Abstract
The human cytomegalovirus (HCMV) UL112-113 promoter represents a useful model for studying temporal regulation of viral gene expression. Stimulation of this promoter by the 86-kDa immediate-early protein (IE86) is controlled by sequences between nucleotides −113 and −59, which include both an ATF/CREB and an IE86 binding site. In transient assays, the ATF/CREB site is essential, and the IE86 site, although nonessential, can enhance transcription. With recombinant viruses, we have assessed the function of these promoter elements in the context of the viral genome. Transcription from the inserted UL112-113 promoter shows the same temporal pattern as the endogenous promoter, including the switch to an upstream RNA start site late in infection. Deletion of sequences containing the IE86 site results in a decrease in the level of early transcription and elimination of late transcription. In contrast, when the ATF/CREB site is deleted, early RNA synthesis is almost completely abolished, but late transcription is comparable to that of the wild type, with repositioning of the RNA start site downstream by the number of nucleotides deleted. Replacement of sequences between −108 and −95 with the HCMV cis-repression signal from the major immediate-early promoter had no effect on the level of late RNAs but resulted in the repositioning of the RNA start site 39 nucleotides upstream. These results suggest that the ATF/CREB site is functional only at early times, while sequences containing the IE86 site modulate the level of early RNAs and may be required for activating late transcription in a distance-dependent manner.
Human cytomegalovirus (HCMV), a member of the betaherpesvirus family, is an important opportunistic pathogen in immunocompromised individuals and is recognized as a major viral cause of birth defects in newborns (2). As for other herpesviruses, HCMV genes are expressed in a temporal pattern upon infection (5, 18, 29, 34, 35). Members of the immediate-early (IE) class of genes are expressed upon viral infection and do not require de novo protein synthesis for production of their RNAs. Several of the IE proteins are transactivators of the products of the next class of genes, the early genes. Concomitant with viral DNA replication, there is expression of the late class of viral genes, whose protein products are generally involved in viral genome packaging and virion maturation. Since several of the early gene protein products are essential for viral DNA replication, understanding the control of early viral gene expression is important for designing therapeutic strategies to inhibit viral replication.
In order to understand the mechanisms that control early viral gene expression, several laboratories have studied a number of different early viral promoters by various types of transcription assays (for reviews, see references 20 and 26). To date, the most common assay to study viral promoter activity has been the transient expression assay. In this assay, a plasmid containing a reporter gene under control of an early viral promoter is transfected into cells along with expression plasmids encoding HCMV transcriptional regulatory proteins. These assays have proven useful in determining which promoter sequences are important for gene expression as well as which HCMV proteins behave as transcriptional regulators. In fact, these assays have identified the HCMV IE2 86-kDa protein (IE86) and the IE1 72-kDa protein (IE72) as the major transregulators of early viral gene expression (for reviews, see references 20 and 26). However, transient expression assays are limited in that they do not provide accurate information about the regulatory events that occur during a normal viral infection. Several labs have attempted to circumvent this issue by transfecting cells with plasmid and subsequently superinfecting with virus (3, 10, 25, 27, 32). Although this allows one to study gene expression in infected cells, there remain template-specific differences between plasmid DNA and viral DNA. In particular, the late induction of the 1.2-kb RNA promoter, normally observed in infected cells, does not occur when the promoter is located on a plasmid in transfection-infection assays unless an HCMV origin of replication, oriLyt, is also included on the plasmid (33). In addition, it was shown that at least two genes, UL83 and UL99, with early-late and true late kinetics, respectively, were activated earlier and to higher levels in transfection-infection assays than observed with the endogenous genes during a normal infection (14). In an attempt to study early and late viral gene expression without the inaccuracies associated with transfection assays, Kohler et al. have used a gene replacement strategy based on the finding that a portion of the unique short region of the HCMV genome is dispensable for viral growth (14). To this end, they have constructed recombinant viruses in which a viral promoter linked to a reporter gene is inserted between the US9 and US10 genes and have shown that the inserted promoters exhibit kinetics similar to those of the endogenous viral promoters.
As a model for early HCMV gene expression, our laboratory has studied the expression of the 2.2-kb class of RNAs (open reading frame UL112-113) which encode a family of nuclear phosphoproteins (13, 27, 28, 36, 37). Although the function of these proteins is unclear, there is evidence that they behave as transcriptional activators and are required for viral DNA replication (7, 10, 21). By transient expression analysis, our laboratory has shown that activation of the UL112-113 promoter is mediated through a major regulatory domain between nucleotides −113 and −59 (23, 24). This region contains a weak binding site for the HCMV transactivator IE86 (−113 to −85) and a consensus ATF/CREB binding site (−71 to −66) (15, 23, 24). In addition, the UL112-113 promoter contains three other binding sites for IE86 (−286 to −257, −248 to −218, and −148 to −120) (1, 24). Results of mutational analyses of the UL112-113 promoter suggest that in transfection assays, the ATF/CREB site is the major regulatory element upstream of the TATA box, whereas the IE86 binding sites play an accessory role (1, 15, 23, 24).
The goal of this study was to determine the functions of various sequence elements within the UL112-113 promoter at different times during HCMV infection. To this end, we have further defined the roles of the sequences containing the ATF/CREB binding site and the weak IE86 binding site within the UL112-113 promoter at early and late times during infection. We have constructed recombinant viruses in which various mutations of the UL112-113 promoter, linked to the chloramphenicol acetyltransferase (CAT) gene, were inserted between the US9 and US10 genes in the viral genome and have analyzed UL112-113 promoter–CAT activity at different times during the infection. We find that the kinetics of expression of the UL112-113 promoter from the US9/10 locus are identical to that of the endogenous viral promoter, including the switch to a different RNA start site late in infection (28). Consistent with transient expression data, our results demonstrate that the sequences containing the ATF/CREB site play a major role in UL112-113 promoter activity early during viral infection, whereas the region containing the weak IE86 binding site has a moderate effect on transcription. However, at late times in the infection, we find that the ATF/CREB site plays little if any role in expression, but the sequences between −113 and −85, which include the weak IE86 binding site, are required for transcription from the late RNA start site within the UL112-113 promoter. Furthermore, replacement of the region between nucleotides −108 and −95 with the HCMV cis-repression signal (CRS) indicated that the sequences between −108 and −95 play a role in distance-dependent, and possibly orientation-dependent, late transcription initiation from the UL112-113 promoter. These data underscore the value of using recombinant viruses to study viral promoter activity and demonstrate that different promoter regulatory elements are employed at early and late times in the infection.
MATERIALS AND METHODS
Cells and virus.
Human foreskin fibroblasts (FF cells) were maintained in minimum essential medium with Earle’s salts containing 10% fetal bovine serum. HCMV Towne strain was obtained from American Type Culture Collection. Methods for cell culture and viral infection have been described elsewhere (30). All infections were performed with a multiplicity of infection of 3 to 10.
Molecular cloning.
Sequences used to facilitate homologous recombination into the HCMV US9/10 locus were derived from a 3.4-kb EcoRI-HindIII fragment from pHCMV-EcoRI-B (30). The 3.4-kb fragment was cut with SalI and end repaired with Klenow enzyme, and ligated to HindIII linkers, and the resulting 2.7-kb fragment was ligated into pGem-3Z at HindIII to give pGem-US9/10. Two oligonucleotides (5′-CAGATCTGCGGCCGCAGGCC-3′ and 5′-TGCGGCCGCAGATCTGGGCC-3′) were annealed, resulting in a 20-bp ApaI fragment containing internal BglII and NotI sites which was subsequently ligated into the ApaI site of pGem-US9/10. The resulting plasmid was cut with BglII, and a 4.8-kb BamHI fragment from pON855 (31), containing the lacZ and guanine phosphoribosyltransferase (gpt) genes, was inserted to give rise to pUS9/10-lacZ/gpt.
