Abstract
BACKGROUND
Supraorbital keyhole approaches (SKAs) have garnered criticism for a limited surgical exposure, restrictive surgical freedom, blind spots, and the learning curve. This retrospective study of patients who underwent SKA aims to explore the outcomes, technical nuances, and the learning curve reflected in a single surgeon’s experience in the initial 3 years of practice.
OBSERVATIONS
A total of 20 SKA operations were performed in 19 patients. Gross- or near-total resection was achieved in 14 of 17 tumor cases. The mean blood loss was 80.5 mL, the mean duration of surgery was 5 hours, and the median stay was 3 days. Endoscopic augmentation was used in 11 cases in which additional tumor removal occurred in 8 of the 11 cases. There were no cases of cerebrospinal fluid leakage or wound infection. A 30-day readmission and typical narcotics after discharge were seen in one patient each. When comparing two halves of a neurosurgery practice over 3 years, the duration of surgery was significantly longer in the later year, which is likely due to operating on a larger tumor size as the years progressed. No cases required static retractors or conversion to larger craniotomies.
LESSONS
Careful case selection and respecting the learning curve allows the safe incorporation of SKA in the early stages of neurosurgical practice.
Keywords: supraorbital keyhole, craniotomy, outcomes, learning curve
ABBREVIATIONS: CN = cranial nerve, CSF = cerebrospinal fluid, FLAIR = fluid-attenuated inversion recovery, GBM = glioblastoma multiforme, GTR = gross-total resection, KPS = Karnofsky performance status, MRI = magnetic resonance imaging, NTR = near-total resection, SKA = supraorbital keyhole approach, WHO = World Health Organization
The concept of “keyhole surgery” was introduced by Donald Wilson in 1971, and, since then, keyhole approaches have been refined with smaller focused incisions, low-profile keyhole instruments, virtual reality augmentation, and a high-definition endoscope.1,2 The primary goal of this minimally invasive craniotomy is to achieve the precise exposure essential for tumor removal. There has been substantial debate regarding satisfactory exposure in comparison with that achieved with the classic pterional approach and its variants.3–10 Nevertheless, potential advantages lie in its minimally invasive nature, resulting in less damage to surrounding structures and improved postoperative outcomes in terms of pain management and cosmesis.11–20
Supraorbital keyhole approaches (SKAs) have been criticized for their restricted surgical exposure, limited surgical freedom, blind spots, and associated learning curve.21,22 No prior study has described the use of this approach in the early years of neurosurgery practice. Hence, the goal of this study was to extensively detail indications, surgical resectability, complications, length of stay, patient performance status, readmissions, typical narcotics use, and technical nuances.
Study Description
Patient Population and Data Collection
All consecutive patients between October 2020 and September 2023 who had undergone supraorbital craniotomy approaches by a single surgeon (J.D.T.) in the first 3 years of practice at the University of South Alabama, Mobile, were identified. Primary outcomes included extent of tumor resection (if applicable), blood loss, duration of surgery, complications, length of hospitalization, new cranial nerve (CN) deficits, and changes in Karnofsky performance status (KPS). A favorable KPS at a recent follow-up was defined as an improved or unchanged status from the preoperative KPS. Resection rates were categorized as gross-total resection (GTR), near-total resection (NTR; 90% tumor resection), or subtotal resection (<90%). The size of the tumor was calculated as the maximum diameter on magnetic resonance imaging (MRI).
Secondary outcomes included analysis of 30-day readmission rates and use of typical narcotics in the perioperative phase. We also assessed the utility of endoscopic removal of residual tumors if applicable. New postoperative fluid-attenuated inversion recovery (FLAIR)/T2 changes in bilateral frontal orbital areas were studied to assess intraoperative brain injury on axial plane comparative MRI. Last, the two different halves of the initial 3-year surgical periods were assessed to highlight the overall learning curve for transciliary SKAs.
Supraorbital Craniotomy and Modifications
Our supraorbital eyebrow exposure is done in a fashion similar to what was described earlier by the senior author.3,4 The patient is placed supine in a 20° reverse Trendelenburg position to facilitate venous drainage and positioned with 20°–30° of neck extension to allow the frontal lobe to fall away from the anterior cranial fossa floor with gravity. According to the location of the pathology, the degree of head rotation toward the contralateral side is determined: a 15°–30° head rotation for ipsilateral lesions and a 45°–60° rotation for lesions of the anterior fossa, olfactory groove, and contralateral lesions. The eyebrows are not shaved. We prefer to make the incision within the eyebrow just inferior to its superior margin in an angled fashion, avoiding a direct cut across the hair follicles. Typical opening is performed as described in our previous publications (Fig. 1A).1,12 For modifications in the supraorbital approach, see Fig. 2 and its legend for details.
