Abstract
Herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) have evolved specific anatomic tropisms and site-dependent rates of reactivation. To determine whether reactivation rates depend on distinct abilities of HSV-1 and -2 to establish latency and to express latency-associated transcripts (LATs), virulent strains of each virus were studied in the guinea pig genital model. Following infection with equivalent titers of virus, the quantities of latent HSV-2 genomes and LATs were higher in lumbosacral ganglia, and HSV-2 infections recurred more frequently and lasted longer than HSV-1 infections. In contrast, if the inoculum of HSV-1 was 10 times that of HSV-2, the quantity of HSV-1 DNA and LATs increased correspondingly and HSV-1 infections were as likely to recur as those with HSV-2. The quantity of latent virus DNA correlates with and may be a major determinant of the site-specific patterns and rates of reactivation of HSV-1 and -2.
Herpes simplex virus type 1 (HSV-1) and HSV-2 are remarkably similar in their abilities to infect mucosal surfaces and to latently infect and reactivate from sensory nerve ganglia, despite their well-characterized genomic and antigenic differences (22). It cannot be coincidence, however, that has segregated the majority of HSV-1 infections to the oral-labial region in humans and HSV-2 to the genital region. HSV-1 and HSV-2 display distinct phenotypic patterns with regard to their rates of symptomatic reactivation at each anatomical site (8, 18, 20). By some estimates, patients with concurrent primary oral-labial and genital HSV-1 infections are nearly sixfold more likely to develop oral-labial rather than genital recurrences. Conversely, those with simultaneous oral-labial and genital HSV-2 infections are about 400-fold more likely to experience genital rather than oral recurrences (11).
Few of the viral factors that could be associated with this anatomic predilection have been compared directly in parallel studies of HSV-1 and HSV-2. Work with animal models has shown that HSV-1 and HSV-2 are equally adept at causing acute infection (12, 20). Both are transported axonally from peripheral sites to infect the central nervous system, although HSV-2 is clearly more neurovirulent than HSV-1 (6, 7, 15, 19). A comparison of HSV-1 and HSV-2 in the mouse vaginal model has shown that both viruses establish latency (1, 20). Both viruses can reactivate from facial and genital sites of inoculation, although in humans, the rates of reactivation vary according to sites of infection and virus type (11).
Recent work suggests that tissue-specific rates of virus reactivation are influenced by sequences in an HSV gene that is expressed during latency (23). In latently infected animal or human sensory neurons, HSV-1 and HSV-2 express only one abundant family of transcripts, termed latency-associated transcripts (LATs). Studies of HSV mutants showed that LATs are not necessary for effective establishment of latency; however, they do influence rates of viral reactivation. Strains that are engineered to express little or no LAT reactivate 1/2 to 1/10 as well as the parental strains from which they derive (2, 4, 9, 13, 17). Moreover, replacement of HSV-2 LAT region sequences with those of HSV-1 transfers a higher rate of ocular reactivation; restoration of the HSV-2 LAT sequences reestablishes the higher rate of genital reactivation (23). Thus, the LAT region influences site-specific reactivation. We sought other, more general attributes of these viruses that would determine their rates of reactivation from latency. Virulent strains of HSV-1 (strain 17 syn+) and HSV-2 (strain 333) were inoculated intravaginally into guinea pigs, and their relative abilities to establish latency, to express LATs, and to reactivate were determined.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were grown in Dulbecco’s modified Eagle medium (Quality Biological, Inc., Gaithersburg, Md.) supplemented with 10% fetal calf serum (Sigma Chemical Co., St. Louis, Mo.) and 1% l-glutamine–aureomycin–streptomycin–penicillin (Quality Biological, Inc.) in a 5% CO2 humidified chamber at 37°C. Primary rabbit kidney cells (Biowhittaker, Walkersville, Md.) were grown in accordance with the supplier’s instructions. Stocks of HSV-1 strain 17 syn+ and HSV-2 strain 333 were prepared in Vero cells and divided into cell-free aliquots, their titers were determined, and they were stored at −80°C until use.
Guinea pigs.
