Abstract
Glucagon-like peptide 1 (GLP-1), a gastrointestinal peptide and central mediator of glucose metabolism, is secreted by L cells in the intestine in response to food intake. Postprandial secretion of GLP-1 is triggered by nutrient-sensing via transporters and G-protein-coupled receptors (GPCRs). GLP-1 secretion may be lower in adults with obesity/overweight (OW) or type 2 diabetes mellitus (T2DM) than in those with normal glucose tolerance (NGT), but these findings are inconsistent. Because of the actions of GLP-1 on stimulating insulin secretion and promoting weight loss, GLP-1 and its analogs are used in pharmacologic preparations for the treatment of T2DM. However, physiologically stimulated GLP-1 secretion through the diet might be a preventive or synergistic method for improving glucose metabolism in individuals who are OW, or have impaired glucose tolerance (IGT) or T2DM. This narrative review focuses on fasting and postprandial GLP-1 secretion in individuals with different metabolic conditions and degrees of glucose intolerance. Further, the influence of relevant diet-related factors (e.g., specific diets, meal composition, and size, phytochemical content, and gut microbiome) that could affect fasting and postprandial GLP-1 secretion are discussed. Some studies showed diminished glucose- or meal-stimulated GLP-1 response in participants with T2DM, IGT, or OW compared with those with NGT, whereas other studies have reported an elevated or unchanged GLP-1 response in T2DM or IGT. Meal composition, especially the relationship between macronutrients and interventions targeting the microbiome can impact postprandial GLP-1 secretion, although it is not clear which macronutrients are strong stimulants of GLP-1. Moreover, glucose tolerance, antidiabetic treatment, grade of overweight/obesity, and sex were important factors influencing GLP-1 secretion. The results presented in this review highlight the potential of nutritional and physiologic stimulation of GLP-1 secretion. Further research on fasting and postprandial GLP-1 concentrations and the resulting metabolic consequences under different metabolic conditions is needed.
Keywords: glucagon-like peptide 1, type 2 diabetes mellitus, glucose tolerance, postprandial metabolism, meal challenge, human
Introduction
Type 2 diabetes mellitus (T2DM), a major lifestyle-related disease, has been increasing in prevalence globally. T2DM is associated with several physical and psychological comorbidities and is a major health concern [1,2]. Obesity, particularly excessive fat accumulation in the abdomen, is an important risk factor for T2DM. Therefore, fighting obesity and T2DM has become a global goal, leading to increased research in this field [3,4]. Nutrition plays a crucial role in addressing this challenge. This is because energy deficits can restore normal body weight and improve health. Moreover, a targeted selection of foods or nutrients can address specific therapeutic goals through various pathways. One potential mechanism through which diet can influence obesity and T2DM is through gastrointestinal peptide hormones, such as glucagon-like peptide 1 (GLP-1) [5]. GLP-1 has been shown to positively affect several factors related to obesity and T2DM, including pancreatic β-cell function, blood glucose homeostasis, satiety, and food intake [[6], [7], [8], [9]]. These physiologic actions of GLP-1 indicate its high therapeutic potential. This, together with studies showing impaired GLP-1 secretion in T2DM, has led to the exploration of GLP-1 as a treatment option for T2DM over the last few decades. Although GLP-1 secretion seems to be reduced in obesity and T2DM, intravenous administration of GLP-1 analogs elicits metabolic responses similar to that of healthy individuals, suggestive of a preserved GLP-1 sensitivity in obesity and T2DM. Therefore, augmentation of endogenous GLP-1 secretion and administration of exogenous GLP-1 have become research targets. GLP-1 analogs [and dipeptidylpeptidase-4 (DPP-IV) inhibitors] have been successfully included in diabetes treatment for several years [7,[9], [10], [11], [12], [13]].
The secretion of GLP-1 is partly mediated by nutrient binding to G-protein-coupled receptors (GPCRs) or by absorption via membrane transporters, which are expressed by enteroendocrine L cells in the gastrointestinal tract. Thus, postprandial GLP-1 secretion also has therapeutic potential. Adjusting the diet in a way that increases the interaction with these molecules could enhance GLP-1 secretion and amplify its beneficial effects.
This review presents recent evidence on glucose- and food-dependent secretion of GLP-1 in individuals with different metabolic conditions, including obesity, impaired glucose tolerance (IGT), and T2DM, as well as its potential modulation through short- and long-term dietary approaches.
Physiologic functions of GLP-1 inside the gut-brain-pancreas axis
GLP-1 is mainly synthesized and secreted by enteroendocrine L cells of the gastrointestinal tract, which are considered the key components of the gut-brain-pancreas axis [14]. Plasma concentrations of GLP-1 are low after an overnight fast and increase after food intake. Its postprandial secretion is partly mediated by direct nutrient sensing by GPCRs which may be activated by peptides, amino acids, monounsaturated fatty acids, polyunsaturated fatty acids, and SCFAs [15]. GLP-1 has also been shown to be triggered by monosaccharide substrates of the apical sodium-dependent glucose co-transporter (SGLT1) [16,17]. Results from in vitro and animal models have shown that nutrients and metabolites (e.g., SCFAs) derived from bacterial fermentation of dietary fiber can stimulate the secretion of GLP-1 via GPCR41 and GPCR43, also termed free-fatty acid receptors (FFAR) 2 and FFAR 3 [[18], [19], [20]]. In humans, nondigestible and fermentable dietary fibers have been shown to increase GLP-1 secretion [[21], [22], [23]]; however, the role of SCFAs remains unclear [[24], [25], [26], [27]].
An important function of GLP-1 is as an incretin. The so-called incretin effect stimulates insulin secretion mediated by hormones released from the gastrointestinal tract—the incretin hormones, GLP-1, and the glucose-dependent insulinotropic polypeptide (GIP). This effect was observed when glucose was administered orally but not after intravenous infusion (which did not stimulate the secretion of incretin hormones) [28]. The magnitude of the “incretin effect” depends on the amount of glucose ingested [29]. Moreover, GLP-1 attenuates glucagon release, increases pancreatic β-cell mass, and regulates gastrointestinal motility by slowing down gastric emptying and enhancing satiety [[30], [31], [32], [33]]. Therefore, an impaired GLP-1 secretion might contribute to the development of IGT and T2DM. However, the extent to which impaired GLP-1 secretion is involved in disease progression remains unclear.
Current studies suggest that GLP-1 plays a modulatory role in the regulation and maintenance of cognitive function, and a postprandial increase in GLP-1 concentrations seems to contribute to neuroprotection. Hence, the physiologic stimulation of GLP-1 release by nutrients, diet, or changes in microbially produced metabolites may contribute to improved brain health [34]. These neuroprotective properties make GLP-1 an interesting target for nutritional intervention and further scientific investigation.
GLP-1 secretion among individuals with different metabolic profiles
GLP-1 secretion has been widely studied in individuals with different metabolic profiles owing to its multiple beneficial properties for glucose homeostasis. Basal- and food-dependent GLP-1 secretion has been hypothesized to differ between healthy adults and adults with IGT or T2DM; however, the results are conflicting. Some studies have shown reduced basal or postprandial GLP-1 concentrations in patients with obesity or T2DM [[35], [36], [37], [38]]. Simon et al. [37] found that the glucose-stimulated secretion of GLP-1 was lower in individuals with obesity than in their age- and sex-matched lean controls. Similar findings were reported in a large study involving 1400 individuals [39]. However, a recent meta-analysis of 18 studies showed that variations in fasting and postprandial GLP-1 secretion in people with and without T2DM were generally small and heterogeneous [40], although most studies suggested impaired secretion in individuals with obesity. Therefore, it is important to investigate GLP-1 secretion in individuals with different metabolic properties and degrees of glucose tolerance. To assess this association, both fasting and postprandial concentrations of glucose- and GLP-1 responses should be included.
Methods
A literature search was performed according to standard procedures. We searched for human intervention studies published between 1996 and 2023 in PubMed that investigated glucose- or food-stimulated total GLP-1 secretion in 5 groups of participants; adults with normal weight, overweight/obesity (OW), normal glucose tolerance, impaired glucose tolerance, or T2DM. Studies were included only if they compared at least 2 of these groups. In addition, studies assessing the role of meal composition in GLP-1 secretion or the effects of short-, medium-, and long-term nutritional interventions, and probiotic, prebiotic, and synbiotic interventions on GLP-1 secretion in at least one of the listed groups of participants were included. The search terms are listed in Supplemental Table 1. Filters applied were humans and clinical trials.
The following data were extracted according to data availability to assess the studies: fasting values of GLP-1, time, and concentration of the maximum values of postprandial GLP-1, and AUC and/or incremental area under the curve (iAUC) for participants grouped as healthy, OW, prediabetes, and diabetes. Data regarding AUC and/or iAUC were extracted from the studies when available. Studies that assessed fasting GLP-1 concentration and/or postprandial GLP-1 secretion after oral glucose tolerance test (OGTT), mixed meal tolerance test (MMTT), or challenge meals were included. In addition, studies assessing these outcomes before and after medium- or long-term interventions were included. The data used in this review are mainly original values that were provided by the corresponding authors (marked as #original data in the tables). If the original data values were not available and the authors could not be successfully contacted, GLP-1 values were estimated from graphs provided in articles, partly with the help of WebPlotDigitizer (web-based Plot Digitizer, Copyright 2010-2020 Ankit Rohatgi, https://apps.automeris.io/wpd/).
Glucose-stimulated GLP-1 response
Because GLP-1 secretion is consistently stimulated by glucose, the OGTT is an adequate tool for examining postprandial GLP-1 response. In this review, only studies that used the standard 75-g-glucose OGTT were included. Five different groups were considered for analysis; adults with normal weight (NW) or OW, NGT, IGT [including impaired fasting glucose (IFG)], and T2DM.
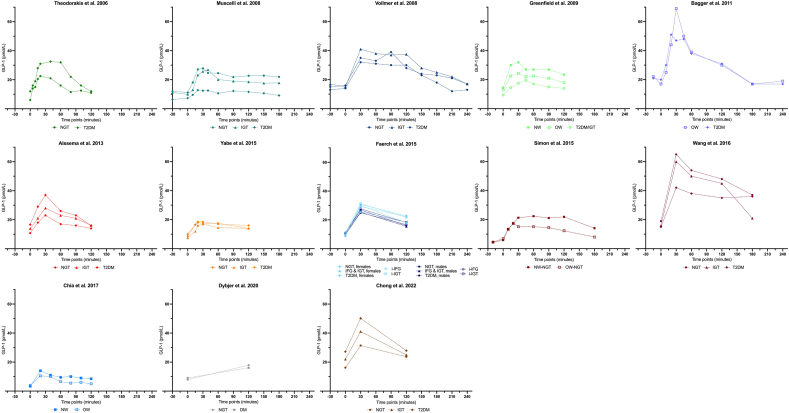
Table 1 [[41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]] provides an overview of the results of the 13 studies included on fasting and peak values of glucose-stimulated GLP-1, the delta between both values, the time at which peak concentration is achieved, GLP-1 concentrations at selected time points, and the AUC or iAUC values (the GLP-1 concentrations measured at all time points are shown in Supplemental Table 2). In addition, details of the assays used for GLP-1 measurements are presented. Eight studies applied the same standardized assay based on radioimmunoassay (RIA) methodology [41]. Overall, across all participants and studies, the time taken to reach the maximum postprandial concentrations after the OGTT was between 20 and 90 min, and ranged from 10.5 and 69 pmol/L. In the NGT group, the time taken to reach maximum peak concentrations was between 20 [42,43] and 30 min [39,[44], [45], [46], [47]], whereas in the IGT group, the time was between 30 [39,44,46,47] and 45 min [48], in TD2M group, the time was between 30 and 90 min (most studies observed a peak at 30 min) [39,[44], [45], [46], [47],49]. The results of these studies are shown as postprandial GLP-1 concentration curves in Figure 1. Theodorakis et al. [42] reported increased GLP-1 fasting values and AUC in T2DM compared with those in the NGT group [42]. These results were confirmed by Alssema et al. [44] and Chong et al. [50].
TABLE 1.
