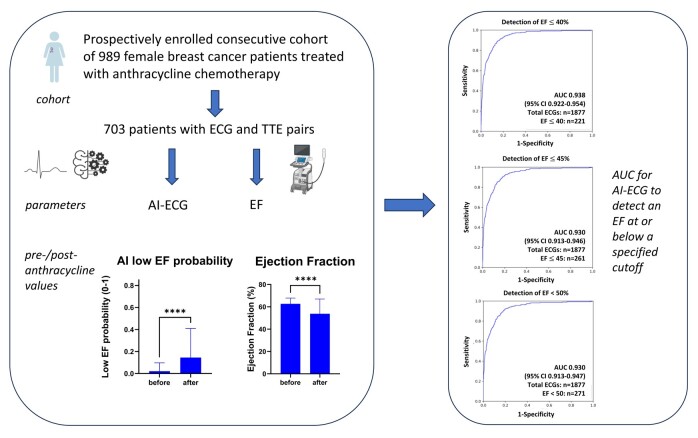
Abstract
Aims
Cardiotoxicity is a serious side effect of anthracycline treatment, most commonly manifesting as a reduction in left ventricular ejection fraction (EF). Early recognition and treatment have been advocated, but robust, convenient, and cost-effective alternatives to cardiac imaging are missing. Recent developments in artificial intelligence (AI) techniques applied to electrocardiograms (ECGs) may fill this gap, but no study so far has demonstrated its merit for the detection of an abnormal EF after anthracycline therapy.
Methods and results
Single centre consecutive cohort study of all breast cancer patients with ECG and transthoracic echocardiography (TTE) evaluation before and after (neo)adjuvant anthracycline chemotherapy. Patients with HER2-directed therapy, metastatic disease, second primary malignancy, or pre-existing cardiovascular disease were excluded from the analyses as were patients with EF decline for reasons other than anthracycline-induced cardiotoxicity. Primary readout was the diagnostic performance of AI-ECG by area under the curve (AUC) for EFs < 50%. Of 989 consecutive female breast cancer patients, 22 developed a decline in EF attributed to anthracycline therapy over a follow-up time of 9.8 ± 4.2 years. After exclusion of patients who did not have ECGs within 90 days of a TTE, 20 cases and 683 controls remained. The AI-ECG model detected an EF < 50% and ≤ 35% after anthracycline therapy with an AUC of 0.93 and 0.94, respectively.
Conclusion
These data support the use of AI-ECG for cardiotoxicity screening after anthracycline-based chemotherapy. This technology could serve as a gatekeeper to more costly cardiac imaging and could enable patients to monitor themselves over long periods of time.
Keywords: Cardio-oncology, Anthracyclines, Cardiotoxicity, Breast cancer, Artificial intelligence
Graphical Abstract
Graphical abstract.
See the editorial comment for this article ‘Artificial intelligence–assisted electrocardiography: a new and easily accessible approach for diagnosing cancer therapy–related cardiac dysfunction', by G. Halasz et al., https://doi.org/10.1093/eurjpc/zwad390.
Introduction
Advances in cancer therapies have improved survival but also increased awareness of cardiovascular side effects.1 Anthracyclines remain the example par excellence for cardiotoxicity, manifesting as a decline in left ventricular ejection fraction (EF) with or without heart failure, referred to as anthracycline-induced cardiomyopathy (AIC).2–4
Early detection of AIC is important as prompt treatment increases the probability of EF recovery.5 The 2022 European Society of Cardiology (ESC) guidelines recommend a risk level-based surveillance approach using transthoracic echocardiography (TTE) and serum biomarkers6; an electrocardiogram (ECG) is recommended only at baseline. There might, however, be merit in obtaining ECGs even in follow-up, especially if used in combination with emerging artificial intelligence (AI) technologies.
