Abstract
Dendritic cells (DCs) can develop from CD14+ peripheral blood monocytes cultured in granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4). By 6 days in culture, the cells have the characteristics of immature DCs and can be further induced to mature by inflammatory stimuli or by monocyte-conditioned medium. After infection with macrophagetropic (M-tropic) human immunodeficiency virus type 1 (HIV-1), monocytes and mature DCs show a block in reverse transcription and only form early transcripts that can be amplified with primers for the R/U5 region. In contrast, immature DCs cultured for 6 or 11 days in GM-CSF and IL-4 complete reverse transcription and show a strong signal when LTR/gag primers are used. Blood monocytes and mature DCs do not replicate HIV-1, whereas immature DCs can be productively infected, but only with M-tropic HIV-1. The virus produced by immature DCs readily infects activated T cells. Although mature DCs do not produce virus, these cells transmit both M- and T-tropic virus to T cells. In the cocultures, both DCs and T cells must express functional chemokine coreceptors for viral replication to occur. Therefore, the developmental stage of DCs can influence the interaction of these cells with HIV-1 and influence the extent to which M-tropic and T-tropic virus can replicate.
Dendritic cells (DCs) are antigen-presenting cells that are found at sites involved in the transmission of human immunodeficiency virus type 1 (HIV-1), such as blood and several body surfaces (reviewed in references 8, 25,, and 31). At these sites, the DCs are immature, lacking costimulatory molecules like CD80 and CD86 as well as the potent T-cell-stimulatory function that is typical of DCs. Immature DCs capture antigens that enter at body surfaces and then mature, e.g., express CD80 and CD86, as they migrate to lymphoid tissues to initiate the immune response. We have used a tissue culture model to study the capacity of immature and mature DCs to replicate HIV-1 and to transfer virus to T cells. We now show two ways whereby HIV-1 can take advantage of the DC pathway for enhancing viral replication. Immature DCs selectively replicate M-tropic HIV-1 and therefore can account for the “bottleneck” that selects for this type of virus during transmission (27, 32). However, when maturation takes place, both macrophagetropic (M-tropic) and T-tropic virus can be transmitted by DCs to T cells.
An important role for DCs during the course of HIV-1 infection is indicated by the evidence that DCs have the ability to spread HIV-1 to T cells, promoting extensive replication and leading to the death of CD4+ T cells (4–6, 19, 28). On the other hand, there are divergent findings with respect to the direct contribution of purified DCs to HIV-1 replication (3, 12, 29). There is evidence that DCs replicate virus (14, 18, 26) and that HIV-1 impairs their antigen-presenting cell function (10, 16), while other reports demonstrate that DCs are not productively infected but nevertheless promote viral replication upon interaction with T cells (5, 20). These discrepancies could be due to several parameters, one of which is the state of DC maturation, as we now report.
MATERIALS AND METHODS
Cells.
Dendritic cells were generated from the blood of normal donors (2, 23). Peripheral blood mononuclear cells PBMC were isolated by sedimentation in Ficoll-Hypaque, and 5 × 107 PBMC were plated per 100-mm-diameter culture plastic dish in RPMI (Gibco) supplemented with 1% heat-inactivated autologous plasma. After 1 h, the floating cells were removed and the adherent cells were incubated for 6 days with granulocyte-macrophage colony-stimulating factor (GM-CSF) (100 IU/ml; Leukine; Immunex) and interleukin 4 (IL-4) (1,000 U/ml; Genzyme). Cells were fed on days 2, 4, and 6 of culture with the same dose of cytokines. At day 6, most of the nonattached cells are immature DCs, being CD14−, CD4+, HLA-DR+, and CD86+ but lacking or weak in the CD83, p55, and CD25 markers of mature, more-stimulatory DCs. The DCs were studied immediately, following purification as large CD3−, CD20− cells by cell sorting. In some experiments the CD14 marker was also included to exclude macrophages. The results obtained were similar. To mature, the cells were returned to culture for 5 days in monocyte-conditioned medium (MCM). To do so, 106 cells were plated in 3 ml of GM-CSF–IL-4, but half of this volume was replaced with MCM. These mature DCs upregulate the surface expression of several molecules and possess strong antigen-presenting capacity even at ratios of stimulator-effector cells of 1/300 to 1/900. Macrophages were plastic adherent monocytes cultured for 11 days. T blasts were generated by stimulation of PBMC with phytohemagglutinin (1 μg/ml; Burroughs Wellcome) for 3 days. MCM was generated by plating 1.5 × 108 PBMC on a gamma globulin (10 μg/ml; Calbiochem)-coated petri dish (30 min at room temperature). Cells were incubated for 45 min at 37°C on the immunoglobulin-coated dishes, the nonadherent cells were washed out, and then the adherent cells were incubated for 24 h in 1% human plasma to produce the MCM.
