Abstract
Background
Vedolizumab has since 2021 been available as a subcutaneous formulation. We aimed to assess 18-month drug persistence and possible predictive factors associated with discontinuation, safety, serum drug profile, drug dosing, and disease activity in a real-world cohort of patients with inflammatory bowel disease switched from intravenous to subcutaneous vedolizumab maintenance treatment.
Methods
Eligible patients were switched to subcutaneous vedolizumab and followed for 18 months or until discontinuation of subcutaneous treatment. Data on preferred route of administration, adverse events, drug dosing, serum-vedolizumab, disease activity, fecal calprotectin, and C-reactive protein were collected. Persistence was described using Kaplan–Meier analysis. The impact of clinical and biochemical variables on persistence was analyzed with Cox proportional hazard models.
Results
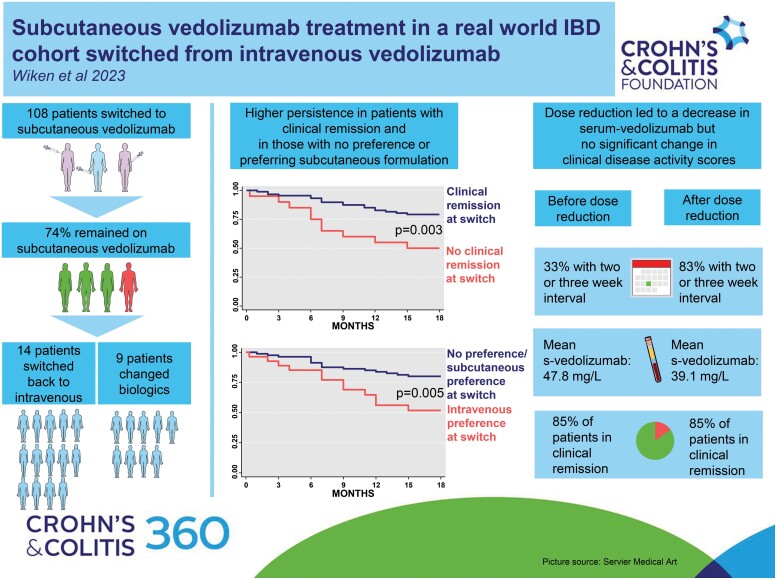
We included 108 patients, and the estimated 18-month drug persistence was 73.6% (95% CI [64.2–80.1]). Patients in clinical remission at switch were less likely to discontinue SC treatment (HR = 0.34, 95% CI [0.16–0.73], P = .006), and patients favoring intravenous treatment at switch were almost 3 times more likely to discontinue (HR = 2.78, 95% CI [1.31–5.90], P = .008). Four patients discontinued subcutaneous vedolizumab due to injection site reactions. At 18 months, 88% of patients administered subcutaneous vedolizumab with an interval of ≥ 14 days, and serum-vedolizumab was 39.1 mg/L. Disease activity was stable during follow-up.
Conclusions
Three of the four patients remained on subcutaneous vedolizumab after 18 months, a large proportion received treatment at standard dosing intervals, and disease activity remained stable. This indicates that switching from intravenous to subcutaneous vedolizumab treatment is convenient and safe.
Keywords: vedolizumab, subcutaneous, therapeutic drug monitoring, Crohn’s disease, ulcerative colitis
Graphical Abstract
Graphical Abstract.
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC), and Crohn’s disease (CD), is a chronic inflammatory disease of the gastrointestinal tract. As no cure is available for IBD, many patients need continuous medical treatment and the proportion of patients on biological drugs has been steadily increasing.1–3
Vedolizumab (VDZ), a humanized monoclonal IgG1-antibody, is one of the biological drugs used in moderate to severe IBD. VDZ was first introduced for intravenous (IV) administration. Since 2021, VDZ has been available for subcutaneous (SC) administration in Norway, after approval by the European Commission in May 2020.4–8 The US Food and Drug Administration (FDA) approved SC VDZ in September 2023.9 Patients seem to prefer SC to IV treatment, mainly relates to the convenience and independency of self-administration including fewer hospital visits.10–12 Additionally, SC administration may reduce direct as well as indirect costs in the health-care system.13,14
The VISIBLE 1 and VISIBLE 2 phase 3 clinical trials examined SC VDZ in UC and CD patients, respectively, and demonstrated that SC VDZ is both effective and safe in VDZ naïve IBD patients.4,5 Patients already established on IV maintenance treatment were not included in these studies. Data on switching from IV to SC VDZ maintenance treatment have not been available until four recent studies with up to 6 months of follow-up reported that such switching is safe and feasible.15–18 In these studies, the proportions of patients remaining on SC VDZ at 6 months ranged from 88.1% to 95.5%. So far, no studies with prolonged observation time have been published.
There are no established recommendations for therapeutic drug monitoring (TDM) in VDZ treatment, and the importance of strictly defined target serum levels on SC treatment is not clarified.19,20 The VISIBLE 1 trial demonstrated that patients on IV VDZ treatment with higher serum-VDZ (s-VDZ) levels had higher rates of clinical remission.4 This observation was not as clear in the SC treatment group. Nevertheless, a recent real-world study on patients switching from IV to SC VDZ demonstrated that the proportion of patients in clinical and biochemical remission was significantly higher with increasing s-VDZ levels.16 In Norway, s-VDZ concentration measurements are available at low cost and widely used in clinical practice, allowing real-world evaluation of a TDM approach.
A recent multi-stakeholder position statement regarding the use of SC VDZ, concluded that most stakeholders welcomed SC VDZ.21 However, according to this statement, more data were requested to be able to identify the right patients for switching. Additionally, SC VDZ is reported to be safe with few adverse events (AEs).4,5,15–18 Yet, recently published reports disclosed that some patients with AEs on SC VDZ, experienced AEs also when switching back to IV treatment.22,23 Thus, further safety data when switching back to IV VDZ are needed.
