Abstract

Broad-band ultraviolet photolysis (λ > 200 nm) of (cyanomethylene)cyclopropane (5) in an argon matrix at 20 K generates 1-cyano-2-methylenecyclopropane (7), a previously unknown compound. This product was initially identified by comparison of its infrared spectrum to that predicted by an anharmonic MP2/6-311+G(2d,p) calculation. This assignment was unambiguously confirmed by the synthesis of 1-cyano-2-methylenecyclopropane (7) and observation of its authentic infrared spectrum, which proved identical to that of the observed photoproduct. We investigated the singlet and triplet potential energy surfaces associated with this isomerization process using density functional theory and multireference calculations. The observed rearrangement of compound 5 to compound 7 is computed to be endothermic (3.3 kcal/mol). We were unable to observe the reverse reaction (7 → 5) under the photochemical conditions.
Introduction
(Cyanomethylene)cyclopropane (5) and 1-cyano-2-methylenecyclopropane (7) are structurally intriguing C5H5N isomers. They are substantially higher in energy than pyridine (ca. 50 kcal/mol, B3LYP/6-311+G(2d,p))1 due, in part, to the absence of aromaticity and the presence of a strained, three-membered cyclopropyl ring with an exocyclic π bond. Compound 5 has been identified previously,1−3 but compound 7 is presented for the first time in this work. The synthesis and spectroscopic investigation of these species are part of a long-standing pursuit by this group of the chemistry and spectroscopy of the pyridine isomers shown in Figure 1.1,4−9
Figure 1.
C5H5N isomers that have been studied by rotational spectroscopy: (E)-1-cyano-1,3-butadiene (E-1),4,5 (Z)-1-cyano-1,3-butadiene (Z-1),4,5 4-cyano-1,2-butadiene (2),4,5 2-cyano-1,3-butadiene (3),6 4-cyano-1-butyne (4),8 (cyanomethylene)cyclopropane (5),1 1-cyanocyclobutene (6),7 1-cyano-2-methylenecyclopropane (7),9 and pyridine (8).10
Our motivation for studying the spectroscopy of these nitrile-containing pyridine isomers is two-fold: the absence of the detection of pyridine in the interstellar medium (ISM)11,12 and the prevalence of nitriles among the molecules detected in interstellar environments. Despite several published searches,11−16 nitrogen-containing aromatic heterocycles, e.g., pyridine, pyrimidine, pyridazine, and pyrrole, which all have permanent dipole moments, have eluded radioastronomical detection in the ISM. Recently, an isomer of pyridine, E-1-cyano-1,3-butadiene (E-1), has been detected in Taurus Molecular Cloud-1 (TMC-1).17 This detection suggests the possibility that other pyridine isomers (C5H5N) are components of interstellar environments. Organic nitriles constitute about 20% of the ca. 300 molecules detected in the ISM.12,18 The cyano moiety imparts a large permanent dipole moment in these organic nitriles, which facilitates their detection via radioastronomy. Nitriles are also found in planetary atmospheres of our solar system, such as that of Saturn’s moon Titan.19,20 The possibility has been discussed that vinyl nitriles, such as E-1-cyano-1,3-butadiene, could even self-assemble into polarity-inverted membranes in conditions similar to those on Titan.21,22 Expanding the family of nitrile-containing pyridine isomers for which laboratory rotational spectra are available facilitates future investigations of the reactivity of these small molecules and searches for these species in extraterrestrial environments. Astronomical detection of any of these molecules is of fundamental interest and could illuminate new astrochemical pathways toward complex aromatic species.
The unique thermal and photochemical properties of methylenecyclopropane and its derivatives have received extensive study for over a century.23 As early as 1893, Feist prepared methylenecyclopropane-trans-2,3-dicarboxylic acid (Feist’s acid),24 though its structure as the first methylenecyclopropane derivative was not elucidated until the 1950s.25,26 Even without a clear identification of its structure, Kon and Nanji reported in 1932 that its ester underwent an irreversible thermal isomerization (Scheme 1a).27 Studying the pyrolysis conditions and products of this thermal rearrangement, Ullman determined that racemization occurred via a reversible equilibration with a planar, substituted trimethylenemethane (TMM) intermediate but did not correctly identify the electronic structure.28 In contemporaneous studies, Dowd observed the triplet ground state of the parent (unsubstituted) TMM by EPR spectroscopy (Scheme 1b)29 and Crawford and Cameron measured a kinetic isotope effect consistent with a TMM intermediate in the thermal decomposition of 4-methylene-Δ1-pyrazoline (Scheme 1c).30 Expanding beyond the studies of thermal isomerization reactions, Kende et al.31 studied photoinduced isomerization of methylenecyclopropane derivatives,31 demonstrating a reversible interconversion of the phenyl-substituted species when irradiated in dilute acetonitrile solution (Scheme 1d).
Scheme 1. Reactions of Methylenecyclopropane and Trimethylenemethane (TMM) Derivatives.
(a) Pyrolysis of Feist’s ester.26,28 (b) Formation of TMM via photolysis of 4-methylene-Δ1-pyrazoline.29 (c) Formation of methylenecyclopropane via pyrolysis from 4-methylene-Δ1-pyrazoline.30 (d) Reversible photochemical rearrangement.31 (e) Formation of TMM via γ-irradiation of methylenecyclopropane.32 (f) Photochemical rearrangement of methylenecyclopropane in a halogen-doped Xe matrix.33,34
Generation of TMM from methylenecyclopropane, rather than from an azo precursor, was first described by Yamaguchi et al.32 (Scheme 1e). γ-Irradiation was needed for the conversion of methylenecyclopropane to trimethylenemethane, presumably due to the weak absorption of methylenecyclopropane in the UV range.35 The vacuum–UV spectrum of methylenecyclopropane, as reported by Wiberg and Wang,35 shows the onset of absorption around 220 nm with a peak near 180 nm. Maier et al. reported both the photoisomerization of methylenecyclopropane to TMM in a halogen-doped Xe matrix and the subsequent photolysis of TMM to its isomeric conjugated diene, 1,3-butadiene (Scheme 1f).33,34 Collectively, these studies establish the expected isomerization of methylenecyclopropane derivatives under photochemical conditions, indicating the possibility of further isomerization to a butadiene derivative, and demonstrate the suitability of matrix-isolation spectroscopy to investigate these reactions.
The current photoisomerization study of methylenecyclopropane derivative 5 in matrix-isolation conditions adds to a growing body of work on the chemistry of pyridine isomers under astrochemically relevant conditions. Morales et al.36 demonstrated that a kinetically controlled reaction of cyano radical and 1,3-butadiene produces 1-cyano-1,3-butadiene and a small amount of pyridine. The C5H5N and C5H6N• potential energy surfaces have been extensively explored, in the context of the reaction of the cyano radical with 1,3-butadiene, by Sun et al.37 This computational study included several three-membered-ring-containing C5H6N• isomers but did not include any of the analogous C5H5N species that would result from the loss of a hydrogen atom. Jamal and Mebel performed a similar computational study of the reaction of cyano radical with 1- and 2-butyne, suggesting the likely formation of three cyano-1,3-butadiene isomers (E-1, Z-1, and 3), depending on which butyne precursor was used.38 Tsukamoto and Lichtin39 observed the formation of E-1 and Z-1 from 1,3-butadiene and nitrogen atoms generated via microwave discharge. Similarly, McCarthy et al.40 identified, via rotational spectroscopy, a multitude of products generated by the discharge of benzene and molecular nitrogen that included E-1 and Z-1. Zwier and co-workers detected Z-1 from a gas-phase pyrolysis of trans-3-pentenenitrile.41 It remains to be determined whether the three-membered-ring-containing C5H5N isomers are are accessible from or can be produced by photochemical or thermal reactions from the open-chain species.