Glucose-stimulated GLP-1 response (OGTT)
| Author, (y), n | GLP-1 assay | Participant characteristics | GLP-1 values (pmol/L) at time points (min) |
TTP (min) | Δ Peak (pmol/L) | GLP-1 AUC/iAUC | Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 10 | 15 | 20 | 30 | 40 | 45 | 60 | 90 | 120 | 180 | ||||||||
| Theodorakis et al. 2006 [42] n = 53 | RIALinco1 | NGT (n = 36, BMI: 27 kg/m2) | 6 | 15 | 21 | 22 | 21 | 16 | 11 | 20 | 16 | 120 min: AUC: 1874 (pmol/L∗min) # |
↑ fasting values and AUC in T2DM compared with NGT | |||||
| T2DM (n = 17, BMI: 30 kg/m2) | 12 | 19 | 28 | 31 | 33 | 32 | 12 | 40 | 21 | 120 min: AUC: 2866 (pmol/L∗min) # |
||||||||
| Muscelli et al. 2008 [45] n = 51 | RIA2 | NGT (n = 24, BMI: 33.1 kg/m2)# | 11 | 18 | 27 | 28 | 25 | 25 | 22 | 23 | 23 | 30 | 17 | 180 min: AUC: 4100 (pmol/L∗h) # |
- ↓ GLP-1 response in T2DM compared with IGT and NGT - no significant differences between NGT and IGT - inverse relationship between GLP-1 response and BMI |
|||
| IGT (n = 17, BMI: 35.9 kg/m2)# | 10 | 13 | 23 | 26 | 27 | 20 | 19 | 19 | 18 | 40 | 17 | 180 min: AUC: 3400 (pmol/L∗h)# |
||||||
| T2DM (n = 10, BMI: 35.5 kg/m2)# | 7 | 10 | 13 | 12 | 13 | 11 | 12 | 12 | 9 | 20 40 | 6 | 180 min: AUC: 2000 (pmol/L∗h)# |
||||||
| Vollmer et al. 2008 [49] n = 48 |
RIA2 | NGT (n = 14, BMI: 27.5 kg/m2) | 14 | 32 | 31 | 30 | 30 | 18 | 30 | 18 | n.a. | - ↔ - positive relation between GLP-1 and age; negative association between GLP-1 concentrations and BMI - GLP-1 concentrations higher in females than in males |
||||||
| IGT (n = 17, BMI: 29.5 kg/m2) | 15 | 41 | 38 | 37 | 38 | 25 | 30 | 26 | ||||||||||
| T2DM (n = 17, BMI: 32.1 kg/m2) | 16 | 35 | 33 | 39 | 28 | 23 | 90 | 23 | ||||||||||
| Greenfield et al. 2009 [48] n = 24 | RIA2 | NW (n = 8, BMI: 22 kg/m2) | 14 | 30 | 32 | 27 | 27 | 27 | 24 | 30 | 18 | 120 min: AUC: 3192 iAUC: 1242 (pmol/L∗120 min)# |
↓ fasting values and AUC in T2DM/ IGT compared with NW/ OW ↔ iAUC |
|||||
| OW (n = 8, BMI: 34.5 kg/m2) | 13 | 22 | 24 | 22 | 23 | 21 | 18 | 30 | 11 | 120 min: AUC: 2550 iAUC:1082 (pmol/L∗ 120 min)# |
||||||||
| T2DM/IGT (n = 8, BMI: 38.5 kg/m2) | 9 | 15 | 18 | 20 | 17 | 15 | 14 | 45 | 11 | 120 min: AUC: 1846 iAUC: 746 (pmol/L∗ 120 min)# |
||||||||
| Bagger et al. 2011 [53] n = 16 |
RIA2 | OW (n = 8, BMI: 29 kg/m2) | 17 | 25 | 44 | 69 | 50 | 39 | 30 | 17 | 30 | 52 | AUC: 7200# | ↔ fasting/ peak values, AUC | ||||
| T2DM (n = 8, BMI: 29 kg/m2) | 20 | 30 | 51 | 47 | 48 | 38 | 31 | 17 | 20 | 31 | AUC: 6900# (4h∗ pmol/L) | |||||||
| Alssema et al. 2013 [44] n = 203 | RIA2 | NGT (n = 163, BMI: 26.7 kg/m2) | 11 | 18 | 23 | 17 | 16 | 14 | 30 | 12 | AUC: 18 iAUC: 7.3 (pmol/L per hour) | ↑ fasting values and AUC per hour in T2DM compared with NGT | ||||||
| IGT (n = 20, BMI: 28.5 kg/m2) | 14 | 21 | 28 | 23 | 21 | 16 | 30 | 14 | AUC: 21 iAUC: 6.7 (pmol/L per hour) | |||||||||
| T2DM (n = 20, 33.1 kg/m2) | 17 | 29 | 37 | 26 | 23 | 16 | 30 | 20 | AUC: 23 iAUC: 6.5 (pmol/L per hour) | |||||||||
| Yabe et al. 2015 [47] n = 102 |
RIA2 | NGT (n = 54, BMI: 21.3 kg/m2) | 9 | 17 | 19 | 19 | 17 | 16 | 20 30 | 10 | 120 min: AUC: 2004 (pmol/L∗min) |
↔ | ||||||
| IGT (n = 20, BMI: 22.5 kg/m2) | 8 | 12 | 16 | 17 | 15 | 14 | 30 | 9 | 120 min: AUC: 1701 (pmol/L∗min) |
|||||||||
| T2DM (n = 28, BMI: 23.5 kg/m2) | 10 | 17 | 18 | 18 | 18 | 14 | 20 30 40 | 8 | 120 min: AUC: 1923 (pmol/L∗min) |
|||||||||
| Færch et al. 2015 [39] n = 1462 |
RIA2 | NGT (n = 774, BMI: 26.0 kg/m2)# | F | 9 | 31 | 23 | 30 | 22 | 120 min: AUC: 3124 (pmol/L∗min)# |
- ↓GLP-1 response in IFG and T2DM (- up to25%) compared with NGT (females) - ↓120-min concentrations in IFG and T2DM (-16-21%) compared with NGT - ↓ GLP-1 response in persons with obesity (-20%) and OW (up to -8%) compared with NW participants |
||||||||
| M | 10 | 26 | 18 | 30 | 16 | 120 min: AUC: 2612 (pmol/L∗min)# |
||||||||||||
| IGT (n = 525), 3 subgroups# | ||||||||||||||||||
| i-IFG (BMI: 27.7 kg/m2) | F | 11 | 29 | 22 | 30 | 18 | 120 min: AUC: 3029 (pmol/L∗min)# |
|||||||||||
| M | 10 | 25 | 17 | 30 | 15 | 120 min: AUC: 2479 (pmol/L∗min)# |
||||||||||||
| i-IGT (BMI: 27.4 kg/m2) | F | 11 | 31 | 22 | 30 | 20 | 120 min: AUC: 3131 (pmol/L∗min)# |
|||||||||||
| M | 11 | 28 | 18 | 30 | 17 | 120 min: AUC: 2680 (pmol/L∗min)# |
||||||||||||
| IFG&IGT (BMI: 28.8 kg/m2) | F | 10 | 26 | 18 | 30 | 16 | 120 min: AUC: 2591 (pmol/L∗min)# |
|||||||||||
| M | 10 | 25 | 16 | 30 | 15 | 120 min: AUC: 2466 (pmol/L∗min)# |
||||||||||||
| T2DM (n = 163, BMI: 29.7 kg/m2)# | F | 9 | 30 | 18 | 30 | 21 | 120 min: AUC: 2849 (pmol/L∗min)# |
|||||||||||
| M | 11 | 27 | 16 | 30 | 16 | 120 min: AUC: 2582 (pmol/L∗min)# |
||||||||||||
| Simon et al. 2015 [43] n = 21 |
RIA2 | NW-NGT (n = 11, BMI: 23.6 kg/m2)# | 6 | 14 | 17 | 21 | 22 | 21 | 22 | 14 | 60 | 16 | n.a. | ↓ GLP-1 response in OW-NGT compared with NW-NGT | ||||
| OW-NGT (n = 10, BMI: 35.5 kg/m2)# | 7 | 13 | 18 | 15 | 15 | 14 | 12 | 8 | 20 | 11 | ||||||||
| Wang et al. 2016 [46] n = 80 (Han Chinese adults) |
ELISA (Westang)3 | NGT (n = 23, BMI: 25.6 kg/m2) | 19 | 65 | 54 | 48 | 37 | 30 | 46 | 180 min: AUC: 192 (pmol/L∗min) |
- ↓ fasting values in T2DM compared with NGT - ↓ peak values and AUC in T2DM compared with NGT/ IGT - no significant differences between NGT/ IGT |
|||||||
| IGT (n = 22, BMI: 26.0 kg/m2) | 16 | 60 | 50 | 45 | 21 | 30 | 44 | 180 min: AUC: 182 (pmol/L∗min) |
||||||||||
| T2DM (n = 35, BMI: 26.5 kg/m2) | 15 | 42 | 38 | 35 | 26 | 30 | 27 | 180 min: AUC: 129 (pmol/L∗min) |
||||||||||
| Chia et al. 2017 [52] n = 40 |
ELISA (Alpco)4 | NW (n = 20, BMI: 23.6 kg/m2) | 3 | 14 | 11 | 10 | 9 | 20 | 11 | 120 min: AUC: 1150 (pmol/L∗min)# |
↓ AUC in OW | |||||||
| OW (n = 20, BMI: 35.6 kg/m2) | 4 | 11 | 10 | 7 | 5 | 20 | 7 | 120 min: AUC: 831 (pmol/L∗min)# |
||||||||||
| Dybjer et al. 2020 [51] n = 3001 | RIALinco1 | NGT (n = 2453, BMI: 26.3 kg/m2)# | 8 | 18 | 120 | 10 | n.a. | - ↑ fasting levels in DM compared with NGT - ↓ postprandial levels in DM compared with NGT |
||||||||||
| DM (n = 548, BMI: 28.7 kg/m2)# | 9 | 16 | 120 | 7 | ||||||||||||||
| Chong et al. 2022 [50] n = 174 |
ELISA (Millipore)5 | NGT (n = 58, BMI: 24 kg/m2)# | 16 | 31 | 24 | 30 | 15 | 120 min: AUC: 3266 (pmol/L∗min)# |
- ↑ levels at fasting, after 30 min and AUC in T2DM compared with IGT and NGT - ↑ levels at fasting, after 30 min and AUC in IGT compared with NGT |
|||||||||
| IGT (n = 54, BMI: 26.2 kg/m2)# | 22 | 41 | 25 | 30 | 19 | 120 min: AUC: 3994 (pmol/L∗min)# |
||||||||||||
| T2DM (n = 62, BMI: 26.7 kg/m2)# | 27 | 50 | 28 | 30 | 23 | 120 min: AUC: 4698 (pmol/L∗min)# |
||||||||||||
GLP-1 concentrations for selected time points of blood sampling, time-to-peak as well as peak (peak concentration – fasting concentration), and—if available—AUC or iAUC values are listed. Under “results,” only significant results are listed. Time point 0 describes fasting concentrations, and time points >0 are reporting postprandial concentrations.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups, ↔ no differences of GLP-1 secretion between groups. Supplemental Table 2 shows GLP-1 values for all measured time points. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: AUC, the area under the curve; CV, coefficient of variation; DM, diabetes mellitus; F, female; GLP-1, glucagon-like peptide 1; IFG, impaired fasting glucose; i-IFG, isolated impaired fasting glucose; IGT, impaired glucose tolerance; i-IGT, isolated impaired glucose tolerance; LOD, limit of detection; M, male; n.a., not available; NGT, normal glucose tolerance; NW, normal weight; OGTT, oral glucose tolerance test; OW, overweight; RIA, radioimmunoassay; TTP, time-to-peak; T2DM, type 2 diabetes mellitus.
RIALinco (Linco Research), polyclonal antiserum no. 89390, LOD: 3 pM, intra-assay CV: 5%, inter-assay CV: 17%, specificity: 100% for GLP-1 (7–36) and GLP-1 (9–36);
RIA: standardized assay (41), polyclonal antiserum no. 89390, LOD: 1 pM, intra-assay CV: 6%, specificity: 100% for GLP-1 (7–36) 89% for GLP-1 (9–36);
ELISA (Westang Biological Technology), sensitivity: <0.1 pM, intra- and inter-assay CV: <10.3%;
ELISA (Alpco Diagnostics), intra-assay CV: 3.7%–4.7%, inter-assay CV: 6.2–9.5%;
ELISA (EMD Millipore), LOD: 1.5 pM, intra-assay CV: <2% inter-assay CV: <12%.
FIGURE 1.
Glucagon-like peptide 1 concentrations during oral glucose tolerance tests in different metabolic conditions. Mean fasting and postprandial values of participants with normal glucose tolerance, impaired glucose tolerance, isolated impaired glucose tolerance, impaired fasting glucose or isolated impaired fasting glucose, type 2 diabetes mellitus or diabetes mellitus, normal weight, and overweight in 13 studies [39,[42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53]].
Dybjer et al. [51] also reported higher fasting GLP-1 concentrations in participants with unclassified T2DM than in nondiabetic controls; however, glucose-stimulated concentrations after 120 min were lower in participants with diabetes than in nondiabetic patients. Similarly, Muscelli et al. [45] showed reduced GLP-1 secretion in T2DM compared with that in IGT and NGT, whereas no significant differences were observed between the IGT and NGT groups. Given that GLP-1 and BMI are inversely related, a lack of significant differences in GLP-1 secretion between the IGT and NGT groups could be due to a similar mean BMI of the participants in these groups [45]. Wang et al. [46] also showed a comparable impaired GLP-1 response in individuals with NGT, IGT, or T2DM. In that study, the T2DM group showed distinctly reduced fasting/ peak values and AUC (≤33%) than the NGT group. No significant differences were observed between the NGT and IGT groups. Færch et al. [39] found ≤25% reduced GLP-1 secretion in females with IFG or T2DM compared with females with NGT. In addition, in both males and females with IFG or T2DM, GLP-1 concentrations were reduced by 16%–21% after 120 min, independent of age and BMI. In this cohort, the GLP-1 response in individuals with obesity was reduced by 20% and in individuals with overweight by ≤8% compared with NW participants, independent of their glucose tolerance status. The authors concluded that impaired GLP-1 response can occur before developing obesity or T2DM. In the present study, higher GLP-1 concentrations were associated with better insulin sensitivity, older age, and a lower degree of OW. These findings aligned with the results of a study by Chia et al. [52], who found a reduced GLP-1 AUC in individuals who had OW compared with those who were of NW, although the fasted and peak values of GLP-1 did not differ between the NW and OW groups [52]. Similar findings were reported by Simon et al. [43]. Greenfield et al. [48] also showed a trend toward reduced fasted GLP-1 values and a lower AUC in the OW group compared with lean-matched controls. Moreover, this effect was more distinct in groups with T2DM/IGT compared with NW and OW groups.
Vollmer et al. [49] detected no differences among the NGT, IGT, and T2DM groups. They described a positive association between GLP-1 response and increasing age and a negative association with higher BMI. Furthermore, higher concentrations of GLP-1 were found in females than in males. Two other studies found no differences in fasting/peak values and AUC between groups with NGT/OW and IGT/T2DM [47,53].
Of the 13 studies that were reviewed, a marked difference was observed in the time taken to reach peak GLP-1 concentrations after OGTT ingestion. Overall, the evidence shows that individuals with NGT attain a GLP-1 peak earlier than individuals with IGT or T2DM. Although OGTT is a highly standardized procedure, the results of the reviewed studies did not provide a conclusive answer as to whether fasting and postprandial GLP-1 values differed or were similar in individuals with disturbed glucose control than in healthy controls. Therefore, further research is needed to account for confounding factors such as BMI and sex.
Food-stimulated GLP-1 response
Owing to the food-stimulated secretion of GLP-1, MMTTs, as a test meal containing all macronutrients, or a meal challenge are widely used in clinical research to monitor postprandial GLP-1 secretion [54]. In this part of the review, studies that used an MMTT with liquid or small-to-large solid challenge meals which were consumed within 10–15 min (as far as information was available) in participants with NGT, IGT, or T2DM were analyzed. Table 2 [[55], [56], [57], [58]] presents the fasted and peak GLP-1 values, delta, time of reaching peak concentrations, AUC or iAUC values, and GLP-1 concentrations at selected time points after food ingestion (the GLP-1 concentrations at any available time points are shown in Supplemental Table 3), and information on the GLP-1 assays. Six of the 7 studies used the same RIA-based assay [41].
TABLE 2.
