Abstract
Microplastics are small plastic pieces with sizes less than 5 mm. They are becoming a global concern due to the potential risk to human health. The potential risks of microplastics may be greater for infants because they do not have sufficiently developed metabolizing enzymes, have less ability to remove microplastics, and have highly sensitive target organs. Infants should be breastfed for the first six months of life. Breast milk is considered to be the most complete and suitable source of nutrition. However, if breastfeeding during this period is not possible, it is necessary to use formulas designed for infant initial feeding. Infants may be exposed to higher levels of MPs through infant foods or plastic products. The aim of this study is to describe the possible sources of exposure to microplastics such as the human placenta, plastic feeding bottles, and toys as well as the presence of released microplastics in infant feces, breast milk, and infant formulas. There is still not enough data available for this study area. Therefore, it is necessary to pay increased attention to minimizing the negative effects of microplastics on human health.
Keywords: microplastics, infants, breast milk, baby bottle, infant formula, feces
1. Introduction
Plastic products entered the market in the 1950s. The production of plastics in Europe reached 58 million tons in 2014. More than 40% of plastic is single-use packaging. It is predicted that the amount of plastic waste will reach 13% by 2060 and will continue to increase. It is estimated that by 2040, about 250 million metric tons of plastic waste will enter the water system and about 460 million metric tons will enter the soil system. It is very difficult to imagine life without plastics, which have become part of our everyday life [1]. They are used in a lot of applications related to packaging, the automotive industry, aquaculture, fisheries, agriculture, building, furniture, transportation, personal care products, textiles, clothing, etc. [2]. Plastics are often used as materials in various industries due to their low production costs, easy transportation, low weight, and high resistance. Important environmental challenges include the necessity to reduce the amount of contaminants in the environment and eliminate and suppress their effects. Plastic pollution in all components of the environment such as water, air, soil, and biota, and their negative impact on human health are still a misunderstood research area [3,4].
The huge consumption of plastics and the associated large amount of plastic waste cause their constant release into the environment, where they can degrade into smaller pieces, microplastics (MPs), which are considered contaminants [5,6]. MPs are a synthetic material that contains a high proportion of polymers. MPs come from multiple sources, interact with different components of the environment, and have different routes of transport and transformation. Currently, microplastic pollution is an urgent worldwide problem due to environmental pollution. Statistics show that about 3 million tons of plastic is produced annually, and most of it is waste. Due to the influence of various external factors (UV radiation, weather effects, etc.), plastics degrade into smaller particles, MPs. As they are highly persistent substances that are not biodegradable and that accumulate in various environments, they represent a potential risk to human health. However, it should be kept in mind that MPs also serve as carriers of heavy metals, persistent organic pollutants that can have toxic effects on the human body [7].
The constant increase in plastics and their degradation products leads to their presence in components of the environment, including the food chain [2,8]. Pollution by MPs is a great concern because they can be transported over long distances and spread into soil, air, and water, but they are also found in various foods such as tap water, bottled water, seafood, honey, and salt. Air, indoor dust, and contaminated food and water have been considered the main sources of MP exposure. Recently, information about other possible sources of exposure to MPs, such as baby bottles, has been increasingly emerging, while studies on human stool, placentas, and fetuses provide evidence of exposure to MPs in infants and children. However, research in this area is still insufficient [9,10].
Scientific studies deal with their presence, distribution, and potential effects on the environment and living organisms. Monitoring and understanding the sources and pathways of MPs are key to developing strategies for their negative impact on the life of organisms on the planet [4].
The main goal of this review is to summarize the possible exposure of infants to MPs as well as the detection of possible released MPs in infant feces, breast milk, and infant formulas.
2. Characterization of Microplastics
A large amount of plastics, such as cosmetic products, personal care products, clothes, plastic bags, and bottles, enters the environment through various routes, for example, households, hospitals, and human, industrial, and agricultural activities. These products gradually degrade in the environment and turn into MPs depending on their molecular weight, chemical structure, crystallinity, additives, and functional groups. Although plastic degradation is a difficult process due to its properties, it is effective in the fight against plastic pollution [11,12].
MPs are usually defined as plastic particles and fibers smaller than 5000 µm. They are small enough to be easily overlooked, but they can have significant environmental and potential health consequences. MPs are divided into primary and secondary groups [13].
Primary MPs in the environment arise from plastic pellets or balls. They are used for commercial purposes and created by industrial activity in the production of personal care products, for example, shampoos, soaps, toothpaste, hair gel, etc. They are secondary to the degradation of plastic products by physical, chemical, and biological processes [14,15,16].
Secondary MPs arise as a result of the degradation of plastics (packaging, paints from various types of plastic products, fibers from textiles, etc.) by various physical, chemical, and biological processes including erosion, corrosion, photooxidation, and biological transformation. The most common way of MP formation is photodegradation [17,18]. Due to their small size, MPs can be consumed by marine animals, terrestrial organisms, and humans, which leads to concerns about their impact on ecosystems and human health.
Concerns about human health are growing as people are constantly exposed to MPs, in particular through the animal or plant food chain, food additives, beverages, and plastic packaging for food [17,18,19].
The mechanism of the degradation of primary microplastics to secondary ones is still not well known, but both groups have harmful effects on the environment and human health [20]. About 80% of MPs in the environment correspond to fibers and fragments [3,11,21,22]. The chemical formulas and structures of the most common primary MPs are listed in Table 1.
Table 1.
The most common primary microplastics.
| Polymer | Chemical Formula |
Chemical Structure | Polymer | Chemical Formula |
Chemical Structure |
|---|---|---|---|---|---|
| PE | (C2H4)n |
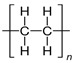
|
PET | (C10H8O4)n |
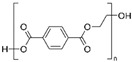
|
| PP | (C3H6)n |
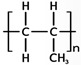
|
PPA | C42H63NO7 |
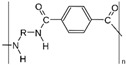
|
| PVC | C2H3Cl |
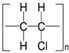
|
PC | (C16O3H14)n |
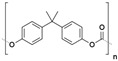
|
| PS | (C8H8)n |
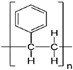
|
PU | (C27H36N2O0) |
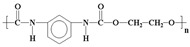
|
| PES | (C10H8O4)n |
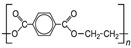
|
PLA | (C3H6O3)n |
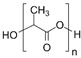
|
| PA | (C6H11NO)n |
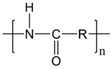
|
PMMA | (C5O2H8)n |
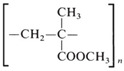
|
| PAN | (C3H3N)n |
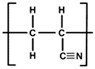
|
PE—polyethylene; PP—polypropylene; PVC—polyvinyl chloride; PS—polystyrene; PES—polyester; PA—polyamide; PAN—polyacrylonitrile; PET—polyethylene terephthalate; PPA—polyphthalamide; PC—polycarbonate; PU—polyurethane; PLA—polylactic acid; PMMA—poly (methyl methacrylate).
Due to the fact that plastics and MPs are hydrophobic and contain covalent bonds and functional groups resistant to attack, these substances are difficult to degrade. The degradation of plastics can take place mechanically, chemically, and physically but also biologically [23]. The different ways of degradation are shown in Table 2.
The degradation process of (micro)plastics can be influenced by their internal properties (composition, material, structure, etc.) as well as external environmental conditions (pH, temperature, humidity, catalysts, enzymes, etc.) [24].
Table 2.