Indeed, AI applied to standard 12-lead ECG has retrospectively and prospectively identified patients with a reduced EF ≤ 35% or < 40% in the general population.7,8 This begets the question if such AI-ECG models can identify patients with a reduction in EF after cardiotoxic therapy, i.e. AIC. The goal of this study was to address if the AI-ECG algorithm developed for the detection of a reduced EF in the general population could be used to detect an abnormal EF in cancer patients after anthracycline therapy utilizing ECG data from an outside institution.
Methods
Study population
Since 2000, all breast cancer patients visiting the Leuven Multidisciplinary Breast Cancer Center (MBC, University Hospitals Leuven, Belgium) have been entered systematically into a comprehensive clinical registry. This registry forms the foundation for an ongoing cardio-oncology breast cancer database and this study. By treatment and era, patients for this study were identified inquiring a cancer clinic database of patients with early breast cancer treated with FEC (5-Fluorouracil (5-FU), epirubicin, and cyclophosphamide) between 2000 and 20109 and the MBC database for patients treated with FEC/AC/EC/CAF (AC = Adriamycin cyclophosphamide, EC = epirubicin cyclophosphamide, CAF = cyclophosphamide Adriamycin and 5FU) between 2011 and 2018 (see Supplementary material online, Figure S1). At the University Hospitals Leuven, all such patients routinely receive a baseline TTE and follow-up TTEs based on applicable guidelines at that point in time. Data after February 2021 were censored for this analysis.
A total of 1783 patient Electronic Medical Records (EMR) were available for review including baseline and follow-up TTEs, gender, cardiovascular risk factors, oncological diseases, including metastatic disease, and significant comorbidities. Baseline and follow-up TTE were defined as a TTE before the start and after completion of anthracycline treatment. All cases of a decline in EF from baseline were reviewed by two independent cardiologists. If no other explanation, a EF < 50% at follow-up coming from a value of ≥ 60% at baseline would meet the definition of cardiotoxicity used in National Cancer Institute-funded clinical trials in breast cancer patients, cardiotoxicity was defined as a 10% reduction in EF from baseline in asymptomatic patients or 5% in symptomatic patients to a final EF < 50%.10 Family history of cardiac disease was defined by the diagnosis of ischaemic heart disease in a first degree relatives before age 70. Obesity was defined as a BMI > 30 kg/m2.
Patients were excluded if they were male, had incomplete TTE data, were treated with Human Epidermal growth factor Receptor 2 (HER2)-directed therapy, had pre-existing cardiovascular disease and/or a EF < 60% at baseline, a significant comorbidity (such as pulmonary hypertension and chronic obstructive pulmonary disease), metastatic disease (data were censored at the time of diagnosis of metastatic disease), or a second malignancy.
Inclusion of electrocardiograms
Electrocardiograms from the University Hospitals Leuven were exported to Mayo Clinic Rochester, MN, USA for AI-ECG analysis via the MUSE Cardiology Information System (GE Medical Systems, Menomonee Falls, WI, USA) using a dedicated export tool in the EMR. The following data were exported: automated measurements and protocol (GE Marquette 12SL™ ECG Analysis Program, GE Medical Systems), raw ECG data as .CSV files, and ECG screenshots as .PNG files for quality assurance.
Only ECGs taken from patients with a TTE performed within 90 days were used to validate the existing model. Artificial intelligence analysis was conducted using an AI algorithm that was developed using a convolutional neuronal network as previously reported.11 Optimal thresholds for the AI-ECG models were chosen to maximize sensitivity and specificity.
Endpoints
The primary outcome parameter was EF on the post-cancer therapy TTE. Optimal thresholds for the AI-ECG models were chosen to maximize sensitivity and specificity for the detection of an EF ≤ 35%, ≤ 40%, ≤ 45% or < 50%. Definitions for cardiotoxicity were not only in keeping with the definitions used in NCI-funded clinical trials (see above) but also the 2022 ESC-ICOS cardio-oncology practice guideline.12
Ethical considerations
Our study complied with the Declaration of Helsinki, and collection of patient data was approved by the local ethics committee (S65509, University Hospitals Leuven). All patients included in the study gave written informed consent.