The main aim of the present study was to assess 18-month drug persistence including identification of possible predictive factors associated with discontinuation in a real-world cohort of IBD patients switched from IV to SC VDZ maintenance treatment. Secondary aim was to study clinical and biochemical disease activity, s-VDZ-levels, changes in drug dosing, and safety of switching back to IV treatment during the follow-up period.
Materials and Methods
Study Design and Population
This is a prospective single-center study conducted at the Department of Gastroenterology, Oslo, Norway. Between February 15th and June 3rd, 2021, adult IBD patients (>18 years) on IV VDZ maintenance treatment were switched to SC VDZ. Data were collected prospectively and included in the locally approved IBD registry at the department (PVO 19/02408). Patients had signed a broad consent for inclusion in the IBD registry, and the project protocol was approved by the data protection officer at the hospital (PVO 21/00119). Results from the first 6 months of follow-up of this study have been published previously.15
All patients on established IV VDZ treatment (more than 6 months of treatment) were eligible for switching to SC, regardless of disease activity or preference. Exclusion criteria were planned surgery within the next 3 months, planned change of IBD treatment, ongoing IBD relapse requiring corticosteroids (active disease without the need for intervention was not an exclusion criterion), or ongoing investigations for other significant diseases.
Patients were followed prospectively with visits at switch (week 0), at time of their fourth injection (median 6 weeks, range 3 to 9 weeks), at 3 months, and then every 3 months. This is in accordance with the standard visit schedule for patients on biologic SC treatment followed at the department. Data were collected at switch, 6 (+/−2), 12 (+/−2), and 18 range (+/−3) months after switch. Baseline data included demographic data, disease phenotype according to the Montreal classification, previous surgeries and previous medical treatment including line of targeted drugs, current medical treatment (concomitant use of immunosuppressives, steroids, and if combining several biologics), and patient preference of treatment administration (no preference, preference to SC or to IV) acquired by questionnaire. Follow-up data included injection intervals, laboratory data (C-reactive protein [CRP], ferritin, fecal calprotectin [FC], and serum-VDZ [s-VDZ]), disease activity assessed by Harvey Bradshaw Index (HBI) for CD and Partial Mayo Score without physician's assessment (PMS) for UC, injection site reactions (ISRs) and reasons for discontinuation of SC treatment.
Algorithm for SC Dosing and Adjustment
At our department, patients on IV VDZ were followed with a proactive treatment approach with the aim of a s-VDZ level > 20 mg/L.24 Thus, patients received infusions with 300 mg VDZ at treatment intervals ranging from 4 to 12 weeks before the switch. To continue individual dosing also on SC VDZ, patients were switched to treatment intervals on SC VDZ according to an arithmetical algorithm developed at our hospital assuming that standard IV dosage of 300 mg every eighth week equaled standard SC dosing of 108 mg every second week. As a result, patients receiving IV VDZ every 4 weeks were converted to weekly SC VDZ injections, whereas patients receiving IV VDZ every 12 weeks were converted to SC injections every 3 weeks. The IV intervals ranged from 4 to 12 weeks. Consequently, the SC intervals based on this algorithm ranged from every 7th to 21st day. More details on this conversion from IV to SC VDZ have been reported elsewhere.15
During the first 6 months after switch, patients remained on the algorithm-based intervals. From 6 months after switch, we intended to convert dosing intervals to full week intervals (every 7th, 14th, 21st, or 28th day) whilst obtaining s-VDZ concentrations between 25 and 45 mg/L. This s-VDZ interval was chosen based on the pharmacokinetic characteristics of SC VDZ presented in the VISIBLE trials.4,5
Measurement of s-VDZ and Antibodies
S-VDZ was measured with a validated 3-step immunofluorometric method performed in streptavidin-coated 96-well microplates. The assay utilizes 2 in-house generated anti-VDZ monoclonal antibodies (Mabs D130, D136.2) and is fully automated on the AutoDELFIA (PerkinElmer, Waltham, MA, USA) immunoassay platform. In the method, biotinylated F(abʹ)2 fragments of Mab D130 are used to trap s-VDZ onto the streptavidin microwell surface. After a wash step, captured drug is then quantified using europium-labeled Mab D136.2 and time-resolved fluorometry (manuscript in preparation). Antibodies were not measured.
Study Outcomes and Definitions
The primary outcome was SC persistence defined as the cumulative incidence of patients remaining on SC VDZ from the time of switch and to the end of follow-up. Possible predictive factors associated with discontinuation of SC VDZ were assessed at switch and included patient characteristics, patient preference, and clinical and biochemical disease activity and remission. Clinical disease activity was assessed using HBI for CD and PMS for UC. Biochemical disease activity was assessed using CRP and/or FC. Clinical remission was defined as HBI ≤ 4 for CD patients and PMS ≤ 1 for UC patients. Biochemical remission was defined as CRP < 5 mg/L and FC < 250 mg/kg. FC was included if the sampling date was +/−2 months from the date of follow-up. Combined remission was defined as both clinical and biochemical remission.
Secondary outcomes were changes in clinical and biochemical disease activity during follow-up, changes in s-VDZ and VDZ dosing during follow-up, and finally safety of switching back to IV formulation.