Types of abiotic and biotic degradation of microplastics.
| Degradation | Degradation Methods | Consequences of Degradation | Ref. | |
|---|---|---|---|---|
|
Photodegradation |
Visible, infrared, UV light Reaction through light and reactive species involvement with a catalyst |
- Chain scission - Cross-linking - Flexibility loss, color change - Oxidative decomposition - Generation of reactive oxygen species - Complete mineralization to H2O, CO2 |
[25,26,27,28] | |
|
ABIOTIC |
Thermal degradation |
1. Identification of MPs by pyrolysis gas 2. High-temperature-production H, CO, CH4, or fuel oil 3. As a pretreatment technique for MP degradation at low temperatures |
- Conformational changes - Depolymerization of fragments - Breakage of polymeric backbone, molecular deterioration, changes in tensile strength, alteration of crystallinity, reduction in durability, cracks and color changes |
[29,30,31] |
| Hydrolytic degradation (hydrogen ions in acidic or alkaline media) |
- Chain breakage - Surface corrosion of polyesters - Cross-linking - Chain breakage - Chain breaking, formation of products - Mineralization of MPs |
[31,32] | ||
| Chemical degradation | Oxidative degradation (heat, light, atmospheric oxygen) | |||
| Advanced oxidation processes | ||||
| Bacterial degradation |
- Enzymatic oxidation - Hydrolysis - Chain scission |
[33,34,35] | ||
| Fungal degradation | ||||
| BIOTIC | Biodegradation | Enzymatic biodegradation | [36] | |
| Combined biodegradation (by multiple bacteria) | [36] | |||
| Algae degradation | [37] | |||
Each type of polymer has different properties and, thus, they can be applied in various applications [10]. MPs have a different polymer structure composed of different monomers, which are responsible for physical or chemical properties [38]. The most commonly used is PE, which has been commonly used in packaging films, garbage and food bags, and many household items for a long time. PET is used for the production of water bottles, and caps are usually made from PP. Beverage cartons are usually made from PE, while PS is suitable for disposable food packaging with insulating properties [19]. According to Directive 2019/904 of the European Parliament and the Council, food and beverage containers made from PS have been restricted since 2021 [39]. MPs are present not only in the environment but also in households. Various disposable or reusable food containers, bottles, jars, cups, caps, and other types of plastic packaging are often used, which are usually made of PET, PP, PVC, and PS. Chemically, MPs have a polymeric structure and contain two main elements, carbon and hydrogen. Some other substances may contain bromide, chlorine, and oxygen. Chemical additives are typical for MPs. Their toxicity is related to the size of the MPs. It is true that the smaller the MP, the more toxic it is [12].
It is necessary to pay more attention to the toxic effects of MPs on the environment. Most publications are focused on the presence of MPs in the environment, methods of exposure to MPs, and their toxic effects on aquatic organisms. In terms of the toxicity of plastics and MPs, we can distinguish the following: 1. Self-toxic effects—these manifest in animals by stopping their growth, changing the structure of their intestinal microflora, and damaging their intestinal tissues. In addition, additives that are added to plastics (plasticizers, UV stabilizers, heat stabilizers, hardeners, biocides, pigments, etc.) are easily released into the environment in unstable conditions (for example, strong UV radiation, weathering, etc.) and damage different organisms in the environment. 2. The effect of load toxicity—due to the large specific surface and small particle size of MPs, there is strong adsorption of substances from the environment, such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), persistent organic pollutants (POPs), heavy metals, antibiotics, etc. These substances can indirectly support the production of toxic effects [40]. MPs absorbed by organisms in the environment can enter the human body through the food chain (for example, through fish) but also through canned food and bottled water and frequently cause various inflammatory reactions and induce oxidative stress [41].
MP exposure to the human body can occur through various routes, such as inhalation, through the skin, or by the ingestion of contaminated food [6,16]. The ingestion of contaminated food, which may contain dangerous substances such as PAHs or PCBs, occurs most often through the gastrointestinal tract [42]. Some MPs are excreted from the body through stool, in which PET has been observed. Its concentration in infant feces was found to be 10 times higher than in adult samples [43].
The problem of MP food contamination cannot be solved without taking environmental pollution into account. The accumulation of MPs has been detected in seas, oceans, surface and groundwater, soils, arctic snow and antarctic ice, and beaches. They are commonly spread through wastewater treatment plants, from which they can enter natural waters, groundwater, or terrestrial ecosystems [44,45,46,47].
People are in close contact with plastics and their degradation products, especially MPs, which have a negative impact on human health due to their complex physical and chemical properties (e.g., polymer, size, shape, charge). The vulnerability of children and pregnant women to these exposures must be taken into account [48].
It is very important to monitor the group (fetuses, newborns, infants, children) that is very sensitive to toxic substances because they do not have sufficiently developed metabolizing enzymes, have a lower ability to remove toxins, and have highly sensitive target organs [1].
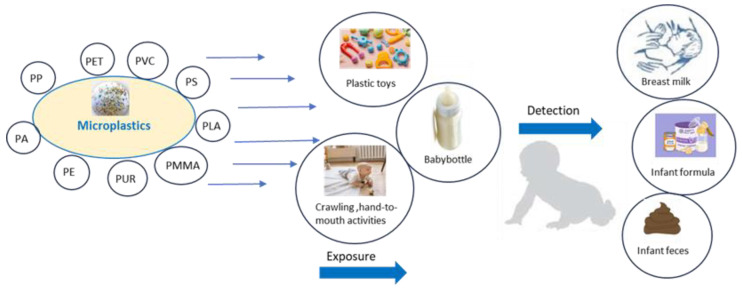
MPs most often enter children’s bodies through toys, pacifiers, and contaminated food, but also by crawling on carpets and floors made of plastic. Baby bottles are an important source of MPs [49]. This will be discussed more in Section 3.1.
3. Sources of Exposure to Microplastics
Research on plastics is mostly focused on the main sources of exposure, namely, water and food, and the transfer of MPs to humans through the food chain, but there is little information on direct MP exposure through plastic products. Monitoring and understanding the sources and pathways of MPs are key to developing strategies for their negative impact on the life of organisms on the planet. MPs can enter food and drink from a variety of sources, including the environment, food packaging, and the manufacturing process. Some studies have shown that MPs can be present in food that is either packed in plastic packaging or that comes into contact with plastic materials in the production process [50].
Organizations such as the World Health Organization (WHO) [51] and the European Food Safety Agency (EFSA) [52] monitor this issue and carry out scientific studies to assess the possible risks associated with the presence of MPs in food. Some of the following studies prove exposure to MPs in infants and pregnant women.
A review article by Kadac-Czapska et al. [13] dealt in detail with current knowledge in the field of microplastics and their impact on human health. It characterized the routes of exposure, defines the sources of pollution, points out the most frequently occurring microplastics in food, and identifies the types of food contaminated with MPs.
Ragusa et al. [53] were the first to address the issue of the presence of MPs in the human placenta. They examined six placentas, four of which contained 12 MPs of various shapes. Based on these results in their research, Liu et al. [54] tested not only placentas but also infant feces, meconium, breast milk, and baby formula.
The exposure of fetuses to MPs through pregnant mothers is a potential risk to newborn children. Aghaei et al. [55] investigated the effect of polystyrene microspheres on the fetus and placenta in laboratory mice, with microplastic particles 50 nm–5 μm in size causing growth problems.
Hu et al. [56] also investigated the effects of exposure to polystyrene MPs in mice and found that these particles can adversely affect pregnancy by disrupting the immune system.
Studies of animal models have found that nanopolystyrene particles can translocate from the maternal lung across the placenta to a fetus and its kidneys, heart, lungs, liver, and brain in late pregnancy [57,58,59]. In addition, using an ex vivo placental perfusion system, nanopolystyrene particles were found to be able to pass from the maternal uterine circulation to the fetal circulation via the placenta [60]. Recently, several microplastic fragments (5 to 10 μm in size) were found for the first time in four human placentas, suggesting that these MPs can pass into the placental tissue [61]. Based on these findings, there is growing concern about the potential risks of MPs to the embryo during pregnancy. However, there is the potential for the translocation of MPs from mother to fetus and the storage or accumulation of MPs in the fetus/embryo [59,60].
Infants, children, and pregnant women constantly come into contact with plastics. The most studied areas of the occurrence of MPs are primarily air, food, and beverages, although exposure in infants can also occur through the placenta and breast milk and materials that come into contact with food [48,49]. These sources of exposure are still not well studied (Figure 1).
Figure 1.
Sources of exposure to microplastics for infants.
In addition to the human placenta [59], MPs have also been detected in children’s stool [62], but the sources of these plastics are relatively difficult to detect. Early exposures can occur through the placenta, during breastfeeding and the administration of infant formula, when a child breathes dust or licks and chews plastic toys and textiles, through baby bottles, etc. [49].
A review by Calatayud Arroyo et al. [1] described the interplay between the microbiota of the mother and the child—xenobiotics—as well as diet during pregnancy and in the perinatal period. Maternal exposure to metals, persistent organic substances, and food additives can cause changes in the microbiota of infants, and exposure can also result in the modulation of mother-to-child transmission of microorganisms during childbirth and breastfeeding [9]. A study focused on the initial evidence of MPs and additives in human amniotic fluid and placenta samples. The identified materials were chlorinated polyethylene and calcium zinc PVC stabilizers. The placenta acted as a partial barrier against the entry of MPs into the amniotic fluid and the fetus.
Similarly, tea bags are among the sources of exposure to MPs. During 5 min of exposing a tea bag to hot water (95 °C), 11.6 trillion MPs were released per cup of tea [61].
Given the high exposure and to avoid potential adverse effects, it is important to identify the main sources of microplastics to reduce exposure [63].