Female Hartley guinea pigs (500 g) were housed in American Association for Laboratory Animal Care-approved facilities and studied in accordance with approved protocols. Guinea pigs were anesthetized with ketamine and xylazine and inoculated intravaginally with virus in a 25- to 100-μl volume as previously described (5). In the second experiment, 25 mg of acyclovir (Burroughs Wellcome Co., Research Triangle Park, N.C.) was given once daily by intraperitoneal injection on days 1 through 7 to animals infected with HSV-2 to reduce the high (30 to 50%) mortality rates.
Scoring of acute and latent genital lesions.
Guinea pig genitalia were scored daily on a scale of 0 to 4 following inoculation as previously described (16). Recurrences were recorded from day 15 or the time of lesion resolution, whichever came later, until day 50.
Determination of the titers of vaginal swabs.
Guinea pigs were swabbed vaginally with Dacron swabs during the acute infection. Swabs were immediately placed into 1 ml of Dulbucco’s modified Eagle medium on ice. Dilutions were plated onto Vero cells in duplicate, and following incubation for 1 h to allow adherence, cells were washed and overlaid with medium containing 0.5% human immunoglobulin. Plaques were counted 2 days later.
Viral titers in tissues.
At desired times after infection, three surviving animals from each group were sacrificed for quantitation of virus in particular anatomical sites. Sacral dorsal root ganglia and spinal cords were dissected free of surrounding tissue and placed into 1 ml of Dulbecco’s modified Eagle medium on ice. Tissues were homogenized by using a Tissumizer (Tekmar, Cincinnati, Ohio) and frozen and thawed once. Homogenized tissues were spun briefly in a microcentrifuge, and dilutions of the supernatant were plated onto primary rabbit kidney cell monolayers in duplicate. Following incubation for 1 h to allow adherence, cells were washed and overlaid with medium containing 0.5% human immunoglobulin. Plaques were counted 2 days later.
Supernatants from some samples (200 μl) were also extracted for quantitative competitive DNA PCR assays.
Extraction of nucleic acids from ganglia.
Guinea pigs were sacrificed by carbon dioxide inhalation, and their sacral dorsal root ganglia were removed with sterile instruments. Ganglia were placed into 300 μl of cell lysis solution (0.001% sodium dodecyl sulfate–0.0001% Triton X-100 in buffer containing Tris-HCl at 10 mM and EDTA at 1 mM) containing 0.6-mg/ml proteinase K (Sigma Chemical Co.) and incubated overnight at 56°C. DNA was extracted by using a Puregene kit (Gentra Systems, Minneapolis, Minn.) in accordance with the manufacturer’s instructions. RNA was extracted by using the RNAgents kit (Promega, Madison, Wis.) in accordance with the manufacturer’s instructions following mechanical dispersion of the ganglia with 16- to 25-gauge needles and syringes in succession. DNA was stored in buffer containing Tris-HCl at 10 mM and EDTA at 1 mM at 4°C. Ganglion RNA was stored in diethylpyrocarbonate-treated water at −80°C. The yields of genomic RNA and DNA were calculated based on their UV spectrographic A260.
Quantitative competitive DNA PCR.
Competitor plasmid constructs were made by insertion of an irrelevant “stuffer” sequence internal to the primers in the LAT region of the genome (see Fig. 3A). Known copy numbers of the competitor plasmids were added to each reaction tube containing 5 μl of 10× PCR buffer (Life Technologies, Gibco, Gaithersburg, Md.), 0.25 μM each primer, 0.15 mM each triphosphorylated deoxynucleotide, 1.5 mM MgCl2, 5% glycerol, 27 μl of sterile water, and 100 ng of genomic DNA. Taq polymerase (Life Technologies, Gibco), 1.5 U per reaction tube, was added during the first annealing cycle. Primer sequences are as follows: HSV-1 LAT upstream, 5′-gccttacgtgaacaagacta; HSV-1 LAT downstream, 5′-tcatccagaggctgttccac; HSV-2 LAT upstream, 5′-gccagacgtgcgtgctctgc; HSV-2 LAT downstream, 5′-tgttggtctttatcatagaacag. Samples were amplified in a Perkin Elmer T1000 thermal cycler. The HSV-1 cycle program was 94°C for 3 min, 55°C for 5 min (hot start), and 72°C for 3 min, followed by 20 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, followed by 40 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s. HSV-2 samples were similarly cycled, except with an annealing temperature of 60°C. PCR products were resolved on 2% agarose gels with ethidium bromide staining. All quantitative competitive DNA PCR studies were accompanied by simultaneous studies of positive and negative controls and a control panel containing known amounts of wild-type genomic sequences and known dilutions of the competitors. Interassay variation proved to be about 0.5 log, or threefold.