A 1.95-kb BamHI fragment from p358-CAT (27) was end repaired with Klenow enzyme, ligated to NotI linkers, and inserted at the NotI site of pUS9/10-lacZ/gpt to give pUS9/10-358-CAT. p148-CAT (27), p119-CAT (previously referred to as D [23]), and p148(Δ84-58)-CAT (previously referred to as Del [23]) were digested with HindIII and BamHI, end repaired with Klenow enzyme, ligated to NotI linkers, and inserted at the NotI site of pUS9/10-lacZ/gpt to give pUS9/10-148-CAT, pUS9/10-119-CAT, and pUS9/10-148(Δ84-58)-CAT, respectively.
p148(Δ84-58)CRS-CAT and p148(Δ84-58)IICRS-CAT were constructed by using a QuickChange site-directed mutagenesis kit (Stratagene). p148(Δ84-58)CRS-CAT was constructed according to the manufacturer’s protocol, using the two oligonucleotides 5′-CTAGAGTACCAGTCGTTTAGTGAACCGTACTGTTTAAGGG-3′ and 5′-CCCTTAAACAGTACGGTTCACTAAACGACTGGTACTCTAG-3′ and the plasmid p148(Δ84-58)-CAT. p148(Δ84-58)IICRS-CAT was constructed similarly, using the two oligonucleotides 5′-GTTTAGTGAACCGTTTAGTGAACGGGTGTTGCTAGG-3′ and 5′-CCTAGCAACACCCGTTCACTAAACGGTTCACTAAAC-3′ and the plasmid p148(Δ84-58)CRS-CAT. HindIII-BamHI fragments from p148(Δ84-58)CRS-CAT and p148(Δ84-58)IICRS-CAT were end repaired with Klenow enzyme, ligated to NotI linkers, and inserted into the NotI site of pUS9/10-lacZ/gpt to give pUS9/10-148(Δ84-58)CRS-CAT and pUS9/10-148(Δ84-58)IICRS-CAT, respectively.
Recombinant virus construction.
All plasmids derived from pUS9/10-lacZ/gpt were linearized with HindIII, and 30 μg was electroporated into 4 × 106 FF cells. Twenty-four hours after electroporation, the cells were infected with wild-type HCMV (Towne strain) at a multiplicity of infection of 5 to 10. Infections were allowed to proceed for 5 days, and virus was harvested by clarifying the culture supernatant at 1,500 rpm for 5 min. Virus was passaged three times under selection as follows. Briefly, cells were infected, and the inoculum was removed 3 h later and replaced with media containing 150 μM mycophenolic acid and 10 μM xanthine. After 5 days, the supernatant was collected, the cells were sonicated to release membrane-associated virus, and the supernatants were combined. Virus was then added to fresh cells, and plaques were stained with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; 0.5 mg/ml) to visualize recombinants. Recombinant viruses were subsequently plaque purified two to three times. The purified virus was then used to infect 5 × 105 cells. The viral supernatant was used to further amplify the virus, and genomic DNA was harvested from the cells to verify virus genotype by Southern blot analysis.
Southern blot analysis.
Genomic DNA from mock or virus-infected cells was isolated by using a QIAamp blood kit (Qiagen) as described in the manufacturer’s protocol. To confirm the presence of the CAT gene in the recombinant viruses, genomic DNA was digested with NotI and subjected to Southern blot analysis by using standard methods. Blots were hybridized to a 32P-labeled, 551-bp HindIII-to-NcoI DNA fragment isolated from pSV2-CAT (American Type Culture Collection) and subjected to autoradiography. To test for purity of recombinant viruses and to confirm insertion into the US9/10 locus, genomic DNA was digested with EcoRI and NcoI, and Southern blot analysis was performed with a 32P-labeled, 4.5-kb EcoRI-to-NcoI DNA fragment isolated from pHCMV-EcoRI-B.
Western blot analysis.
FF cells were infected with HCMV recombinants and harvested at the indicated times by lysing directly in Laemmli sample buffer. Lysates (104 cell equivalents) were electrophoresed on 10% polyacrylamide protein gels and transferred to Immobilon membranes (Millipore). Blocked membranes were incubated with primary antibody BSA 2-9 (37), followed by incubation with a horseradish peroxidase-coupled secondary antibody and detection with chemiluminescence (Pierce) according to standard methods.
CAT enzyme assays and RNA primer extension analysis.
FF cells were infected with HCMV recombinants, harvested at the indicated times, and assayed for CAT enzyme as previously described (27). CAT assays were quantitated by scintillation counting or phosphorimager analysis.
For primer extension analysis, 7 × 106 FF cells were infected with recombinant HCMV and harvested at the indicated times by trypsinization. Total RNA was isolated from cell pellets by using a Qiagen RNeasy Midi kit. Primer extension reactions were performed as described previously by using a 32P-end-labeled oligonucleotide complementary to CAT RNA (positions +15 to +53 relative to the ATG codon) and 50 μg of total RNA (22).
RESULTS
Construction of recombinant HCMV.
The HCMV UL112-113 promoter has been extensively studied by using plasmid transfections, in vitro transcription, and DNase I footprint analyses (1, 13, 15, 23, 24, 27, 28, 36). However, these assays are limited in that certain aspects of infection-specific viral gene regulation are absent. In particular, late viral transcription is inaccurately represented in plasmid transfections (14). To better understand the regulation of the UL112-113 promoter during infection, we constructed recombinant HCMV in which the UL112-113 promoter, linked to the CAT gene, was inserted into the viral genome. The recombinant viruses were then used to analyze the role of various UL112-113 promoter elements throughout the infection.
The region containing the US1 to the US11 genes has been shown to be dispensable for viral growth (8, 9). Stable recombinant HCMV can be constructed by insertion of sequences between the US9 and US10 genes through homologous recombination (14). Therefore, we have used this technique to insert the UL112-113 promoter–CAT sequences into the viral genome. We constructed several different recombinant viruses containing previously uncharacterized site-directed mutations as well as promoter mutations that have been previously analyzed by transient expression analysis (Fig. 1). Recombinant viruses were propagated under GPT selection, and putative recombinants were identified by X-Gal staining as described in Materials and Methods.
