Abstract
Rotaviruses are the major cause of severe diarrhea in infants and young children worldwide. Due to their restricted site of replication, i.e., mature enterocytes, local intestinal antibodies have been proposed to play a major role in protective immunity. Whether secretory immunoglobulin A (IgA) antibodies alone can provide protection against rotavirus diarrhea has not been fully established. To address this question, a library of IgA monoclonal antibodies (MAbs) previously developed against different proteins of rhesus rotavirus was used. A murine hybridoma “backpack tumor” model was established to examine if a single MAb secreted onto mucosal surfaces via the normal epithelial transport pathway was capable of protecting mice against diarrhea upon oral challenge with rotavirus. Of several IgA and IgG MAbs directed against VP8 and VP6 of rotavirus, only IgA VP8 MAbs (four of four) were found to protect newborn mice from diarrhea. An IgG MAb recognizing the same epitope as one of the IgA MAbs tested failed to protect mice from diarrhea. We also investigated if antibodies could be transcytosed in a biologically active form from the basolateral domain to the apical domain through filter-grown Madin-Darby canine kidney (MDCK) cells expressing the polymeric immunoglobulin receptor. Only IgA antibodies with VP8 specificity (four of four) neutralized apically administered virus. The results support the hypothesis that secretory IgA antibodies play a major role in preventing rotavirus diarrhea. Furthermore, the results show that the in vivo and in vitro methods described are useful tools for exploring the mechanisms of viral mucosal immunity.
There is significant evidence indicating that secretory immunoglobulin A (sIgA) antibodies are associated with protection against mucosal pathogens (5, 22, 23, 33, 37, 38, 44, 46). However, while sIgA antibodies form a first line of defense against many pathogens, the actual mechanisms of how they protect are not well understood. Proposed mechanisms include prevention of contact of pathogens with epithelial surfaces, formation of immune complexes, clearance by peristalsis, transcytosis of immune complexes, and intracellular neutralization with or without neutralizing antibodies (1, 3, 4, 18, 21, 29, 32).
Rotavirus is the most important etiologic agent of severe diarrhea in young children and is estimated to be responsible for 870,000 deaths per year in children under 5 years of age (20). The virus is composed of a core surrounded by VP6, the major inner capsid protein. The outer capsid layer of infectious particles contains two proteins, VP4 and VP7, one of which is subjected to cleavage by proteolytic enzymes (VP4 is cleaved into VP5 and VP8) and the other of which is an endoplasmic reticulum glycoprotein (6). The two outer capsid proteins are associated with stimulation of serotype-specific antibodies and protection in vivo and neutralization in vitro (11, 12, 15, 19, 35, 43). Although antibodies with protein specificity other than VP4 and VP7 may participate in protection against rotavirus infection, protection from clinical disease appears to rely mainly on the stimulation of neutralizing antibodies against outer capsid proteins VP4 and VP7 (26, 36, 45). It is therefore tempting to believe that neutralizing sIgA antibodies play a crucial role in mucosal defense. Several studies have also shown a strong correlation between protection in vivo and serum and intestinal IgA responses (2, 7, 25, 31). However, apart from one recent interesting study showing that two nonneutralizing VP6-specific IgA monoclonal antibodies (MAbs) were capable of preventing primary infection and resolving chronic murine rotavirus infection (3), no qualitative data on the mechanism of how sIgA antibodies protect against and clear a rotavirus infection have been reported.
We recently reported the production and characterization of murine IgA MAbs directed against rhesus rotavirus (RRV) (9). In the present study, we used several of these IgA MAbs to examine if a single MAb secreted onto mucosal surfaces via the normal epithelial transport pathway can protect mice from rotavirus diarrhea. We also studied if IgA antibodies can be transcytosed in a biologically active form through filter-grown Madin-Darby canine kidney (MDCK) cells expressing the polymeric immunoglobulin receptor (pIgR) (14) and, upon apical arrival, neutralize apically administered virus. Both experiments were performed to obtain information about the mechanisms involved in protection against rotavirus diarrhea and to evaluate if the methods used can be applied to the study of viral mucosal immunity.
MATERIALS AND METHODS
Virus production and purification.
Plaque-purified RRV was used throughout the study. A single virus stock was produced for the entire study by infecting MA104 cells with RRV at a multiplicity of infection (MOI) of 0.1 in serum-free M199 medium (Gibco Laboratories, Grand Island, N.Y.) containing 0.5 μg of trypsin (Sigma Chemical Co., St. Louis, Mo.) per ml. When the cytopathogenic effect reached approximately 75% of the monolayer, cells were freeze-thawed twice and cell lysates were cleared by low-speed centrifugation. The virus suspension was divided into aliquots and stored at −80°C until use. Determination of virus titers was performed by an immunoperoxidase focus reduction test (42) (see below).
RRV antigen for use in enzyme-linked immunosorbent assays (ELISA) (see below) was prepared from infected cell lysates by ultracentrifugation in a Beckman 45 Ti rotor at 35,000 rpm for 2 h at 4°C. The pellet was resuspended in 10 mM Tris–100 mM NaCl–2 mM CaCl2 (pH 7.4) (TNC) and layered onto a 25% sucrose cushion in TNC. After centrifugation in a Beckman SW41 rotor at 35,000 rpm for 2 h at 4°C, the virus pellet was resuspended in 1 to 2 ml of TNC and stored at 4°C until use.
Polarized epithelial cell monolayers.
MDCK cells stably transfected with cDNA encoding the rabbit pIgR (pIgR+MDCK cells) (14) were cultured in M199 medium containing 10% fetal calf serum (Gibco). Confluent pIgR+MDCK cell monolayers on permeable supports were obtained by seeding 4 × 105 cells in 1.5 ml of medium onto 24-mm (0.4-μm-pore-size) Transwell-Col filters (Costar, Cambridge, Mass.). The basolateral chambers in six-well plates (Costar) were then filled with 3 ml of the same medium. Media were replaced every other day until the monolayers developed transepithelial electrical resistance of >1,000 Ω. Transepithelial electrical resistance was measured with a Millicell-ERS resistance apparatus (Millipore, Bedford, Mass.) as described previously (42). Electrical resistance values obtained in the absence of cells were considered background values. The net resistance was calculated by subtracting the background values and multiplying the resulting resistance by the area of the filter.