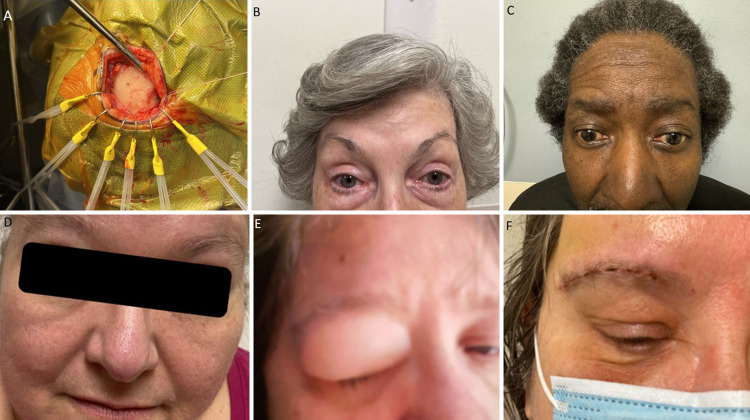
FIG. 1.
A: Transciliary supraorbital approach exposure. B–D: Postoperative cosmesis after the supraorbital craniotomy. E: Preoperative photograph of a patient with an orbital encephalocele. F: Postoperative photograph after supraorbital craniotomy repair for an orbital encephalocele.
FIG. 2.
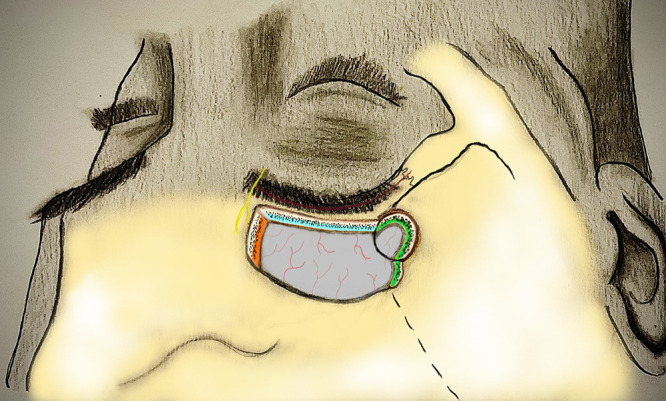
Illustration depicting the transciliary incision (red), supraorbital nerve (yellow), and the margins of the supraorbital ridge osteotomy (blue), medial edge of the osteotomy (orange), and sphenoid wing of the osteotomy (green). In addition to drilling of the orbital rim overhang, the modifications include drilling of the medial edge of the inner table to enhance the cross-court corridor for better visualization of the contralateral anterior cranial fossa in large anterior cranial base tumors. Additionally, drilling the inner table of the lateral limit of craniotomy facilitates sphenoid wing undercutting, which increases the surgical freedom to split the sylvian fissure and increase the exposure over the middle fossa in addition to the anterior skull base. Orbital rim can also be completely removed. However, in our case series, we did not see the need for complete removal of the orbital rim.
The initial intradural step involves the meticulous microscope-guided wide opening of the proximal sylvian fissure (Video 1), as well as the prechiasmatic, opticocarotid, and carotid-oculomotor cisterns. This is undertaken to facilitate the release of cerebrospinal fluid (CSF), promote brain relaxation, and serve as primary working windows for the subfrontal approach.
Once the pathology is addressed under the microscope, a handheld 30° 2.9-mm- or 4-mm-diameter rigid endoscope is introduced into the working space to enhance the surgeon’s visual perspective. By manipulating the angled endoscope laterally, medially, superiorly, or inferiorly, a comprehensive view of the entire anterior and middle cranial fossa floor can be obtained. Endoscopes are used to visualize the notable blind spots often encountered in keyhole approaches.
VIDEO 1. Clip highlighting the importance of and technical steps for splitting fissures in a third-ventricle tumor via a supraorbital craniotomy. Click here to view.
Patient Selection
Indications (Table 1) for using eyebrow approaches in the first 3 years of practice included olfactory groove meningiomas, planum meningiomas (Video 2), tuberculum sella meningiomas, frontal glioblastoma multiforme (GBM), frontal low-grade glioma, third-ventricle tumors, suprasellar residuals from endonasal resection of giant pituitary macroadenomas, and CSF leakage/encephalocele/anterior skull base defect.
TABLE 1.