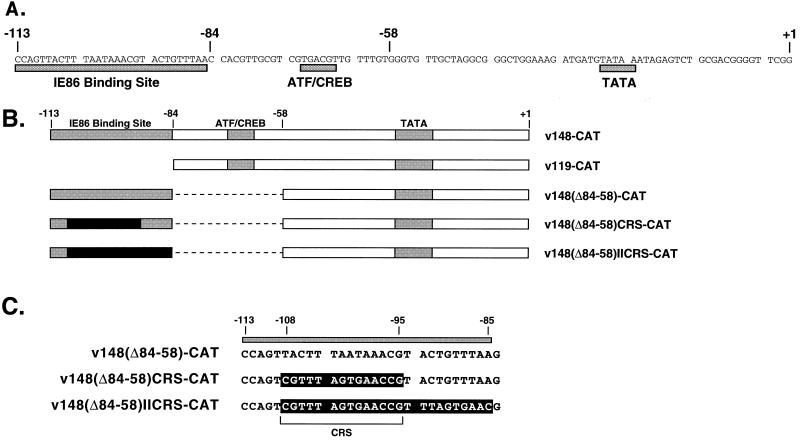
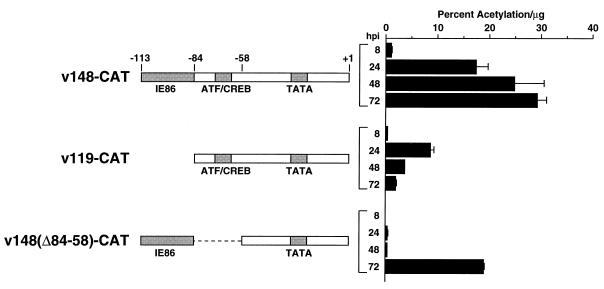
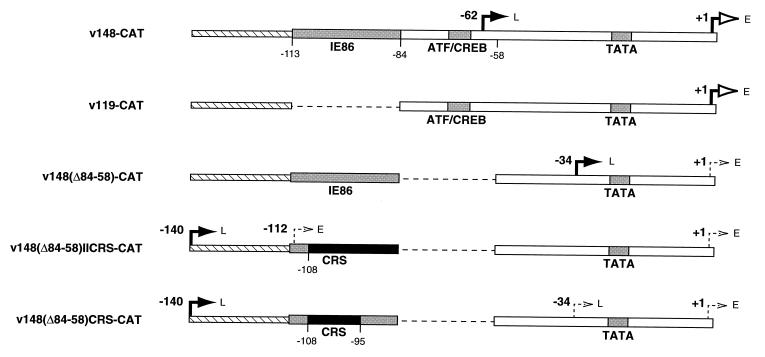
FIG. 1.
UL112-113 promoters inserted into recombinant viruses. (A) Sequences of the HCMV UL112-113 promoter from −113 to +1. Previously identified regulatory elements are indicated by the shaded boxes beneath the sequence. The IE86 binding site represents the sequences protected by IE86 in a DNase I footprint analysis (24), whereas the CREB/ATF and TATA sites are the respective consensus sequences. (B) Map of UL112-113 promoter mutations used in this study. The name of each recombinant virus is indicated at the right. The weak IE86 binding site, CREB/ATF site, and TATA box are indicated by shaded boxes. The black box indicates the replacement of the weak IE86 binding site and/or adjacent A-T rich sequences with the HCMV CRS from the major IE promoter. Dashed lines denote deleted sequences. (C) Sequence between −113 and −85 in v148(Δ84-58)-CAT, v148(Δ84-58)CRS-CAT, and v148(Δ84-58)IICRS-CAT. Sequences protected by IE86 in a DNase I footprint are indicated by the shaded box above the sequence. Sequences outlined in black are those replaced in v148(Δ84-58)CRS-CAT and v148(Δ84-58)IICRS-CAT. The CRS is indicated by the bracket.
The genotype of the recombinant viruses was confirmed by Southern blot analysis (Fig. 2). To confirm the presence of CAT sequences, genomic DNA preparations from infected cells were digested with NotI, which should yield a fragment containing the CAT gene and the UL112-113 promoter. The size of this fragment varies from 1.8 to 2.1 kb, depending on the promoter mutation inserted. Southern blots were probed with a 32P-labeled, 551-bp HindIII-NcoI DNA fragment containing only CAT sequences. As expected, CAT sequences were not detected in wild-type- or mock-infected cells (Fig. 2C, lanes 1 and 2). However, a single specific band between 1.8 and 2.1 kb was detected in all recombinant viruses, confirming the presence of the CAT gene (Fig. 2C, lanes 3 to 7). Furthermore, the size of each band was consistent with the length of the UL112-113 promoter mutation inserted.
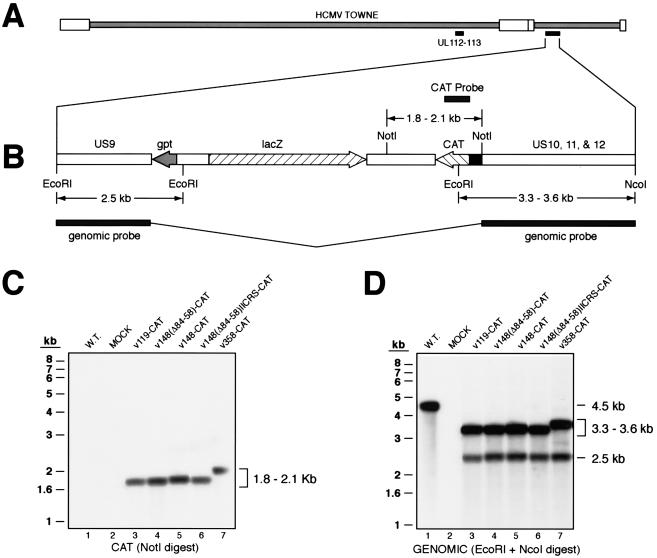
FIG. 2.
Site of recombination in the HCMV genome and Southern blot analysis of recombinant viruses. (A) Schematic representation of the site of recombination within the HCMV genome. Shown is the HCMV Towne strain. White boxes denote the terminal repeat sequences. Black bars beneath the genome indicate the location of the endogenous UL112-113 genes and the site of insertion within the region between the US9 and US12 genes. (B) Expanded map of the region between the US9 and US12 genes in recombinant HCMV. Where indicated, the direction of the open reading frame is shown with an arrow. Sizes of fragments expected to hybridize to the CAT probe in Southern blots are shown above the map; sizes of fragments expected to hybridize to the genomic probe are shown below the map. Angled lines between the shaded bars of the genomic probe indicate that the probe is contiguous. (C) Southern blot of genomic DNA from mock- and virus-infected cells, using the CAT probe for detection. Viruses analyzed are indicated above the autoradiogram. The expected size range of the hybridized DNA fragment is indicated on the right. Positions of molecular weight markers are indicated on the left. W.T., wild type. (D) Southern blot of genomic DNA from mock- and virus-infected cells, using the genomic probe for detection. Viruses analyzed are indicated above the autoradiogram. The expected size ranges of the hybridized DNA fragments are indicated on the right. Positions of molecular weight markers are indicated on the left.
To test for purity of recombinant viruses and to confirm proper insertion into the US9/10 locus, genomic DNA was digested with EcoRI and NcoI, which, in wild-type virus, yields a 4.5-kb fragment spanning the US9-US12 genes. This 4.5-kb sequence was also used as a probe for Southern blots. The 4.5-kb fragment was detected in cells infected with wild-type virus, whereas no signal was detected in mock-infected cells (Fig. 2D, lanes 1 and 2). In recombinant viruses, an EcoRI/NcoI digest gave a pattern different from that of wild-type virus due to the presence of two additional EcoRI sites within the inserted sequences (Fig. 2B). Therefore, the 4.5-kb probe detected two fragments flanking the insertion site; a 2.5-kb fragment containing the US9 and gpt genes, and a fragment containing the US10-12 genes, the UL112-113 promoter, and CAT sequences (Fig. 2D, lanes 3 to 7). The size of the latter fragment varied from 3.3 to 3.6 kb, depending on the promoter mutation. The pattern shown in Fig. 2D confirmed the site of insertion between the US9 and US10 genes. The purity of the recombinants was verified by the absence of a 4.5-kb band on longer exposures (data not shown).
Analysis of viral protein production in recombinant virus infection.
The US9/10 region has been shown to accept the insertion of exogenous DNA without affecting viral growth (11, 14). However, it was important to verify that all constructed recombinant viruses had normal growth characteristics. To assess the growth characteristics of the recombinant viruses and to confirm that the endogenous UL112-113 promoter behaved normally, we analyzed the kinetics of expression of the endogenous UL112-113 family of proteins during recombinant virus infection. In a wild-type virus infection, a 43-kDa protein is detected by 8 h after infection; by 48 to 72 h after infection, the 34-, 50-, and 84-kDa proteins are also detected (37). By Western blot analysis, we determined that the pattern of expression of the UL112-113 family of proteins in the recombinant viruses was similar to that in wild-type Towne virus at both early and late time points (Fig. 3). In addition, all recombinant viruses grew to titers similar to that of wild-type Towne virus (data not shown). These results suggest that insertion of sequences between the US9 and US10 genes had no effect on viral growth or endogenous UL112-113 promoter activity.
