Abstract
The nucleus accumbens (NAc) core is involved in regulating stress and shaping reward seeking behaviours. Multiple neuromodulators, including dynorphin/kappa opioid receptor (KOR) and dopamine systems, converge in this area to influence behavioural outcomes. KOR activation acutely inhibits dopamine release and chronically depresses overall dopamine transmission. Recently, studies in the NAc shell have revealed that the impact of KOR activation on behaviour is regionally specific, and these rostro-caudal differences are likely driven by greater control of KORs over dopamine inhibition in the caudal compared with rostral subregion. Given the importance of NAc core, particularly the interaction between KORs and dopamine in regulating reward seeking behaviours, we examined the impact of KOR activation on dopamine release and uptake along the rostro-caudal axis in the NAc core of male and female mice. Using ex vivo fast scan cyclic voltammetry, we observed that KOR mediated inhibition of dopamine release was significantly greater in caudal compared with rostral NAc core with no significant sex differences observed. These data suggest that KORs regulate dopamine release differentially along the rostro-caudal axis, providing a new axis on which to examine the process by which the KOR/dopamine system controls reward encoding.
Keywords: dopamine, kappa opioid receptors, nucleus accumbens core, rostro-caudal, U50, 488
1 |. INTRODUCTION
The nucleus accumbens (NAc) core heavily contributes to the regulation of responses to negative and positive stimuli, thereby affecting homeostatic and affective states. It is a heterogeneous region, which receives glutamatergic inputs from the basolateral amygdala, prefrontal cortex, ventral hippocampus and the paraventricular thalamus,1–3 serotonergic inputs from the dorsal raphe4 and dopaminergic inputs from the ventral tegmental area (VTA). The VTA dopaminergic projection to the NAc core shapes reinforcement learning and motivated behaviours,5–8 with both reinforcing and aversive stimuli facilitating dopamine (DA) release in the NAc.9 The NAc core also contains dynorphin producing cell bodies and axon terminals.10 Dynorphin is a neuropeptide released in response to stress, drugs and drug withdrawal11–13; it is also the endogenous ligand of the inhibitory G-protein coupled kappa opioid receptor (KOR).14,15 In the NAc core, KORs are located on pre-synaptic DA terminals16–18 and exhibit a profound inhibitory control over DA release.19–24 KORs are colocalized with DA transporters (DATs) and modulate DAT function, augmenting the reuptake of DA and further reducing DA release.18,25,26 Previous studies have shown that KORs and DATs can exist together in protein complexes, with activation of KORs promoting the formation of these complexes and ultimately augmenting DAT activity in an ERK 1/2 dependent manner.25 Overall, KOR activation inhibits DA transmission, leading to a hypodopaminergic state, which may contribute to anhedonia. It is important to note that KORs are also located post-synaptically on striatal medium spiny neurons and exert additional downstream effects on neurotransmission and therefore behavioural outcomes.3,27
The mesolimbic DA system and the dynorphin/KOR system both regulate affective states and addictive behaviours.23,28,29 Predominantly, studies have shown that chronic KOR activation produces aversion and promotes drug seeking and taking behaviours.20,21,24,28,30 Animal models of repeated or prolonged stress exposure promote excessive drug seeking and taking, which can be reversed by the inhibition of KORs. For example, augmented cocaine seeking after repeated forced swim stress exposure was attenuated following the administration of the KOR antagonist nor-binaltorphimine in rats.31 Similarly, adolescent social isolation23 and chronic intermittent ethanol induced increases in ethanol consumption were reduced by KOR antagonists.24 Interestingly, however, these KOR-blockade mediated reductions in drug responding and consumption were only observed in animals previously exposed to drugs or stress, suggesting that these exposures result in changes to KOR function, which were crucial in the drug seeking and taking behaviours.
In contrast to the relatively consistent findings of beneficial effects of KOR antagonists on drug- and alcohol-related behaviours, KOR agonist effects are highly variable. The KOR agonist U50,488 has been shown to both increase and decrease drug self-administration, depending on the dose and the timing of the exposure.28,29,32 Acute KOR activation is known to rapidly produce anhedonia, which can influence the reinforcing properties of drugs of abuse, including cocaine. Thus, when a misused drug is experienced during acute anhedonia, close to the time of agonist administration, there may be either enhanced or reduced propensity to take the drug, depending on the behavioural assay.28,32 At later time points, typically one or more hours after agonist exposure,28 findings are more consistent and tend to mimic chronic stress-associated KOR activation, such as increased reinforcing effects of the drug and self-administration behaviours, although variables such sex,33,34 and drug dose24,35 affect the results. The temporal effects of KOR activation may be a result of differential temporal dynamics of KOR interaction with various proteins that regulate DA transmission, potentially affecting perceived reward value. The combined effects of chronic stress or drug exposure on DA and KOR systems likely alter the interaction between these systems, further affecting reward value perception and thus influencing behavioural outcomes.
Recent studies have reported opposing behavioural effects of dynorphin/KOR system activation in different subregions of the NAc shell.36,37 Optical stimulation of dynorphinergic neurons in dorsal NAc shell was positively reinforcing and induced conditioned place preference; in contrast, optical stimulation of these neurons in the ventral NAc shell elicited robust conditioned place aversion.37 Similarly, activation of KORs in the rostral NAc shell stimulated hedonic orofacial responses to sucrose and produced conditioned place preference whereas activation of KORs in the caudal NAc suppressed these hedonic responses and produced conditioned place aversion.36 The opposing behaviour observed following selective KOR activation of the subregions in the NAc shell may be linked to greater modulation of DA by KORs in caudal versus rostral NAc shell.38 Despite the growing body of literature on rostro-caudal differences in the NAc shell, the adjacent region of the core remains to be examined for similar subregional effects.