In the current study, we sought to probe the photochemistry of (cyanomethylene)cyclopropane (5) to determine whether irradiation would lead to the production of open-chain cyanobutadienes, other isomers of pyridine, fragments, or a combination of products. The only identifiable photoproduct in our matrix-isolation study is the isomeric methylenecyclopropane derivative, 1-cyano-2-methylenecyclopropane (7), which we were subsequently able to synthesize and characterize.
Experimental and Computational Methods
Synthesis
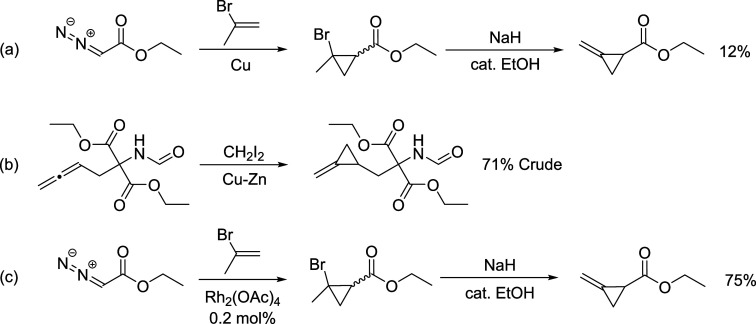
The sample of (cyanomethylene)cyclopropane (5) used in this work was prepared by the Wittig reaction of 1-ethoxycyclopropanol and (cyanomethyl)triphenyl-phosphonium chloride, as described previously.1,3 The synthesis of 1-cyano-2-methylenecyclopropane (7) followed the synthetic methodology that was first developed for the synthesis of hypoglycin A, a natural product found in unripe fruit from the Ackee tree.42 Carbon et al. synthesized ethyl methylenecyclopropanecarboxylate by adding ethyl diazoacetate to 2-bromopropene in the presence of a copper–bronze catalyst followed by a sodium hydride-induced elimination (Scheme 2a).43,44 Black and Landor utilized a zinc–copper-catalyzed reaction to generate the methylenecyclopropane moiety in a much higher yield (Scheme 2b).45 More recently, Lai and Liu improved the yield of the ethyl diazoacetate route by utilizing a rhodium acetate catalyst (Scheme 2c).46 Our synthesis of 7 closely follows the established route of Lai and Liu.
Scheme 2. Methylenecyclopropane Synthetic Methodology.
(a) Synthesis of ethyl methylenecyclopropanecarboxylate by Carbon et al.44 (b) A portion of the synthesis of (±)-hypoglycin A by Black and Landor.45 (c) Synthesis of ethyl methylenecyclopropanecarboxylate by Lai and Liu.46
Our synthesis of 1-cyano-2-methylenecyclopropane (7) is presented in Scheme 3.47 The sequence begins with a rhodium acetate-catalyzed cyclopropanation reaction of 2-bromopropene with ethyl diazoacetate using a procedure modified from Scott et al.,48 followed by a sodium hydride-induced elimination of bromide from 9 to generate the methylenecyclopropane moiety. Ester 10 is converted to amide 11(49) and then treated with phosphoric anhydride to produce nitrile 7 through dehydration of 11.
Scheme 3. Synthesis of 1-Cyano-2-methylenecyclopropane (7).
Matrix-Isolation Spectroscopy
(Cyanomethylene)cyclopropane (5) and 1-cyano-2-methylenecyclopropane (7) have vapor pressures measured with a capacitance manometer of 1.2 and 1.1 Torr at room temperature, respectively. Each was premixed with argon prior to depositing in a ratio of ∼1:1000 and ∼1:700 for 5 and 7, respectively. The sample was deposited on a CsI window maintained at a temperature of 30 K by using a Lake Shore Cryotronics temperature controller (model 331). The infrared spectra of the matrix-isolated samples were obtained from 4000 to 400 cm–1 using a Thermo Nicolet Nexus 870 FT-IR instrument (MCT-B detector). Sample irradiation was performed using a Xe arc lamp (300 W, ILC Technology LX300UV). A λ > 237 nm cutoff filter (Schott glass) was used for one irradiation experiment. The matrix-isolation apparatus and technique have been described previously.50−52
Computational Chemistry
Electronic structure calculations were carried out with Gaussian 1653 using the WebMO interface.54 Optimized geometries at the unrestricted B3LYP/6-311+G(2d,p) and MP2/6-311+G(2d,p) levels were obtained by using “verytight” convergence criteria and an “ultrafine” integration grid, and subsequent anharmonic vibrational frequency calculations were used to obtain predicted frequencies and IR intensities. Electronic transitions were computed using time-dependent density functional theory (TDDFT) (wB97XD/aug-cc-pVDZ), similar to the work by Hernández et al.55 Transition-state optimizations were carried out at the B3LYP/6-311+G(2d,p) level to investigate the reaction pathway for the isomerization, and frequency calculations were completed to show that these transition-state geometries had a single imaginary vibrational frequency. Intrinsic reaction coordinate (IRC) calculations were performed to confirm the transition states connected to the stationary points of interest. Reaction pathways were explored on both triplet and singlet surfaces. Trimethylenemethane diradicals were investigated using complete-active-space methods as implemented in OpenMolcas.56−58 Optimized geometries were obtained using unrestricted CASPT2/aug-cc-pVTZ. Computational data can be found in the Supporting Information.
Results and Discussion
Photochemistry of (Cyanomethylene)cyclopropane (5)
Figure 2a provides the matrix-isolation infrared spectrum of (cyanomethylene)cyclopropane (5). Upon irradiation with λ > 237 nm (20 h), there was no observed change in the IR spectrum, establishing that 5 displays no photoreactivity under these conditions. Subsequent irradiation at a shorter wavelength (λ > 200 nm, 37 h) led to the gradual appearance of new IR signals and the simultaneous disappearance of signals associated with 5 (Figure 2).
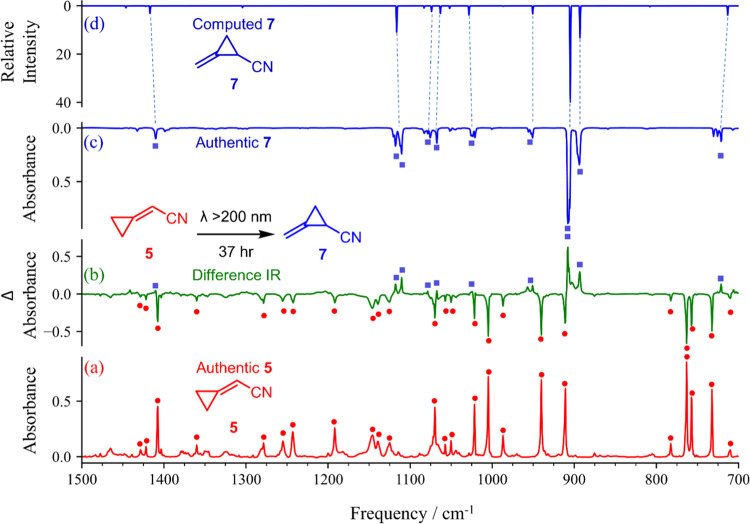
Figure 2.