Hybridoma cell cultures.
The production and characterization of the murine anti-RRV IgG and IgA MAbs used in this study (Table 1) have been reported elsewhere (9, 40). Four hybridoma cell clones producing IgA MAbs were selected; these accounted for at least three distinct neutralization epitopes on outer capsid protein VP4 (all in the VP8 tryptic peptide of VP4). Three nonneutralizing IgA-secreting hybridomas to VP6, randomly chosen from a total of five available cell lines, were also included. A neutralizing IgA MAb to Sabin 1 poliovirus (3C10) was used as a control (8). In all cases, IgA-secreting cell lines were shown to produce mostly polymeric forms of immunoglobulin, based on nonreducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis (8, 9). Hybridoma cells were grown in Dulbecco’s minimal essential medium (DMEM; Gibco) supplemented with 15% fetal calf serum, 1 mM sodium pyruvate, 2 mM l-glutamine, 50 IU of penicillin per ml, and 50 μg of streptomycin per ml.
TABLE 1.
Determination of anti-RRV IgA and IgG MAbs transcytosed through pIgR+MDCK cells by an ELISA
| Hybridoma | Isotype | Antigena | Immunoglobulin concn in media (range)b
|
|
|---|---|---|---|---|
| Apical (ng/ml) | Basolateral (μg/ml) | |||
| 1E4 | IgA | VP4 (ND) | 852 (755–990) | 35 (29–46) |
| 2A10 | IgA | VP4 (133) | 485 (385–560) | 27 (23–33) |
| 2B12 | IgA | VP4 (148) | 333 (20–405) | 11 (10–14) |
| 4B6 | IgA | VP4 (190) | 357 (290–470) | 11 (9–14) |
| 1D4 | IgA | VP6 (ND) | 541 (415–710) | 45 (36–59) |
| 2C5 | IgA | VP6 (ND) | 800 (620–1,040) | 32 (23–39) |
| 2F8 | IgA | VP6 (ND) | 733 (590–810) | 3 (1–6) |
| 1A9 | IgG | VP4 (100) | 76 (70–80) | 135 (105–170) |
| 7A12 | IgG | VP4 (188) | 85 (70–100) | 56 (42–76) |
| 3C10 | IgA | Sabin 1 poliovirus | 492 (430–570) | 33 (28–40) |
Numbers in parentheses indicate positions of amino acid changes in neutralization escape virus variants selected for by each MAb. ND, not determined.
Average of three independent experiments.
Transcytosis of anti-RRV MAbs.
Transcytosis of polymeric IgA was performed essentially as described previously (14). Briefly, hybridoma cells were washed with serum-free DMEM and resuspended in a collagen solution containing 8 volumes of collagen (Vitrogen 100; Celtrix, Santa Clara, Calif.); 1 volume of M199 medium (10×); 0.1 volume each of 1 M HEPES (pH 7.1), l-glutamine, nonessential amino acids, sodium pyruvate, and Nutridoma-SP (Boehringer GmbH, Mannheim, Germany); and 0.3 volume of 7% sodium bicarbonate. The cell-collagen mixture (3 × 106 cells in 1.7 ml of collagen solution/well) was poured into six-well plates and incubated at 37°C for 1 h to allow collagen polymerization. One milliliter of DMEM supplemented with 1% Nutridoma-SP, l-glutamine, sodium pyruvate, nonessential amino acids, and antibiotics was then added, and the plates were incubated at 37°C for 4 days, with the medium being changed every other day. One day before the experiment, pIgR+MDCK-cell containing filters were placed on top of the hybridoma cell cultures and fresh DMEM–Nutridoma-SP containing 1 μM dexamethasone (Sigma) was added to both chamber sides to stimulate the expression of pIgR (14). Nutridoma-SP and dexamethasone were included in both test and control wells in all experiments.
Immunoperoxidase staining of RRV-infected cells.
Cells grown in plastic wells or on permeable filters were processed for immunoperoxidase staining essentially as described previously (42). MA104 or MDCK cell monolayers were fixed with 1% paraformaldehyde in phosphate-buffered saline (PBS) for 1 h at 4°C. After being washed with PBS, cells were permeabilized by incubation with 0.5% Triton X-100 in PBS for 10 min at room temperature. Rotavirus-infected cells were identified by an immunoperoxidase staining method with an anti-VP7 nonneutralizing IgG MAb (m60) and a peroxidase-conjugated goat anti-mouse IgG (heavy and light chains) antibody (Sigma). The reaction was developed with aminoethylcarbazole (1 mg/ml) in 0.1 M acetate buffer (pH 5.2), and stained cells were counted under a light microscope. Background staining in the absence of virus replication and de novo synthesis of VP7 was found to be low with this procedure (data not shown).
Virus neutralization assay with pIgR+MDCK-hybridoma cell cocultures.
MDCK cell monolayers in Transwell units (Costar) were infected from the apical side by adding 4 × 103 PFU of RRV (0.2 ml) to the apical medium (1.5 ml), corresponding to an MOI of approximately 0.002. Virus was trypsin activated (5 μg/ml, 1 h, 37°C) prior to dilution, and trypsin was omitted from the infection medium to avoid multiple cycles of viral replication. In experiments concerning intracellular neutralization, the apical medium was first removed, MDCK cell monolayers were then washed twice, and fresh medium was added immediately prior to virus inoculation. After binding at 37°C for 90 min, the apical medium was aspirated, cells were washed once, and fresh medium was added. Plates were then incubated at 37°C for 18 h in a CO2 atmosphere. Monolayers were fixed and stained as described above, and the number of infected cells was determined. Collagen-filled wells with no hybridoma cells were included as controls.