Modulation of KORs alters drug-induced elevations in DA release in both shell and core NAc.22,23,28,29 Given the opposing influence of KORs in the subregions of the shell, it is possible that KORs differentially regulate DA release along the rostro-caudal axis in the core as well. Thus, in this study, we have examined the interaction between the KOR and DA systems in the rostral and caudal NAc core in both male and female mice. Based on previous literature, we anticipate that KOR-induced inhibition of DA release is greater in caudal compared with rostral NAc core.
2 |. MATERIAL AND METHODS
2.1 |. Subjects
Adult male and female C57Bl/6J mice (N = 12; six males and six females bred at Wake Forest School of Medicine) were pair housed in AAALAC-accredited facilities on a 12-h light/dark cycle (lights on at 6 AM). Mice received ad libitum chow (Laboratory Rodent Diet 5001, Lab Diet, St. Louis, MO, USA) and water. Experiments were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine and followed the NIH Guide for the Care and Use of Laboratory Animals (protocol number: A19–104). The study was conducted in accordance with the Basic & Clinical Pharmacology & Toxicology policy for experimental and clinical studies.39
2.2 |. Ex vivo fast scan cyclic voltammetry (FSCV)
To determine the effects of KOR stimulation on DA dynamics in the rostral compared with caudal NAc core, ex vivo FSCV was used to measure DA release under baseline conditions and in the presence of a selective KOR agonist, (−)-U50,488 hydrochloride (Tocris, Minneapolis, MN, USA).40,41 Male and female mice (n = 6/group) were sacrificed 3 h into the light phase, and their brains were rapidly removed and prepared as previously described.42 A vibrating tissue slicer was used to prepare 300 μm thick coronal brain sections containing NAc core—One rostral slice (+1.34 to +1.10 from Bregma)43 and one caudal slice (+0.86 to +0.62 from Bregma)43 was taken from each animal, resulting in a within-subject design. These slices were immersed in oxygenated artificial cerebrospinal fluid (aCSF; 32°C) containing: 126 mM NaCl, 2.5 mM KCl, 1.2 mM NaH2PO4, 2.4 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 11 mM glucose and 0.4 mM L-ascorbic acid, and the pH was adjusted to 7.4. Endogenous DA release was evoked by single electrical pulse stimulation (monophasic+, 4 ms, 350 μA) applied to the tissue every 5 min, and DA was detected using a triangular scanning waveform (−0.4 to +1.2 and back to 0.4 V, Ag vs AgCl) at the rate of 400 V/s. Electrode placements for recordings were distributed throughout the NAc core (lateral, medial and ventral) over the experiments. After stable DA responses were obtained, cumulative concentrations of U50,488 (0.01–1.0 μM)23,24,38,44 were bath applied to rostral and caudal NAc core slices acquired from the same animal. Subsequently, increasing concentrations of U50,488 were bath applied once DA release to the previous concentration stabilized. Recording electrodes were calibrated upon termination of each experiment with known concentrations of DA (3 μM). All ex vivo FSCV data were analysed for stimulated DA release, and the uptake portion of the curve was fitted to an exponential decay model and expressed as tau or time to 33% of the peak height (unit in seconds).45,46 These analyses were carried out using the peak and decay function of the Demon Voltammetry and Analysis software.45
2.3 |. Statistical analysis
Statistical analysis of the FSCV data was conducted using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA). Baseline DA release and uptake data for male and female mice were analysed together using repeated measures (RM) two-way analysis of variance (ANOVA), with sex and region as the independent variables and DA release or uptake (tau, exponential decay constant) as the dependent variables. The U50,488 concentration response curves were analysed using an RM two-way ANOVA. The dependent variables were μM DA release or tau, and the independent variables were rostral/caudal subregions of NAc core and U50,488 concentration. First, the analysis for U50,488 effects on DA release and tau were conducted separately in male and female mice. Regional differences in efficacy and potency were analysed using paired two-tailed Student’s t-test. To analyse sex differences in KOR modulation of DA transmission, a mixed model two-way ANOVA was used (sex, between-subject; subregion, within-subject). Significant effects from ANOVAs were followed up by Sidak’s pairwise post-hoc analysis. All data are reported as mean ± standard error of the mean. Significance was set at p < 0.05. Changes in release and tau from baseline to a single U50,488 dose were compared using paired two-tailed Student’s t-test. The paired two-tailed Student’s t-test was used to evaluate the percent baseline of tau from 10 nM to 30 nM of U50,488. FSCV data analysis was performed under blinded conditions. Sample size was selected based on previous studies examining the KOR system function in the NAc core.22–24
3 |. RESULTS
3.1 |. Stimulated DA release and tau in rostral and caudal NAc core
To determine whether stimulated DA release was comparable or different along the rostro-caudal axis in the NAc core, a single pulse electrical stimulation was applied in rostral or caudal NAc core from female and male mice (n = 6/group), and DA release and rate of uptake (using exponential decay constant, tau) were measured using ex vivo FSCV. Representative DA signals are shown in Figure 1A (rostral, top; caudal, bottom; male, blue; female, pink). Shaded areas within each section indicate general electrode placements. A two-way ANOVA comparing DA release in rostral and caudal NAc core (RM) in female and male mice revealed no significant differences in region [F (1,10) = 0.5564, p = 0.4729], or of sex [F (1, 10) = 0.1866, p = 0.6749], and no interaction between the variables [F (1, 10) = 0.03705, p = 0.8512] (Figure 1B). Comparison of DA uptake rate (tau) between the two subregions (RM) in the two sexes also did not reveal significant differences (region: [F (1, 10) = 2.187, p = 0.1700]; sex: [F (1, 10) = 2.119, p = 0.1762] and interaction: [F (1, 10) = 1.305, p = 0.2800]; Figure 1C).
FIGURE 1.