(a) IR spectrum of (cyanomethylene)cyclopropane (5) at 20 K in an ∼1:1000 argon matrix. (b) Experimental difference IR spectrum showing changes after irradiation (λ > 200 nm). The positive bands are due to the formation of 1-cyano-2-methylenecyclopropane (7) (solid blue squares). The negative bands are due to the consumption of (cyanomethylene)cyclopropane (5) (solid red circles). (c) IR spectrum of 1-cyano-2-methylenecyclopropane (7) at 20 K in an ∼1:700 argon matrix. (d) IR spectrum of 1-cyano-2-methylenecyclopropane (7) computed at the anharmonic MP2/6-311+G(2d,p) level.
The signals arising from the irradiation of 5 were compared to authentic IR spectra of C5H5N isomers (E)-1-cyano-1,3-butadiene (E-1),4 (Z)-1-cyano-1,3-butadiene (Z-1),4 4-cyano-1,2-butadiene (2),4 2-cyano-1,3-butadiene (3),4 4-cyano-1-butyne (4),59 1-cyano-1-butyne (17),60 1-cyanocyclobutene (6),7 2-azabicyclo[2.2.0]hexa-2,5-diene (Dewar pyridine),61 and pyridine (8).61,62 None of these known C5H5N isomers was observable in our photolysis of 5. The photoproduct spectrum was also compared to authentic IR spectra of plausible fragmentation products, HCN,63 C4H4 isomers (methylenecyclopropene,64 vinylacetylene,64 butatriene,64 and cyclobutadiene65), cyanoacetylene,66 and ethylene.67 The absence of these fragmentation products and the lack of a match to the IR spectra of known C5H5N isomers indicate that the species being generated is likely a novel isomer. As part of our ongoing studies of the structure, spectroscopy, and photochemistry of pyridine isomers, we have compiled a database of computational data on 48 isomers of pyridine (see the Supporting Information). We drew on this database to compare calculated anharmonic B3LYP and MP2/6-311+G(2d,p) vibrational frequencies and IR intensities of pyridine isomers, which led to the tentative identification of the photoproduct as 1-cyano-2-methylenecyclopropane (7). This assignment was subsequently validated by the independent chemical synthesis of compound 7 (Figure 2). Chemically, structure 7 represents a logical photochemical rearrangement product of 5, as precedented by the known rearrangement chemistry of other substituted methylenecyclopropane derivatives (Scheme 1d).31
The IR spectra of (cyanomethylene)cyclopropane (5) and its photoproduct, 1-cyano-2-methylenecyclopropane (7), display many similarities. Both 5 and its photoproduct exhibit an absorption characteristic of a C≡N nitrile stretch (2250 and 2257 cm–1, respectively). The nitrile signal in the photoproduct is shifted higher in frequency compared with 5, which can be attributed to the loss of conjugation of the nitrile group with the C−C π bond. That the shift is small in magnitude is attributable to the interaction of the π system of the nitrile with the Walsh orbitals of the cyclopropane ring in 7.68 Both isomers feature signals indicative of either a =CH2 or =C–H wag (908 cm–1 for the photoproduct). A key feature that distinguishes the IR spectrum of photoproduct 7 from that of compound 5 is the signal at 893 cm–1, a typical location and intensity of an absorption caused by a terminal =CH2 out-of-plane twisting motion. In terms of the computed IR spectra, the spectrum of (cyanomethylene)cyclopropane (5) is predicted reasonably well by both the B3LYP and MP2 methods. The IR spectrum calculated for 1-cyano-2-methylenecyclopropane (7) (Figure 2d; MP2) corresponds very well to the experimental spectrum of the photoproduct (Figure 2b). To confirm this assignment, we developed an independent synthesis of 7 and measured the IR spectrum under similar matrix-isolation conditions. The authentic IR spectrum of 7 (Figure 2c) matches that of the photoproduct and also that of the computational prediction, unambiguously establishing the assignment of 7 as the photoproduct. Obtaining a sample of 7 also allowed us to measure the laboratory rotational spectrum of this species—an additional pyridine isomer, which is reported in a companion manuscript.9
To further investigate the photochemistry of this system, we irradiated 7 to determine if 5 could be generated from 7 or if other photoproducts could be identified. We irradiated a matrix-isolated sample of 1-cyano-2-methylenecyclopropane (7) (λ > 200 nm, up to 130 h), but the formation of 5 was not observed during this experiment. We observed a 20% reduction in the intensity of the 908 cm–1 signal, possibly due to matrix degradation caused by the full output of the Xe arc lamp and the appearance of several IR spectral features we have yet to identify, some of which are also obtained upon prolonged irradiation (λ > 200 nm) of (cyanomethylene)cyclopropane (5) (see the Supporting Information). As determined by comparison with experimental or computed IR spectra, these unassigned IR absorptions are not associated with 2-cyanocyclobutylidene (13), 1-cyanocyclobutene (6), cyano-substituted trimethylenemethane (12), ethylene, cyanoacetylene, or HCN (see below).
The UV–vis absorption spectra of (cyanomethylene)cyclopropane (5) and 1-cyano-2-methylenecyclopropane (7) in an acetonitrile solution are depicted in Figure 3. It is clear that (cyanomethylene)cyclopropane (5) absorbs much more strongly than 1-cyano-2-methylenecyclopropane (7) under the irradiation conditions used in the matrix-isolation experiments. The electronic absorption of 5 is red-shifted relative to the unsubstituted methylenecyclopropane (178 nm)35 and an alkyl-substituted derivative, 9-isopropylidenebicyclo[6.1.0]nonane (end absorption only; no absorption maximum at λ > 200 nm).69 The electronic absorption of 7 seems to be rather similar to those of methylenecyclopropane35 and 9-isopropylidenebicyclo[6.1.0]nonane, although a careful comparison is not possible without access to the vacuum–UV spectra. The red shift in the electronic absorption spectrum of 5, relative to 7, may be attributed to the π-conjugation of the nitrile and alkene groups in 5 and, in fact, the absorption spectrum of 5 is similar to that of H2C = CHCN.70 TDDFT calculations of the electronic transitions of these species are in good accord with the experimental observations. The predicted lowest-energy excitations with significant oscillator strength are methylenecyclopropane (180 nm, f = 0.34), (cyanomethylene)cyclopropane (5; 200 nm, f = 0.47), and 1-cyano-2-methylenecyclopropane (7; 174 nm, f = 0.13, 177 nm, f = 0.20). Additional details of the TDDFT calculations are available in the Supporting Information. We initially hypothesized that isomers 5 and 7 might exist in a photostationary state that substantially favors the formation of 7, as governed by the significant difference in absorption by the two species under the experimental conditions. That irradiation of 7 does not yield a detectable quantity of 5, however, casts doubt on that interpretation. Under the experimental conditions (λ > 200 nm), the conversion of 5–7 appears to be irreversible.
Figure 3.

(a) UV–vis absorption spectrum of (cyanomethylene)cyclopropane (5) in acetonitrile at room temperature. (b) UV–vis absorption spectrum of 1-cyano-2-methylenecyclopropane (7) in acetonitrile at room temperature.