Progeny virus production in pIgR+MDCK-hybridoma cell cocultures.
MDCK-hybridoma cell cocultures were infected as described above at an MOI of approximately 0.02. At 18 h postinfection, filters were moved to new six-well plates and washed on both sides five times with fresh medium to remove extracellular antibody. Monolayers were then freeze-thawed twice, and filters were cut apart and placed into a centrifuge tube along with the last washing medium (2 ml). RRV was extracted by vortexing cells in the presence of 1,1,2-trichloro-trifluoro-ethane (Sigma) for 2 min, and the aqueous phase was recovered after low-speed centrifugation. Infectious virus titers were then determined with MA104 cell monolayers grown in microtiter plates by immunoperoxidase staining of infected cells as described above (42).
Determination of total IgA and IgG in cell culture supernatants, sera, and stools.
The concentrations of antibodies in basolateral and apical media were determined by an ELISA. Briefly, microtiter ELISA plates were coated with a 1:5,000 dilution of goat anti-mouse immunoglobulin (Sigma) in PBS overnight at 4°C. Plates were blocked with 3% bovine serum albumin (BSA) for 2 h at 37°C and washed with PBS containing 0.1% Tween 20 (PBS/T). Serial twofold dilutions of test media in PBS–0.5% BSA were added to each well in duplicate and incubated for 2 h at 37°C. Plates were then rinsed four times with PBS/T and further incubated with a 1:2,000 dilution of a goat anti-mouse IgA (α chain) or anti-mouse IgG (heavy and light chains) antibody conjugated with biotin (Sigma) for 1 h at 37°C. After being washed, plates were incubated with a 1:5,000 dilution of streptavidin conjugated with alkaline phosphatase (Sigma) for 30 min at 37°C. p-Nitrophenyl phosphate (Sigma 104; 1 mg/ml) in 10 mM diethanolamine (pH 9.5) was added to wells. Plates were read with an ELISA reader (Bio-Rad Laboratories, Hercules, Calif.) at a wavelength of 405 nm. Antibody concentrations were determined from standard curves constructed with purified preparations of mouse IgA (318 μg/ml) and IgG1 (852 μg/ml) antibodies. These were included in each test plate to monitor plate-to-plate variations.
Stools and sera were collected from mouse pups used in protection experiments at the time of oral challenge with RRV. Stools were diluted to 10% in PBS and centrifuged to remove debris. Stool supernatants and sera were examined for total IgA and IgG by an ELISA as described above.
Determination of anti-RRV antibodies in sera and stools.
The amounts of both neutralizing and nonneutralizing antibodies (i.e., anti-VP6) secreted into the blood samples and stools of mice carrying hybridoma “backpack tumors” (see below) were determined by an RRV-specific ELISA. Briefly, ELISA plates were coated with a 1:200 dilution of purified RRV in PBS at 4°C overnight. Plates were blocked with 3% BSA–PBS, and stool or serum samples were added in twofold dilutions. After incubation for 2 h at 37°C, plates were washed with PBS/T and further incubated with a 1:2,000 dilution of an anti-mouse IgA (α chain) or IgG (heavy and light chains) antibody conjugated with biotin for 1 h at 37°C. Absorbance values equal to or higher than three times the background values were considered positive.
Determination of neutralizing anti-RRV antibodies in sera and stools.
Sera and stools obtained from pups were also tested by a microneutralization assay as described previously (9). Briefly, serial twofold dilutions (100 μl) of serum or stool extracts in M199 medium were incubated with a trypsin-activated RRV suspension (100 μl) containing approximately 200 PFU for 2 h at 37°C. Mixtures were then inoculated into MA104 cell monolayers grown in 96-well plates and incubated for 2 h at 37°C. After being washed with M199 medium, monolayers were refed with fresh M199 medium and incubated at 37°C in a CO2 atmosphere for 18 h. Monolayers were fixed and stained with immunoperoxidase as described above. A reduction in the number of RRV-infected cells of greater than 60% with respect to the number in control wells was considered to indicate neutralization. Neutralizing titers were expressed as the reciprocal of the highest dilution of sera or stools yielding neutralization.
Hybridoma backpack tumors and in vivo protection.
At day 1 after birth, 106 hybridoma cells in 70 μl of DMEM were injected subcutaneously into the upper back of BALB/c mice to generate IgA-secreting hybridoma tumors as described by Winner et al. (46). After 6 to 8 days, when a tumor was visible, mice were given 2 × 107 PFU of RRV orally with a gavage needle. Mice were then examined for the onset of diarrhea daily for 1 week by gentle abdominal palpation.
RESULTS
Transcytosis of IgA and IgG through filter-grown pIgR+MDCK cells.
The amounts of IgA and IgG MAbs present in the basolateral media and transcytosed into the apical media were quantified by an ELISA (Table 1). At 24 h after dexamethasone stimulation, the concentrations of IgG and IgA antibodies in the basolateral medium ranged from 3 to 135 μg/ml. During the same period, IgA was transported into the apical medium at concentrations of 333 to 852 ng/ml. Although IgA and IgG MAbs were produced in comparable amounts by hybridoma cells in the basolateral medium, the corresponding amounts of transcytosed IgG were 4 to 11 times lower, i.e., 76 ng/ml for MAb 1A9 and 85 ng/ml for MAb 7A12 (Table 1), indicating that IgG was not actively transported by pIgR through the epithelium. Further support for this result derives from control experiments with nontransfected MDCK cells, in which IgG and IgA concentrations in the apical medium were similar and as low as the apical medium IgG concentration in transfected cells during the same study period (data not shown).
Transcytosed anti-VP8 IgA antibodies neutralize apically administered RRV.