3.1. Microplastics in Baby Feeding Bottles
PP-based plastic products are often used for food preparation and storage. Such products also include lunch boxes and baby feeding bottles [64]. Baby feeding bottles and plastic packaging for baby food must be considered as potential sources of MPs. In [65], the possible exposure of infants to MPs through the contamination of infant formula in PP feeding bottles was investigated. A 21-day test was conducted on infants up to 12 months of age and in 48 regions in the central Amazon. The effects of water temperature, sterilization, and repeated use over a 21-day period on MP release levels were assessed.
During the preparation of infant formula, feeding bottles are exposed to temperatures up to 100 °C in accordance with WHO guidelines. To assess the effect of temperature on MP release, they exposed bottles to deionized water at temperatures of 25, 40, 70, and 95 °C. The measured values of MP released were in the range of 14,600–4,550,000 particles per capita per day, depending on the region. The sterilization of bottles and exposure to high-temperature water during sterilization significantly increased the release of MPs. As a result of the prevalence of PP products used in the preparation of infant formula, the exposure of infants is higher than expected and, therefore, it is necessary to assess whether exposure to MPs at this level poses a risk to the health of infants [65].
MPs of irregular shapes released from baby bottles can cause increased cytotoxicity. A study by Xu et al. analyzed the thermal-oxidative processes of the aging of plastics and what kind of inflammatory response in human intestinal cells (Caco-2) can be induced by these irregular MPs. The exposure of intestinal cells to PP from a baby bottle triggered oxidative stress, which caused a decrease in glutathione levels, increased lipid peroxidation, and the release of reactive oxygen species. The levels of pro-inflammatory cytokines (IL-6 and TNFα), which are markers of the inflammatory process, increased and were even more intense during the disinfection of bottles with boiling water or when using microwave heating [66].
A Chinese study tested the release of MPs during the opening/closing process of a baby bottle, finding that 53–393 particles. mL−1 were released during 100 opening and closing cycles. In addition, they found that the type and brand of bottles, whether plastic or glass, affected the release of microparticles, suggesting that high-quality plastic and glass bottles release fewer microparticles and are more suitable for the health of infants and children [67].
Considering the results of this study, there is a growing need to focus more intensively on the potential of possible health risks arising from the use of baby bottles with PP contents.
Bisphenol A (BPA) is used in the production of PC plastics, which are used to make many products, including baby bottles. Despite the fact that most manufacturers of PC baby bottles declare that the products do not contain BPA, residual amounts of BPA have been detected in some products [68,69]. In a study, the authors tested 15 PC bottles with a clear label of BPA-free/safe/clear, which they subjected to a stress test (cleaning and rinsing). The results showed that some products contained a residual amount of BPA that did not exceed the tolerable daily intake (TDI) [68]. The EFSA established 50 g/kg/bw/day as the TDI of BPA based on non-observable adverse effect levels [70]. The amount of BPA increases with repeated use, cleaning, and sterilization. Therefore, it is recommended that parents change baby bottles more often and use a cleaning agent that reduces the risk of BPA leakage. The EU prohibited the use of PC plastics in infant feeding bottles. Since the safety of infants is of the utmost importance, parents should be aware of all the health risks associated with the use of plastic baby bottles [71].
3.2. Microplastics in Human Feces
Due to the properties of MPs, such as their persistence and slow degradation in the environment, their bioavailability for various organisms increases. The presence of MPs in an organism can cause the growth and reproduction of other organisms and achieve entry into the food chain. Most organisms use the process of excretion in feces to remove indigestible MPs. A study by Perez-Guevara et al. [62] from 2018 was the first study to identify microplastics in human feces. Studies that dealt with the extent of the contamination of feces with MPs began to appear more often. Schwabl et al. published a study where they assigned microplastics to nine different types of polymers in human feces. Currently, there is still little knowledge about the consequences of the behavior of MPs in feces that enter the environment. Research on MPs in feces continues to grow and it is important to understand the analytical methods available to identify and quantify MPs [72].
There are few studies that address the toxic effects of MP on humans, although some studies on laboratory animals have shown adverse health effects [73,74]. It is known that humans are exposed to MPs, but the degree of exposure is poorly understood. Some studies provide a dose range of exposure to MPs calculated using controversial empirical models; others report MP intake through various sources and pathways. But, research describing the burden of MPs on the human body is still lacking. One limitation is also the lack of reliable analytical methods for determining MPs in biological matrices. Since particles larger than 150 µm are reported to be excreted in feces, the determination of MPs in stool could indicate the degree of burden. The occurrence of MPs in the placenta has been proven, but there is no information about the presence of MPs in meconium or children’s stool. A USA study measured PET and PC, which are mainly used in the production of textile fibers, water bottles, and mobile phones, in samples of meconium and infant and adult feces. A depolymerization method followed by LC-MS/MS was used, with PET and PC doses assessed from fecal concentrations. A concentration of 36,000 ng.g−1 PET and 78 ng.g−1 PC was detected in the feces of six infants. In stool samples from eight adults, values of 2600 ng.g−1 for PET and 110 ng.g−1 for PC were detected in 10 stool samples. The concentration of PET in adults was an order of magnitude lower than in infants; the results for PC were approximately the same. High concentrations of MPs in infant feces can come from several sources of exposure, mainly the use of plastic products, such as baby feeding bottles, tableware, plastic teethers and toys, and plastic containers for baby food. A one-year-old child often puts plastic products in their mouth, sucks cloths, and crawls on carpeted surfaces. In the study, there was observed a higher content of MPs in infant feces than in adults. The first reason they cited was the possibility of the transmission of MPs from mother to child. Infants who consumed more than 600 mL of breast milk had a higher MP content than those who received a mixed diet. Infants who received a complementary diet (more than 50 g per day) had a lower MP content. From this, it follows that exposure to MPs probably occurs through breast milk. However, there may be more reasons. Some women use a breast pump and store their milk in breast milk storage bags, which can lead to milk contamination. Another source of exposure can be the use of baby bottles. MP contamination was detected in infants who consumed infant formula five times a day and infants who received breast milk from a bottle. Since MP content was detected in both milk and infant formula, the bottle was probably the potential source of exposure [54].
3.3. Microplastics in Breast Milk and Infant Formula
Nutrition plays an important role in the first few months of a child’s life for their physical and cognitive development. According to the WHO, a child should be exclusively breastfed for at least the first 6 months of life because breast milk is unique, complex, optimal, and irreplaceable in its composition. It represents the ideal form of nutrition for a newborn and is adapted to its needs. However, if the mother cannot breastfeed, it is necessary to replace breast milk with artificial milk intended for the initial feeding of infants. Although all breast milk substitutes differ from breast milk, it is necessary that these products provide a comparable rate of growth and metabolism observed in exclusively breastfed infants. For infants with specific health problems, formula must be adapted to eliminate or at least minimize the health problems [49].
Bisphenol analogs were detected in 62 breast milk samples and 54 infant formula samples. The median concentration (0.56 ng.g−1) of bisphenol F (BPF) was the highest in infant formula, while, in breast milk, the median concentration (0.01 ng.mL−1) of bisphenol S (BPS) was the highest [75].
A study by Liu et al. investigated the presence of MPs in 18 mother–infant pairs and assessed exposure in the placenta, meconium, infant feces, breast milk, and infant formula. Infant feces, breast milk, and infant formula samples were collected in the first 6 months of age. More than 74% of MPs had dimensions of 20–50 μm and were PA- and PU-dominated. For women, the sources of exposure could be cleaning products or toothpaste, while baby bottles and plastic toys could be the sources for infants. The total amount of released PA, PU, and PE in infants who also received complementary food > 50 g per day was significantly lower than in infants who did not. An interesting finding was that the way plastic toys were washed affected the amount of PET and PVC in the infants’ feces. In addition, higher consumption of breast milk in infants and a higher frequency of use of baby bottles caused an increase in the number of released MP particles. The number of MPs in feces was also higher in infants who sucked plastic toys [54].
Infant formula is packaged in plastic materials, which represent additional sources of MP exposure through dietary intake or oral activities in infants. Sealable disposable plastic bags that are sterile are often used to store freshly expressed breast milk. The US Centers for Disease Control and Prevention (CDC) recommend storing maternal medicine in these bags for 4 h at room temperature of 25 °C or 4 days in a refrigerator (4 °C). Given that these products are now often used and come into direct contact with breast milk, more detailed research on the risk of infant exposure is necessary [43,76]. For the study, the six most commonly used types of baby food packaging were selected, and the aim was to characterize the size, amount, and composition of microplastic particles released from these products. Large amounts of microparticles (<300 μm) and fragments (1–50 μm) were released, which were identified as PE, PET, and nylon-6 using Raman spectroscopy. The amount of released MPs was in the range of 0.61–0.89 mg.day−1 due to the average daily intake of breast milk for infants [43].