Reaction Pathways
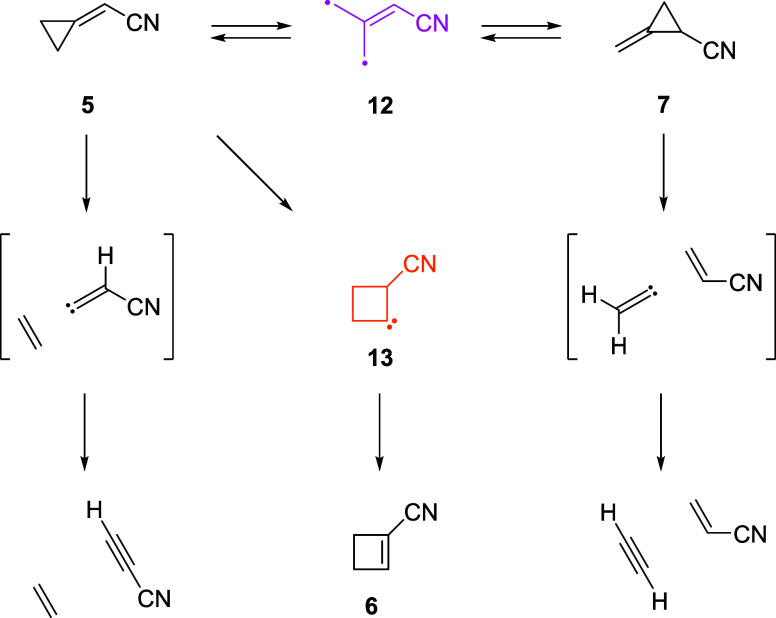
Although the thermal rearrangement chemistry of methylenecyclopropane derivatives is well studied, the photochemistry and photophysics of these compounds are not. Thermal (ground-state) rearrangements typically involve trimethylenemethane (TMM) diradicals, while photochemical rearrangements have been variously postulated to proceed via TMM (diradical or zwitterion),31 fragmentation,69 or cyclobutylidene71 (Scheme 4). Using B3LYP/6-311+G(2d,p) calculations, we explored the potential energy surface to gain insight into the general features of the potential energy surface involving 5 and 7. Several reaction pathways for isomerization were considered, as shown in Figure 4. On the singlet potential energy surface, two transition states (1TS-A and 1TS-B), only 0.8 kcal/mol apart, were found to connect 5–7. The two transition states arise as a consequence of the fact that there are nonequivalent bonds that may migrate in the rearrangement. The ground-state rearrangement is shown in greater detail in Figure 5.
Scheme 4. Reaction Pathways.
Figure 4.
Triplet (blue) and singlet (red) potential energy surfaces of the isomerization of (cyanomethylene)cyclopropane (5) to 1-cyano-2-methylenecyclopropane (7). Relative energy in kcal/mol (B3LYP/6-311+G(2d,p)). ZPVE was not included.
Figure 5.
Ground-state isomerization pathways of (cyanomethylene)cyclopropane (5) to 1-cyano-2-methylenecyclopropane (7). Relative energy in kcal/mol (B3LYP/6-311+G(2d,p)).
On the triplet surface, a two-step reaction pathway between 35 and 37 occurs through a triplet cyano-trimethylenemethane intermediate (312). Four different triplet transition-state structures were found to connect 312 to four distinct triplet stationary points. The possible involvement of a cyano-substituted trimethylenemethane derivative invites closer scrutiny as trimethylenemethane is a classic reactive intermediate in organic chemistry. The electronic structure of this open-shell diradical was investigated using multireference computational methods.72 Structures for 112 and 312 were optimized at CASPT2(6,6)/aug-cc-pVTZ (Figure 6). As with the parent (unsubstituted) trimethylenemethane, both singlet and triplet states of cyano-trimethylenemethane (12) are computed to be planar diradicals, with the triplet lower in energy by 14.8 kcal/mol. These results are consistent with other studies involving trimethylenemethane and its derivatives.29,72,73 The singlet 2-cyanocyclobutylidene intermediate (113) is only ca. 13 kcal/mol higher in energy than singlet cyano-trimethylenemethane (112). In terms of the photochemical pathway, we do not have sufficient evidence to discriminate among various mechanistic possibilities. Aspects of the excited states and photochemistry of methylenecyclopropane have been investigated computationally by Koseki et al.,74 who identified an avoided crossing between S1 and S0 states of methylenecyclopropane as a mechanism for deactivation of the excited state and interpreted product formation in terms of competitive branching in a ground-state cyclobutylidene intermediate.
Figure 6.

Computed structures and relative energies of (a) triplet ground state and (b) singlet cyano-substituted trimethylenemethane (12) (CASPT2/aug-cc-pVTZ).
Conclusions
We present the photoisomerization of (cyanomethylene)cyclopropane (5) and conclusively identify the novel pyridine isomer 1-cyano-2-methylenecyclopropane (7) as the photoproduct of this reaction. The identity of 7 as the photoproduct, first suggested by literature precedent (Scheme 1) and by the nitrile and terminal alkene IR signals in the photoproduct IR spectrum, was confirmed by the chemical synthesis of 7 and comparison with the matrix-isolation IR spectrum of the authentic sample. 1-Cyano-2-methylenecyclopropane (7) does not absorb strongly under the experimental conditions. Isomer 7 does not appear to photoequilibrate with (cyanomethylene)cyclopropane (5), and we were unable to identify the low-abundance photoproducts that did arise from the photolysis of 7.
The potential energy surface was theoretically modeled using B3LYP, providing mechanistic insights into this transformation by proposing plausible reaction pathways. A photodynamic simulation, similar to that recently described by Lopez and co-workers,55,75,76 would provide detailed insight into the photochemical transformation and may also shed light on the IR signals observed from the photolysis of 7 that could not be assigned in this work. An improved computational study will be the subject of further investigation and may afford additional insight concerning this isomerization of a substituted methylenecyclopropane derivative.
General Experimental Methods
1H NMR spectra (400 MHz) and 13C{1H} NMR spectra (100 MHz) were obtained in CDCl3; chemical shifts (δ) are reported as ppm downfield from internal SiMe4. Mass spectra and exact mass measurement (EMM) were obtained on a time-of-flight instrument using electrospray ionization (ESI) or atmospheric solid analysis probe (ASAP). UV–vis spectra of 5 and 7, dissolved in acetonitrile at room temperature, were obtained on a Varian Cary 5000 UV–vis–NIR spectrophotometer. Starting materials and reagents were purchased from commercial sources.
(Cyanomethylene)cyclopropane (5)
Prepared by the method described previously.1 UV (CH3CN), λmax = 205 nm (ε = 6765 M–1 cm–1).
2-Bromo-2-methyl-cyclopropanecarboxylic Acid Ethyl Ester (9)48
A three-neck round-bottom flask containing rhodium acetate dimer Rh2(OAc)4 (43.9 mg, 0.199 mmol Rh, 0.2 mol %) was fitted with septa and a nitrogen bubbler. 2-Bromopropene (25.2 g, 0.204 mol, 2.09 equiv) was added via a syringe. While stirring, a solution of ethyl diazoacetate (13 wt % in CH2Cl2; 12 mL, 12.8 g, 0.0975 mol, 1 equiv) was added to the flask slowly over 48 h with a syringe pump. A short-path distillation apparatus was then attached, and the flask was heated to 30 °C with an oil bath. CH2Cl2 and excess 2-bromopropene were then removed via vacuum distillation as the pressure was gradually reduced to 1 Torr. The pressure in the flask was further reduced to 0.2 Torr, and the oil bath was gradually heated to 60 °C. 2-Bromo-2-methyl-cyclopropanecarboxylic acid ethyl ester (9) (16.0 g, 0.0780, mol 80% yield) was isolated as a clear, colorless liquid via vacuum distillation (45–57 °C, 0.2 Torr) as a mixture of cis and trans isomers. 1H NMR (400 MHz, CDCl3) δ 4.18 (qd, J = 7.1, 2.8 Hz, 2H cis), 4.15 (q, J = 7.2 Hz, 2H trans), 2.29 (dd, J = 9.3, 6.8 Hz, 1H trans), 1.86 (s, 3H trans), 1.83 (s, 3H cis), 1.79 (dd, J = 8.6, 6.7 Hz, 1H cis), 1.71 (t, J = 6.4 Hz, 1H cis), 1.58 (dd, J = 9.4, 6.2 Hz, 1H trans), 1.41 (t, J = 6.5 Hz, 1H trans), 1.28 (t, J = 7.0, 3H cis), 1.28 (t, J = 7.1, 3H trans), 1.23 (dd, J = 8.6, 6.1 Hz, 1H cis). 13C{1H} NMR (100 MHz, CDCl3) δ 169.81, 168.87, 60.93, 60.87, 33.10, 32.91, 30.70, 29.46, 28.61, 24.18, 23.81, 22.72, 14.39, 14.29 (mixture of cis and trans isomers).