Food-stimulated GLP-1 response (MMTT/ challenge meals)
| Author, (y), n | GLP-1 assay | Test meal (Duration, nutrient composition) | Participant characteristics | GLP-1 values (pmol/L) at time points (min) |
TTP (min) | Δ Peak (pmol/L) | GLP-1 AUC/iAUC | Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 90 | 120 | 150 | 180 | |||||||||
| Solid meals | |||||||||||||||||
| Rask et al. 2001 [57] n = 33 males |
RIA1 | Western-styled breakfast (10 min) (424 kcal, 49% CH, 38% F, 13% P) |
High IS (HIS, n = 11, BMI:: 23.9 kg/m2) | 11 | 19 | 17 | 14 | 15 | 16 | 15 | 14 | 15 | 8 | 45 min: 771 (pmol/L∗min)# |
- ↔ fasting levels, total AUC, iAUC - ↓ 15min values in LIS compared with HIS - ↓ overall and early GLP-1 response (AUC) in LIS compared with HIS |
||
| Medium IS (MIS, n = 11, BMI:: 26.4 kg/m2) | 8 | 13 | 16 | 14 | 15 | 15 | 15 | 13 | 30 | 8 | n.a. | ||||||
| Low IS (LIS, n = 11, BMI:: 30.4 kg/m2) | 6 | 8 | 14 | 11 | 10 | 11 | 13 | 10 | 30 | 8 | 45 min: 482 (pmol/L∗min)# |
||||||
| Toft-Nielsen et al. 2001 [38] n = 102 | RIA1 | Western-styled breakfast (10-15 min) (537 kcal, 41% CH, 42% F, 17% P) |
NGT (n = 33, BMI:: 29.6 kg/m2) | 5 | 14 | 18 | 18 | 16 | 11 | 60 80 | 13 | 240 min: AUC: 3101 iAUC: 1927 (pmol/L∗min)# |
- ↓ AUC and iAUC in T2DM compared with NGT (and IGT upon ANOVA correcting for BMI: and sex) - ↑ fasting values in T2DM compared with NGT; ↔ upon ANOVA |
||||
| IGT (n = 15, BMI:: 35 kg/m2) | 5 | 13 | 16 | 14 | 13 | 10 | 60 | 11 | 240 min: AUC: 2765 iAUC: 1587 (pmol/L∗min)# |
||||||||
| T2DM (n = 54, BMI:: 30.2 kg/m2) | 7 | 13 | 14 | 12 | 11 | 9 | 60 | 7 | 240 min: AUC: 2482 iAUC: 907 (pmol/L∗min)# |
||||||||
| Vilsbøll et al. 2003 [56] n = 24 | RIA1 | Western-styled breakfast (10 min) (48% CH, 33% F, 19% P) Small (S) (260 kcal) Large (L) (520 kcal) |
NGT-NW (n = 8, BMI:: 22.5 kg/m2) | S | 14 | 23 | 31 | 30 | 31 | 25 | 21 | 18 | 17 | 60 | 17 | 180 min: 3336 (pmol/L∗ min)# | - ↓ AUC, iAUC in T2DM compared with matched NGT (small and large meal) - ↓ late phase values in T2DM compared with matched NGT (small meal) |
| L | 18 | 21 | 35 | 31 | 30 | 33 | 30 | 28 | 25 | 30 | 17 | 180 min: 4197 (pmol/L∗ min)# | |||||
| NGT-OW (n = 8, BMI:: 32.5 kg/m2) | S | 17 | 26 | 36 | 33 | 34 | 33 | 27 | 27 | 26 | 30 | 19 | 180 min: 4301 (pmol/L∗ min)# | ||||
| L | 17 | 29 | 42 | 40 | 40 | 37 | 36 | 32 | 29 | 30 | 25 | 180 min: 5030 (pmol/L∗ min)# | |||||
| T2DM (n = 8, BMI:: 32.1 kg/m2) | S | 18 | 19 | 29 | 30 | 27 | 20 | 19 | 17 | 18 | 45 | 12 | 180 min: (pmol/L∗ min)# | ||||
| L | 18 | 19 | 31 | 32 | 34 | 33 | 30 | 23 | 20 | 60 | 16 | 180 min: 4008 (pmol/L∗ min)# | |||||
| Ryskjær et al. 2006 [58] n = 16 |
RIA1 | Western-styled breakfast (15 min) (566 kcal, 47% CH, 34% F, 19% P) |
NGT (n = 8, BMI:: 30.9 kg/m2) | 20 | 21 | 28 | 28 | 29 | 31 | 28 | 27 | 26 | 90 | 11 | 180 min: AUC: 4877 iAUC: 1296 (pmol/L∗ min)#: |
↔ AUC, iAUC | |
| T2DM (n = 8, BMI:: 33 kg/m2) | 23 | 27 | 38 | 39 | 34 | 35 | 33 | 31 | 33 | 45 | 16 | 180 min: AUC: 5950 iAUC: 1735 (pmol/L∗ min)# |
|||||
| Vollmer et al. 2008 [49] n = 48 |
RIA1 | Western-styled breakfast (15 min) (820 kcal, 44% CH, 43% F, 13% P) |
NGT (n = 14, BMI:: 27.5 kg/m2) | 14 | 24 | 30 | 33 | 31 | 34 | 29 | 150 | 20 | n.a. | ↔ | |||
| IGT (n = 17, BMI: 29.5 kg/m2) | 19 | 35 | 38 | 41 | 34 | 36 | 37 | 90 | 22 | ||||||||
| T2DM (n = 17, BMI:: 32.1 kg/m2) | 14 | 37 | 33 | 37 | 31 | 34 | 29 | 30 90 | 23 | ||||||||
| Alssema et al. 2013 [44] n = 203 |
RIA1 | Western-styled breakfast (n.a.) (833 kcal, 36% CH, 52% F, 12% P) |
NGT (n = 163, BMI: 26.7 kg/m2) | 11 | 14 | 17 | 16 | 18 | 20 | 17 | 120 | 9 | AUC: 17 iAUC: 5.8 (pmol/L per hour) | - ↓GLP-1 response in T2DM compared with NGT/IGT - ↔ AUC - ↓ iAUC in T2DM compared with NGT/ IGT - no differences between NGT/ IGT |
|||
| IGT (n = 20, BMI:: 28.5 kg/m2) | 13 | 15 | 21 | 18 | 22 | 22 | 18 | 90 120 | 9 | AUC: 20 iAUC: 5.5 (pmol/L per hour) | |||||||
| T2DM (n = 20, BMI:: 33.1 kg/m2) | 16 | 18 | 22 | 21 | 21 | 20 | 18 | 30 | 6 | AUC: 20 iAUC: 3 (pmol/L per hour) | |||||||
| Solid + liquid meals | |||||||||||||||||
| Ruetten et al. 2018 [55] n = 62 |
RIA (Merck Millipore)2 | Test meal (10 Min) 237ml Boost (Nestlé) + 1 PowerBar (Nestlé) (470 kcal, 66% CH, 18% F, 16% P) |
NGT (n = 23, BMI:: 31.5 kg/m2) | 5 | 13 | 8 | 9 | 30 | 8 | 120 min: iAUC: 512 (pmol∗min/mL) | -↑ fasting values in T2DM compared with NGT/ IGT -↑ iAUC in T2DM compared with NGT/ IGT |
||||||
| IGT (n = 17, BMI:: 35 kg/m2) | 5 | 10 | 9 | 8 | 30 | 5 | 120 min: iAUC: 384 (pmol∗min/mL) | ||||||||||
| T2DM (n = 22, BMI:: 32.8 kg/m2) | 9 | 20 | 17 | 12 | 30 | 11 | 120 min: iAUC: 785 (pmol∗min/mL) | ||||||||||
GLP-1 concentrations for selected time points of blood sampling, time-to-peak as well as peak (peak concentration – fasting concentration) and —if available—AUC or iAUC values are listed. Under “results” only significant results are listed. Time point 0 describes fasting concentrations, time points >0 are reporting postprandial concentrations. Under “results” only significant results are listed.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups, ↔ no differences of GLP-1 secretion between groups.
Supplemental Table 3 shows GLP-1 values for all measured time points. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: AUC, area under the curve; CH, carbohydrate; CV, coefficient of variation; F, fat; GLP-1, glucagon-like peptide 1; HIS, high insulin sensitivity; iAUC: incremental area under the curve; IGT, impaired glucose tolerance; IS, insulin sensitivity; L, large; LIS, low insulin sensitivity; LOD, limit of detection; MIS, medium insulin sensitivity; MMTT, mixed meal tolerance test; n.a., not available; NGT, normal glucose tolerance; NW, normal weight; OW, overweight; P, protein; RIA, radioimmunoassay; S, small; TTP, time-to-peak; T2DM, type 2 diabetes mellitus.
RIA [standardized assay (41)], polyclonal antiserum no. 89390, LOD: 1 pM, intra-assay CV: 6%, specificity: 100% for GLP-1 (7–36) 89% for GLP-1 (9–36);
RIA (Merck Millipore), sensitivity: 3 pM, specificity: 100% for GLP-1 (7–36) and GLP-1 (9–36), intra-assay CV: 22–36%, inter-assay CV: 10–23%.
The maximum postprandial GLP-1 concentrations were reached between 15 and 150 min after MMTT or challenge meal ingestion and varied between 9.7 and 42 pmol/L.
Only one study used an MMTT (470 kcal) consisting of both a liquid plus a solid meal [237 mL Boost Drink (Nestlé) and a Power Bar (Nestlé)]. Fasting GLP-1 concentrations were 75 % higher in adults with T2DM than in those with NGT/IGT. The iAUC of total GLP-1 was higher in T2DM than in IGT (1200 pg∗min/mL compared with 2600 pg∗min/mL) but did not differ between NGT and IGT, or NGT and T2DM [55].
In contrast, several studies that used a solid meal for MMTT or challenge meal tests have reported impaired GLP-1 response in patients with (pre)diabetes. Vilsbøll et al. [56], investigated GLP-1 response after consumption of a small (260 kcal) compared with a large (520 kcal) western-styled breakfast meal consisting of a glass of milk and white and black bread with margarine, cheese, and jam. A reduced GLP-1 response was observed in T2DM patients when compared with matched NGT controls after both small and large meals [56]. These results were confirmed in the study by Alssema et al. [44] in which a reduced GLP-1 response was found in T2DM after consuming a western-styled breakfast consisting of 2 croissants with butter and cheese, full-fat milk, and a yogurt drink with soluble carbohydrates (maltose) (833 kcal) when compared with NGT/IGT. Moreover, there were no differences in GLP-1 response between the NGT and IGT groups. Rask et al. [57], also reported an impaired early GLP-1 response after the ingestion of a western-styled breakfast meal consisting of bread with butter, cheese, and jam (424 kcal) in insulin-resistant males when compared with matched nondiabetic controls. Fifteen minutes after meal ingestion, GLP-1 concentrations in the insulin-resistant participants reached 44% of the GLP-1 concentrations of their matched controls. Additionally, the iAUC30min was 45% lower than that in the insulin-sensitive controls. Furthermore, an association was found between the degree of insulin resistance and impaired GLP-1 response [57]. Toft-Nielsen et al. [38], reported important results after conducting a western-styled challenge breakfast meal test (537 kcal). Although lower AUC and iAUC values were observed in the T2DM group than in the NGT and IGT groups, higher fasting values were observed in the T2DM group than in the NGT group, whereas there were no significant differences between the three groups. The AUC for the IGT group ranged between those of the NGT and T2DM groups. Furthermore, the AUC in male participants was lower than that in female participants and decreased with increasing BMI [38].
In addition to the studies that showed increased or decreased GLP-1 response in patients with (pre)diabetes, some studies that compared healthy, glucose-tolerant individuals and individuals with IGT/T2DM reported no significant difference in GLP-1 secretion. Likewise, Ryskjær et al. [58] and Vollmer et al. [49] did not find differences in GLP-1 response between NGT/IGT and T2DM groups after consuming a western-styled breakfast meal [58].
A review of 7 studies investigating the MMTT-stimulated GLP-1 response showed that the differences in GLP-1 peak time and concentrations were larger than those in the OGTT studies. This could be due to the heterogeneity of the applied test meals, or also because of the additional protein and fat from the test meals. However, most of the reviewed studies reported an impaired GLP-1 response in patients with (pre)diabetes. Additional research in well characterized, highly comparable cohorts with different metabolic conditions is needed to evaluate test meal-stimulated GLP-1 response.
Role of meal composition for GLP-1 secretion
Because human nutrition predominantly consists of meals prepared by combining foods, analyzing the effect of whole meals or meal patterns on GLP-1 secretion, in addition to the OGTT and MMTT, is an important approach. Among other things, meal composition could also influence the results of MMTT or challenge meal studies. In this review, 14 intervention studies compared GLP-1 responses to different liquid or solid tests and challenge meals or foods; see Table 3 [[59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71]] and Supplemental Table 4. Although for most studies no information on meal duration was provided, the meal durations in the remaining studies were 10–20 min. These studies varied in the methods used to measure the GLP-1 concentrations.
TABLE 3.
GLP-1 response and meal composition
| Author, (y), n | GLP-1 assay | Test meal (Duration, nutrient composition) | Participant characteristics | GLP-1 values (pmol/L) at time points (min) |
TTP (min) | Δ Peak (pmol/L) | GLP-1 AUC/iAUC | Results | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 15 | 30 | 45 | 60 | 90 | 120 | 180 | 240 | |||||||||
| Solid meals | |||||||||||||||||
| Rijkelijkhuizen et al. 2010 [69] n = 24 RCT cross-over |
RIA1 | Test meals (10 min) Small CH-rich meal (SCH, 460 kcal. 66% CH, 18% F, 16% P) Large CH-rich meal (LCH, 680 kcal, 66% CH, 18% F, 16% P) Fat-rich meal (FRM, 833 kcal, 36% CH, 52% F, 12% P) |
NGT (n = 6, BMI:: 26.9 kg/m2) | SCH | 5 | 11 | 13 | 14 | 120 | 9 | 120 min: iAUC: 13.5 (pmol/L∗h) | ↔ absolute values - in NGT values still rising after first 2 h, in T2DM stabilized -↑ iAUC after LCH compared with FRM in T2DM |
|||||
| LCH | 7 | 15 | 17 | 22 | 120 | 15 | 120 min: iAUC: 16.8 (pmol/L∗h) | ||||||||||
| FRM | 4 | 15 | 13 | 16 | 120 | 12 | 120 min: iAUC: 19.1 (pmol/L∗h) | ||||||||||
| T2DM (n = 18, BMI:: 28.9 kg/m2) | SCH | 3 | 13 | 15 | 14 | 60 | 12 | 120 min: iAUC: 18.3 (pmol/L∗h) | |||||||||
| LCH | 3 | 18 | 19 | 19 | 60 120 | 16 | 120 min: iAUC: 23.8 (pmol/L∗h) | ||||||||||
| FRM | 3 | 13 | 12 | 12 | 30 | 10 | 120 min: iAUC: 13.6 (pmol/L∗h) | ||||||||||
| Törrönen et al. 2012 [63] n = 12 RPCT cross-over |
ELISA (Millipore)2 | Test meal (n.a.): IG: 35 g sugar + berries (150 g) CG: placebo |
NGT (BMI:: 24.3 kg/m2) | IG | 26 | 32 | 34 | 28 | 27 | 26 | 25 | 30 | 8 | 120 min: AUC: 312 (pmol/L∗min)# |
↑ GLP-1 secretion (overall difference) borderline significant in IG compared with CG | ||
| CG | 25 | 29 | 30 | 26 | 26 | 26 | 25 | 30 | 5 | 120 min: AUC: 216 (pmol/L∗min)# |
|||||||
| Belinova et al. 2014 [65] n = 100 RCT cross-over |
Multiplex immunoanalysis xMAP technology3 | Test meal (n.a.): VE: Vegan (455 kcal, 52% CH, 37% F, 11% P) ME: processed meat meal (455 kcal, 27% CH, 52% F, 21% P) |
NGT (n = 50, BMI:: 24.4 kg/m2) | VE | 3.7 | 3.9 | 4 | 3.8 | 3.7 | 60 | 0.3 | n.a. | ↑ postprandial GLP-1 concentration in T2DM in VE compared with ME | ||||
| ME | 3.6 | 4.3 | 4.2 | 3.9 | 3.8 | 30 | 0.7 | ||||||||||
| T2DM (n = 50, BMI:: 33.3 kg/m2) | VE | 4.5 | 11 | 7.5 | 5 | 4.5 | 30 | 6.5 | |||||||||
| ME | 4 | 4.5 | 4.7 | 4.4 | 4.2 | 60 | 0.7 | ||||||||||
| Carnevale et al. 2017 [61] n = 30 RCT cross-over |
ELISA (Sigma Aldrich)4 | Test meal (n.a.): (725 kcal, 53-54% CH, 28-30% F, 16-19% P) IG: with CG: without olive oil |
IFG (BMI:: 31.3 kg/m2)# | IG | 2 | 5 | 12 | 120 | 10 | n.a | ↑ GLP-1 secretion in IG compared with CG | ||||||
| CG | 2 | 3 | 6 | 120 | 4 | ||||||||||||
| Alyami et al. 2019 [66] n = 26 RCT cross-over |
ELISA (Millipore)2 | Breakfast meal (15 min): SOP: Scottish oats porridge (220 kcal, 42 g CH, 4.4 g F) PMP: Pearl millet porridge (220 kcal, 44 g CH, 3 g F) |
NGT (BMI:: 23.4 kg/m2)# | SOP | 27 | 39 | 37 | 29 | 25 | 24 | 22 | 15 | 12 | 120 min: iAUC: 3670 (pmol/L∗min)# | ↔ | ||
| PMP | 29 | 35 | 37 | 29 | 24 | 21 | 22 | 30 | 8 | 120 min: iAUC: 3467 (pmol/L∗min)# | |||||||