Demographics and clinical characteristics of patients undergoing supraorbital craniotomies
| Case No. | Age (yrs) | Sex | Pathology | Location | New Deficit | FU (mos) | LOS (days) | Place of Discharge | Readmission (30 days) | KPS Difference | ASA Score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
67 |
F |
Meningioma |
TS |
No |
12 |
3 |
Home |
No |
+10 |
3 |
| 2 |
67 |
F |
Meningioma |
OG |
No |
3.5 |
4 |
Home |
No |
0 |
3 |
| 3 |
67 |
F |
GBM |
Fronto-orbital |
No |
4.5 |
3 |
Home |
No |
−70 |
4 |
| 4 |
67 |
F |
GBM |
Fronto-orbital |
No |
9 |
2 |
Home |
No |
+10 |
3 |
| 5 |
71 |
F |
Meningioma |
CS |
No |
4 |
3 |
Home |
No |
0 |
3 |
| 6 |
73 |
M |
Meningioma |
OG |
No |
12 |
2 |
Home |
No |
+10 |
3 |
| 7 |
77 |
F |
Meningioma |
TS |
No |
1.5 |
4 |
Home |
No |
0 |
3 |
| 8 |
78 |
M |
Meningioma |
Frontal |
No |
7 |
3 |
Home |
No |
+20 |
3 |
| 9 |
90 |
F |
Meningioma |
Planum |
No |
4 |
3 |
Home |
No |
+10 |
3 |
| 10 |
43 |
F |
Meningioma |
OG |
No |
12 |
2 |
Home |
No |
−20 |
3 |
| 11 |
43 |
F |
Suprasellar residual pituitary adenoma |
Suprasellar |
No |
4 |
4 |
Home |
No |
0 |
2 |
| 12 |
45 |
M |
CSF leak |
ASB |
No |
6 |
3 |
Home |
No |
+20 |
3 |
| 13 |
47 |
F |
Meningioma |
Planum |
Yes* |
1 |
3 |
Home |
No |
+10 |
3 |
| 14 |
50 |
F |
Meningioma |
Planum |
No |
9 |
2 |
Home |
No |
+10 |
3 |
| 15 |
58 |
M |
GBM |
3rd ventricle |
No |
Hospice |
2 |
Home |
Yes† |
0 |
3 |
| 16 |
58 |
F |
Meningioma |
TS |
No |
14 |
10 |
Home |
No |
+10 |
3 |
| 17 |
62 |
F |
CSF leak |
ASB |
No |
4 |
2 |
Home |
No |
+10 |
3 |
| 18 |
64 |
F |
CSF leak |
ASB |
No |
5 |
2 |
Home |
No |
+10 |
3 |
| 19 |
64 |
M |
Low-grade glioma |
Fronto-orbital |
No |
12 |
3 |
Home |
No |
+10 |
3 |
| 20 | 64 | M | Meningioma | Planum | No | 12 | 2 | Home | No | +10 | 3 |
ASA = American Society of Anesthesiologists; ASB = anterior skull base; CS = cavernous sinus; LOS = length of stay; OG = olfactory groove; TS = tuberculum sella.
* CN I.
† Obstructive hydrocephalus.
VIDEO 2. Clip showing the technical steps for a planum meningioma via the supraorbital approach. Click here to view.
For anterior skull base meningiomas, our preferred route was the supraorbital approach. Our maximum tumor diameter was 6.7 cm; however, the overall mean tumor diameter was 3 cm. For relatively larger planum/tuberculum meningiomas with substantial superior extension into the interhemispheric fissure, clinoidal meningiomas with a substantial middle fossa component, tumors with circumferential compression of the middle cerebral artery, or vascular pathology, we did not use the eyebrow approach; instead, a standard pterional craniotomy with or without an orbitozygomatic variant was used. In tumors with prior surgery, our selective approach did not use supraorbital approaches. Although we believe some of the anterior skull base meningiomas could have been addressed via an endonasal route, the operating surgeon felt more comfortable with an eyebrow approach. The reasons for the latter include (1) the availability of an ear, nose, and throat surgeon able to perform the extended approach, (2) longer operating hours for extended endonasal approaches in comparison with the eyebrow approach with potentially higher chances of CSF leakage, and (3) surgeon preference. We performed optic canal decompression (unilateral and bilateral) for the resection of circumferential meningiomas invading the optic canal. Tumor invading the optic canal did not factor towards selecting endonasal approach.
FLAIR Imaging Changes
Axial FLAIR/T2 images were assessed on postoperative day 1 or 2 for the presence or absence of new or increased signal changes in the bilateral orbitofrontal regions around the resection area and approach trajectory.
Statistical Analysis
R statistical software (version 4.3.2, R Foundation for Statistical Computing) was used for all analyses. Univariate analysis of categorical variables was conducted using the Pearson chi-square test. Differences between the means of the two groups were compared using the Student t test to determine statistical significance, which was established at an alpha level of less than 0.05. Furthermore, z-score analysis was employed using the same subcohorts to evaluate the trends of various outcomes within the reference population (n = 20).
Results
Description of Patients
In total, 19 patients (mean age 62.75 ± 12.52 years, 68% female) underwent 20 supraorbital craniotomies (Table 1). Pathologies included World Health Organization (WHO) grade 1 (60%) and WHO grade 2 (10%) meningioma, GBM (15%), suprasellar residual pituitary macroadenoma (5%), CSF leakage (15%), and low-grade glioma (5%). One patient underwent additional surgery for the recurrence of GBM. The mean follow-up was 7.42 ± 4.7 months.