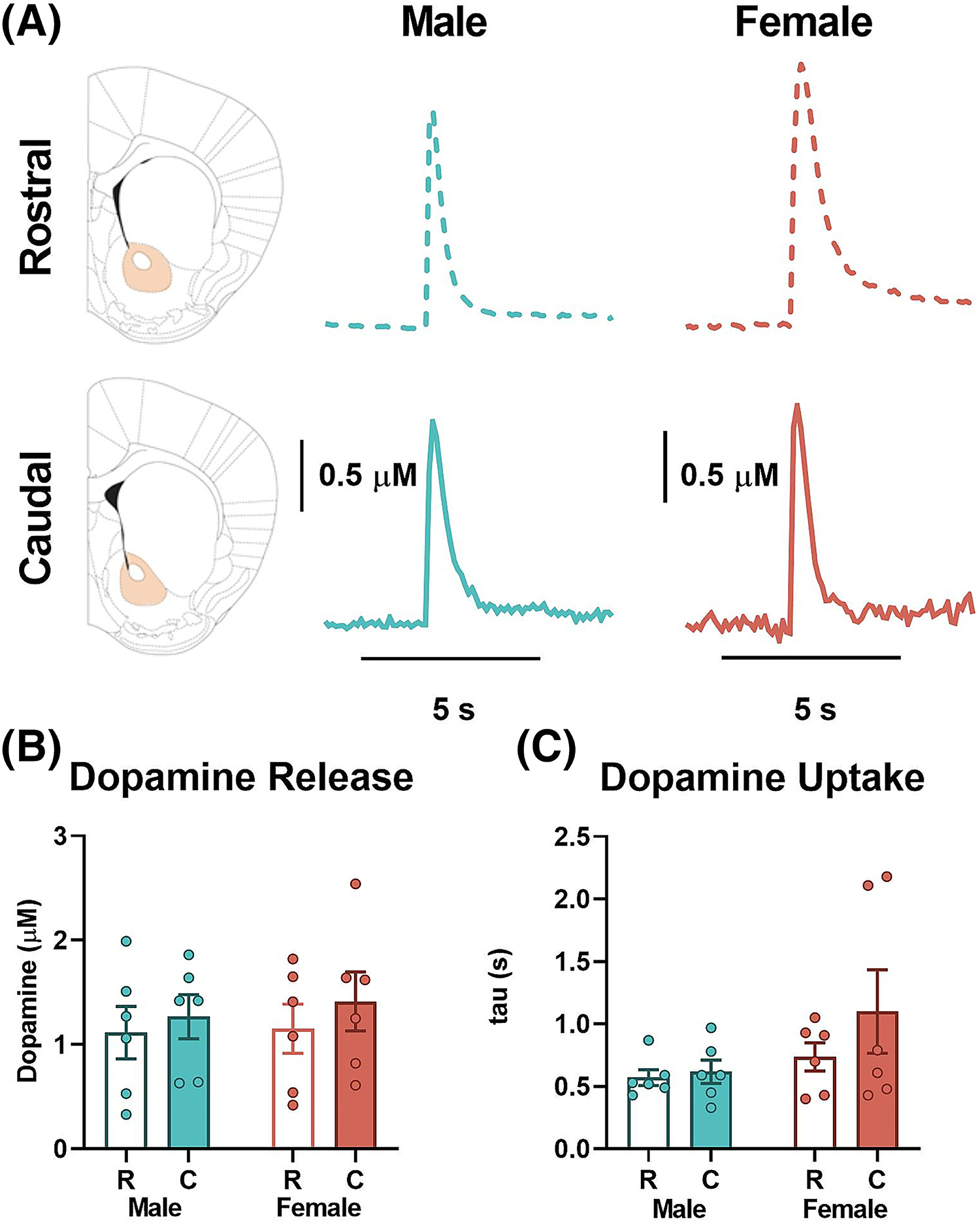
Evoked dopamine release and uptake in rostral and caudal nucleus accumbens (NAc) core. (A) Representative traces of transient dopamine signals in response to single pulse electrical stimulation in rostral (top, dashed line) and caudal (bottom, solid line) NAc core of male (middle, blue) and female (right, pink) mice with the target area highlighted on the coronal slices diagram (left). (B) Stimulated dopamine release. There was no significant difference in baseline dopamine release in rostral compared with caudal NAc core in female (white/pink—rostral; solid pink—caudal) or male (white/ blue—rostral; solid blue—caudal) mice. There were also no significant sex differences in evoked dopamine release. (C) Dopamine uptake as measured by tau was also not different between the rostral and caudal NAc core in female (white/pink—rostral; solid pink—caudal) or male (white/ blue—rostral; solid blue—caudal) mice. No sex differences were observed in uptake. C, caudal; KOR, kappa opioid receptor; R, rostral.
