Abstract
This communication describes the Pd-catalyzed C(sp3)–H functionalization of a tropane derivative to generate products with functionalization at two (β/γ) or three (β/γ/β) different sites on the alicyclic amine core. These reactions proceed via an initial dehydrogenation to generate an alkene product that can react further to form a Pd(I) alkene-bridged dimer. Functionalization of this dimer affords β/γ/β-functionalized allylic arylation and allylic acetoxylation products.
Grahical Abstract
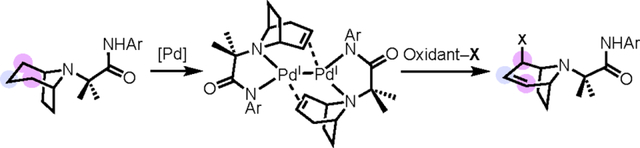
Six-membered alicyclic amines are the single most common heterocycle in pharmaceutically relevant architectures.1–2 As such, there is significant interest in approaches for the selective C(sp3)–H functionalization of these scaffolds. To date, synthetic methods have been identified to target each of the individual C(sp3)–H sites on the core (Scheme 1a–c).3–5 Most relevant to this report, our group has developed a Pd-catalyzed g-selective C(sp3)–H functionalization of alicyclic amines (Scheme 1c) in which the amine nitrogen and an appended directing group bind the catalyst and enable selective transannular Cγ–H activation.6 A complementary approach (Scheme 1d) would be to functionalize multiple sites on an alicyclic amine in a single transformation.7 This would enable the rapid generation of derivatives for biological evaluation.
Scheme 1.
(a-c) C(sp3)–H functionalization reactions that selectively target the α-, β-, and γ-C(sp3)–H bonds of 6-membered alicyclic amines. [R, R1 = hydrogen, alkyl, aryl, or directing group (DG), depending on the transformation.] (d) This work: C(sp3)–H functionalization of the β/γ/β sites in a single transformation. [R = directing group, DG]
In this communication, we demonstrate the realization of this goal in the context of the Pd-catalyzed triple C–H functionalization of tropane substrate 1.8 We show that selective β/γ/β functionalization can be achieved by the transannular dehydrogenation of 1 followed by further functionalization of the resulting alkene. An alkene-bridged Pd(I) dimer was isolated from the stoichiometric reaction of 1 with Pd(OAc)2, and this complex reacts with oxidants to form β/γ/β functionalized products in high yield and selectivity.
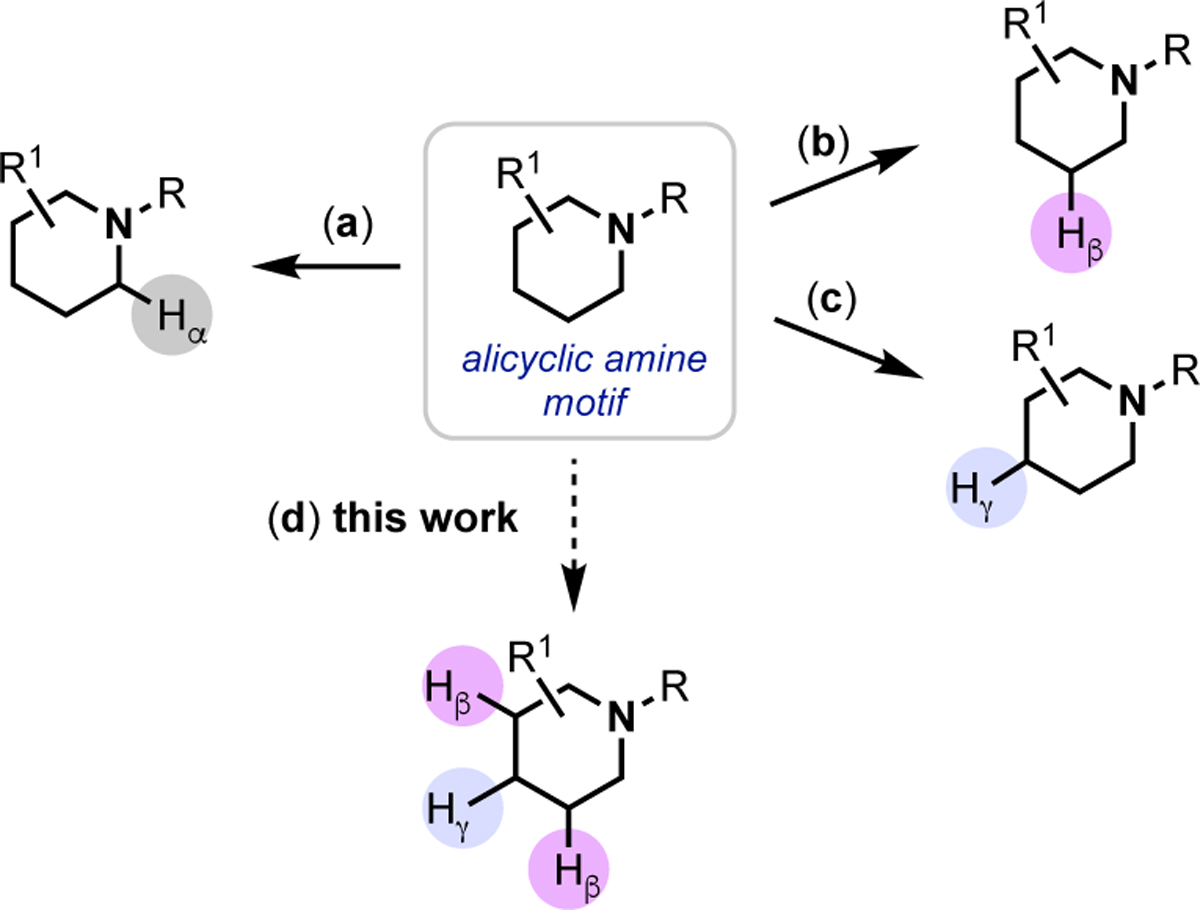
This work commenced with studies of the Pd(OAc)2-catalyzed reaction between tropane substrate 1 and PhI using conditions previously reported by our group for Cγ–H arylation (Scheme 2).6b The expected γ-arylation product 2 was obtained in 63% yield after 18 h at 140 °C. However, careful inspection of the crude reaction mixture revealed the minor side product 3, derived from functionalization at three different sites β/γ/β on the tropane core (9% yield). The structure and stereochemistry of 3 were confirmed by NMR spectroscopy and x-ray crystallography (see SI for details). Variation of the solvent, base, and picolinic acid derivative led to conditions where 3 is the major product (Scheme 2b). However, even under these optimized conditions, significant quantities of 2 were formed (~9%). Furthermore, the yield of 3 was variable, ranging from 27–64% from run-to-run.9
Scheme 2.