Primary Outcomes
Case Characteristics
Of all the supraorbital craniotomies performed between October 2020 and September 2023, 17 (85%) were performed for tumors (Table 1). Of these tumors, 12 (70.59%) were WHO grade 1 meningiomas, 2 (11.76%) were WHO grade 2 meningiomas, 3 (17.65%) were GBMs, 1 (5.88%) was a suprasellar residual pituitary adenoma, and 1 (5.88%) was a low-grade glioma. Of the remaining cases, 15% involved the use of a supraorbital craniotomy for CSF leaks. Meningiomas were most often located in the planum sphenoidale, accounting for 33.33% of cases (n = 4), followed by the olfactory groove at 25% (n = 3), tuberculum sellae at 25% (n = 3), cavernous sinus at 8.33% (n = 1), and the fronto-orbital region with olfactory groove involvement at 8.33% (n = 1). Two patients underwent resection for GBM. Of these two, one required a second surgery for a recurrence in the fronto-orbital region (after GTR), whereas the other had a tumor in the third ventricle extending into the basal ganglia and midbrain and later presented with hydrocephalus. Additionally, one patient underwent a supraorbital craniotomy for a residual pituitary adenoma in the suprasellar region via a recent endonasal approach. One patient had a low-grade glioma in the left fronto-orbital region with proximity to the hypothalamus and basal ganglia (Supplementary Table 1).
Surgical Learning Curve
The overall mean duration of surgery was 5.1 hours with an average blood loss of 80.1 mL. In 7 (35%) of 20 cases, a fat graft was used. In 7 (35%) of 20 cases, the frontal sinus was exposed. When dividing the patients into two halves of the 3-year neurosurgery practice on the basis of median surgery date, we found the mean duration of surgery was significantly longer in the second half (6.0 ± 1.2 hours) than in the first (4.2 ± 1.0 hours; p = 0.002). An increase in mean blood loss was also noted in the second half (121.7 mL) compared with the first (46.8 mL; p = 0.28). However, the mean tumor diameter was higher in the second half of the practice than in the first (28.4 ± 18.1 mm versus 22.5 ± 12.9 mm, p = 0.79; Table 2). Calculated z-scores for each of the established subcohorts support this numerical trend (Supplementary Fig. 1).
TABLE 2.
Differences in clinical parameters and outcomes after supraorbital craniotomies in the first and second half of neurosurgery practice
| Variable | First Half | Second Half | p Value |
|---|---|---|---|
| No. of patients |
11 |
9 |
|
| Readmission |
1/11 (0.09%) |
0 (0%) |
0.85 |
| Duration of op in hrs |
4.2 (1.0) |
6.0 (1.2) |
0.002 |
| Tumor diameter in mm |
22.5 (12.9) |
28.4 (18.1) |
0.79 |
| Blood loss in mL |
46.8 (22.4) |
121.7 (152.1) |
0.28 |
| New deficit |
0 (0%) |
1 (0.11%) |
1.0 |
| KPS difference | 10 (10.6) | 10 (8.3) | 0.89 |
Values are expressed as number (%) or mean (standard deviation), unless indicated otherwise.
As mentioned, 17 (85%) of 20 supraorbital craniotomies within our cohort were performed for elective tumor removal. GTR or NTR was achieved in 14 (82.4%) of the 17 tumor cases. The first half of patients accounted for 6 (42.8%) of 14 GTRs or NTRs, whereas the second half accounted for the remaining 8 (57.1%) of 14, indicating a 14.3% increase in GTR or NTR between the first and second halves of supraorbital tumor resection (p = 0.84). Subtotal resection was achieved in three cases, including one for GBM, one for cavernous meningioma with sphenoidal extension, and one for a low-grade myxoid glioneuronal tumor (Table 2).
Clinical Outcomes
There were no major complications. Minor complications included hyposmia in one patient and the development of frontalis palsy in another. Three patients experienced transient frontal numbness, which resolved within 3 months of follow-up. No patients developed a mucocele, sinusitis, wound infection, CSF leak, or stroke. Preoperatively, five patients had CN I deficits, and six patients had CN II deficits. One patient had CN III, CN IV, and partial CN VI palsy. Postoperatively, three of the five patients with CN I deficits showed improvement, whereas one patient’s CN I deficit worsened. Four of the six patients with CN II deficits showed improvement (Supplementary Table 2).
Functional Outcomes and 30-Day Readmissions
All patients were discharged home, and no patients needed inpatient rehabilitation or a skilled nursing facility. The median length of stay was 3 days. One patient was readmitted within 30 days because of obstructive hydrocephalus and underwent ventriculoperitoneal shunt placement. Ultimately, because of aggressive pathology (GBM in the brainstem, third ventricle, and basal ganglia), the family opted for hospice care. Two patients showed a decline in KPS from baseline to follow-up. One patient died of aggressive malignancy and location (as mentioned above), and another showed some decline due to seizures after 1 year of follow-up, although they still maintained a KPS >70 (Table 1).