| Schönknecht et al. 2020 [60] n = 60 RCT cross-over |
RIA1 | Breakfast challenge meal (20 min): WDHF: Western diet high-fat (1010 kcal, 37% CH, 53% F, 10% P) WDHC: Western diet high-CH (1013 kcal, 58% CH, 31% F, 10% P) MED: Mediterranean Diet (1012 kcal, 53% CH, 36% F, 10% P) |
OW (BMI:: 30.9 kg/m2)# | WDHF | 29 | 44 | 44 | 30 | 37 | 60 120 | 15 | 300 min: iAUC: 46 (pmol/L∗h)# | ↔ fasting/ postprandial values, iAUC | ||||
| WDHC | 30 | 44 | 46 | 41 | 37 | 120 | 16 | 300 min: iAUC: 44 (pmol/L∗h)# | |||||||||
| MED | 29 | 44 | 44 | 40 | 38 | 60 120 | 15 | 300 min: iAUC: 45 (pmol/L∗h)# | |||||||||
| Di Mauro et al. 2021 [68] n = 12 RCT cross-over |
ELISA (Millipore)2 | Test meal (n.a.): MED: Mediterranean diet (665 kcal, 32% CH, 45% F, 23% P) HVF: High-fiber Vegetarian diet (704 kcal, 78% CH, 8% F, 15% P) |
T2DM (BMI:: 34.4 kg/m2) | MED | 13 | 18 | 17 | 17 | 17 | 15 | 30 | 5 | 210 min: AUC: 11359 (pg/mL∗min)# |
- ↑ AUC in MED compared with HFV ↔ iAUC (trend for ↑ in MED) |
|||
| HFV | 12 | 16 | 14 | 15 | 13 | 13 | 30 | 4 | 210 min: AUC: 9576 (pg/mL∗min)# |
||||||||
| Muangchan et al. 2021 [62] n = 6 RCT cross-over |
ELISA (Millipore)2 | Test meal (n.a.): 100 g steamed rice with microwaved labeled egg + water IG: Riceberry rice (370 kcal, 75.5 g CH, 3.6 g F, 8.6 g P, 4.1 g fiber) CG: white rice (350 kcal, 79.4 g CH, 1.2 g F, 5.7 g P, 1.7 g fiber) |
NW (BMI:: 21.5 kg/m2) | IG | 38 | 39 | 37 | 35 | 43 | 21 | 120 | 5 | 180 min: iAUC: 7094 (pmol/L∗min) | - ↔ iAUC - trend for ↑ values in IG after 30 and 60 min |
|||
| CG | 32 | 31 | 28 | 27 | 33 | 38 | 180 | 6 | 180 min: iAUC: 5687 (pmol/L∗min) | ||||||||
| Dicks et al. 2022 [64] n = 22 RCT cross-over |
RIA1 | Test meal (15 min): IG: Enriched with 20 g powder of oyster mushroom (456 kcal, 67.9 g CH, 21.8 g F, 7.1 g P) CG: without enrichment (403 kcal, 52.5 g CH, 21.5 g F, 5.0 g P) |
IGT (BMI:: 31.3 kg/m2) | IG | 10 | 18 | 22 | 25 | 29 | 29 | 27 | 18 | 11 | 60 90 | 19 | 240 min: AUC: 5380 (pmol/L∗min) |
- ↑ concentration at 180 min in IG compared with CG - ↑ AUC in IG compared with CG |
| CG | 9 | 17 | 21 | 25 | 28 | 25 | 23 | 13 | 9 | 60 | 19 | 240 min: AUC: 4612 (pmol/L∗min) |
|||||
| Bajka et al. 2023 [71] n = 20 RCT cross-over |
Electrochemilumin-escent multiplexed assay (Mesoscale Discovery)5 | Test meal (n.a.): bread with jam + water IG1: wheat bread with 30% cellular chickpea powder (360 kcal, 48.3 g CH, 5.6 g F, 17.7 g P, 6.2 g fiber) IG2: wheat bread with 60% cellular chickpea powder (435 kcal, 48.2 g CH, 8.8 g F, 27.2 g P, 10.7 g fiber) CG: wheat bread (310 kcal, 48.1 g CH, 3.3 g F, 12.9 g P, 2.6 g fiber) |
NW (BMI: ∼. 23.5 kg/m2) | IG1 | 0 | 15 | 29 | 25 | 29 | 25 | 20 | 10 | 6 | 30 60 | 29 | 120 min: iAUC: 2825 (pmol/L∗min)# | - ↑ iAUC and postprandial values in IGs compared with CG, especially during late postprandial period. IG2 lead to higher secretion than IG1 - ↔ maximum values, but peaks were reached ∼ 40 min later in IG2 |
| IG2 | 0 | 12 | 26 | 25 | 27 | 33 | 31 | 22 | 15 | 90 | 33 | 120 min: iAUC: 3051 (pmol/L∗min)# | |||||
| CG | 0 | 14 | 25 | 21 | 17 | 15 | 11 | 3 | 2 | 30 | 25 | 120 min: iAUC: 1966 (pmol/L∗min)# | |||||
| Nakamura et al. 2023 [67] n = 17 RCT cross-over |
ELISA (Yanaihara Institute Inc.)6 | Test meal (n.a.): IG: 150 g cooked OIST rice (222 kcal, 45 g CH, 2.1 g F, 5.9 g P, 7.1 g resistant starch) CG: 150 g cooked white rice (210 kcal, 48.5 g CH, 0.5 g F, 3 g P, 1.4 g resistant starch) |
T2DM (BMI:: 25.9 kg/m2) | IG | 13 | 18 | 15 | 14 | 15 | 30 | 5 | 240 min: AUC: 3964 iAUC: 505 (pmol/L∗min)# |
↔ (i)AUC | ||||
| CG | 14 | 20 | 19 | 17 | 17 | 30 | 6 | 240 min: AUC: 3970 iAUC: 608 (pmol/L∗min)# |
|||||||||
| Liquid (+ solid) meals | |||||||||||||||||
| Lamiquiz-Moneo et al. 2022 [59] n = 10 RCT cross-over |
Human Metabolic Hormone Magnetic Bead Panel (Merck)7 | Test drinks: Isoglucidic interventions (25 g CH) (n.a.) IG1: Regular alcohol-free beer (AFB) IG2: CH-modified AFB + isomaltulose (2.5 g/100 ml) + resistant maltodextrin (0.8 g/100 ml) IG3: CH-modified AFB + resistant maltodextrin (2.0 g/100 ml) CG: glucose (25 g CH) |
NW (BMI:: 23.4 kg/m2) | IG1 | 51 | 56 | 46 | 38 | 38 | 42 | 21 | 15 | 5 | 120 min: AUC: 17402 |
- ↑ AUC in all IGs compared with CG - ↔ iAUC - ↔ maximum increase over baseline value |
||
| IG2 | 52 | 52 | 45 | 40 | 37 | 41 | 43 | 0 15 | 0 | 120 min: AUC: 16929 |
|||||||
| IG3 | 50 | 49 | 40 | 40 | 38 | 41 | 42 | 0 | -1 | 120 min: AUC: 16633 |
|||||||
| CG | 47 | 51 | 40 | 32 | 29 | 29 | 31 | 15 | 4 | 120 min: AUC: 13580 |
|||||||
| Lamiquiz-Moneo et al. 2022 [59] n = 20 RCT cross-over |
Human Metabolic Hormone Magnetic Bead Panel (Merck)7 | Test meals + drinks: Isoglucidic interventions (64.3 g CH) (n.a.) 50 g CH from white bread + 14.3 g CH from IG1: AFB IG2: CH-modified AFB + isomaltulose (2.5 g/ 100 mL) + resistant maltodextrin (0.8 g /100 mL) IG3: CH-modified AFB + resistant maltodextrin IG4: Extra white bread + water CG: + water (50 g CH) |
NW (BMI:: 24.4 kg/m2) | IG1 | 59 | 67 | 59 | 53 | 50 | 48 | 46 | 15 | 8 | 120 min: AUC: 20,813 |
- ↑ AUC in all IGs compared with CG - ↑ AUC in IG1 compared with IG2 - ↔ iAUC - ↔ maximum increase over baseline value |
||
| IG2 | 57 | 58 | 55 | 53 | 48 | 44 | 42 | 15 | 1 | 120 min: AUC: 18,338 |
|||||||
| IG3 | 49 | 57 | 50 | 46 | 44 | 46 | 40 | 15 | 8 | 120 min: AUC: 19,560 |
|||||||
| IG4 | 50 | 60 | 59 | 53 | 50 | 42 | 39 | 15 | 10 | 120 min: AUC: 19,443 |
|||||||
| CG | 45 | 49 | 48 | 44 | 40 | 36 | 36 | 15 | 4 | 120 min: AUC: 16,286 |
|||||||
| Smith et al. 2023 [70] n = 18 RCT cross-over |
ELISA (Millipore)2 | Test meal (15 min): cereal + milk (387 kcal, 58% CH, 27% F, 15% P) IG: test meal + pre-meal whey protein drink (100 kcal, 15.6 g P) CG: test meal + pre-meal placebo shot (35 kcal, <.1 g P) |
T2DM (BMI:: 32.7 kg/m2) | IG | 47 | 58 | 62 | 57 | 55 | 51 | 45 | 38 | 35 | 30 | 15 | 240 min: iAUC: 14.6 (pmol/L∗min)# | ↑ iAUC and postprandial values in IG compared with CG |
| CG | 35 | 44 | 46 | 46 | 43 | 39 | 33 | 29 | 31 | 30 45 | 11 | 240 min: iAUC: 4.4 (pmol/L∗min)# | |||||
GLP-1 concentrations for selected time points of blood sampling, time-to-peak as well as Δ peak (peak concentration – fasting concentration) and —if available—AUC or iAUC values are listed. Under “results” only significant results are listed. Time point 0 describes fasting concentrations, time points >0 are reporting postprandial concentrations.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups, ↔ no differences of GLP-1 secretion between groups.
Supplemental Table 4 shows GLP-1 values for all measured time points. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: AFB, alcohol-free beer; approx., approximately; AUC, area under the curve; CH, carbohydrates; CG, control group; CV, coefficient of variation; F, fat; FRM, fat-rich meal; GLP-1, glucagon-like peptide 1; HFV, high-fiber vegetarian diet; iAUC, incremental area under the curve; IFG, impaired fasting glucose; IG, intervention group; IGT, impaired glucose tolerance; LCH, large carbohydrate-rich meal; LOD, limit of detection; ME, meat meal; MED, Mediterranean diet; n.a., not available; NGT, normal glucose tolerance; NW, normal weight, OW, overweight; P, protein; PMP, pearl millet porridge; RIA, radioimmunoassay; RPCT, randomized placebo-controlled trial; SCH, small carbohydrate-rich meal; SOP, Scottish oats porridge; TTP, time-to-peak; T2DM, type 2 diabetes mellitus; VE, vegan meal; WDHC, western diet high-carbohydrate; WDHF, western diet high-fat.
RIA [standardized assay (41)], polyclonal antiserum no. 89390, LOD: 1 pM, intra-assay CV: 6%, specificity: 100% for GLP-1 (7–36) 89% for GLP-1 (9-36);
ELISA (Merck Millipore), LOD: 1.5 pM, intra-assay CV: < 5%, inter-assay CV: < 12%;
Multiplex immunoanalysis xMAP technology, MILLIPEX MAP Human Gut Hormone Panel (Millipore), sensitivity: 13.7 pg/mL;
ELISA (Arbor Assay, Sigma Aldrich), sensitivity: 1.5 pM, intra-assay CV: 1%–2%, inter-assay CV: < 12%;
Electrochemilumin-escent multiplexed assay (Mesoscale Discovery), n.a.;
ELISA (YK 161, Yanaihara Institute Inc.), sensitivity: 1.5 pM, intra-assay CV: 2%–5.4%, inter-assay CV: 2.2%–3.8%, cross-reactivity: 100% for GLP-1 (7–36) and for GLP-1 (9–36);
Human Metabolic Hormone Magnetic Bead Panel MILLIPLEX MAP Kits (Cat. # HMHEMAG-34K, Merck), sensitivity: 7.3 pg/mL, intra-assay CV: < 10%, inter-assay CV: < 15%.
Across all the studies and participants, the maximum postprandial GLP-1 concentrations were reached between 15 [59] and 180 [[60], [61], [62]] min after the ingestion of the test meal.
Adding plant-based foods such as berries [63], virgin olive oil [61], or mushroom powder [64] to the test meals compared with nonfortified test meals resulted in increased postprandial GLP-1 secretion in individuals who were of NW or OW groups, or had IFG. In another study, a vegan test meal increased the postprandial GLP-1 response in patients with T2DM compared with an isoenergetic test meal containing processed meat. Further, the GLP-1 peak concentration was more than twice as high after the vegan test meal, indicating the possible clinical relevance of this acute change. However, similar effects have not been demonstrated in healthy or glucose-tolerant individuals [65]. Another study examining the effect of Scottish oats compared with that of isoenergetic pearl millet porridge on the postprandial GLP-1 response in healthy NW adults showed no differences between the 2 test meals [66]. In addition, a comparison of two test meals with either white rice or riceberry rice (rich in antioxidant bioactive compounds and high in protein and fiber) in 6 healthy men did not reveal significant differences in postprandial GLP-1 secretion, whereas there was a slight trend for higher values after consuming riceberry rice [62]. Moreover, in 17 participants with T2DM, a test meal with a newly developed rice variety rich in resistant starch (OIST rice) did not lead to higher GLP-1 AUC or iAUC values than a control meal of regular white rice [67].
When investigating the impact of 3 isocaloric test meals representing 3 different dietary patterns (high-fat western diet, high-carbohydrate western diet, and Mediterranean diet) on 60 participants who were OW, no meal-induced differences in postprandial GLP-1 values were found [60]. In contrast to these results, a recent study of 12 individuals with T2DM found that a Mediterranean test meal led to ∼20% higher GLP-1 AUC210min than a high-fiber vegetarian test meal. However, when expressed as the iAUC, the difference was not statistically significant [68]. Rijkelijkhuizen et al. [69] investigated the GLP-1 response after a test meal with a small (460 kcal) and large carbohydrate-rich challenge meal (680 kcal) or a fat-rich meal (833 kcal) in adults with NGT and T2DM. There were no differences in the GLP-1 response (absolute values) detected between both the groups; however, in individuals with NGT, postprandial GLP-1 concentrations kept increasing even 2 h after the meal, whereas in individuals with T2DM, the concentrations returned to baseline levels. Moreover, a large carbohydrate-rich meal caused a ∼30% higher iAUC when compared with the fat-rich meal only in adults with T2DM.
A study focusing on the macronutrient composition of test meals found that a meal tolerance test with a liquid pre-meal whey protein shot led to an augmented postprandial GLP-1 response when compared with a placebo shot in 18 patients with T2DM [70]. Bajka et al. [71] found that higher amounts of cellular chickpea flour (60% compared with 30%; meaning higher amounts of protein, fiber, and fat content) led to increased GLP-1 secretion, especially in the late postprandial period in 20 healthy participants. Results on the effect of carbohydrate composition on GLP-1 secretion are discordant. Lamiquiz-Moneo et al. [59] investigated the effects of a liquid (plus solid) test meal and found higher GLP-1 AUCs in NW individuals after a single intake of 3 different complex carbohydrate-containing drinks (regular alcohol-free beer, carbohydrate-modified alcohol-free beer + isomaltulose + resistant maltodextrin, or carbohydrate-modified alcohol-free beer + resistant maltodextrin) compared with consuming an isoglycemic glucose-based control beverage, with no differences between intervention groups. However, when combining the different drinks with white bread, significant differences in the AUC between the intervention groups were observed, in addition to a difference from the control group. The iAUCs and maximum increase in GLP-1 concentrations did not differ significantly between the groups in the 2 studies [59].
After reviewing 14 studies that used single-test meals, we found the results to be inconclusive. However, the results suggest that higher fiber and protein content might substantially increase GLP-1 secretion, with the potential to evoke metabolic alterations.
GLP-1 secretion after short-, mid-, and long-term dietary interventions
To date, evidence from controlled human intervention studies investigating the short-term, mid-term or long-term dietary effects on GLP-1 secretion is rare. In this review, fasting and postprandial GLP-1 secretion in humans and controlled intervention studies investigating the effect of short-term to long-term dietary interventions on glucose- (OGTT) or food-stimulated (MMTT test or challenge meals) were examined (see Table 4 for studies assessing dietary intervention effects on glucose- or food-stimulated GLP-1 secretion, Table 5 for studies assessing effects on fasting levels [[72], [73], [74], [75], [76], [77], [78], [79]] and Supplemental Table 5 for GLP-1 concentrations measured at all time points). Different assays were used to measure GLP-1 concentrations in these studies.
TABLE 4.