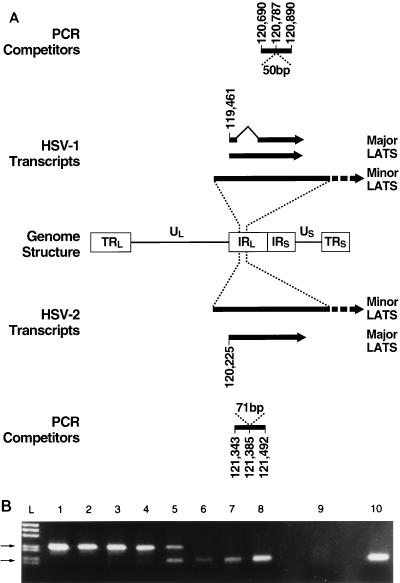
FIG. 3.
Quantitative competitive PCR for viral DNA and RNA. (A) Diagram of the HSV genome depicting the long and short terminal (TRL, TRS) and internal (IRL, IRS) repeats and unique long (UL) and short (US) regions with schematic drawings of structures and known sequence base numbers pertinent to the present studies of HSV-1 (above) and HSV-2 (below). Lines: 1, HSV-1 PCR competitors indicating the 50-bp insert for DNA and RNA PCR; 2, HSV-1 major and minor LAT species; 3, genome structure; 4, HSV-2 major and minor LATs; 5, HSV-2 competitors showing the 71-bp insert for DNA and RNA PCR. (B) RT-PCR quantitation of LATs produced in latently HSV-2-infected ganglia. Lanes 1 to 8 are samples from RT-PCRs each containing 200 ng of genomic RNA and dilutions of in vitro-synthesized competitor transcripts (100 fg, 30 fg, 10 fg, 3 fg, 1 fg, 300 ag, 100 ag, and 30 ag). Competition in the experiment shown occurs at 1 fg of competitor (lane 5). Lane 9 shows the RT-PCR products of a reaction with no added template, and lane 10 contains the product of a reaction with no added competitor. Lane L contains a marker ladder, and the top and bottom arrows point to the expected sizes of the competitor (221-bp) and native (150-bp) products, respectively.
Quantitative competitive RT-PCR.
Competitor RNA transcripts were generated in accordance with the manufacturer’s (Promega) instructions, by in vitro transcription of plasmid constructs made by PCR extension of the wild-type sequences internal to primers. Known copy numbers of competitor transcripts were added to each reaction tube containing 200 ng of sample RNA and reverse transcribed by using the downstream primer. HSV-1 and HSV-2 RNA PCR was performed by first generating cDNA in a reaction mixture containing 6 μl of 5× reverse transcriptase (RT) buffer (Life Technologies, Gibco), 40 U of RNasin (Boehringer Mannheim, Indianapolis, Ind.), 1 mM dithiothreitol, 2.5 μM 3′ primer, 0.5 mM each triphosphorylated deoxynucleotide, and 9.5 μl of diethylpyrocarbonate-treated sterile water. HSV-1 cDNA was generated by using avian myeloblastosis virus RT (Boehringer Mannheim) at 20 u per reaction for 1 h at 42°C, followed by 5 min at 94°C. HSV-2 cDNA was generated by using 600 U of Moloney murine leukemia virus RT (Life Technologies, Gibco) for 1 h at 39°C, followed by 5 min at 94°C. Products of both the HSV-1 and HSV-2 reverse transcription reactions were amplified and electrophoretically separated on 2% agarose gels. All RNA samples were treated with RNase-free DNase 1 (Boehringer Mannheim) for 40 min at 37°C and then inactivated for 20 min at 65°C in buffer containing 3 mM MgCl2 prior to transcription. All quantitative competitive RT-PCR studies were accompanied by simultaneous assay of positive and negative controls, RT-negative controls, and a panel of control specimens containing known amounts of wild-type, in vitro-generated RNA and known dilutions of competitor RNAs (see Fig. 3B). Interassay variation was estimated to be 0.5 log, or about threefold.