Secondary Outcomes
Acute MRI Changes
Among 20 surgeries, postoperative day 1 MRI showed a regional increase in FLAIR/T2 signal changes in seven patients (36%). In all patients, changes were seen in the frontal orbital region. On analysis, FLAIR changes were significantly associated with an increase in tumor size (p = 0.019). The duration of surgery, blood loss, and patient age did not contribute to any significant increase in FLAIR changes in our selected cohort (p = 0.735, p = 0.368, p = 0.23, respectively). No patients with increased FLAIR changes had neurological deficits because of their FLAIR changes (Table 3, Supplementary Table 3).
TABLE 3.
Surgical and clinical characteristics in patients undergoing supraorbital craniotomies
| Case No. | Diagnosis | Op & Modification | Use of Endoscope | Additional Tumor Removed w/ Endoscope | Frontal Sinus Opened | Use of Fat Graft | Extent of Resection | Postop MRI FLAIR Change |
|---|---|---|---|---|---|---|---|---|
| 1 |
Meningioma |
SOR+SW |
Yes |
Yes |
No |
No |
GTR |
No |
| 2 |
Meningioma |
SOR+MIT |
No |
No |
No |
No |
GTR |
No |
| 3 |
GBM |
SOR+MIT |
No |
No |
No |
No |
NTR |
Yes |
| 4 |
GBM |
SOR+MIT |
No |
No |
No |
No |
GTR |
Yes |
| 5 |
Meningioma |
SOR+SW |
No |
No |
No |
No |
STR (biopsy + optic nerve decompression) |
Yes |
| 6 |
Meningioma |
SOR+MIT |
Yes |
Yes |
No |
No |
GTR |
No |
| 7 |
Meningioma |
SOR+SW |
No |
No |
No |
No |
NTR |
No |
| 8 |
Meningioma |
SOR+MIT |
Yes |
Yes |
Yes |
Yes |
NTR |
Yes |
| 9 |
Meningioma |
SOR |
No |
No |
Yes |
Yes |
GTR |
No |
| 10 |
Meningioma |
SOR+MIT |
Yes |
Yes |
Yes |
Yes |
GTR |
No |
| 11 |
Suprasellar residual pituitary adenoma |
SOR+SW |
No |
No |
No |
No |
GTR |
No |
| 12 |
CSF leak |
SOR+MIT |
Yes |
No |
No |
No |
No resection |
No |
| 13 |
Meningioma |
SOR+MIT |
Yes |
Yes |
Yes |
No |
GTR |
No |
| 14 |
Meningioma |
POR+MIT |
Yes |
Yes |
No |
No |
GTR |
No |
| 15 |
GBM |
SOR+SW |
Yes |
Yes |
No |
Yes |
STR |
Yes |
| 16 |
Meningioma |
SOR+SW |
Yes |
Yes |
Yes |
Yes |
GTR |
Yes |
| 17 |
CSF leak |
SOR |
No |
No |
No |
No |
No resection |
No |
| 18 |
CSF leak |
SOR+MIT |
Yes |
No |
Yes |
Yes |
No resection |
No |
| 19 |
Low-grade glioma |
SOR |
No |
No |
No |
No |
STR |
No |
| 20 | Meningioma | SOR+MIT | Yes | No | Yes | Yes | GTR | Yes |
MIT = medial edge inner table osteotomy; SOR = supraorbital ridge osteotomy; STR = subtotal resection; SW = sphenoid wing osteotomy.
Use of Endoscopy
Endoscopic augmentation was used in 11 (55.0%) of 20 of our supraorbital cases and was useful in the additional tumor removal in 8 (72.7%) of 11 cases in which it was used. Endoscopy was also used in two of three nontumor supraorbital craniotomies (66.7%, all CSF leakage repairs). Of the cases with additional endoscopic tumor removal, seven (87.5%) of eight resulted in GTR, whereas the remaining case resulted in NTR. Of the eight tumor cases in which endoscopy was not used as an adjunct with the supraorbital craniotomy approach, only four resulted in GTR (50.0%). Endoscopic assistance was used in 63.6% of cases in the first half of our cohort compared with 44.4% of cases in the second half (Table 3).
Opioid Use
Among the 20 surgeries in 19 patients, 1 patient needed typical narcotic pain medicine in the 90-day perioperative period. That patient was previously on a narcotic pain regimen at some point before surgery for other reasons.
Patient Informed Consent
The necessary patient informed consent was obtained in this study.
Discussion
Observations
Transition to Independent Practice: Outcomes, Challenges, and Lessons Learned
In the first 3 years of practice, we used the supraorbital eyebrow approach mainly for meningiomas located in the anterior skull base; however, the approach was very well suited for frontobasal gliomas, third-ventricle tumors, and unilateral anterior skull base defects resulting in an encephalocele or CSF leaks. The most common location of tumors operated on in this case series was the planum sphenoidale (33.33%), followed by the olfactory groove and tuberculum sellae (25% and 25%). The anterior skull base meningioma cases, which were approached with traditional skull base routes, included redo meningiomas, meningiomas involving distal anterior cerebral artery vasculature, clinoidal meningiomas with middle fossa extension, bilateral skull base defects causing CSF leaks, an encephalocele needing bilateral cranialization of the frontal sinus, or any vascular case (dural arteriovenous fistulas, arteriovenous malformation, or aneurysms).