Results
Study population
A total of 989 patients met the inclusion and exclusion criteria with baseline characteristics as outlined in the Supplementary material online, Table S1. Over an average follow-up time of 9.8 ± 4.2 years, 49 (5%) patients developed a reduction in EF to < 50% (from a baseline EF ≥ 60%). In 22 of these cases (2.2% of the total population), no factor other than anthracycline exposure was identified to account for the decline in cardiac function (average time from anthracycline therapy 6.5 ± 5.5 years); for the remaining 27 patients, the causes for EF decline are illustrated in the Supplementary material online, Figure S2 and include ischaemic heart disease, tachycardiomyopathy, and valvular heart disease. Among the AIC cases, 5 and 17 patients (23 and 77% of AIC cases) met the criteria for moderate and severe CRTCD according to the 2022 ESC-ICOS definition, i.e. a reduction in EF by more than 10% points from a baseline of ≥ 60% to an EF of 40–49% or < 40%, respectively.12 Patients with cardiotoxicity were older and had received more cycles of anthracyclines.
Artificial intelligence electrocardiogram analysis
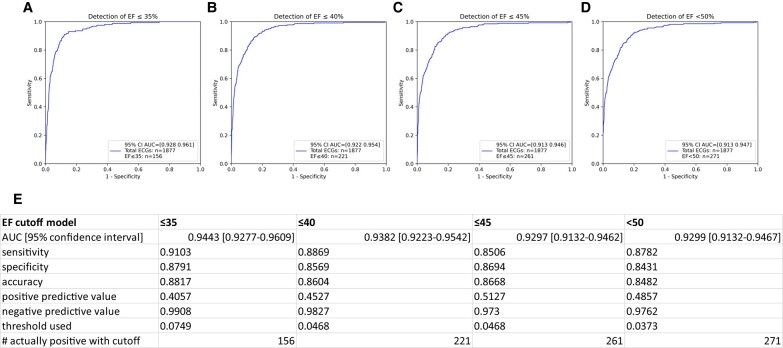
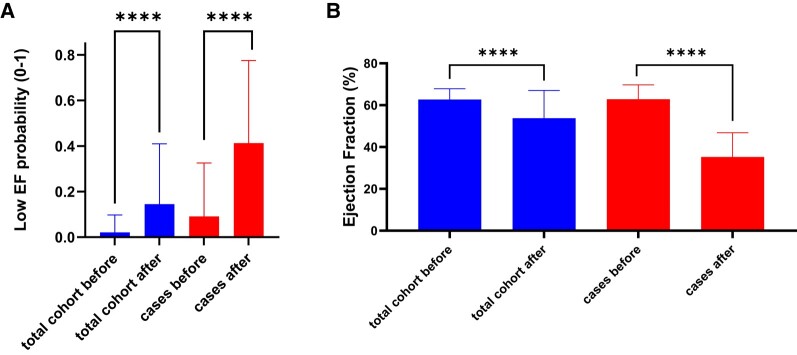
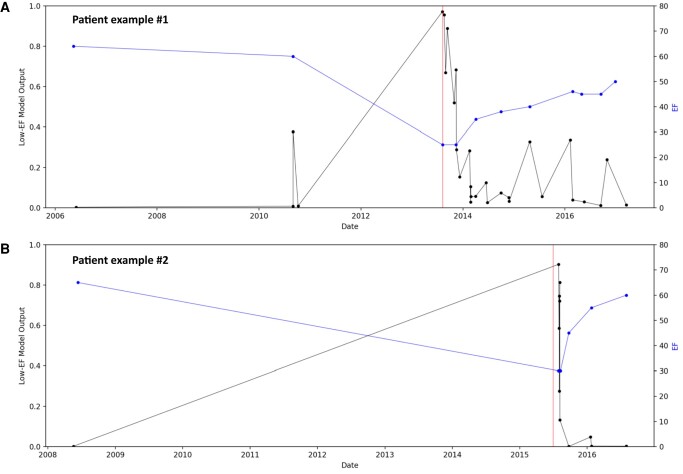
Of the eligible study population of interest, 286 patients did not have ECGs within 90 days of a TTE, leaving 703 unique patients (20 cases and 683 controls) with a total of 1877 ECGs available for analysis (see Supplementary material online, Figure S3). The baseline characteristics of this population can be found in Table 1. The AI-ECG performance at different EF cut-off levels is shown in Figure 1 and was rather consistent across the EF spectrum. Pointing out the ends of this spectrum, the AI-ECG model predicted an EF < 50% with an area under the curve (AUC) of 0.93, and an EF ≤ 35% with an AUC of 0.94. Ejection fraction values in the total cohort from before to after anthracycline therapy declined (62.7 ± 5.1 vs. 53.8 ± 13.2, P < 0.001) and correspondingly an increase in low EF probability (based on the original AI algorithm) was noted (0.02 ± 0.08 vs. 0.15 ± 0.26, P < 0.001) (Figure 2). Figure 3 illustrates the AI-ECG model performance in two patients with serial TTEs and ECGs and corresponding changes of EF and low EF (≤ 35%) probability by the AI-ECG algorithm. As evident in these patients, the AI-ECG output can track EF function changes over time.
Table 1.
Baseline characteristics studied cohort
