followed by adjuvant radiotherapy.6 However, cerebral parenchymal involvement, tumor vascularity, dural sinus involvement, and critical regional anatomy present obstacles to radical and complete resection, highlighting the role for radiotherapy to control residual tumor.2 Despite the appearance of initial success following the resection of HPCs, recurrences can appear more than a decade following treatment, making long-term follow-up critical to the management of these tumors and an important factor in survival.3,7 Mean survival following initial diagnosis of HPC is 84 months.8
We detail our experience managing patients with giant intracranial HPCs who underwent aggressive resection followed by adjuvant radiotherapy. A retrospective single-center review was conducted analyzing cases from 2014 to 2023. Four consecutive patients with intracranial HPC were identified in this case series, and their electronic medical records were reviewed (Table 1). This study was conducted in accordance with the CARE guidelines.9 We aimed to describe the treatment course and outcomes and propose a general management strategy. We also reviewed the literature and discuss the strengths and drawbacks of other reported treatment protocols for the management of HPC.
TABLE 1.
Clinical, radiographic, and operative variables
| Case No. | Age (yrs)/Sex | Preop Findings | Tumor size (cm): CC, ML, AP | Location | Approach | Extent of Resection | Pathology | Time to Progression (mos) | Postop Findings | Radiation Therapy | FU from Initial Resection (mos) | Recurrence at Last FU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
54/M |
Altered mental status, lt visual field loss |
8.0, 7.3, 7.2 |
Ant fossa |
Stage 1: EEA, stage 2: lt-sided orbitozygomatic craniotomy |
STR |
WHO grade III hemangiopericytoma |
7 |
PE, blood loss anemia, hypothyroidism |
None |
36; stable lt-sided vision loss, patient died 90 mos after initial procedure |
No |
| Seizure,* residual on MRI* |
3.1, 3.5, 3.5† |
Ant fossa |
Stage 1‡: EEA, stage 2‡: rt-sided frontal orbital craniotomy |
NTR |
WHO grade III hemangiopericytoma |
NA |
Seizure |
50.4 Gy fractionated radiation (30 fractions) starting 6 wks postop |
||||
| 2 |
44/M |
Severe headaches, blurry vision, papilledema |
7.8, 5.0, 5.9 |
Occipital |
Rt suboccipital/occipital craniotomy |
GTR |
WHO grade II hemangiopericytoma |
84 |
NA |
None |
96; neurologically intact |
No |
| Lt homonymous hemianopsia* |
5.5, 3.7, 4.8† |
Occipital |
Rt suboccipital/occipital craniotomy‡ |
GTR |
WHO grade III hemangiopericytoma |
NA |
Pseudomeningocele, epidural abscess, PE, resolution of homonymous hemianopsia |
60 Gy fractionated radiation (30 fractions) starting 4 mos postop |
||||
| 3 |
35/M |
Hearing loss, dysphagia, seizure |
4.1, 5.4, 5.5 |
Pst fossa |
Translabyrinthine/ transmastoid approach |
STR |
WHO grade II hemangiopericytoma |
1 |
Dysphagia improved, persistent hearing loss |
None |
74; no evidence of intracranial or spine tumor progression |
No |
| Progression of residual on MRI* |
3.5, 2.4, 4.1† |
Pst fossa |
Translabyrinthine/ retrosigmoid‡ |
GTR |
WHO grade III hemangiopericytoma |
1 intracranially; 48 to spine |
Pseudomeningocele |
60 Gy fractionated radiation starting 2 mos postop |
||||
| Lower-extremity weakness, numbness, & tingling* |
5.5, 5.4, 4.3† |
Paraspinal (T10) |
Paraspinal mass resection & T8–12 fusion‡ |
GTR |
WHO grade III hemangiopericytoma |
NA |
Improved bilat lower-extremity numbness & strength |
30 Gy fractionated radiation starting 1 mo postop |
||||
| 4 | 60/M | Headaches, dizziness, syncope, cognitive decline, memory loss, ventriculomegaly |
5.4, 3.3, 3.9 |
Pineal region (supra- & infratentorial) |
Stage 1: supracerebellar infratentorial, stage 2: occipital interhemispheric |
STR |
WHO grade II hemangiopericytoma |
39 |
Improved alertness & balance |
59.4 Gy fractionated radiation starting 3 mos postop |
68; declining performance status | Yes |
| Asymptomatic | 2.8, 1.9, 2.0† | Pineal/thalamus region (supratentorial) | Occipital interhemispheric‡ | STR | WHO grade II hemangiopericytoma | 6 | New rt foot drop, steadily improving | 25 Gy fractionated radiation starting 1 mo postop |
ant = anterior; FU = follow-up; NA = not applicable; pst = posterior.
* Preoperative symptoms/findings associated with progression of residual tumor or recurrence of tumor.