Next, we explored if antibodies could be trancytosed in a biologically active form from the basolateral side through pIgR+MDCK cells and neutralize apically administered RRV. The experiment was performed by adding 4 × 103 PFU of RRV to the apical side of monolayers 24 h after dexamethasone stimulation. After 18 h of infection, cells were fixed and the number of infected cells was determined. The data in Fig. 1 represent the percentages of infected cells compared to controls (collagen with no hybridoma cells) and show that all four anti-VP8 IgA MAbs neutralized RRV approximately 10-fold. No or a limited neutralization effect was observed in this assay with anti-VP6 IgA MAbs (zero of three), anti-VP8 IgG MAbs (zero of two), or antipoliovirus IgA (zero of one).
FIG. 1.
RRV neutralization in polarized pIgR+MDCK cells cocultured with hybridoma cells in Transwell-Col filter chambers. Filter-grown confluent MDCK cell monolayers laying on top of hybridoma cell-containing collagen layers were infected with 4 × 103 PFU of RRV from the apical side after 24 h of dexamethasone stimulation. After virus binding at 37°C for 90 min, the apical medium was replaced and cells were further incubated at 37°C for 18 h. Monolayers were fixed and immunostained as described in Materials and Methods, and RRV-infected cells were counted. Columns indicate the percentages of infected cells compared to control monolayers infected in the absence of hybridoma cells. Data represent the averages of three independent experiments (bars indicate ranges). 1E4, 2A10, 2B12, and 4B6 were IgA MAbs to VP4; 1D4, 2C5, and 2F8 were IgA MAbs to VP6; 1A9 and 7A12 were IgG MAbs to VP4; and 3C10 was an IgA MAb to Sabin 1 poliovirus.
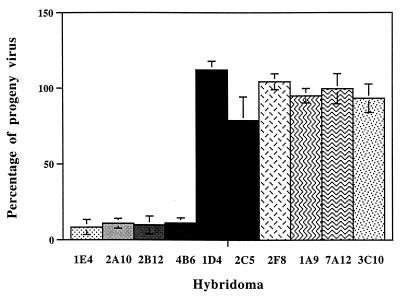
The effect of trancytosed IgA MAbs on the yield of progeny virus in polarized MDCK cells was investigated by determining the infectivity of cell lysates after 18 h of infection with RRV. The results of these experiments are reported in Fig. 2. In agreement with the neutralization experiments, IgA MAbs directed at outer capsid protein VP4 caused a significant reduction in the yield of infectious virions produced by the cells. Since the number of infected cells between monolayers was different depending on the hybridoma cell line, the ratio between progeny virus yield and infected MDCK cells was determined with duplicate cell monolayers. In all cases, infected cells appeared to yield similar amounts of infectious progeny virus, indicating that virions escaping neutralization by extracellular antibodies underwent normal replication in the cells. Output virus was neutralized by the corresponding MAb used in these experiments (data not shown), thus ruling out the possibility that neutralization-resistant virus mutants might have been selected during the experiments. In contrast to the results obtained with VP8-specific MAbs, the yield of progeny RRV was unaffected by IgA MAbs directed at inner capsid protein VP6 of RRV or directed at poliovirus.
FIG. 2.
Inhibition of progeny virus production in polarized pIgR+MDCK cells cocultured with hybridoma cells in Transwell-Col filter chambers. Filter-grown confluent MDCK cell monolayers laying on top of hybridoma cell-containing collagen layers were infected with 4 × 103 PFU of RRV from the apical side after 24 h of dexamethasone stimulation. After virus binding at 37°C for 90 min, the apical medium was replaced and cells were further incubated at 37°C for 18 h. Filters were removed and extensively washed to eliminate extracellular antibodies. Cells were disrupted by freeze-thawing and extraction with 1,1,2-trichloro-trifluoro-ethane to release intracellular virions. Infectious progeny virus titers were determined by infection of MA104 cell monolayers and immunoperoxidase staining as described in detail in Materials and Methods. Columns indicate the percentages of recovered infectious virus compared to control MDCK cell monolayers infected in the absence of hybridoma cells. Data represent the averages of three independent experiments (bars indicate ranges). 1E4, 2A10, 2B12, and 4B6 were IgA MAbs to VP4; 1D4, 2C5, and 2F8 were IgA MAbs to VP6; 1A9 and 7A12 were IgG MAbs to VP4; and 3C10 was an IgA MAb to Sabin 1 poliovirus.
It has recently been proposed that IgA can inactivate virus by a mechanism called intracellular neutralization (29). To examine if the neutralizing effect observed with the anti-VP8 IgA MAbs could be attributed to intracellular rather than extracellular neutralization, the apical side of the filters was washed twice (no residual IgA could be detected) and fresh medium was added immediately before the addition of virus. In this set of experiments, no difference in the number of infected cells could be observed between filters grown in the presence or absence of hybridoma cells. We concluded that intracellular neutralization did not contribute to the neutralizing effect observed with the anti-VP8 IgA antibodies examined.
Transepithelium-transported anti-VP8 IgA antibodies protect newborn mice from rotavirus diarrhea.
To test whether IgA and IgG MAbs could protect mice from rotavirus diarrhea in vivo, hybridoma backpack tumors (46) were implanted in 1-day-old BALB/c mice derived from rotavirus antibody-free dams. The tumor-bearing mice were challenged orally 6 to 8 days later with 2 × 107 PFU of RRV, a virus dose which induces diarrhea in at least 90% of antibody-naive mice. At least two different litters were used to test each MAb. The results of these assays are summarized in Table 2. Hybridoma tumors secreting four different IgA MAbs to RRV VP8 were found to prevent the onset of diarrhea in virus-challenged mice. Conversely, backpack tumors secreting two IgG MAbs to RRV VP8 proved unable to protect pups, despite the ability of these antibodies to neutralize RRV in MA104 cell cultures. None of the pups bearing hybridoma tumors secreting anti-VP6 IgA MAbs or an IgA MAb directed against poliovirus were protected from RRV infection in these experiments. The onset of diarrhea in these mice occurred at day 1 or 2 after challenge, and loose stools lasted for 2 to 4 days, as in control mice, which did not receive hybridoma cell implants.
