Learning points for clinicians.
This report identified a novel missense Krüppel-like transcription factor 11 (KLF11) variant in a family in which two patients were diagnosed with diabetes mellitus, while one patient had hyperinsulinemia. This report highlights molecular diagnosis of maturity-onset diabetes of the young (MODY) is crucial for effectively treating, predicting outcomes and providing genetic counseling for individuals with monogenic diabetes. MODY7 patients may benefit from the administration of oral antidiabetic drugs, subcutaneous insulin injections and dietary intervention.
Case presentation
A 43-year-old woman presented at our hospital’s ophthalmology clinic complaining of unexplained dry mouth, excessive thirst and blurry vision with impaired sight in her right eye for 1 day. An ophthalmological examination revealed bloody vitreous opacity, scattered retinal hemorrhage and exudation in the right eye. As a result, the patient was transferred to the endocrinology department for further treatment. Upon admission to the endocrinology department, the patient’s random blood sugar level was 22.40 mmol/l, and the glycated hemoglobin A1c level was 13.30%.
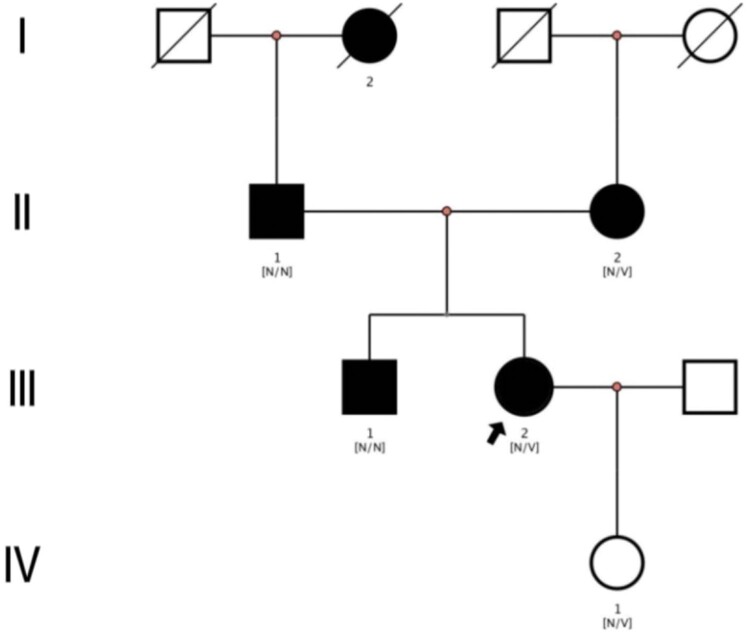
Remarkably, the proband’s father, mother, brother and grandmother have all been diagnosed with diabetes, indicating a potential genetic component in the family (Figure 1). Consequently, the clinical data of other family members were gathered and analyzed. Among the family members, three individuals exhibited heterozygous mutations in the Krüppel-like transcription factor 11 (KLF11) gene at the site c.820C > T, thereby resulting in an alteration in the amino acid p.P274S (Figure 2). Two patients displayed diabetes mellitus, while one patient exhibited hyperinsulinemia. Table 1 summarizes the specific clinical information and biochemical data of the proband and family members.
Figure 1.
Pedigree of the family with MODY showing affected cases (fully shaded). N: normal; V: the KLF11 c.820C > T (p.P274S) variant. Arrow indicates the proband.
Figure 2.
DNA sequence chromatograms of c.820C > T (p.P274S) variant in the KLF11 gene. (A) Sequencing result of the proband. (B) Sequencing result of the mother. (C) Sequencing result of the daughter. (D) Sequencing result of the father. (E) Sequencing result of the brother.
Table 1.
Clinical indicators and laboratory examination results of the family.