Statistical methods.
Viral DNA and LAT contents in tissues were examined by one-way analysis of variance using log-transformed data. Medians and distributions were compared by the Wilcoxon two-sample test using the Bonferroni method for adjustment to P values for multiple testing. The proportions of guinea pigs without lesions in the two groups were compared by the Fisher exact test. Comparisons of the Kaplan-Meier estimated proportions of animals experiencing a recurrence were done by the log rank test.
RESULTS
Phenotypic differences between HSV-1 and HSV-2 during acute infection of guinea pigs with equivalent titers of virus.
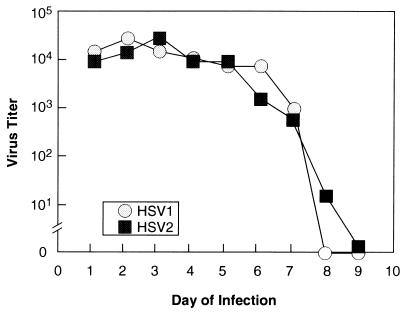
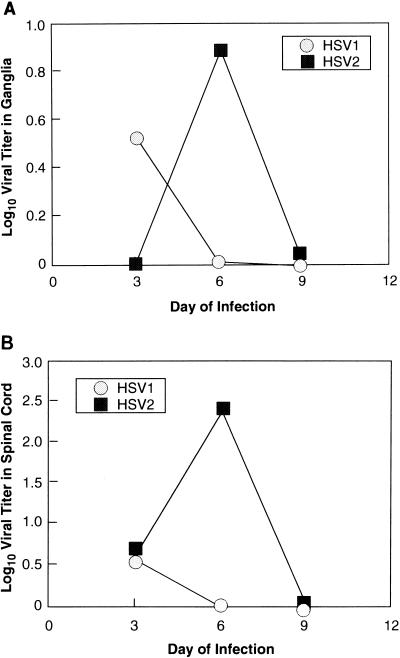
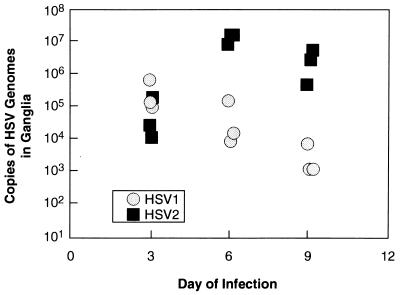
In initial experiments, animals were infected with identical titers of HSV-1 or -2 (5 × 105 PFU) and examined daily for the characteristic appearance of acute disease and the titers of daily vaginal swabs were determined on Vero cell monolayers. Animals infected with HSV-1 developed somewhat milder local and systemic disease (data not shown) than did those infected with HSV-2, peaking in intensity earlier than those infected with HSV-2. There were no significant differences, though, between the titers of HSV-1 and HSV-2 shed from the vaginal mucosa (Fig. 1), indicating that both viruses replicate efficiently at mucosal surfaces and are available for axonal transport to the dorsal root ganglia. HSV-2, however, achieved higher titers in the dorsal root ganglia (statistically insignificant) and spinal cord (P = 0.03 for day 6 data) during the acute infection than did HSV-1 (Fig. 2). Quantitative competitive DNA PCR using amplimers in the LAT region (Fig. 3A), a far more sensitive assay than titration of virus from homogenized ganglia, corroborated the general trends of the infectivity data, with HSV-1 genome levels peaking in the ganglia on day 3 of infection and HSV-2 DNA peaking on day 6, at levels approximately 2 logs higher than those of HSV-1 (P = 0.003 and 0.002 for day 6 and day 9 comparisons, respectively; Fig. 4). Clearly, more virus and viral DNA were present in the dorsal root ganglia and spinal cord during acute infection in animals inoculated with HSV-2 than in those of animals inoculated with HSV-1. Infected animals were then monitored prospectively for the next 8 weeks for genital herpes recurrences. Following that, the animals were sacrificed and their lumbosacral ganglia were recovered for analysis.