† Size at recurrence.
‡ Surgery due to progression of residual tumor or recurrence of tumor.
Illustrative Cases
Case 1
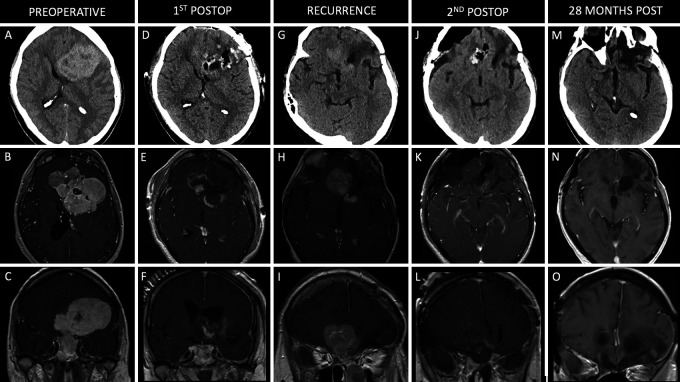
A 54-year-old male with a medical history including myocardial infarction, pulmonary embolism (PE), stage 3 chronic kidney disease, and seizures presented with an altered mental status, as well as bilateral visual field loss that had progressed to left-eye blindness over several months. Computed tomography (CT) scanning of the head showed a large anterior skull base mass involving the sinonasal cavities with significant mass effect. Magnetic resonance imaging (MRI) revealed a large enhancing mass centered along the anterior cranial fossa measuring approximately 8.0 × 7.3 × 7.2 cm (craniocaudal [CC] × mediolateral [ML] × anteroposterior [AP]; Fig. 1A–C). A multistage approach was planned, consisting of an endoscopic endonasal approach (EEA) to decompress the optic nerves bilaterally and to debulk and devascularize the tumor, followed by a left-sided orbitozygomatic craniotomy to complete the resection. Biopsy from the EEA confirmed a World Health Organization (WHO) grade III HPC. The transcranial surgery was performed 5 days later, resulting in subtotal resection (STR), given the proximity of the tumor to eloquent structures and functional parenchyma. The postoperative course was complicated by a PE and anemia. Despite confirming residual tumor on postoperative imaging, given the recent symptomatic PE, a 3-month period on anticoagulation therapy was recommended before considering reoperation. The patient ultimately elected not to proceed with surgery for removal of the residual tumor. Seven months after the initial procedure, he presented with a seizure and significant progression of the residual component, measuring approximately 3.1 × 3.5 × 3.5 cm (Fig. 1G–I). The patient returned to the operating room for resection of the residual tumor via an EEA followed by a right-sided cranio-orbital approach. Final pathology confirmed an anaplastic HPC, WHO grade III. Near-total resection (NTR) was achieved with a small amount of residual tumor along the inferior margin of the left frontal horn of the ventricle, because the risk of injuring a perforating vessel to the internal capsule was believed to be prohibitive (Fig. 1J–L). The patient tolerated the second surgery well. He then completed radiation treatment of 50.4 Gy in 30 fractions, which began 6 weeks after surgery. The patient remained blind in his left eye and had persistent decreased vision in the right eye. Surveillance MRI performed 28 months after the second surgery (36 months after initial procedure; Fig. 1M–O) showed tumor stability with no signs of progression of the residual component. The patient was lost to follow-up after 12 months and ultimately died of unknown causes 90 months after his initial procedure.
FIG. 1.
Case 1. Preoperative axial noncontrast CT (A) and axial (B) and coronal (C) contrast-enhanced T1-weighted MRI showing a large, enhancing anterior skull base lesion. Postoperative axial noncontrast CT (D) and axial (E) and coronal (F) contrast-enhanced T1-weighted MRI following staged EEA and left-sided orbitozygomatic craniotomy with STR of the lesion. Postoperative axial noncontrast CT (G) and axial (H) and coronal (I) contrast-enhanced T1-weighted MRI showing recurrence of the anterior skull base tumor. Axial noncontrast CT (J) and axial (K) and coronal (L) contrast-enhanced T1-weighted MRI following combined EEA followed by a right-sided frontal orbital temporal craniotomy for NTR of the lesion. Axial noncontrast CT (M) and axial (N) and coronal (O) contrast-enhanced T1-weighted MRI 28 months after the second surgery (36 months after the index surgery).