| Baseline characteristics | Total population (n = 703) | Controls (n = 683) | Cases (n = 20) | P-value |
|---|---|---|---|---|
| Age (mean ± SD) | 64.89 years (±11.22) | 64.81 years (±11.28) | 68.99 years (±8.93) | 0.25 |
| Tumour characteristics | ||||
| – ER positivity | 69.1% (n = 486) | 69% (n = 471) | 59.7% (n = 408) | 0.81 |
| – PR positivity | 59.6% (n = 419) | 59.7% (n = 408) | 55% (n = 11) | 0.65 |
| Pathological stage (data available n = 547) |
0.12 | |||
| – IA/IB | 12.4% (n = 68)/3.3% (n = 18) | 9.5% (n = 65)/2.6% (n = 18) | 15% (n = 3)/0% (n = 0) | |
| – IIA/IIB | 26.9% (n = 147)/31.3% (n = 171) | 21.2% (n = 145)/24.5% (n = 167) | 10% (n = 2)/20% (n = 4) | |
| – IIIA/IIIB/IIIC | 19.9% (n = 109)/0% (n = 0)/5.9% (n = 32) | 15.8% (n = 108)/0% (n = 0), 4.2% (n = 29) | 5% (n = 1)/0% (n = 0)/15% (n = 3) | |
| – IV | 0.3% (n = 2) | 0.3% (n = 2) | 0% (n = 0) | |
| Grade | 0.10 | |||
| – 1 | 3.6% (n = 25) | 3.7% (n = 25) | 0% (n = 0) | |
| – 2 | 40.7% (n = 286) | 41.3% (n = 282) | 20% (n = 4) | |
| – 3 | 55% (n = 387) | 54.5% (n = 372) | 75% (n = 15) | |
| Setting | 0.78 | |||
| – Adjuvant | 80.1% (n = 563) | 79.9% (n = 546) | 85% (n = 17) | |
| – Neoadjuvant | 19.9% (n = 140) | 20.1% (n = 137) | 15% (n = 3) | |
| Oncological treatment | 0.14 | |||
| – FEC (5FU, epirubicin, cyclophosphamide) | 65.6% (n = 461) | 64.9% (n = 443) | 90% (n = 18) | |
| – EC (epirubicin, cyclophosphamide) | 32.9% (n = 231) | 33.5% (n = 229) | 10% (n = 2) | |
| – AC (Adriamycin, cyclophosphamide) | 1.3% (n = 9) | 1.3% (n = 9) | 0% (n = 0) | |
| – CAF (cyclophosphamide, Adriamycin, 5FU) | 0.3% (n = 2) | 0.3% (n = 2) | 0% (n = 0) | |
| Number of cycles with anthracyclines received | 3.79 (±1.10) | 3.75 (±1.06) | 5.25 (±1.33) | <0.01 |
| Radiotherapy | 94.5% (n = 664) | 94.4% (n = 645) | 95% (n = 19) | >0.99 |
| Cardiovascular risk factors | ||||
| – Smoking (data available n = 641) | 38.7% (n = 248) | 38.6% (n = 240) | 42.1% (n = 8) | 0.81 |
| – Hypertension (data available n = 702) | 27.9% (n = 196) | 28.3% (n = 193) | 15% (n = 3) | 0.31 |
| – Diabetes (data available n = 703) | 5.8% (n = 41) | 5.7% (n = 39) | 10% (n = 2) | 0.33 |
| – Obesity (data available n = 700) | 25.2% (n = 177) | 12.4% (n = 172) | 25% (n = 5) | 0.16 |
| – Hypercholesterolaemia (data available n = 701) | 30.2% (n = 212) | 30.2% (n = 206) | 30% (n = 6) | >0.99 |
| – Family history of cardiovascular disease (data available n = 282) |
31.6% (n = 89) | 32.8% (n = 87) | 13.3% (n = 2) | 0.16 |
| Baseline EF (±SD) | 63 ± 5% | 63 ± 6% | 63 ± 5% | 0.44 |