FIG. 1.
HSV-1 and -2 shedding from guinea pig vaginal mucosa during acute infection. Female 500-g Hartley guinea pigs were inoculated with equivalent doses (5 × 105 PFU) of HSV-1 strain 17 syn+ or HSV-2 strain 333 and cultured intravaginally daily thereafter. Titers of swabs were determined on Vero cell monolayers.
FIG. 2.
Infectious yields of HSV-1 and -2 from acutely infected dorsal root ganglia (A) and spinal cords (B). Sacral dorsal root ganglia and spinal cords from acutely infected guinea pigs were homogenized, frozen, and thawed, and the titers of supernatants were determined on primary rabbit kidney cells. Although the infectious yields of the ganglia in these assays are relatively low, the general trends were confirmed by quantitative PCR (see Fig. 4).
FIG. 4.
Quantities of HSV-1 and -2 DNAs in acutely infected dorsal root ganglia. DNA extracted from acutely infected ganglia was quantitated by using a competitive DNA assay. These results mirrored those obtained by virus titering methods; however, this assay is more sensitive, showing HSV-2 DNA content peaking on day 6 at least 1 log higher than the HSV-1 DNA content, which peaked on day 3. These results validated those of the competitive quantitative PCR assay.
HSV-2 reactivates more frequently than HSV-1 after infection with equivalent titers of virus.
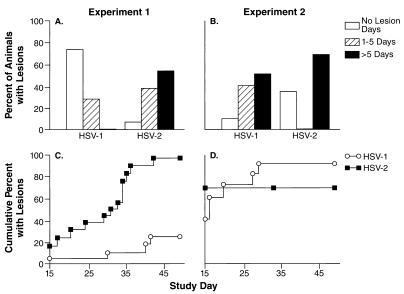
Despite equivalent inocula of virus and similar rates of shedding from the vaginal mucosa during acute infection, both the duration of lesions and the number of spontaneously appearing genital recurrences over the ensuing months were significantly higher for guinea pigs infected with HSV-2 than for those infected with HSV-1 (experiment 1 in Fig. 5A and C). Notably, the median numbers of recurrences per animal were 0 (range, 0 to 3) and 2 (range, 0 to 6) for HSV-1 and HSV-2, respectively (P < 0.001). The median numbers of days until lesions healed were 0 (range, 0 to 5) for HSV-1-infected animals and 6 (range, 0 to 19) for HSV-2-infected animals (P < 0.001; Fig. 5A); only 4 (27%) of 15 guinea pigs infected with HSV-1 had lesions, while 15 of 16 infected with HSV-2 had lesions (P < 0.001; Fig. 5C).
FIG. 5.
Recurrent genital lesions in independent experiments in which animals were infected with equivalent titers of HSV-1 or HSV-2 (experiment 1 in A and C) or with 10-fold higher inocula of HSV-1 (experiment 2 in B and D). Following resolution of the acute infections, the presence of lesions was noted daily from day 15 through day 50. The data are displayed at the top as the percentages of animals with no lesions, with lesions for 1 to 5 days, and with lesions for more than 5 days in the study interval (A and B). Below (C and D), the data indicate the cumulative percentage of animals experiencing a first genital recurrence in the study interval (Kaplan-Meier curves).
Important differences were also seen in virion genome and LAT copy numbers in latently infected ganglia from these animals, as quantitated by competitive DNA and RNA PCR assays (an example is shown in Fig. 3B). The geometric mean number of latent HSV-2 DNA copies per 200 ng of ganglion DNA was over three times that of latent HSV-1 DNA (P = 0.11; Table 1). Dorsal root ganglia from guinea pigs experiencing recurrent outbreaks contained greater numbers of latent HSV DNA than did those from animals without recurrences, regardless of the viral type (geometric mean latent viral DNA copy numbers per 200 ng of ganglion DNA of 8 × 100 for animals without recurrences and 4.9 × 101 for animals with recurrences, P < 0.02). Ganglia latently infected with HSV-2 also contained 15-fold more copies of HSV-2 than HSV-1 LATs (P < 0.01; Table 1). These results suggested that the burden of latent viral DNA and the levels of LAT expression may be important determinants of recurrence frequency. To further test this hypothesis, we analyzed recurrence rates in animals bearing a different ratio of HSV-1 to HSV-2 DNA and LAT copy numbers.