Glucose- or food-stimulated GLP-1 secretion after short-, mid-, and long-term dietary interventions
| Author, (y), n | GLP-1 assay | Intervention Participant characteristics |
GLP-1 values (pmol/L) at time points (min) |
TTP (min) | Δ Peak (pmol/L) | GLP-1 AUC/iAUC (pmol/L ∗ min) | Results | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | 150 | 180 | |||||||||
| Glucose-stimulated GLP-1 secretion (OGTT) before and after dietary intervention | |||||||||||||||
| Otten et al. 2019 [72] n = 70 RCT |
RIA1 | 2-y intervention: Paleo diet (PD) Healthy Nordic Diet (ND) (slight energy reduction) NGT (BMI: 32.3 kg/m2) females |
ND# | 0 mo | 10 | 18 | 20 | 18 | 17 | 60 | 10 | 120 min: AUC: 2424 iAUC: 1084 |
- ↑ AUC, iAUC in PD and ND compared with baseline - ↑ fasting values in ND compared with baseline |
||
| 6 mo | 10 | 21 | 21 | 19 | 19 | 30 60 |
11 | 120 min: AUC: 2769 iAUC: 1449 |
|||||||
| 24 mo | 12 | 27 | 26 | 24 | 22 | 30 | 15 | 120 min: AUC: 2826 iAUC: 1572 |
|||||||
| PD# | 0 mo | 11 | 23 | 21 | 22 | 20 | 30 | 12 | 120 min: AUC: 2113 iAUC: 933 |
||||||
| 6 mo | 11 | 25 | 26 | 25 | 24 | 60 | 15 | 120 min: AUC: 2287 iAUC: 1036 |
|||||||
| 24 mo | 11 | 26 | 26 | 26 | 24 | 30 60 90 |
15 | 120 min: AUC: 2907 iAUC: 1488 |
|||||||
| Stentz et al. 2021 [74] n = 24 RCT |
Human Metabolic Hormone Magnetic Bead Panel (Merck)2 |
6-mo intervention: Calorie restricted diets (-500 kcal) High-Protein Diet (HP, 40% CH, 30% F, 30% P) High-Carbohydrate Diet (HC, 55% CH, 30% F, 15% P) OW-IGT (BMI: 39.0 kg/m2) |
HP | 0 mo | 13 | 68 | 44 | 30 | 27 | 30 | 55 | n.a. |
↑ AUC in HP compared with HC |
||
| 6 mo | 17 | 85 | 56 | 38 | 29 | 30 | 68 | ||||||||
| HC |
0 mo | 15 | 67 | 41 | 29 | 24 | 30 | 52 | |||||||
| 6 mo |
15 |
67 |
48 |
29 |
24 |
30 |
52 |
||||||||
| Food-stimulated GLP-1 secretion (test meal) before and after dietary intervention | |||||||||||||||
| Samkani et al. 2018 [78] n = 16 RCT cross-over |
RIA1 | 2-d intervention: High-CH (HC, 54% CH, 30% F, 16% P) CH-reduced high-protein (CRHP, 31% CH, 40% F, 29% P) Breakfast test meal on day 2 T2DM (BMI:: 30 kg/m2) |
HC Day 2 |
11 | 24 | 30 | 26 | 26 | 28 | 25 | 60 | 19 | n.a. | - ↑ postprandial value (120 min) in CRHP compared with HC - ↔ netAUC |
|
| CRHP Day 2 |
11 | 18 | 28 | 30 | 34 | 32 | 28 | 120 | 23 | ||||||
| Samkani et al. 2018 [77] n = 14 RCT cross-over |
RIA1 | 2-d intervention: HC (54% CH, 30% F, 16% P) CRHP (31% CH, 40% F 29% P) Breakfast test meal on day 2 NW to OW (BMI:: 32 kg/m2) |
HC Day 2 |
0 | 11 | 12 | 12 | 16 | 14 | 13 | 120 | 16 | n.a. | ↑ postprandial/ peak, netAUC values in CRHP compared with HC | |
| CRHP Day 2 |
0 | 9 | 14 | 15 | 20 | 20 | 18 | 120 150 | 20 | ||||||
| Fuglsang-Nielsen et al. 2021 [76] n = 73 RCT |
RIA1 | 12 wks intervention: Whey Protein (WP, 60 g/d) or Maltodextrin (MD, 60 g/d) combined with High-Fiber (HF, 30 g/d) or Low-Fiber diet (LF, 10 g/d) High-fat mixed meal test at baseline and endline (360 min) OW (BMI:: 29.4 kg/m2) |
WP-HF | 0 wk | 19.3# | 360 min: iAUC: 6143# |
↔ fasting values, iAUC | ||||||||
| 12 wks | 20.2# | 360 min: iAUC: 3791# |
|||||||||||||
| WP-LF | 0 wk | 21.5# | 360 min: iAUC: 4174# |
||||||||||||
| 12 wks | 19.6# | 360 min: iAUC: 4264# |
|||||||||||||
| MD-HF | 0 wk | 21.2# | 360 min: iAUC: 3896# |
||||||||||||
| 12 wks | 21.1# | 360 min: iAUC: 4676# |
|||||||||||||
| MD-LF | 0 wk | 21# | 360 min: iAUC: 3471# |
||||||||||||
| 12 wks | 20.7# | 360 min: iAUC: 3062# |
|||||||||||||
| Oliveira et al. 2022 [75] n = 43 RCT cross-over |
V-PLEX (Meso Scale)3 | 32-h intervention: IG: High-protein total diet replacement (35% CH, 25% F, 40% P) CG: (55% CH, 30% F, 15% P) Lunch test meal on day 1 NW (BMI:: 22.0 kg/m2) |
IG# | Day 1 | 1.6 | 4.2 | 120 | 2.6 | n.a. | ↑ postprandial values in IG compared with CG on day 1 ↑ fasting values in CG compared with IG on day 2 |
|||||
| Day 2 | 1.2 | ||||||||||||||
| CG# | Day 1 | 1.6 | 2.6 | 120 | 1 | ||||||||||
| Day 2 | 1.5 | ||||||||||||||
| Day 2 | 6.7# | 12 | 10 | 9 | 8 | 10 | 9 | 30 | 5.3 | 180 min: AUC: 440 |
|||||
GLP-1 concentrations for selected time points of blood sampling, time-to-peak as well as peak (peak concentration – fasting concentration), and —if available—AUC or iAUC values are listed. Under “results,” only significant results are listed. Time point 0 describes fasting concentrations, and time points >0 are reporting postprandial concentrations. Under “results,” only significant results are listed.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups, ↔ no differences of GLP-1 secretion between groups.
Supplemental Table 5 shows GLP-1 values for all measured time points. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: AUC, area under the curve; CH, carbohydrates; CG, control group; CRHP, carbohydrate-reduced-high-protein; CV, coefficient of variation; ELISA, enzyme-linked immunosorbent assay; F, fat; GLP-1, glucagon-like peptide 1; HC, high-carbohydrate; HF, high fiber; HP, high protein; iAUC, incremental area under the curve; iFG, impaired fasting glucose; IG, intervention group; IGT, impaired glucose tolerance; LF, low fiber; LOD, limit of detection; MD, maltodextrin; min, minutes; n.a., not available; ND, healthy nordic diet; NGT, normal glucose tolerance; NW, normal weight, OW, overweight; P, protein; PD, paleo diet; RCT, randomized controlled trial; RIA, radioimmunoassay; RPCT, randomized placebo-controlled trial; RT, randomized trial; TTP, time-to-peak; T2DM, type 2 diabetes mellitus; WP, whey protein.
RIA [standardized assay (41)], polyclonal antiserum no. 89390, LOD: 1 pM, intra-assay CV: 6%, specificity: 100% for GLP-1 (7–36) 89% for GLP-1 (9–36);
Human Metabolic Hormone Magnetic Bead Panel MILLIPLEX MAP Kits (Cat. # HMHEMAG-34K, Merck), sensitivity: 7.3 pg/mL, intra-assay CV: < 10%, inter-assay CV: < 15%.
Electro-chemiluminescence V-PLEX (Meso Scale Discovery), specificity: 0.017 pM.
TABLE 5.
Fasting GLP-1 values before and after dietary intervention
| Author, (y), n | GLP-1 assay | Intervention Participant characteristics |
Fasting GLP-1 values (pmol/L) |
Δ | Results | ||
|---|---|---|---|---|---|---|---|
| Baseline | Endline | ||||||
| Ohlsson et al. 2018 [79] n = 30 |
Bio-Plex Pro Human Diabetes 10-plex panel (Bio-Rad Laboratories)1 | 12 wks Okinawa-based ND (slight energy reduction) T2DM (BMI:: 29.9 kg/m2) |
0.81# | 0.54# | - 0.27 | ↓ fasting values compared with baseline | |
| Arjmand et al. 2022 [73] n = 37 RCT |
ELISA (Bioassay Technology Laboratory)2 | 12 wks Calorie restricted MIND diet (n = 22) vs. hypocaloric diet (n = 15) (1500 kcal/day, 50%–55% CH, 30% F, 15%–20% P) OW (BMI:: 32 kg/m2) |
MIND diet | 12.8# | 14.7# | 1.9 | ↑ fasting values after the MIND diet compared with baseline and hypocaloric diet |
| Hypocaloric diet | 8.8# | 7.4# | -1.4 | ||||
Fasting GLP-1 concentrations as well as Δ (endline value – baseline value) before and after dietary intervention are listed. Under “results” only significant results are listed.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: CH, carbohydrates; CV, coefficient of variation; F, fat; GLP-1, glucagon-like peptide 1; MIND, Mediterranean-DASH Intervention for Neurodegenerative Delay; ND, healthy Nordic diet; OW, overweight; P, protein; RCT, randomized controlled trial; T2DM: type 2 diabetes mellitus.
n.a.;
sensitivity: 1.3 pM; intra-assay CV: 2.8%–5.2%.
In a randomized controlled trial (RCT), the effects of a 2-y paleo compared with healthy Nordic diet in 70 healthy NW females on glucose-stimulated GLP-1 secretion were examined. Here, the Paleo diet was characterized by higher protein and fat content and lower carbohydrate content than the healthy Nordic diet, which was based on the Nordic Nutrition Recommendations. In both groups, iAUCs of GLP-1 after OGTT increased significantly in the Paleolithic group by 45% after 24 mo and in the Nordic group by 59%. Fasting GLP-1 concentrations increased only in the Nordic group. Participants in the Paleolithic group lost on average 10% of their body weight in 24 mo (11% in the first 6 mo), and females in the Nordic group lost 6%. The increase in postprandial GLP-1 concentrations was not associated with the macronutrient composition of the diet but with weight loss. However, even after 6 mo, when body weight remained stable, the postprandial GLP-1 concentrations increased [72]. Similar results were shown in a 12-wk human intervention study comparing a calorie-restricted Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet with a hypocaloric control diet. In 37 women who were OW, a significant increase in fasting GLP-1 concentrations was observed only after the MIND diet. In both groups, a significant reduction in BMI and fat mass was seen, and this decrease was greater in the MIND-diet group. The MIND diet mainly consists of green leafy vegetables, legumes, berries, nuts, fish, poultry, and olive oil, and is similar to the Nordic Diet [73]. Furthermore, in participants who were OW or had IGT, a high-protein diet (40% carbohydrates, 30% fat, and 30% protein) resulted in greater postprandial GLP-1 secretion both during OGTT and MMTT when compared with a high-carbohydrate (HC) diet (55% carbohydrates, 30% fat, and 15% protein). Importantly, the drop-out rate within this study was 36%, which might weaken the findings [74]. This is in line with results from a 32-h RCT in which a high-protein total diet replacement (35% carbohydrates, 25% fat, and 40% protein) led to higher postprandial GLP-1 concentrations 2 h after lunch meal compared with an isocaloric low-protein diet (55% carbohydrates, 30% fat, and 15% protein) [75]. When comparing the effects of maltodextrin (60 g/d) and whey protein (60 g/d) in combination with high-fiber (30 g/d) or low-fiber (30 g/d) in a 12-wk randomized controlled intervention trial in individuals who were OW, no effects on fasting GLP-1 concentrations and meal-stimulated GLP-1 response (iAUC) were observed. In this study, compliance with the study diet was high (88%–94%) and the drop-out rate was low (11%), which might strengthen the findings [76].
Samkani et al. [77] investigated the food-stimulated GLP-1 response in 14 adults who were NW or OW after consuming an HC and carbohydrate-reduced high-protein (CRHP) diet for 2 d. The breakfast meals of the 2 diets also served as the MMTT on day 2. The HC breakfast meal was composed of bread, cheese, ham, eggs, and yogurt, whereas the CRHP breakfast consisted of bread, cheese, jam, eggs, apples, almonds, and milk. The HC lunch consisted of chicken, vegetables, bread, milk, and pasta, whereas the CRHP lunch consisted of chicken, vegetables, chickpeas, feta cheese, and bread. After consumption of the CRHP meal, GLP-1 peak concentration was 17% and net AUC was 27% higher than that after HC meal; both changes were statistically significant. The authors also investigated the effects of similar HC and CRHP breakfast and lunch meals on patients with T2DM. The postprandial concentration at 120 min was significantly higher in the CRHP group than in the HC group, but there was no significant difference in the GLP-1 net AUC between the 2 test meals [78]. However, the results of a longer-term intervention from an uncontrolled human study contrast these findings. Ohlsson et al. [79] investigated the health effects of a 12-wk Okinawan-based Nordic Diet with moderately low-carbohydrate, high-fat, and high-protein content in 30 individuals with T2DM and showed a significant decrease in fasting GLP-1. The authors attributed the decrease in GLP-1 concentrations to the low-carbohydrate and high-protein content of the diet. In addition, weight loss was observed after 12 wks. Within this study, a comparably high drop-out rate occurred (23%), which might, in combination with the uncontrolled study design, weaken the findings [79].
To summarize, long-term, health-promoting dietary interventions might have the potential to enhance physiologic GLP-1 secretion both in the fasting and postprandial states. In addition, as seen with the ingestion of single-test meals, it seems that increased protein content in the diet may contribute to a sustained enhancement of GLP-1 secretion. Undoubtedly, there is a limited comparative value of 2-d [75,77,78] interventions and interventions with a duration of weeks or months [73,74,76,79]; however, even very short-term clinical trials can promote an understanding of the underlying mechanisms of diet-induced alterations in GLP-1 secretion, especially as the reported dynamics might recur multiple times of the day after food intake.
The effect of probiotic, prebiotic, and synbiotic interventions on GLP-1 secretion
To date, the effects of probiotic, prebiotic, and synbiotic interventions on gut hormone secretion in humans have only been examined in a few clinical trials. Moreover, there is high heterogeneity in the methodological approaches regarding the interventions and the GLP-1 assays (see Table 6 for studies assessing intervention effects on glucose- or food-stimulated GLP-1 secretion and Table 7 [[80], [81], [82], [83]] for studies assessing effects on fasting levels and Supplemental Table 6 for GLP-1 concentrations at all time points). We found 2 studies investigating the effects of different probiotic interventions on GLP-1 secretion. Simon et al. [43] showed that a 4-wk probiotic supplementation with Lactobacillus reuteri increased GLP-1 concentrations during OGTT by 76% and increased fasting GLP-1 concentrations when compared with the placebo group in 21 glucose-tolerant adults who were either NW or OW. In lean participants, the maximal response to GLP-1 increased in the intervention group. Rondanelli et al. [80] investigated the effect of a 60-d probiotic intervention (500 mg/d Saccharomyces cerevisiae variant boulardii, strain DBVPG 6763 [5.0 × 109 colony-forming units (CFU)] in combination with 1000 international units (IU) superoxide dismutase (which is believed to be diminished by an increase of adipose tissue) compared with placebo on fasting GLP-1 concentration in OW individuals. In both groups, the participants followed an energy-restricted diet (-800 kcal of their daily requirement) for the duration of the study. Both groups showed a significant decrease in GLP-1 fasting values but no significant difference was observed between the intervention and placebo groups, which is likely attributable to energy reduction.
TABLE 6.
