Abstract
Increasing evidence points to a role of the mitogenic Ras/Raf/MEK/ERK signaling cascade in regulation of human immunodeficiency virus type 1 (HIV-1) gene expression. Stimulation of elements of this pathway leads to transactivation of the HIV-1 promoter. In particular, the NF-κB motif in the HIV long terminal repeat (LTR) represents a Raf-responsive element in fibroblasts. Regulation of the Raf kinase in T cells differs from findings with a variety of cell lines that the catalytic domain of Raf (RafΔ26–303) shows no activity. In this study, we restored the activity of the kinase in T cells by fusing its catalytic domain to the CAAX motif (-Cx) of Ras, thus targeting the enzyme to the plasma membrane. Constitutive activity of Raf was demonstrated by phosphorylation of mitogen-activated protein kinase kinase (MEK) and endogenous mitogen-activated protein kinase 1/2 (ERK1/2) in A3.01 T cells transfected with RafΔ26–303-Cx. Membrane-targeted Raf also stimulates NF-κB, as judged by κB-dependent reporter assays and enhanced NF-κB p65 binding on band shift analysis. Moreover, we found that active Raf transactivates the HIVNL4-3 LTR in A3.01 T lymphocytes and that dominant negative Raf (C4) blocked 12-O-tetradecanoylphorbol-13-acetate induced transactivation. When cotransfected with infectious HIVNL4-3 DNA, membrane-targeted Raf induces viral replication up to 10-fold over basal levels, as determined by the release of newly synthesized p24gag protein. Our study clearly demonstrates that the activity of the catalytic domain of Raf in A3.01 T cells is dependent on its cellular localization. The functional consequences of active Raf in T lymphocytes include not only NF-κB activation and transactivation of the HIVNL4-3 LTR but also synthesis and release of HIV particles.
Transcriptional control of human immunodeficiency virus type 1 (HIV-1) in T lymphocytes involves a complex interaction between cellular and viral regulatory proteins and their target sequences within the long terminal repeat (LTR) (15). Enhancement of HIV-1 replication can be induced by external stimuli that activate T lymphocytes, such as cytokines, or by T-cell receptor engagement, indicating that these factors can trigger cellular signaling pathways leading to viral gene expression (11). We and others have identified cellular proteins belonging to the NF-κB family of transcription factors and other NF-κB-binding proteins as important stimulating factors (3–5, 13, 15, 16, 28). Specifically, the NF-κB-binding motif in the HIV LTR is a Raf-responsive element (8, 12). Moreover, in monocytes, HIV infection activates mitogen-activated protein kinase kinase (MEK), a downstream target of Raf-1, and this activation participates in NF-κB stimulation (14). Taken together, these data suggest a direct link between the Raf/MEK/ERK intracellular signaling pathway and HIV-1 transcriptional activation.
The serine/threonine kinase Raf is a member of the mitogen-activated protein kinase pathway. This cascade transmits and amplifies signals generated by a variety of stimuli, including growth factors and phorbol esters (6, 9, 34). In lymphoid cells, Raf-1 kinase is activated upon T-cell receptor engagement, interleukin treatment, CD4 cross-linking, or binding of HIV-1 gp120 to CD4 surface receptors (25, 30, 33, 35, 36). Activation of Raf-1 kinase is a complex multistep regulated process involving changes in phosphorylation events, subcellular localization, and protein interactions (27). Receptor tyrosine kinase signaling through Ras leads to Raf-1 activation, which in turn phosphorylates and stimulates the dual-specificity kinase MEK, which transmits the signal to mitogen-activated protein kinase (ERK). The latter has been shown to phosphorylate and to activate several proteins, including other protein kinases, transcription factors, and cytoskeletal proteins (9, 29).
The Raf protein can be subdivided into two functional domains: the kinase domain, located in the C terminus (residues 330 to 627), and a negative regulatory domain, located in approximately the first third of the protein (residues 51 to 149). Deletion of the N-terminal domain leads to a constitutively active kinase in a variety of cell lines such as fibroblasts and human embryonic kidney cells (7, 20, 22, 39); however, in T lymphocytes, such truncated versions of Raf do not exhibit catalytic activity (43). The reasons for this apparent (down)regulation of Raf activity in T cells are not clear.
The N-terminal region is further distinguished by containing the elements necessary for Ras binding (44). The interaction of this region with GTP-bound p21ras at the plasma membrane is thought to be necessary for Raf kinase activity within a cellular environment (40). This is supported by experiments where Raf was targeted to the plasma membrane by adding the farnesylation signal of p21K-ras to the C-terminal region (37). This modified form of Raf is recruited to the plasma membrane independently of Ras and is thereby locally activated (23). Thus, this type of recruiting functions to bring Raf into close contact with its relevant physiological activators and/or substrates.
In this study, we overcome the regulation of expressed N-terminally truncated Raf in T cells by membrane targeting with the farnesylation signal of K-Ras. We used this construct to investigate the consequences of Raf/MEK/ERK signaling on NF-κB activation and stimulation of HIV-1 replication in a CD4+ T-lymphoblast cell line. In this report, we provide evidence that constitutively active Raf not only is involved in HIV-1 transactivation but also triggers κB-dependent gene expression and HIV-1 replication in T cells.
MATERIALS AND METHODS
DNA constructs and cloning.