| Individual | Proband (July 2022) | Proband (July 2023) | Father | Mother | Brother | Daughter |
|---|---|---|---|---|---|---|
| Base change | c.820C > T | No | c.820C > T | No | c.820C > T | |
| Amino acid change | p.P274S | No | p.P274S | No | p.P274S | |
| Diagnosis | Diabetes | Diabetes | Diabetes | Diabetes | Hyperinsulinemia | |
| Age (years) | 43 | 44 | 68 | 69 | 47 | 16 |
| DKA | No | No | No | No | No | No |
| Height (cm) | 160.0 | 160.0 | 164.00 | 148.00 | 179.00 | 162.00 |
| Weight (kg) | 58.80 | 60.00 | 67.50 | 59.50 | 62.50 | 59.50 |
| BMI (kg/m2) | 22.96 | 23.44 | 25.10 | 27.20 | 19.50 | 22.70 |
| WC (cm) | 80 | 76 | 84.00 | 90.00 | 77.00 | 78.00 |
| HC (cm) | 93 | 92 | 98.00 | 96.00 | 85.00 | 95.00 |
| VFA (cm2) | 62.00 | 48.00 | 81.00 | 81.00 | 24.00 | 62.00 |
| ASFA (cm2) | 137.00 | 131.00 | 181.00 | 247.00 | 87.00 | 212.00 |
| SBP (mmHg) | 139 | 129 | 148 | 137 | 117 | 123 |
| DBP (mmHg) | 79 | 68 | 78 | 75 | 73 | 69 |
| FPG (mmol/l) | 5.80 | 5.68 | 4.01 | 5.53 | 4.80 | 4.48 |
| 0.5-h glucose (mmol/l) | 8.70 | 6.89 | 7.28 | 10.82 | 9.05 | 9.46 |
| 1-h glucose (mmol/l) | 12.27 | 11.16 | 9.58 | 13.58 | 11.26 | 8.84 |
| 2-h glucose (mmol/l) | 18.30 | 16.60 | 12.58 | 6.97 | 10.76 | 5.06 |
| 3-h glucose (mmol/l) | 17.50 | 17.01 | N/A | N/A | N/A | N/A |
| HbA1c (%) | 13.30 | 7.70 | 7.40 | 6.60 | 7.20 | 5.60 |
| FINS (μU/ml) | 0.75 | 1.85 | 3.79 | 8.45 | 2.11 | 35.00 |
| 0.5-h insulin (μU/ml) | 1.85 | 2.12 | 6.32 | 60.20 | 3.57 | 214.00 |
| 1-h insulin (μU/ml) | 2.87 | 4.44 | 7.94 | 107.00 | 7.77 | 266.00 |
| 2-h insulin (μU/ml) | 4.83 | 9.81 | 18.00 | 98.60 | 8.79 | 101.00 |
| 3-h insulin (μU/ml) | 5.41 | 12.00 | N/A | N/A | N/A | N/A |
| F-Cp (ng/ml) | 0.40 | 0.51 | 1.36 | 2.12 | 0.60 | 3.81 |
| 0.5-h Cp (ng/ml) | 0.64 | 0.57 | 1.70 | 6.11 | 0.93 | 11.90 |
| 1-h Cp (ng/ml) | 0.79 | 0.87 | 1.99 | 10.80 | 1.61 | 14.40 |
| 2-h Cp (ng/ml) | 1.33 | 1.81 | 3.86 | 13.60 | 2.30 | 9.67 |
| 3-h Cp (ng/ml) | 1.62 | 2.65 | N/A | N/A | N/A | N/A |
| HOMA-IR | 0.19 | 0.47 | 0.68 | 2.08 | 0.45 | 6.97 |
| Right baPWV (cm/s) | N/A | 1396.00 | 1741.00 | 1781.00 | 1188.00 | 1154.00 |
| Left baPWV (cm/s) | 1553.00 | 1436.00 | 1923.00 | 1803.00 | 1191.00 | 1130.00 |
| Right ABI | N/A | 1.13 | 1.00 | 1.19 | 1.16 | 0.91 |
| Left ABI | 1.20 | 1.11 | 0.95 | 1.07 | 1.16 | 0.97 |
| LSM (kPa) | N/A | 4.60 | 6.90 | 11.9 | 7.20 | 4.00 |
| CAP (db/m) | N/A | 182.40 | 263.40 | 293.80 | 219.10 | 241.10 |
| HR (bpm) | 84 | 74 | 65 | 67 | 57 | 78 |
| IA-2A (U/ml) | 0.01 | <0.70 | 1.40 | <0.70 | <0.70 | <0.70 |
| GAD-ab (U/ml) | 0.01 | 0.95 | 0.66 | 0.75 | 0.76 | 0.64 |
| ICA (COI) | Negative | 0.07 | 0.08 | 0.06 | 0.05 | 0.06 |
| IAA (COI) | Negative | 0.10 | 0.12 | 0.13 | 0.13 | 0.49 |
| ZnT8A (AU/ml) | N/A | <1.00 | N/A | N/A | N/A | N/A |
| BUN (mmol/l) | 3.91 | 5.17 | 6.30 | 6.63 | 7.16 | 5.11 |
| Cr (mmol/l) | 27.00 | 27.50 | 75.00 | 44.00 | 60.00 | 46.00 |
| UA (mmol/l) | 236.60 | 282.20 | 342.70 | 286.10 | 338.90 | 344.40 |