Glucose- or food-stimulated GLP-1 secretion after pro-, pre-, and synbiotic interventions
| Author, (y), n | GLP-1 assay | Intervention Participant characteristics |
GLP-1 values (pmol/L) at time points (min) |
TTP (min) | Δ Peak (pmol/L) | GLP-1 AUC (pmol/L∗min) | Results | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 20 | 30 | 45 | 60 | 90 | 120 | 180 | |||||||||||
| Glucose-stimulated GLP-1 secretion (OGTT) before and after interventionrowhead | ||||||||||||||||||
| Simon et al. 2015 [43] n = 21 RPCT |
RIA1 |
4 wks IG: Probiotics (1010 b.i.d. L. reuteri SD5865) CG: Placebo NW-NGT (n = 11, BMI:: 23.6 kg/m2) OW-NGT (n = 10, BMI:: 35.5 kg/m2) |
IG | NW baseline# | 6 | 17 | 24 | 21 | 22 | 22 | 13 | 30 | 18 | n.a. |
- ↑postprandial values, AUC in IG compared with baseline and CG - ↑ΔAUC in IG compared with CG |
|||
| NW 4 wks# | 9 | 34 | 37 | 37 | 29 | 5 | 16 | 30 60 |
28 | |||||||||
| OW baseline# | 6 | 16 | 12 | 15 | 14 | 11 | 6 | 20 | 10 | |||||||||
| OW 4 wks# | 6 | 17 | 19 | 20 | 15 | 18 | 11 | 60 | 14 | |||||||||
| CG |
NW baseline# | 7 | 18 | 20 | 24 | 21 | 23 | 16 | 60 | 17 | ||||||||
| NW 4 wks# | 8 | 17 | 21 | 20 | 20 | 17 | 12 | 30 | 13 | |||||||||
| OW baseline# | 8 | 19 | 18 | 15 | 15 | 14 | 10 | 20 | 11 | |||||||||
| OW 4 wks# |
5 |
14 |
14 |
13 |
14 |
13 |
8 |
20 30 60 |
9 |
|||||||||
| Food-stimulated GLP-1 secretion (test meal) before and after interventionrowhead | ||||||||||||||||||
| Roshanravan et al. 2017 [23] n = 60 RPCT |
ELISA (ZellBio GmbH, Berlin, Germany)2 | 45 d Prebiotic supplements A) sodium butyrate B) inulin C) sodium butyrate + inulin D) placebo Breakfast test meal T2DM (BMI:: 33.3 kg/m2) |
A | Baseline | 27 | n.a. | n.a. | n.a. | ↑ postprandial values in A + C compared with D | |||||||||
| 45 d | 32 | |||||||||||||||||
| B | Baseline | 21 | ||||||||||||||||
| 45 d | 26 | |||||||||||||||||
| C | Baseline | 29 | ||||||||||||||||
| 45 d | 32 | |||||||||||||||||
| D | Baseline | 22 | ||||||||||||||||
| 45 d | 21 | |||||||||||||||||
| Müller et al. 2020 [81] n = 48 RPCT |
RIA1 | 12 wks IG: Prebiotics (15 g/d Arabinoxylan-Oligosaccharide) CG: Placebo Breakfast test meal: 2 slices of white bread, fried egg, chocolate milk (412 kcal, 52% CH, 27% F, 19% P) NGT (BMI:: 24.5 kg/m2) |
IG | Baseline | 24 | 32 | 31 | 31 | 31 | 29 | 30 | 8 | 90 min: 1767 | ↓ early AUC (0-90 min) in IG compared with CG | ||||
| 12 wks | 23 | 24 | 26 | 31 | 30 | 25 | 90 | 8 | 90 min: 1487 | |||||||||
| CG | Baseline | 23 | 31 | 32 | 30 | 31 | 28 | 60 | 9 | 90 min: 1729 | ||||||||
| 12 wks | 23 | 32 | 29 | 33 | 28 | 26 | 90 | 10 | 90 min: 1754 | |||||||||
| Birkeland et al. 2021 [82] n = 25 RPCT cross-over |
RIA1 | 6 wks IG: Prebiotics (16 g/d inulin-type fructans) CG: Placebo Mixed meal test (Fresubin Drink vanilla + Jucy Drink apple, 550 kcal, 78.5 g CH, 15.6 g F, 24 g P) T2DM (BMI:: 29.1 kg/m2) |
IG | Baseline | 28 | 50 | 47 | 40 | 42 | 39 | 37 | 30 | 22 | 180 min: 7180# | - ↔ AUC - ↓ decrease in postprandial excursion in IG compared with CG |
|||
| 6 wks | 27 | 44 | 45 | 40 | 40 | 36 | 36 | 45 | 18 | 180 min: 6979# | ||||||||
| CG | Baseline | 27 | 47 | 47 | 40 | 41 | 37 | 36 | 30 45 | 20 | 180 min: 6998# | |||||||
| 6 wks | 28 | 58 | 54 | 45 | 45 | 39 | 36 | 30 | 30 | 180 min: 7596# | ||||||||
GLP-1 concentrations for selected time points of blood sampling, time-to-peak as well as peak (peak concentration – fasting concentration), and —if available—AUC values are listed. Under “results” only significant results are listed. Time point 0 describes fasting concentrations, and time points >0 are reporting postprandial concentrations. Under “results” only significant results are listed.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups, ↔ no differences of GLP-1 secretion between groups. Supplemental Table 6 shows GLP-1 values for all measured time points. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: AUC, area under the curve; CH, carbohydrates; CG, control group; CV, coefficient of variation; ELISA, enzyme-linked immunosorbent assay; F, fat; GLP-1, glucagon-like peptide 1; iAUC, incremental area under the curve; IG, intervention group; LOD, limit of detection; min, minutes; n.a., not available; NGT, normal glucose tolerance; NW, normal weight, P, protein; RIA, radioimmunoassay; RPCT, randomized placebo-controlled trial; TTP, time-to-peak; T2DM, type 2 diabetes mellitus.
RIA [standardized assay (41)], polyclonal antiserum no. 89390, LOD: 1 pM, intra-assay CV: 6%, specificity: 100% for GLP-1 (7–36) 89% for GLP-1 (9–36);
ELISA (ZellBio GmbH, Berlin, Germany), sensitivity: 8.2 pg/mL.
TABLE 7.
Fasting GLP-1 values before and after pro- and synbiotic interventions
| Author, (y), n | GLP-1 assay | Intervention Participant characteristics |
Fasting GLP-1 values |
Δ | Results | ||
|---|---|---|---|---|---|---|---|
| Baseline | Endline | ||||||
| Rabiei et al. 2018 [83] n = 46 RPCT |
ELISA1 | 3 mo IG: Synbiotics CG: Placebo + energy-restricted diet (-500 kcal/day) MetS (BMI:: 32.3 kg/m2) |
IG | 8.1 (ng/mL)# | 10.2 (ng/mL)# | 2.1 | ↑ fasting values compared with baseline and CG |
| CG | 5.9 (ng/mL)# | 6.3 (ng/mL)# | 0.4 | ||||
| Rondanelli et al. 2021 [80] n = 25 RPCT |
ELISA (Invitrogen, ThermoFischer Sci)2 | 60 d IG: Probiotics CG: Placebo + energy-restricted diet (-800 kcal/d) OW (BMI: 34.8 kg/m2) |
IG | 10.9 (pmol/l)# | 8.6 (pmol/l)# | -2.3 | ↓ fasting values in IG and CG compared with baseline |
| CG | 7.3 (pmol/l)# | 5.3 (pmol/l)# | -2 | ||||
Fasting GLP-1 concentrations as well as Δ (endline value – baseline value) before and after pro- or synbiotic intervention are listed. Under “results” only significant results are listed.
#original data, ↑ higher GLP-1 secretion in comparison to referred groups, ↓ lower GLP-1 secretion in comparison to referred groups. Details on GLP-1 assays as stated in the publications or according to manufacturer instructions:
Abbreviations: CG, control group; CV, coefficient of variation; ELISA, enzyme-linked immunosorbent assay; GLP-1, glucagon-like peptide 1; IG, intervention group; MetS, metabolic syndrome; OW, overweight; RPCT, randomized placebo-controlled trial.
n.a.;
intra- and inter-assay CV: 3.6%.
Prebiotic interventions were examined in 3 studies. A recent study investigated the effect of a 12-wk prebiotic intervention with 15 g/d wheat bran extract arabinoxylan-oligosaccharide on meal-stimulated GLP-1 secretion in 48 participants with NGT. Compared with the placebo group, the early postprandial GLP-1 response (AUC0-90min) was decreased in the prebiotic group after consumption of a solid test meal consisting of 2 slices of white bread, a fried egg, and 250 mL chocolate milk (27% fat, 52% carbohydrates, 19% protein, 412 kcal) [81]. Recently, Birkeland et al. [82] also found no effects of a 6-wk prebiotic treatment with 16 g/d inulin-type fructans on the GLP-1 response to a mixed meal test (300 mL of 2 nutritional drinks containing 550 kcal, 78.5 g carbohydrates, 15.6 g fat, and 24 g protein) in participants with T2DM. Roshanravan et al. [23] conducted a double-blind RCT on the effects of prebiotic butyrate and inulin supplementation over 45 d on food-stimulated GLP-1 concentrations in 60 patients with T2DM. The participants were randomly assigned into 1 of 4 groups: Group A received sodium butyrate capsules, group B received inulin supplement powder, group C was exposed to the concomitant use of inulin and sodium butyrate, and group D received a placebo for 45 consecutive days. Butyrate (group A) and butyrate combined with inulin (group C) supplementation led to significant increases in postprandial GLP-1 concentrations 120 min after nonstandardized breakfast when compared with the placebo group. Intragroup comparisons between baseline and after 45 d showed a trend for higher GLP-1 values after butyrate, inulin, and butyrate + inulin supplementation. Within all groups, there were no significant changes in body weight in any of the groups after 45 d [23].
In a triple-blind RCT, Rabiei et al. [83] investigated the effects of synbiotic (a combination of a probiotic and a prebiotic) supplementation accompanied by a weight-loss diet in 46 adults with metabolic syndrome over 12 wks. All participants followed a weight-loss diet and were randomly assigned to receive either a synbiotic supplement (consisting of 7 probiotic strains (2×108 CFU for all strains) plus 125 mg fructooligosaccharides as prebiotics) or a placebo. Although the fasting GLP-1 concentrations increased in both groups, the increase in the synbiotic group was significantly greater than that in the placebo group [83].
After reviewing these 6 human intervention studies with different methodological approaches, the data regarding the effect of prebiotic, probiotic, and synbiotic interventions on GLP-1 secretion appear inconclusive. It is important to mention that in all 6 studies, the drop-out rate was low (<15%).
Discussion
We analyzed several studies investigating fasting-, glucose-, or food-stimulated GLP-1 secretion under different metabolic conditions. Both fasting and postprandial concentrations of GLP-1, including AUC/iAUC values, the time, and delta-to-peak concentrations, were extracted and evaluated in detail, allowing us to map the physiologic course of the GLP-1 response in numerous studies. Based on the large number of studies analyzed, it is possible to assess the relationship between GLP-1 secretion and metabolic factors, as well as to estimate the impact of dietary interventions on GLP-1 secretion.
Although some studies showed decreased GLP-1 secretion in patients with IGT and T2DM after both OGTT [39,45,46,48] and MMTT or challenge meals [44,56], others reported an increased [42,44,50,55] or unchanged [47,49,58,69] GLP-1 response in the IGT and T2DM groups after OGTT or MMTT/challenge meals. The conflicting nature of the results is also evident from studies reporting increased fasting but decreased meal- or glucose-related GLP-1 concentrations in T2DM [38] and nonspecified diabetes [51], or unchanged fasting but reduced early postprandial GLP-1 concentrations in participants with low insulin sensitivity than in those with high insulin sensitivity [57]. Here, it is important to mention that studies that also include NW/NGT individuals [47,50] can provide a more comprehensive picture of GLP-1 secretion, and possible step-wise alterations, across different metabolic stages than the studies that only compare OW and T2DM [53].
When comparing fasting GLP-1 concentrations between groups with different metabolic conditions, or before and after dietary intervention, it is important to note that fasting levels are often close to the detection limits of the respective assays and are not well characterized. Only clear changes in fasting levels may be related to metabolic alterations; the mechanisms underlying these changes remain unknown. Therefore, it is important to evaluate critically whether statistically significant changes have any metabolic consequences. It cannot be excluded that an increase in the fasting state is associated with metabolic effects, although this has not been experimentally established. Population-based studies have reported that elevated fasting total GLP-1 concentrations are predictive of increased liver fat [84] and cardiometabolic risks [85], whereas higher physical activity is associated with lower fasting GLP-1 concentrations. The underlying mechanisms still need to be elucidated because it is not clear whether elevated fasting GLP-1 values precede the increase in insulin and subsequent glucometabolic changes (including weight gain) [85] or vice versa. Enhancing GLP-1 activity and targeting GLP-1R activation are key areas of interest for the management of OW and T2DM. High postprandial GLP-1 secretion is beneficial for insulin-dependent glucose control and appetite inhibition and satiety, facilitating its use for the prevention and treatment of OW [86,87]. Although diet-induced enhancement of GLP-1 secretion seems to hold potential, it is important to emphasize that it does not represent an alternative to pharmacologic treatments for T2DM, but rather a preventative or supplementary approach. As shown in a recent meta-analysis, GLP-1R agonist-based treatments have convincing effects on glucometabolic outcomes in T2DM [88]. The new promising drug retatrutide, a synthetic triagonist targeting receptors for GLP-1, GIP, and glucagon, showed comparable effects in a phase 2 trial in which different dosages were administered along with diet and exercise treatment [89]. The American Diabetes Association and the European Association for the Study of Diabetes also highlight the importance of body weight management through diet and physical exercise which play an integral role in a holistic approach to T2DM management [90]. In some of the dietary intervention studies, effects on glucometabolic outcomes were measured in addition to the impact on GLP-1 concentrations, and in some cases, comparable effects have been shown [72,74,77]. However, comparing diet-induced effects to those of pharmacologic treatments has limitations, as the study designs are heterogeneous.
These conflicting results indicate that the relationship between altered GLP-1 secretion and OW and IGT, and more importantly, the direction of this association cannot be conclusively established. This may, in part, be due to various influencing factors, such as the metabolic profile, sex, gut microbiome, diet, glucose tolerance, medication, and BMI (Figure 2). Moreover, when interpreting the GLP-1 values from the studies, the variability in the methods of GLP-1 measurement needs to be considered because different assays differ in their sensitivity, specificity, and coefficients of variation, which could lead to divergent results [91, 92]. However, although absolute GLP-1 values may vary among different assays, the pattern of postprandial secretion appears to be similar [92]. Yet, most reported studies used a standardized assay based on RIA methodology [41]. Studies measuring active GLP-1 were not included because active GLP-1 is low in abundance, which can lead to high variability and low accuracy [31]. In addition, the time taken to reach peak GLP-1 concentration is only comparable to a limited extent, as blood collection was performed at different time points and, so far, no continuous GLP-1 measurement is possible.
FIGURE 2.
Main influencing factors of GLP-1 secretion. GLP-1 secretion may be modulated by several factors, such as the gut microbiome, eating behavior, glucose tolerance, metabolic profile, sex, medication, and body mass index (indicated by dark blue arrows). GLP-1 also affects these factors (except sex and medication), in addition to its neuroprotective properties (indicated by light blue arrows). GLP-1, glucagon-like peptide 1.
Impact of BMI
BMI is an important factor influencing GLP-1 secretion, even after correcting for metabolic differences, with GLP-1 responses decreasing with increasing BMI [38, 45]. The bivariate analysis of the study population stratified by OW convincingly showed an independent contribution of BMI on insulin release, β-cell glucose sensitivity, and GLP-1 response, whereas GLP-1 responses were gradually depressed across increasing degrees of OW [45]. Interestingly, when receiving an equivalent dose of a GLP-1 analog, individuals who were OW exhibited metabolic and appetite responses similar to those of their healthy matched controls [93], suggesting that sensitivity to GLP-1 is maintained in OW. Because OW and T2DM often occur simultaneously, separating the impact of elevated body weight from impaired glucose control is challenging. As BMI in the OW study groups ranged between 29 kg/m2 [53] and 39 kg/m2 [74], the grade of OW within the study cohorts could have contributed to the contradictory results of this review.
In addition to BMI, weight loss may also influence the GLP-1 response. Studies have reported that the meal response of GLP-1 increases after weight loss [72,94,95] and even during weight maintenance [72]. Regarding fasting GLP-1 concentrations, data are more inconsistent because some studies showed unchanged levels during acute and maintained weight loss [74,95], whereas others reported decreased values [79,80]. In addition, although in some studies higher fasting GLP-1 concentrations were observed after an energy-restricted diet, it is important to note that these occurred only in the intervention groups combining a calorie restriction with an additional intervention such as synbiotic supplement [83], MIND diet [73] or healthy nordic diet [72], whereas there was no effect in the control groups. These results indicate that weight loss during hypoenergetic intervention studies [[72], [73], [74],79] may also influence GLP-1 secretion when compared with isoenergetic dietary approaches [76]. However, the effects of the specific diets or alternative interventions seem to be important, and additional differences in study designs, such as intervention duration, extent of calorie reduction, and participant characteristics, need to be considered.
Impact of glucose tolerance
The effect of GLP-1 on glucose tolerance has been the focus of numerous studies. The observation that GLP-1 responses may be blunted in T2DM [29,38,96,97] has led to the notion that an impaired incretin effect contributes to the β-cell incompetence found in diabetes [29]. Clinical data showing that GLP-1 analogs can normalize glycemia by stimulating insulin secretion in T2DM strengthen the incretin hypothesis [98,99]. Besides, in a few studies, impaired GLP-1 secretion and impaired GIP secretion have been found in individuals with IGT or T2DM [38]. The incretin effect of GLP-1 and GIP has been demonstrated in animal studies, and now, with the help of antagonists for both receptors, the importance of incretin hormones for the maintenance of NGT has been demonstrated [31,[100], [101], [102]]. Therefore, impaired incretin secretion may contribute to the development and clinical worsening of diabetes mellitus.