Baby food can be contaminated with various substances, such as inorganic and organic substances, drug residues, pesticides, and even MPs. Given that little is known about the extent of food contamination with MPs, there are knowledge gaps that prevent effective action on plastics. A Mexican study found an MP concentration in a milk sample of 6.5 ± 2.3 MPs.L−1. The study conducted in Mexico found the presence of MPs in 23 samples of branded milk with an average of 6.5 ± 2.3 particles.L−1. Evidence on children’s exposure to microplastics is quite limited. It is very important to know which products are contaminated and to what extent children are exposed to MPs. The most vulnerable period for MP exposure is the first few months of a child’s life because immunological, metabolic, cardiovascular, and neurobehavioral developmental processes are taking place. Children are more exposed to the environment than adults (crawling, hand-to-mouth activities) and eat and drink more per unit of body weight, which again increases exposure to environmental contaminants [77].
In a Polish study, 30 types of infant formula from pharmacies, drugstores, and supermarkets were tested. The products were intended for healthy babies as well as for infants with digestive problems. The infant formula samples came from six manufacturers. The contamination of infant formula is incompatible with food safety standards; the results of this study pointed to the presence of MPs in infant formula. Daily consumption in infants from birth to 6 months of age was 49 ± 32 MP particles.100 g−1. They assumed that one of the reasons for the high concentration of plastic particles in products is the type of packaging. The most common polymers identified were PE (63% of packaging) and PP (37% of packaging). Sources to which infants may be exposed are breast milk, plastic baby bottles, plastic toys, and disposable breast milk storage bags. In canned infant formula from the Chinese market, MPs were detected with fewer particles by one order of magnitude (4 ± 3 MPs.100 g−1) than products from the Polish market (51 ± 8 MP.100 g−1). Compared to the well-studied area of MP food contamination, the presence of MPs in baby food packaging is still not well studied [50]. A study by Liu et al. reported the release of large amounts of micro and submicron particles, flakes (<300 μm), and fragments (1–50 μm) when using commercially available disposable breast milk storage bags. Among the released particles, PE, PET, and nylon-6 predominated, which were identified by Raman spectroscopy. The weight of MPs released from the bags was 0.61–0.89 mg.day−1 with an average intake of breast milk. Infant formula is packaged in plastic material, which can be another source of MP exposure for infants through eating or food sources [76].
In a study by Li et al., it is stated that infant formula prepared in a PP bottle will release up to millions of MPs [54]. PET and PC were quantified in the feces of infants, the concentrations of which were significantly higher than in adults [44,54]. In infants who consumed more than 50 g of complementary food per day, the amount of total MPs, PA, and PU was significantly lower than in infants who did not receive complementary food. The method of washing plastic toys influenced the amount of released PET and PVC in infant feces. The more breast milk the infants drank, the greater the amount of MPs released into the feces. The amount of released MPs increased with the increasing frequency of drinking milk from feeding bottles. The amount of MPs in feces was higher in children who had a habit of sucking plastic toys. This study was the first study to comprehensively examine MP exposure in pregnant women, fetuses, and infants. The presence of MPs in breast milk and infant formula was detected. In children at an early age, exposure to MPs occurred through breastfeeding and the use of baby bottles and plastic toys. The content of PA was higher in meconium than in placentas [54]. Experimental studies investigating how MPs reach the embryo and fetus are still lacking. The increased content of MPs in meconium may be related to the long-term accumulation of MPs in meconium, which accumulates in the fetus from the 16th week of pregnancy and is not excreted until delivery. Apart from the mentioned study, only one other study found the MP contamination of breast milk. Infants’ exposure to MPs, especially PET and PC, in their daily diet is higher than that of adults. Children are more susceptible and come into contact with various plastic objects, such as plastic feeding bottles, toys, plastic dishes, etc. [78]. However, there is still a lack of information on neonatal exposure. Estimates so far are based on general food intake assumptions but do not take into account exposure in children with specific requirements. For infants who cannot be breastfed, the main component of the diet is powdered milk, which is packed in special packaging. However, there is no research to determine if powdered milk contains MPs. Contamination with MPs in milk powder does not only come from the milk powder but can also be released from the baby bottle and when the milk powder is brewing. In addition, MPs are commonly found in the air and in clothing. Few studies have correctly understood the need to consider all sources of exposure that may contaminate milk powder. The authors Zhang et al. conducted a comprehensive study of potential sources of contamination of powdered infant milk, namely, whether it comes from the packaging, powdered milk, baby bottles, or the preparation of the milk itself. They studied 13 different types of powdered milk with different types of packaging, milk preparation methods, and milk sources. There were more MPs in milk in boxes than that in cans. They assumed that the main source was the inner packaging of the boxes, which consisted of three layers: the outer special cardboard, the middle aluminum foil, and the inner PE layer. The exposure from powdered milk itself was low, exposure from baby bottles was 6.8 times higher, and exposure from milk preparation was 1.7 times higher [43].
4. Analytical Methods for the Identification and Quantification of Microplastics
Considering that MPs represent a great risk, it is necessary to put into practice developed general standard protocols (currently, they do not exist) that relate to the collection, characterization, and quantification of MPs. Analytical techniques relate mainly to sample collection and preparation and the identification and quantification of MPs. In the research field, there is still a deficit of standardized methods for the extraction of MPs from different samples, especially from sediment, air, and biological tissues [10,79].
Analytical methods for the identification and detection of MPs are described in various studies. An overview of some methods for the identification and detection of MPs is given in Table 3.
Table 3.
Examples of determination of polymers in various samples using different pretreatment techniques as well as methods for identification and detection.
| Sample | Polymers | Sample Pretreatment | Method | Ref. |
|---|---|---|---|---|
| Water, sediment |
PES, PA, PS | Catalytic wet peroxidation with H2O2, NaClO, and Fenton reagents, |
SEM, FTIR | [80] |
| Sand | PE, PET, PS, PA, PVC | Wet peroxidation with H2O2 5.3 M NaCl Filtration |
μ-FTIR | [81] |
| Sand, sediment | PE, PS, PET, PP, PVC, Silicone, Nylon 6, Polyimide, Polysulfone | Drying, sieving Oxidation with H2O2 and Fenton reagents (H2O2 + Fe2+) |
FTIR Raman spectroscopy |
[82] |
| Indoor dust | PET, PA, PP, PE | Digestion with 30% H2O2 and ZnCl2 Filtration through metal filter, sonication with ethanol |
LDIR LC-MS/MS |
[83] |
| Surface water | PE, PP, PS, PVC, PET, PDMS, CPE | Oxidation with 30% H2O2 + 0.05 M FeSO4·7H2O 5 M NaCl Filtration |
Pyr-GC/MS FTIR |
[84] |
| Atmospheric aerosol Atmospheric deposition |
PET, PC | Sieving, digestion, density separation, filtration, drying | Microscope, SEM, FTIR, Raman spectroscopy, LC-MS/MS | [85] |
| Surface water | PE, PP | Sieving, oxidation with 30% and 0.05 M Fe(II) 5 M NaCl Filtration, drying |
SEM, FTIR | [86] |
| Marine fish (gastrointestinal tract) |
PET, PP, PAN, PE, PVAc, PA, PS, PB, PC | Digestion with 10% KOH and 30% H2O2 Filtration, drying |
SEM, FTIR | [40] |
| Water, sediment, tadpoles | PES, PP | Digestion with 30% H2O2 Filtration, drying |
SEM, FTIR | [87] |
| Sea water | LDPE, PA, PET, PVC | Resuspension water SDS (150 g.L−1), ultrasonication, filtration |
Raman spectroscopy | [88] |
| Indoor and outdoor air | PC, PE, PET, PS, PVC, PP | Raman spectroscopy | [89] |
SDS—sodium dodecylsulfate; PDMS—polydmethylsiloxane; CPE—chlorinated polyethylene, PVAc—polyvinyl acetate; PB—polybutene; LDPE—low-density polyethylene.
Visual observation using a microscope is the most commonly used method because it does not require complex techniques and is based on the observation of particle size, shape, color, surface, and transparency. But, it appears to be an ineffective method of identification as the results obtained have often been overestimated or underestimated [10,79,90].
Infrared spectroscopy with Fourier transform (FTIR) is the most popular and widespread technique to identify the type of plastic of a MP found in the environment. It is a very precise, clean, and reliable method in which plastics can be easily distinguished from natural materials thanks to specificities that are shown in the spectra. Single-clean plastics have a specific structure and are therefore different from pure plastics. They do not have the same infrared spectra, which allows for unambiguous identification. FTIR is more sensitive for polar groups [79,90].