TABLE 1.
Geometric mean numbers of latent HSV-1 and HSV-2 DNA copies and LAT copies in lumbosacral gangliaa
| Expt and virus | DNA copy no. | LAT copy no. |
|---|---|---|
| 1, Equal-titer inocula | ||
| HSV-1 (n = 6) | 1.5 × 101 (6.6 × 100– 3.2 × 101) | 7.4 × 104 (3.2 × 104– 1.7 × 105) |
| HSV-2 (n = 6) | 4.9 × 101 (1.6 × 101– 1.5 × 102) | 1.1 × 106 (3.4 × 105– 3.4 × 107) |
| 2, 10-fold higher HSV-1 inoculum | ||
| HSV-1 (n = 4) | 2.2 × 102 (1.4 × 102– 3.5 × 102) | 1.1 × 107 (8.7 × 106– 3.0 × 107) |
| HSV-2 (n = 5) | 1.7 × 102 (4.2 × 101– 7.0 × 102) | 1.1 × 106 (2.9 × 105– 4.0 × 106) |
Number of copies per 200 ng of ganglion DNA or RNA are shown. Geometric means are shown with 95% confidence intervals in parentheses.
Latent HSV infection and recurrent genital herpes in guinea pigs infected with a 10-fold higher inoculum of HSV-1 and HSV-2.
It was postulated that HSV-1 infection would recur as frequently as HSV-2 infection if the input inoculum of HSV-1 was sufficiently increased to achieve levels of latent HSV-1 DNA equivalent to or higher than those of latent HSV-2 DNA. Guinea pigs were infected intravaginally with either 106 PFU of HSV-1 (five times the amount used in the first experiment) or only 105 PFU of HSV-2 (half of the amount used in the first experiment). In this experiment, suboptimal acyclovir therapy was given to the HSV-2-infected animals for the first 7 days with the goal of reducing somewhat the mortality that results from the primary infection. Prior studies showed that this extent of therapy does not alter genital recurrence frequency (15). Daily observations revealed that the disease recurred in similar proportions of animals infected with HSV-1 and HSV-2 (P > 0.5; Fig. 5D). Although the median number of recurrences per animal with HSV-1 was three times greater than that of animals with HSV-2, three (range, zero to six) and one (range, zero to four), respectively, the distributions of recurrences were not statistically significantly different (P = 0.22; Fig. 5B). The median numbers of days with lesions, 5.5 days (range, 0 to 33) for HSV-1 and 9.5 days (range, 0 to 23) for HSV-2, were also not statistically significantly different (P > 0.5), nor were the numbers of days until the first recurrence (P > 0.5). In comparing experiment 1 (Fig. 5A and C) with experiment 2 (Fig. 5B and D), it was noted that the 10-fold elevation in the HSV-1 inoculum led to a significantly enhanced likelihood of disease recurrence (P < 0.01). The likelihood of HSV-2 recurrence was unchanged (P = 0.34) by decreasing its inoculum by half.
In accord with the increased likelihood of HSV-1 recurrence with a greater inoculum, quantitation of DNA and LAT contents in latently infected ganglia demonstrated corresponding increases in the latent HSV-1 genome and LAT contents to levels that were higher than those found in HSV-2-infected ganglia (P = 0.01 for LAT copies; Table 1).
DISCUSSION
Genital herpes recurrence rates are influenced by the quantity of latent virus in the ganglia. We found that the number of copies of latent viral DNA and LATs were higher, often significantly so, in the groups of animals experiencing higher rates of genital outbreaks. When the titer of virus with which the animals were infected was increased, the levels of latent viral DNA and RNA increased and there were corresponding increases in the likelihood and duration of recurrences.