Case 2
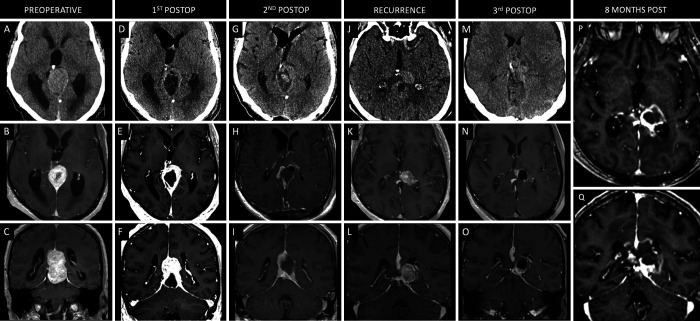
A 44-year-old male with no past medical history presented with several weeks of severe headaches and bilateral blurry vision. He underwent ophthalmological evaluation, which showed papilledema. Neuroimaging revealed a large extra-axial mass in the right occipital region measuring 7.8 × 5.0 × 5.9 cm (CC × ML × AP), with invasion of the transverse sinus and extension into the posterior fossa (Fig. 2A–C). A preoperative angiogram revealed that the transverse sinus was obliterated on the right side. A right occipital/paramedian suboccipital approach was selected. Given tumor invasion into the right transverse sinus, it was ligated intraoperatively to enable GTR. An intraoperative frozen section demonstrated a solitary fibrous tumor. The patient tolerated the procedure well, and final pathology confirmed a WHO grade II HPC. The patient’s headaches resolved, and his vision improved. Surveillance MRI 36 months postoperatively showed no recurrence. The patient was lost to follow-up and presented 76 months postoperatively with a mild left homonymous hemianopsia. Neuroimaging revealed tumor recurrence measuring 5.5 × 3.7 × 4.8 cm (Fig. 2G–I). He was taken back to the operating room for GTR using the same approach (Fig. 2J–L). Pathology confirmed WHO grade III anaplastic HPC. The patient returned to the hospital 11 days after discharge with dizziness, nausea, and headaches and was found to have a large saddle PE with acute cor pulmonale, which was managed via endovascular thrombectomy and inferior vena cava filter placement and required a 19-day hospital stay. Following discharge, the patient returned to the hospital 21 days later in sepsis and was found to have a pseudomeningocele and a Klebsiella aerogenes wound infection, as well as an epidural abscess that required a return to the operating room for washout and bone flap removal. He completed a course of cefepime and metronidazole and underwent titanium mesh cranioplasty. He ultimately recovered well with resolution of his left homonymous hemianopsia and underwent radiation therapy consisting of 60 Gy in 30 fractions 4 months postoperatively. MRI performed 12 months after the second surgery for tumor resection (96 months after the initial procedure) showed no evidence of tumor progression.
FIG. 2.
Case 2. Preoperative axial noncontrast CT (A) and axial (B) and coronal (C) contrast-enhanced T1-weighted MRI showing a large enhancing lesion centered along the right occipital convexity. Postoperative axial noncontrast CT (D) and axial (E) and coronal (F) contrast-enhanced T1-weighted MRI following initial right occipital craniotomy for GTR of the lesion. Axial contrast-enhanced CT angiogram (G) and axial (H) and coronal (I) contrast-enhanced T1-weighted MRI showing recurrence of the right occipital lobe lesion 76 months after the initial resection. Axial noncontrast CT (J) and axial (K) and coronal (L) contrast-enhanced T1-weighted MRI following right occipital/suboccipital craniotomy for GTR of the recurrent lesion. Axial noncontrast CT (M) and axial (N) and coronal (O) contrast-enhanced T1-weighted MRI scans 12 months after the second surgery (96 months after initial procedure).