The calculated average diameter of tumors in the second half of the practice was larger than in the first half, although the difference was not statistically significant (28.4 ± 18.1 mm versus 22.5 ± 12.9 mm, p = 0.79). Previous research describing the learning curve for skull base surgeons using the endonasal transsphenoidal approach did not note a trend or significant difference over time concerning the size of the tumor undergoing resection.23 Given the relatively larger (although not statistically significantly different) tumors resected in the second half, the blood loss and operative time were higher in the second half, as expected. However, the complication rate or readmission rate did not differ in the two halves and remained low.
The use of a rigid endoscope along with the operative microscope can provide a synergistic effect with the supraorbital craniotomy and subfrontal approach.24 In our case series, using endoscopy often aided in maximizing resection. Three of the 17 tumor patients undergoing elective tumor surgeries underwent subtotal resection, which was performed in clinoidal-cavernous meningioma (goals of surgery were optic canal decompression and tissue diagnosis), third-ventricle GBM with extension into the basal ganglia and brainstem, and low-grade myxoid glioneuronal tumor extending close to the hypothalamus and basal ganglia (Table 3, Supplementary Table 1).
Because the incision is on the eyebrow, which is very visible, good cosmetic outcomes are of paramount importance. Often, surgeons prefer facial plastic surgery closure assistance. In our series, our neurosurgery team closed the incision primarily. With permission, we have highlighted some cosmetic outcomes in Fig. 1. Eighteen of 19 patients were happy with their cosmetic outcome. One patient with frontobasal GBM had a favorable cosmetic result after the first surgery; however, a redo eyebrow approach for a recurrence after radiation and chemotherapy led to unexpected tearing of the skin edges with skin retractors, which was difficult to repair. The patient healed with no infection or dehiscence, but the scar was not cosmetically pleasing. We believe redo surgeries can lead to more tissue contraction, and patients with thin eyebrow hair follicles will often have poor cosmesis.
In our series, one patient needed typical opioids (e.g., oxycodone, codeine) following discharge. We used a combination of Tylenol, ibuprofen, and tramadol at discharge. Of the 19 patients, one developed worsening hyposmia, 11 had no change in CN function, and 7 had improvements in CN functioning. Permanent frontalis palsy was seen in one patient, possibly due to injury to the frontotemporal branch of the facial nerve while harvesting the pericranial flap.
Learning Curve in Skull Base Surgery
Research on learning curves in skull base surgeons has focused primarily on endonasal transsphenoidal approaches, with several studies demonstrating a steep initial learning curve.25–28 Younus et al.29 further suggested that improvements in surgical outcomes and reductions in complications extend beyond the initial learning curve and into the “plateau” or “tail end” of the learning curve for the endonasal transsphenoidal approach. We agree with this, because the surgeon takes on more challenging and complicated pathologies through similar approaches.
Impact of Using Eyebrow Approaches on Neurosurgical Boards
The senior author had logged eyebrow craniotomies and other keyhole approaches for his American Board of Neurological Surgery oral board examination in 2023. On the basis of his subjective experience, he did not believe that using keyhole approaches had any impact on outcome. He did not experience any negative feedback for using keyhole approaches early in his career. That said, the case selection was very stringent, and the key component in defining the goals of surgery was patient safety. Additionally, the author had comfortable exposure during his residency training and fellowship and was mentored toward practicing independently.1,11,13,30,31
Study Bias and Limitations
The major limitations of this study are its limited sample size, retrospective approach, and selection bias in reviewing only 20 supraorbital approaches. However, this study represents the early experience of a single surgeon and hence is expected to have a smaller sample size. Additionally, given the early experience, the study has a shorter follow-up, which we hope to extend further to analyze the long-term efficacy of supraorbital approaches.
Lessons
Supraorbital keyhole surgery with careful patient selection can be done safely in the early years of practice with good outcomes and low complication and readmission rates while lowering the narcotic burden in the community. Dedicated residency and fellowship programs with hands-on mentorship play a vital role in harnessing these skills.
Acknowledgments
We thank Parker Gavlin for her contribution in creating an illustration for our paper.
Author Contributions
Conception and design: Thakur, Shahid. Acquisition of data: Shahid, Butler, Bassett, Hummel, Chason. Analysis and interpretation of data: Shahid, Butler, Dyess. Drafting the article: Shahid, Butler, Dyess, Hummel. Critically revising the article: Thakur, Shahid, Butler. Reviewed submitted version of manuscript: Thakur, Shahid, Harris. Approved the final version of the manuscript on behalf of all authors: Thakur. Statistical analysis: Butler, Dyess. Administrative/technical/material support: Shahid, Harris, Hummel. Study supervision: Thakur, Shahid. Patient care: Chason. Institutional review board compliance: Harris. Surgical first assistant: Hummel.