TABLE 2.
Protection of infant mice from RRV diarrhea by IgG and IgA MAb-secreting hybridoma backpack tumors
| Hybridoma | Isotype | Antigena | No. of mice with diarrhea/no. tested |
|---|---|---|---|
| 1E4 | IgA | VP8 (ND) | 0/7 |
| 2A10 | IgA | VP8 (133) | 0/5 |
| 2B12 | IgA | VP8 (148) | 0/9 |
| 4B6 | IgA | VP8 (190) | 1/10 |
| 1D4 | IgA | VP6 (ND) | 9/10 |
| 2C5 | IgA | VP6 (ND) | 10/10 |
| 2F8 | IgA | VP6 (ND) | 8/9 |
| 1A9 | IgG | VP8 (100) | 10/11 |
| 7A12 | IgG | VP8 (188) | 10/10 |
| 3C10 | IgA | Sabin 1 poliovirus | 7/7 |
| None | 20/22 |
See Table 1, footnote a.
Secretion of IgA and IgG MAbs into the intestinal lumens of mice.
Mice bearing IgA- or IgG-secreting hybridoma tumors or naive mice were examined for the presence of either total or RRV-specific antibodies in their serum and gut contents by an ELISA (Table 3). Total IgA antibody concentrations ranged from approximately 400 to 1,050 μg/ml of serum in IgA-secreting tumor-bearing mice, whereas serum IgA concentrations in control and IgG-secreting tumor-bearing pups varied from approximately 1 to 11 μg/ml. The concentrations of serum IgG antibodies ranged from approximately 1,200 to 1,500 μg/ml in mice injected with IgG-secreting hybridoma cells, whereas the serum IgG concentrations in control and IgA-secreting tumor-bearing mice varied from approximately 125 to 230 μg/ml. The amount of IgA antibodies varied from undetectable (<0.1 μg) to 3.6 μg/ml of stools and did not differ significantly between IgA- and IgG-secreting tumor-bearing mice and controls, whereas no appreciable level of intestinal IgG antibodies was revealed even in mice injected with IgG-secreting hybridoma cells.
TABLE 3.
Determination of total IgA and IgG and anti-RRV antibodies in mouse sera and stools by an ELISA
| Backpack tumor | Isotype | Antigena | IgA concn (μg/ml) in:
|
IgG concn (μg/ml) in:
|
Anti-RRV antibody concnb in:
|
|||
|---|---|---|---|---|---|---|---|---|
| Serum | Stool | Serum | Stool | Serum | Stool | |||
| 1E4 | IgA | VP4 (ND) | 640 | 1.4 | 200 | —c | 16,000 | 80 |
| 2A10 | IgA | VP4 (133) | 820 | 230 | — | 32,000 | 80 | |
| 2B12 | IgA | VP4 (148) | 410 | 3.6 | 125 | — | 8,000 | 40 |
| 4B6 | IgA | VP4 (190) | 950 | 1.1 | 180 | — | 32,000 | 40 |
| 1D4 | IgA | VP6 (ND) | 710 | 0.9 | 140 | — | 64,000 | 160 |
| 2C5 | IgA | VP6 (ND) | 1,050 | 0.7 | 190 | — | 64,000 | 160 |
| 2F8 | IgA | VP6 (ND) | 460 | 1.6 | 160 | — | 32,000 | 160 |
| 1A9 | IgG | VP4 (100) | 3.2 | 1,540 | — | 128,000 | <10 | |
| 7A12 | IgG | VP4 (188) | 0.8 | 0.6 | 1,230 | — | 64,000 | <10 |
| 3C10 | IgA | Sabin 1 poliovirus | 510 | 1.2 | 190 | — | <100 | <10 |
| None | 11.2 | 1.2 | 210 | — | <100 | <10 | ||
See Table 1, footnote a.
Reciprocal of the last positive dilution in the ELISA.
—, The concentration was less than 0.1 μg/ml of stools.
The concentrations of RRV-specific antibodies in serum and intestinal fluid samples from mice are also shown in Table 3. Data are expressed as the reciprocal of the last dilution of samples giving an optical density higher than three times the background staining in the absence of antibody. Sera from IgG-secreting tumor-bearing mice were positive at dilutions ranging from 1:64,000 to 1:128,000, while sera from IgA-secreting tumor-bearing mice were positive at dilutions ranging from 1:8,000 to 1:64,000. No antibody against RRV was detected even at a 1:100 dilution of sera from either uninjected pups or dams in these assays. RRV-specific IgA antibody was detected in stool samples from mice with IgA-secreting hybridoma tumors at dilutions ranging from 1:40 to 1:160, whereas no intestinal virus-specific IgG or IgA was found in IgG-secreting tumor-bearing or control mice.
The anti-RRV activity of mouse sera and stools was also assayed by a microneutralization test. Neutralizing titers ranged from 1:4,000 and 1:32,000 in sera from mice carrying both anti-VP8 IgA- and IgG-secreting hybridoma tumors, whereas no virus-neutralizing activity was found in mice with anti-VP6 IgA-secreting tumor-bearing or control mice. Only stool suspensions from two mice carrying anti-VP8 IgA-secreting tumors (2A10 and 2B12, respectively) exhibited a low level of neutralizing activity at a 1:100 dilution; lower dilutions of stools could not be tested because of toxicity for cell cultures.
DISCUSSION
While the antigenic determinants involved in protection from rotavirus diarrhea have been widely investigated, primarily with murine IgG and IgM MAbs (26, 27, 36), the exact mechanisms and which effector cells participate in mounting protective immunity remain largely unresolved. There is, however, a significant amount of information suggesting that IgA plays a crucial role in protecting the epithelial mucosa from rotavirus infections (2, 7, 25, 31).