Viruses and infection of cells.
In most of the experiments, cells were infected with the Ba-L isolate, grown in mitogen-stimulated PBMC. Other viruses were obtained by transfection of 293 T cells. For each HIV-1 isolate, cells were infected with equal amounts of p24 antigen (2 ng for 105 cells). To reduce the amount of HIV-1 DNA associated with virus-containing supernatants, the latter were filtered and treated with RNase-free DNase (50 U/ml; Boehringer Mannheim) for 30 min at room temperature in the presence of 10 mM MgCl2. Irradiation of DCs was accomplished in a cesium irradiator by exposing DCs in the cold at 3,000 rads. The cells were washed before infection and then infected for 90 min at 37°C in 1% autologous plasma. The nonadsorbed viruses were extensively washed, and the cells were trypsinized (0.25% trypsin for 7 min at 37°C) to remove residual virus. This trypsinization removed the CD4 epitope recognized by the Leu 3a monoclonal antibody (11). The DCs after infection were resuspended in the original medium until the end of the experiment. In coculture experiments, noninfected cells were added as indicated in the figure legends.
Detection of HIV-1 in infected cells. (i) PCR.
All reagents used in the PCR were tested to ensure that no HIV-1 DNA contamination was present. To analyze viral DNA, infected cells were collected at the indicated times, washed twice with phosphate-buffered saline, and lysed in lysis buffer (10 mM Tris HCl [pH 8], 1 mM EDTA, 0.001% Triton X 100-sodium dodecyl sulfate, and proteinase K [1 mg/ml]). The samples were incubated at 60°C for 1 h and then placed in a boiling water bath to inactivate the protease. Reverse transcripts were amplified by PCR using R/U5 (sense, 5′-GGCTAACTAGGGAACCCACGT-3′; antisense, 5′-CTGCTAGAGTTTTCCCACTGAC-3′) and LTR/gag (sense, 5′-GGCTAACTAGGGAACCCACGT-3′; antisense, 5′-CCTGCGTCGAGAGAGCTCCTCTGG-3′) primers as described previously (11). Amplified products were resolved on nondenaturing 8% polyacrylamide gels and visualized by direct autoradiography of the dried gels.
(ii) RTase assay.
At different times after infection of the cells, triplicate 10-μl aliquots of culture supernatants were harvested and stored at −20°C. Samples were assayed for reverse transcriptase (RTase) activity by a microtiter method (5).
RESULTS
Recently, methods for generating immature and mature DCs from precursors in human blood have been described (2, 23). We have adapted this methodology to study HIV-1 infection during DC differentiation. The DCs develop from CD14+ blood monocytes when cultured with IL-4 and GM-CSF and then complete their differentiation and maturation in the presence of MCM (2, 17, 23) that includes products of inflammation like IL-1 and tumor necrosis factor alpha (21). The DCs generated do not divide; the cells differentiated only. Cells are in G0/G1 as determined by propidium iodide and fluorescence-activated cell sorting (FACS) (see Fig. 3C). Also, these cells do not incorporate thymidine and are negative for Ki-67 (Dako) staining (not shown). Our in vitro model uses, as immature DCs, T-cell-depleted mononuclear cells that have been cultured for 6 or 11 days with GM-CSF and IL-4 and, as mature DCs, the same cells supplemented with MCM from day 7 to 11. In each of the following experiments, which have been repeated at least twice, the DCs were depleted of lymphocytes by cell sorting (11).
FIG. 3.