Raman spectroscopy is, together with infrared spectroscopy, the most widely used technique for the characterization of MPs. It can analyze Raman spectroscopy samples larger than 1 μm and allows for the tracking of very small differences in the molecular conformation of polymers, degree crystallinity with respect to amorphous regions, and stereoregularity of the polymer. Compared to FTIR spectroscopy, Raman spectroscopy achieves a better response of non-polar symmetric bonds [10,46,91].
Scanning electron microscopy (SEM) is suitable for MP identification and it is able to provide clear images of the vital physical properties of a particle. There is also the combination of SEM and X-ray dispersion spectroscopy (SEM-EDS), which is appropriate for determining the content of additives in plastics [92,93].
Pyrolysis-gas chromatography-mass spectrometry (Pyro-GC-MS) is a method for the characterization of MPs from the point of view of analysis of the degradation products of the given polymer. It consists of thermal degradation up to the pyrolysis of the polymer, which takes place in an inert atmosphere and breaks the chemical bonds of the polymer. Molecules with a lower molecular weight are formed, which are further separated by gas chromatography and detected by mass spectrometry. The sample is fragmented at a temperature between 500 and 1400 °C in the presence of helium and at low pressure, creating fragments that are introduced into the GC system. No sample pretreatment is required and the amount of sample can be within the range of 5–200 μg [91,94,95]. This methodo-logy makes it possible to simultaneously identify the type of polymer and organic filler and determine the chemical composition of MPs using spectral libraries and databases. It provides quantitative results with high accuracy, sensitivity, and selectivity. A small amount of sample is required and it allows for an analysis of the entire MP, not just its surface. The main disadvantage of this technique is that it is destructive and no information can be obtained about the color, number, size, or shape of the particle. It is not possible to analyze more MPs simultaneously as the analysis time is extended. Although this technique is highly effective in identifying different types of polymers, it is unable to distinguish between low- and high-density MPs [79].
Nuclear magnetic resonance (NMR) spectroscopy is used to identify MPs because it provides information about the molecular structure, crystallinity, and branching of monomers in copolymer compounds [10,91,95]. The principle of NMR is that the magnetic properties of atomic nuclei are used to obtain chemical information. NMR is used to calculate molar concentrations and monitor molecular changes in a sample, identify compounds present in samples, and study degradation [96].
Various new approaches are emerging in the field of detection and identification. For example, some authors describe fast identification using neural networks with one hidden layer, the so-called extreme learning machines (ELMs) [10,97]. A nonlinear vibration imaging technique—coherent anti-Stokes Raman scattering (CARS) microscopy—is used to identify ultrafine MPs [98]. A deep learning method has been developed, which uses a faster convolutional neural network model Faster R-CNN, where the residual network-50 (ResNet-50) and the pyramidal network module (FPN) are used [99].
5. Conclusions
The production of plastics was originally aimed at simplifying and improving human life, whether in industry, agriculture, or the home. However, this production has gotten out of control over the decades, and we have started to face unwanted consequences in the form of microplastics. These microparticles, which are created by the degradation of plastics, have spread to all areas of the environment, with a negative impact on human health and the overall ecosystem. Our review is based on scientific studies to clarify the extent of the presence of microplastics in various aspects of life, including breast milk, baby bottles, toys, and milk forms. On the basis of the finding that contact with microplastics occurs fromy young age through baby bottles and toys, it is found that there is a strong need for urgent measures. It is essential to examine their impact on children’s health and take measures to minimize exposure to these harmful particles. This significant problem requires immediate attention and action to ensure the healthy and safe development of children. Through further research and a systematic approach to tracking the sources of MPs, we will be able to create foundations for the development of effective strategies and minimize the negative impact of these microparticles on the environment and human health from an early age.
Author Contributions
All authors have contributed to this review equally. Conceptualization, C.M., M.V. and Z.S.; methodology, C.M., M.V. and Z.S.; software, C.M., M.V. and Z.S.; validation, C.M., M.V. and Z.S.; formal analysis, C.M., M.V. and Z.S.; investigation, C.M., M.V. and Z.S.; resources, C.M., M.V. and Z.S.; data curation, C.M., M.V. and Z.S.; writing—original draft preparation, C.M., M.V. and Z.S.; writing—review and editing, C.M., M.V. and Z.S.; visualization, C.M., M.V. and Z.S.; supervision, C.M., M.V. and Z.S.; project administration, C.M., M.V. and Z.S.; funding acquisition, C.M., M.V. and Z.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by projects of the Slovak internal grant (SVG) numbers 06/2021, 07/2021, and 08/2021 of the Slovak Medical University in Bratislava.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Calatayud Arroyo M., García Barrera T., Callejon Leblic B., Arias Borrego A., Collado M.C. A review of the impact of xenobiotics from dietary sources on infanthealth: Early life exposures and the role of the microbiota. Environ. Poll. 2021;269:115994. doi: 10.1016/j.envpol.2020.115994. [DOI] [PubMed] [Google Scholar]
- 2.Rose P.K., Jain M., Kataria N., Sahoo P.K., Garg V.K., Yadav A. Microplastics in multimedia environment: A systematic review on its fate, transport, quantification, health risk, and remedial measures. Groundw. Sustain. Develop. 2023;20:100889. doi: 10.1016/j.gsd.2022.100889. [DOI] [Google Scholar]
- 3.Cox K.D., Covernton G.A., Davies H.L., Dower J.F., Juanes F., Dudas S.E. Human Consumption of Microplastics. Environ. Sci. Technol. 2019;53:7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- 4.Su L., Xiong X., Zhang Y., Wu C., Xu X., Sun C., Shi H. Global transportation of plastics and microplastics: A critical review of pathways and influences. Sci. Total Environ. 2022;831:154884. doi: 10.1016/j.scitotenv.2022.154884. [DOI] [PubMed] [Google Scholar]
- 5.Wo’zniak-Budych M., Staszak K., Wieszczycka K., Bajek A., Staszak M., Roszkowski S., Giamberini M., Tylkowski B. Microplastic label in microencapsulation field—Consequence of shell material selection. J. Hazard. Mat. 2024;465:133000. doi: 10.1016/j.jhazmat.2023.133000. [DOI] [PubMed] [Google Scholar]
- 6.Huang J., Chen H., Zheng Y., Yang Y., Zhang Y., Gao B. Microplastic pollution in soils and groundwater: Characteristics, analytical methods and impacts. Chem. Engin. J. 2021;425:131870. doi: 10.1016/j.cej.2021.131870. [DOI] [Google Scholar]
- 7.Jadhav E.B., Sankhla M.S., Bhat R.A., Bhagat D.S. Microplastics from food packaging: An overview of human consumption, health threats, and alternative solutions. Environ. Nanotech. Monit. Manag. 2021;16:100608. doi: 10.1016/j.enmm.2021.100608. [DOI] [Google Scholar]