(a) Pd-catalyzed reaction of 1 with PhI affords products functionalized at the γ (2) and β/γ/β positions (3). (b) Optimized conditions afford 3 as the major product, but selectivity is modest, and yield is variable.
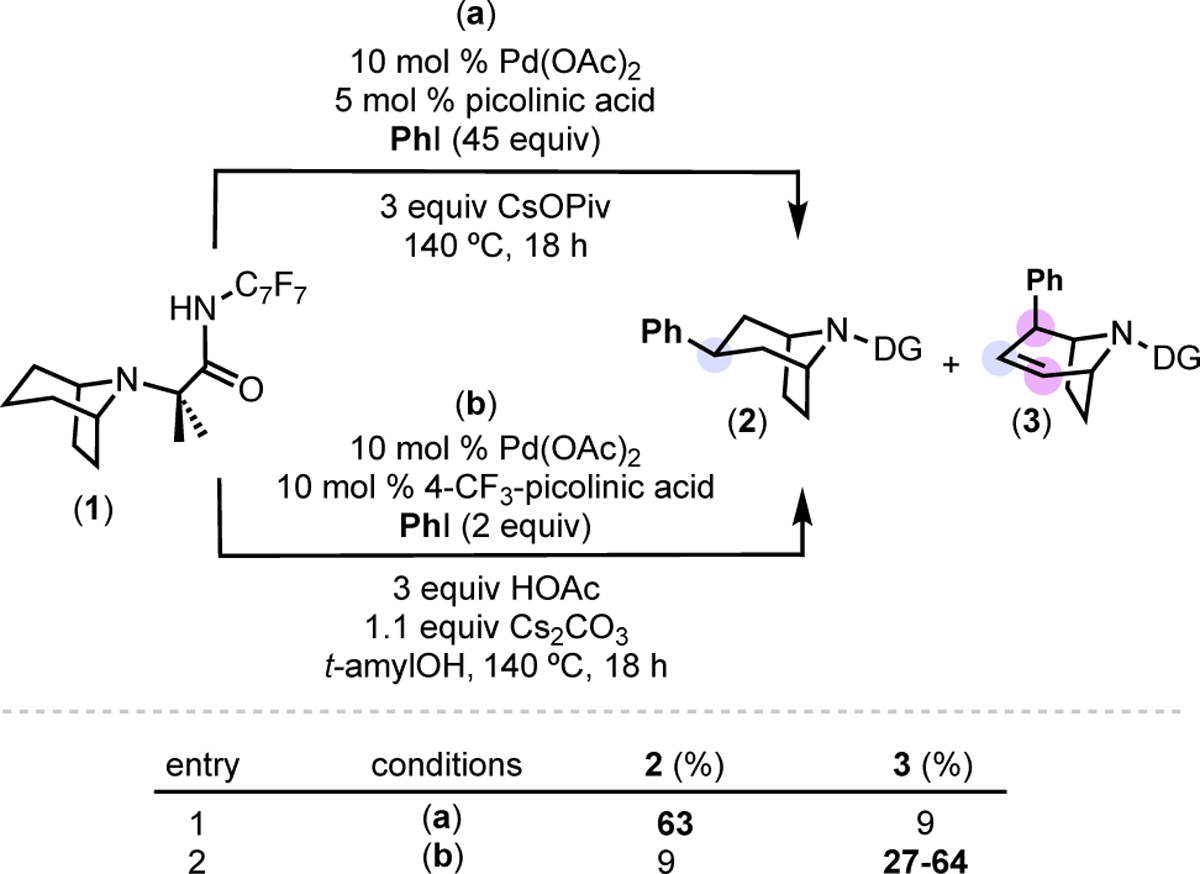
In an effort to address these issues, we interrogated the pathway to the β/γ/β functionalization product, 3. We hypothesized that 3 is formed via sequential Pd-catalyzed dehydrogenation followed by allylic arylation (Scheme 3a). Notably, Yu and coworkers have reported related Pd(OAc)2-catalyzed oxazoline-10 and carboxylic acid-directed11 dehydrogenations of alkanes to afford alkenes.12 Furthermore, Pd-catalyzed Heck-type reactions between cyclic alkenes and aryl iodides to form allylic arylation products are well precedented.13
Scheme 3.
(a) Proposed pathway to 3. (b) Alkene 4 is formed in the absence of PhI. (c) Resubjecting 4 to the reaction conditions affords 3.