We believe that the quantity of LATs merely reflects the level of latent viral DNA and is not, by itself, an efficient determinant of reactivation rates. In fact, our recent analyses of a series of HSV-2 mutants that produce high, intermediate, or very low levels of LATs in guinea pig ganglia showed that only very profound (>5-log) reductions in LAT expression but unchanged levels of latent viral DNA result in modest (50 to 90%) reductions in the rates of disease recurrence (21). Although HSV mutants deficient in LAT expression showed reduced rates of reactivation, these mutants have not always been rigorously assessed for the levels of latent DNA that they achieve in sensory ganglia (2, 4, 9, 13). The present data also do not negate the recent findings that the type specificity of the LATs influences the rate of reactivation, since latent DNA levels were not quantitated precisely in those studies (23). A recent study by Maggioncalda et al. verified decreased numbers of latently infected mouse neurons and rates of induced reactivation by explant cultivation with selected LAT region mutants of HSV-1 (14).
The present results have implications regarding antiviral therapy and vaccine development for HSV infections. Were one able to reduce the quantity of virus that can establish latency, the likelihood and rate of disease reactivation should decrease. However, multiple trials have proven that acyclovir is not initiated sufficiently early in the course of first episodes of genital herpes to alter subsequent-recurrence rates (10), and vaccines have failed to induce protective immunity in humans (3), but more potent antiviral drugs and more immunogenic vaccines may prove effective.
More immediately, the present data may explain the disproportionate rates at which HSV-1 and -2 cause recurrent genital herpes outbreaks in humans (11). Although there might be tissue-specific or immunologic obstacles to virus reactivation at a particular anatomic site, the lower rate at which HSV-1 genital infections recur could simply reflect a lower burden of latent virus in the lumbosacral ganglia. The present data establish that the quantity of latent virus correlates with the rate at which HSV infections recur and suggest that it is one of its major determinants.
ACKNOWLEDGMENTS
We gratefully acknowledge Philip Krause, Jeffrey Cohen, and Rhonda Kost for helpful discussions and Brenda Rae Marshall and Sara Kaul for editorial assistance. Claire Hallahan and Rona LeBlanc assisted with statistical analysis.
REFERENCES
- 1.Asher L V S, Walz M A, Notkins A L. Effect of immunization on the development of latent ganglionic infection in mice challenged intravaginally with herpes simplex virus types 1 and 2. Am J Obstet Gynecol. 1978;131:788–791. doi: 10.1016/0002-9378(78)90248-x. [DOI] [PubMed] [Google Scholar]
- 2.Bloom D C, Devi-Rao G B, Hill J M, Stevens J G, Wagner E K. Molecular analysis of herpes simplex virus type 1 during epinephrine-induced reactivation of latently infected rabbits in vivo. J Virol. 1994;68:1283–1292. doi: 10.1128/jvi.68.3.1283-1292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burke R L. Current developments in herpes simplex virus vaccines. Semin Virol. 1993;4:187–197. [Google Scholar]
- 4.Devi-Rao G B, Bloom D C, Stevens J G, Wagner E K. Herpes simplex virus type 1 DNA replication and gene expression during explant-induced reactivation of latently infected murine sensory ganglia. J Virol. 1994;68:1271–1282. doi: 10.1128/jvi.68.3.1271-1282.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler S L, Harrison C J, Myers M G, Stanberry L R. Outcome of herpes simplex virus type 2 infection in guinea pigs. J Med Virol. 1992;36:303–308. doi: 10.1002/jmv.1890360413. [DOI] [PubMed] [Google Scholar]
- 6.Gologan R, Mutiu A, Wecsler P, Lupu R, Gruia M. Comparative studies on some herpes simplex virus strains with different locations. Rev Roum Virol. 1973;19:39–45. [PubMed] [Google Scholar]
- 7.Gressens P, Martin J R. In situ polymerase chain reaction: localization of HSV-2 DNA sequences in infections of the nervous system. J Virol Methods. 1994;46:61–83. doi: 10.1016/0166-0934(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 8.Hanna L, Ostler H B, Keshishyan H. Observed relationship between herpetic lesions and antigenic type of herpesvirus hominis. Surv Ophthalmol. 1976;21:110–114. doi: 10.1016/0039-6257(76)90088-6. [DOI] [PubMed] [Google Scholar]