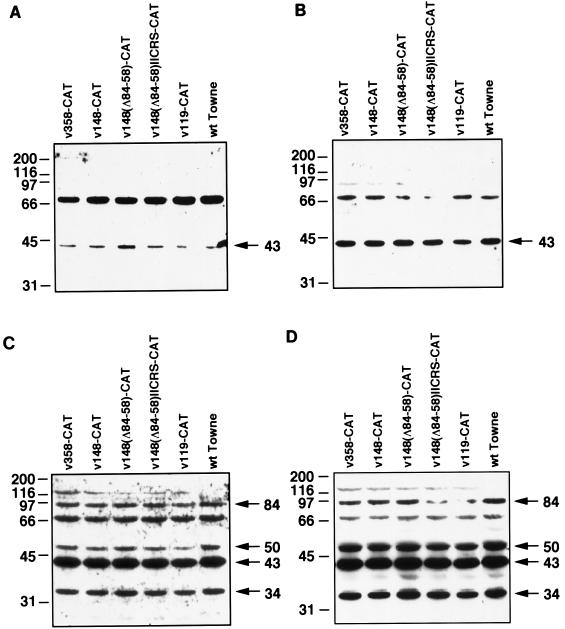
FIG. 3.
Western blot analysis of the UL112-113 proteins during infection with recombinant viruses. Western blot analysis was performed with total protein isolated from infected cells at 8 (A), 24 (B), 48 (C), and 72 (D) h after infection as described in Materials and Methods. The recombinant viruses used are indicated above each blot. Sizes of the UL112-113 proteins are indicated in kilodaltons on the right of each blot; positions of molecular weight markers are indicated in kilodaltons on the left of each blot. The band detected just above 66 kD appears to be a cross-reactive protein and has been previously observed in some experiments (36). The band near 116 kDa is detected only with recombinant viruses and may represent LacZ expressed from the human β-actin promoter. wt, wild type.
Analysis of CAT protein and RNA expression from the US9/10 locus in the recombinant virus v358-CAT.
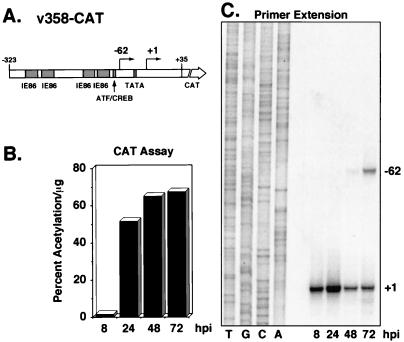
The recombinant virus designated v358-CAT contains wild-type UL112-113 promoter sequences from −323 to +35 (Fig. 4A). These promoter sequences contain four binding sites for the HCMV transactivator protein IE86 and one ATF/CREB binding site upstream of the TATA box (1, 15, 23, 24). v358-CAT was used to test the pattern of expression of the UL112-113 promoter when inserted into the US9/10 locus. CAT assays performed on lysates harvested at 8, 24, 48, and 72 h after infection showed low levels of protein at 8 h after infection followed by an accumulation of higher levels of protein starting at 24 h after infection (Fig. 4B). This was similar to the accumulation of protein levels of the endogenous UL112-113 family of proteins at the same times after wild-type virus infection (Fig. 3) (37). UL112-113 promoter expression from the US9/10 locus was also examined by primer extension analysis using a primer that hybridizes to sequences within the CAT gene (Fig. 4C). By using total RNA isolated at 8, 24, 48, and 72 h after infection, it was demonstrated that the previously mapped early mRNA start site for the UL112-113 promoter (28) was also used when the promoter was inserted in the US9/10 region. Beginning at 48 h after infection, transcription from the early (+1) start site declined and transcription initiated from a new start site at −62. These kinetics, including the switch in transcriptional start sites at late times, mimic that of the endogenous UL112-113 promoter (28), demonstrating that insertion of promoter sequences within the US9/10 locus represents a valid method to study UL112-113 promoter activity in the context of a viral infection.
FIG. 4.
Analysis of CAT RNA and protein expression during infection with v358-CAT recombinant virus. (A) Map of the UL112-113 promoter and CAT gene inserted into v358-CAT. Sequences from −323 to +35 were linked to the CAT gene and inserted into a recombinant virus as described in Materials and Methods. Arrows indicate the location and direction of transcription of RNA from the early start site (+1) and the late start site (−62). Previously identified regulatory regions are indicated by the shaded boxes. (B) CAT activity detected in lysates from v358-CAT-infected cells. Protein lysates from infected cells were harvested at 8, 24, 48, and 72 h after infection with v358-CAT. CAT activity was determined as described in Materials and Methods and is represented as percent acetylation per microgram of lysate used in the reaction. Time points are indicated below the histogram. (C) Primer extension analysis of total RNA isolated from v358-CAT-infected cells. Total RNA was isolated from infected cells at 8, 24, 48, and 72 h after infection, and primer extension analysis was performed as described in Materials and Methods, using a primer which detects CAT mRNA. Extension products were separated by denaturing polyacrylamide gel electrophoresis and subjected to autoradiography. Products were electrophoresed adjacent to a sequencing ladder (lanes T, G, C, and A) generated with plasmid p148-CAT and the identical CAT primer to identify transcriptional start sites. Locations of start sites are indicated at the right. Time points are indicated below the autoradiogram. hpi, hours postinfection.
CAT activity from UL112-113 recombinant viruses.
Previous transient expression assays have shown that the three IE86 binding sites between −323 and −113 have very little effect on UL112-113 promoter activity (1, 15, 23, 24). These experiments also identified the ATF/CREB site between −71 and −66 as the major element upstream of the TATA box contributing to promoter activity. Therefore, in determining which UL112-113 promoter sequences are important during viral infection, we have concentrated on the sequences between −113 and +35, which contain, in addition to the ATF/CREB site and the TATA box, a weak IE86 binding site (Fig. 1A) (24). The recombinant virus containing the sequences between −113 and +35 is designated v148-CAT (Fig. 1B). CAT assays performed with lysates harvested at 8, 24, 48, and 72 h after infection with v148-CAT showed kinetics similar to that of v358-CAT (compare Fig. 5 to Fig. 4B). CAT levels at each time point were about twofold lower in v148-CAT infections compared to v358-CAT, confirming that the three upstream IE86 binding sites in combination have only a moderate effect on promoter activity.
FIG. 5.
Analysis of CAT protein expression during infection with recombinant viruses containing UL112-113 promoter deletions. Protein lysates were prepared at 8, 24, 48, and 72 h postinfection (hpi), and CAT activity was determined as described in Materials and Methods. Activity is represented as percent acetylation per microgram of lysate used in the reaction. Solid bars represent the average of two independent infections; error bars represent the range of the two values. Promoter deletions contained in the recombinant viruses analyzed are indicated on the left.
Our initial analysis also included two recombinant viruses containing promoter deletions that were previously analyzed in transient expression assays (23). Recombinant virus v119-CAT contains sequences from −84 to +35 and is missing all IE86 binding sites but maintains the ATF/CREB site and TATA box (referred to as D in reference 23). Recombinant virus v148(Δ84-58)-CAT is deleted for sequences between −84 and −58 and contains the weak IE86 binding site and the TATA box but lacks the ATF/CREB site (referred to as Del in reference 23).