RafΔ22–303 and epitope-tagged (HA)-RafΔ22–303 have been described previously (41, 43) and carry an in-frame deletion of amino acids 22 to 303 (7, 17, 21). The construct RafΔ22–303-Cx (containing the C-terminal, membrane-targeting 17 amino acids of Ki-Ras fused to the kinase domain of Raf) was created by fusing the C-terminal part of RafΔ22–303-Cx to hemagglutinin (HA)-tagged RafΔ22–303 by using the BglI-XbaI restriction sites. RafΔ22–303-KD and RafΔ22–303-KD-Cx have a K-to-W substitution at position 375 in the ATP-binding site of Raf, which abolishes kinase activity (kinase-dead mutants [KD]). HA-tagged Raf-C4 is a dominant negative carboxy-terminal deletion mutant of Raf-1 and contains the Ras-binding domain (7). All cDNAs are subcloned into the multiple-cloning site of pSRSPA (10). The 3×κB-tk luciferase plasmid contains three tandem copies of the κB motif cloned upstream of a minimal thymidine kinase promoter reporter gene and was obtained from T. Wirth, University of Wuerzburg, Wuerzburg Germany. Two different NL4-3 clones of infectious HIV-1 DNA were used (obtained from A. Rethwilm and O. Kutsch, National Institutes of Health AIDS Research and Reference Reagent Program). The NL4-3 luciferase plasmid contains nucleotide sequences from −150 to +70 of the HIVNL4-3 LTR and was obtained from F. Kirchhoff, University of Erlangen, Erlangen, Germany. The HIV-LTR-wt plasmid was described previously (13) and subcloned into a luciferase vector. The HIV-LTR-κBmt plasmid contains point mutations (GGG to GCT) and (GGG to CTC) in both NF-κB sites of the HIV LTR.
Antibodies.
Monoclonal anti-p24gag antibody was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. ERK1, ERK2, and phospho-ERK specific antibodies were purchased from Santa Cruz Biotechnology, Inc. Anti-p65 rabbit antiserum was provided by Nancy Rice, Frederick, Md. Monoclonal antibodies against HA-tag (12CA5) were produced and purified by standard methods.
Cell culture, DNA transfection, and reporter gene assay.
A3.01 T cells were maintained in RPMI 1640 (Gibco BRL) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, streptomycin, and penicillin. The cells were cultured routinely to a density of 0.5 × 106 to 1.0 × 106 cells per ml. Briefly, cells (3 × 105 to 6 × 105 per six-well dish) were transfected with 0.5 to 2.0 μg of pRSPA expressing Raf kinases by a modified transfection procedure from GIBCO-BRL. For luciferase assays, T cells were transfected with combinations of 0.4 μg of reporter construct, 0.25 to 1.5 μg of HIV LTR, 0.5 to 1.5 μg of pRSPA expressing Raf kinases, and 0.1 to 0.5 μg of pRSPA-HIV-tat expression vector as indicated in the figure legends. The cells were incubated for 5 h in an incubator at 37°C under 7.5% CO2 in the presence of the DMRIE-C reagent (GIBCO-BRL) nucleic acid complexes, and 1.5 ml of growth medium was added. For luciferase assays, total-cell extracts were prepared 24 to 42 h later. Briefly, cells from each well were harvested in 100 μl of lysis buffer (50 mM sodium morpholineethanesulfonate [pH 7.8], 50 mM Tris-HCl [pH 7.8], 10 mM dithiothreitol, 2% Triton X-100). The crude cell lysates were cleared by centrifugation, 50 μl of precleared cell extracts was added to 50 μl of luciferase assay buffer (125 mM sodium MES [pH 7.8], 25 mM magnesium acetate, 2 mg of ATP per ml), and the activity was measured after injection of 50 μl of 1 mM d-luciferin (AppliChem) in a Berthold Lumat luminometer. The total protein concentration was measured by the Bradford technique (Bio-Rad). Results are presented as luciferase units normalized to protein concentration and mock transfection with empty expression vectors. Each experiment was done in triplicate and is representative of at least three different sets of experiments.
Immunocomplex kinase assay and immunoblotting.
For immunocomplex kinase assays, cells were harvested 42 h after DNA transfection, washed once in phosphate-buffered saline (PBS) and lysed with a modified radioimmunoprecipitation buffer (RIPA) (25 mM Tris-HCl [pH 8.0], 137 mM NaCl, 10% [vol/vol] glycerol, 0.1% [vol/vol] sodium dodecyl sulfate [SDS], 0.5% [vol/vol] deoxycholate, 1% [vol/vol] Nonidet P-40, 2 mM EDTA, 1 mM Pefabloc, 1 mM Na3VO3, 0.15 U of aprotinin per ml, 20 μM leupeptin) at 4°C for 10 min. Cell debris was removed by centrifugation at 2,000 × g for 10 min. The supernatant was then incubated with monoclonal anti-HA (clone 12CA5) antibodies at 4°C for 2 h. The immunocomplexes were precipitated with protein A-agarose and extensively washed with RIPA buffer. They were either boiled in electrophoresis sample buffer for 3 min or used for in vitro kinase assays as previously described by Flory et al. (13). After SDS-polyacrylamide gel electrophoresis (PAGE), 10% polyacrylamide gels were electroblotted onto an Immobilon polyvinylidene difluoride or Nitrocellulose BAS-85 membrane (Schleicher & Schuell) and analyzed by autoradiography and Western blot analysis. For Western blot analysis, the membranes were incubated in blocking buffer (nonfat dry milk)-Tris-buffered saline and Tween 20 (TBST) and washed in TBST as described previously (13). As a secondary antibody protein A-peroxidase (Amersham) was used. This step was followed by the standard enhanced chemiluminescence reaction.
Nuclear extraction and electrophoretic gel mobility shift assay.