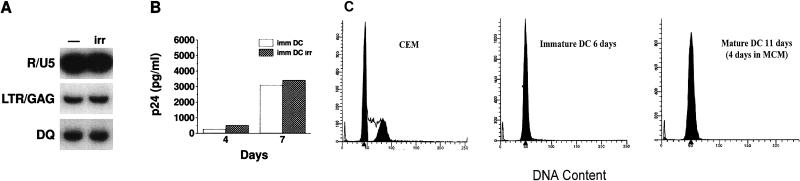
Effect of irradiation on HIV-1 replication in DCs. DCs cultured for 11 days in cytokines were irradiated (3,000 rads) or not and then pulsed for 1.5 h with Ba-L. (A) Cell lysates were prepared from 5 × 104 cells 72 h after infection, and reverse transcripts were amplified by PCR. DQ sequences were amplified to control for DNA imput. −, immature DCs; irr, irradiated DCs. (B) p24 was measured 4 and 7 days after infection of immature DCs (imm DC) or irradiated DCs (imm DC irr). Both immature and mature DCs are in G0/G1 phase. (C) DNA contents of the indicated cell lines as determined by FACS with PI staining.
Selective HIV-1 replication in immature DCs.
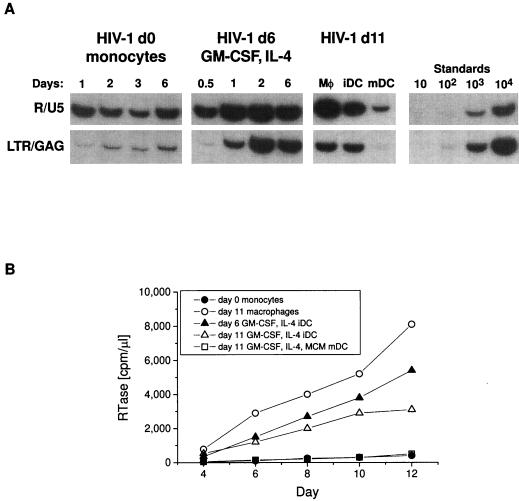
Freshly isolated, T-cell-depleted PBMC were infected by HIV-1, because early R/U5-bearing transcripts were found. However, the cells did not complete reverse transcription (Fig. 1A, left) or replicate virus (Fig. 1B). When these same cells were cultured for 6 days in GM-CSF and IL-4, they acquired the characteristics of immature DCs (HLA-DR+, CD14low, CD83low, p55low, and CD25low) (9). At this stage of maturation, HIV-1 entered and replicated extensively, forming large numbers of full-length, LTR/gag-containing sequences (Fig. 1A, right) and releasing RTase (Fig. 1B). Moreover, the virus produced by the immature DCs was highly infectious when used to infect stimulated PBMC (Fig. 2). Next, three different populations of cells were compared for HIV-1 infectivity. These were macrophages (11 days old) and DCs kept in GM-CSF–IL-4 for 11 days as immature and mature DCs. Figure 1 shows that viral replication occurs in macrophages and immature DCs but not in mature DCs.
FIG. 1.
HIV-1 infection of monocytes, immature DCs (iDC), and mature DCs (mDC). (A) Early and late stage of reverse transcription. The different cell populations were pulsed with HIV-1 Ba-L (2 ng of p24 for 105 cells [11]). Cell lysates were prepared from 5 × 104 cells at the indicated times postinfection and reverse transcripts were amplified by PCR and compared to graded doses of ACH-2 cells. The left panel has T- and B-depleted blood cells, primarily monocytes, infected at day 0; the second panel has the same cells cultured for 6 days in GM-CSF and IL-4 to form immature DCs; the third panel has 11-day-cultured cells: macrophages (Mφ), immature DCs (iDC), and mature DCs (mDC) infected and analyzed at day 14. (B) RTase activity secreted in the culture media by different cell types that were pulsed with virus as described above and returned to culture for 12 days. The monocytes used were freshly isolated PBMC depleted of T and B cells. The macrophages used were plastic adherent monocytes kept in culture for 11 days prior to infection. At different time points after exposure to HIV-1, triplicate 10-μl aliquots of culture supernatant were collected and assayed for RTase activity.
FIG. 2.
The HIV-1 that is produced by immature DCs is infectious. (A) Immature DCs (2 × 105) were infected with HIV-1 Ba-L, and the supernatants (200 μl), collected at the indicated days, were used to infect 105 T blasts. After 72 h, T blast lysates were analyzed by PCR. (B) RTase activity released by T blasts infected by HIV-1 produced by immature DCs after 11 days of infection.