In transient assays, it was previously shown that deletion of the weak IE86 binding site resulted in a twofold drop in promoter activity (23). Analysis of CAT activity derived from v119-CAT infection showed a similar twofold drop in CAT activity relative to v148-CAT when assayed at 8 and 24 h after infection. However, at 48 and 72 h after infection, there were decreases of 6.8- and 15.8-fold, respectively (Fig. 5). These data suggested that although sequences containing the IE86 binding site play a modest role early infection, those sequences play a greater role in UL112-113 promoter activity late in infection.
It was also shown by transient assays that deletion of the ATF/CREB site results in a 20-fold drop in UL112-113 promoter activity (23). Analysis of CAT activity derived from v148(Δ84-58)-CAT infection showed a similar dependence on the ATF/CREB site early during infection. At 8, 24, and 48 h after infection, deletion of the ATF/CREB site resulted in 19.6-, 51.2-, and 68.9-fold decreases in CAT activity, respectively, relative to v148-CAT. However, between 48 and 72 h after infection with v148(Δ84-58)-CAT, CAT levels were stimulated approximately 52.8-fold. Furthermore, CAT levels 72 h after infection with v148(Δ84-58)-CAT were only twofold lower than that detected 72 h after infection with v148-CAT. Although the ATF/CREB site is a major regulatory element early in the infection, these data suggest that this site plays little if any role at late times in the infection. Taken together, these results demonstrate that separate DNA elements differentially regulate the UL112-113 promoter at early and late times in the infection.
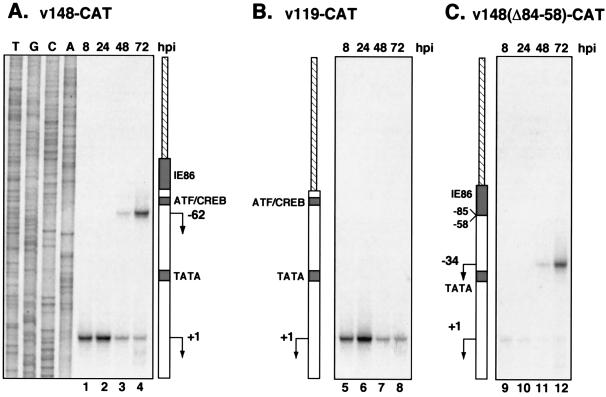
Primer extension analysis of UL112-113 recombinant viruses.
Since the ATF/CREB site and sequences containing the weak IE86 binding site seem to have different effects on promoter activity depending on the stage of the infection, we sought to determine what role these sequences played in the initiation of transcription from the early (+1) and late (−62) RNA start sites. To this end, primer extension analysis was performed on RNA isolated at 8, 24, 48, and 72 h after infection with v148-CAT, v119-CAT, and v148(Δ84-58)-CAT (Fig. 6). During infection with v148-CAT, transcription initiation from the +1 site increased up to 24 h after infection and then declined by 48 and 72 h (Fig. 6A). As with v358-CAT, a shift in the transcriptional start site to −62 was first observed at 48 h after infection and increased between 48 and 72 h. The levels of extended product mapping to +1 were slightly lower with v148-CAT than with v358-CAT. However, the levels mapping to −62 were almost identical, suggesting that the IE86 binding sites upstream of −113 have no effect on transcription from the late, −62 mRNA initiation site.
FIG. 6.
Analysis of CAT RNA expression during infection with recombinant viruses containing UL112-113 promoter deletions. Primer extension analysis of total RNA isolated from cells infected with v148-CAT (A), v119-CAT (B), and v148(Δ84-58)-CAT (C) was performed as described in the legend to Fig. 4. Promoter deletions contained in the recombinant viruses analyzed are indicated adjacent to each panel. Arrows indicate the position of the transcriptional start sites detected in the assay. Products were electrophoresed adjacent to a sequencing ladder (lanes T, G, C, and A) generated with the plasmid p148-CAT and the identical CAT primer to identify transcriptional start sites. Each panel as well as Fig. 4C was derived from the same autoradiogram but was cropped for display purposes. hpi, hours postinfection.
CAT activity observed during infection with v119-CAT suggested that the sequences from −113 to −85, containing the IE86 binding site, had little effect on promoter activity early during infection but had a more dramatic role late. By primer extension analysis of RNA from v119-CAT infections, we found that transcription from the +1 site was identical to that seen with v148-CAT (Fig. 6B). However, mRNA initiation from −62 was completely abolished in v119-CAT infections. Since the −62 initiation site is still present in v119-CAT, these results suggested that sequences upstream from −62, which included the IE86 binding site, were important in directing the initiation of transcription late in infection.
Primer extension analysis was also performed with RNA isolated from v148(Δ84-58)-CAT infections. Deletion of the ATF/CREB site (which also deleted the −62 position) inhibited transcription from the +1 site, providing further evidence for the role of the ATF/CREB site in early UL112-113 promoter activity. The deletion of promoter sequences from −84 to −58 placed the sequences containing the IE86 binding site 26 nucleotides closer to the TATA box. In turn, it was observed that the initiation site detected at 48 and 72 h after v148-(Δ84-58)-CAT infection was shifted 28 nucleotides downstream of the original −62 position to the −34 position (Fig. 6C, lanes 11 and 12). Taken together, these data suggest that the site of transcription initiation from the UL112-113 promoter late in infection is determined not by the sequence of the initiation site but by upstream sequences, which contain the IE86 binding site, in a distance-dependent manner. Furthermore, since the level of extended product detected at 48 and 72 h after infection with v148(Δ84-58)-CAT was comparable to that detected after v148-CAT infection (compare Fig. 6C, lanes 11 and 12 with Fig. 6A, lanes 3 and 4), the ATF/CREB binding site was not required for late transcription from the UL112-113 promoter.
Late transcription initiation from the UL112-113 promoter.
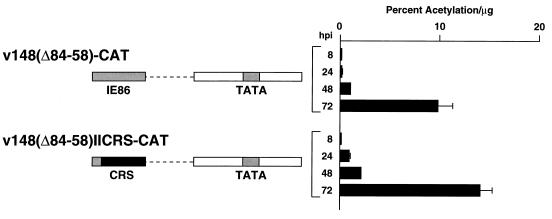
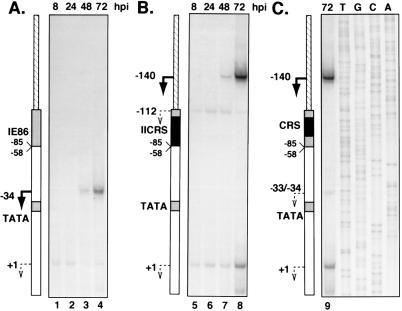
The UL112-113 promoter sequences between −113 and −85, which seem to be responsible for distance-dependent initiation of transcription late in infection, contain a weak IE86 binding site. In addition, two A-T-rich sequences are present within this region (Fig. 1C). To determine which sequences within −113 to −85 were involved in directing late transcription initiation, we constructed two recombinant viruses containing site-directed mutations between −113 and −85 in the context of v148(Δ84-58)-CAT. In v148(Δ84-58)IICRS-CAT, both A-T-rich sequences have been deleted and the CRS from the HCMV major IE promoter has been inserted between −108 and −95 in an attempt to retain IE86 binding to the promoter. A second virus, v148(Δ84-58)CRS-CAT, contains the CRS but leaves intact the A-T-rich sequences between −94 and −85 (Fig. 1C). In v148(Δ84-58)IICRS-CAT infections, CAT levels at all time points tested were comparable to the levels detected in cells infected with v148(Δ84-58)-CAT (Fig. 7). Thus, the original interpretation was that the mutations had no effect on late transcription. However, primer extension analysis of RNA isolated from v148(Δ84-58)IICRS-CAT infections showed that transcription from the normal late start site [−34 with v148(Δ84-58)-CAT] was abolished, and a new RNA start site, with identical kinetics, was detected upstream at −140 (Fig. 8). Of particular interest was the observation that this new start site was located approximately 39 nucleotides upstream from the center of the CRS element, similar to the distance between the normal late start site and the IE86 binding site in v148-CAT. In addition, a weaker start site with kinetics similar to transcription from the +1 early site was detected at −112. Primer extension analysis of RNA isolated 72 h after infection with v148(Δ84-58)CRS-CAT showed a pattern similar to that of v148(Δ84-58)IICRS-CAT, but with low levels of transcripts also initiating at or near −34.