Statistical Analyses
Number of patients on IV VDZ treatment at the department eligible for switch determined the sample size. Continuous data are presented as mean (standard deviation [SD]) when normally distributed or median (range or interquartile range [IQR]) for variables with skewed distribution. Categorical data are presented as counts and percentages. Pairs of categorical data were compared using the Chi-squared test or Fisher’s exact test when appropriate. Continuous variables measured at 2-time points on the same individuals were compared using paired samples t-test. Changes over time after drug switch were analyzed using linear mixed models for repeated measures. The effect of time was estimated as a fixed effect, and gender and age were included in the regression model as possible confounders. For the main analysis, all patients were included. In addition, we performed sensitivity analyses on patients who had data for the entire follow-up, eg, no missing data as missing data can be considered as informative censoring in this sample. When the whole follow-up was considered, the overall effect of a given covariate on the outcome was expressed using the P-values from the F-test. In addition, the results are presented as estimated marginal means with 95% confidence intervals.
To obtain the SC VDZ persistence curves, the cumulative incidences were depicted using the Kaplan–Meier method and groups were compared with the log-rank test. The event was defined as discontinuation of SC VDZ. Follow-up was defined as the time from switch to the date of discontinuation (in months) or study end (18 months), whichever occurred first. Patients lost to follow-up were censored according to survival type of analysis principles. Hazard ratios (HR) for discontinuation were estimated using univariate and multivariate Cox regression models. Competing risk analysis was used to depict cumulative incidence of termination of SC VDZ due to switching back to IV VDZ (main event) when considering all other causes for discontinuation (competing event). Sensitivity and specificity of s-VDZ cutoff values at all time points (switch, 6, 12, and 18 months) to predict outcomes (defined as clinical, biochemical, and combined remission) at all time points were assessed using receiver-operating characteristic curves. As the study was considered exploratory, P-values < 0.05 were considered statistically significant and no correction for multiple testing was performed. All analyses were conducted using SPSS version 26 (SPSS Inc, Chicago, IL, USA) or Stata version 17 (StataCorp LLC, TX, USA).
Ethics
The study was approved by the local data protection officer (PVO 21/00119), based on a written broad informed consent given by the participants. Except for the questionnaires, only data from standard clinical follow-up were included in the study database.
Results
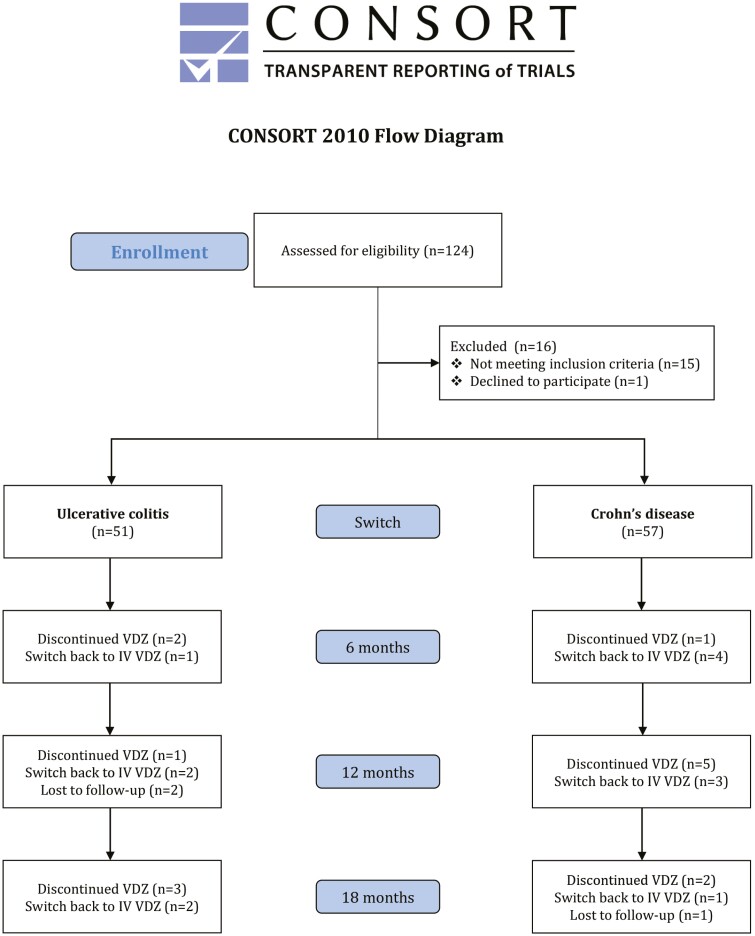
In total, 124 patients were assessed for eligibility, of which 108 (57 CD and 51 UC) were switched (Figure 1). Median time on IV VDZ treatment before switch was 3.8 years (range 0.6–10.3). The majority (93.5%, n = 101) had received one or more biologics before initiating VDZ, and 53.7% (n = 58) had experience with SC treatment from other biologics. Before the first SC VDZ injection, 25.3% (n = 27) preferred IV VDZ, 28.0% (n = 30) preferred SC VDZ, whereas 46.7% (n = 50) did not have any preference. There was no difference in disease activity at switch between those preferring IV and those preferring SC or having no preference (Supplementary Table S1). Detailed patient characteristics have been reported previously.15
Figure 1.
CONSORT flow diagram of the patient cohort at time of switch, at 6, 12, and 18 months follow-up. VDZ vedolizumab; SC subcutaneous; IV intravenous.