Case 3
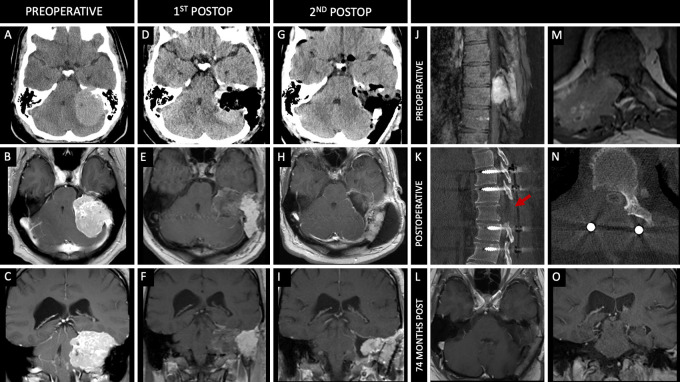
A 35-year-old male presented with decreased left-sided hearing, dysphagia, and generalized tonic-clonic seizure. A CT scan revealed a large left-sided cerebellopontine angle lesion measuring 4.1 × 5.4 × 5.5 cm (CC × ML × AP) with mass effect on the occipital lobe (Fig. 3A–C). Preoperative endovascular embolization of the left middle meningeal and occipital arterial pedicles was performed. The next day, the patient was taken for resection of the lesion via a translabyrinthine/transmastoid approach for STR (Fig. 3D–F). Pathology demonstrated WHO grade II HPC. The patient’s dysphagia improved, although his hearing remained impaired. One-month postoperative MRI showed progression of the residual tumor. The patient underwent a subsequent surgery 3 months after the initial surgery via a combined translabyrinthine and retrosigmoid approach for resection of the remaining tumor; GTR was achieved (Fig. 3G–I). The postoperative course was complicated by a pseudomeningocele, which required a return to the operating room for wound washout and revision. One month later, surveillance MRI showed progression of tumor in the jugular foramen, for which he underwent radiation therapy with 60 Gy in 30 fractions starting 2 months postoperatively. The patient’s dysphagia resolved, but he was left with persistent left-sided hearing loss. Fifty-five months after the initial procedure, surveillance MRI showed no tumor recurrence; however, the patient reported 4–6 weeks of progressive bilateral lower-extremity weakness along with numbness and tingling. Subsequent MRI of the spine revealed a large paraspinal mass at T10 measuring 5.5 × 5.4 × 4.3 cm (CC × ML × AP; Fig. 3J and M). This spinal lesion was completely resected, and the patient underwent T8–12 fusion (Fig. 3K and N). Pathology confirmed WHO grade III HPC. Numbness and leg strength returned to baseline 1 week following surgery. One month after resection of the spinal tumor, he underwent adjuvant radiotherapy with 30 Gy in five fractions. The patient’s latest follow-up imaging, 74 months from the time of initial surgery, showed no evidence of intracranial or spine tumor progression, although he continued to have mild imbalance.
FIG. 3.
Case 3. Preoperative axial noncontrast CT (A) and axial (B) and coronal (C) contrast-enhanced T1-weighted MRI showing a large enhancing lesion along the left side of the posterior fossa. Postoperative axial noncontrast CT (D) and axial (E) and coronal (F) contrast-enhanced T1-weighted MRI following a translabyrinthine/transmastoid approach for STR of the lesion. Axial noncontrast CT (G) and axial (H) and coronal (I) contrast-enhanced T1-weighted MRI following a combined translabyrinthine/transmastoid and retrosigmoid approach 3 months after the initial resection. Sagittal (J) and axial (M) contrast-enhanced CT showing a large metastatic lesion at T10. Sagittal (K) and axial (N) contrast-enhanced CT immediately after excision of the T10 lesion and T8–12 posterior fusion. Red arrow indicates vertebral level of the resected metastasis on postoperative imaging. Axial (L) and coronal (O) contrast-enhanced T1-weighted MRI showing no recurrence of the intracranial lesion 74 months following initial surgery.
Case 4
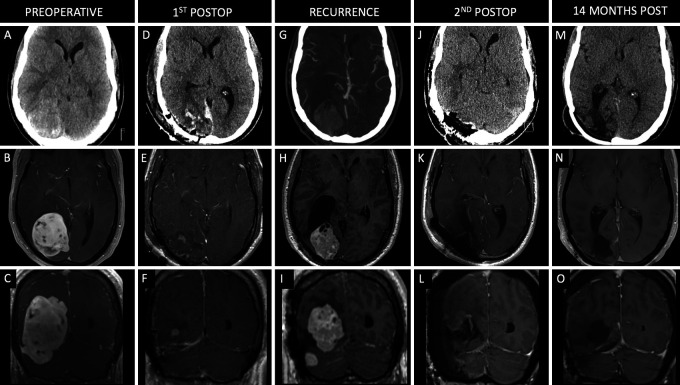
A 60-year-old male presented to the emergency department with 6–8 weeks of progressive temporal headaches, dizziness, and syncopal events. The patient’s family and friends also reported cognitive decline and short-term memory loss. Head CT revealed ventriculomegaly and a 5.4 × 3.3 × 3.9–cm (CC × ML × AP; Fig. 4A–C) lesion involving the splenium of the corpus callosum. A right frontal ventriculoperitoneal shunt was placed, and the patient underwent stage 1 resection 1 month later. A left-sided paramedian supracerebellar infratentorial approach was used to achieve STR, with residual tumor noted in the supratemporal and corpus callosum regions (Fig. 4D–F). Pathology revealed a WHO grade II HPC. Stage 2 was performed 10 weeks later using a left-sided occipital interhemispheric approach to achieve STR of the residual tumor (Fig. 4G–I). Postoperatively, the patient reported improved alertness and balance. Three months after his second surgery, the patient began radiation treatment of the residual tumor and received a total of 59.4 Gy in 33 fractions over 1.5 months. He remained asymptomatic for 39 months, but surveillance scans revealed progression of disease in the left thalamic region measuring 2.8 × 1.9 × 2.0 cm (Fig. 4J–L). The patient elected to undergo a posterior occipital interhemispheric approach, and STR was achieved (Fig. 4M–O). Pathology confirmed a WHO grade II HPC. The patient developed a new right foot drop postoperatively. One month postoperatively, he received an additional 25 Gy of radiation in five fractions over 4 days. During a 6-month surveillance MRI, there was concern for tumor progression, so he was enrolled in a clinical trial during which he received nivolumab for 8 months but was then transitioned off trial due to a decline in performance status, including cognitive decline and motor dysfunction, and was switched to bevacizumab.