Figure 1.
Predictive value of the AI-ECG model for the detection of reduced left ventricular ejection fraction (EF) at stated cut-offs. (A) Detection of EF ≤ 35%; (B) detection of EF ≤ 40%; (C) detection of EF ≤ 45%; (D) detection of EF < 50%; (E) predictive values for each of the models. Threshold used refers to the optimal threshold of low EF probability value identified by AUC for the specific EF cutoff level. EF, Ejection Fraction; AUC, Area Under the Curve; ECG, Electrocardiogram.
Figure 2.
(A) Change in artificial intelligence (AI) low ejection fraction (EF) probability from before to after anthracycline treatment in the total cohort and cases, and (B) the corresponding changes in EF. **** p < 0.001.
Figure 3.
Examples of serial changes in left ventricular ejection fraction (EF) and AI-ECG-based probability for low EF (≤ 35%). Blue line represents the EF, black line the output of the AI-ECG model. Red vertical line is the moment of AIC diagnosis. A: female, born in 1950, who developed cardiotoxicity in 2013, 7 years after six cycles of anthracycline therapy; lowest EF was 25%, which partially recuperated; corresponding changes in AI-ECG readouts are seen. B: female, born in 1940, who developed cardiotoxicity in 2015, 9 years after three cycles of anthracycline therapy; lowest EF was 30%, which fully recuperated; again, corresponding changes in AI-ECG readouts are seen. EF, ejection fraction.
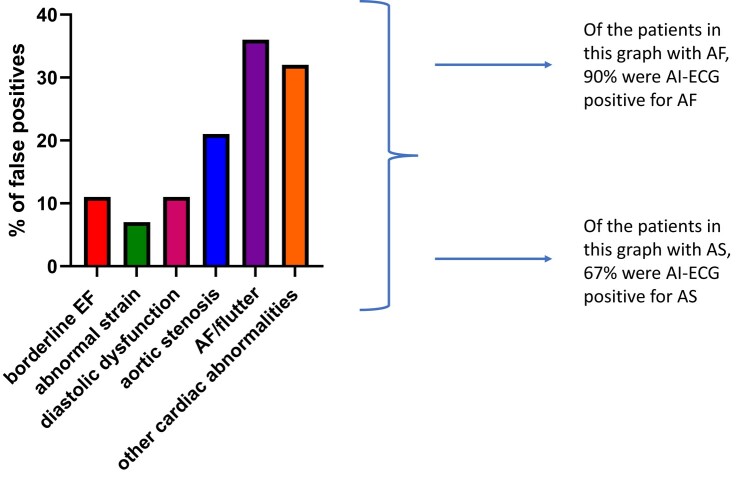
False positives
A thorough review was conducted of all 27 cases with an EF of 50% or higher in which, however, the AI-ECG indicated a high probability for an abnormal EF. Even though with a formally preserved EF, 71% of all false positives and 80% of false positives with a probability score above 0.50 had some kind of cardiovascular disease or abnormality, as detailed in Figure 4. So even if the AI-ECG is deemed ‘false positive’ for an EF below the conventional lower limit of normal, a cardiovascular evaluation can still be useful to screen for other cardiovascular abnormalities and diseases including aortic stenosis (AS) and atrial fibrillation (AF). To further analyse and leverage the ability of AI-ECG to predict moderate/severe AS and AF, we conducted additional studies to evaluate if AI-ECG could detect AS or AF in the false positives. In the false positives with known AF, the AI-ECG AF model could detect 90% of cases (threshold of 0.089) and 67% of known AS could be identified by the AI-ECG AS model (threshold of 0.41).