Treatment Persistence, Safety, and Patient Outcomes
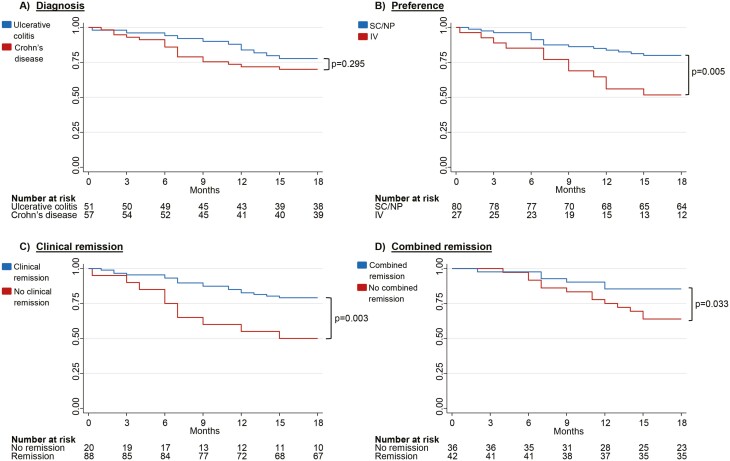
Within 18 months, 73.6% (95% CI [64.2–80.1]) remained on SC VDZ; 70.1% (95% CI [56.3–80.2]) in the CD group and 77.8% (95% CI [63.4–87.0]) in the UC group (Figure 2). Three patients moved and were censored due to loss of follow-up. Fourteen patients (13%; 9 CD, 5 UC) switched back to IV treatment due to either ISRs (n = 4), preference/discomfort with injections (n = 8), or low s-VDZ (n = 2, one patient due to incorrect injection technique and one patient unexplained). Amongst patients switching back to IV, median time to discontinuation was 7 months (range 2–15), and the majority (11/14 patients) switched back during the first 9 months (cumulative incidence 10.0% (95% CI [5.0–16.1]), Supplementary Figure S1). Half (7/14) of the patients who switched back to IV VDZ, favored IV at switch.
Figure 2.
Kaplan Meier Survival Curves for continuing subcutaneous (SC) vedolizumab (VDZ) with 18-month follow-up. The Y-axis depicts the cumulative probability of continuing on SC VDZ. The X-axis depicts time from switch to 18 months. (A) Kaplan–Meier drug persistence curve stratified by diagnosis. (B) Kaplan–Meier drug persistence curve stratified by preference at switch (no preference or SC preference versus IV preference) (C) Kaplan–Meier drug persistence curve stratified by clinical remission and no clinical remission at switch. Clinical remission is defined as Harvey Bradshaw Index ≤ 4 for Crohn’s disease and Partial Mayo Score without physician's assessment ≤ 1 for Ulcerative colitis. D) Kaplan Meier drug persistence curve stratified by combined remission and no combined remission at switch. Combined remission is defined as clinical remission and C-reactive protein < 5 mg/L and Fecal Calprotectin < 250 mg/kg. SC, subcutaneous; NP, No preference; IV, intravenous.
Three out of four patients with ISRs at injection site on SC VDZ developed erythema, pruritus, and swelling at the previous injection site after switching back to IV, in one case leading to discontinuation of IV VDZ due to an additional development of acute generalized infusion reaction (Table 1). In the 7 patients who switched back to IV VDZ due to preference or discomfort with injections, and without ISRs on SC treatment, the switch was successful without any recorded AEs.
Table 1.
Characteristics of patients who discontinued subcutaneous vedolizumab due to injection site reactions and switched back to intravenous treatment.
| IBD type | Montreal classification | Previous treatments | Duration of IV VDZ treatment (months) |
Duration of SC VDZ treatment (months) |
Type of reaction to SC injection | Type of reaction to IV injection after switch back | S-VDZ at first infusion with reactions(mg/L) | VDZ discontinuation |
|---|---|---|---|---|---|---|---|---|
| CD | L2B1 | Azathioprine Infliximab |
25 | 2 | Erythema, swelling and pruritus at injection site | Erythema, swelling and pruritus at former injection site the first 2 infusions | 30.2 | No |
| CD | L3B3 | Azathioprine Methotrexate Adalimumab Infliximab |
49 | 4 | Pruritus at injection site | None | 30.9 | No |
| UC | E3 | 5-ASA Golimumab Infliximab |
53 | 3 | Erythema, swelling and pruritus at injection site | Erythema, swelling and pruritus at former injection site first 2 infusions Rhinorrhoea, pruritus in the throat and red eyes shortly after third and fourth infusion, and during the fifth infusion. pretreatment IV hydrocortisone without effect |
41.6 | Yes |
| UC | E3 | 5-ASA Azathioprine Infliximab |
32 | 6 | Erythema, swelling and pruritus at injection site | Erythema, swelling and pruritus at former injection site at all infusions after switch, decreasing severity but ongoing 18 months after switch | 26.9 | No |
Abbreviations: IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; Montreal classification (CD): L1 ileum, L2 colon, L3 ileocolon, B1 non-structuring non-penetrating, B3 penetrating, (UC): E3 involvement proximal to the splenic flexure; IV, intravenous; VDZ, vedolizumab; SC, subcutaneous; S-VDZ, serum-vedolizumab.
Ten patients (9%; 7 CD, 3 UC) changed treatment to either ustekinumab (n = 9) or filgotinib (n = 1) due to secondary loss of response (LOR) to VDZ (Supplementary Table S2). Median time to change of treatment was 7.5 months (range 1–15, Supplementary Figure S1). Half of the changes occurred within the first 6 months (one patient after 1 month, 4 patients after 6 months).
Four patients stopped using VDZ due to either remission (n = 1), no need for combination treatment with infliximab (n = 1), surgery (n = 1), or a recurrent Clostridium difficile infection (n = 1).
There was no change in disease activity scores throughout the 18-month follow-up, neither for CD nor for UC patients with median HBI of 2 (range 0–16) and median PMS of 0 (range 0–3) at switch, respectively, and median HBI of 2 (range 0–11) and median PMS of 0 (range 0–2) at 18 months, respectively. CRP, Ferritin, and FC did not show any significant changes over time, and these findings were confirmed by sensitivity analysis when comparing all 108 patients and the 77 patients followed for 18 months exclusively (Supplementary Table S3).