FIG. 4.
Case 4. Preoperative axial noncontrast CT (A) and axial (B) and coronal (C) contrast-enhanced T1-weighted MRI showing a large enhancing lesion along the splenium of the corpus callosum and pineal region. Postoperative axial noncontrast CT (D) and axial (E) and coronal (F) contrast-enhanced T1-weighted MRI following a stage 1 supracerebellar infratentorial approach for partial resection of the lesion. Axial noncontrast CT (G) and axial (H) and coronal (I) contrast-enhanced T1-weighted MRI following a stage 2 interhemispheric occipital approach for STR of the lesion 2.5 months after stage 1. Axial noncontrast CT (J) and axial (K) and coronal (L) contrast-enhanced T1-weighted MRI showing recurrence of the hemangiopericytoma along the pineal region. Axial noncontrast CT (M) and axial (N) and coronal (O) contrast-enhanced T1-weighted MRI following a posterior occipital interhemispheric approach for STR of the lesion. Axial (P) and coronal (Q) contrast-enhanced T1-weighted MRI 8 months after the third surgery.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Intracranial HPCs are rare and aggressive neoplasms with a propensity to metastasize and recur despite GTR and adjuvant radiotherapy. We report our experience with intracranial HPCs in a case series of four patients (Table 1). Intracranial HPCs, unlike meningiomas, more commonly affect males than females.10 This trend was also noted in our series, which was composed entirely of males. Intracranial HPC, as compared with meningioma, also has a lower mean age at presentation, ranging from 42 to 51.2 years, which is similar to the average age of 48.3 years in our series.10,11 The vast majority of intracranial HPCs occur in adults, with an estimated 10% occurring in the pediatric population.12,13 HPCs can arise anywhere along the CNS but tend to be located intracranially, with the majority of lesions being supratentorial.14–16 A small subset of HPCs has been reported to expand across the tentorium with both supra- and infratentorial components.17 In our series, preoperative imaging revealed two patients (50%) who had tumors located in the supratentorial compartment, one patient (25%) with an infratentorial tumor confined to the posterior fossa, and one patient (25%) with a tumor with both infratentorial and supratentorial components.
HPCs can be classified according to the 2016 WHO criteria as grade I, II, or III on the basis of histological features, including mitotic index, necrosis, cellularity, and nuclear atypia.18 Extant literature reports a higher prevalence of grade II than grade III HPCs, with notable differences in the clinical course between the two groups.15,19 Grade II HPCs are associated with better survival and lower rates of recurrence and metastasis than grade III HPCs.14,20–23 In a series of 49 cases, Apra et al.23 reported, for the first time, malignant transformation of five WHO grade I/II HPCs to WHO grade III within a span of 3–13 years. Notably, three of these patients had histological progression without prior radiation. The authors noted that a longer follow-up would likely have revealed more cases of histological progression.23 In our series, three patients (75%) were originally diagnosed with WHO grade II HPC on the basis of histopathological analysis of intraoperative specimens at the initial resection, two of which converted to grade III at the time of subsequent resections prior to receiving any radiotherapy. The remaining patient was diagnosed with WHO grade III HPC at the index surgery.