Cytosolic and nuclear fractions were extracted as described previously (13). Double-stranded oligonucleotide probes for electrophoretic mobility shift assay experiments were labeled in a reaction mixture containing 200 ng of double-stranded DNA probe, [α-32P]dCTP, 1 mM dATP, 1 mM dGTP, 1 mM dTTP, 500 mM Tris-HCl (pH 7.5), 100 mM MgCl2, and 2 U of Klenow fragment. After a 30-min incubation at 37°C, oligonucleotides were separated on a G-25 Sephadex spin column and finally resuspended in Tris-EDTA buffer (15,000 cpm/μl). For typical binding reactions, 3- to 5-μg samples of nuclear extracts were incubated at room temperature for 20 min in the absence or presence of competitor DNA in a 20-μl reaction mixture containing 60 mM HEPES (pH 7.9), 3 mM EDTA, 3 mM dithiothreitol, 150 mM KCl, 1 μg of bovine serum albumin, 12% (vol/vol) Ficoll, and 1.5 μg of poly(dI-dC) (Boehringer); 30,000 dpm of labeled oligonucleotide was added, and the mixture was loaded onto a 5% nondenaturing polyacrylamide gel equilibrated with 0.25× Tris-borate-EDTA (TBE) and electrophoresed for 4 to 6 h at 170 V at room temperature. The gels were then dried and visualized by autoradiography.
Flow cytometry analysis and p24 ELISA.
A total of 5 × 105 cells were sedimented by centrifugation, washed in PBS, and fixed with 3.5% formalin–PBS for 45 min at 4°C, and intracellular p24gag staining was performed with a FACSCalibur flow cytometer (Becton Dickinson) as previously described by Heinkelein et al. (18). For enzyme-linked immunosorbent assay (ELISA), culture supernatants were collected 24 to 120 h posttransfection and stored at −70°C. HIV-1 p24 antigen in culture supernatant was detected by the Abbott Laboratories HIVAG-1 enzyme immunoassay as specified by the manufacturer. The p24 concentration (in picograms per milliliter) was calculated from the Abbott Laboratories quantification ELISA. The cutoff value was an optical density at 420 nm of 0.063, which represents 240 pg of p24 per ml.
RESULTS
Plasma membrane-targeted RafΔ26–303 in T cells phosphorylates MEK in vitro.
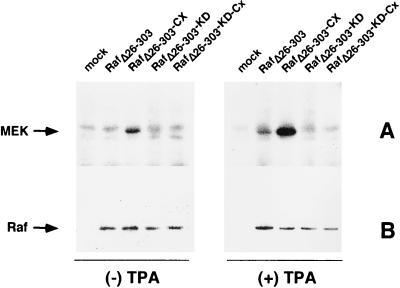
To detect the effects of Raf activation in A3.01 T cells, we first established a system to measure Raf kinase activity by using transient transfection with epitope-tagged (HA) versions of the catalytic domain of Raf (RafΔ26–303). In this experiment, we also measured the consequences of membrane targeting of RafΔ26–303 by fusing the C-terminal CAAX domain of Ki-Ras to this protein (RafΔ26–303-Cx). These C-terminal amino acids of Ras are sufficient to target a heterologous cytoplasmic protein to the plasma membrane (23, 37). Figure 1 shows that RafΔ26–303-Cx expression in T lymphocytes is catalytically active toward its substrate MEK in in vitro kinase assays. In contrast, RafΔ26–303 without the membrane-targeting signal has no detectable activity in the same cell system. Stimulation of transfected cells with phorbol ester induces enhanced kinase activity of both versions of RafΔ26–303. ATP-binding-site mutants (375W) of RafΔ26–303, either RafΔ26–303-KD or RafΔ26–303-KD-Cx, showed no kinase activity when used as a negative control (Fig. 1). These results indicate that only membrane-targeted RafΔ26–303 represents a constitutively active kinase in the A3.01 T-lymphocyte environment. We next investigated the functional consequences of this activity with regard to the mitogenic signaling cascade, NF-κB activity, and HIV-1 replication.
FIG. 1.
(A) RafΔ26–303-Cx phosphorylates MEK in an in vitro kinase assay. A3.01 T cells were transiently transfected with expression plasmids containing RafΔ26–303, RafΔ26–303-Cx, kinase-dead mutants (RafΔ26–303-KD, RafΔ26–303-KD-Cx) or with the empty expression vector as a control (mock). At 42 h posttransfection, cells were stimulated with TPA (25 ng/ml) for 20 min or left untreated. The cells were lysed in RIPA buffer, epitope-tagged Raf kinases were immunopurified with anti-HA (clone 12CA5) antibodies, and an in vitro kinase assay was performed with recombinant MEK as the substrate (for details, see Materials and Methods). Proteins were separated by SDS-PAGE and visualized by autoradiography. (B) Corresponding immunoblots demonstrating that equal amounts of the Raf kinase mutants are expressed in A3.01 T cells.
RafΔ26–303-Cx leads to phosphorylation of endogenous ERK in T cells.
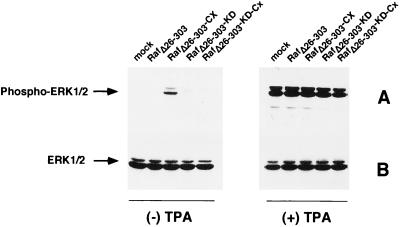
Western blot analysis of whole lysates of transfected A3.01 cells with a phospho-ERK-specific antibody demonstrates that 12-O-tetradecanoylphorbol-13-acetate (TPA) stimulation of these cells induces the phosphorylation of proteins with molecular sizes of 44 and 42 kDa (Fig. 2A, right side). By using Western blot analysis, these proteins were identified as ERK1 and ERK2, respectively (Fig. 2B, right side). Activation of endogenous ERKs by phorbol esters is in accordance with previously published data (39). Similar to our results on MEK phosphorylation, only RafΔ26–303-Cx is able to induce endogenous ERK1/2 activation in the absence of TPA stimulation (Fig. 2A, left side). Neither RafΔ26–303-KD-Cx, RafΔ26–303, nor RafΔ26–303-KD showed any catalytic activity. These findings demonstrate that activation of MEK by transfection of membrane-targeted RafΔ26–303 is transmitted to endogenous ERK.
FIG. 2.