Risk for Discontinuation of SC VDZ
In univariate analysis, patients in clinical remission at switch had significantly lower risk for discontinuation compared to patients with clinical disease activity at switch (HR = 0.34, 95% CI [0.16–0.73], P = 0.006). There was an increased risk for discontinuation with higher clinical scores at switch (HR = 1.12, 95% CI [1.01–1.26], P = 0.039 (HBI) and HR = 1.92, 95% CI [1.12–3.29], P = 0.017 [PMS]; Table 2). CRP assessed at switch was not associated with any risk for discontinuation, whereas patients with FC ≥ 250 mg/kg at switch had almost a 3-fold increased risk for discontinuation (HR = 2.76, 95% CI [1.09–7.02], P = 0.033). Patients in combined clinical and biochemical remission at switch had a 60% lower risk for discontinuation (HR = 0.39, 95% CI [0.14–0.97], P = 0.043). Furthermore, higher VDZ dosage (mg/day) was associated with a higher risk for discontinuation, and an increase of 1mg per day increased the risk by 20% (HR = 1.20, 95% CI [1.05–1.37], P = 0.009). Patients favoring IV treatment were almost 3 times more likely to discontinue SC treatment (HR = 2.78, 95% CI [1.31–5.90], P = 0.008) than those with no preference or preferring SC at switch. Duration of IV treatment, co-medication, and line of treatment had no impact on SC VDZ persistence. In the multivariate analysis when adjusted for clinical remission, FC (as a categorical variable), SC VDZ dose (mg/day) and preference, clinical remission, and IV preference remained independent predictive factors for discontinuation (Table 2).
Table 2.
Regression analysis showing univariate and multivariate associations between discontinuation of subcutaneous vedolizumab and covariates at switch
| Univariate | |||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Age at switch | 0.99 | 0.98–1.02 | .984 |
| Diagnosis | 1.5 | 0.70–3.18 | .303 |
| Harvey Bradshaw Index, (0–16) | 1.12 | 1.01–1.26 | .039 |
| Partial Mayo Score, (0–3) | 1.92 | 1.12–3.29 | .017 |
| Fecal Calprotectin ≥ 250 mg/kg | 2.76 | 1.08–7.02 | .033 |
| C-reactive protein ≥ 5.0 mg/L | 1.23 | 0.50–3.04 | .649 |
| Serum vedolizumab | 1.02 | 0.96–1.09 | .521 |
| Subcutaneous VDZ dose (mg/day) | 1.20 | 1.05–1.37 | .009 |
| Clinical remissiona | 0.34 | 0.16–0.73 | .006 |
| Biochemical remissionb | 0.43 | 0.17–1.06 | .067 |
| Combined remissionc | 0.39 | 0.14–0.97 | .043 |
| Preference No preference/subcutaneous (reference) |
1 | - | - |
| Intravenous | 2.78 | 1.31–5.90 | .008 |
| Multivariate | |||
| Clinical remission | 0.27 | 0.09–0.81 | .019 |
| Fecal Calprotectin ≥ 250 mg/kg | 2.97 | 0.72–12.23 | .131 |
| Subcutaneous VDZ dose (mg/day) | 0.96 | 0.76–1.23 | .772 |
| Intravenous preference | 3.84 | 1.46–10.06 | .006 |
Italic numbers indicate statistical significance.
aPartial Mayo Score without physician's assessment ≤ 1 for Ulcerative colitis and Harvey Bradshaw Index ≤ 4 for Crohn’s disease.
bC-reactive protein < 5 mg/L and Fecal Calprotectin <250 mg/kg.
cClinical and biochemical remission.
Serum VDZ and Treatment Interval
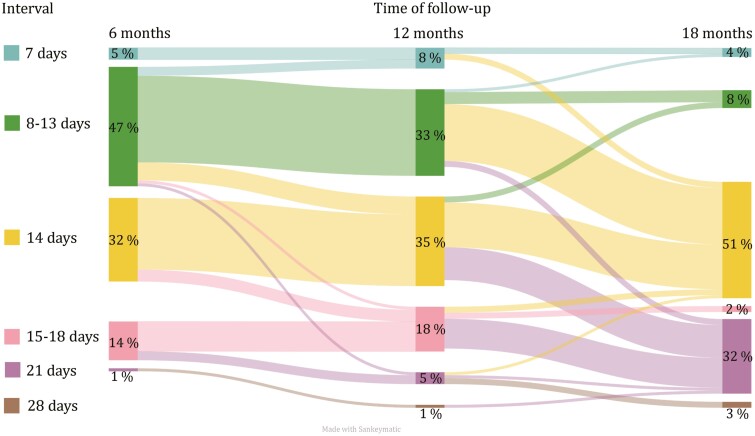
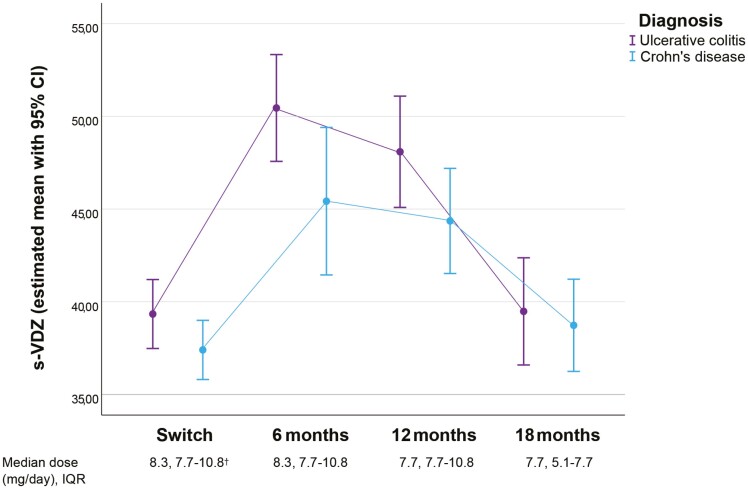
During the 18 months follow-up period after switch, 61 patients underwent dose adjustments (Figure 4). Of these, 41 patients (67.2%) adjusted the interval once during the 18-month follow-up, 17 patients (25%) had 2 adjustments and 3 patients (5%) had 3 adjustments. In total, 83 adjustments were implemented and 88% (n = 73) of these adjustments represented increased intervals due to either high s-VDZ and/or the intention to administer injections in full-week intervals. The remaining 12% of the changes were related to a decrease in interval length. The adjustments resulted in a statistically significant VDZ dose reduction from median of 8.3 mg/day (IQR 7.7–10.8) at 6 months to 7.7 mg/day (IQR 5.1–7.7) at 18 months. Mean s-VDZ decreased from 47.8 mg/L (95% [45.3–50.3]) at 6 months to 39.1 mg/L (95% CI [37.2–41.0]) at 18 months (P < 0.001). Estimated s-VDZ means for CD and UC are depicted in Figure 3. At 18 months, 90% of the patients used SC VDZ in full-week intervals; every 7th (4%), 14th (51%), 21st (32%), or 28th (3%) day (Figure 4). Receiver-operating characteristic curve analysis did not reveal any acceptable level of sensitivity and specificity for s-VDZ cutoff values regarding any of the investigated outcomes (data not shown).