Radiotherapy for intracranial HPC has been described primarily as a means of adjuvant therapy following both index surgery and surgery for recurrence, demonstrating increased tumor control, progression-free survival, and overall survival.24–28 In a large multi-institutional study, Lee and colleagues29 found that postoperative radiotherapy was significantly associated with improved local control and progression-free survival in both the STR and GTR groups. In their study, 64% of patients received postoperative radiotherapy, and 98% of these patients underwent radiotherapy within 3 months of surgery. The most favorable outcomes were seen in patients who had undergone GTR followed by radiotherapy, and the least favorable outcomes were seen in patients who had undergone STR with no adjuvant radiotherapy. These findings highlight the role of postoperative radiotherapy, regardless of the extent of resection achieved.29 Gamma Knife radiosurgery enables high-precision delivery of radiation with a lower rate of radiotherapy-induced morbidity. In a cohort of 15 patients (28 tumors) with residual, recurrent, or metastatic HPCs, Huang et al.30 reported complete tumor disappearance in 7 tumors (25%), reduction in 14 (50%), stability in 1 (3.57%), and recurrence in 6 (21.4%). Proton therapy uses beams of protons rather than X-rays and allows precise targeting by controlling the direction and depth of energy emitted, minimizing damage to surrounding tissues.31 Emerging evidence suggests that proton therapy is safe and efficacious in patients with HPC and offers a lower side-effect profile than traditional forms of radiation, particularly when lesions are close to critical intracranial structures.32,33
In a study of 40 patients, the authors found that a maximal tumor diameter ≥6 cm was associated with recurrence due to reduced rates of GTR because of the difficulty in obtaining adequate exposure and more extensive involvement of critical neurovascular structures.5 The anatomical location of the HPC can also be a factor in clinical outcomes, especially in cases of posterior fossa tumors, which have been shown to have greater morbidity.2,10,11 This may be attributed to the abundance of cranial nerves, critical vasculature, and brainstem structures in this region, increasing the technical difficulty of achieving a safe total extirpation. In our series, upfront GTR appeared to be superior to STR, with an average time to recurrence of 84 months (n = 1) versus 15.7 months (n = 3), respectively. One patient exhibited progression of his residual tumor that was treated with follow-up resection after an initial excision. It is important to acknowledge that residual tumor progression was in part mediated by a delay in surgical management, given the patient’s reluctance to return for completion surgery. Of note, there was no local tumor progression in patients who received radiotherapy following NTR or GTR.
Even if GTR is achieved and postoperative radiotherapy is used, it is often not enough to prevent recurrence.4 Recurrence has been documented as early as 7 months after resection but can occur more than 10 years following initial resection.3,34 In our series, the average follow-up interval was 68.5 months (5.7 years) after initial resection. All four patients required a return to the operating room for resection of residual or recurrent tumor. Three patients (75%) had local tumor recurrence, two following GTR and one following STR and adjuvant fractionated radiation. In our second case, the patient initially did well but had a delayed recurrence 7 years postoperatively, thus emphasizing the importance of prolonged serial surveillance imaging in all patients, regardless of GTR and initially reassuring follow-up. It is also important to consider the possibility of remote disease progression, as evidenced by our patient with a paraspinal WHO grade III HPC 4 years following surgery for resection of their posterior fossa WHO grade II HPC. Although GTR was achieved at surgery, this case raises the question whether serial imaging of extracranial anatomy is justified to identify remote disease progression and avoid permanent neurological deficits. In a series of 21 patients who received postoperative radiotherapy, Dufour et al.4 found that adjuvant radiotherapy reduced the risk of local recurrence but was not protective against peripheral metastasis. On the basis of the circumstances of the aforementioned case and available evidence in the literature, the use of global serial MRI can be considered in the event of new neurological decline to optimize the care of patients and monitor for peripheral disease; however, we do not yet have data to support prophylactic/preventative MRI. Additionally, studies using radiotracers such as whole-body 68Ga-DOTATOC positron emission tomography (PET)/CT have demonstrated the ability to detect components of tumors that may be undetectable using traditional MRI or CT scanning. This imaging modality may be of particular benefit in the setting of HPC, given the lesion’s likelihood of recurrence and metastasis.35
Limitations
Intracranial HPCs are rare tumors, and low incidence rates compounded by the heterogeneity in treatment between institutions limits the power of any associative statements made about treatment paradigms and clinical outcomes. The limited number of cases in this series allows us only to share our experience with intracranial HPCs in the context of the existing literature. Additional cases and multi-institutional studies will allow more robust conclusions to be made about the nuances of managing these unique neoplasms. Last, adjuvant radiotherapy was used following initial resection in only one patient in this series. This is important to consider in the context of discussing benefits of adjuvant therapy in cases of NTR or STR.
Lessons
Intracranial HPCs are rare and aggressive lesions with a propensity to recur locally and form distant metastases despite GTR and adjuvant radiotherapy. Evidence from the existing literature indicates that achieving GTR confers the greatest survival benefit. However, GTR may not always be attainable with a single surgery/approach. As described in our series, some patients can require staged surgeries to achieve GTR or NTR followed by radiation therapy within 3 months after surgery. This strategy appears to be superior to STR with radiation therapy for local control of the disease. Given the possibility of recurrence and metastasis, providers should maintain a high index of suspicion in patients presenting with new or recurrent neurological symptoms, with a low threshold for performing cranial and global imaging. Last, given the high probability of tumor recurrence, even years after resection, patient compliance is fundamental to long-term management; providers should stress the importance of long-term follow-up evaluations and serial imaging with whole-body 68Ga-DOTATOC PET/CT.