FIG. 7.
Analysis of CAT protein expression during infection with v148(Δ84-58)IICRS-CAT. Protein lysates were prepared at 8, 24, 48, and 72 h after infection with either v148(Δ84-58)-CAT or v148(Δ84-58)IICRS-CAT, and CAT activity was determined as described in Materials and Methods. Activity is represented as percent acetylation per microgram of lysate used in the reaction. Solid bars represent the average of two independent infections; error bars represent the range of the two values. Promoter deletions and site-directed mutations contained in the recombinant viruses analyzed are indicated on the left. hpi, hours postinfection.
FIG. 8.
Analysis of CAT RNA expression during infection with v148(Δ84-58)-CAT, v148(Δ84-58)CRS-CAT, and v148(Δ84-58)IICRS-CAT. Primer extension analysis of total RNA isolated from cells infected with v148(Δ84-58)-CAT (A), v148(Δ84-58)IICRS-CAT (B), and v148(Δ84-58)CRS-CAT (C) was performed as described in the legend to Fig. 4 except that only the 72-h time point was analyzed for v148(Δ84-58)CRS-CAT. Promoter deletions contained in the recombinant viruses analyzed are indicated adjacent to each panel. Arrows indicate positions of the transcriptional start sites detected in the assay. Numbers represent the positions of the nucleotides relative to the sequence of the promoter in v148-CAT. Each panel was derived from the same autoradiogram but was cropped for display purposes. hpi, hours postinfection.
Thus, replacement of the sequences between −108 and −95 with the CRS had no effect on the kinetics of late transcription or on the distance of the late start site from the center of the element between −108 and −95. However, when the CRS was inserted, the late start site was now positioned approximately 39 nucleotides upstream of the element rather than downstream. Taken together, these data suggest that the sequences between −108 and −95 play a role in directing late transcription from the UL112-113 promoter, in a distance-dependent and possibly orientation-dependent manner.
DISCUSSION
The goal of this study was to more accurately define the roles of specific sequence elements within the HCMV UL112-113 promoter during infection. The success of the recombinant virus approach depended on the ability of the UL112-113 promoter inserted into a different region of the genome to function similarly to the endogenous promoter. We found that when inserted between the US9 and US10 genes, the UL112-113 promoter showed identical kinetics of RNA expression as the endogenous promoter located in the UL region. As with the endogenous promoter, the inserted UL112-113 promoter was active both early and late in infection. In particular, a shift in the RNA start site late in infection was also observed from the inserted UL112-113 promoter. Taken together, our data indicated that expression of the UL112-113 promoter was accurately represented when located at an alternate position in the genome and in the opposite orientation relative to the endogenous promoter. Thus, by inserting the UL112-113 promoter-CAT sequences into the HCMV genome, we were able to determine which promoter elements were functional at various times after infection.
UL112-113 promoter activity late in infection.
The results presented here from early time points (8 and 24 h) during recombinant virus infection are in agreement with data previously obtained from transient expression analysis (1, 15, 23, 24). Namely, for the UL112-113 promoter, the ATF/CREB site played a major role early in infection, whereas the sequences containing the IE86 binding site (−113 to −85) played an accessory role. However, the most intriguing finding in this study was that late in infection, the relative importance of the ATF/CREB site and the sequences containing the IE86 binding site was reversed. By 72 h after infection, the ATF/CREB site was no longer functional in transcriptional activation, whereas the sequences between −113 to −85 were required for the switch to the late RNA start site and wild-type levels of late transcription. This phenomenon was not detected by transient expression analysis and demonstrates the importance of using recombinant viruses to accurately determine temporal regulation of viral promoters during infection.
Two experiments indicated that the ATF/CREB site was no longer functional late in infection. First, infection with v148(Δ84-58)-CAT, which is deleted for the ATF/CREB site, and v148-CAT, which contains the ATF/CREB site, resulted in a similar induction of CAT activity by 72 h (Fig. 5). Second, during infection with a recombinant virus containing only the ATF/CREB site and TATA box (v119-CAT), CAT levels steadily declined late in infection despite the increase in viral template DNA. Furthermore, v119-CAT showed CAT levels at 72 h after infection similar to those of a virus containing only the TATA box (data not shown). Since CREB is the major protein that binds to the UL112-113 ATF/CREB site in uninfected cells (23), our data suggest that CREB activity is downregulated at late times in the infection. Although it has been known for many years that some early HCMV promoters are downregulated late in infection (for a review, see reference 20), this study provides the first evidence to suggest that one mechanism of early promoter shutoff is due to down regulation of specific host transcription factor activity as the infection proceeds.
Although CREB binds to the UL112-113 ATF/CREB site in uninfected cell extracts, it is not known whether CREB still binds to this promoter late in the infection. The binding activity of another member of the ATF/CREB family, ATF-1, is induced at late times during infection (11). However, the UL112-113 ATF/CREB site binds little if any ATF-1 in uninfected cells (23), and it is not known if ATF-1 can bind to the UL112-113 ATF/CREB site late in infection. If ATF-1 can bind to the UL112-113 ATF/CREB site late in infection, our data suggest that this binding cannot lead to transcriptional activation since the UL112-113 ATF/CREB site was not functional in late transcription.
There are several possible mechanisms for the downregulation of CREB activity during infection, including, but not limited to, degradation, dephosphorylation, or alternative modification of CREB. CREB activity is normally regulated through phosphorylation on Ser-133 (6) which is required for interaction with the CREB binding protein (4). Preliminary results from our laboratory indicate that CREB protein levels remain constant throughout infection (19). However, it appears that Ser-133 becomes dephosphorylated as the infection proceeds and that there are additional modifications to CREB. Thus, it is possible that HCMV encodes or modifies functions to regulate host transcription factor activity so that certain viral genes are expressed at specific times during the infection. Studies are in progress to address these questions.
Although the sequences between −113 and −85, which contain a weak IE86 binding site, are dispensable for early transcriptional activity, results from experiments with two recombinant viruses suggested that these sequences are required for normal levels of late transcription as well as the change in RNA start site observed at 48 and 72 h after infection. First, deletion of the sequences between −113 and −85 in v119-CAT abolished transcription initiation from the −62 position late in infection. Second, deletion of the sequences between −84 to −58 in v148(Δ84-58)-CAT had no effect on the levels of late transcription, but the late RNA initiation site was moved 28 bp downstream to −34, a distance similar to the number of deleted nucleotides between −84 and −58 (Fig. 6). This putative distance-dependent late transcription initiation seems to be unique to the sequences around the weak IE86 site, since no transcription initiation is detected near the upstream IE86 binding sites (Fig. 4C). However, at present we do not know whether the three upstream IE86 sites can contribute to transcription initiation either late in infection in the absence of the sequences containing the weak IE86 binding site or early in infection in the absence of the ATF/CREB site.