- 8.Cverenkárová K., Valachovičová M., Mackul’ak T., Žemlička L., Bírošová L. Microplastics in the Food Chain. Life. 2021;11:1349. doi: 10.3390/life11121349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kannan K., Vimalkumar K. A Review of Human Exposure to Microplastics and Insights Into Microplastics as Obesogens. Front. Endocrinol. 2021;12:724–989. doi: 10.3389/fendo.2021.724989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Tran T., Jalil A.A., Nguyen T.M., Nguyen T.T.T., Duyen W.D., Nguyen T.C. A review on the occurrence, analytical methods, and impact of microplastics in the environment. Environ. Toxicol. Pharmacol. 2023;102:104248. doi: 10.1016/j.etap.2023.104248. [DOI] [PubMed] [Google Scholar]
- 11.Lee J., Jeong S. Approach to an answer to “How dangerous microplastics are to the human body”: A systematic review of the quantification of MPs and simultaneously exposed chemicals. J. Hazard. Mat. 2023;460:132404. doi: 10.1016/j.jhazmat.2023.132404. [DOI] [PubMed] [Google Scholar]
- 12.Sridhar A., Kannan D., Kapoor A., Prabhakar S. Extraction and detection methods of microplastics in food and marine systems: A critical review. Chemosphere. 2022;286:131653. doi: 10.1016/j.chemosphere.2021.131653. [DOI] [PubMed] [Google Scholar]
- 13.Kadac-Czapska K., Knez E., Grembecka M. Food and human safety: The impact of Microplastics. Crit. Rev. Food Sci. Nutr. 2022 doi: 10.1080/10408398.2022.2132212. [DOI] [PubMed] [Google Scholar]
- 14.Sewwandi M., Wijesekara H., Rajapaksha A.U., Soysa S., Vithanage M. Microplastics and plastics-associated contaminants in food and beverages; Global trends, concentrations, and human exposure. Environ. Poll. 2023;317:120747. doi: 10.1016/j.envpol.2022.120747. [DOI] [PubMed] [Google Scholar]
- 15.Hwang J., Choi D., Han S., Yong Jung S., Choi J., Hong J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020;10:7391. doi: 10.1038/s41598-020-64464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olewnik-Kruszkowska E., Nowaczyk J., Kadac K. Effect of ozone exposure on thermal and structural properties of polylactide based composites. Polym. Test. 2016;56:299–307. doi: 10.1016/j.polymertesting.2016.10.030. [DOI] [Google Scholar]
- 17.Periyasamy A.P., Tehrani-Bagha A. A review on microplastic emission from textile materials and its reduction techniques. Polym. Degr. Stab. 2022;199:109901. doi: 10.1016/j.polymdegradstab.2022.109901. [DOI] [Google Scholar]
- 18.Yang X., Ma J.B., Wong M.H., Owen R.B., Chow K.L. Environmental health impacts of microplastics exposure on structural organization levels in the human body. Sci. Total Environ. 2022;825:154025. doi: 10.1016/j.scitotenv.2022.154025. [DOI] [PubMed] [Google Scholar]
- 19.Kadac-Czapska K., Knez E., Gierszewska M., Olewnik-Kruszkowska E., Grembecka M. Microplastics Derived from Food Packaging Waste-Their Origin and Health Risks. Materials. 2023;16:674. doi: 10.3390/ma16020674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vethaak A.D., Legler J. Microplastics and human health. Science. 2021;371:672–674. doi: 10.1126/science.abe5041. [DOI] [PubMed] [Google Scholar]
- 21.Wua P., Huang J., Zheng Y., Yang Y., Zhang Y., Chen H., He F., Chen H., Quan G., Yan J., et al. Environmental occurrences, fate, and impacts of microplastics. Ecotox. Environ. Saf. 2019;184:109612. doi: 10.1016/j.ecoenv.2019.109612. [DOI] [PubMed] [Google Scholar]
- 22.Grafmueller S., Manser P., Diener L., Diener P.-A., Maeder-Althaus X., Maurizi L., Jochum W., Krug H.F., Buerki-Thurnherr T., von Mandach U., et al. Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model. Environ. Health Perspect. 2015;123:1280–1286. doi: 10.1289/ehp.1409271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emisha L., Nishitha W., Kavitha S., Gopinath H., Dibyajyoti H., Anil K.P., Reeta R.S., Ashok P. Biodegradation of microplastics: Advancement in the strategic approaches towards prevention of its accumulation and harmful effects. Chemosphere. 2024;346:140661. doi: 10.1016/j.chemosphere.2023.140661. [DOI] [PubMed] [Google Scholar]
- 24.Liu L., Xu M., Ye Y., Zhang B. On the degradation of (micro)plastics: Degradation methods, influencing factors, environmental impacts. Sci. Total Environ. 2022;806:151312. doi: 10.1016/j.scitotenv.2021.151312. [DOI] [PubMed] [Google Scholar]
- 25.Delre A., Goudriaan M., Morales V.H., Vaksmaa A., Tintswalo Ndhlovu R., Baas M., Keijzer E., de Groot T., Zeghal E., Egger M., et al. Plastic photodegradation under simulated marine conditions. Mar. Poll. Bul. 2023;187:114544. doi: 10.1016/j.marpolbul.2022.114544. [DOI] [PubMed] [Google Scholar]
- 26.Lin J., Yan D., Fu J., Chen Y., Ou H. Ultraviolet-C and vacuum ultraviolet inducing surface degradation of microplastics. Water Res. 2020;186:116360. doi: 10.1016/j.watres.2020.116360. [DOI] [PubMed] [Google Scholar]
- 27.Nabi I., Bacha A.U.R., Li K., Cheng H., Wang T., Liu Y., Ajmal S., Yang Y., Feng Y., Zhang L. Complete photocatalytic mineralization of microplastic on TiO2 nanoparticle film. iScience. 2020;23:101326. doi: 10.1016/j.isci.2020.101326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X., Zheng H., Zhao J., Luo X., Wang Z., Xing B. Photodegradation elevated the toxicity of polystyrene microplastics to grouper (Epinephelus moara) through disrupting hepatic lipid homeostasis. Environ. Sci. Technol. 2020;54:6202–6212. doi: 10.1021/acs.est.9b07016. [DOI] [PubMed] [Google Scholar]
- 29.Arpia A.A., Chen W.H., Ubando A.T., Nagvi S.R., Culaba A.B. Microplastic degradation as a sustainable concurrent approach for producing biofuel and obliterating hazardous environ- mental effects: A state-of-the-art review. J. Hazard. Mat. 2021;2017:126381. doi: 10.1016/j.jhazmat.2021.126381. [DOI] [PubMed] [Google Scholar]
- 30.Toapanta T., Okoffo E.D., Ede S., O’Brien S., Burrows S.D., Ribeiro F., Gallen M., Colwell J., Whittaker A.K., Kaserzoin S., et al. Influence of surface oxidation on the quantification of polypropylene microplastics by pyrolysis gas chromatography mass spectrometry. Sci. Total Environ. 2021;796:148835. doi: 10.1016/j.scitotenv.2021.148835. [DOI] [PubMed] [Google Scholar]
- 31.Du H., Xie Y., Wang J. Microplastic degradation methods and corresponding degradation mechanism: Research status and future perspectives. J. Hazard. Mat. 2021;418:126377. doi: 10.1016/j.jhazmat.2021.126377. [DOI] [PubMed] [Google Scholar]
- 32.Liu F., Xu Y., Zhang B., Liu Y., Zhang H. Heterogeneous degradation of organic contaminant by peroxydisulfate catalyzed by activated carbon cloth. Chemosphere. 2020;238:124611. doi: 10.1016/j.chemosphere.2019.124611. [DOI] [PubMed] [Google Scholar]
- 33.Matjašič T., Simčič T., Medvešček N., Bajt O., Dreo T., Mori N. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci. Total Environ. 2021;752:141959. doi: 10.1016/j.scitotenv.2020.141959. [DOI] [PubMed] [Google Scholar]
- 34.Peng B.Y., Chen Z., Chen J., Yu H., Zhou X., Criddle C.S., Wu W.-M., Zhang Y. Biodegradation of polyvinyl chloride (PVC) in Tenebrio molitor (Coleoptera: Tenebrionidae) larvae. Environ. Int. 2020;145:106106. doi: 10.1016/j.envint.2020.106106. [DOI] [PubMed] [Google Scholar]
- 35.Zhang J., Gao D., Li Q., Zhao Y., Li L., Lin H., Bi Q., Zhao Y. Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci. Total Environ. 2020;704:135931. doi: 10.1016/j.scitotenv.2019.135931. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J., Ma J., Sun Y., Zhou T., Zhao Y., Yu F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020;715:136968. doi: 10.1016/j.scitotenv.2020.136968. [DOI] [PubMed] [Google Scholar]
- 37.Sameh A., Elsamahy S., Al-Tohamy R., Zhu D., Mahmoud J.A.-G., Koutra E., Metwally M.A., Kornaros M., Sun J. Plastic wastes biodegradation: Mechanisms, challenges and future prospects. Sci. Total Environ. 2021;780:146590. doi: 10.1016/j.scitotenv.2021.146590. [DOI] [PubMed] [Google Scholar]
- 38.Muhib I., Uddin K., Rahman M., Malafaia G. Occurrence of microplastics in tap and bottled water, and food packaging: A narrative review on current knowledge. Sci. Total Environ. 2023;865:161274. doi: 10.1016/j.scitotenv.2022.161274. [DOI] [PubMed] [Google Scholar]
- 39.European Union Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Off. J. Eur. Union. 2019;155:1–19. [Google Scholar]