DCs derived from monocytes in cytokine medium do not divide, as judged by several criteria (FACS with propidium iodide [Fig. 3C] and Ki-67 staining [not shown]). To rule out the involvement of trace replicating cells in the cultures, immature DCs (day 6 or 11) were irradiated (3,000 rads) before infection. No significant difference in virus entry and virus replication (Fig. 3A and B) was noted under these conditions. These results indicate that cell replication does not contribute to HIV-1 replication in these cultures.
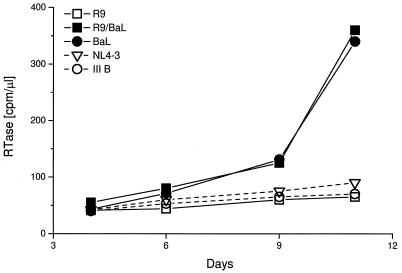
Virus replication in immature DCs occurred preferentially with M-tropic HIV-1 or with T-cell line-adapted viruses that carry an M-tropic envelope (compare R9 with R9/Ba-L in Fig. 4). Other M-tropic isolates that replicated in immature DCs were JR-FL and ADA. This selectivity for M-tropic over T-tropic viruses is paradoxical, since both HIV-1 coreceptors, CCR5 for M-tropic viruses and CXCR4 for T-tropic viruses, are expressed and signal calcium fluxes in immature DCs (9). The behavior of immature DCs was strikingly different from that of mature DCs, which did not support the formation of LTR/gag sequences and produced little or no RTase (5, 11, 20, 30) (Fig. 1).
FIG. 4.
Immature DCs selectively replicate virus with M-tropic envelopes. IIIB and NL4-3 (T-tropic) and Ba-L (M-tropic) viruses were grown in activated PBMC. The other viruses were obtained by transfection of 293 cells. R9 is a chimera of NL4-3 and HxB2 T-tropic virus. R9/BaL, T-tropic envelope has been replaced with Ba-L envelope.
Mature DCs efficiently transmit HIV-1 to T cells.
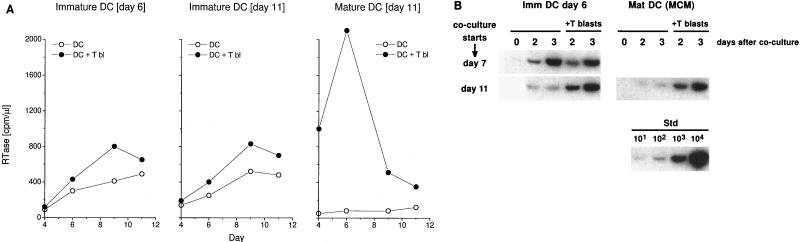
To pursue the capacity of immature and mature DCs to disseminate HIV-1, each type of DC was pulsed with virus and washed, and 24 h later, T blasts were added. Culture supernatants were assayed for RTase activity for 12 days. Immature DCs (day 6 and day 11 in cytokines) replicated virus in the absence of T cells and gave a modest increase in RTase when T blasts were added (Fig. 5A). In contrast, mature DCs did not release RTase when exposed to HIV-1 but replicated virus actively in association with T blasts (Fig. 5A).
FIG. 5.
Enhancement of viral replication in DC-T blast cocultures primarily occurs with mature rather than immature DCs. (A) RTase release by day 6 and day 11 immature DCs and mature DCs. DCs were pulsed 1.5 h at 37°C with Ba-L. After 24 h, 5 × 104 infected DCs were cocultured with 5 × 104 T blasts (T bl). Supernatants were collected at the indicated times and assayed for RTase. (B) Rapid expansion of proviral DNA in DC-T blast cocultures. Immature (Imm) DCs or mature (Mat) DCs were pulsed 1.5 h at 37°C with Ba-L and cultured for 24 h prior to mixing with an equal number of T blasts. In the left panel, the infected DCs in the lower line were also matured 4 days with MCM prior to mixing with T blasts. Full-length reverse transcripts (LTR/gag primers) were then amplified in the DCs cultured alone or with T blasts for 2 and 3 days. Std, standard.