The meal-stimulated GLP-1 response of individuals with IGT ranged between healthy participants and patients with T2DM, independent of sex and BMI [38]. This suggests that GLP-1 response correlates strongly with glucose tolerance. At the same time, an unexpected positive correlation between fasting blood glucose concentrations and GLP-1 secretion in T2DM has been described, which led to the hypothesis that hyperglycemia is unlikely to be responsible for the impaired GLP-1 response in T2DM. However, in multiple regression analyses, diabetes was found to be a decisive determinant of GLP-1 secretion [38]. These results are consistent with the findings in monozygotic twins discordant for T2DM, where the new dimension of the “incretin defect” was added to the complicated and self-perpetuating process involved in the development of noninsulin-dependent diabetes [96]. Moreover, the incretin effect on total insulin secretion and β-cell glucose sensitivity, when investigated separately, and the GLP-1 response to oral glucose were reduced in T2DM compared with NGT or IGT. Further, the GLP-1 response was inversely related to glucose tolerance (plasma glucose concentrations after 2 h) [45]. In twin pairs discordant and concordant for OW, an acquired unhealthy pattern of OW, characterized by liver fat accumulation and insulin resistance, was closely related to an impaired glucose-stimulated GLP-1 response [103]. An additional explanation for the difference in GLP-1 secretion between healthy individuals and patients with T2DM is that the number of incretin-expressing enteroendocrine cells in the jejunum containing both GIP and GLP-1 [104,105] were increased in the diabetic state in some studies [42]. Therefore, the reciprocal influence of GLP-1 secretion and glucose homeostasis is of great interest when evaluating glucose- and food-dependent GLP-1 responses. Additionally, the BMI of the T2DM study groups varied between 23.5 kg/m2 [47] and 38.5 kg/m2 [48], which led to a heterogeneous group of participants across the studies despite having the same co-morbidity.
An increasing body of clinical evidence suggests that antidiabetic agents directly affect GLP-1 secretion. Metformin treatment increases fasting and postprandial GLP-1 concentrations, both over the short-term [[106], [107], [108]] and over the mid-term to long-term [[109], [110], [111], [112]] and in patients with [106,109,112] and without T2DM [107,108,[110], [111], [112]]. In human gut cells, metformin is a direct secretagogue for GLP-1 release from L cells [106]. In addition, bile acid sequestrants may increase fasting- and meal-stimulated GLP-1 secretion in patients with T2DM [113]. Furthermore, an altered gastric emptying rate after short and long-term metformin administration in T2DM has been reported [114,115]. Although the results were contrary, these studies highlight the potential influence of metformin therapy on gastric emptying and GLP-1 secretion [116]. In addition, other antidiabetic agents, such as GLP-1 receptor agonists or DPP-IV inhibitors, delay gastric emptying in individuals who have OW [117, 118] or T2DM [119]. However, some studies have reported no change in gastric emptying [120,121]. Therefore, it is important to consider the impact of prevailing diabetes control when investigating GLP-1 secretion in patients with and without diabetes, especially when antidiabetic agents are administered on the day the meal tests are conducted. Different inclusion and exclusion criteria of the studies and different approaches on the examination days could have resulted in the observed discrepancy in results in participants with and without T2DM—although some studies in this review excluded patients receiving antidiabetic therapy [47,64,71,76], others included them [65,79]. Furthermore, study populations with the same BMI but different stages of glucose tolerance are highly useful to investigate the influence of glucose tolerance independent of BMI [46,53], in contrast to study groups where BMI and glucose tolerance vary simultaneously.
In summary, the current evidence emphasizes the importance of a detailed assessment of glucose metabolism and diabetes control in study participants when examining GLP-1 secretion. A recent study also showed that the increase in GLP-1 concentrations after ingestion of a glucose solution compared with a sucrose solution depended on the insulin sensitivity of the participants [122]. This emphasizes that the degree of glucose tolerance, separately and in combination with the nutrient composition and type of carbohydrates, influences carbohydrate-stimulated GLP-1 secretion. The relationship between impaired incretin secretion and impaired glucose tolerance is of great scientific interest and certainly a key element in further studies.
Impact of meal composition or size
As described above, several studies have suggested that meal composition and size are important factors influencing the postprandial GLP-1 response. Although it is reasonable to assume that macronutrients and their digestive products specifically stimulate GLP-1 secretion, we have only begun to understand the underlying mechanisms in humans, and the possible mechanisms of nutrient-stimulated GLP-1 secretion from various experiments have recently been reviewed elsewhere [123].
Glucose, protein, and fat have been described to be strong GLP-1 secretagogues after they have been orally ingested or directly administered into the intestine [32,[124], [125], [126]]; with carbohydrate and proteins eliciting earlier peaks in GLP-1 secretion (30–60 min postprandially) than lipids (>120 min postprandially) [32,127]. However, the role of macronutrient composition in GLP-1 secretion in OW or IGT, or T2DM has not been fully elucidated. Some evidence points to a major influence of carbohydrate concentrations (including fiber), wherein a carbohydrate-rich test led to higher postprandial GLP-1 secretion than test meals containing less carbohydrates in T2DM [65,69]. Already in 1955, it was demonstrated that sucrose stimulates GLP-1 secretion through both early and late mechanisms involving luminal contact [128]. Apart from the amount of carbohydrates, the type of simple carbohydrate appears to play an important role in GLP-1 secretion. A study in healthy individuals with OW showed that ingestion of a 75-g sucrose load provoked a less robust postprandial rise in GLP-1 concentration compared with an equicaloric 75-g glucose load [122]. Similarly, test drinks with a modified carbohydrate composition [regular alcohol-free beer, alcohol-free beer with the fermentation of the regular carbohydrates and enriched with resistant maltodextrin (and isomaltulose)] led to a higher GLP-1 response than the regular test drink (containing glucose) [59]. Some study results illustrate the confusion in this field, as a reduced amount of carbohydrates, as a possible explanation for reduced GLP-1 concentrations, contrasts with the higher fiber content of the Okinawan-based Nordic Diet, examined by Ohlsson et al. [79].
In addition, for protein and amino acids, stimulation of GLP-1 secretion by a higher content than control meals has long been demonstrated in vitro and in vivo in the short and long-term and in both NW or OW groups and healthy individuals, and with T2DM [68,74,78,[129], [130], [131], [132]]. Although the interaction of proteins with intestinal endocrine cells is still under investigation, amino acids (the breakdown products) have been more thoroughly studied. The amino acid composition of proteins seems to be relevant as amino acids differ in their GLP-1 stimulatory potential [133], which may partly explain the heterogeneous study results. Some amino acids appear to simulate L cell secretion from the luminal side, whereas others may interact basolaterally with receptors following absorption [133,134]. Moreover, protein hydrolysates stimulated ileal GLP-1 secretion in male rats [135].
Even if the cell density of intestinal GLP-1-releasing L cells, which are expressed in the entire small intestine and colon, is highest in the ileum and colon, a considerable number of L cells are also found in the duodenum and proximal jejunum, thus leaving the possibility of a common mechanism for carbohydrates, proteins, and fat involving intestinal L cells releasing GLP-1 as a response to direct nutrient contact [42,136,137]. Experimental studies have shown that the proximal part of the gut is responsible for most early GLP-1 responses to meal ingestion [135]. Multiple studies showing a gastric emptying time of <1 h [138] underline the possibility that nutrients, especially after a fast transit of liquid meals or glucose solutions, can reach the duodenum in a short time, leading to GLP-1 release from L cells. This could explain the observed peaks in GLP-1 concentrations, even in short-term postprandial protocols with a duration of ∼3 h. As summarized in the same review, the shortest small intestine transit time reported was 3.3 h, leaving a short time for the luminal content to come in contact with L cells located in the jejunum or even the ileum and stimulate the chemo-sensing machinery, leading to the modulation of GLP-1 release. Moreover, according to the results of a very early study, the presence of appropriate nutrients in the upper small intestine could induce very early postprandial GLP-1 release (∼15 min after meal ingestion) via an indirect pathway, possibly involving enteroendocrine nerves. Furthermore, sparse GLP-1-producing cells present in the upper intestinal tract may be sufficient to produce the increment observed in the early postprandial phase [128]. The colonic contribution to postprandial L cell secretion is unknown, and secretion from the colon is probably not normally related to nutritional stimulation but rather to malabsorbed bile acids and microbial metabolites [139].
The sweet taste receptor expressed by human duodenal L cells, which binds to sugars, sweeteners, sweet amino acids, and sweet proteins has been suggested to be involved in GLP-1 secretion [140,141]. In rodents, it has been shown that taste cells also express GLP-1, which may enter the circulation [142]. Even if the concentration of GLP-1 released from taste cells is limited, because of the near absence of DPP-IV compared with GLP-1 released from L cells in the distal intestine, it may exert a larger effect than expected [143]. As it seems possible that glucose may reach the duodenum during the 3-h test of an OGTT, it might be speculated that sweet taste receptors may be involved in postprandial GLP-1 elevations during OGTTs. However, further studies are warranted to elucidate the function of taste receptors in glucose-stimulated GLP-1 secretion in vivo.
As it is undisputable that liquids empty from the stomach more quickly than solid meals, the physical state of the test meal applied in the reviewed studies had a decisive effect on L cell-mediated GLP-1 secretion [138]. In addition, the size of the meal seemed to matter; the late GLP-1 response (30–180 min) to a large meal (520 kcal) was shown to be more pronounced than that to a small meal (260 kcal), possibly due to lower exposure of stimulatory nutrients to the L cells following the small meal [56]. The secretory capacity of the distal small intestine is thought to be related to the role of GLP-1 as an “ileal brake” hormone, which is part of an inhibitory feedback mechanism that signals nutrient abundance to the brain, promotes satiety, and inhibits upper gastrointestinal motility and secretion [139,144,145]. Therefore, the distal part of the small intestine appears to be more involved in responses to larger meals as well as in connection with changes in intestinal transit times [146]. However, other studies reported that the quantity of a meal is less influential than its composition [69]. The effects of meal size on GLP-1 secretion have been reviewed and discussed in more detail elsewhere [[147], [148], [149]].
As carbohydrates, fats, and proteins have different cephalic phase responses and effects on gastric emptying when ingested in isolation [150], it is important to consider gastric emptying and transit time when analyzing the impact of meal composition on GLP-1 secretion. It is also long known that GLP-1 secretion is directly influenced by the rate of gastric emptying. This indicates that food selection is particularly important for GLP-1 secretion in patients with (pre)diabetes. Although the inhibitory effect of GLP-1 on appetite and satiety is well documented, first proposals suggest that alterations in gut hormone secretion could also lead to changes in macronutrient-specific food preferences, favoring lower-calorie foods [151]. This is supported by the observation that elevated fasting and postprandial GLP-1 secretion after bariatric surgery are associated with the magnitude of weight loss [152, 153]. Elevated nutrient delivery to the distal small intestine is likely the predominant mechanism behind the exaggerated GLP-1 response after gastric bypass [123,154]. Although complex physiologic changes occur after bariatric surgery, which is not the topic of this review, this highlights the idea that diets could be designed to enhance L cell sensing and satiety through nutrients that stimulate GLP-1 and other gut hormones.
Moreover, other dietary factors, such as secondary plant compounds, can influence postprandial GLP-secretion, for example, by counteracting oxidative stress and the associated upregulation of DPP-IV activity [61,155,156]. In addition, it is speculated that the degree of food processing influences GLP-1 secretion and glucose metabolism, for example, via the impact of altered starch properties or food additives including antimicrobial preservatives and monosodium glutamate in ultra-processed foods [[157], [158], [159], [160]]. Food processing also affects digestibility, tolerance, and nutrient accessibility [161]. Considering the content of secondary plant compounds and the grade of processing of the test meals applied in the studies reviewed could explain the contrary results, especially when applying westernized test diets containing processed foods [38,44,49,[56], [57], [58],60,65] or test diets with a high percentage of fruits and vegetables [63,65,68,72,73,79].
Impact of sex
In some studies, sex was a significant determinant of GLP-1 secretion. Male participants had ≤20% smaller postprandial GLP-1 response to the test meals than females [38,49,96,122]. Interestingly, in one study, this effect was observed in individuals with NGT, but not in those with IGT or T2DM [39]. Supporting an enhancing effect of estrogens on incretin responses, estradiol was described to positively regulate oral glucose-induced GLP-1 response in mouse and human α- and L cells [162]. In addition, an impact of the menstrual cycle was reported to influence GLP-1 secretion in women. It was shown that gastric emptying of glucose was slower and GLP-1 concentrations were lower during the follicular compared with the luteal phase [163]. Therefore, the sex distribution and lack of consideration of the menstrual cycle phase for female participants within the study cohort could also have influenced the study results. A few studies discussed in this review including only women [72,73] or men [57] allow a sex-independent evaluation of GLP-1 secretion, whereas this is not possible in studies including both sexes.
Impact of the gut microbiota
Alterations in the gut microbiota (dysbiosis) may be associated with the pathogenesis of metabolic diseases [[164], [165], [166], [167]]. Recent studies have demonstrated the impact of the gut microbiota on the therapeutic effect of metformin in patients with diabetes [168]. Although causal relationships have been well demonstrated in animal models, studies investigating the underlying functional pathways in humans are required [166,169]. GLP-1 has various physiologic actions, including anorectic and neuroprotective effects, and has been suggested to play a central role in the microbiota-gut-brain axis and disease pathogenesis [9,[170], [171], [172]]. One link between GLP-1 and the gut microbiota may be microbial metabolites, such as SCFAs from bacterial fermentation of fiber, which may stimulate GLP-1 secretion via FFAR2 and FFAR3; however, as discussed above, this may not apply to humans. Moreover, as the effect of bacterial metabolites would presumably be time delayed, it is likely that they affect fasting GLP-1 concentrations or secretion in the long run and might not be observed in postprandial protocols of 3-h duration [173]. This makes gut microbiota a potential target for dietary and pharmaceutical interventions aimed at modulating basal GLP-1 secretion, thereby preventing or treating T2DM and other metabolic disorders. Conversely, GLP-1 receptor agonists may affect the gut microbiome composition in mice and in patients with T2DM consuming liraglutide. The underlying mechanisms are yet to be elucidated in detail but modifications of gastric emptying, along with changes in caloric intake, and modifications in gut pH levels and nutrient availability are being evaluated [174].
Altogether, the results from the mid- to long-term human trials presented in this review support the outlined relevance of the interaction between diet and gut microbiota for GLP-1 secretion. Moreover, they indicated that a longer intervention duration might be necessary to achieve the desired effects on GLP-1 concentrations. It also became obvious that, in addition to uncontrolled studies [79] or studies with a control group that also experienced an alteration of the habitual dietary behavior, e.g., through an energy restriction [[72], [73], [74]], long-term dietary interventions with a control group that does not change its habitual diet are highly needed. Three studies directly targeting the gut microbiota with synbiotic, prebiotic, and probiotic supplements showed significant positive effects on GLP-1 concentrations and other metabolic parameters (e.g., synbiotic’s effect on insulin resistance, probiotic’s effect on insulin secretion, and prebiotic’s (inulin and butyrate) effect on fasting glucose [23,43,83]). Unfortunately, only Simon et al. [43] directly examined the effects of the intervention on gut microbiota composition and found differences in the supplemented bacterial strain L. reuteri (whereas there were no differences in other bacteria). When interpreting the results of these studies, it is important to consider the differences in methodologies. Probiotic, prebiotic, and synbiotic interventions are fundamentally different approaches, and the reported studies vary in the bacterial strains and prebiotic substances implemented, duration and type of the intervention (e.g., additional energy-restricted diets), and variations in using several types of GLP-1 assays. These are the general limitations of clinical probiotic-, prebiotic-, and synbiotic intervention studies leading to heterogeneous and sometimes inconclusive results that need to be addressed in microbiome research [175]. Furthermore, the difference in drop-out rates of <20% [23,43,81,82] compared with >20% [72,74,79] could have had a decisive impact on the results.