Supplemental Information
Videos
Video 1. https://vimeo.com/908705938.
Video 2. https://vimeo.com/908711589.
Online-Only Content
Supplementary material is available with the online version of the article.
Supplementary Tables and Figure. https://thejns.org/doi/suppl/10.3171/CASE23744.
Previous Presentations
A portion of this abstract was presented at the Southern Neurosurgical Society Meeting 2024 in Orlando, FL, March 6–9, 2024, as an e-poster.
References
- 1. Thakur JD, Mallari RJ, Corlin A, et al. Critical appraisal of minimally invasive keyhole surgery for intracranial meningioma in a large case series. PLoS One. 2022;17(7):e0264053. doi: 10.1371/journal.pone.0264053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altay T, Couldwell WT. The frontotemporal (pterional) approach: an historical perspective. Neurosurgery. 2012;71(2):481–492. doi: 10.1227/NEU.0b013e318256c25a. [DOI] [PubMed] [Google Scholar]
- 3. Mourier KL, Cophignon J, D’Hermies F, Clay C, Lot G, George B. Superolateral approach to orbital tumors. Minim Invasive Neurosurg. 1994;37(1):9–11. doi: 10.1055/s-2008-1053441. [DOI] [PubMed] [Google Scholar]
- 4. Al-Mefty O, Fox JL. Superolateral orbital exposure and reconstruction. Surg Neurol. 1985;23(6):609–613. doi: 10.1016/0090-3019(85)90012-6. [DOI] [PubMed] [Google Scholar]
- 5. Delashaw JB, Jr, Tedeschi H, Rhoton AL. Modified supraorbital craniotomy: technical note. Neurosurgery. 1992;30(6):954–956. doi: 10.1227/00006123-199206000-00028. [DOI] [PubMed] [Google Scholar]
- 6. Delashaw JB, Jr, Jane JA, Kassell NF, Luce C. Supraorbital craniotomy by fracture of the anterior orbital roof. Technical note. J Neurosurg. 1993;79(4):615–618. doi: 10.3171/jns.1993.79.4.0615. [DOI] [PubMed] [Google Scholar]
- 7. Delfini R, Raco A, Artico M, Salvati M, Ciappetta P. A two-step supraorbital approach to lesions of the orbital apex. Technical note. J Neurosurg. 1992;77(6):959–961. doi: 10.3171/jns.1992.77.6.0959. [DOI] [PubMed] [Google Scholar]
- 8. Zabramski JM, Kiriş T, Sankhla SK, Cabiol J, Spetzler RF. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89(2):336–341. doi: 10.3171/jns.1998.89.2.0336. [DOI] [PubMed] [Google Scholar]
- 9. Andaluz N, Romano A, Reddy LV, Zuccarello M. Eyelid approach to the anterior cranial base. J Neurosurg. 2008;109(2):341–346. doi: 10.3171/JNS/2008/109/8/0341. [DOI] [PubMed] [Google Scholar]
- 10. Dare AO, Landi MK, Lopes DK, Grand W. Eyebrow incision for combined orbital osteotomy and supraorbital minicraniotomy: application to aneurysms of the anterior circulation. Technical note. J Neurosurg. 2001;95(4):714–718. doi: 10.3171/jns.2001.95.4.0714. [DOI] [PubMed] [Google Scholar]
- 11. Avery MB, Mallari RJ, Barkhoudarian G, Kelly DF. Supraorbital and mini-pterional keyhole craniotomies for brain tumors: a clinical and anatomical comparison of indications and outcomes in 204 cases. J Neurosurg. 2021;136(5):1314–1324. doi: 10.3171/2021.6.JNS21759. [DOI] [PubMed] [Google Scholar]
- 12. Mallari RJ, Thakur JD, Rhee JH, et al. Endoscopic endonasal and supraorbital removal of tuberculum sellae meningiomas: anatomic guides and operative nuances for keyhole approach selection. Oper Neurosurg (Hagerstown). 2021;21(2):E71–E81. doi: 10.1093/ons/opab138. [DOI] [PubMed] [Google Scholar]
- 13. Mallari RJ, Thakur JD, Griffiths C, et al. Tuberculum sellae meningiomas in pregnancy: 3 cases treated in the second trimester and literature review. World Neurosurg. 2020;143:268–275. doi: 10.1016/j.wneu.2020.07.198. [DOI] [PubMed] [Google Scholar]
- 14. Ormond DR, Hadjipanayis CG. The supraorbital keyhole craniotomy through an eyebrow incision: its origins and evolution. Minim Invasive Surg. 2013;2013:296469. doi: 10.1155/2013/296469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson DH. Limited exposure in cerebral surgery. Technical note. J Neurosurg. 1971;34(1):102–106. doi: 10.3171/jns.1971.34.1.0102. [DOI] [PubMed] [Google Scholar]