To test the feasibility of an initial dehydrogenation, we conducted the reaction from Scheme 2a in the absence of PhI, substituting trifluorotoluene as an inert aromatic solvent. This afforded alkene 4 as the major product in 48% yield (Scheme 3b). The conditions were optimized (by varying the solvent, base, temperature, and picolinic acid derivative) to afford alkene 4 in 63% isolated yield (see SI for details). An isolated sample of 4 was then re-subjected to the original Pd-catalyzed C–H arylation conditions. After 18 h at 140 °C, the reaction afforded 3 in 30% yield, consistent with 4 as an intermediate en route to 3.14
Our previous work has shown that transannular Cγ–H functionalization of related alicyclic amine substrates can be achieved in enhanced yield and selectivity via stoichiometric reactions of palladium-amine coordination complexes.15 For example, as shown in Scheme 4a, we demonstrated that PdII complex A forms under mild conditions from the reaction between Pd(OAc)2/pyridine and alicyclic amines bearing fluoroarylamide directing groups. A then reacts with oxidants (FG in Scheme 4a) to afford γ-functionalized products.16 In some instances, the analogous organic products were formed in poor yield under catalytic conditions. Thus, we hypothesized that an analogous stoichiometric sequence might provide cleaner access to the target β/γ/β-functionalized product 3 and analogues thereof.
Scheme 4.
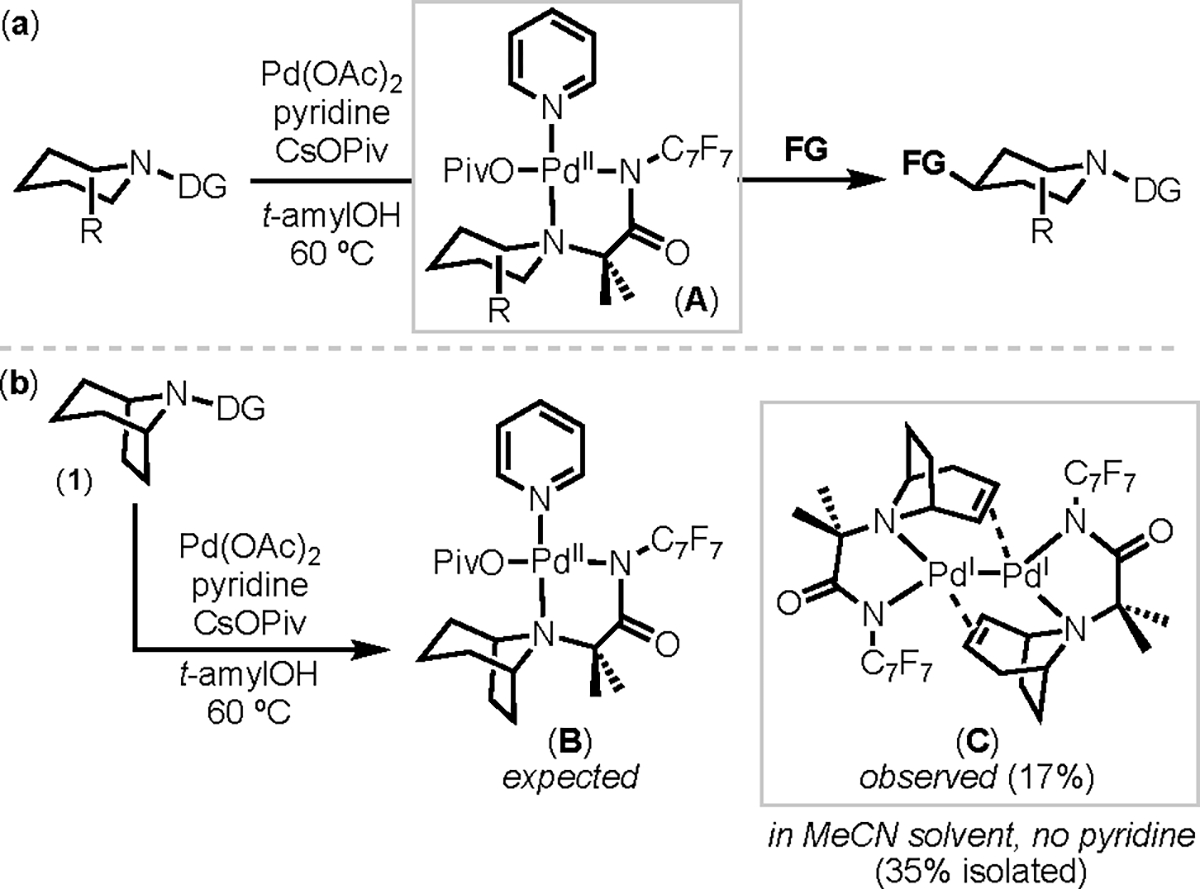
(a) Previous work on isolation and selective C–H functionalization of A. (b) Stoichiometric reaction of 1 with Pd(OAc)2 forms alkene-bridged dimer C
In the event, the reaction of tropane substrate 1 with Pd(OAc)2 and pyridine (identical conditions to those in Scheme 4a) did not afford the expected coordination complex B. Instead, it resulted in dehydrogenation of 1 and formation of the PdI alkene-bridged dimer C as the major distinguishable organometallic product.17 This dimer, which shows four diagnostic 1H NMR resonances between 5.28 and 3.79 ppm, was formed in 17% yield as determined by 1H NMR spectroscopic analysis of the crude reaction mixture. Modifying the reaction conditions (by changing the solvent from t-amyl alcohol to MeCN and removing the pyridine) resulted in a 36% crude yield of C. Purification by column chromatography on silica gel afforded an analytically pure sample of C in 35% isolated yield. X-ray quality crystals were obtained from a dichloromethane/hexanes solution at room temperature. The x-ray crystal structure is provided in Figure 1 and shows that this is a dimeric complex with the alkene ligands bridging two palladium(I) centers. The Pd–Pd distance is 2.445 Å, which is comparable to that in structurally similar PdI dimers.18
Figure 1.