(A) Overexpression of RafΔ26–303-Cx leads to phosphorylation of endogenous ERK1/2. A3.01 T cells were transfected with either RafΔ26–303, RafΔ26–303-Cx the corresponding kinase-dead mutant (RafΔ26–303-KD-Cx), or empty expression vector as a control (mock). Unstimulated or TPA-stimulated (25 ng/ml for 20 min) T cells were lysed in RIPA buffer 42 h after transfection, proteins were separated by SDS-PAGE, and immunoblots were prepared with phospho-ERK specific antibodies. (B) Corresponding immunoblots obtained with anti-panERK antiserum to demonstrate that there were equal amounts of protein kinase.
Expression of constitutively active, membrane-targeted RafΔ26–303 stimulates NF-κB activity in T cells.
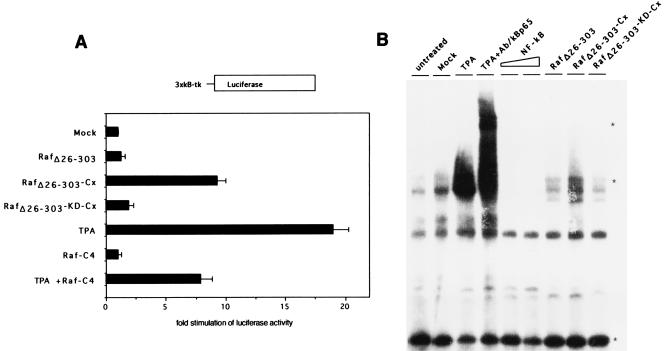
Previously, we and others have shown that active Raf transactivates κB-containing promoter elements in NIH 3T3 cells (8, 12). Since only membrane-targeted RafΔ26–303 is constitutively active in T cells, we investigated whether this catalytic activity is sufficient to trigger further downstream signaling effects, including NF-κB activation. Therefore, we used a 3×κB-luciferase reporter construct in transactivation assays. Figure 3A shows that stimulation with TPA resulted in an approximately 20-fold increase in luciferase gene expression compared to unstimulated A3.01 T cells. Transfection of RafΔ26–303-Cx alone, but not its kinase-dead mutant (RafΔ26–303-KD-Cx) or wild-type RafΔ26–303, transactivates a κB-dependent promoter ninefold over that shown for control transfected T cells. Moreover, expression of dominant-negative Raf-C4 reduced TPA-stimulated NF-κB activation by more than half. To further strengthen these results, we prepared nuclear extracts from untransfected or transfected A3.01 T cells and performed electrophoretic mobility shift assays with an NF-κB oligonucleotide as a probe. TPA stimulation for 30 min resulted in an increased binding of NF-κBp65 compared that for untreated, mock, unstimulated RafΔ26–303-KD-Cx- or RafΔ26–303-transfected cells (Fig. 3B). In the absence of TPA treatment, enhanced p65 binding to the NF-κB probe was apparent only in RafΔ26–303-Cx-transfected cells (Fig. 3B). The specificity of p65 binding was demonstrated by using excess unlabeled NF-κB probe as a competitor or by using p65-specific antibodies in supershift experiments (Fig. 3B). Taken together, these data demonstrate that RafΔ26–303-Cx is constitutively active and induces downstream signaling events in T cells, including ERK phosphorylation and transactivation of specific promoter elements.
FIG. 3.
Overexpression of constitutively active RafΔ26–303-Cx activates κB-dependent transcription. (A) A3.01 cells were cotransfected with 3×κB-tk luciferase construct together with either RafΔ26–303, RafΔ26–303-Cx, the corresponding kinase-dead mutant (RafΔ26–303-KD-Cx), dominant-negative Raf-C4, or empty expression vector as a control (mock). At 24 h posttransfection, luciferase assays were performed as described in Materials and Methods. Relative luciferase activities are based on the vector control (see Materials and Methods for details). A3.01 cells, stimulated with TPA (10 ng/ml) for 16 h or left untreated, were used as a positive control. (B) Overexpression of constitutively active RafΔ26–303-Cx stimulates enhanced binding of NF-κBp65 (lane RafΔ26–303-Cx). A3.01 T cells were untreated or transfected with different expression vectors as described for panel A, and electrophoretic mobility shift assays were performed with a radiolabelled κB oligonucleotide with 3-μg nuclear extracts as described in Materials and Methods. For competition assays, 10- and 100-fold molar excesses of unlabelled NF-κB oligonucleotides were added to the binding-reaction mixture (lanes NF-κB) and supershift experiments were performed with anti-p65 specific antiserum (lane TPA + Ab/κBp65). Free probe, NF-κBp65-containing protein complexes, and shifted NF-κBp65 complexes are indicated by asterisks.
Consequences of Raf activation for HIVNL4-3 promoter transactivation and viral replication.
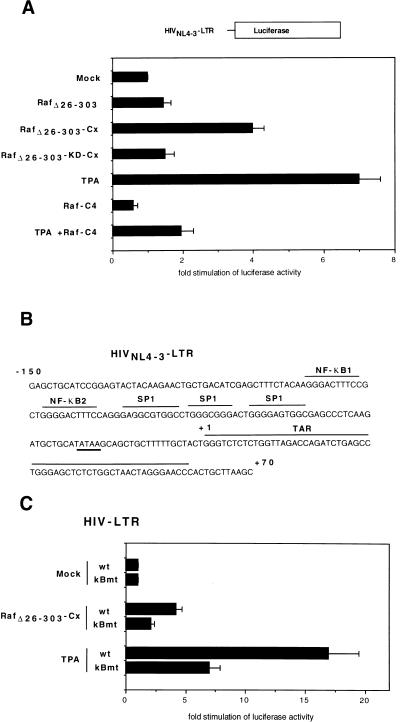
We next investigated how constitutively active Raf kinase acts on induction of the HIV-1 promoter in A3.01 T cells. To mimic the situation in HIVNL4-3-infected T lymphocytes, we used an amplified region of the HIVNL4-3 LTR spanning from nucleotides −150 to +70 including the TAT-responsive region and the NF-κB element (Fig. 4B) (19). Figure 4A shows that stimulation with phorbol ester resulted in a sevenfold stimulation of HIVNL4-3 LTR-driven gene expression compared to that in unstimulated T cells. Similar to the experiments investigating κB-dependent promoter activity, only RafΔ26–303-Cx was able to induce the transcriptional activity of the HIV-1 promotor (Fig. 4A). Transfections with RafΔ26–303-KD-Cx or RafΔ26–303 showed no detectable HIVNL4-3 LTR transactivation, and expression of dominant negative Raf-C4 (Fig. 4A) or dominant negative ERK (data not shown) impaired phorbol ester-stimulated HIV LTR activity. Interestingly, point mutations in both NF-κB sites of the HIV LTR reduced both the TPA and RafΔ26–303-Cx HIV LTR-driven gene expression (Fig. 4C). These data indicate that constitutively active RafΔ26–303-Cx stimulates HIV-1 promoter activity in A3.01 T cells and that functional NF-κB sites in the HIV LTR are important for this effect.