| TG (mmol/l) | 1.30 | 0.88 | 0.99 | 0.97 | 1.39 | 1.33 |
| TC (mmol/l) | 8.76 | 4.71 | 4.31 | 5.89 | 6.03 | 4.01 |
| HDL-c (mmol/l) | 1.31 | 1.26 | 1.12 | 1.54 | 1.69 | 1.11 |
| LDL-c (mmol/l) | 6.88 | 2.97 | 2.81 | 4.23 | 4.21 | 2.74 |
| VLDL-c (mmol/l) | 0.57 | 0.48 | 0.38 | 0.12 | 0.13 | 0.16 |
| CRP (mmol/l) | 1.36 | 0.69 | 1.13 | 0.69 | 0.16 | 0.19 |
| AST (U/l) | 12.00 | 10.00 | 18.00 | 17.00 | 17.00 | 13.00 |
| ALT (U/l) | 14.00 | 15.00 | 12.00 | 22.00 | 18.00 | 10.00 |
| GGT (U/l) | 20.00 | 13.00 | 21.00 | 29.00 | 16.00 | 16.00 |
| Urine glucose | Positive | Negative | Negative | Negative | Positive | Negative |
| Urine protein | Negative | Negative | Negative | Negative | Negative | Negative |
| Urine Cr (mmol/l) | 4.59 | 3.63 | 6.95 | 2.27 | 13.07 | 26.39 |
| UMA (mg/l) | 52.20 | 15.70 | 15.70 | 8.70 | 9.90 | 28.10 |
| UACR (mg/mmol) | 11.37 | 4.33 | 2.26 | 3.83 | 0.76 | 1.06 |
| RBC (×1012/l) | 4.80 | 4.05 | 3.80 | 4.60 | 5.06 | 4.36 |
| WBC (×109/l) | 7.57 | 4.89 | 6.90 | 6.51 | 6.29 | 5.93 |
| Plt (×109/l) | 371.00 | 315.00 | 283.00 | 240.00 | 287.00 | 340.00 |
| Hb (g/l) | 139.00 | 117.00 | 118.00 | 134.00 | 150.00 | 122.00 |
ABI, ankle-brachial index; ALT, alanine aminotransferase; ASFA, abdominal subcutaneous fat area; AST, aspartate aminotransferase; baPWV, brachial ankle pulse wave velocity; BMI, body mass index; BUN, blood urea nitrogen; CAP, controlled attenuation parameter; Cr, creatinine; CRP, C-reactive protein; DBP, diastolic blood pressure; DKA, diabetic ketoacidosis; F-Cp, fasting C-peptide; FINS, fasting serum insulin; FPG, fasting plasma glucose; N/A, not applicable; GADA, glutamic acid decarboxylase antibodies; GGT, gamma-glutamyl transferase; Hb, hemoglobin; HbA1c, hemoglobin A1c; HC, hip circumference; HDL-c, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; HR, heart rate; IA-2A, insulinoma-associated antigen-2 antibodies; IAA, insulin antibodies; ICA, Islet cell antibodies; LDL-c, low-density lipoprotein cholesterol; LSM, liver stiffness measurement; Plt, platelet; RBC, red blood cell; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid; UACR, urine albumin-to-creatinine ratio; UMA, urine microalbumin; VFA, visceral fat area; VLDL-c, very-low-density lipoprotein cholesterol; WBC, white blood cell; WC, waist circumferences; ZnT8A, zinc transporter 8 antibodies.
The proband was treated with insulin (7/U insulin glargine for basal/before bedtime bolus doses and 3/U insulin lispro pre-meal bolus doses) and oral hypoglycemic agent (acarbose 1 capsule before each meal). In addition, calcium dobesilate capsules were used to improve circulation, epalrestat tablets to improve diabetic nerve damage, mecobalamin for nerve nutrition, atorvastatin calcium tablets to regulate lipids and aspirin enteric-coated tablets as an antiplatelet agent. Moreover, the patient was given a low-salt and low-fat diet, instructed to exercise appropriately, and advised to monitor blood glucose.