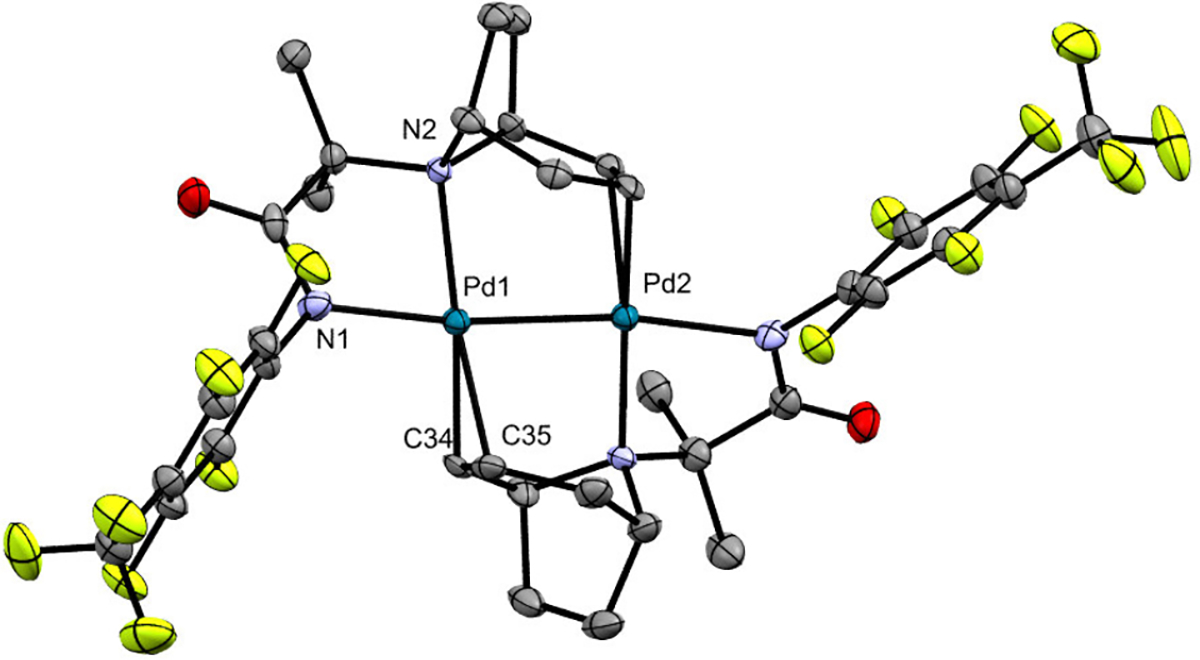
X-ray crystal structure of C. Selected bond distances (Å): N1−Pd1 2.113, N2−Pd1 2.139, C34−Pd1 2.131, C35−Pd1 2.184, Pd1–Pd2 2.445. Hydrogen atoms are omitted for clarity.
With C in hand, we investigated stoichiometric reactions of this complex with oxidants to generate β/γ/β-functionalized products. As shown in Scheme 5, the treatment of C with phenyl iodide at 100 °C afforded 3 in 72% yield.19a–b In contrast to the catalytic reactions in Schemes 1 and 2, this transformation was reproducible and high yielding. Analogous reactivity was observed using 3,5-dimethylphenyl iodide, providing 5 in 77% yield.19a
Scheme 5.
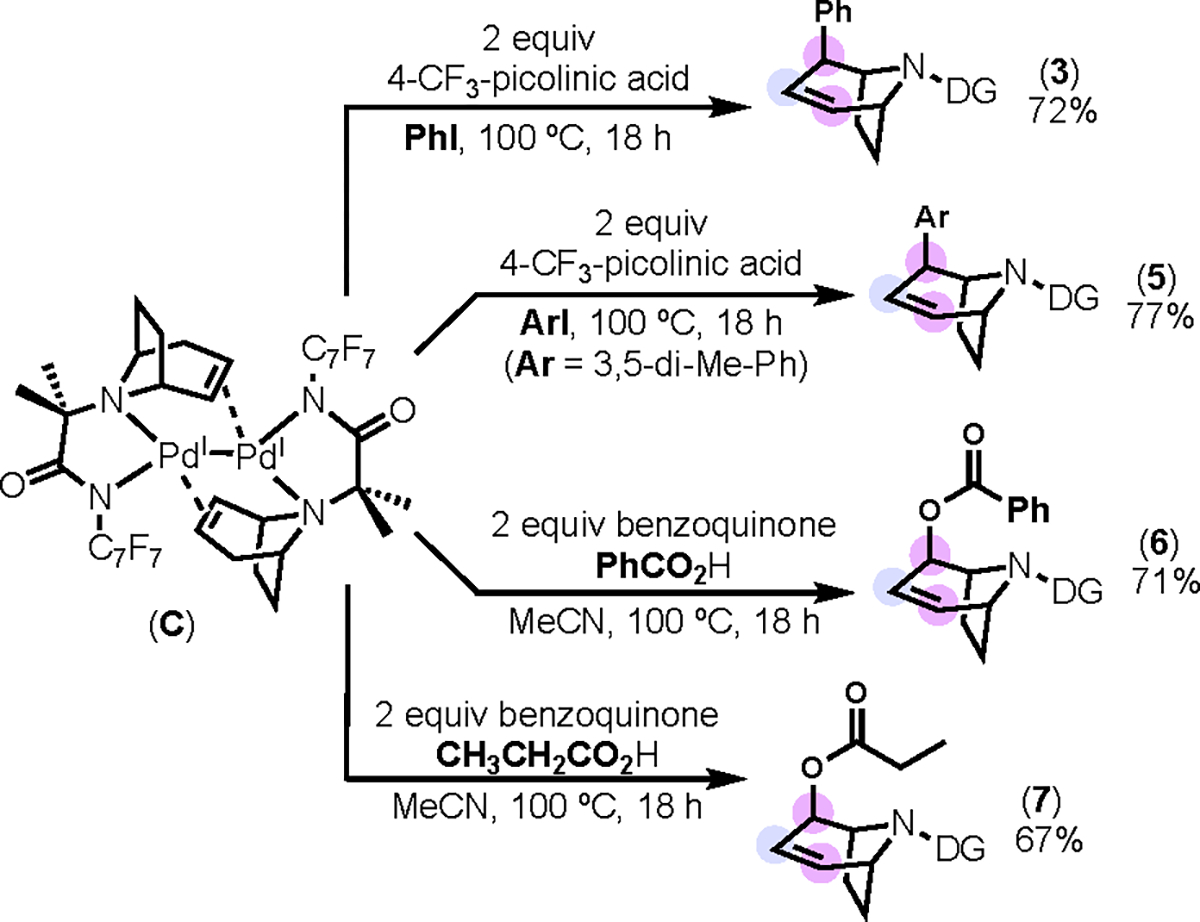
High yielding functionalization of dimer C to form 3 and 5–7.
Based on literature reports from White20 and others,21 we hypothesized that allylic oxygenation might also be feasible from C using carboxylic acids in conjunction with benzoquinone (BQ). Indeed, the treatment of C with benzoic acid and 2 equiv of BQ formed the allylic benzoylation product 6 in 71% yield.19a This reaction proceeded similarly with propionic acid to afford 7 in 67% yield.19a The structure and stereochemistry of the latter product was confirmed via x-ray crystallography (see SI for details). Overall, the stoichiometric formation and subsequent functionalization of C offers a clean, selective, reproducible, and high yielding route to the tri-functionalized products 3 and 5–7.
In summary, this report describes a route to β/γ/β-functionalized tropane derivatives via dehydrogenation/functionalization. The Pd(OAc)2-catalyzed reaction requires high temperatures (≥140 °C) and exhibits poor reproducibility, yield, and product selectivity. To address these challenges, we developed a stoichiometric sequence involving initial dehydrogenation to form a dimeric Pd(I) intermediate followed by subsequent functionalization of this complex. This sequence proceeds under relatively mild conditions (60–100 °C) with high reproducibility. Furthermore, it provides a route to diverse C–C and C–O coupled products in good yield/selectivity. These studies highlight the value of interrogating stoichiometric reactions between metal and substrate as a pathway to achieving selective C–H functionalization reactions.
Supplementary Material
ACKNOWLEDGMENT
We acknowledge financial support from NIH NIGMS (R35GM1361332). EYA also thanks Rackham Graduate School and NSF for graduate fellowships. We thank Jeff W. Kampf for carrying out x-ray crystallographic analysis. Dr. Melissa Lee, Dr. Pablo Cabrera, and Dr. Michael Bellas are all acknowledged for preliminary studies of the Pd-catalyzed formation of alkene 4.
Footnotes
The authors declare no competing financial interest.
REFERENCES
- 1.(a) Taylor RD; MacCoss M; Lawson ADG Rings in Drugs. J. Med. Chem. 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]; (b) Top 200 Pharmaceutical Products by Prescription in 2016; University of Arizona, 2016. http://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/2016Top200PharmaceuticalPrescriptionSalesPosterLowResV2.pdf (accessed January 2023). [Google Scholar]