Figure 4.
Cardiac abnormalities in patients with false positive AI-ECGs (total population n = 28). EF, Ejection Fraction; AF, Atrial Fibrillation; AI, Artificial Intelligence; ECG, Electrocardiogram; AS, Aortic Stenosis.
Discussion
This study shows that an AI algorithm developed in the general population to detect a reduced EF based on 12-lead ECGs can identify breast cancer patients who develop an abnormal cardiac function after anthracycline-based therapy.
Cardiotoxicity is a serious side effect of anthracycline treatment, and early recognition and treatment have been advocated for best possible outcomes.13 In agreement, the 2022 ESC guidelines recommend a biomarker and echocardiography-based surveillance approach with an emphasis on closer follow-up within the first year after treatment.6,14,15 Global longitudinal strain (GLS) is an additional technique that can detect cardiotoxicity early on in specific cases, but has more an adjunctive role.12,16,17 Since GLS was only implemented in our hospital in 2014, it was not possible to include GLS analysis in our study. A number of challenges, however, remain. First of all, patients remain at risk over their lifetime but long-term screening recommendations are either not defined (ASCO practice guidelines) or lack defining data (ESC practice guidelines). Second, there is a void of robust, convenient, and cost-effective screening modalities. Cardiac imaging studies take centre stage, but remain demanding in logistics, efforts, and costs, especially in various health care systems. Third, while large populations are at risk, only a relatively small fraction of patients will eventually develop a decline in cardiac function, especially those with lower anthracycline dose exposure such as breast cancer patients. This poses a tremendous challenge when defining screening modalities and intervals. A potential solution would be the availability of a technique that would allow for screening of every patient exposed with high sensitivity so that a negative result effectively rules a condition out, and with a specificity high enough so that a positive result would not generate a high volume of unnecessary testing. One contender for such ideal screening tool may have been identified in the AI-ECG as outlined in this study.
Indeed, for the first time to our knowledge, we show that the AI-ECG can detect a newly abnormal cardiac function in patients exposed to anthracyclines. Importantly, the diagnostic performance of AI-ECG was consistent across the entire spectrum of EF cut-off levels in this cancer patient cohort, even at a detection level of an EF < 50%, though developed originally for the detection of an EF ≤ 35% in the general population. Indeed, based on the design of this study, requiring all patients to have an EF of 60% or higher and cases to have an EF of < 50%, common definitions of cardiotoxicity and the most recent ESC-ICOS definition of cancer therapy-related cardiac dysfunction (CTRCD) are met. Importantly, the degree of CRTCD was severe based on the new ESC-ICOS criteria in 77% of the AIC cases. This degree of severity has been deemed prognostically relevant.18 Accordingly, AI-ECG may yield a clinical difference, i.e. allowing for prompt recognition and proper triaging to complete work-up and therapy.