We recently showed that purified human IgA is capable of neutralizing rotavirus in vitro and that a majority of human antirotavirus IgA antibodies are directed against inner capsid proteins VP2 and VP6 (16, 17). Furthermore, an interesting recent study showed that some nonneutralizing VP6-specific IgA MAbs are capable of preventing and resolving a chronic murine rotavirus infection (3). This finding raises interesting questions about novel mechanisms of neutralization and protection mediated by IgA antibodies.
It has been difficult to directly assess the protective contribution of sIgA antibodies against mucosal invasion, partly because of technical obstacles against obtaining pure IgA from local mucosal secretions. One approach to overcoming this problem has been to generate dimeric IgA MAbs that can be delivered to the mucosa either passively (30) or via the normal receptor-mediated epithelial transport system (3, 33, 34, 38, 46). These studies have shown that IgA antibodies can indeed protect against a variety of microbial infections. In particular, the hybridoma backpack tumor model (46) has proven valuable in overcoming the limiting factor that passive application of antibodies to mucosal surfaces does not accurately reproduce the distribution of sIgA antibodies in animals, in which effector molecules are secreted following receptor-mediated transcytosis across mucosal and glandular epithelial cells (41).
We recently reported the production of IgA MAbs to RRV from orally immunized mice and showed that most of them are directed against antigenic sites on the VP8 tryptic fragment of VP4, including epitopes which are distinct from those recognized by antibodies raised by parenteral immunization (9). To study the protective capacity and mechanisms of these IgA antibodies, we established a murine backpack hybridoma tumor model essentially as described by Winner et al. (46). To gain insight into the neutralization mechanisms, we explored if antibodies could be transcytosed in a biologically active form from the basolateral to the apical domain through filter-grown MDCK cells expressing pIgR (14). We found that a significant amount of IgA but not IgG was transported through transfected MDCK cell monolayers by receptor-mediated transcytosis. The amounts of transcytosed IgA were similar to those in previous reports with this cell system (14, 18, 29) and most likely reflect the maximum transport capacity of the cells. This result, together with the observation that neutralizing anti-VP8 IgG antibodies did not cross the tight MDCK cell monolayers at biologically active concentrations, indicates the suitability of the cell system for studying transcytosis and intraluminal effects of secretory antibodies.
Only anti-VP8 IgA MAbs could neutralize rotavirus added to the apical side of cells, despite the fact that all of the IgA and IgG MAbs examined here have similar activities toward RRV in standard neutralization assays (9, 39). Neutralization appeared to occur after antibody release from the apical surface of cells, since replacement of the apical culture medium prior to virus inoculation completely abolished neutralization. The fact that anti-rotavirus VP8 MAbs have been found to block infection by preventing virus binding to cells (39), together with the comparable rates of neutralization (1 to 2 logs) observed in this study and in our previous investigations of RRV neutralization (9, 39), further suggests that rotavirus was inactivated by extracellular mechanisms.
The similar ratio between the progeny virus titer and the number of infected cells in the presence or absence of neutralizing antibodies also indicated that newly formed virions did not undergo intracellular neutralization by anti-VP8 IgA MAbs, at least not to detectable levels. This novel protective mechanism has been reported to occur with Sendai and influenza viruses (28, 29) which, however, have entry, uncoating, and assembly processes significantly different from those of rotavirus.
We did not observe neutralization of rotavirus with any of the examined anti-VP6 MAbs. In fact, MAbs directed to inner capsid protein VP6 of rotavirus have never been shown to neutralize rotavirus in cell cultures, extracellularly or intracellularly. A few anti-VP6 MAbs have, however, been reported to block the transcription of rotavirus genomic double-stranded RNA by binding to single-shelled particles in vitro (10), suggesting that certain antibodies with critical epitope specificities and correct intracellular localization might be capable of inhibiting the intracellular replication of virus. In line with this hypothesis, anti-VP6 IgA antibodies to a murine strain of rotavirus were recently shown to protect adult mice from virus shedding in a hybridoma backpack tumor model (3). However, none of the anti-VP6 IgA MAbs examined by us was capable of protecting infant mice from diarrhea. The discrepancies between our observations and those of Burns and coworkers (3) might be attributed to the use of different animal models for protection (disease versus virus shedding). Infant mice (<9 days of age) develop clinical diarrhea despite restricted replication of heterologous RRV. With this animal model, rotavirus may therefore induce diarrhea by cytolytic or toxic mechanisms rather than by lysis after extensive replication. In fact, there is no evidence that viral replication per se induces diarrhea, and the pathogenic mechanism of rotavirus diarrhea is still unresolved. Adult mice (2 months), however, can both become infected and shed homologous rotavirus in the absence of clinical diarrhea. This model may therefore monitor protection from shedding by antibody interference with viral replication. However, the most reasonable explanation for the discrepancies regarding VP6 between our observations and those of Burns et al. (3) is that our anti-VP6 MAbs did not recognize the critical epitopes on VP6 required for protection. Unfortunately, no data are available to describe the epitope map of inner capsid protein VP6 of rotavirus.
Our observations that VP8-specific IgA MAbs protected mice from diarrhea contrast with the observations of Burns et al. (3), who found none of their VP4-specific IgA MAbs to be effective in the homologous adult mouse model. This discrepancy may reflect differences in the epitopes recognized by the respective MAbs, but as no information regarding epitope specificity is available for these MAbs, no comparison can be made. However, the fact that all anti-VP8 neutralizing IgA MAbs examined by us could protect mice from clinical disease indicates that several neutralizing VP8 epitopes contribute to making this protein a suitable target for protective immune responses in the gut. We previously showed that these IgA antibodies recognize at least three distinct epitopes on the VP8 portion of VP4, at amino acid positions 132 to 135, 148, and 190 (9). The epitope at amino acid position 148 was previously described for RRV, and an IgM MAb directed toward this epitope was previously shown to protect infant mice when administered via the oral route (27). Of particular interest is the observation that while IgA MAb 4B6 and IgG MAb 7A12 recognized the same epitope located between amino acids 188 and 194 of VP4 (9, 24), only the former antibody proved protective in mice, thus stressing the critical role of the isotype in mucosal protective immunity.