2-Methylenecyclopropanecarboxylic Acid, Ethyl Ester (10)48
To a three-neck round-bottom flask fitted with septa, a reflux condenser, and a nitrogen bubbler, a slurry of 60 wt % of sodium hydride dispersion in mineral oil (3.89 g, 0.0972 mol, 1.82 equiv) in 70 mL of diethyl ether was added via a syringe. By a syringe, 2-bromo-2-methyl-cyclopropanecarboxylic acid ethyl ester (9) (11.0 g, 0.0533 mol, 1 equiv) was added to the flask, which was subsequently heated to reflux in an oil bath set to 45 °C. Ethanol (0.7 mL, 0.0119 mol, 0.225 equiv) was slowly added to the flask via a syringe, and the solution was stirred at reflux for 15 h. The solution was cooled to room temperature and filtered through a fritted filter with a pad of celite, and the flask was rinsed three times with 10 mL of diethyl ether. The diethyl ether was removed from the filtrate solution at room temperature using rotary evaporation. 2-Methylenecyclopropanecarboxylic acid, ethyl ester (10) (6.0789 g, 0.0482 mol, 90% yield) was isolated as a clear colorless liquid via vacuum distillation (<28 °C, 1.6 Torr). 1H NMR (400 MHz, CDCl3) δ 5.55–5.46 (m, 2H), 4.14 (q, J = 7.1 Hz, 2H), 2.31–2.21 (m, 1H), 1.84–1.77 (m, 1H), 1.62 (tt, J = 8.8, 2.4 Hz, 1H), 1.26 (t, J = 7.2 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ: 172.04, 130.41, 104.54, 60.72, 18.14, 14.25, 11.45.
2-Methylenecyclopropane-1-carboxamide (11)49
In a heavy-walled pressure tube, 2-methylenecyclopropanecarboxylic acid, ethyl ester (10) (11.7 g, 0.0952 mol), and a cold solution of 25% aqueous ammonia (40 mL) were combined. The vessel was sealed and allowed to warm to room temperature. The two clear, colorless, immiscible liquids were stirred vigorously at room temperature in the dark for 6 days, by which time the flask contents had become a homogeneous clear, colorless liquid with a white solid floating precipitate. This mixture was concentrated under reduced pressure at room temperature, and the white solid was isolated via vacuum filtration with a fritted filter to afford 2-methylenecyclopropane-1-carboxamide (11) (5.02 g, 0.0517 mol, 54% yield). 1H NMR (CDCl3, 400 MHz): δ (ppm) 6.05 (s, 1H), 5.70 (s, 1H), 5.56–5.54 (m, 2H), 2.20–2.14 (m, 1H), 1.74 (ddt, J = 9.6, 4.9, 2.5 Hz 1H), 1.64 (tt, J = 9.1, 2.5 Hz 1H). 13C{1H} NMR (100 MHz, CDCl3) δ: 174.06, 130.65, 105.29, 19.49, 11.46.
1-Cyano-2-methylenecyclopropane (7)
To a 50 mL round-bottom flask, 2-methylenecyclopropane-1-carboxamide (11) (2.74 g, 0.0282 mol, 1 equiv) was added. Approximately 15 g of 30-mesh sea sand was added on top of the amide. Phosphoric anhydride (P4O10) (9.61 g, 0.0339 mol, 1.2 equiv) was added to the flask, and the contents were thoroughly mixed with a spatula. The 50 mL round-bottom flask was connected to a 25 mL round-bottom receiving flask via a glass tube and a vacuum adaptor, and the system was placed under vacuum (0.006 Torr). The receiving flask was cooled to −78 °C with a dry ice–acetone bath, and the flask containing the sand mixture was gradually heated to 100 °C with an oil bath. The contents of the reaction vessel gradually turned dark brown as it was heated. The flask was heated until 1 h after the visible effervescence in the sand mixture stopped; the total duration of heating was approximately 2 h. Over the duration of the reaction, 1-cyano-2-methylenecyclopropane (7) (1.02 g, 0.0129 mol, 46% yield) was collected as a clear, colorless oil in the receiving flask. 1H NMR (CDCl3, 400 MHz): δ (ppm) 5.77 (dd, J = 4.5, 2.5 Hz 1H), 5.68 (dd, J = 4.5, 2.1 Hz 1H), 2.04–1.98 (m, 1H), 1.83 (t, J = 2.5 Hz 1H), 1.81 (dd, J = 4.0, 2.0 Hz 1H). 13C{1H} NMR (100 MHz, CDCl3) δ: 125.02, 119.57, 107.71, 11.39, 0.47. HRMS (ASAP) m/z: [MH]+ calcd for C5H6N+ 80.0495, found, 80.0494. UV (CH3CN), end absorption only, λ = 200 nm (ε = 1590 M–1 cm–1).
Acknowledgments
The authors gratefully acknowledge financial support from the National Science Foundation (CHE-1954270 and CHE-1664912). The authors thank William Karney and Jingbai Li for their assistance with the CASSCF calculations. The authors thank the following organizations and individuals for support of shared departmental facilities: Bruker AVANCE 400 NMR spectrometer (NSF CHE-1048642), Bruker AVANCE 500 NMR spectrometer (gift from Paul J. and Margaret M. Bender), and Thermo Scientific Q Exactive Plus mass spectrometer (NIH 1S10 OD020022-1).
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jpca.3c08001.