The utility and suitability of AI-ECG to screen and monitor patients undergoing cardiotoxic anthracycline-based therapy throughout their cancer journey are supported by available serial data that indicate that AI-ECG correlates with/tracks EF over time. The positive predictive value of AI-ECG for an EF < 50% of nearly 0.5 compares favourably with other widely used medical tests, including BNP.13,21 In fact, there is currently no other test in this cohort of patients that would have a better or even similar performance. Even more, with a negative predictive value of nearly 1, the AI-ECG is well suited as an initial screening effort, guiding the pursuit of more costly tests (gatekeeper role). Patients with a negative readout can remain reassured unless other noteworthy signs or symptoms of cardiovascular disease emerge. If these are present, or AI-ECG no longer indicates low likelihood of an abnormality, these patients can present to their provider for further assessment. This approach becomes even more attractive considering the development of mobile devices. Indeed, we have recently shown that the same AI-ECG algorithm that was developed for 12-lead ECGs to detect a reduced EF can be applied to single lead ECGs obtained from a smart watch with a comparable diagnostic AUC of 0.89.8 This consolidates the possibility for patients who were exposed to cardiotoxic therapies to conveniently monitor themselves over long periods of time.
Last but not least, herein we describe the successful cross-institutional transfer application of an AI-ECG algorithm that was developed for the development of a reduced EF in an unselected general population at one institution and then applied to 12-lead ECGs from an unbiased anthracycline-treated breast cancer population at another institution for the detection of a newly reduced EF post-therapy, i.e. cardiotoxicity/AIC. These aspects support the general applicability and scalability of this technique, with 12-lead ECG and conceivably mobile devices.
Our study is best understood in the context of its limitations. Firstly, because of the retrospective design, ECGs are not taken at predefined timepoints, but based on the clinical need, thereby possibly introducing a selection bias. However, proof-of-principle is made for the detection of reduced EF in affected patients with a robust comparison group. Secondly, the study population is derived from a single tertiary care cancer referral centre, all patients were female and mainly white. It is unclear how precisely this model would perform in other populations including other malignancies, male patients, and other ethnicities, even though generalizability in these aspects has previously been shown for the AI-ECG algorithm used herein.19,20
In conclusion, AI-ECG analysis of serial ECGs can be a valuable and cost-effective screening method for the long-term follow-up of patients after cardiotoxic medications. Further research is needed to validate these findings in other cohorts in the real-life setting.
Authors’ contributions
J.H., Z.I.A., B.V., L.V.A., S.J., and J.E.J.J. contributed to study design. H.W. collected the patient data in the oncological registry. J.-U.V. collected echo data. B.V. and R.W. collected ECG data. J.E.J.J. and L.V.A. created the database and collected the cardiovascular parameters. G.G., K.E.M., and Z.I.A. ran and analysed the AI model. G.G., K.E.M., and J.E.J.J. performed statistics. G.G. and J.E.J.J. designed figures and tables. J.H., Z.I.A., B.V., L.V.A., S.J., P.F., H.W., R.W., G.G., and J.E.J.J. contributed to data interpretation. J.H. and J.E.J.J. wrote the manuscript. All authors contributed to critical review of the manuscript.
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology.
Supplementary Material
Contributor Information
Johanna E J Jacobs, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA; Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Grace Greason, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Kathryn E Mangold, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Hans Wildiers, Department of Oncology, University Hospitals Leuven, Leuven, Belgium.
Rik Willems, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Stefan Janssens, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Peter Noseworthy, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Francisco Lopez-Jimenez, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Jens-Uwe Voigt, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Paul Friedman, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Lucas Van Aelst, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Bert Vandenberk, Department of Cardiovascular Sciences, KU Leuven, Leuven, Belgium.
Zachi Itzhak Attia, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Joerg Herrmann, Department of Cardiovascular Medicine, Mayo Clinic, 200 First St. SW, Rochester, MN 55905, USA.