Since the sequences from −113 to −85 are protected by IE86 in a DNase I footprint, it is possible that IE86 binding to this sequence plays a role in late transcription initiation from the UL112-113 promoter. Alternatively, there are two A-T-rich sequences within that region which could behave as TATA-like sequences to direct late transcription in a distance dependent manner. To distinguish between these possibilities, we constructed two recombinant viruses that contained substitution mutations between −113 and −85, v148(Δ84-58)CRS-CAT and v148(Δ84-58)IICRS-CAT. Although the sequences from −113 to −85 are protected by IE86 in a DNase I footprint, this sequence diverges somewhat from the consensus IE86 binding site. Based on footprint analyses, a consensus IE86 binding site consists of an A-T-rich 10-nucleotide sequence bounded by CG dinucleotides (1, 16, 24). The sequence between −113 and −86 contains a CG with A-T rich sequences on either side but lacks a complementary CG dinucleotide (Fig. 1). The best approximation of the IE86 binding site within the −113 to −84 sequence, based on the DNase I protection pattern (24), would be nucleotides −108 to −95. Therefore, we replaced that sequence with the CRS in v148(Δ84-58)CRS-CAT to determine if the IE86 binding site was involved in late transcription. We also constructed another virus, v148(Δ84-58)IICRS-CAT, which included mutation of the TATA-like sequences from −88 to −84 in addition to the substituted CRS.
The results of the primer extension analyses on all recombinant viruses are summarized in Fig. 9. Substitution of the sequences between −108 and −85 resulted in several interesting observations. First, transcription from the late RNA start site at −34 was abolished in v148(Δ84-58)IICRS-CAT, suggesting that the sequences from −108 to −85 were involved in directing late transcription initiation at the downstream site. We also noted that there was a very low level of steady-state RNA initiating near −34 in v148(Δ84-58)CRS-CAT infection. One interpretation of these data is that the TATA-like sequence between −88 and −84 may play a role in determining the site of late transcription initiation and that the upstream sequences between −108 to −95 are important for high-level expression. However, because close inspection of the gels suggests that this RNA may actually be initiating at −33, it is possible that the substituted sequences have activated a weak cryptic site for initiation at late times. Nevertheless, it appears that the wild-type sequences containing the weak IE86 binding site between −108 and −95 are involved in downstream late transcription initiation.
FIG. 9.
Summary of RNA start sites from recombinant viruses and schematic representation of promoter mutations used in this study. Names of the viruses containing the promoters are indicated at the left. Major early (E) RNA start sites are denoted by open arrowheads, whereas major late (L) RNA start sites are denoted by closed arrowheads. Minor RNA start sites are shown as dashed arrows. Sequence elements are as described in the legend to Fig. 1. Numbers represent the positions of the nucleotides relative to the sequence of the promoter in v148-CAT.
One of the most interesting observations from the primer extension analysis of v148(Δ84-58)CRS-CAT and v148(Δ84-58)IICRS-CAT infections was the emergence of two new RNA start sites upstream of the inserted CRS. A weak start site with early kinetics was detected initiating from −112. However, since this site is observed only in v148(Δ84-58)IICRS-CAT, it is likely the result of mutation of the sequences between −94 and −85. The strongest of the two new sites initiated at −140 and displayed late kinetics. Thus, late transcription was abolished from a position downstream of the IE86 binding site and instead initiated at a novel position upstream. Interestingly, the novel late RNA start site initiated within the polylinker sequence which had been inserted as a result of cloning the UL112-113 promoter into the transfer vector used for recombinant virus construction. Since this novel late RNA start site was detected during infection with both v148(Δ84-58)CRS-CAT and v148(Δ84-58)IICRS-CAT, insertion of the CRS was responsible for the appearance of this site. It is interesting that the distance from the novel late start site (−140) to the center of the inserted CRS (between nucleotides and −102 and −101) is almost identical to the distance from the center of the putative weak IE86 binding site (between nucleotides and −102 and −101) to the wild-type late start site (−62) detected in v148-CAT infection. This observation raises the possibility that the orientation of the CRS, and of the weak IE86 binding site within the wild-type UL112-113 promoter, determines the positioning of the late transcriptional start site. Since IE86 binds to the minor groove of the DNA (17), the orientation of the IE86 binding site relative to other surrounding sequences may result in certain structural effects on IE86 binding that determine the position of late transcription initiation. Alternatively, unidentified sequences within the CRS, and within the UL112-113 promoter sequences between −108 and −95, may be involved in orientation-dependent, late transcription initiation independent of IE86 binding. At present, we also cannot rule out the possibility that the mutations cause activation of an upstream cryptic promoter which results in initiation of transcription at position −140. Further experiments are under way to determine the precise sequences required, the dependence on orientation and distance, and whether IE86 binding to these sequences is essential.
UL112-113 promoter activity early in infection.
Analysis of CAT activity and mRNA levels at 8 and 24 h after infection with v148(Δ84-58)-CAT, which is deleted for the ATF/CREB site, suggested that the ATF/CREB site is required for high levels of promoter activity early in infection, whereas the sequences containing the IE86 binding site are less important. Since deletion of the ATF/CREB site positioned the IE86 binding site closer to the TATA box, one can argue that the IE86 site is no longer functional due to the change in its position. However, in transient expression assays, site-directed mutation of the ATF/CREB site without altering the position of the IE86 binding site showed that the IE86 site could not compensate for the loss of the ATF/CREB site (23). Since our results suggest that UL112-113 promoter activity in transient expression assays mimics that of early time points during infection, we expect that the IE86 site in its normal location also would not compensate for the loss of the ATF/CREB site early in infection.
Since the ATF/CREB site is functional only early during infection and since the host transcription factor CREB binds to this site within the UL112-113 promoter, HCMV, like many viruses, employs the strategy of utilizing cellular transcription factors in the initial phases of the infection. However, only weak expression from the UL112-113 promoter is observed when viral protein synthesis is inhibited (27), suggesting that other viral proteins, such as IE86, are required for maximum levels of transcription. Our results also suggested that the sequences containing the weak IE86 binding site played only a modest role early in infection. Similarly, in transient expression assays, deletion of the weak IE86 binding site reduced UL112-113 promoter activity only about twofold (23). However, in the transient expression experiments the IE86 protein is required for promoter activity even in the absence of an IE86 binding site on the promoter. Thus, IE86 may function through protein-protein contacts without the requirement for DNA binding, or at least without the need for a consensus IE86 binding site. Indeed, IE86 can interact with the CREB binding protein (CBP) and weakly with a truncated form of CREB, ΔCREB (15, 23). Since IE86 is expressed in the recombinant virus infections, these studies do not allow us to distinguish the role of the IE86 protein at the promoter early in infection. We can only conclude that the sequences containing the weak IE86 binding site are not essential for early UL112-113 promoter activity.
The results presented here suggest that for specific promoters there are different mechanisms governing early and late viral transcription. It is likely that many early promoters use existing cellular transcription factors, as well as virus-encoded factors, to enhance transcription under low-template conditions prior to viral DNA replication. However, as the infection proceeds, certain cellular factors may be either downregulated or upregulated to alter the expression pattern of specific viral genes. Although there is an increase in viral DNA template late in infection due to DNA replication, transcription from the +1 position in the UL112-113 promoter decreases. The mechanism governing this downregulation of transcription from one site and upregulation from a different site late in infection is unclear. Further analysis of other early and late HCMV promoters is needed to fully understand the regulation of viral gene expression during infection.