- 16. Brock M, Dietz H. The small frontolateral approach for the microsurgical treatment of intracranial aneurysms. Neurochirurgia (Stuttg). 1978;21(6):185–191. doi: 10.1055/s-0028-1090343. [DOI] [PubMed] [Google Scholar]
- 17. Reisch R, Marcus HJ, Hugelshofer M, Koechlin NO, Stadie A, Kockro RA. Patients’ cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg. 2014;121(3):730–734. doi: 10.3171/2014.4.JNS13787. [DOI] [PubMed] [Google Scholar]
- 18. Park HH, Sung KS, Moon JH, et al. Lateral supraorbital versus pterional approach for parachiasmal meningiomas: surgical indications and esthetic benefits. Neurosurg Rev. 2020;43(1):313–322. doi: 10.1007/s10143-019-01147-8. [DOI] [PubMed] [Google Scholar]
- 19. Andrade-Barazarte H, Jägersberg M, Belkhair S, et al. The extended lateral supraorbital approach and extradural anterior clinoidectomy through a frontopterio-orbital window: technical note and pilot surgical series. World Neurosurg. 2017;100:159–166. doi: 10.1016/j.wneu.2016.12.087. [DOI] [PubMed] [Google Scholar]
- 20. Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57(4) suppl:242–255. doi: 10.1227/01.neu.0000178353.42777.2c. [DOI] [PubMed] [Google Scholar]
- 21. Eroglu U, Shah K, Bozkurt M, et al. Supraorbital keyhole approach: lessons learned from 106 operative cases. World Neurosurg. 2019;124:e667–e674. doi: 10.1016/j.wneu.2018.12.188. [DOI] [PubMed] [Google Scholar]
- 22. Fonseca RB, Correia AO, Vieira RS, et al. Comparative study between minimally invasive supraorbital craniotomy and pterional craniotomy for treating anterior circulation cerebral aneurysms in a low-resource setting. Sci Rep. 2021;11(1):5555. doi: 10.1038/s41598-021-85115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zumofen DW, Rychen J, Roethlisberger M, et al. A review of the literature on the transciliary supraorbital keyhole approach. World Neurosurg. 2017;98:614–624. doi: 10.1016/j.wneu.2016.10.110. [DOI] [PubMed] [Google Scholar]
- 24. Marcus HJ, Pratt P, Hughes-Hallett A, et al. Comparative effectiveness and safety of image guidance systems in surgery: a preclinical randomised study. Lancet. 2015;385(suppl 1):S64. doi: 10.1016/S0140-6736(15)60379-8. [DOI] [PubMed] [Google Scholar]
- 25. Bokhari AR, Davies MA, Diamond T. Endoscopic transsphenoidal pituitary surgery: a single surgeon experience and the learning curve. Br J Neurosurg. 2013;27(1):44–49. doi: 10.3109/02688697.2012.709554. [DOI] [PubMed] [Google Scholar]
- 26. Boetto J, Joitescu I, Raingeard I, et al. Endoscopic transsphenoidal surgery for non-functioning pituitary adenoma: learning curve and surgical results in a prospective series during initial experience. Front Surg. 2022;9:959440. doi: 10.3389/fsurg.2022.959440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Candy NG, Ovenden C, Jukes AK, Wormald PJ, Psaltis AJ. The learning curve for endoscopic endonasal pituitary surgery: a systematic review. Neurosurg Rev. 2023;46(1):241. doi: 10.1007/s10143-023-02136-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chi F, Wang Y, Lin Y, Ge J, Qiu Y, Guo L. A learning curve of endoscopic transsphenoidal surgery for pituitary adenoma. J Craniofac Surg. 2013;24(6):2064–2067. doi: 10.1097/SCS.0b013e3182a24328. [DOI] [PubMed] [Google Scholar]
- 29. Younus I, Gerges MM, Uribe-Cardenas R, et al. How long is the tail end of the learning curve? Results from 1000 consecutive endoscopic endonasal skull base cases following the initial 200 cases. J Neurosurg. 2020;134(3):750–760. doi: 10.3171/2019.12.JNS192600. [DOI] [PubMed] [Google Scholar]
- 30. Nanda A, Thakur JD, Sonig A, Missios S. Microsurgical resectability, outcomes, and tumor control in meningiomas occupying the cavernous sinus. J Neurosurg. 2016;125(2):378–392. doi: 10.3171/2015.3.JNS142494. [DOI] [PubMed] [Google Scholar]
- 31. Ansari SF, Eisenberg A, Rodriguez A, Barkhoudarian G, Kelly DF. The supraorbital eyebrow craniotomy for intra- and extra-axial brain tumors: a single-center series and technique modification. Oper Neurosurg (Hagerstown). 2020;19(6):667–677. doi: 10.1093/ons/opaa217. [DOI] [PubMed] [Google Scholar]