One year later, the patient was readmitted to screen for diabetes-related complications. The clinical indicators and laboratory examination results were significantly improved (Table 1). The hypoglycemic regimen was adjusted to insulin glargine 16/U basal dose/bedtime bolus dose. The other treatment regimens were unchanged.
Discussion
Maturity-onset diabetes of the young (MODY) encompasses a diverse set of inherited endocrine disorders, exhibiting autosomal dominance in transmission. These disorders contribute to approximately 1–5% of all diabetes cases. They typically manifest at an early age, primarily before 35 years old, and particularly in individuals younger than 25. MODY is not progressively mild, characterized by persistent fasting hyperglycemia, presence of residual pancreatic function, negative for diabetes-associated antibodies, non-insulin-dependent and involvement across multiple generations within a family.1,2
MODY7 arises from a mutation in the KLF11 gene. The clinical manifestations associated with MODY vary significantly, and diagnosis is primarily reliant on identifying distinct clinical features. Unfortunately, some individuals with MODY may remain undiagnosed for extended periods. Consequently, molecular diagnosis of MODY is crucial for effectively treating, predicting outcomes and providing genetic counseling for individuals with monogenic diabetes. In this particular study, the researchers identified a mutation (c.820C > T) in the KLF11 gene within a specific family, thus expanding our understanding of the genotype and clinical spectrum of MODY7.
Oral sulfonylureas are generally recommended for individuals with MODY7 as they have proven to be effective.3,4 A previous study supported the administration of insulin therapy at the early stage of MODY7,2,5,6 which aligns with the treatment received by the proband in our study. In this particular case, better control of the proband’s blood glucose levels was achieved by substituting sulfonylureas with insulin, acarbose and metformin. In addition, a stable total energy intake was maintained by reducing high-sugar and high-fat foods. Hence, a combination of oral antidiabetic drugs, subcutaneous insulin injections and dietary intervention may contribute to glycemic control in diabetic patients with KLF11 mutations. However, considering the limited number of reported MODY7 cases, further evidence is required to evaluate long-term microvascular complications and determine the optimal pharmacological management.
Acknowledgements
We thank the participants involved in the study.
Contributor Information
Y Wang, Department of Endocrinology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, China.
X Ye, Department of Endocrinology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, China.
X Chen, Department of Endocrinology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, China.
H Zang, Department of Endocrinology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, China.
Q Shen, Department of Endocrinology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, China.
L Chen, Department of Endocrinology, The Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou 215000, China.
Author contributions
Yalin Wang (Data curation [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Writing—original draft [equal]), Xuan Ye (Data curation [equal], Investigation [equal], Validation [equal], Visualization [equal], Writing—original draft [equal]), Xinxin Chen (Investigation [equal], Resources [equal]), Huanhuan Zang (Investigation [equal], Resources [equal]), Qiong Shen (Investigation [equal], Resources [equal], Supervision [equal], Visualization [equal]) and Lei Chen (Conceptualization [lead], Formal analysis [equal], Funding acquisition [lead], Methodology [lead], Supervision [equal], Visualization [lead], Writing—review & editing [lead])
Funding
The study was supported by the Suzhou Industrial Technology Innovation Special Project, Project of Suzhou Science and Technology Bureau and Special Subject of Clinical Trials for Demonstration Drugs in Endocrinology Department.
Declarations
Approval of the research protocol: The ethical committee of The Affiliated Suzhou Hospital of Nanjing Medical University.
Informed consent: Informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Registry and the registration: N/A.
Animal studies: N/A.
Conflict of interest
None declared.
References
- 1. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care 2023; 46:S19–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skoczek D, Dulak J, Kachamakova-Trojanowska N.. Maturity onset diabetes of the young-new approaches for disease modelling. Int J Mol Sci 2021; 22:7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jang KM. Maturity-onset diabetes of the young: update and perspectives on diagnosis and treatment. Yeungnam Univ J Med 2020; 37:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes 2019; 12:1047–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun Y, Qu J, Wang J, Zhao R, Wang C, Chen L, et al. Clinical and functional characteristics of a novel KLF11 Cys354Phe variant involved in maturity-onset diabetes of the young. J Diabetes Res 2021; 2021:7136869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ushijima K, Narumi S, Ogata T, Yokota I, Sugihara S, Kaname T, et al. ; Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes. KLF11 variant in a family clinically diagnosed with early childhood-onset type 1B diabetes. Pediatr Diabetes 2019; 20:712–9. [DOI] [PubMed] [Google Scholar]