3.2 |. KOR mediated inhibition of DA is enhanced in the caudal NAc core subregion
To determine the magnitude of KOR mediated inhibition of DA release along the rostro-caudal axis in NAc core, cumulative concentrations of U50,488 were bath applied to slices containing rostral or caudal NAc core from female and male mice. In males, an RM two-way ANOVA comparison of rostral and caudal subregions of the NAc core (Figure 2A) revealed main effects of U50,488 concentration [F (4, 20) = 53.41, p < 0.0001] and subregion [F (1, 5) = 14.07, p = 0.0133], but there are no significant interaction between U50,488 concentration and subregion [F (4, 20) = 2.167, p = 0.1099]. Post-hoc Sidak’s pairwise comparisons revealed that U50,488 significantly inhibited DA release to a greater extent in the caudal compared with rostral NAc shell at the 0.03 (p = 0.0003), 0.1 (p < 0.0001), 0.3 (p = 0.0058) and 1.0 μM (p = 0.0238) concentrations. A paired t-test comparison of the EC50 between rostral and caudal regions also showed a significant difference [t = 3.992, df = 5, p = 0.0104] (Figure 2B). The maximal effect of U50,488-induced inhibition of DA release was trending towards being significantly greater in the caudal compared with rostral subregion of the NAc core [t = 2.343, df = 5, p = 0.0661] (Figure 2C). An RM two-way ANOVA comparison of the rostral and caudal subregions of the NAc core of females (Figure 2D) revealed main effects of U50,488 concentration [F (4, 20) = 170.6, p < 0.0001] and subregion [F (1, 5) = 11.46, p = 0.0196], but no significant interaction between U50,488 concentration and subregion [F (4, 20) = 0.6904, p = 0.6072]. Post-hoc Sidak’s pairwise comparisons revealed that U50,488 significantly inhibited DA release to a greater extent in the caudal compared with rostral NAc core at 0.01 (p = 0.0006), 0.03 (p = 0.0002), 0.1 (p < 0.0001) 0.3 (p < 0.0001) and 1.0 μM (p < 0.0001) concentrations. A paired t-test comparison of the EC50 between rostral and caudal subregions also showed a significant difference [t = 3.024, df = 5, p = 0.0293] (Figure 2E). Moreover, the maximal effect of U50,488-induced inhibition of DA release was significantly greater in the caudal compared with rostral subregion of the NAc core [t = 2.640, df = 5, p = 0.0460] (Figure 2F).
FIGURE 2.
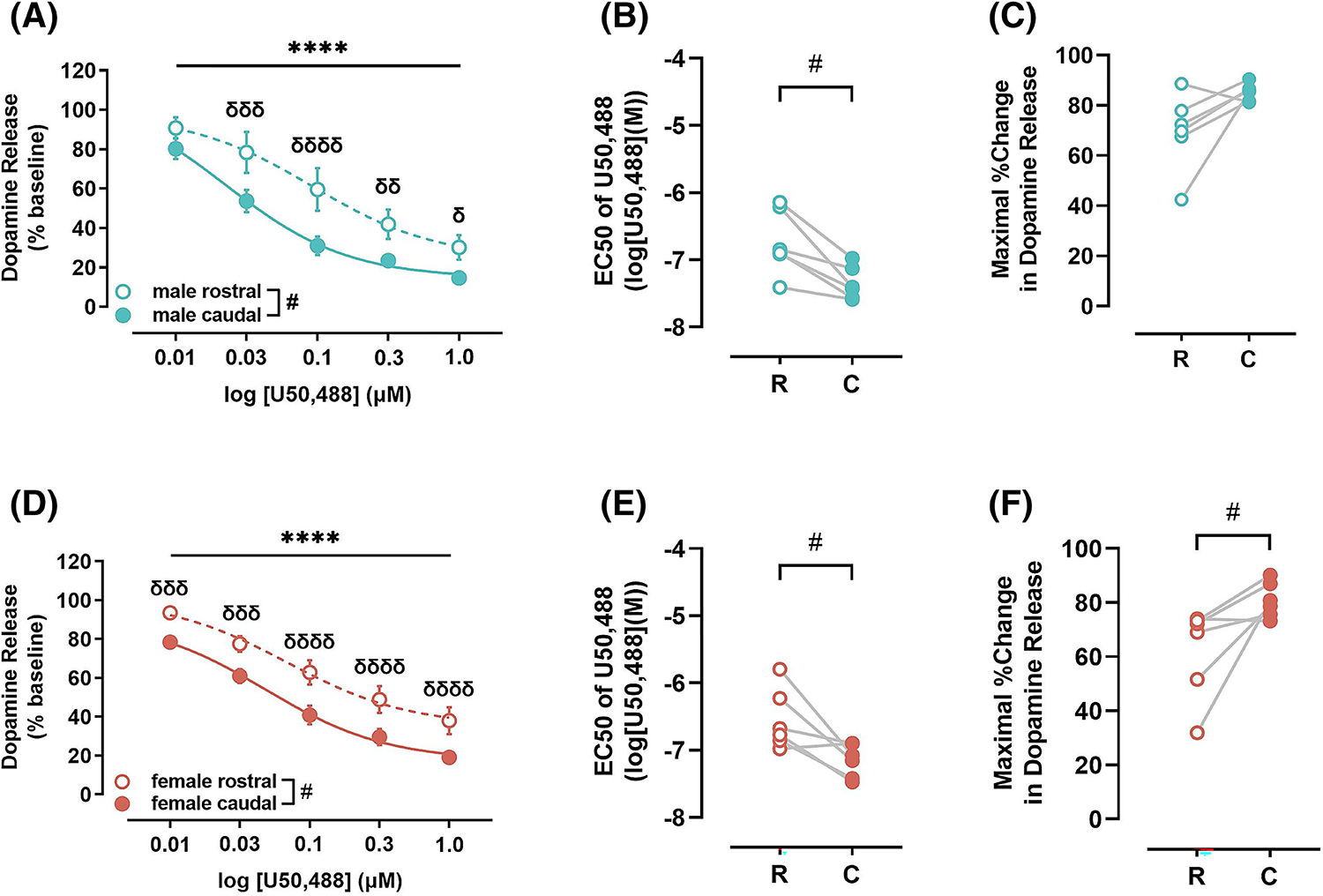
Impact of kappa opioid receptor (KOR) activation on dopamine release in rostral and caudal nucleus accumbens (NAc) core. (A) KOR activation mediated inhibition of dopamine release was greater in caudal (closed circles and solid line) compared with rostral (open circles and dotted line) NAc core in male mice. (B) Potency of KORs (EC50) was greater in the caudal (closed circles), indicated by lower EC50, compared with rostral (open circles) NAc core of male mice. (C) Maximal KOR-mediated reduction in dopamine release, efficacy, was statistically trending to be greater in the caudal (closed circles) compared with rostral (open circles) NAc core of male mice. (D) In female mice, KOR activation mediated inhibition of dopamine release was greater in caudal (closed circles and solid line) compared with rostral (open circles and dotted line) NAc core. This augmented function was driven by greater potency (E) and efficacy (F) of KORs in the caudal (closed circles) compared with rostral (open circles) NAc core. U50,488, KOR agonist; C, caudal; EC50, concentration of drug that results in the half-maximal response in the tissue; KOR, kappa opioid receptor; NAc, nucleus accumbens; R, rostral. **** p < 0.0001, main effect of U50,488 concentration; # p < 0.0001, δδδ p < 0.001, δδ p < 0.01, δ p < 0.05, post hoc pairwise comparison.