Matrix-isolation IR spectra, 1H and 13C{1H} NMR spectra of synthesized compounds; computed energies, harmonic or anharmonic vibrational frequencies, infrared intensities, electronic transitions, and Cartesian coordinates of computed structures explicitly considered in this article (PDF)
Database of computational data of 48 isomers of pyridine (C5H5N), including computed energies, anharmonic vibrational frequencies, infrared intensities, and Cartesian coordinates (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Esselman B. J.; Kougias S. M.; Zdanovskaia M. A.; Woods R. C.; McMahon R. J. Synthesis, Purification, and Rotational Spectroscopy of (Cyanomethylene)Cyclopropane–An Isomer of Pyridine. J. Phys. Chem. A 2021, 125, 5601–5614. 10.1021/acs.jpca.1c03246. [DOI] [PubMed] [Google Scholar]
- Wilson C. L. 17. Reactions of Furan compounds. Part IV. High-Temperature Decomposition of the Vapours of Tetrahydrofuronitrile and Methyl Tetrahydrofuroate to Give 2,3-Dihydrofuran and Cyclopropanealdehyde. J. Chem. Soc. 1945, 58–61. 10.1039/jr9450000058. [DOI] [Google Scholar]
- Mauduit M.; Kouklovsky C.; Langlois Y. Cyanomethylene Cyclopropane, a Useful Dipolarophile and Dienophile in [2 + 3] and [2 + 4] Cycloadditions. Tetrahedron Lett. 1998, 39, 6857–6860. 10.1016/S0040-4039(98)01496-8. [DOI] [Google Scholar]
- Kougias S. M.; Knezz S. N.; Owen A. N.; Sanchez R. A.; Hyland G. E.; Lee D. J.; Patel A. R.; Esselman B. J.; Woods R. C.; McMahon R. J. Synthesis and Characterization of Cyanobutadiene Isomers—Molecules of Astrochemical Significance. J. Org. Chem. 2020, 85, 5787–5798. 10.1021/acs.joc.9b03388. [DOI] [PubMed] [Google Scholar]
- Zdanovskaia M. A.; Dorman P. M.; Orr V. L.; Owen A. N.; Kougias S. M.; Esselman B. J.; Woods R. C.; McMahon R. J. Rotational Spectra of Three Cyanobutadiene Isomers (C5H5N) of Relevance to Astrochemistry and Other Harsh Reaction Environments. J. Am. Chem. Soc. 2021, 143, 9551–9564. 10.1021/jacs.1c03777. [DOI] [PubMed] [Google Scholar]
- Zdanovskaia M. A.; Esselman B. J.; Kougias S. M.; Patel A. R.; Woods R. C.; McMahon R. J. The 130–360 GHz rotational spectrum of syn-2-cyano-1,3-butadiene (C5H5N) – a molecule of astrochemical relevance. Mol. Phys. 2021, 119, e1964629 10.1080/00268976.2021.1964629. [DOI] [Google Scholar]
- Smith H. H.; Kougias S. M.; Esselman B. J.; Woods R. C.; McMahon R. J. Synthesis, Purification, and Rotational Spectroscopy of 1-Cyanocyclobutene (C5H5N). J. Phys. Chem. A 2022, 126, 1980–1993. 10.1021/acs.jpca.2c00384. [DOI] [PubMed] [Google Scholar]
- Dorman P. M.; Esselman B. J.; Changala P. B.; Kougias S. M.; McCarthy M. C.; Woods R. C.; McMahon R. J. Rotational spectrum of anti- and gauche-4-cyano-1-butyne (C5H5N) – An open-chain isomer of pyridine. J. Mol. Spectrosc. 2022, 385, 111604 10.1016/j.jms.2022.111604. [DOI] [Google Scholar]
- Jean D. R.; Wood S. A.; Esselman B. J.; Woods R. C.; McMahon R. J.. Rotational Spectroscopy of 1-Cyano-2-methylenecyclopropane (C5H5N) – A Newly Synthesized Pyridine Isomer. J. Phys. Chem. A 2024, 128, 10.1021/acs.jpca.3c08002. [DOI] [PubMed] [Google Scholar]
- Ye E.; Bettens R. P. A.; De Lucia F. C.; Petkie D. T.; Albert S. Millimeter and submillimeter wave rotational spectrum of pyridine in the ground and excited vibrational states. J. Mol. Spectrosc. 2005, 232, 61–65. 10.1016/j.jms.2005.02.004. [DOI] [Google Scholar]
- Charnley S. B.; Kuan Y.-J.; Huang H.-C.; Botta O.; Butner H. M.; Cox N.; Despois D.; Ehrenfreund P.; Kisiel Z.; Lee Y.-Y.; et al. Astronomical searches for nitrogen heterocycles. Adv. Space Res. 2005, 36, 137–145. 10.1016/j.asr.2005.09.005. [DOI] [Google Scholar]
- McGuire B. A. 2021 Census of Interstellar, Circumstellar, Extragalactic, Protoplanetary Disk, and Exoplanetary Molecules. Astrophys. J., Suppl. Ser. 2022, 259, 30 10.3847/1538-4365/ac2a48. [DOI] [Google Scholar]
- Barnum T. J.; Siebert M. A.; Lee K. L. K.; Loomis R. A.; Changala P. B.; Charnley S. B.; Sita M. L.; Xue C.; Remijan A. J.; Burkhardt A. M.; et al. A Search for Heterocycles in GOTHAM Observations of TMC-1. J. Phys. Chem. A 2022, 126, 2716–2728. 10.1021/acs.jpca.2c01435. [DOI] [PubMed] [Google Scholar]
- McGuire B. A.; Burkhardt A. M.; Kalenskii S.; Shingledecker C. N.; Remijan A. J.; Herbst E.; McCarthy M. C. Detection of the aromatic molecule benzonitrile (c-C6H5CN) in the interstellar medium. Science 2018, 359, 202–205. 10.1126/science.aao4890. [DOI] [PubMed] [Google Scholar]
- Kuan Y.-J.; Yan C.-H.; Charnley S. B.; Kisiel Z.; Ehrenfreund P.; Huang H.-C. A search for interstellar pyrimidine. Mon. Not. R. Astron. Soc. 2003, 345, 650–656. 10.1046/j.1365-8711.2003.06975.x. [DOI] [Google Scholar]
- Kutner M. L.; Machnik D. E.; Tucker K. D.; Dickman R. L. Search for interstellar pyrrole and furan. Astrophys. J. 1980, 242, 541–544. 10.1086/158488. [DOI] [Google Scholar]
- Cooke I. R.; Xue C.; Changala P. B.; Toru Shay H.; Byrne A. N.; Tang Q. Y.; Fried Z. T. P.; Lee K. L. K.; Loomis R. A.; Lamberts T.; et al. Detection of Interstellar (E)-1-cyano-1,3-butadiene in GOTHAM Observations of TMC-1. Astrophys. J. 2023, 948, 133 10.3847/1538-4357/acc584. [DOI] [Google Scholar]
- McGuire B. A.; mcguiregroup . bmcguir2/astromol: Added n-methyl formamide detection. bmcguir2/astromol, 2024 10.5281/zenodo.5541828(accessed January 11, 2024). [DOI]
- Raulin F.; Brassé C.; Poch O.; Coll P. Prebiotic-like chemistry on Titan. Chem. Soc. Rev. 2012, 41, 5380–5393. 10.1039/c2cs35014a. [DOI] [PubMed] [Google Scholar]
- Palmer M. Y.; Cordiner M. A.; Nixon C. A.; Charnley S. B.; Teanby N. A.; Kisiel Z.; Irwin P. G. J.; Mumma M. J. ALMA detection and astrobiological potential of vinyl cyanide on Titan. Sci. Adv. 2017, 3, e1700022 10.1126/sciadv.1700022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson J.; Lunine J.; Clancy P. Membrane alternatives in worlds without oxygen: Creation of an azotosome. Sci. Adv. 2015, 1, e1400067 10.1126/sciadv.1400067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandström H.; Rahm M. Can polarity-inverted membranes self-assemble on Titan?. Sci. Adv. 2020, 6, eaax0272 10.1126/sciadv.aax0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd D.Feist’s Acid. In Topics in Carbocyclic Chemistry; Lloyd D., Ed.; Springer US: Boston, MA, 1969; Vol. 1, pp 251–270. [Google Scholar]
- Feist F. Ueber den Abbau des Cumalinringes. Ber. Dtsch. Chem. Ges. 1893, 26, 747–764. 10.1002/cber.189302601152. [DOI] [Google Scholar]