The findings were then analyzed further at the level of copy numbers of full-length proviral DNA (Fig. 5B). Immature DCs (cultured 6 days with GM-CSF and IL-4) were pulsed with HIV-1, washed, cultured for one day (corresponding to day 7), and then cultured alone or with T blasts. Full-length DNA transcripts were then amplified with LTR/gag primers 0, 2, or 3 days later. The amounts of full-length HIV-1 DNA in immature DCs without or with T blasts were comparable (Fig. 5B, left). In contrast, if the infected immature DCs were first allowed to mature until day 11 with MCM and then the T blasts were added, replication of viral DNA became dependent on the presence of T cells (Fig. 5B, bottom left). Likewise, if the DCs were first matured in the presence of MCM and then exposed to virus as mature cells, the virus did not replicate unless T blasts were added (Fig. 5B, right). The efficiency of viral replication in the DC-T cell cocultures was substantial, since the DCs were being infected with ∼100 infectious units per 5 × 104 cells, and then we added these DCs to an equal number of T blasts to obtain several thousand copies of proviral DNA 2 days later.
Relevance of CCR5 coreceptors for transmission of virus from DCs to T cells.
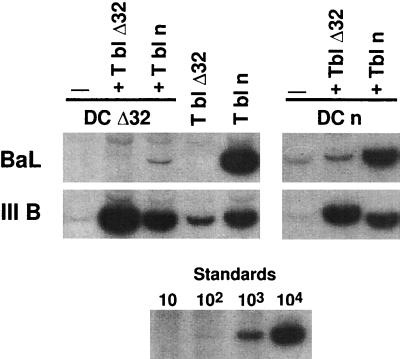
We next tested cells with a CCR5 coreceptor for M-tropic virus that was nonfunctional as a result of a 32-bp deletion in the CCR5 gene (15). By preparing DCs and T blasts from these donors, we could analyze the role of HIV-1 viral entry into each cell type in DC-T cell cocultures. In a prior study, we found that infection of mature DCs with M-tropic but not T-tropic virus was dependent on CCR5 (11). When mutant cells were tested, the M-tropic Ba-L isolate was not infectious if either the DC or T blast was from a CCR5 mutant donor, whereas the IIIB isolate replicated well, yielding thousands of copies of full-length viral DNA in just 2 days of coculture (Fig. 6, left, first three lanes). When DCs were pulsed with virus and cocultured with T cells, the copy numbers of new virus were similar or greater than those when virus was added directly to the T blasts (Fig. 6, left, compare first three lanes with next two lanes). When DCs were obtained from normal CCR5-competent donors, the DCs would not replicate M-tropic virus if cultured with mutant T blasts (Fig. 6, right, second lane). However Δ32 mature DCs could transmit IIIB T-tropic virus to normal and Δ32 T blasts, much like normal DCs (Fig. 5, right). These experiments indicate that M-tropic virus must gain entry to both DCs and T cells via the CCR5 coreceptor in order for virus to replicate in the cocultures.
FIG. 6.
Both DCs and T cells must express functioning CCR5 coreceptor to replicate M-tropic HIV-1. For the panels on the left, mature DCs were prepared from blood of CCR5 Δ32 bp (DC Δ32) and infected with Ba-L (M tropic) or IIIB (T tropic). After 90 min, HIV-1 was removed by washing and the DCs were cultured with T blasts from a normal individual (T bl n) or T blasts from a Δ32 bp individual (T bl Δ32). In parallel, mature DCs from a normal individual (DCn) were analyzed. LTR/gag reverse transcripts were amplified by PCR. Signals can be compared to those of graded doses of ACH-2 cells, i.e., one copy of viral DNA per cell.