- 2.(a) Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [DOI] [PubMed] [Google Scholar]; (b) Källström S; Leino R Synthesis of Pharmaceutically Active Compounds Containing a Disubstituted Piperidine Framework. Bioorg. Med. Chem. 2008, 16, 601–635. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wencel-Delord J; Glorius F C–H Bond Activation Enables the Rapid Construction and Late-Stage Diversification of Functional Molecules. Nature Chem 2013, 5, 369–375. [DOI] [PubMed] [Google Scholar]; (b) Cernak T; Dykstra KD; Tyagarajan S; Vachal P; Krska SW The Medicinal Chemist’s Toolbox for Late-Stage Functionalization of Drug-like Molecules. Chem. Soc. Rev. 2016, 45, 546–576. [DOI] [PubMed] [Google Scholar]
- 4.For examples of α–functionalization of alicyclic amines, see: [Google Scholar]; (a) Pastine SJ; Gribkov DV; Sames D sp3 C–H Bond Arylation Directed by Amidine Protecting Groups: α-Arylation of Pyrrolidines and Piperidines. J. Am. Chem. Soc. 2006, 128, 14220–14221. [DOI] [PubMed] [Google Scholar]; (b) Campos KR Direct sp3 C–H Bond Activation Adjacent to Nitrogen in Heterocycles. Chem. Soc. Rev. 2007, 36, 1069–1084. [DOI] [PubMed] [Google Scholar]; (c) Mitchell EA; Peschiulli A; Lefevre N; Meerpoel L; Maes BUW Direct α-Functionalization of Saturated Cyclic Amines. Chem. Eur. J. 2012, 18, 10092–10142. [DOI] [PubMed] [Google Scholar]; (d) Shi L; Xia W Photoredox Functionalization of C–H Bonds Adjacent to a Nitrogen Atom. Chem. Soc. Rev. 2012, 41, 7687–7697. [DOI] [PubMed] [Google Scholar]; (e) He J; Hamann LG; Davies HML; Beckwith REJ Late-Stage C–H Functionalization of Complex Alkaloids and Drugs Molecules via Intermolecular Rhodium-Carbenoid Insertion. Nat. Commun. 2015, 6, 5943. [DOI] [PubMed] [Google Scholar]; (f) Spangler JE; Kobayashi Y; Verma P; Wang D-H; Yu J-Q α-Arylation of Saturated Azacycles and N-methylamines via Palladium(II)-Catalyzed C(sp3)–H Coupling. J. Am. Chem. Soc. 2015, 137, 11876–11879. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen W; Ma L; Paul A; Seidel D Direct α-C–H Bond Functionalization of Unprotected Cyclic Amines. Nat. Chem. 2017, 10, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Nagaraaj P; Vijayakumar V Oxidation of Amine α-Carbon to Amide: A Review on Direct Methods to Access the Amide Functionality. Org. Chem. Front. 2019, 6, 2570–2599. [Google Scholar]; (i) Paul A; Seidel D α-Functionalization of Cyclic Secondary Amines: Lewis Acid Promoted Addition of Organometallics to Transient Imines. J. Am. Chem. Soc. 2019, 141, 8778–8782. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Copper-Catalyzed Late-Stage C(sp3)–H Functionalization of Nitrogen Heterocycles. Nature Commun. 2021, 12, article number 4342. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Yu F; Valles DA; Chen W; Daniel SD; Ghiviriga I; Seidel D Regioselective α-Cyanation of Unprotected Alicyclic Amines. Org. Lett. 2022, 24, 6364–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.For examples of β–functionalization of alicyclic amines, see: Ye S; Yang W; Coon T; Fanning D; Neubert T; Stamos D; Yu J-Q N-Heterocyclic Carbene Ligand-Enabled C(sp3)–H Arylation of Piperidine and Tetrahydropyran Derivatives. Chem. Eur. J. 2016, 22, 4748–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Affron DP; Bull JA Palladium-Catalyzed Directed C(sp3)-H Arylation of Saturated Heterocycles at C-3 Using a Concise Optimization Approach: Pd-Catalyzed Directed C(sp3)-H Arylation. Eur. J. Org. Chem. 2016, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Van Steijvoort BF; Kaval N; Kulago AA; Maes BUW Remote Functionalization: Palladium-Catalyzed C5(sp3)-H Arylation of 1-Boc-3-Aminopiperidine through the Use of a Bidentate Directing Group. ACS Catal. 