- 40.Wang Q., Zhu X., Hou C., Wu Y., Teng J., Zhang C., Tan H., Shan E., Zhang W., Zhao J. Microplastic uptake in commercial fishes from the Bohai Sea, China. Chemosphere. 2021;263:127962. doi: 10.1016/j.chemosphere.2020.127962. [DOI] [PubMed] [Google Scholar]
- 41.Osman A.I., Hosny M., Eltaweil A.S., Omar S., Elgarahy A.M., Farghali M., Yap P.-S., Wu Y.-S., Nagandran S., Batumalaie K., et al. Microplastic sources, formation, toxicity and remediation: A review. Environ. Chem. Lett. 2023;21:2129–2169. doi: 10.1007/s10311-023-01593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumari A., Upadhyay V., Kumar S. A critical insight into occurrence and fate of polycyclic aromatic hydrocarbons and their green remediation approaches. Chemosphere. 2023;329:138579. doi: 10.1016/j.chemosphere.2023.138579. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Wang L., Trasande L., Kannan K. Occurrence of Polyethylene Terephthalate and Polycarbonate Microplastics in Infant and Adult Feces. Environ. Sci. Technol. Lett. 2021;8:989–994. doi: 10.1021/acs.estlett.1c00559. [DOI] [Google Scholar]
- 44.Zurier H.S., Goddard J.M. Biodegradation of microplastics in food and agriculture. Curr. Opinion Food Sci. 2021;37:37–44. doi: 10.1016/j.cofs.2020.09.001. [DOI] [Google Scholar]
- 45.Yusuf A., Sodiq A., Giwa A., Eke J., Pikuda O., Eniola J.O., Ajiwokewu B., Sambudi N.S., Bilad M.R. Updated review on microplastics in water, their occurrence, detection, measurement, environmental pollution, and the need for regulatory standards. Environ. Poll. 2022;292:118421. doi: 10.1016/j.envpol.2021.118421. [DOI] [PubMed] [Google Scholar]
- 46.Schymanski D., Goldbeck C., Humpf H.-U., Fürst P. Analysis of microplastics in water by micro-Raman spectroscopy: Release of plastic particles from different packaging into mineral water. Water Res. 2018;129:154–162. doi: 10.1016/j.watres.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Möller J.N., Löder M.G.J., Laforsch C. Finding Microplastics in Soils: A Review of Analytical Methods. Environ. Sci. Technol. 2020;54:2078–2090. doi: 10.1021/acs.est.9b04618. [DOI] [PubMed] [Google Scholar]
- 48.Sripada K., Wierzbicka A., Abass K., Grimalt J.O., Erbe A., Röllin H.B., Weihe P., Jiménez Díaz G., Singh R.R., Visnes T., et al. Children’s Health Perspective on Nano- and Microplastics. Environ. Health Perspect. 2022;130:015001-15. doi: 10.1289/EHP9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kadac-Czapska K., Jutrzenka Trzebiatowska P., Mazurkiewicz M., Kowalczyk P., Knez E., Behrendt M., Mahlik S., Zaleska-Medynska A., Grembecka M. Isolation and identification of microplastics in infant formulas—A potential health risk for children. Food Chem. 2024;440:138246. doi: 10.1016/j.foodchem.2023.138246. [DOI] [PubMed] [Google Scholar]
- 50.Feng Y., Tu C., Li R., Wu D., Yang J., Xia Y., Peijnenburg W.J.G.M., Luo Y. A systematic review of the impacts of exposure to micro- and nano-plastics on human tissue accumulation and health. Eco-Environ. Health. 2023;2:195–207. doi: 10.1016/j.eehl.2023.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.World Health Organization Healthy Diet. 2020. [(accessed on 27 February 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet.
- 52.EFSA Panel on Dietetic Products, Nutrition, and Allergies Scientific opinion on dietary reference values for water. EFSA J. 2010;8:1459. [Google Scholar]
- 53.Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., Papa F., Rongioletti M.C.A., Baiocco F., Draghi S., et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- 54.Liu S., Guo J., Liu X., Yang R., Wang H., Sun Y., Chen B., Dong R. Detection of various microplastics in placentas, meconium, infant feces, breastmilk and infant formula: A pilot prospective study. Sci. Total Environ. 2023;854:158699. doi: 10.1016/j.scitotenv.2022.158699. [DOI] [PubMed] [Google Scholar]
- 55.Aghaei Z., Sled J.G., Kingdom J.C., Baschat A.A., Helm P.A., Jobst K.J., Cahill L.S. Maternal exposure to polystyrene micro- and nanoplastics causes fetal growth restriction in mice. Environ. Sci. Technol. Lett. 2022;9:426–430. doi: 10.1021/acs.estlett.2c00186. [DOI] [Google Scholar]
- 56.Hu J., Qin X., Zhang J., Zhu Y., Zeng W., Lin Y., Liu X. Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 2021;106:42–50. doi: 10.1016/j.reprotox.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Wick P., Malek A., Manser P., Meili D., Maeder-Althaus X., Diener L., Diener P.-A., Zisch A., Krug H.F., von Mandach U. Barrier capacity of human placenta for nanosized materials. Environ. Health Perspect. 2010;118:432–436. doi: 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fournier S.B., D’Errico J.N., Adler D.S., Kollontzi S., Goedken M.J., Fabris L., Yurkow E.J., Stapleton P.A. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Part. Fibre Toxicol. 2020;17:55. doi: 10.1186/s12989-020-00385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jeong B., Baek J.Y., Koo J., Park S., Ryu Y.-K., Kim K.-S., Zhang S., Chung C., Dogan R., Choi H.-S., et al. Maternal exposure to polystyrene nanoplastics causes brain abnormalities in progeny. J. Hazard Mat. 2022;426:127815. doi: 10.1016/j.jhazmat.2021.127815. [DOI] [PubMed] [Google Scholar]
- 60.D’Errico J.N., Fournier S.B., Stapleton P.A. Ex vivo perfusion of the rodent placenta. J. Vis. Exp. 2019;147:e59412. doi: 10.3791/59412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernandez L.M., Xu E.G., Larsson H.C.E., Tahara R., Maisuria V.B., Tufenkji N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019;53:12300–12310. doi: 10.1021/acs.est.9b02540. [DOI] [PubMed] [Google Scholar]
- 62.P’erez-Guevara F., Kutralam-Muniasamy G., Shruti V. Critical review on microplastics in fecal matter: Research progress, analytical methods and future outlook. Sci. Total Environ. 2021;778:146395. doi: 10.1016/j.scitotenv.2021.146395. [DOI] [PubMed] [Google Scholar]
- 63.Halfar J., Čabanová K., Vávra K., Delongová P., Motyka O., Špaček R., Kukutschová J., Šimetka O., Hevianková S. Microplastics and additives in patients with preterm birth: The first evidence of their presence in both human amniotic fluid and placenta. Chemosphere. 2023;343:140301. doi: 10.1016/j.chemosphere.2023.140301. [DOI] [PubMed] [Google Scholar]
- 64.Li D., Yang L., Kavanagh R., Xiao L., Shi Y., Kehoe D.K., Gun’ko Y.K., Boland J.J., Wang J.J. Sampling, Identification and Characterization of Microplastics Release from Polypropylene Baby Feeding Bottle during Daily Use. J. Vis. Exp. 2021;173:e62545. doi: 10.3791/62545. [DOI] [PubMed] [Google Scholar]
- 65.Li D., Shi Y., Yang L., Xiao L., Kehoe D.K., Gun’ko Y.K., Boland J.J., Wang J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food. 2020;1:746–754. doi: 10.1038/s43016-020-00171-y. [DOI] [PubMed] [Google Scholar]
- 66.Xu Z., Shen J., Lin L., Chen J., Wang L., Deng X., Wu X., Lin Z., Zhang Y., Yu R., et al. Exposure to irregular microplastic shed from baby bottles activates the ROS/NLRP3/Caspase-1 signaling pathway, causing intestinal inflammation. Environ. Int. 2023;181:108296. doi: 10.1016/j.envint.2023.108296. [DOI] [PubMed] [Google Scholar]
- 67.Song K., Ding R., Sun C., Yao L., Zhang W. Microparticles and microplastics released from daily use of plastic feeding and water bottles and plastic injectors: Potential risks to infants and children in China. Environ. Sci. Poll. Res. 2021;28:59813–59820. doi: 10.1007/s11356-021-14939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ali M., Jaghbir M., Salam M., Al-Kadamany G., Damsees R., Al-Rawashdeh N. Testing baby bottles for the presence of residual and migrated bisphenol A. Environ. Monit. Assess. 2019;191:7. doi: 10.1007/s10661-018-7126-0. [DOI] [PubMed] [Google Scholar]