Direct comparison of KOR activation-mediated inhibition of DA release between males and females in the rostral NAc core using a two-way ANOVA revealed a main effect of U50,488 (RM) [F (4, 40) = 65.04, p < 0.0001], but not sex [F (1, 10) = 0.2113, p = 0.6556], and there are no interactions between the two variables [F (4, 40) = 0.3528, p = 0.8406] (Figure 3A). Similar analysis of the caudal NAc core showed a main effect of U50,488 (RM) [F (4, 40) = 189.3, p < 0.0001], but no effect of sex [F (1, 10) = 1.298, p = 0.2811] or interaction effect [F (4, 40) = 1.432, p = 0.2411] (Figure 3B).
FIGURE 3.
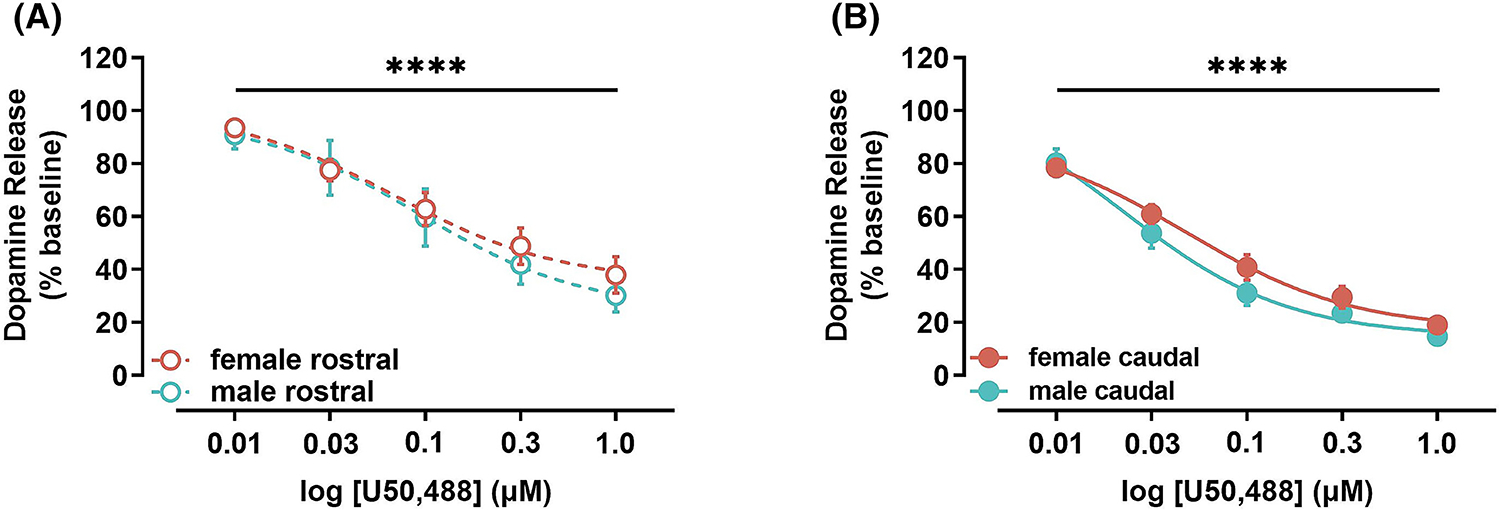
Impact of kappa opioid receptor (KOR) activation on dopamine release in males vs females in the rostral and caudal nucleus accumbens (NAc) core. (A) KOR activation mediated inhibition of dopamine release was not significantly different in the rostral NAc core of female (pink) and male (blue) mice. (B) Likewise, KOR activation mediated inhibition of dopamine release was not significantly different in the caudal NAc core of female (pink) and male (blue) mice. U50, 488, KOR agonist. **** p < 0.0001.
3.3 |. KOR did not alter DA uptake in any of the NAc core subregions
To determine the impact of KOR activation on DAT function, we quantified percent of baseline tau as a measure of uptake at the 10 and 30 nM doses of U50,488, as tau cannot be reliably quantified when DA release is very low, which occurs at higher concentrations of U50,488. The percent change in tau from the 10 to 30 nM doses were examined using a paired t-test and revealed that tau was not significantly changing across the two doses of U50,488 (male rostral [t = 0.06185, df = 5, p = 0.9531]; male caudal [t = 0.3770, df = 5, p = 0.7217]; female rostral [t = 0.7163, df = 5, p = 0.5059]; female caudal [t = 1.755, df = 5, p = 0.1396]) (Figure 4).
FIGURE 4.

Impact of kappa opioid receptor (KOR) activation in the rostral and caudal nucleus accumbens (NAc) core on uptake of dopamine. The percent change in tau at both the 10 and 30 nM doses of U50,488 did not show consistent changes in any of the groups analysed. F, female; M, male; R, rostral; C, caudal.