The absence of IgG antibodies and the relatively small amount of intestinal IgA antibodies detected in the stools of tumor-bearing mice, despite a high antibody concentration in sera, strongly support the occurrence of isotype-specific secretion of antibodies into the gut lumen in the animal model adopted. Also, the lack of RRV-directed antibodies in the sera of dams indicates that the presence of IgA antibodies in the intestinal lumens of pups cannot be ascribed to passive transfer of secretory antibodies via milk.
In agreement with our findings, Haneberg and coworkers (13) recently reported that mice with IgA-secreting hybridoma tumors showed normal levels of IgA in the intestinal lumens. These authors also addressed the question of whether IgA present in the gut was transported through the bile duct rather than through the intestinal epithelium. They observed (13) that neither MAb nor total IgA levels on mucosal surfaces were altered by bile duct ligation, concluding that sIgA antibodies originated mainly from local secretions and not from bile. Together, these data strongly suggest that the backpack tumor model closely mimics normal sIgA antibody protection in the gut and is well suited to studying the mechanisms of protection on mucosal surfaces.
In conclusion, the present study clearly shows that in the backpack tumor model, IgA but not IgG antibodies are effective in preventing rotavirus diarrhea in mice. Studies of the induction of protection from clinical disease should address the elicitation of secretory antibody responses at the intestinal level, involving VP8 epitopes in addition to VP7 and VP5 antigenic determinants.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This research project received financial support from the WHO/UNDP Programme for Vaccine Development, the Swedish Medical Council (K97-06x-10392-05A), the European Community (ERBIC18CT960027), and the Swiss National Science Foundation (31-37612.93).
We are grateful to Harry B. Greenberg for supplying 1A9, 7A12, and m60 hybridoma cell lines.
REFERENCES
- 1.Brandzaeg P. Overview of the mucosal immune system. Curr Top Microbiol Immunol. 1989;146:13–25. doi: 10.1007/978-3-642-74529-4_2. [DOI] [PubMed] [Google Scholar]
- 2.Burns J W, Krishnaney A A, Vo P T, Rouse R V, Anderson L J, Greenberg H B. Analyses of homologous rotavirus infection in the mouse model. Virology. 1995;207:143–153. doi: 10.1006/viro.1995.1060. [DOI] [PubMed] [Google Scholar]
- 3.Burns J W, Siadat-Pajouh M, Krishnaney A A, Greenberg H B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science. 1996;272:104–107. doi: 10.1126/science.272.5258.104. [DOI] [PubMed] [Google Scholar]
- 4.Childers N, Bruce M, McGhee J. Molecular mechanisms of immunoglobulin A defence. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- 5.Davidson G, Hogg R, Kirubakaran C. Serum and intestinal immune response to rotavirus enteritis in children. Infect Immun. 1983;40:447–452. doi: 10.1128/iai.40.2.447-452.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estes M K. Rotaviruses and their replication. In: Fields B, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1324–1352. [Google Scholar]
- 7.Feng N, Burns J W, Bracy L, Greenberg H B. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–7773. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiore L, Ridolfi B, Genovese D, Buttinelli G, Lucioli S, Lahm A, Ruggeri F M. Poliovirus Sabin type 1 neutralization epitopes recognized by immunoglobulin A monoclonal antibodies. J Virol. 1997;71:6905–6912. doi: 10.1128/jvi.71.9.6905-6912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giammarioli A M, Mackow E R, Fiore L, Greenberg H B, Ruggeri F M. Production and characterization of murine IgA monoclonal antibodies to the surface antigens of rhesus rotavirus. Virology. 1996;225:97–110. doi: 10.1006/viro.1996.0578. [DOI] [PubMed] [Google Scholar]
- 10.Ginn D I, Ward R L, Hamparian V V, Hughes J H. Inhibition of rotavirus in vitro transcription by optimal concentrations of monoclonal antibodies specific for rotavirus VP6. J Gen Virol. 1992;73:3017–3022. doi: 10.1099/0022-1317-73-11-3017. [DOI] [PubMed] [Google Scholar]
- 11.Greenberg H B, Flores J, Kalica A R, Wyatt R G, Jones R. Gene coding assignments for growth restriction, neutralization and subgroup specificities of the W and DS-1 strains of human rotavirus. J Gen Virol. 1983;64:313–320. doi: 10.1099/0022-1317-64-2-313. [DOI] [PubMed] [Google Scholar]
- 12.Greenberg H B, Valdesuso J, van Wyke K, Midthun K, Walsh M, McAuliffe V, Wyatt R G, Kalica A R, Flores J, Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983;47:267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haneberg B, Kendall D, Apter M, Neutra M. Distribution of monoclonal antibodies in intestinal and urogenital secretions of mice bearing hybridoma “backpack” tumours. Scand J Immunol. 1997;45:151–159. doi: 10.1046/j.1365-3083.1997.d01-383.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirt R, Hughes G, Frutiger S, Michetti P, Perregaux C, Poulain-Godefroy O, Jeanguenat N, Neutra M, Kraehenbuhl J. Trancytosis of the polymeric Ig receptor requires phosphorylation of serine 664 in the absence but not the presence of dimeric IgA. Cell. 1993;74:245–255. doi: 10.1016/0092-8674(93)90416-n. [DOI] [PubMed] [Google Scholar]
- 15.Hoshino Y, Sereno M M, Midthun K, Flores J, Kapikian A Z, Chanock R M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci USA. 1985;82:8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johansen K, Granqvist L, Karlen K, Stintzing G, Uhnoo I, Svensson L. Serum IgA immune response to individual rotavirus polypeptides in young children with rotavirus infection. Arch Virol. 1994;138:247–259. doi: 10.1007/BF01379129. [DOI] [PubMed] [Google Scholar]