2016, 6, 4486–4490. [Google Scholar]; (d) Zhou M-J; Zhu S-F; Zhou Q-L Copper-Catalyzed Mannich-Type Oxidative β-Functionalization of Tertiary Amines. Chem. Commun. 2017, 53, 8770–8773. [DOI] [PubMed] [Google Scholar]; (e) Griffiths RJ; Kong WC; Richards SA; Burley GA; Willis MC; Talbot EPA Oxidative β-C–H Sulfonylation of Cyclic Amines. Chem. Sci. 2018, 9, 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen W; Paul A; Abboud KA; Seidel D Rapid Functionalization of Multiple C–H Bonds in Unprotected Alicyclic Amines. Nature Chem. 2020, 12, 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chang Y; Cao M; Chan JZ; Zhao C; Wang Y; Yang R; Wasa M Enantioselective Synthesis of N-Alkylamines through β-Amino C–H Functionalization Promoted by Cooperative Actions of B(C6F5)3 and a Chiral Lewis Acid Co-Catalyst. J. Am. Chem. Soc. 2021, 143, 2441–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Holmberg-Douglas N; Choi Y; Aquila B; Huynh H; Nicewicz DA β-Functionalization of Saturated Aza-Heterocycles Enabled by Organic Photoredox Catalysis. ACS Catal. 2021, 11, 3153–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Piticari A-S; Antermite D; Higham JI; Moore HJ; Webster MP; Bull JA Stereoselective Palladium-Catalysed C(sp3)–H Mono-Arylation of Piperidines and Tetrahydropyrans with a C(4) Directing Group. Adv. Syn. Catal. 2022, 364, 1488–1497. [Google Scholar]
- 6.(a) Topczewski JJ; Cabrera PJ; Saper NI; Sanford MS Palladium-Catalysed Transannular C–H Functionalization of Alicyclic Amines. Nature 2016, 531, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cabrera PJ; Lee M; Sanford MS Second Generation Palladium Catalyst System for Transannular C–H Functionalization of Azabicycloalkanes. J. Am. Chem. Soc. 2018, 140, 5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Lee M; Adams A; Cox PB; Sanford MS Access to 3D Alicyclic Amine-Containing Fragments through Transannular C–H Arylation. Synlett 2019, 30, 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Li Z; Dechantsreiter M; Dandapani S Systematic Investigation of the Scope of Transannular C–H Heteroarylation of Cyclic Secondary Amines for Synthetic Application in Medicinal Chemistry. J. Org. Chem. 2020, 85, 6747–6760. [DOI] [PubMed] [Google Scholar]; (e) Daugulis O; Roane J; Tran LD Bidentate, Monoanionic Auxiliary-Directed Functionalization of Carbon-Hydrogen Bonds. Acc. Chem. Res. 2015, 48, 1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For examples of difunctionalization of piperidine derivatives, see:Xu G-Q; Xu J-T; Feng Z-T; Liang H; Wang Z-Y; Qin Y; Xu P-F Dual C(sp3)–H Bond Functionalization of N-Heterocycles through Sequential Visible-Light Photocatalyzed Dehydrogenation/[2 + 2] Cycloaddition Reactions. Angew. Chem., Int. Ed. 2018, 57, 5110–5114.Visible-Light-Induced α,γ-C(sp3)–H Difunctionalization of Piperidines. Org. Lett. 2022, 24, 2894–2898. Colson E; Andrez J; Dabbous A; Denes F; Maurel V; Mouesca J-M; Renaud P Tropane and Related Alkaloid Skeletons via a Radical [3+3]-Annulation Process. Commun. Chem. 2022, 5, 57.