4 |. DISCUSSION
Here, we have documented a rostral-to-caudal gradient of KOR activity, with less DA inhibition via KOR in the rostral compared with the caudal area of the NAc core. This sub-regional based dichotomy appears to be a function of anatomical location, that is, independent of magnitude of DA innervation, as we observed comparable DA release in rostral and caudal NAc core in males and females, suggesting that the dopaminergic projection from the VTA is equivalent across the rostro-caudal axis and between sexes. Indeed, a recent tract tracing study showed that the mesolimbic projection is equally dense across the subregions, although the projection neurons themselves may be distinct.47 A previous study using rats has shown sex differences in magnitude of DA release between males and females, with evoked accumbal DA release being greater in females compared with males because of greater DA synthesis in females.33,48,49 However, these studies were all done in rats, whereas our work here and others carried out in mice have found no sex differences.50 The lack of differences in DA uptake across the rostro-caudal axis and across sexes suggests that there is no difference in DAT expression or function. The greater variability observed in our female data could be, in part, because of the oestrous cycling effects or dispersed electrode placements within the NAc core.
Although no differences were observed in baseline DA release and uptake, KOR activation was shown to inhibit DA release to a greater extent in the caudal subregion of the NAc core in female and male mice. KORs inhibit DA release because of terminal hyperpolarization via increased potassium channel conductance.51 The increased inhibition of DA in the caudal subregion could be driven by differential expression levels of KORs on DA terminals in the rostral and caudal accumbens core. To date, there have been no studies that have examined these differences. The differential magnitude of KOR-activation induced inhibition of DA release appears to be driven by a difference in potency, demonstrated by a reduction in EC50, as well as in efficacy, shown by greater maximal effect in inhibition of DA release. Although the mechanisms underlying the differential KOR agonist effects are unknown, ligand affinity can be influenced by many factors that influence G-protein coupling, including properties of the lipid bilayer and intracellular milieu.52,53 Thus, the intracellular cascade triggered by U50,488 binding may be different along the rostro-caudal axis of the NAc core. For example, individual Gα-subunits differentially affect conformation and ligand affinity of the KORs.53 It is possible that these various Gα-subunits are expressed in distinct proportions in DA terminals in rostral and caudal NAc core, leading to the unequal efficacy and potency in the two subregions.
The regional differences in KOR control over DA release are comparable in both sexes. Previous studies have shown sex differences in KOR-mediated DA inhibition and associated behaviours, with females exhibiting less DA inhibition and behavioural responses following KOR activation, but no differences in KOR mRNA.33,34,54 It is important to note that these are in vivo studies, and KOR agonist was administered intraperitoneally. Thus, it is possible that the observed sex differences are driven by differential KOR expression and/or function in brain areas other than the NAc core. Another study examining the effect of KOR activation on aversion, analgesia and DA transmission showed that KOR-mediated inhibition of DA release in the NAc is blunted in female mice.55 This blunting of KOR inhibitory control over DA release occurs via oestradiol mediated phosphorylation of G-protein receptor protein kinase 2, ultimately reducing G-protein signalling and is thus dependent on the oestrous cycle.55 In the work presented herein, we did not track the female’s cycles, as we aimed to examine the effects of acute KOR activation and avoid any repeated stress. It is possible that it is the reason that we do not see sex differences, as our sample size could be spread across different stages of the oestrous cycle. However, we do not anticipate that the intracellular signalling pathway by which oestradiol mediated G-protein signalling to be any different across the rostro-caudal axis.
In addition to DA release, we also evaluated the exponential decay of the uptake portion of the curve by the measure tau.45,46 At very low DA release concentrations, such as those caused by U50,488, the uptake is obscured as return to baseline appears to be slower than at the higher release concentrations. To limit this effect from affecting our assessment of uptake, we evaluated tau at only the two lowest doses of U50,488. We did not observe a significant directional change from the 10 to the 30 nM dose of U50,488, suggesting that the uptake was not consistently being affected in either direction. Previous studies showed KOR activation rapidly increased DAT activity; however, they were conducted using an in vitro analysis using rat tissue and a single exposure of the agonist.25 The data presented herein used an ex vivo method in mice following a cumulative concentration response curve; thus, the assay preparation, agonist dose, species differences and KOR activation time could potentially explain the discrepancy.
In this study, we have shown that although DA release and uptake rate are not different across the rostro-caudal axis within NAc core, KOR control over these measures is significantly enhanced in the caudal versus rostral subregion. Activation of KORs in rostral NAc shell has been reported to promote conditioned place preference, whereas KOR activation in the caudal shell subregion produces conditioned place avoidance.36 Given the involvement of the DA system in shaping hedonic and anhedonic states, it is possible that these behavioural differences are produced because of, at least in part, differential impact of KORs over DA transmission across the rostro-caudal axis. Particularly, KOR activation inhibits DA release significantly more in the caudal compared with the rostral NAc core. The differences in KOR regulation of DA release along the rostro-caudal axis observed in the current study likely gate behavioural outcomes as reported in previous studies.36,38 KORs are ubiquitously present on afferents into the NAc and may bias activity towards direct or indirect pathways in the NAc, further influencing behavioural outcomes.56 More research is necessary to pinpoint exact mechanisms that may be involved in the differential interaction between KORs and DATs across the rostro-caudal axis in NAc core and its impact on motivated and affective behaviours.
ACKNOWLEDGEMENTS
We thank Steven E. Albertson for his excellent technical assistance.
Funding information
This research was supported by the National Institutes of Health under Award Numbers K01AA023874, R01AA028228 (A.N.K.), T32AA007565 (A.M.W.), U01AA014091, P50AA026117, R01DA048490, R01DA054694 and T32DA041349 (S.R.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST STATEMENT
None.