ACKNOWLEDGMENTS
We thank Ruth Schwartz and Ed Mocarski for plasmids used in this study. We also thank Roopashree Dwarakanath, Elizabeth Fortunato, Anita McElroy, Chris Morello, and Bryan Salvant for helpful discussions and critical reviews of the manuscript.
This investigation was supported by NIH grant CA 34729 (D.H.S.) and NIH training grant AI-07384 (S.M.R.).
REFERENCES
- 1.Arlt H, Lang D, Gebert S, Stamminger T. Identification of binding sites for the 86-kilodalton IE2 protein of human cytomegalovirus within an IE2-responsive viral early promoter. J Virol. 1994;68:4117–4125. doi: 10.1128/jvi.68.7.4117-4125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Britt W, Alford C. Cytomegalovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2493–2523. [Google Scholar]
- 3.Chang C-P, Malone C L, Stinski M F. A human cytomegalovirus early gene has three inducible promoters that are regulated differentially at various times after infection. J Virol. 1989;63:281–290. doi: 10.1128/jvi.63.1.281-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 5.DeMarchi J M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981;114:23–28. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez G A, Montminy M R. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 7.Iskenderian A C, Huang L, Reilly A, Stenberg R M, Anders D G. Four of eleven loci required for transient complementation of human cytomegalovirus DNA replication cooperate to activate expression of replication genes. J Virol. 1996;70:383–392. doi: 10.1128/jvi.70.1.383-392.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones T R, Muzithras V P. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J Virol. 1992;66:2541–2546. doi: 10.1128/jvi.66.4.2541-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones T R, Muzithras V P, Gluzman Y. Replacement mutagenesis of the human cytomegalovirus genome; US10 and US11 gene products are nonessential. J Virol. 1991;65:5860–5872. doi: 10.1128/jvi.65.11.5860-5872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerry J A, Priddy M A, Jervey T Y, Kohler C P, Staley T L, Vanson C D, Jones T R, Iskenderian A C, Anders D G, Stenberg R M. Multiple regulatory events influence expression of human cytomegalovirus DNA polymerase (UL54) expression during viral infection. J Virol. 1996;70:373–382. doi: 10.1128/jvi.70.1.373-382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerry J A, Priddy M A, Staley T L, Jones T R, Stenberg R M. The role of ATF in regulating the human cytomegalovirus DNA polymerase (UL54) promoter during viral infection. J Virol. 1997;71:2120–2126. doi: 10.1128/jvi.71.3.2120-2126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klucher K M, Rabert D K, Spector D H. Sequences in the human cytomegalovirus 2.7-kilobase RNA promoter which mediate its regulation as an early gene. J Virol. 1989;63:5334–5343. doi: 10.1128/jvi.63.12.5334-5343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klucher K M, Sommer M, Kadonaga J T, Spector D H. In vivo and in vitro analysis of transcriptional activation mediated by the human cytomegalovirus major immediate-early proteins. Mol Cell Biol. 1993;13:1238–1250. doi: 10.1128/mcb.13.2.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler C P, Kerry J A, Carter M, Muzithras V Z, Jones T R, Stenberg R M. Use of recombinant virus to assess human cytomegalovirus early and late promoters in the context of the viral genome. J Virol. 1994;68:6589–6597. doi: 10.1128/jvi.68.10.6589-6597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lang D, Gebert S, Arlt H, Stamminger T. Functional interaction between the human cytomegalovirus 86-kilodalton IE2 protein and the cellular transcription factor CREB. J Virol. 1995;69:6030–6037. doi: 10.1128/jvi.69.10.6030-6037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lang D, Stamminger T. The 86-kilodalton IE-2 protein of human cytomegalovirus is a sequence-specific DNA-binding protein that interacts directly with the negative autoregulatory response element located near the cap site of the IE-1/2 enhancer-promoter. J Virol. 1993;67:323–331. doi: 10.1128/jvi.67.1.323-331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang D, Stamminger T. Minor groove contacts are essential for an interaction of the human cytomegalovirus IE2 protein with its DNA target. Nucleic Acids Res. 1994;22:3331–3338. doi: 10.1093/nar/22.16.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonough S H, Spector D H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983;125:31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- 19.McElroy, A. K., and D. H. Spector. Unpublished data.
- 20.Mocarski E S. Cytomegaloviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Field’s virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2447–2492. [Google Scholar]
- 21.Pari G S, Anders D G. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J Virol. 1993;67:6979–6988. doi: 10.1128/jvi.67.12.6979-6988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodems S M, Friesen P D. The hr5 transcriptional enhancer stimulates early expression from the Autographa californica nuclear polyhedrosis virus genome but is not required for virus replication. J Virol. 1993;67:5776–5785. doi: 10.1128/jvi.67.10.5776-5785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz R, Helmich B, Spector D H. CREB and CREB-binding proteins play an important role in the IE2 86-mediated transactivation of the human cytomegalovirus 2.2-kb RNA promoter. J Virol. 1996;70:6955–6966. doi: 10.1128/jvi.70.10.6955-6966.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz R, Sommer M H, Scully A, Spector D H. Site-specific binding of the human cytomegalovirus IE2 86-kilodalton protein to an early gene promoter. J Virol. 1994;68:5613–5622. doi: 10.1128/jvi.68.9.5613-5622.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaete R R, Mocarski E S. Regulation of cytomegalovirus gene expression: α and β promoters are trans-activated by viral functions in permissive fibroblasts. J Virol. 1985;56:135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spector D H. Activation and regulation of human cytomegalovirus early genes. Intervirology. 1996;39:293–301. doi: 10.1159/000150507. [DOI] [PubMed] [Google Scholar]
- 27.Staprans S I, Rabert D K, Spector D H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988;62:3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Staprans S I, Spector D H. 2.2-Kilobase class of early transcripts encoded by cell-related sequences in human cytomegalovirus strain AD169. J Virol. 1986;57:591–602. doi: 10.1128/jvi.57.2.591-602.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stinski M F. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J Virol. 1978;26:686–701. doi: 10.1128/jvi.26.3.686-701.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamashiro J C, Hock L J, Spector D H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169) J Virol. 1982;42:547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viera J, Farrell H E, Rawlinson W D, Mocarski E S. Genes in the HindIII J fragment of the murine cytomegalovirus genome are dispensable for growth in cultured cells; insertion mutagenesis with the lacZ/gpt cassette. J Virol. 1994;68:4837–4846. doi: 10.1128/jvi.68.8.4837-4846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade E J, Klucher K M, Spector D H. An AP-1 binding site is the predominant cis-acting regulatory element in the 1.2 kilobase early RNA promoter of human cytomegalovirus. J Virol. 1992;66:2407–2417. doi: 10.1128/jvi.66.4.2407-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wade E J, Spector D H. The human cytomegalovirus origin of DNA replication (oriLyt) is the critical cis-acting sequence regulating the replication-dependent late induction of the viral 1.2-kb promoter. J Virol. 1994;68:6567–6577. doi: 10.1128/jvi.68.10.6567-6577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wathen M W, Stinski M F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982;41:462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wathen M W, Thomsen D R, Stinski M F. Temporal regulation of human cytomegalovirus transcription at immediate early and early times after infection. J Virol. 1981;38:446–459. doi: 10.1128/jvi.38.2.446-459.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright D A, Spector D H. Posttranscriptional regulation of a class of human cytomegalovirus phosphoproteins encoded by an early transcription unit. J Virol. 1989;63:3117–3127. doi: 10.1128/jvi.63.7.3117-3127.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright D A, Staprans S I, Spector D H. Four phosphoproteins with common amino termini are encoded by human cytomegalovirus AD169. J Virol. 1988;62:331–340. doi: 10.1128/jvi.62.1.331-340.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]