Author Contributions
Conception and design: Vignolles-Jeong, Finger, Beaumont, Prevedello. Acquisition of data: Vignolles-Jeong, McGahan, Beaumont, Weber, Prevedello. Analysis and interpretation of data: Vignolles-Jeong, Finger, McGahan, Beaumont, Wu, Prevedello. Drafting the article: Vignolles-Jeong, Finger, Beaumont, Weber, Wu. Critically revising the article: Vignolles-Jeong, Finger, McGahan, Weber, Wu, Prevedello. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Vignolles-Jeong. Statistical analysis: Vignolles-Jeong. Administrative/technical/material support: Vignolles-Jeong, McGahan. Study supervision: Vignolles-Jeong, Beaumont, Wu, Prevedello.
Supplemental Information
Previous Presentations
An abstract of this work was presented at the Congress of Neurological Surgeons Annual Meeting on September 9–13, 2023, in Washington, DC.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rutkowski MJ, Sughrue ME, Kane AJ, et al. Predictors of mortality following treatment of intracranial hemangiopericytoma. J Neurosurg. 2010;113(2):333–339. doi: 10.3171/2010.3.JNS091882. [DOI] [PubMed] [Google Scholar]
- 3.Rutkowski MJ, Bloch O, Jian BJ, et al. Management of recurrent intracranial hemangiopericytoma. J Clin Neurosci. 2011;18(11):1500–1504. doi: 10.1016/j.jocn.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Dufour H, Métellus P, Fuentes S, et al. Meningeal hemangiopericytoma: a retrospective study of 21 patients with special review of postoperative external radiotherapy. Neurosurgery. 2001;48(4):756–763. doi: 10.1097/00006123-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 5. Rutkowski MJ, Jian BJ, Bloch O, et al. Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer. 2012;118(6):1628–1636. doi: 10.1002/cncr.26411. [DOI] [PubMed] [Google Scholar]
- 6. Tashjian VS, Khanlou N, Vinters HV, Canalis RF, Becker DP. Hemangiopericytoma of the cerebellopontine angle: a case report and review of the literature. Surg Neurol. 2009;72(3):290–295. doi: 10.1016/j.surneu.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 7. Matsushige T, Nakaoka M, Yahara K, et al. Single-stage operation for a giant haemangiopericytoma following intracranial feeder embolization. J Clin Neurosci. 2007;14(2):162–167. doi: 10.1016/j.jocn.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 8. Kumar R, Wani AA. Unusual tumors of the posterior fossa skull base. Skull Base. 2006;16(2):75–84. doi: 10.1055/s-2006-934103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Agha RA, Sohrabi C, Mathew G, Franchi T, Kerwan A, O’Neill N. The PROCESS 2020 guideline: updating consensus Preferred Reporting Of CasESeries in Surgery (PROCESS) guidelines. Int J Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 10. Fountas KN, Kapsalaki E, Kassam M, et al. Management of intracranial meningeal hemangiopericytomas: outcome and experience. Neurosurg Rev. 2006;29(2):145–153. doi: 10.1007/s10143-005-0001-9. [DOI] [PubMed] [Google Scholar]
- 11. Guthrie BL, Ebersold MJ, Scheithauer BW, Shaw EG. Meningeal hemangiopericytoma: histopathological features, treatment, and long-term follow-up of 44 cases. Neurosurgery. 1989;25(4):514–522. [PubMed] [Google Scholar]
- 12. Cavalheiro S, Sparapani FV, Moron AF, da Silva MC, Stávale JN. Fetal meningeal hemangiopericytoma. Case report. J Neurosurg. 2002;97(5):1217–1220. doi: 10.3171/jns.2002.97.5.1217. [DOI] [PubMed] [Google Scholar]
- 13. Cole JC, Naul LG. Intracranial infantile hemangiopericytoma. Pediatr Radiol. 2000;30(4):271–273. doi: 10.1007/s002470050738. [DOI] [PubMed] [Google Scholar]
- 14. Schiariti M, Goetz P, El-Maghraby H, Tailor J, Kitchen N. Hemangiopericytoma: long-term outcome revisited. Clinical article. J Neurosurg. 2011;114(3):747–755. doi: 10.3171/2010.6.JNS091660. [DOI] [PubMed] [Google Scholar]
- 15. Kim BS, Kim Y, Kong DS, et al. Clinical outcomes of intracranial solitary fibrous tumor and hemangiopericytoma: analysis according to the 2016 WHO classification of central nervous system tumors. J Neurosurg. 2018;129(6):1384–1396. doi: 10.3171/2017.7.JNS171226. [DOI] [PubMed] [Google Scholar]
- 16. Shetty PM, Moiyadi AV, Sridhar E. Primary CNS hemangiopericytoma presenting as an intraparenchymal mass—case report and review of literature. Clin Neurol Neurosurg. 2010;112(3):261–264. doi: 10.1016/j.clineuro.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 17. Fukutome K, Kimura R, Okumura Y, Ohta Y. Solitary fibrous tumor/hemangiopericytoma expanding the superior and inferior cerebellar tentorium: a case report. Interdiscip Neurosurg. 2019;15:1–5. [Google Scholar]