- Ettlinger M. G. Structure of Feist’s Methylcyclopropene-Dicarboxylic Acid. J. Am. Chem. Soc. 1952, 74, 5805–5806. 10.1021/ja01142a537. [DOI] [Google Scholar]
- Ullman E. F. Mechanism of Hydrogenation of Unsaturated Cyclopropanes. J. Am. Chem. Soc. 1959, 81, 5386–5392. 10.1021/ja01529a034. [DOI] [Google Scholar]
- Kon G. A. R.; Nanji H. R. 380. The structure of the glutaconic acids and esters. Part VII. Derivatives of 3-methylcyclopropene-1:2-dicarboxylic acid. J. Chem. Soc. 1932, 2557–2568. 10.1039/jr9320002557. [DOI] [Google Scholar]
- Ullman E. F. Thermal Rearrangement of Feist’s Ester. A New Type of Intermediate. J. Am. Chem. Soc. 1960, 82, 505–506. 10.1021/ja01487a074. [DOI] [Google Scholar]
- Dowd P. Trimethylenemethane. J. Am. Chem. Soc. 1966, 88, 2587–2589. 10.1021/ja00963a039. [DOI] [Google Scholar]
- Crawford R. J.; Cameron D. M. Evidence for Trimethylenemethane as an Intermediate in a Pyrolysis Reaction. J. Am. Chem. Soc. 1966, 88, 2589–2590. 10.1021/ja00963a040. [DOI] [Google Scholar]
- Kende A. S.; Goldschmidt Z.; Smith R. F. Photochemical methylenecyclopropane rearrangement. J. Am. Chem. Soc. 1970, 92, 7606–7607. 10.1021/ja00729a027. [DOI] [Google Scholar]
- Yamaguchi T.; Irie M.; Yoshida H. Electron Paramagnetic Resonace of Trimethylenemethane Formed by Radiolysis of Methylenecyclopropane. Chem. Lett. 1973, 2, 975–978. 10.1246/cl.1973.975. [DOI] [Google Scholar]
- Maier G.; Reisenauer H. P.; Lanz K.; Tross R.; Jürgen D.; Hess B. A. Jr.; Schaad L. J. Detection of Trimethylenemethane by IR Spectroscopy: The Result of an Unexpected Photoisomerization of Methylenecyclopropane in a Halogen-Doped Xe Matrix. Angew. Chem., Int. Ed. 1993, 32, 74–76. 10.1002/anie.199300741. [DOI] [Google Scholar]
- Maier G.; Jürgen D.; Tross R.; Reisenauer H. P.; Hess B. A.; Schaad L. J. Detection of trimethylenemethane by IR spectroscopy: result of the photoisomerization of methylenecyclopropane in a halogen-doped xenon matrix. Chem. Phys. 1994, 189, 383–399. 10.1016/0301-0104(94)00266-5. [DOI] [Google Scholar]
- Wiberg K. B.; Wang Y.-g. Electronically Excited States of Methylenecycloalkanes. J. Phys. Chem. A 2004, 108, 9417–9422. 10.1021/jp0404450. [DOI] [Google Scholar]
- Morales S. B.; Bennett C. J.; Le Picard S. D.; Canosa A.; Sims I. R.; Sun B. J.; Chen P. H.; Chang A. H. H.; Kislov V. V.; Mebel A. M.; et al. A Crossed Molecular Beam, Low-Temperature Kinetics, and Theoretical Investigation of the Reaction of the Cyano Radical (CN) with 1,3-Butadiene (C4H6). A Route to Complex Nitrogen-Bearing Molecules in Low-Temperature Extraterrestiral Environments. Astrophys. J. 2011, 742, 26 10.1088/0004-637X/742/1/26. [DOI] [Google Scholar]
- Sun B. J.; Huang C. H.; Chen S. Y.; Chen S. H.; Kaiser R. I.; Chang A. H. H. Theoretical Study on Reaction Mechanism of Ground-State Cyano Radical with 1,3-Butadiene: Prospect of Pyridine Formation. J. Phys. Chem. A 2014, 118, 7715–7724. 10.1021/jp5056864. [DOI] [PubMed] [Google Scholar]
- Jamal A.; Mebel A. M. Theoretical Investigation of the Mechanism and Product Branching Ratios of the Reactions of Cyano Radical with 1- and 2-Butyne and 1,2-Butadiene. J. Phys. Chem. A 2013, 117, 741–755. 10.1021/jp3091045. [DOI] [PubMed] [Google Scholar]
- Tsukamoto A.; Lichtin N. N. Reactions of Active Nitrogen with Organic Substrates. I. The Monomeric Products of the Reaction with 1,3-Butadiene. J. Am. Chem. Soc. 1962, 84, 1601–1605. 10.1021/ja00868a018. [DOI] [Google Scholar]
- McCarthy M. C.; Lee K. L. K.; Carroll P. B.; Porterfield J. P.; Changala P. B.; Thorpe J. H.; Stanton J. F. Exhaustive Product Analysis of Three Benzene Discharges by Microwave Spectroscopy. J. Phys. Chem. A 2020, 124, 5170–5181. 10.1021/acs.jpca.0c02919. [DOI] [PubMed] [Google Scholar]
- Mishra P.; Fritz S. M.; Herbers S.; Mebel A. M.; Zwier T. S. Gas-phase pyrolysis of trans 3-pentenenitrile: competition between direct and isomerization-mediated dissociation. Phys. Chem. Chem. Phys. 2021, 23, 6462–6471. 10.1039/D1CP00104C. [DOI] [PubMed] [Google Scholar]
- Hassall C. H.; Reyle K.; Feng P. Hypoglycin A,B: Biologically Active Polypeptides from Blighia sapida. Nature 1954, 173, 356–357. 10.1038/173356b0. [DOI] [PubMed] [Google Scholar]
- Newman M. S.; Merrill S. H. Synthesis of a Series of Substituted Phenylpropiolic Acids. J. Am. Chem. Soc. 1955, 77, 5549–5551. 10.1021/ja01626a033. [DOI] [Google Scholar]
- Carbon J. A.; Martin W. B.; Swett L. R. Synthesis of α-Amino-methylenecyclopropanepropionic Acid (Hypoglycin A). J. Am. Chem. Soc. 1958, 80, 1002. 10.1021/ja01537a066. [DOI] [Google Scholar]
- Black D. K.; Landor S. R. Allenes. Part XIX. Synthesis of (±)-hypoglycin A and configuration of the natural isomer. J. Chem. Soc. C 1968, 288–290. 10.1039/J39680000288. [DOI] [Google Scholar]
- Lai M. T.; Liu H. W. Inactivation of general acyl-CoA dehydrogenase by enantiomerically pure (methylenecyclopropyl)acetyl-CoA and its implication for this enzyme-catalyzed reaction. J. Am. Chem. Soc. 1990, 112, 4034–4035. 10.1021/ja00166a048. [DOI] [Google Scholar]
- For the record, 1-cyano-2-methylenecyclopropane (7) is a chiral compound. Our synthesis and experiments were conducted with racemic material
- Scott M. E.; Tseng N.-W.; Lautens M. 2-Methylenecyclopropanecarboxylic Acid Ethyl Ester. Org. Syn. 2008, 85, 172–178. 10.1002/0471264229.os085.18. [DOI] [Google Scholar]
- Nishimura A.; Ohta M.; Kato H. Deamination of Methylenecyclopropylcarbinylamine. The Formation of a Vinyl Cation by Carbon-Carbon Bond Cleavage. Bull. Chem. Soc. Jpn. 1970, 43, 1530–1534. 10.1246/bcsj.43.1530. [DOI] [Google Scholar]
- Pharr C. R.; Kopff L. A.; Bennett B.; Reid S. A.; McMahon R. J. Photochemistry of Furyl- and Thienyldiazomethanes: Spectroscopic Characterization of Triplet 3-Thienylcarbene. J. Am. Chem. Soc. 2012, 134, 6443–6454. 10.1021/ja300927d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon R. J.; Chapman O. L.; Hayes R. A.; Hess T. C.; Krimmer H. P. Mechanistic studies on the Wolff rearrangement: the chemistry and spectroscopy of some α-keto carbenes. J. Am. Chem. Soc. 1985, 107, 7597–7606. 10.1021/ja00311a063. [DOI] [Google Scholar]
- Seburg R. A.; McMahon R. J. Photochemistry of matrix-isolated diazoethane and methyldiazirine: ethylidene trapping?. J. Am. Chem. Soc. 1992, 114, 7183–7189. 10.1021/ja00044a034. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Petersson G. A.; Nakatsuji H., et al. Gaussian 16; Gaussian, Inc.: Wallingford, CT, 2016. [Google Scholar]
- Schmidt J. R.; Polik W. F.. WebMO Enterprise, 17.0.012e; WebMO LLC: Holland, MI, 2013; www.webmo.net.