- 9.Krause P R, Stanberry L R, Bourne N, Connelly B, Kurawadwala J F, Patel A, Straus S E. Expression of the herpes simplex virus type 2 latency-associated transcript enhances spontaneous reactivation of genital herpes in latently infected guinea pigs. J Exp Med. 1995;181:297–306. doi: 10.1084/jem.181.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krause P R, Straus S E. The treatment, management and prevention of genital herpes. In: Stanberry L R, editor. Genital and neonatal herpes. New York, N.Y: John Wiley & Sons, Inc.; 1996. pp. 139–178. [Google Scholar]
- 11.Lafferty W E, Coombs R W, Benedetti J, Critchlow C, Corey L. Recurrences after oral and genital herpes simplex virus infection. N Engl J Med. 1987;316:1444–1449. doi: 10.1056/NEJM198706043162304. [DOI] [PubMed] [Google Scholar]
- 12.Landry M L, et al. The effect of acyclovir on genital infection with herpes simplex virus types 1 and 2 in the guinea pig. Am J Med. 1982;73:143–150. doi: 10.1016/0002-9343(82)90080-8. [DOI] [PubMed] [Google Scholar]
- 13.Leib D A, Bogard C L, Kosz-Vnenchak M, Hicks K A, Coen D M, Knipe D M, Schaffer P A. A deletion mutant of the latency-associated transcript of herpes simplex virus type 1 reactivates from the latent state with reduced frequency. J Virol. 1989;63:2893–2900. doi: 10.1128/jvi.63.7.2893-2900.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maggioncalda J, Mehta A, Su Y H, Fraser N W, Block T M. Correlation between herpes simplex virus type 1 rate of reactivation from latent infection and the number of infected neurons in trigeminal ganglia. Virology. 1996;225:72–81. doi: 10.1006/viro.1996.0576. [DOI] [PubMed] [Google Scholar]
- 15.Plummer G, Waner J L, Phuangsab A, Goodheart C R. Type 1 and type 2 herpes simplex viruses: serological and biological differences. J Virol. 1970;5:51–59. doi: 10.1128/jvi.5.1.51-59.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanberry L R, Kern E R, Richards J T, Abbott T M, Overall J C. Genital herpes in guinea pigs: pathogenesis of the primary infection and description of recurrent diseases. J Infect Dis. 1982;146:397–404. doi: 10.1093/infdis/146.3.397. [DOI] [PubMed] [Google Scholar]
- 17.Steiner I, Spivack J G, Lirette R P, Brown S M, MacLean A R. Herpes simplex virus type 1 latency associated transcripts are evidently not essential for latent infection. EMBO J. 1989;8:505–511. doi: 10.1002/j.1460-2075.1989.tb03404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald A, Zeh J, Selke S, Ashley R L, Corey L. Virologic characteristics of subclinical and symptomatic genital herpes infections. N Engl J Med. 1993;333:770–775. doi: 10.1056/NEJM199509213331205. [DOI] [PubMed] [Google Scholar]
- 19.Walz M A, Price R W, Hayashi K, Katz B J, Notkins A L. Effect of immunization on acute and latent infections of vagino-uterine tissue with herpes simplex virus types 1 and 2. J Infect Dis. 1977;135:744–752. doi: 10.1093/infdis/135.5.744. [DOI] [PubMed] [Google Scholar]
- 20.Walz M A, Price R W, Notkins A L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neuronectomy. Science. 1974;184:1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- 21.Wang K, Pesnicak L, Straus S E. Mutations in the 5′ end of the herpes simplex virus type 2 latency-associated transcript (LAT) promoter affect LAT expression in vivo but not the rate of spontaneous reactivation of genital herpes. J Virol. 1997;71:7903–7910. doi: 10.1128/jvi.71.10.7903-7910.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whitley R J. Herpes simplex viruses. In: Fields B N, et al., editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven Press, Inc.; 1996. pp. 2207–2342. [Google Scholar]
- 23.Yoshikawa T, Hill J M, Stanberry L R, Bourne N, Kurawadwala J F, Krause P R. The characteristic site-specific reactivation phenotypes of HSV-1 and HSV-2 depend upon the latency-associated transcript region. J Exp Med. 1996;184:659–664. doi: 10.1084/jem.184.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]