- 69.Moghadama Z.A., Mirlohi M., Pourzamani H., Malekpour A., Amininoor Z., Reza Merasi M. Exposure assessment of Bisphenol A intake from polymeric baby bottles in formula-fed infants aged less than one year. Toxicol. Rep. 2015;2:1273–1280. doi: 10.1016/j.toxrep.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Siddique S., Zhang G., Coleman K., Kubwabo C. Investigation of the migration of bisphenols from baby bottles and sippy cups. Curr. Res. Food Sci. 2021;4:619–626. doi: 10.1016/j.crfs.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen D., Kannan K., Tan H., Zheng Z., Feng Y.L., Wu Y., Widelka M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity-A review. Environ. Sci. Technol. 2016;50:5438–5453. doi: 10.1021/acs.est.5b05387. [DOI] [PubMed] [Google Scholar]
- 72.Schwabl P., Köppel S., Königshofer P., Bucsics T., Trauner M., Reiberger T., Liebmann B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Int. Med. 2019;171:453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- 73.Deng Y., Zhang Y., Qiao R., Bonilla M.M., Yang X., Ren H., Lemos B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus) J. Hazard. Mat. 2018;357:348–354. doi: 10.1016/j.jhazmat.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 74.Yang Y.F., Chen C.Y., Lu T.H., Liao C.M. Toxicity-based toxicokinetic/toxicodynamic assessment for bioaccumulation of polystyrene microplastics in mice. J. Hazard. Mat. 2019;366:703–713. doi: 10.1016/j.jhazmat.2018.12.048. [DOI] [PubMed] [Google Scholar]
- 75.Zheng Q., Xiao J., Zhang D., Li X., Xu J., Ma J., Xiao Q., Fu J., Guo Z., Zhu J., et al. Bisphenol analogues in infant foods in south China and implications for infant exposure. Sci. Total Environ. 2024;910:168509. doi: 10.1016/j.scitotenv.2023.168509. [DOI] [PubMed] [Google Scholar]
- 76.Liu L., Zhang X., Jia P., He S., Dai H., Deng S., Han J. Release of microplastics from breastmilk storage bags and assessment of intake by infants: A preliminary study. Environ. Pollut. 2023;323:121197. doi: 10.1016/j.envpol.2023.121197. [DOI] [PubMed] [Google Scholar]
- 77.Kutralam-Muniasamy G., Pérez-Guevara F., Elizalde-Martínez I., Shruti C. Branded milks—Are they immune from microplastics contamination? Sci. Total Environ. 2020;714:136823. doi: 10.1016/j.scitotenv.2020.136823. [DOI] [PubMed] [Google Scholar]
- 78.Marie C., Vendittelli F., Sauvant-Rochat M.-P. Obstetrical outcomes and biomarkers to assess exposure to phthalates: A review. Environ. Int. 2015;83:116–136. doi: 10.1016/j.envint.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 79.Dong H., Wang X., Niu X., Zeng J., Zhou Y., Suona Z., Yuan Y., Chen X. Overview of analytical methods for the determination of microplastics: Current status and trends. TrAC. 2023;167:117261. doi: 10.1016/j.trac.2023.117261. [DOI] [Google Scholar]
- 80.Talukdar A., Kundu P., Bhattacharya S., Dutta N. Microplastic contamination in wastewater: Sources, distribution, detection and remediation through physical and chemical-biological methods. Sci. Total Environ. 2024;916:170254. doi: 10.1016/j.scitotenv.2024.170254. [DOI] [PubMed] [Google Scholar]
- 81.Rathore C., Saha M., Gupta P., Kumar M., Naik A., de Boer J. Standardization of micro-FTIR methods and applicability for the detection and identification of microplastics in environmental matrices. Sci. Total Environ. 2023;888:164157. doi: 10.1016/j.scitotenv.2023.164157. [DOI] [PubMed] [Google Scholar]
- 82.Soursou V., Campo J., Picó Y. A critical review of the novel analytical methods for the determination of microplastics in sand and sediment samples. TrAC. 2023;166:117190. doi: 10.1016/j.trac.2023.117190. [DOI] [Google Scholar]
- 83.Peng C., Zhang X., Zhang X., Liu C., Chen Z., Sun H., Wang L. Bacterial Community under the Influence of Microplastics in Indoor Environment and the Health Hazards Associated with Antibiotic Resistance Genes. Environ. Sci. Technol. 2022;56:422–432. doi: 10.1021/acs.est.1c04520. [DOI] [PubMed] [Google Scholar]
- 84.Hendrickson E., Minor E.C., Schreiner K. Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018;52:1787–1796. doi: 10.1021/acs.est.7b05829. [DOI] [PubMed] [Google Scholar]
- 85.Luo X., Wang Z., Yang L., Tao G., Zhang Y. A review of analytical methods and models used in atmospheric microplastic research. Sci. Total Environ. 2022;828:154487. doi: 10.1016/j.scitotenv.2022.154487. [DOI] [PubMed] [Google Scholar]
- 86.Rose D., Webber M. Characterization of microplastics in the surface waters of Kingston Harbour. Sci.Total Environ. 2019;664:753–760. doi: 10.1016/j.scitotenv.2019.01.319. [DOI] [PubMed] [Google Scholar]
- 87.Hu L., Chernick M., Hinton D.E., Shi H. Microplastics in Small Waterbodies and Tadpoles from Yangtze River Delta, China. Environ. Sci. Technol. 2018;52:8885–8893. doi: 10.1021/acs.est.8b02279. [DOI] [PubMed] [Google Scholar]
- 88.Lenz R., Enders K., Stedmon C.A., Mackenzie D.M.A., Nielsen T.G. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Marine Poll. Bull. 2015;100:82–91. doi: 10.1016/j.marpolbul.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 89.Levermore J.M., Smith T.E.L., Kelly F.J., Wright S.L. Detection of Microplastics in Ambient Particulate Matter Using Raman Spectral Imaging and Chemometric Analysis. Anal. Chem. 2020;92:8732–8740. doi: 10.1021/acs.analchem.9b05445. [DOI] [PubMed] [Google Scholar]
- 90.Shruti V.C., Perez-Guevara F., Elizalde-Martínez I., Kutralam-Muniasamy G. Toward a unified framework for investigating micro(nano)plastics in packaged beverages intended for human consumption. Environ. Poll. 2021;268:115811. doi: 10.1016/j.envpol.2020.115811. [DOI] [PubMed] [Google Scholar]
- 91.Martín-Gómez B., Elmore J.S., Valverde S., Ares A.M., Bernal J. Recent applications of chromatography for determining microplastics and related compounds (bisphenols and phthalate esters) in food. Microchem J. 2024;197:109903. doi: 10.1016/j.microc.2024.109903. [DOI] [Google Scholar]
- 92.Fu W., Min J., Jiang W., Li W., Zhang W. Separation, characterization and identification of microplastics and nanoplastics in the environment. Sci. Total Environ. 2020;721:137561. doi: 10.1016/j.scitotenv.2020.137561. [DOI] [PubMed] [Google Scholar]
- 93.Chellasamy G., Ramasundaram S., Veerapandian M., Chandran M., Dhanasekaran B., Oh T.H., Govindaraju S., Yun K. Systematic review on fate and behavior of microplastics towards the environment. TrAC. 2023;169:117390. doi: 10.1016/j.trac.2023.117390. [DOI] [Google Scholar]
- 94.da Costa J.P., Avellan A., Mouneyrac C., Duarte A., Rocha-Santos T. Plastic additives and microplastics as emerging contaminants:Mechanisms and analytical assessment. TrAC. 2023;258:116898. [Google Scholar]
- 95.Jiménez-Skrzypek G., Ortega-Zamora O., González-Sálamoa J., Hernández-Sánchez C., Hernández-Borges J. The current role of chromatography in microplastic research: Plastics chemical characterization and sorption of contaminants. J. Chromatogr. Open. 2021;1:100001. doi: 10.1016/j.jcoa.2021.100001. [DOI] [Google Scholar]
- 96.Kadac-Czapska K., Trzebiatowska P., Knez E., Zaleska-Medynska A., Grembecka M. Microplastics in food—A critical approach to definition, sample preparation, and characterisation. Food Chem. 2023;418:135985. doi: 10.1016/j.foodchem.2023.135985. [DOI] [PubMed] [Google Scholar]
- 97.Xue Q., Dong Y., Lu F., Yang H., Yu G. ELM combined with differential Raman spectroscopy for the detection of microplastics in organisms. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2024;312:124039. doi: 10.1016/j.saa.2024.124039. [DOI] [PubMed] [Google Scholar]
- 98.Rhee H., Jeong S., Lee H., Cho M.G., Choi D.S. Rapid detection and identification of microplastics from nonchemically treated soil with CARS microspectroscopy. Environ. Poll. 2024;342:123080. doi: 10.1016/j.envpol.2023.123080. [DOI] [PubMed] [Google Scholar]
- 99.Wang J., Dong J., Tang M., Yao J., Li X., Kong D., Zhao K. Identification and detection of microplastic particles in marine environment by using improved faster R–CNN model. J. Environ. Manag. 2023;345:118802. doi: 10.1016/j.jenvman.2023.118802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.