Figure 4.
Distribution of dosing intervals at 6, 12, and 18 months follow-up. The diagram demonstrates the flow of patients who changed intervals between the different time-points. Intervals displayed are 7, 8–13, 14, 15–20, 21, and 28 days. Percentages describe proportion of patients in each group during the 3 time points.
Figure 3.
Serum-vedolizumab from switch to 18 months of follow-up and median s-vedolizumab dose. S-VDZ s-vedolizumab. IQR inter quartile range. †dose at switch calculated on planned interval during the first month after switch.
Discussion
In this real-world cohort study of IBD patients switching from IV to SC VDZ treatment with 18 months follow-up, 3 out of 4 patients remained on SC VDZ. Higher disease activity and preference for IV administration at switch were associated with reduced drug persistence. Except for 1 case, switch back to IV did not result in discontinuation of VDZ treatment. Furthermore, a dose reduction from 8.3 mg/day at 6 months to 7.7 mg/day at 18 months followed by a corresponding decrease in serum drug levels did not result in any change in disease activity scores or biochemical markers. After 18 months, 88% of the patients had a dosing interval of 2 or more weeks.
In the 2 previous randomized clinical trials on SC VDZ (VISIBLE 1 and VISIBLE 2), discontinuation rates were 27.4% (UC) and 38.9% (CD) within 52 weeks after induction of VDZ treatment.4,5 In our cohort, 22.3% (UC) and 30.0% (CD) discontinued SC VDZ within 18 months. In the VISIBLE trials, only patients with clinical response after 2 infusions were continued on to treatment with SC VDZ. In our study, patients were on long-term maintenance treatment at the time of switch, and discontinuation was mainly caused by a switch back to IV (14/28) or LOR to VDZ (10/28). Amiot et al studied treatment persistence in 294 IBD patients treated with IV VDZ induction therapy and found that persistence on IV VDZ after 3 years was 71.3% (UC) and 57.1% (CD) in patients still on VDZ 12 months after induction.25 Furthermore, the LOR rate decreased the longer patients were treated with VDZ. Our patients were on established IV VDZ treatment with a median duration of 3.8 years before switch, and to the best of our knowledge, no studies have examined VDZ persistence in a comparable cohort. A LOR rate of 9%; however, suggests that this rate is independent of the type of administration.
In the VISIBLE trials, treatment response was evaluated by a decrease in total Mayo score for UC patients and CD Activity Index (CDAI) for patients with CD.4,5 In the present study, SC VDZ persistence was significantly higher in patients with lower clinical disease activity scores, and patients in clinical and both clinical and biochemical remission were more likely to continue SC VDZ treatment throughout follow-up. Our findings suggest that patients in remission assessed by clinical disease activity scores and biochemical markers can be switched safely to SC VDZ.
In the present study, patients were switched to individual treatment intervals of SC VDZ. At switch, all patients were followed by TDM with an intention of keeping IV VDZ serum levels > 20 mg/L. The dosing of SC VDZ at switch was calculated to maintain treatment comparable to the IV dosing, and we have previously reported an increase in s-VDZ levels from 6 months before switch to 6 months after switch.15 The recorded mean s-VDZ of 48.2 mg/L 6 months after switch is high compared to other real-world studies, suggesting that our initial dosing of SC VDZ maintained or even increased VDZ exposure in our patients.16,17 It might thus be questioned whether the dosing intervals at switch could have been longer. Furthermore, as the majority of patients (61%) had an inconvenient injection interval (interval not in full weeks) at 6 months and the median s-VDZ levels were high, we intended to reduce dosage by extending injection intervals to full weeks. At 18 months, only 12% of the patients received a SC VDZ dose higher than the recommended standard dosage with injections every second week.
Even though median s-VDZ decreased after extending injection intervals, we observed no significant changes in disease activity scores and biochemical markers of inflammation. This suggests that patients planning to switch from IV to SC treatment may switch safely to the standard dosage of SC VDZ every second week regardless of previous IV treatment intervals. Concerning intervals, the proportion of patients in clinical remission increased with increasing s-VDZ in the VISIBLE trials, and Volkers et al found higher biochemical remission rates with s-VDZ > 37.0 mg/L. In our cohort, however, no such cutoff values for s-VDZ were disclosed, which might be explained by the high s-VDZ levels. Although not being able to disclose any s-VDZ target levels, the TDM approach with monitoring of s-VDZ in our patients regularly, allowed us to decrease VDZ dosage and thus reduced direct drug costs.