- 17.Johansen K, Svensson L. Neutralization of rotavirus and recognition of immunologically important epitopes on vp4 and vp7 by human IgA. Arch Virol. 1997;142:1491–1498. doi: 10.1007/s007050050175. [DOI] [PubMed] [Google Scholar]
- 18.Kaetzel C, Robinson J, Chintalacharavu K, Vaerman J, Lamm M. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: a local defence function for IgA. Proc Natl Acad Sci USA. 1991;88:8796–8800. doi: 10.1073/pnas.88.19.8796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalica A R, Greenberg H B, Wyatt R G, Flores J, Sereno M M, Kapikian A Z, Chanock R M. Genes of human (strain Wa) and bovine (strain UK) rotaviruses that code for neutralization and subgroup antigens. Virology. 1981;112:385–390. doi: 10.1016/0042-6822(81)90285-3. [DOI] [PubMed] [Google Scholar]
- 20.Kapikian A Z, Chanock R M. Rotaviruses. In: Fields B N, editor. Virology. 2nd ed. New York, N.Y: Raven Press; 1990. pp. 1353–1404. [Google Scholar]
- 21.Kerr M. The structure and function of human IgA. Biochem J. 1990;271:285–296. doi: 10.1042/bj2710285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraehenbuhl J, Neutra M. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 23.Losonsky G A, Rennels M B, Lim Y, Krall G, Kapikian A Z, Levine M M. Systemic and mucosal immune responses to rhesus rotavirus vaccine MMU 18006. Pediatr Infect Dis J. 1988;7:388–393. doi: 10.1097/00006454-198806000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Mackow E R, Shaw R D, Matsui S M, Vo P T, Dang M N, Greenberg H B. The rhesus rotavirus gene encoding protein VP3: location of amino acids involved in homologous and heterologous rotavirus neutralization and identification of a putative fusion region. Proc Natl Acad Sci USA. 1988;85:645–649. doi: 10.1073/pnas.85.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matson D O, O’Ryan M L, Herrera I, Pickering L K, Estes M K. Fecal antibody responses to symptomatic and asymptomatic rotavirus infections. J Infect Dis. 1993;167:577–583. doi: 10.1093/infdis/167.3.577. [DOI] [PubMed] [Google Scholar]
- 26.Matsui S M, Mackow E R, Greenberg H B. Molecular determinant of rotavirus neutralization and protection. Adv Virus Res. 1989;36:181–214. doi: 10.1016/s0065-3527(08)60585-0. [DOI] [PubMed] [Google Scholar]
- 27.Matsui S M, Offit P A, Vo P T, Mackow E R, Benfield D A, Shaw R D, Padilla N L, Greenberg H B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J Clin Microbiol. 1989;27:780–782. doi: 10.1128/jcm.27.4.780-782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazanec M B, Nedrud J G, Lamm M E. Immunoglobulin A monoclonal antibodies protect against Sendai virus. J Virol. 1987;61:2624–2626. doi: 10.1128/jvi.61.8.2624-2626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McNeal M M, Broome R L, Ward R L. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology. 1994;204:642–650. doi: 10.1006/viro.1994.1579. [DOI] [PubMed] [Google Scholar]
- 32.Mestecky J. Immunobiology of IgA. Am J Kidney Dis. 1988;12:378–383. doi: 10.1016/s0272-6386(88)80029-5. [DOI] [PubMed] [Google Scholar]
- 33.Michetti P, Mahan J, Slauch J, Mekalanos J, Neutra M. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Michetti P, Porta N, Mahan M J, Slauch J M, Mekalanos J J, Blum A L, Kraehenbuhl J P, Neutra M R. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology. 1994;107:915–923. doi: 10.1016/0016-5085(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 35.Offit P, Blavat G. Identification of two rotavirus genes determining neutralizing specificities. J Virol. 1986;57:376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Offit P A, Shaw R D, Greenberg H B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J Virol. 1986;58:700–703. doi: 10.1128/jvi.58.2.700-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Renegar K, Small P. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renegar K, Small P. Passive transfer of local immunity to influenza virus infection by IgA antibodies. J Immunol. 1991;146:1972–1978. [PubMed] [Google Scholar]
- 39.Ruggeri F, Greenberg H. Antibodies to the trypsin cleavage peptide vp8 neutralize rotavirus by inhibiting binding of virions to target cells in culture. J Virol. 1991;65:2211–2219. doi: 10.1128/jvi.65.5.2211-2219.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw R D, Vo P T, Offit P A, Coulson B S, Greenberg H B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986;155:434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- 41.Solari R, Kraehenbuhl J. The biosynthesis of secretory component and its role in the epithelial transport of IgA dimer. Immunol Today. 1985;6:17–20. doi: 10.1016/0167-5699(85)90163-X. [DOI] [PubMed] [Google Scholar]
- 42.Svensson L, Finlay B B, Bass D, von Bonsdorff C-H, Greenberg H B. Symmetric infection of rotavirus on polarized human intestinal epithelial (Caco-2) cells. J Virol. 1991;65:4190–4197. doi: 10.1128/jvi.65.8.4190-4197.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taniguchi K, Urasawa S, Urasawa T. Preparation and characterization of neutralizing monoclonal antibodies with different reactivity patterns to human rotaviruses. J Gen Virol. 1985;66:1045–1053. doi: 10.1099/0022-1317-66-5-1045. [DOI] [PubMed] [Google Scholar]
- 44.Uhnoo I, Dharakul T, Riepenhoff-Talty M, Ogra P L. Immunological aspects of interaction between rotavirus and the intestine in infancy. Immunol Cell Biol. 1988;66:135–145. doi: 10.1038/icb.1988.17. [DOI] [PubMed] [Google Scholar]
- 45.Ward R L, McNeal M M, Sander D S, Greenberg H B, Bernstein D I. Immunodominance of the VP4 neutralization protein of rotavirus in protective natural infections of young children. J Virol. 1993;67:464–468. doi: 10.1128/jvi.67.1.464-468.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winner L, III, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]