Funding
J.E.J.J. is funded by a research fellowship of the Belgian American Educational Foundation (B.A.E.F.) in 2022–23. J.H. is supported by the National Institutes of Health/National Cancer Institute (R01CA233610), the Miami Heart Research Institute, and the Mayo Center for Biomedical Discovery and Department of Cardiovascular Diseases. R.W. is supported as senior clinical researcher by the Research Foundation Flanders (FWO).
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Ethical approval
S65509—ethical committee University Hospitals Leuven.
References
- 1. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 2020;17:474–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sawicki KT, Sala V, Prever L, Hirsch E, Ardehali H, Ghigo A. Preventing and treating anthracycline cardiotoxicity: new insights. Annu Rev Pharmacol Toxicol 2021;61:309–332. [DOI] [PubMed] [Google Scholar]
- 3. Narezkina A, Narayan HK, Zemljic-Harpf AE. Molecular mechanisms of anthracycline cardiovascular toxicity. Clin Sci (Lond 2021;135:1311–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardinale D, Iacopo F, Cipolla CM. Cardiotoxicity of anthracyclines. Front Cardiovasc Med 2020;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog Cardiovasc Dis 2010;53:121–129. [DOI] [PubMed] [Google Scholar]
- 6. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J 2022;43:4229–4361. [DOI] [PubMed] [Google Scholar]
- 7. Siontis KC, Noseworthy PA, Attia ZI, Friedman PA. Artificial intelligence-enhanced electrocardiography in cardiovascular disease management. Nat Rev Cardiol 2021;18:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Attia ZI, Harmon DM, Dugan J, Manka L, Lopez-Jimenez F, Lerman A, et al. Prospective evaluation of smartwatch-enabled detection of left ventricular dysfunction. Nat Med 2022;28:2497–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vulsteke C, Pfeil AM, Maggen C, Schwenkglenks M, Pettengell R, Szucs TD, et al. Clinical and genetic risk factors for epirubicin-induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat 2015;152:67–76. [DOI] [PubMed] [Google Scholar]
- 10. Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, et al. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol 2019;73:2859–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Attia ZI, Kapa S, Lopez-Jimenez F, McKie PM, Ladewig DJ, Satam G, et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med 2019;25:70–74. [DOI] [PubMed] [Google Scholar]
- 12. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio-Oncology Society (IC-OS) consensus statement. Eur Heart J 2022;43:280–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 14. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J Cardiovasc Imaging 2022;23:e333–e465. [DOI] [PubMed] [Google Scholar]
- 15. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail 2017;19:9–42. [DOI] [PubMed] [Google Scholar]
- 16. Thavendiranathan P, Negishi T, Somerset E, Negishi K, Penicka M, Lemieux J, et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol 2021;77:392–401. [DOI] [PubMed] [Google Scholar]
- 17. Guerra F, Marchesini M, Contadini D, Menditto A, Morelli M, Piccolo E, et al. Speckle-tracking global longitudinal strain as an early predictor of cardiotoxicity in breast carcinoma. Support Care Cancer 2016;24:3139–3145. [DOI] [PubMed] [Google Scholar]
- 18. López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020;41:1720–1729. [DOI] [PubMed] [Google Scholar]
- 19. Noseworthy PA, Attia ZI, Brewer LC, Hayes SN, Yao X, Kapa S, et al. Assessing and mitigating bias in medical artificial intelligence: the effects of race and ethnicity on a deep learning model for ECG analysis. Circ Arrhythm Electrophysiol 2020;13:e007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harmon DM, Carter RE, Cohen-Shelly M, Svatikova A, Adedinsewo DA, Noseworthy PA, et al. Real-world performance, long-term efficacy, and absence of bias in the artificial intelligence enhanced electrocardiogram to detect left ventricular systolic dysfunction. Eur Heart J Digit Health 2022;3:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu X, Zhao Y, Chen C, Han C, Xue L, Xing D, et al. BNP as a marker for early prediction of anthracycline-induced cardiotoxicity in patients with breast cancer. Oncol Lett 2019;18:4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.