DISCUSSION
The present study provides new information on two important roles for DCs during HIV-1 infection. The first is the capacity of DCs to replicate HIV-1. Immature DCs can do so but do so primarily for M-tropic virus even though CXCR4 is functional in these cells (9). HIV-1 produced by immature DCs may augment plasma viremia and thereby disseminate infection to T cells. The best-characterized example of immature DCs in vivo are the Langerhans cells, which are usually isolated from the epidermis, but also are found in the surface epithelium of the vagina, uterine cervix, anus, and oral pharynx, i.e., at potential sites for HIV-1 entry. In a recent study virus was scratched into skin organ cultures, and then the emigrating DCs were analyzed for capture of infectious HIV-1. It was found that only M-tropic virus was captured by DCs in the epidermis (22). Our findings are entirely compatible and provide large numbers of these immature DCs for further study. Selective replication of M-tropic HIV-1 by DCs derived by CD34+ blood progenitors has also been reported (7). When DCs are derived from the CD34+ progenitors, there is extensive cell replication. However, DCs that develop from monocytes with cytokines do not proliferate (Fig. 3C). It is known that there is a bottleneck wherein M-tropic viruses are primarily responsible for HIV-1 transmission (27, 32). Blood monocytes, in contrast to immature DCs, do not support HIV-1 replication (Fig. 1). Also monocytes only migrate to macrophage-rich regions of lymphoid tissues (24), whereas DCs migrate to the T-cell areas of the organs (1, 13).
A second role for DCs is to transfer HIV-1 during their normal interactions with T cells. We find that when DCs mature, they lose the capacity to replicate HIV-1 but can nonetheless transmit both M-tropic and T-tropic virus to T cells. The availability of CCR5 mutant cells now makes it clear that both DCs and T cells must express functioning chemokine coreceptors for viral replication to occur. By replicating M-tropic HIV-1 at sites of viral entry into the body and by maturing to a state where both M- and T-tropic virus can be transported to T cells as in lymphoid organs, immature DCs may be critical in the transmission of HIV-1 in vivo.
ACKNOWLEDGMENTS
This work was supported by grants AI 40045 and AI 40874 from the NIH and by the Direct Effect program. E.D. is a recipient of a fellowship from the Ministerio de Educacion y Cultura, Spain.
REFERENCES
- 1.Austyn J M, Kupiec-Weglinski J W, Hankins D F, Morris P J. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988;167:646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender A, Sapp M, Schuler G, Steinman R M, Bhardwaj N. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J Immunol Methods. 1996;196:121–135. doi: 10.1016/0022-1759(96)00079-8. [DOI] [PubMed] [Google Scholar]
- 3.Cameron P, Pope M, Granelli-Piperno A, Steinman R M. Dendritic cells and the replication of HIV-1. J Leukocyte Biol. 1996;59:158–171. doi: 10.1002/jlb.59.2.158. [DOI] [PubMed] [Google Scholar]
- 4.Cameron P U, Forsum U, Teppler H, Granelli-Piperno A, Steinman R M. During HIV-1 infection most blood dendritic cells are not productively infected and can induce allogeneic CD4+ T cells clonal expansion. Clin Exp Immunol. 1992;88:226–236. doi: 10.1111/j.1365-2249.1992.tb03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron P U, Freudenthal P S, Barker J M, Gezelter S, Inaba K, Steinman R M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- 6.Cameron P U, Pope M, Gezelter S, Steinman R M. Infection and apoptotic cell death of CD4+ T cells during an immune response to HIV-1 pulsed dendritic cells. AIDS Res Hum Retroviruses. 1994;10:61–71. doi: 10.1089/aid.1994.10.61. [DOI] [PubMed] [Google Scholar]
- 7.Canque B, Rosenzwajg M, Camus S, Yagello M, Bonnet M-L, Guigon M, Gluckman J C. The effect of in vitro human immunodeficiency virus infection on dendritic-cell differentiation and function. Blood. 1996;88:4215–4228. [PubMed] [Google Scholar]
- 8.Caux C, Banchereau J. In vitro regulation of dendritic cell development and function. In: Whetton T, Gordon J, editors. Blood cell biochemistry. London, United Kingdom: Plenum Press; 1996. pp. 263–301. [Google Scholar]
- 9.Delgado, E., V. Finkel, M. Baggiolini, I. Clark-Lewis, C. R. Mackay, R. M. Steinman, and A. Granelli-Piperno. Mature dendritic cells respond to SDF-1, but not to several β chemokines. Immunobiology, in press. [DOI] [PubMed]