- 8.Recent report of C–H functionalization approach to β/γ/β functionalized tropanes: Zayene M; Le Bideau F; Retailleau P; Jannet HB; Alami M; Romdhane A; Messaoudi S Site-Selective Palladium(II)-Catalyzed Methylene C(sp3)–H Diarylation of a Tropane Scaffold. J. Org. Chem. 2022, 87, 16399–16409. [DOI] [PubMed] [Google Scholar]
- 9.Product 3 is always observed in these reactions, but its yield is variable. Reactions with lower yields typically show low (<50%) conversion of starting material 1. Despite extensive studies, we have been unable to pinpoint the origin of this poor reproducibility. [Google Scholar]
- 10.Giri R, Maugel N, Foxman BM, Yu J-Q, Dehydrogenation of Inert Alkyl Groups via Remote C–H Activation: Converting a Propyl Group into a π-Allylic Complex. Organometallics 2008, 27, 1667–1670. [Google Scholar]
- 11.Wang Z; Hu L; Chekshin N; Zhuang Z; Qian JX; Yu J-Q Ligand-Controlled Divergent Dehydrogenative Reactions of Carboxylic Acids via C–H Activation. Science 2021, 374, 1281–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For a different mechanism for the Pd-catalyzed desaturation of amines, see: Chuentragool P; Parasram M; Shi Y; Gevorgyan V General, Mild, and Selective Method for the Desaturation of Aliphatic Amines. J. Am. Chem. Soc. 2018, 140, 2465–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Cabri W; Candiani I Recent Developments and New Perspectives in the Heck Reaction. Acc. Chem. Res. 1995, 28, 2–7. [Google Scholar]; (b) Beletskaya IP; Cheprakov AV Heck Reaction as a Sharpening Stone of Palladium Catalysis. Chem. Rev. 2000, 100, 3009–3066. [DOI] [PubMed] [Google Scholar]; (c) Berthiol F; Doucet H; Santelli M Heck Reaction of Aryl Halides with Linear or Cyclic Alkenes Catalysed by a Tetraphosphine/Palladium Catalyst Tetrahedron Lett. 2003, 44, 1221–1225. [Google Scholar]; (d) Zafar MN; Mohsin MA; Danish M Palladium Catalyzed Heck-Mizoroki and Suzuki-Miyaura coupling reactions (Review). Russ. J. Coord. Chem. 2014, 40, 781–800. [Google Scholar]; (e) Biffis A; Centomo P; del Zotto A; Zecca M Pd Metal Catalysts for Cross-Couplings and Related Reactions in the 21st Century: A Critical Review. Chem. Rev. 2018, 4, 2249–2295. [DOI] [PubMed] [Google Scholar]; (f) Li C; Qiang XY; Qi ZC; Cao B; Li JY; Yang SD Pd-Catalyzed Heck-Type Reaction: Synthesizing Highly Diastereoselective and Multiple Aryl-Substituted P-Ligands. Org. Lett. 2019, 21, 7138–7142. [DOI] [PubMed] [Google Scholar]; (g) Xie JQ; Liang RX; Jia YX Recent Advances of Catalytic Enantioselective Heck Reactions and Reductive-Heck Reactions. Chin. J. Chem. 2021, 710–728. [Google Scholar]; (h) Reznikov AN; Ashatkina MA; Klimochkin YN Recent Developments in Asymmetric Heck Type Cyclization Reactions for Constructions of Complex Molecules. Org. Bio. Chem. 2021, 5673–5701. [DOI] [PubMed] [Google Scholar]
- 14.As expected, no allylic arylation of 4 was detected in the absence of Pd(OAc)2. [Google Scholar]
- 15.Aguilera EY; Sanford MS Model Complexes for the Palladium-Catalyzed Transannular C–H Functionalization of Alicyclic Amines. Organometallics 2019, 38, 138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguilera EY; Sanford MS Palladium-Mediated Cγ–H Functionalization of Alicyclic Amines. Angew. Chem. Int. Ed. 2021, 60, 11227–11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.A proposed pathway for the formation of C is outlined in Figure S9. [Google Scholar]
- 18.(a) Nuricumbo-Escobar JJ; Campos-Alvarado C; Ríos-Moreno G; Morales-Morales D; Walsh PJ; Parra-Hake M Binuclear Palladium(I) and Palladium(II) Complexes of Ortho-Functionalized 1,3-Bis(Aryl)Triazenido Ligands. Inorg. Chem. 2007, 46, 6182–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yamaguchi Y; Yamanishi K; Kondo M; Tsukada N Synthesis of Dinuclear (μ‑η3-Allyl)palladium(I) and -platinum(I) Complexes Supported by Chelate-Bridging Ligands. Organometallics 2013, 32, 4837–4842. [Google Scholar]; (c) Chu J; Xie XH; Yang SR; Zhan SZ Synthesis and Electro-Catalytic Properties of a Dinuclear Palladium(I) 1,3-Bis[(2-Chloro)Benzene]Triazenide Complex. Inorganica