FIG. 4.
Overexpression of constitutively active RafΔ26–303-Cx is sufficient to activate HIVNL4-3 LTR-dependent transcription. (A) A3.01 cells were cotransfected with 0.4 μg of HIVNL4-3 LTR luciferase construct together with either RafΔ26–303, RafΔ26–303-Cx expression vectors, the corresponding kinase-dead mutant (RafΔ26–303-KD-Cx), Raf-C4, or empty expression vector as a control (mock). At 24 h posttransfection, the cells were harvested and luciferase assays were performed as described above. A3.01 cells, stimulated with TPA (10 ng/ml) for 16 h or left untreated, were used as a positive control. (B) Sequence of the HIVNL4-3 LTR spanning the region from nucleotides −150 to +70 of the HIVNL4-3 promoter. NF-κB, SP1 binding sites, and the Tat-responsive region (TAR) are indicated. (C) Point mutations in both NF-κB sites of the HIV LTR impaired RafΔ26–303-Cx as well as phorbol ester-stimulated HIV LTR transactivation. A3.01 cells were cotransfected with RafΔ26–303-Cx expression vectors together with either 0.4 μg of wild-type (wt) or κB-mutant (κBmt) HIV LTR luciferase constructs.
After establishing the effect of constitutively active Raf kinase on the transcriptional activity of the HIVNL4-3 LTR, we used two different molecular clones of HIVNL4-3 to study viral replication in the same cell environment. After transfection, viral production of either clone was demonstrated by intracytoplasmic staining with anti p24gag monoclonal antibodies, detection of p24 particles released into the supernatant, and syncytium formation. Titer determinations revealed that both intracytoplasmic p24gag production and release were dependent on the concentration of HIV-1 DNA used for transfection (data not shown). Time course experiments showed that p24gag synthesis correlated with HIV-1 DNA input for up to 48 h (data not shown). At later time points, viral replication dramatically increased, probably due to secondary infections by released viral particles. To test the effect of Raf kinase on HIV replication, we chose 0.25 and 0.5 μg of HIVNL4-3 DNA in time course experiments up to 42 h after transfection.
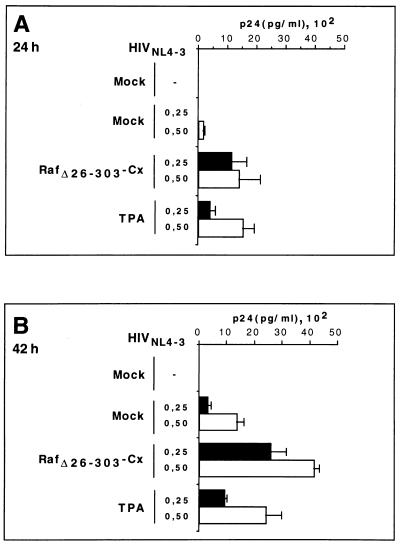
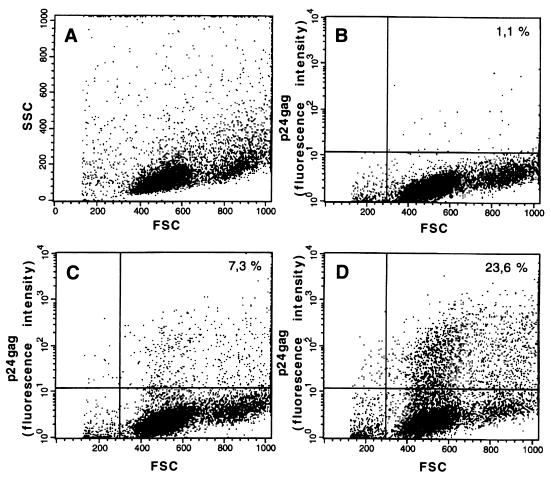
Stimulation of HIVNL4-3-transfected cells with phorbol ester induced significantly increased HIV replication over basal levels, as determined by measurement of the release of p24gag into the supernatant (Fig. 5). This effect was observed as early as 24 h after transfection when using both HIVNL4-3 DNA concentrations. In the absence of phorbol esters, only expression of RafΔ26–303-Cx enhanced HIVNL4-3 replication in cotransfected cells. This effect was both time and dose dependent, with an optimal p24 antigen production response up to 10-fold over basal levels (Fig. 5). At later time points, the effect of membrane-targeted Raf was less significant, most probably due to secondary infections (data not shown). At 64 h posttransfection, these results were confirmed by fluorescence-activated cell sorter flow cytometric experiments measuring intracytoplasmic p24gag expression (Fig. 6). These data clearly show that activation of the mitogenic pathway stimulates HIV-1 replication in infected CD4+ T cells.
FIG. 5.