REFERENCES
- 1.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76(4):790–803. doi: 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma L, Chen W, Yu D, Han Y. Brain-wide mapping of afferent inputs to accumbens nucleus core subdomains and accumbens nucleus subnuclei. Front Syst Neurosci. 2020;14:14. doi: 10.3389/fnsys.2020.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tejeda HA, Wu J, Kornspun AR, et al. Pathway- and cell-specific kappa-opioid receptor modulation of excitation-inhibition balance differentially gates D1 and D2 accumbens neuron activity. Neuron. 2017;93(1):147–163. doi: 10.1016/j.neuron.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993;624(1):188–198. doi: 10.1016/0006-8993(93)90077-Z [DOI] [PubMed] [Google Scholar]
- 5.Aitken TJ, Greenfield VY, Wassum KM. Nucleus accumbens core dopamine signaling tracks the need-based motivational value of food-paired cues. J Neurochem. 2016;136(5):1026–1036. doi: 10.1111/jnc.13494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Chiara G Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res. 2002;137(1–2):75–114. doi: 10.1016/s0166-4328(02)00286-3 [DOI] [PubMed] [Google Scholar]
- 7.Mohebi A, Pettibone JR, Hamid AA, et al. Dissociable dopamine dynamics for learning and motivation. Nature. 2019;570(7759):65–70. doi: 10.1038/s41586-019-1235-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West EA, Carelli RM. Nucleus accumbens core and shell differentially encode reward-associated cues after reinforcer devaluation. J Neurosci. 2016;36(4):1128–1139. doi: 10.1523/JNEUROSCI.2976-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margolis EB, Karkhanis AN. Dopaminergic cellular and circuit contributions to kappa opioid receptor mediated aversion. Neurochem Int. 2019;129:104504. doi: 10.1016/j.neuint.2019.104504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Bockstaele EJ, Gracy KN, Pickel VM. Dynorphin-immunoreactive neurons in the rat nucleus accumbens: ultrastructure and synaptic input from terminals containing substance P and/or dynorphin. J Comp Neurol. 1995;351(1):117–133. doi: 10.1002/cne.903510111 [DOI] [PubMed] [Google Scholar]
- 11.Caputi FF, Caffino L, Candeletti S, Fumagalli F, Romualdi P. Short-term withdrawal from repeated exposure to cocaine during adolescence modulates dynorphin mRNA levels and BDNF signaling in the rat nucleus accumbens. Drug Alcohol Depend. 2019;197:127–133. doi: 10.1016/j.drugalcdep.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 12.Marinelli PW, Lam M, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of dynorphin A(1–8) release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2006;30(6):982–990. doi: 10.1111/j.1530-0277.2006.00112.x [DOI] [PubMed] [Google Scholar]
- 13.Shirayama Y, Ishida H, Iwata M, Hazama GI, Kawahara R, Duman RS. Stress increases dynorphin immunoreactivity in limbic brain regions and dynorphin antagonism produces antidepressant-like effects. J Neurochem. 2004;90(5):1258–1268. doi: 10.1111/j.1471-4159.2004.02589.x [DOI] [PubMed] [Google Scholar]
- 14.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. doi: 10.1126/science.6120570 [DOI] [PubMed] [Google Scholar]
- 15.Cox BM. Recent developments in the study of opioid receptors. Mol Pharmacol. 2013;83(4):723–728. doi: 10.1124/mol.112.083279 [DOI] [PubMed] [Google Scholar]
- 16.Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. Neuropharmacology. 2016;110(Pt A):1901158. doi: 10.1152/jn.2001.85.3.1153 [DOI] [PubMed] [Google Scholar]
- 17.Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc Natl Acad Sci. 1992;89(6):2046–2050. doi: 10.1073/pnas.89.6.2046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svingos AL, Chavkin C, Colago EE, Pickel VM. Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synap N Y N. 2001;42(3):185–192. doi: 10.1002/syn.10005 [DOI] [PubMed] [Google Scholar]
- 19.Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244(3):1067–1080. [PubMed] [Google Scholar]
- 20.Ebner SR, Roitman MF, Potter DN, Rachlin AB, Chartoff EH. Depressive-like effects of the kappa opioid receptor agonist salvinorin A are associated with decreased phasic dopamine release in the nucleus accumbens. Psychopharmacology (Berl). 2010;210(2):241–252. doi: 10.1007/s00213-010-1836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrich JM, Messinger DI, Knakal CR, et al. Kappa opioid receptor-induced aversion requires p38 MAPK activation in VTA dopamine neurons. J Neurosci. 2015;35(37):12917–12931. doi: 10.1523/JNEUROSCI.2444-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karkhanis AN, Huggins KN, Rose JH, Jones SR. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: role of kappa opioid receptors. Neuropharmacology. 2016;110(Pt A):190–197. doi: 10.1016/j.neuropharm.2016.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karkhanis AN, Rose JH, Weiner JL, Jones SR. Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacology. 2016;41(9):2263–2274. doi: 10.1038/npp.2016.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose JH, Karkhanis AN, Chen R, et al. Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int J Neuropsychopharmacol. 2016;19(5):pyv127. doi: 10.1093/ijnp/pyv127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kivell B, Uzelac Z, Sundaramurthy S, et al. Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology. 2014;86:228–240. doi: 10.1016/j.neuropharm.2014.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson AC, Zapata A, Justice JB, Vaughan RA, Sharpe LG, Shippenberg TS. Kappa-opioid receptor activation modifies dopamine uptake in the nucleus accumbens and opposes the effects of cocaine. J Neurosci. 2000;20(24):9333–9340. doi: 10.1523/JNEUROSCI.20-24-09333.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Escobar AP, Gonzalez MP, Meza RC, et al. Mechanisms of kappa opioid receptor potentiation of dopamine D2 receptor function in quinpirole-induced locomotor sensitization in rats. Int J Neuropsychopharmacol. 2017;20(8):660–669. doi: 10.1093/ijnp/pyx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chartoff EH, Ebner SR, Sparrow A, et al. Relative timing between kappa opioid receptor activation and cocaine determines the impact on reward and dopamine release. Neuropsychopharmacology. 