- 18. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 19. Sung KS, Moon JH, Kim EH, et al. Solitary fibrous tumor/hemangiopericytoma: treatment results based on the 2016 WHO classification. J Neurosurg. 2019;130(2):418–425. doi: 10.3171/2017.9.JNS171057. [DOI] [PubMed] [Google Scholar]
- 20. Damodaran O, Robbins P, Knuckey N, Bynevelt M, Wong G, Lee G. Primary intracranial haemangiopericytoma: comparison of survival outcomes and metastatic potential in WHO grade II and III variants. J Clin Neurosci. 2014;21(8):1310–1314. doi: 10.1016/j.jocn.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Ecker RD, Marsh WR, Pollock BE, et al. Hemangiopericytoma in the central nervous system: treatment, pathological features, and long-term follow up in 38 patients. J Neurosurg. 2003;98(6):1182–1187. doi: 10.3171/jns.2003.98.6.1182. [DOI] [PubMed] [Google Scholar]
- 22. Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE. Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol. 1991;22(1):84–91. doi: 10.1016/0046-8177(91)90067-y. [DOI] [PubMed] [Google Scholar]
- 23. Apra C, Mokhtari K, Cornu P, Peyre M, Kalamarides M. Intracranial solitary fibrous tumors/hemangiopericytomas: first report of malignant progression. J Neurosurg. 2018;128(6):1719–1724. doi: 10.3171/2017.1.JNS162593. [DOI] [PubMed] [Google Scholar]
- 24. Chang SD, Sakamoto GT. The role of radiosurgery for hemangiopericytomas. Neurosurg Focus. 2003;14(5):e14. doi: 10.3171/foc.2003.14.5.15. [DOI] [PubMed] [Google Scholar]
- 25. Kano H, Niranjan A, Kondziolka D, Flickinger JC, Lunsford LD. Adjuvant stereotactic radiosurgery after resection of intracranial hemangiopericytomas. Int J Radiat Oncol Biol Phys. 2008;72(5):1333–1339. doi: 10.1016/j.ijrobp.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 26. Olson C, Yen CP, Schlesinger D, Sheehan J. Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat Gamma Knife surgery. J Neurosurg. 2010;112(1):133–139. doi: 10.3171/2009.3.JNS0923. [DOI] [PubMed] [Google Scholar]
- 27. Sheehan J, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for treatment of recurrent intracranial hemangiopericytomas. Neurosurgery. 2002;51(4):905–911. doi: 10.1097/00006123-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Soyuer S, Chang EL, Selek U, McCutcheon IE, Maor MH. Intracranial meningeal hemangiopericytoma: the role of radiotherapy: report of 29 cases and review of the literature. Cancer. 2004;100(7):1491–1497. doi: 10.1002/cncr.20109. [DOI] [PubMed] [Google Scholar]
- 29. Lee JH, Jeon SH, Park CK, et al. The role of postoperative radiotherapy in intracranial solitary fibrous tumor/hemangiopericytoma: a multi-institutional retrospective study (KROG 18-11) Cancer Res Treat. 2022;54(1):65–74. doi: 10.4143/crt.2021.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang L, Bai J, Zhang Y, et al. Treatment of residual, recurrent, or metastatic intracranial hemangiopericytomas with stereotactic radiotherapy using CyberKnife. Front Oncol. 2021;11:577054. doi: 10.3389/fonc.2021.577054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jones B, McMahon SJ, Prise KM. The radiobiology of proton therapy: challenges and opportunities around relative biological effectiveness. Clin Oncol (R Coll Radiol). 2018;30(5):285–292. doi: 10.1016/j.clon.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 32. Ma M, Gong Y, Tang X, et al. Solitary fibrous tumor in the saddle area treated with neuroendoscopic surgery and proton therapy: a case report and literature review. Oncol Lett. 2023;26(6):505. doi: 10.3892/ol.2023.14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gordon K, Gulidov I, Gogolin D, et al. A clinical case of 5 times irradiated recurrent orbital hemangiopericytoma. Case Rep Oncol. 2021;14(1):78–84. doi: 10.1159/000513030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chacko G, Chacko AG, Rajshekhar V, Muliyil JP. Intracranial hemangiopericytomas: correlation of topoisomerase IIalpha expression with biologic behavior. Surg Neurol. 2006;65(1):11–17. doi: 10.1016/j.surneu.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Umana GE, Ferini G, Harikar MM, et al. Detection of “incidentalomas” on brain and body 68Ga-DOTATOC-PET scans: a retrospective study and case illustration. Anticancer Res. 2022;42(12):5867–5873. doi: 10.21873/anticanres.16095. [DOI] [PubMed] [Google Scholar]