Safety concerns have been raised regarding switch back to IV once being established on SC treatment.22,23 In the present study, 4 patients switched back to IV due to ISRs, and 3 of these patients experienced AEs at consecutive infusions, 1 case leading to discontinuation of VDZ. Our observations are consistent with observations made by Volkers et al, where 1 out of 4 patients switching back to IV due to ISRs had to discontinue VDZ. However, in a retrospective case series by Richard et al, almost 60% of patients (4/7) with ISRs while on SC VDZ discontinued IV VDZ due to development of AEs on IV treatment. Further studies with larger numbers are therefore needed to assess safety of alternating between IV and SC formulations of VDZ.
Van Deen et al found that current and past modes of drug administration were strong predictors of preference.26 In our cohort, 50 patients (46.3%) had only experience with IV medication, and IV preference at switch was associated with higher risk for discontinuation of SC VDZ. We have previously reported a change in preference at switch to 6-month follow-up favoring SC formulation, suggesting that preference can change when gaining positive experience with the new formulation.15 Nevertheless, patients’ preferences should be acknowledged when implementing a change in formulation.
Limitations
This study has some limitations. We did not include an IV reference cohort, which would have allowed us to compare persistence between SC and IV VDZ. This would have been interesting, especially concerning secondary LOR and LOR rates. Another limitation is that we did not include assessment of mucosal healing as an outcome of disease activity. Lastly, as the number of patients included was determined by the eligible patients in our department, did we not have enough statistical power (eg, enough patients) to fit larger models allowing us to adjust for competing risks. Thus, we were not able to perform a detailed search for variables associated with the different reasons for discontinuation (eg, switch back to IV, LOR, remission).
Strengths
Patients were prospectively followed with regular visits at the outpatient clinic with measurements of clinical and biochemical markers, including measurement of s-VDZ levels. This resulted in a few missing data. Selection bias was reduced by including almost all patients on IV treatment at our hospital, regardless of treatment interval at baseline.
Conclusion
The present study demonstrates that SC VDZ persistence was high, with 3 out of 4 patients remaining on SC treatment after 18 months. Preference towards IV treatment and disease activity at switch increased the risk for discontinuation of SC VDZ. Noteworthy, patients with ISRs switching back to IV should be monitored during their first infusions due to possible infusion reactions. Furthermore, most patients can be treated with the standard injection interval of 2 weeks regardless of previous IV interval, whilst maintaining acceptable s-VDZ levels and stable disease activity. As we did not identify any association between s-VDZ levels and disease activity, the clinical role of TDM in IBD patients on SC VDZ remains questionable. Nevertheless, TDM can be used to increase treatment intervals in patients in remission. In conclusion, the present 18-month follow-up study shows that a switch to SC VDZ treatment is safe and feasible, and that most patients can receive SC treatment at standard dosing intervals.
Supplementary Material
Acknowledgments
We thank Maren Sjåmo for contributing to data collection.
Contributor Information
Thea H Wiken, Departement of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Marte L Høivik, Departement of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Karoline Anisdahl, Departement of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Lydia Buer, Departement of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway.
David J Warren, Department of Medical Biochemistry, Oslo University Hospital, Radiumhospitalet, Norway.
Nils Bolstad, Department of Medical Biochemistry, Oslo University Hospital, Radiumhospitalet, Norway.
Milada Hagen, Departement of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Faculty of Health Sciences, Oslo Metropolitan University, Oslo, Norway.
Bjørn A Moum, Institute of Clinical Medicine, University of Oslo, Oslo, Norway; Department of FOU and Gastroenterology, Østfold Hospital Trust, Kalnes, Norway.
Asle W Medhus, Departement of Gastroenterology, Oslo University Hospital, Ullevål, Oslo, Norway; Institute of Clinical Medicine, University of Oslo, Oslo, Norway.
Author Contribution
T.H.W. was involved in conceptualization, data curation, formal analysis, investigation, project administration, visualization, drafting, and revising the manuscript. A.W.M. and M.L.H. were involved in conceptualization, funding acquisition, supervision, drafting, and revising the manuscript. L.B. was involved in conceptualization, supervision, and revising of the manuscript. B.M. was involved in conceptualization, supervision, and revising of the manuscript. K.A. was involved in revising the manuscript. D.J.W. and N.B. were involved in providing resources regarding s-VDZ measurements and in revising the manuscript. M.H. was involved in conceptualization, formal analysis, methodology, software, and revising the manuscript. All authors approved the final document.
Funding
This work was supported by Takeda AS [grant number IISR-2021-200115] and Oslo University Hospital.
Conflict of Interest
T.H.W. and K.A. report consultant fees from Takeda outside the submitted work. A.W.M. reports research grant from Takeda. B.M. reports consultant fees from Takeda, Janssen, AbbVie, Pfizer; advisory board Takeda, Janssen, AbbVie, Pfizer, Sandoz, Pharma Cosmos; speaker fees from Takeda, Janssen, AbbVie, Sandoz, Orion Pharma. M.L.H. reports speaker fees from AbbVie, Meda, Tillotts, and Takeda; advisory board Takeda. M.H., L.B., N.B., and D.J.W. report no conflicts of interest.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethical Considerations
The study was approved by the local data protection officer (PVO 21/00119), based on a written broad informed consent given by the participants. Except for one questionnaire, only data from standard clinical follow-up were included in the study database.