Expression of RafΔ26–303-Cx triggers the release of virus into the supernatant. The amounts of p24 antigen detected in the tissue culture media at 24 h (A) and 42 h (B) posttransfection, using 0.25 μg of HIVNL4-3 DNA (solid boxes) and 0.5 μg HIVNL4-3 DNA (open boxes), are shown. A3.01 cells were cotransfected with background vector as a control (mock) or RafΔ22–303-Cx with or without HIVNL4-3 DNA. Stimulation (with 0.5 ng of TPA per ml) was used as a positive control. The results are the means and standard deviations of three independent experiments.
FIG. 6.
Flow cytometric analysis of intracellular HIVNL4-3 expression in A3.01 T cells. The dot plots show A3.01 T cells either cotransfected with background vector as a control (mock) or cotransfected with expression vector with or without 0.25 μg of HIVNL4-3 DNA as described above. Immunofluorescence of the A3.01 total T-cell population (A), mock-transfected cells (B), cells cotransfected with HIVNL4-3 (C), and cells cotransfected with HIVNL4-3 and RafΔ22–303-Cx (D) is shown. Numbers in the dot plots indicate percentage of p24-positive cells (FSC, forward scatter; SSC, right-angle scatter). Intracytoplasmic staining of cells with anti-p24 monoclonal antibodies was performed at 64 h posttransfection as described in Materials and Methods. The figure is representative of three independent experiments.
DISCUSSION
In this study, we investigated the functional consequences of Raf activation in T cells with regard to the classical mitogenic signaling cascade, stimulation of NF-κB activation, and HIV-1 replication. Truncated versions of Raf-1 that lack the N-terminal regulatory domain (RafΔ22–303) are inactive when expressed in Jurkat T cells (43). Additional signals like phorbol ester or anti-CD3 stimulation appear to be necessary for Raf kinase activation in this cell type. We overcame this cell-specific regulation by targeting RafΔ26–303 to the plasma membrane with the addition of the CAAX motif of K-Ras to the C terminus (RafΔ26–303-Cx). Thus, in contrast to the non-membrane-targeted RafΔ26–303, RafΔ26–303-Cx is constitutively active in A3.01 CD4+ T lymphocytes, mediating further downstream signaling via MEK to ERK. Moreover, RafΔ26–303-Cx effectively stimulates NF-κB as well as HIV-1 promoter-dependent reporter gene expression, leading to viral replication.
Membrane targeting of wild-type Raf by CAAX sequences (-Cx) results in activation of the kinase in COS cells (23, 26, 37). These experiments are based on findings that Raf is associated with the plasma membrane in cells expressing oncogenic Ras (23, 38, 42). Although GTP-Ras can associate with Raf at the plasma membrane, it remains controversial whether this interaction is sufficient to achieve maximal Raf activation. In our experiments, we have excluded the influence of Ras by deleting the regulatory domain of Raf, including the Ras-binding domain. The observed effects of this kinase are therefore independent of Ras and may be attributed to its cellular localization. Such regulation of Raf activity by subcellular localization was recently illustrated by studies in which Raf was targeted to the mitochondrial membrane by interaction with Bcl-2 (41). In our experiments, targeting Raf kinase domain to the plasma membrane appears to specifically trigger the MEK/ERK pathway, since neither the SAPK/JNK nor the p38 activating pathway was affected (data not shown). These observations support the notion that membrane targeting of Raf resembles the physiological situation in which Raf activation is achieved mainly at the plasma membrane. As discussed above, regulation of Raf-1 kinase activity in T cells differs from other cell types. The mechanism for this phenomenon remains to be determined. We propose that translocation of cytoplasmic RafΔ22–303 in T cells dissociates the kinase from cytoplasmic (down)regulating proteins, which are connected to downstream effectors like the nuclear shuttle protein ERK.
We and others have reported that Raf-1 stimulates NF-κB-dependent reporter gene expression in fibroblasts (8, 12). Furthermore, in situ immunofluorescence studies with NIH 3T3 cells have shown that the expression of active v-raf leads to elevated nuclear levels of the p65 subunit of NF-κB (24). In the present study, we demonstrate, using NF-κB-dependent reporter gene analysis and electrophoretic mobility shift assays, that NF-κB activity is induced in CD4+ T cells only by membrane-targeted RafΔ22–303, taking place under conditions where JNK/SAPK activity is unaffected. We observed that the binding of p65 in RafΔ22–303-Cx-transfected cells is considerably lower than that observed in TPA-stimulated cells. Whether this observation is due to the low transfection efficiency in T cells (approximately 20% [data not shown]) or whether active Raf is less efficient than TPA in stimulating NF-κB activity is not clear. Nevertheless, our data clearly show that targeting Raf-1 to the plasma membrane is sufficient to stimulate NF-κB promoter activity in T cells.
NF-κB activation is known to play a major role in HIV-1 transactivation as well as in replication (2, 16). Stimulation of NF-κB occurs through a variety of stimuli, which include signals through cytokine and growth factor receptors (3). Some of these cytokines, for example, interleukin-2 and tumor necrosis factor alpha, upregulate transcription of the HIV-1 provirus (11) and stimulate the mitogenic signaling cascade through Raf. Thus, activation of the Ras/Raf/MEK/ERK pathway may further contribute to the first round of transcription of HIV-1 provirus. To test this hypothesis, we investigated whether stimulation through Raf alone was sufficient to promote HIV transactivation and replication.