- 10.Eales L-J, Farrant J, Helbert M, Pinching A J. Peripheral blood dendritic cells in persons with AIDS and AIDS related complex: loss of high intensity class II antigen expression and function. Clin Exp Immunol. 1988;71:423–427. [PMC free article] [PubMed] [Google Scholar]
- 11.Granelli-Piperno A, Moser B, Pope M, Chen D, Wei Y, Isdell F, O’Doherty F, Paxton W, Koup R, Mojsov S, Bhardwaj N, Clark-Lewis I, Baggiolini M, Steinman R M. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–2438. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight S C, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 13.Kudo S, Matsuno K, Ezaki T, Ogawa M. A novel migration pathway for rat dendritic cells from the blood: hepatic sinusoids-lymph translocation. J Exp Med. 1997;185:777–784. doi: 10.1084/jem.185.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langhoff E, Kalland K H, Haseltine W A. Early molecular replication of human immunodeficiency virus type 1 in cultured-blood-derived T helper dendritic cells. J Clin Invest. 1993;91:2721–2726. doi: 10.1172/JCI116512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 16.Macatonia S E, Lau R, Patterson S, Pinching A J, Knight S C. Dendritic cell infection, depletion and dysfunction in HIV infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 17.O’Doherty U, Peng M, Gezelter S, Swiggard W J, Betjes M, Bhardwaj N, Steinman R M. Human blood contains two subsets of dendritic cells, one immunologically mature, and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson S, Macatonia S E, Gross J, Bedford P A, Knight S C. Morphology and phenotype of dendritic cells from peripheral blood and productive and non-productive infection with human immunodeficiency virus type 1. Immunology. 1991;72:361–367. [PMC free article] [PubMed] [Google Scholar]
- 19.Pope M, Betjes M G H, Romani N, Hirmand H, Cameron P U, Hoffman L, Gezelter S, Schuler G, Steinman R M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994;78:389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 20.Pope M, Gezelter S, Gallo N, Hoffman L, Steinman R M. Low levels of HIV-1 in cutaneous dendritic cells initiate a productive infection upon binding to memory CD4+ T cells. J Exp Med. 1995;182:2045–2056. doi: 10.1084/jem.182.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–3646. [PubMed] [Google Scholar]
- 22.Reece, J. C., A. Handley, J. Anstee, W. Morrison, S. M. Crowe, and P. U. Cameron. HIV-1 selection by epidermal dendritic cells during transmission across human skin. Unpublished data. [DOI] [PMC free article] [PubMed]
- 23.Romani N, Reider D, Heuer M, Ebner S, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood: an improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 24.Rosen H, Gordon S. Adoptive transfer of fluorescence-labeled cells shows that resident peritoneal macrophages are able to migrate into specialized lymphoid organs and inflammatory sites in the mouse. Eur J Immunol. 1990;20:1251–1258. doi: 10.1002/eji.1830200609. [DOI] [PubMed] [Google Scholar]
- 25.Steinman R M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 26.Tsunetsugu-Yokota Y, Akagawa K, Kimoto H, Suzuki K, Iwasaki M, Yasuda S, Hausser G, Hultgren C, Meyerhans A, Takemori T. Monocyte-derived cultured dendritic cells are susceptible to human immunodeficiency virus infection and transmit virus to resting T cells in the process of nominal antigen presentation. J Virol. 1995;69:4544–4547. doi: 10.1128/jvi.69.7.4544-4547.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van’t Wout A B, Kootstra N A, Mulder-Kampinga G A, Albrect-van Lent N, Scherpbier H J, Veenstra J, Boer K, Coutinho R A, Miedema F, Schuitemaker H. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J Clin Invest. 1994;94:2060–2067. doi: 10.1172/JCI117560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissman D, Barker T D, Fauci A S. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J Exp Med. 1996;183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weissman D, Fauci A S. Role of dendritic cells in immunopathogenesis of human immunodeficiency virus infection. Clin Microbiol Rev. 1997;10:358–367. doi: 10.1128/cmr.10.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weissman D, Li Y, Ananworanich J, Zhou L-J, Adelsberger J, Tedder T F, Baseler M, Fauci A S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1995;92:826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams L A, Egner W, Hart D N J. Isolation and function of human dendritic cells. Int Rev Cytol. 1994;153:41–104. doi: 10.1016/s0074-7696(08)62188-9. [DOI] [PubMed] [Google Scholar]
- 32.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]