References
- 1. Raine T, Bonovas S, Burisch J, et al. ECCO Guidelines on therapeutics in ulcerative colitis: medical treatment. J Crohn’s Colitis. 2021;16(1):2-17. [DOI] [PubMed] [Google Scholar]
- 2. Torres J, Bonovas S, Doherty G, et al. ECCO Guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2020;14(1):4-22. [DOI] [PubMed] [Google Scholar]
- 3. Anisdahl K, Svatun Lirhus S, Medhus AW, Moum B, Melberg HO, Høivik ML.. First-line biologic treatment of inflammatory bowel disease during the first 12 months after diagnosis from 2010 to 2016: a Norwegian nationwide registry study. Scand J Gastroenterol. 2021;56(10):1163-1168. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):562-572.e12. [DOI] [PubMed] [Google Scholar]
- 5. Vermeire S, D’Haens G, Baert F, et al. Efficacy and safety of subcutaneous vedolizumab in patients with moderately to severely active Crohn’s disease: results from the VISIBLE 2 randomised trial. J Crohn’s Colitis. 2021;16(1):27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699-710. [DOI] [PubMed] [Google Scholar]
- 7. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711-721. [DOI] [PubMed] [Google Scholar]
- 8. Takeda. European Commission Approves Subcutaneous Formulation of Entyvio® (Vedolizumab) for use as Maintenance Therapy in Adults with Moderately to Severely Active Ulcerative Colitis or Crohn’s Disease. https://www.takeda.com/newsroom/newsreleases/2020/european-commission-approves-subcutaneous-entyvio-for-use-as-maintenance-therapy-in-ulcerative-colitis-or-crohns-disease. Accessed November 8, 2023.
- 9. U.S. FDA Approves Subcutaneous Administration of Takeda’s ENTYVIO® (vedolizumab) for Maintenance Therapy in Moderately to Severely Active Ulcerative Colitis. https://www.takeda.com/newsroom/newsreleases/2023/US-FDA-Approves-Subcutaneous-Administration-of-Takeda-ENTYVIO-vedolizumab-for-Maintenance-Therapy-in-Moderately-to-Severely-Active-Ulcerative-Colitis. Accessed November 8, 2023.
- 10. Stoner KL, Harder H, Fallowfield LJ, Jenkins VA.. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient. 2014;8(2):145-153. [DOI] [PubMed] [Google Scholar]
- 11. Chilton F, Collett RA.. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care. 2008;6(1):1-14. [DOI] [PubMed] [Google Scholar]
- 12. Remy C, Caron B, Gouynou C, et al. Inflammatory bowel disease patients’ acceptance for switching from intravenous infliximab or vedolizumab to subcutaneous formulation: the Nancy experience. J Clin Med. 2022;11(24):7296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fenu E, Lukyanov V, Acs A, et al. Cost effectiveness of subcutaneous vedolizumab for maintenance treatment of ulcerative colitis in Canada. PharmacoEcon Open. 2022;6(4):519-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oppe M, Muresan B, Chan K, et al. Budget impact of introducing subcutaneous vedolizumab as a maintenance therapy in biologic-naïve and biologic-experienced patients with ulcerative colitis in France. Expert Rev Pharmacoecon Outcomes Res. 2023;23(2):205-213. [DOI] [PubMed] [Google Scholar]
- 15. Wiken TH, Høivik ML, Buer L, et al. Switching from intravenous to subcutaneous vedolizumab maintenance treatment in patients with inflammatory bowel disease followed by therapeutic drug monitoring. Scand J Gastroenterol. 2023;58(8):863-873. [DOI] [PubMed] [Google Scholar]
- 16. Volkers A, Straatmijer T, Duijvestein M, et al. ; IBD center Amsterdam and the Dutch Initiative on Crohn and Colitis. Real-world experience of switching from intravenous to subcutaneous vedolizumab maintenance treatment for inflammatory bowel diseases. Aliment Pharmacol Ther. 2022;56(6):1044-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergqvist V, Holmgren J, Klintman D, Marsal J.. Real-world data on switching from intravenous to subcutaneous vedolizumab treatment in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;55(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ventress E, Young D, Rahmany S, et al. Transitioning from intRavenous to subcutAneous VEdolizumab in patients with infLammatory bowEl diSeaSe (TRAVELESS). J Crohns Colitis. 2021;16(6):911-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Restellini S, Afif W.. Update on TDM (therapeutic drug monitoring) with ustekinumab, vedolizumab and tofacitinib in inflammatory bowel disease. J Clin Med. 2021;10(6):1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pouillon L, Vermeire S, Bossuyt P.. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019;17(1):89-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fierens L, Liefferinckx C, Hoefkens E, et al. Introduction of subcutaneous infliximab CT-P13 and vedolizumab in clinical practice: a multi-stakeholder position statement highlighting the need for post-marketing studies. J Crohns Colitis. 2022;16(7):1059-1069. [DOI] [PubMed] [Google Scholar]
- 22. Richard N, Vuitton L, Fumery M.. Letter: tricky reactions to switch back from subcutaneous to intravenous vedolizumab in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2023;57(6):741-742. [DOI] [PubMed] [Google Scholar]
- 23. Volkers A, Straatmijer T, Duijvestein M, Löwenberg M, van der Meulen A, D'Haens G.. Letter: tricky reactions to switch back from subcutaneous to intravenous vedolizumab in inflammatory bowel disease patients—authors’ reply. Aliment Pharmacol Ther. 2023;57(6):743-744. [DOI] [PubMed] [Google Scholar]
- 24. Ward MG, Sparrow MP, Roblin X.. Therapeutic drug monitoring of vedolizumab in inflammatory bowel disease: current data and future directions. Therap Adv Gastroenterol. 2018;11(1):175628481877278-175628481877286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amiot A, Serrero M, Peyrin-Biroulet L, et al. ; OBSERV-IBD study group, the GETAID. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi-centre cohort study. Aliment Pharmacol Ther. 2019;50(1):40-53. [DOI] [PubMed] [Google Scholar]
- 26. van Deen WK, Khalil C, Bonthala NN, et al. Inflammatory bowel disease patients’ preferences for subcutaneous versus intravenous therapies: a mixed methods study. Dig Dis. 2022;41(3):412-421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.