2016;41(4):989–1002. doi: 10.1038/npp.2015.226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrich JM, Phillips PEM, Chavkin C. Kappa opioid receptor activation potentiates the cocaine-induced increase in evoked dopamine release recorded in vivo in the mouse nucleus accumbens. Neuropsychopharmacology. 2014;39(13):3036–3048. doi: 10.1038/npp.2014.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28(2):407–414. doi: 10.1523/JNEUROSCI.4458-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groblewski PA, Zietz C, Willuhn I, Phillips PEM, Chavkin C. Repeated stress exposure causes strain-dependent shifts in the behavioral economics of cocaine in rats. Addict Biol. 2015;20(2):297–301. doi: 10.1111/adb.12123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31(6):1241–1248. doi: 10.1038/sj.npp.1300872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conway SM, Puttick D, Russell S, Potter D, Roitman MF, Chartoff EH. Females are less sensitive than males to the motivational- and dopamine-suppressing effects of kappa opioid receptor activation. Neuropharmacology. 2019;146:231–241. doi: 10.1016/j.neuropharm.2018.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chartoff EH, Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front Neurosci. 2015;9:466. doi: 10.3389/fnins.2015.00466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morales M, Anderson RI, Spear LP, Varlinskaya EI. Effects of the kappa opioid receptor antagonist, nor-binaltorphimine, on ethanol intake: impact of age and sex. Dev Psychobiol. 2014;56(4):700–712. doi: 10.1002/dev.21137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34(12):4239–4250. doi: 10.1523/JNEUROSCI.4458-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Hasani R, McCall JG, Shin G, et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87(5):1063–1077. doi: 10.1016/j.neuron.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pirino BE, Spodnick MB, Gargiulo AT, Curtis GR, Barson JR, Karkhanis AN. Kappa-opioid receptor-dependent changes in dopamine and anxiety-like or approach-avoidance behavior occur differentially across the nucleus accumbens shell rostrocaudal axis. Neuropharmacology. 2020;181:108341. doi: 10.1016/j.neuropharm.2020.108341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tveden-Nyborg P, Bergmann TK, Jessen N, Simonsen U, Lykkesfeldt J. BCPT policy for experimental and clinical studies. Basic Clin Pharmacol Toxicol. 2021;128(1):4–8. doi: 10.1111/bcpt.13492 [DOI] [PubMed] [Google Scholar]
- 40.Von Voigtlander PF, Lewis RA. U-50,488, a selective kappa opioid agonist: comparison to other reputed kappa agonists. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6(4–6):467–470. doi: 10.1016/s0278-5846(82)80130-9 [DOI] [PubMed] [Google Scholar]
- 41.Vonvoigtlander PF, Lahti RA, Ludens JH. U-50,488: a selective and structurally novel non-mu (kappa) opioid agonist. J Pharmacol Exp Ther. 1983;224(1):7–12. [PubMed] [Google Scholar]
- 42.John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52(8):1596–1605. doi: 10.1016/j.neuropharm.2007.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. Third ed. Elsevier;2007. [Google Scholar]
- 44.Spodnick MB, Amirault RT, Towner TT, Varlinskaya EI, Spear LP, Karkhanis AN. Adolescent intermittent ethanol exposure effects on kappa opioid receptor mediated dopamine transmission: sex and age of exposure matter. Brain Sci. 2020;10(8):472. doi: 10.3390/brainsci10080472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods. 2011;202(2):158–164. doi: 10.1016/j.jneumeth.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everett AC, Graul BE, Ronström JW, et al. Effectiveness and relationship between biased and unbiased measures of dopamine release and clearance. ACS Chem Nerosci. 2022;13(10):1534–1548. doi: 10.1021/acschemneuro.2c00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poulin JF, Caronia G, Hofer C, et al. Mapping projections of molecularly defined dopamine neuron subtypes using intersectional genetic approaches. Nat Neurosci. 2018;21(9):1260–1271. doi: 10.1038/s41593-018-0203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pitts EG, Stowe TA, Christensen BA, Ferris MJ. Comparing dopamine release, uptake, and D2 autoreceptor function across the ventromedial to dorsolateral striatum in adolescent and adult male and female rats. Neuropharmacology. 2020;175:108163. doi: 10.1016/j.neuropharm.2020.108163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95(4):1061–1070. doi: 10.1016/s0306-4522(99)00500-x [DOI] [PubMed] [Google Scholar]
- 50.Jameson AN, Siemann JK, Melchior J, Calipari ES, McMahon DG, Grueter BA. Photoperiod impacts nucleus accumbens dopamine dynamics. eNeuro. 2023;10(2): ENEURO.0361–22.2023. doi: 10.1523/ENEURO.0361-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhawan BN, Cesselin F, Raghubir R, et al. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48(4):567–592. [PubMed] [Google Scholar]
- 52.Xu W, Yoon SI, Huang P, et al. Localization of the κ opioid receptor in lipid rafts. J Pharmacol Exp Ther. 2006;317(3):12951306. doi: 10.1124/jpet.105.099507 [DOI] [PubMed] [Google Scholar]
- 53.Yan F, Mosier PD, Westkaemper RB, Roth BL. Gα-subunits differentially alter the conformation and agonist affinity of κ-opioid receptors. Biochemistry. 2008;47(6):1567–1578. doi: 10.1021/bi701476b [DOI] [PubMed] [Google Scholar]
- 54.Russell SE, Rachlin AB, Smith KL, et al. Sex differences in sensitivity to the depressive-like effects of the kappa opioid receptor agonist U-50488 in rats. Biol Psychiatry. 2014;76(3):213–222. doi: 10.1016/j.biopsych.2013.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abraham AD, Schattauer SS, Reichard KL, et al. Estrogen regulation of GRK2 inactivates kappa opioid receptor signaling mediating analgesia, but not aversion. J Neurosci. 2018;38(37):8031–8043. doi: 10.1523/JNEUROSCI.0653-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castro DC, Bruchas MR. A motivational and neuropeptidergic hub: anatomical and functional diversity within the nucleus accumbens shell. Neuron. 2019;102(3):529–552. doi: 10.1016/j.neuron.2019.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]