- Hernández F. J.; Cox J. M.; Li J.; Crespo-Otero R.; Lopez S. A. Multiconfigurational Calculations and Photodynamics Describe Norbornadiene Photochemistry. J. Org. Chem. 2023, 88, 5311–5320. 10.1021/acs.joc.2c02758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OpenMolcas - A Quantum Chemistry Package. https://comp.chem.umn.edu/openmolcas/ (accessed January 11, 2024).
- Fdez Galván I.; Vacher M.; Alavi A.; Angeli C.; Aquilante F.; Autschbach J.; Bao J. J.; Bokarev S. I.; Bogdanov N. A.; Carlson R. K.; et al. OpenMolcas: From Source Code to Insight. J. Chem. Theory Comput. 2019, 15, 5925–5964. 10.1021/acs.jctc.9b00532. [DOI] [PubMed] [Google Scholar]
- Li Manni G.; Fdez; Galván I.; Alavi A.; Aleotti F.; Aquilante F.; Autschbach J.; Avagliano D.; Baiardi A.; Bao J. J.; Battaglia S.; et al. The OpenMolcas Web: A Community-Driven Approach to Advancing Computational Chemistry. J. Chem. Theory Comput. 2023, 19, 6933–6991. 10.1021/acs.jctc.3c00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The authentic matrix IR spectrum of 4-cyano-1-butyne was collected in this study and is found in Supporting Information.
- The authentic matrix IR spectrum of 1-cyano-1-butyne was collected in this study and is found in Supporting Information.
- Kudoh S.; Takayanagi M.; Nakata M. Dewar pyridine studied by matrix isolation infrared spectroscopy and DFT calculation. J. Photochem. Photobiol., A 1999, 123, 25–30. 10.1016/S1010-6030(99)00035-0. [DOI] [Google Scholar]
- Johnstone D. E.; Sodeau J. R. Matrix-Controlled Photochemistry of Benzene and Pyridine. J. Phys. Chem. A 1991, 95, 165–169. 10.1021/j100154a033. [DOI] [Google Scholar]
- King C. M.; Nixon E. R. Matrix-Isolation Study of the Hydrogen Cyanide Dimer. J. Chem. Phys. 1968, 48, 1685–1695. 10.1063/1.1668895. [DOI] [Google Scholar]
- Wrobel R.; Sander W.; Cremer D.; Kraka E. Photochemistry of Butatriene – Spectroscopic Evidence for the Existence of Allenylcarbene. J. Phys. Chem. A 2000, 104, 3819–3825. 10.1021/jp9940147. [DOI] [Google Scholar]
- Arnold B. R.; Michl J. Ultraviolet and polarized infrared spectroscopy of matrix-isolated cyclobutadiene and its isotopomers. J. Phys. Chem. A 1993, 97, 13348–13354. 10.1021/j100152a046. [DOI] [Google Scholar]
- Guennoun Z.; Couturier-Tamburelli I.; Piétri N.; Aycard J. P. UV photoisomerisation of cyano and dicyanoacetylene: the first identification of CCNCH and CCCNCN isomers – matrix isolation, infrared and ab initio study. Chem. Phys. Lett. 2003, 368, 574–583. 10.1016/S0009-2614(02)01898-5. [DOI] [Google Scholar]
- Rytter E.; Gruen D. M. Infrared spectra of matrix isolated and solid ethylene. Formation of ethylene dimers. Spectrochim. Acta, Part A 1979, 35, 199–207. 10.1016/0584-8539(79)80137-3. [DOI] [Google Scholar]
- Anslyn E. V.; Dougherty D. A.. Modern Physical Organic Chemistry; University Science Books: Sausalito, CA, 2006; Vol. Chapter 14. [Google Scholar]
- Gilbert J. C.; Luo T. Photochemical Fragmentation of a Methylenecyclopropane. J. Org. Chem. 1981, 46, 5237–5239. 10.1021/jo00338a043. [DOI] [Google Scholar]
- Mullen P. A.; Orloff M. K. The Electronic Spectrum of Acrylonitrile. Theor. Chim. Acta 1971, 23, 278–284. 10.1007/BF00530237. [DOI] [Google Scholar]
- Takahashi Y.; Mori Y.; Nakamura A.; Tomioka H. Intermediacy of cyclobutylidene in photochemical methylenecyclopropane rearrangement. Tetrahedron Lett. 2005, 46, 8415–8418. 10.1016/j.tetlet.2005.09.149. [DOI] [Google Scholar]
- Dong H.; Hrovat D. A.; Quast H.; Borden W. T. Calculations of the Relative Energies of the Low-Lying Electronic States of 2-Methylenedihydrophenalene-1,3-diyl: Effects of a 1,8-Naphtho Bridging Group on Trimethylenemethane and of a Vinylidene Bridging Group on 1,8-Naphthoquinodimethane. J. Phys. Chem. A 2009, 113, 895–901. 10.1021/jp809715v. [DOI] [PubMed] [Google Scholar]
- Wei H.; Hrovat D. A.; Borden W. T. Ab Initio Calculations of the Potential Surface for Rearrangement of 2,2,3,3-Tetrafluoromethylenecyclopropane to 1-(Difluoromethylene)-2,2-difluorocyclopropane. J. Am. Chem. Soc. 2006, 128, 16676–16683. 10.1021/ja065963y. [DOI] [PubMed] [Google Scholar]
- Koseki S.; Haruta M.; Sawada N.; Asada T. Exploring the Reaction Paths on the Potential Energy Surfaces of the S1 and T1 States in Methylenecyclopropane. Photochem. Photobiol. 2021, 97, 126–135. 10.1111/php.13326. [DOI] [PubMed] [Google Scholar]
- Li J.; Lopez S. A. A Look Inside the Black Box of Machine Learning Photodynamics Simulations. Acc. Chem. Res. 2022, 55, 1972–1984. 10.1021/acs.accounts.2c00288. [DOI] [PubMed] [Google Scholar]
- Li J.; Reiser P.; Boswell B. R.; Eberhard A.; Burns N. Z.; Friederich P.; Lopez S. A. Automatic discovery of photoisomerization mechanisms with nanosecond machine learning photodynamics simulations. Chem. Sci. 2021, 12, 5302–5314. 10.1039/D0SC05610C. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its online Supporting Information.