We demonstrate that membrane-targeted RafΔ22–303 is constitutively active with respect to HIV-1 promoter transactivation in T cells. Furthermore, as a specific activator of the mitogenic signaling cascade, RafΔ26–303-Cx also enhanced viral replication. The HIVNL4-3 molecular clone used in the experiments is well characterized and established as a model for the study of HIV replication in T cells (1). We used A3.01 CD4+ T cells for two reasons. First, a derivative line, ACH-2, which represents an established cell line carrying a latent HIV-1 provirus, which can be released upon phorbol ester or TNF stimulation, is available (31, 32). Second, these cells produce mature viral particles after transfection of HIV-1 DNA or infection. HIV-1 DNA titer determination and time course experiments were performed to define the baseline of p24gag release in our cell system. By using large amounts of HIV-1 DNA and/or long time points, HIVNL4-3 alone leads to the release of p24gag due to viral replication and secondary infections. By using phorbol ester as a known trigger of HIV-1 replication in T cells, viral release was significantly increased in a dose- and/or time-dependent fashion compared to basal p24gag levels, an effect which was detectable as soon as 24 h posttransfection. The laboratory strain HIVNL4-3 also replicates much more effectively in A3.01 T cells overexpressing the membrane-targeted version of RafΔ26–303. This observation indicates that induction of the mitogenic signaling pathway not only transactivates the HIV LTR but also enhances viral replication. We correlate this with ERK phosphorylation and NF-κB transactivation as indicators of Raf-1-induced signaling processes. Interestingly, a recent report has shown that reverse transcriptase activity is significantly greater in HIV-infected Jurkat T cells which have been stably transfected with RafΔ22–303 (33). Since RafΔ22–303 is silent with respect to ERK phosphorylation and induction of NF-κB activity in our cell environment, these data would suggest that Raf-induced increases in HIV replication are regulated in at least two ways depending on the cellular localization of the catalytic domain of the kinase. This is supported by previous data demonstrating that RafΔ22–303 targets GA-binding protein, an ets-like transcription factor, which binds the NF-κB element to transactivate the HIV-1 promotor in transfected NIH 3T3 cells (13).
In conclusion, our data demonstrate that the classical mitogenic signaling cascade plays an important role in NF-κB induction and HIV replication in T cells. Our findings also underline the importance of cellular localization for Raf kinase activity. Since CD4+ T cells are the predominant location of viral replication, studying T-cell-specific regulation of Raf kinase is crucial to define connections between signal transduction elements and HIV propagation.
ACKNOWLEDGMENTS
We thank Inge Euler-Koenig for providing excellent technical assistance; Manuela Schorn, Birgit Strobel, and Christiane Koehler for performing the p24 ELISA; and Manuela Schuler for purifying MEK. We thank Nancy Rice, Frank Kirchhoff, and Thomas Wirth for providing reagents. The following reagents were obtained from the NIH AIDS Research and Reference Reagent Program: A3.01 T-cell line, HIV-1p24 antibody (183-H12-5C) and pNL4-3 HIV expression plasmid. We are greatly indebted to Joseph Slupsky for all his contributions to the manuscript. We thank Hartmut Ohnimus and Robert Ehret for specific background questions and/or for flow cytometry. For critical reading of the manuscript, we thank Stephan Ludwig.
This work was supported by the Deutsche Forschungsgemeinschaft Sonderforschungsbereich 165 and DFG grant We 2023/2-1.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of aquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcami J, Lain de Lera T, Folgueira L, Pedraza M A, Jacque J M, Bachelerie F, Noriega A R, Hay R T, Gaynor R B. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 1995;14:1552–1560. doi: 10.1002/j.1460-2075.1995.tb07141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 5.Bell B, Sadowski I. Ras-responsiveness of the HIV-1 LTR requires RBF-1 and RBF-2 binding sites. Oncogene. 1996;13:2687–2697. [PubMed] [Google Scholar]
- 6.Blumer K J, Johnson G L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994;19:236–240. doi: 10.1016/0968-0004(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 7.Bruder J T, Heidecker G, Rapp U R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- 8.Bruder J T, Heidecker G, Tan T-H, Weske J C, Derse D, Rapp U R. Oncogene activation of HIV-LTR-driven expression via the NF-kappa B binding sites. Nucleic Acids Res. 1993;21:5229–5234. doi: 10.1093/nar/21.22.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daum G, Eisenmann-Tappe I, Fries H W, Troppmair J, Rapp U R. Ins and outs of raf kinases. Trends Biochem Sci. 1994;19:474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Dorn P L, DaSilva L, Matarano L, Derse D. Equine infectious anemia virus tat: insights into the structure, function, and evolution of lentivirus trans-activator proteins. J Virol. 1990;64:1616–1624. doi: 10.1128/jvi.64.4.1616-1624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauci A S. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 12.Finco T S, Baldwin A S. κB site-dependent induction of gene expression by diverse inducers of nuclear factor κB requires raf-1. J Biol Chem. 1993;268:17676–17679. [PubMed] [Google Scholar]
- 13.Flory E, Hoffmeyer A, Smola U, Rapp U R, Bruder J T. Raf-1 kinase targets GA-binding protein in transcriptional regulation of the human immunodeficiency virus type 1 promoter. J Virol. 1996;70:2260–2268. doi: 10.1128/jvi.70.4.2260-2268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folgueira L, Algeciras A, Macmorran W S, Bren G D, Paya C V. The Ras-Raf pathway is activated in human immunodeficiency virus-infected monocytes and participates in the activation of NF-κB. J Virol. 1996;70:2332–2338. doi: 10.1128/jvi.70.4.2332-2338.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. AIDS. 1992;6:347–363. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Grilli M, Chiu J J-S, Lenardo M J. NF-kB and Rel: participants in a multiform transcriptional regulatory system. Int Rev Cytol. 1993;143:1–61. doi: 10.1016/s0074-7696(08)61873-2. [DOI] [PubMed] [Google Scholar]