Chim. Acta 2014, 410, 191–194. [Google Scholar]; (d) Correa-Ayala E; Campos-Alvarado C; Chávez D; Hernández-Ortega S; Morales-Morales D; Miranda-Soto V; Parra-Hake M Dipalladium(I) Complexes of Ortho- and Para-Functionalized 1,3-Bis(Aryl)Triazenide Ligands: Synthesis, Structure and Catalytic Activity. Inorganica Chim. Acta 2019, 490, 130–138. [Google Scholar]; (e) Zhan JZ; Xie ZL; Liu QH; Zhan SZ Design, Synthesis, Characterization and Electrocatalytic Behaviour of a Dinuclear Palladium(I) Complex Supported by 1-[(2-Chloro)Benzene]-3-[(2-Carboxmethyl)Benzene]Triazenide Ions. Polyhedron 2019, 163, 108–113. [Google Scholar]
- 19.(a) This yield reflects conversion of both of the alkenes in C into the organic product. [Google Scholar]; (b) C reacts with 4-CF3-picolinic acid at 100 °C in the absence of ArI to release 4 in 62% yield after 18 h (Figure S10). As such, it is unclear whether the formation of 3/5 under these conditions proceeds directly from C or via the intermediacy of 4. [Google Scholar]
- 20.(a) Chen MS; White MC A Sulfoxide-Promoted, Catalytic Method for the Regioselective Synthesis of Allylic Acetates from Monosubstituted Olefins via C–H Oxidation. J. Am. Chem. Soc. 2004, 126, 1346–1347. [DOI] [PubMed] [Google Scholar]; (b) Chen MS; Prabagaran N; Labenz NA; White MC Serial Ligand Catalysis: A Highly Selective Allylic C–H Oxidation. J. Am. Chem. Soc. 2005, 127, 6970–6971. [DOI] [PubMed] [Google Scholar]
- 21.(a) Åkermark B; Hansson S; Rein T; Vågberg J; Heumann A; Bäckvall J-E Palladium-Catalyzed Allylic Acetoxylation: An Exploratory Study of the Influence of Added Acids. J. Organometallic Chem. 1989, 369, 433–444. [Google Scholar]; (b) Pilarski LT; Selander N; Böse D; Szabó KJ Catalytic Allylic C–H Acetoxylation and Benzoyloxylation via Suggested (η3-Allyl)Palladium(IV) Intermediates. Org. Lett. 2009, 11, 5518–5521. [DOI] [PubMed] [Google Scholar]; (c) Lin BL; Labinger JA; Bercaw JE Mechanistic Investigations of Bipyrimidine-Promoted Palladium-Catalyzed Allylic Acetoxylation of Olefins. Can. J. Chem. 2009, 87, 264–271. [Google Scholar]; (d) Henderson WH; Check CT; Proust N; Stambuli JP Allylic Oxidations of Terminal Olefins Using a Palladium Thioether Catalyst. Org. Lett. 2010, 12, 824–827. [DOI] [PubMed] [Google Scholar]; (e) Thiery E; Aouf C; Belloy J; Harakat D; Bras J le; Muzart, J. Palladium-Catalyzed Allylic Acyloxylation of Terminal Alkenes in the Presence of a Base. J. Org. Chem. 2010, 75, 1771–1774. [DOI] [PubMed] [Google Scholar]; (f) Campbell AN; White PB; Guzei IA; Stahl SS Allylic C–H Acetoxylation with a 4,5-Diazafluorenone-Ligated Palladium Catalyst: A Ligand-Based Strategy to Achieve Aerobic Catalytic Turnover. J. Am. Chem. Soc. 2010, 132, 15116–15119. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Mann SE; Aliev AE; Tizzard GJ; Sheppard TD Sulfur-Directed Olefin Oxidations: Observation of Divergent Reaction Mechanisms in the Palladium-Mediated Acetoxylation of Unsaturated Thioacetals. Organometallics 2011, 30, 1772–1775. [Google Scholar]; (h) Kondo H; Yu F; Yamaguchi J; Liu G; Itami K Branch-Selective Allylic C–H Carboxylation of Terminal Alkenes by Pd/Sox Catalyst. Org. Lett. 2014, 16, 4212–4215. [DOI] [PubMed] [Google Scholar]; (i) Liron F; Oble J; Lorion MM; Poli G Direct Allylic Functionalization through Pd-Catalyzed C–H Activation. Eur. J. Org. Chem. 2014, 5863–5883. [Google Scholar]; (j) Malik HA; Taylor BLH; Kerrigan JR; Grob JE; Houk KN; du Bois J; Hamann LG; Patterson AW Non-Directed Allylic C–H Acetoxylation in the Presence of Lewis Basic Heterocycles. Chem. Sci. 2014, 5, 2352–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Xing X; O’Connor NR; Stoltz BM Palladium(II)-Catalyzed Allylic C–H Oxidation of Hindered Substrates Featuring Tunable Selectivity Over Extent of Oxidation. Angew. Chem. Int. Ed. 2015, 54, 11186–11190. [DOI] [PubMed] [Google Scholar]; (l) Li X; Sun B; Zhou J; Jin C; Yu C Regioselective Acetoxylation of Terminal Olefins Using a Palladium(II)–Thiadiazole Catalyst. Eur. J. Org. Chem. 2019, 15, 2635–2638. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