- 17.Heidecker G, Huleihel M, Cleveland J L, Kolch W, Beck T W, Lloyd P, Pawson T, Rapp U R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990;10:2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heinkelein M, Sopper S, Jassoy C. Contact of human immunodeficiency virus type 1-infected and uninfected CD4+ T lymphocytes is highly cytolytic for both cells. J Virol. 1995;69:6925–6931. doi: 10.1128/jvi.69.11.6925-6931.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirchhoff F, Greenough T C, Hamacher M, Sullivan J L, Desrosiers R C. Activity of human immunodeficiency virus type 1 promoter/TAR region and tat1 genes derived from individuals with different rates of disease progression. Virology. 1997;232:319–331. doi: 10.1006/viro.1997.8586. [DOI] [PubMed] [Google Scholar]
- 20.Kolch W, Heidecker G, Kochs G, Hummel R, Vahidi H, Mischak H, Finkenzeller G, Marmé D, Rapp U R. Protein kinase C alpha activates RAF-1 by direct phosphorylation. Nature. 1993;364:249–252. doi: 10.1038/364249a0. [DOI] [PubMed] [Google Scholar]
- 21.Kolch W, Heidecker G, Lloyd P, Rapp U R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991;349:426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- 22.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U R, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 23.Leevers S J, Paterson H, Marshall C J. Requirement of Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Sedivy J M. Raf-1 protein kinase activates the NF-kB transcription factor by dissociating the cytoplasmic NF-κB-IκB complex. Proc Natl Acad Sci USA. 1993;90:9247–9251. doi: 10.1073/pnas.90.20.9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh C, Romeo C, Seed B, Bruder J T, Rapp U, Rao A. Association of Raf with the CD3 δ and γ chains of the T cell receptor-CD3 complex. J Biol Chem. 1994;269:8817–8825. [PubMed] [Google Scholar]
- 26.Marais R, Light Y, Paterson H F, Marshall C J. Ras recruits Raf-1 to the plasma membrane for activation by tyrosine phosphorylation. EMBO J. 1995;14:3136–3145. doi: 10.1002/j.1460-2075.1995.tb07316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison D K. The complexity of Raf-1 regulation. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 28.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:711–713. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 29.Naumann U, Hoffmeyer A, Flory E, Rapp U R. Raf protein serine/threonine kinases. In: Marx F, editor. Protein phosphorylation. Weinheim, Germany: VCH Verlagsgesellschaft mbH; 1996. pp. 203–228. [Google Scholar]
- 30.Owaki H, Varma R, Gillis B, Bruder J T, Rapp U R, Davis L S, Geppert T D. Raf-1 is required for T cell IL2 production. EMBO J. 1993;12:4367–4373. doi: 10.1002/j.1460-2075.1993.tb06121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poli G, Kinter A, Justement J S, Kehrl J, Bressler P, Stanley S, Fauci A S. Tumor necrosis factor alpha functions in an autocrine manner in the induction of human immunodeficiency virus expression. Proc Natl Acad Sci USA. 1990;87:782–785. doi: 10.1073/pnas.87.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pomerantz R J, Trono D, Feinberg M B, Baltimore D. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell. 1990;61:1271–1276. doi: 10.1016/0092-8674(90)90691-7. [DOI] [PubMed] [Google Scholar]
- 33.Popik W, Pitha P. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapp U R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991;6:495–500. [PubMed] [Google Scholar]
- 35.Siegel J N, June C H, Yamada H, Rapp U R, Samelson L E. Rapid activation of C-Raf-1 after stimulation of the T-cell receptor or the muscarinic receptor type 1 in resting T cells. J Immunol. 1993;151:4116–4127. [PubMed] [Google Scholar]
- 36.Siegel J N, Klausner R D, Rapp U R, Samelson L E. T cell antigen receptor engagement stimulates c-raf phosphorylation and induces c-raf-associated kinase activity via a protein kinase C-dependent pathway. J Biol Chem. 1990;265:18472–18480. [PubMed] [Google Scholar]
- 37.Stokoe D, Macdonald S G, Cadwallader K, Symons M, Hancock J F. Activation of raf as a result of recruitment to the plasma membrane. Science. 1994;264:1463–1467. doi: 10.1126/science.7811320. [DOI] [PubMed] [Google Scholar]
- 38.Traverse S, Cohen P, Paterson H, Marshall C, Rapp U, Grand R J. Specific association of activated MAP kinase kinase kinase (Raf) with the plasma membranes of ras-transformed retinal cells. Oncogene. 1993;8:3175–3181. [PubMed] [Google Scholar]
- 39.Troppmair J, Bruder J T, Munoz H, Lloyd P A, Kyriakis J, Banerjee P, Avruch J, Rapp U R. Mitogen-activated protein kinase/extracellular signal-regulated protein kinase activation by oncogenes, serum, and 12-O-tetradecanoylphorbol-13-acetate requires Raf and is necessary for transformation. J Biol Chem. 1994;269:7030–7035. [PubMed] [Google Scholar]
- 40.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;47:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 41.Wang H-G, Rapp U R, Reed J C. Bcl-2 targets the protein kinase Raf-1 to mitochondria. Cell. 1996;87:629–638. doi: 10.1016/s0092-8674(00)81383-5. [DOI] [PubMed] [Google Scholar]
- 42.Wartmann M, Davis R J. The native structure of the activated Raf protein kinase is a membrane-bound multi-subunit complex. J Biol Chem. 1994;269:6695–6701. [PubMed] [Google Scholar]
- 43.Whitehurst C E, Owaki H, Bruder J T, Rapp U R, Geppert T D. The MEK kinase activity of the catalytic domain of RAF-1 is regulated independently of RAS binding in T cells. J Biol Chem. 1995;270:5594–5599. doi: 10.1074/jbc.270.10.5594. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X F, Settleman J, Kyriakis J M, Takeuchi-Suzuki E, Elledge S J, Marshall M S, Bruder J T, Rapp U R, Avruch J. Normal and oncogenic p21ras proteins bind to the amino-terminal regulatory domain of c-Raf-1. Nature. 1993;364:308–313. doi: 10.1038/364308a0. [DOI] [PubMed] [Google Scholar]