Future research needs
The heterogeneity of the subject groups affected the comparability of the cohorts; for instance, not all studies considered the medication of patients with T2DM, even though it has been previously reported that antidiabetic agents directly affect fasting and postprandial GLP-1 secretion and gastric emptying. There was also high variation in the average BMI and range of the study populations. For example, in the NGT groups, the BMI of the participants varied between 21.3 kg/m2 and 35.5 kg/m2, and not all studies had subgrouped participants into lean and having OW. As outlined above, an association between BMI and GLP-1 secretion has been shown in several studies, which probably contributes to conflicting results. Moreover, not all studies evaluated the degree of glucose (in)tolerance in the study population. Therefore, for future studies, it is highly relevant to thoroughly characterize their cohorts according to BMI, sex, ethnicity, age, glucose tolerance, medication, and health status, as well as assess habitual diet and physical activity to improve the comparability of the cohorts.
Regarding the assessment of the impact of meal composition or size on GLP-1 secretion, applying standardized methods for the MMTT and challenge meals would help improve comparability between studies. As for example, the amino acid composition of proteins can affect GLP-1 secretion, future studies need to analyze the composition of the meal tests applied in detail. In addition, it is essential to assess gastric emptying rate and transit time to fully understand the impact of certain test meals on GLP-1 secretion.
Additionally, further mid- to long-term studies should be conducted to investigate the modulation of GLP-1 secretion by dietary pattern interventions or prebiotic, probiotic, and synbiotic interventions after accounting for factors such as glucose tolerance, other metabolic and gut-brain axis-related parameters, and gut microbiota, to generate a more comprehensive understanding of the complex interrelations and consolidate knowledge about the underlying mechanisms.
In summary, the application of highly standardized meal tests with detailed analyses of nutrient composition and gut-related outcomes, especially gastric emptying, in well-defined cohorts is crucial to derive robust conclusions.
Conclusion
This review has demonstrated that studies on fasting GLP-1 concentrations and glucose- and food-stimulated GLP-1 responses in individuals with different metabolic conditions are inconsistent, but there is convincing evidence that these responses may be influenced by the metabolic profile, sex, gut microbiome, glucose tolerance, BMI, antidiabetic medication, eating behavior, and nutrient composition.
Some studies have indicated that GLP-1 secretion is impaired in patients with IGT or T2DM, and consistently more so in individuals who have OW. Considering the relevance of GLP-1 in glucose homeostasis, eating behavior, weight maintenance, and neuroprotection, these results are important for both healthy individuals and patients with metabolic impairments.
In addition, this review highlights that GLP-1 secretion can be modified exogenously through dietary interventions. As more attention is being drawn to dietary habits or food patterns, it has become evident that a holistic approach and studies on the influence of long-term diets on health and GLP-1 release are more important than examining single nutrients or foods. To date, only a few studies have focused on the effects of food patterns on GLP-1 secretion and thus on metabolism, the immune system, and the microbiota-gut-brain axis [79,176,177]. These findings indicate that food selection is particularly important for GLP-1 secretion in patients with diabetes, and has great potential for the prevention and holistic treatment of IGT, T2DM, and OW. However, further nutritional intervention studies are needed to examine over mid- and long-term.
Acknowledgments
The figures were partly created using BioRender.com.
Author contributions
The authors’ responsibilities were as follows – HH, AS: conducted the research and prepared the first draft of the manuscript, which was subsequently finalized in close collaboration with MCS and JJH; all authors: provided substantial content contributions and edited the manuscript; MCS: had the initial idea for this manuscript; AS, MCS: created and edited the figures; and all authors: have read and approved the final version of the manuscript.
Conflict of interest statement
The authors report no conflicts of interest.
Funding
HH, AS, and MCS are supported by funding from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung, BMBF), grant number: 01EA1707. MCS received funding from the German Diabetes Association.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2024.01.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Nouwen A., Winkley K., Twisk J., Lloyd C.E., Peyrot M., Ismail K., et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia. 2010;53(12):2480–2486. doi: 10.1007/s00125-010-1874-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iglay K., Hannachi H., Joseph Howie P., Xu J., Li X., Engel S.S., et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2016;32:1243–1252. doi: 10.1185/03007995.2016.1168291. [DOI] [PubMed] [Google Scholar]
- 3.Blüher M. Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019;15(5):288–298. doi: 10.1038/s41574-019-0176-8. [DOI] [PubMed] [Google Scholar]
- 4.Boles A., Kandimalla R., Reddy P.H. Dynamics of diabetes and obesity: epidemiological perspective. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017;1863(5):1026–1036. doi: 10.1016/j.bbadis.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doucet E., Cameron J. Appetite control after weight loss: what is the role of bloodborne peptides? Appl. Physiol. Nutr. Metab. 2007;32(3):523–532. doi: 10.1139/H07-019. [DOI] [PubMed] [Google Scholar]
- 6.Cheang J.Y., Moyle P.M. Glucagon-like peptide-1 (GLP-1)-based therapeutics: current status and future opportunities beyond type 2 diabetes. ChemMedChem. 2018;13(7):662–671. doi: 10.1002/cmdc.201700781. [DOI] [PubMed] [Google Scholar]
- 7.Nolen-Doerr E., Stockman M.-C., Rizo I. Mechanism of glucagon-like peptide 1 improvements in type 2 diabetes mellitus and obesity. Curr. Obes. Rep. 2019;8(3):284–291. doi: 10.1007/s13679-019-00350-4. [DOI] [PubMed] [Google Scholar]
- 8.Holst J.J. Incretin hormones and the satiation signal. Int. J. Obes. (Lond). 2013;37(9):1161–1168. doi: 10.1038/ijo.2012.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nauck M.A., Meier J.J. Incretin hormones: their role in health and disease. Diabetes. Obes. Metab. 2018;20:5–21. doi: 10.1111/dom.13129. [DOI] [PubMed] [Google Scholar]
- 10.Nauck M.A., Petrie J.R., Sesti G., Mannucci E., Courrèges J.-P., Lindegaard M.L., et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes. Care. 2016;39(2):231–241. doi: 10.2337/dc15-0165. [DOI] [PubMed] [Google Scholar]
- 11.Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 7-36 amide but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J. Clin. Invest. 1993;91(1):301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nathan D.M., Schreiber E., Fogel H., Mojsov S., Habener J.F. Insulinotropic action of glucagon-like peptide I-(7-37) in diabetic and nondiabetic subjects. Diabetes. Care. 1992;15(2):270–276. doi: 10.2337/diacare.15.2.270. [DOI] [PubMed] [Google Scholar]
- 13.Gutniak M., Orskov C., Holst J.J., Ahrén B., Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N. Engl. J. Med. 1992;326(20):1316–1322. doi: 10.1056/NEJM199205143262003. [DOI] [PubMed] [Google Scholar]
- 14.Gunawardene A.R., Corfe B.M., Staton C.A. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int. J. Exp. Pathol. 2011;92(4):219–231. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho Y.M., Fujita Y., Kieffer T.J. Glucagon-like peptide-1: glucose homeostasis and beyond. Annu. Rev. Physiol. 2014;76:535–559. doi: 10.1146/annurev-physiol-021113-170315. [DOI] [PubMed] [Google Scholar]
- 16.Ferraris R.P., Diamond J. Regulation of intestinal sugar transport. Physiol. Rev. 1997;77(1):257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- 17.Sun E.W., de Fontgalland D., Rabbitt P., Hollington P., Sposato L., Due S.L., et al. Mechanisms controlling glucose-induced GLP-1 secretion in human small intestine. Diabetes. 2017;66(8):2144–2149. doi: 10.2337/db17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen L.J., Esterhazy D., Kim S.-H., Lemetre C., Aguilar R.R., Gordon E.A., et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh A., de Vadder F., Kovatcheva-Datchary P., Bäckhed F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell. 2016;165(6):1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 20.Tolhurst G., Heffron H., Lam Y.S., Parker H.E., Habib A.M., Diakogiannaki E., et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarini J., Wolever T.M.S. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl. Physiol. Nutr. Metab. 2010;35(1):9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 22.Freeland K.R., Wilson C., Wolever T.M.S. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br. J. Nutr. 2010;103(1):82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 23.Roshanravan N., Mahdavi R., Alizadeh E., Jafarabadi M.A., Hedayati M., Ghavami A., et al. Effect of butyrate and inulin supplementation on glycemic status, lipid profile and glucagon-like peptide 1 level in patients with type 2 diabetes: a randomized double-blind, placebo-controlled trial. Horm. Metab. Res. 2017;49(11):886–891. doi: 10.1055/s-0043-119089. [DOI] [PubMed] [Google Scholar]
- 24.Piche T., Des Varannes S.B., Sacher-Huvelin S., Holst J.J., Cuber J.C., Galmiche J.P. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124(4):894–902. doi: 10.1053/gast.2003.50159. [DOI] [PubMed] [Google Scholar]
- 25.Ropert A., Cherbut C., Rozé C., Le Quellec A., Holst J.J., Fu-Cheng X., et al. Colonic fermentation and proximal gastric tone in humans. Gastroenterology. 1996;111(2):289–296. doi: 10.1053/gast.1996.v111.pm8690193. [DOI] [PubMed] [Google Scholar]
- 26.Olesen M., Gudmand-Høyer E., Holst J.J., Jørgensen S. Importance of colonic bacterial fermentation in short bowel patients: small intestinal malabsorption of easily digestible carbohydrate. Dig. Dis. Sci. 1999;44(9):1914–1923. doi: 10.1023/a:1018819428678. [DOI] [PubMed] [Google Scholar]
- 27.Christiansen C.B., Veedfald S., Hartmann B., Gauguin A.M., Møller S., Moritz T., et al. Colonic lactulose fermentation has no impact on glucagon-like peptide-1 and peptide-YY secretion in healthy young men. J. Clin. Endocrinol. Metab. 2022;107(1):77–87. doi: 10.1210/clinem/dgab666. [DOI] [PubMed] [Google Scholar]
- 28.Holst J.J., Deacon C.F., Vilsbøll T., Krarup T., Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends. Mol. Med. 2008;14(4):161–168. doi: 10.1016/j.molmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Nauck M.A., Homberger E., Siegel E.G., Allen R.C., Eaton R.P., Ebert R., et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J. Clin. Endocrinol. Metab. 1986;63(2):492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 30.Campbell J.E., Drucker D.J. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell. Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Holst J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 32.Elliott R.M., Morgan L.M., Tredger J.A., Deacon S., Wright J., Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J. Endocrinol. 1993;138:159–166. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 33.Orskov C., Wettergren A., Holst J.J. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand. J. Gastroenterol. 1996;31(7):665–670. doi: 10.3109/00365529609009147. [DOI] [PubMed] [Google Scholar]
- 34.Gejl M., Gjedde A., Egefjord L., Møller A., Hansen S.B., Vang K., et al. In Alzheimer’s disease, 6-month treatment with GLP-1 analog prevents decline of brain glucose metabolism: randomized, placebo-controlled, double-blind clinical trial. Front. Aging. Neurosci. 2016;8:108. doi: 10.3389/fnagi.2016.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carr R.D., Larsen M.O., Jelic K., Lindgren O., Vikman J., Holst J.J., et al. Secretion and dipeptidyl peptidase-4-mediated metabolism of incretin hormones after a mixed meal or glucose ingestion in obese compared to lean, nondiabetic men. J. Clin. Endocrinol. Metab. 2010;95:872–878. doi: 10.1210/jc.2009-2054. [DOI] [PubMed] [Google Scholar]
- 36.Bernsmeier C., Meyer-Gerspach A.C., Blaser L.S., Jeker L., Steinert R.E., Heim M.H., et al. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS. One. 2014;9(1) doi: 10.1371/journal.pone.0087488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simon M.C., Strassburger K., Nowotny B., Zivehe F., Kolb H., Stehle P., et al. Decreased secretion of GLP-1 and GLP-2 after oral glucose in obese versus lean healthy human subjects. Exp. Clin. Endocrinol. Diabetes. 2014;122(3) doi: 10.1055/s-0034-1372112. [DOI] [Google Scholar]
- 38.Toft-Nielsen M.B., Damholt M.B., Madsbad S., Hilsted L.M., Hughes T.E., Michelsen B.K., et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2001;86(8):3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 39.Færch K., Torekov S.S., Vistisen D., Johansen N.B., Witte D.R., Jonsson A., et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: the ADDITION-PRO study. Diabetes. 2015;64:2513–2525. doi: 10.2337/db14-1751. [DOI] [PubMed] [Google Scholar]
- 40.Watkins J.D., Carter S., Atkinson G., Koumanov F., Betts J.A., Holst J.J., et al. Glucagon-like peptide-1 secretion in people with versus without type 2 diabetes: a systematic review and meta-analysis of cross-sectional studies. Metabolism. 2023;140 doi: 10.1016/j.metabol.2022.155375. [DOI] [PubMed] [Google Scholar]
- 41.Orskov C., Rabenhøj L., Wettergren A., Kofod H., Holst J.J. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43(4):535–539. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 42.Theodorakis M.J., Carlson O., Michopoulos S., Doyle M.E., Juhaszova M., Petraki K., et al. Human duodenal enteroendocrine cells: source of both incretin peptides, GLP-1 and GIP. Am. J. Physiol. Endocrinol. Metab. 2006;290(3):E550–E559. doi: 10.1152/ajpendo.00326.2004. [DOI] [PubMed] [Google Scholar]
- 43.Simon M.-C., Strassburger K., Nowotny B., Kolb H., Nowotny P., Burkart V., et al. Intake of Lactobacillus reuteri improves incretin and insulin secretion in glucose-tolerant humans: a proof of concept. Diabetes. Care. 2013;38(10):1827–1834. doi: 10.2337/dc14-2690. [DOI] [PubMed] [Google Scholar]
- 44.Alssema M., Rijkelijkhuizen J.M., Holst J.J., Teerlink T., Scheffer P.G., Eekhoff E.M.W., et al. Preserved GLP-1 and exaggerated GIP secretion in type 2 diabetes and relationships with triglycerides and ALT. Eur. J. Endocrinol. 2013;169(4):421–430. doi: 10.1530/EJE-13-0487. [DOI] [PubMed] [Google Scholar]
- 45.Muscelli E., Mari A., Casolaro A., Camastra S., Seghieri G., Gastaldelli A., et al. Separate impact of obesity and glucose tolerance on the incretin effect in normal subjects and type 2 diabetic patients. Diabetes. 2008;57:1340–1348. doi: 10.2337/db07-1315. [DOI] [PubMed] [Google Scholar]
- 46.Wang X.-L., Ye F., Li J., Zhu L.-Y., Feng G., Chang X.-Y., et al. Impaired secretion of glucagon-like peptide 1 during oral glucose tolerance test in patients with newly diagnosed type 2 diabetes mellitus. Saudi. Med. J. 2016;37(1):48–54. doi: 10.15537/smj.2016.1.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yabe D., Kuroe A., Watanabe K., Iwasaki M., Hamasaki A., Hamamoto Y., et al. Early phase glucagon and insulin secretory abnormalities, but not incretin secretion, are similarly responsible for hyperglycemia after ingestion of nutrients. J. Diabetes. Complications. 2015;29:413–421. doi: 10.1016/j.jdiacomp.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 48.Greenfield J.R., Farooqi I.S., Keogh J.M., Henning E., Habib A.M., Blackwood A., et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am. J. Clin. Nutr. 2009;89(1):106–113. doi: 10.3945/ajcn.2008.26362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vollmer K., Holst J.J., Baller B., Ellrichmann M., Nauck M.A., Schmidt W.E., et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678–687. doi: 10.2337/db07-1124. [DOI] [PubMed] [Google Scholar]
- 50.Chong S.C., Sukor N., Robert S.A., Ng K.F., Kamaruddin N.A. Fasting and stimulated glucagon-like peptide-1 exhibit a compensatory adaptive response in diabetes and pre-diabetes states: a multi-ethnic comparative study. Front. Endocrinol. (Lausanne). 2022;13 doi: 10.3389/fendo.2022.961432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dybjer E., Engström G., Helmer C., Nägga K., Rorsman P., Nilsson P.M. Incretin hormones, insulin, glucagon and advanced glycation end products in relation to cognitive function in older people with and without diabetes, a population-based study. Diabet. Med. 2020;37(7):1157–1166. doi: 10.1111/dme.14267. [DOI] [PubMed] [Google Scholar]
- 52.Chia C.W., Carlson O.D., Liu D.D., González-Mariscal I., Santa-Cruz Calvo S., Egan J.M. Incretin secretion in humans is under the influence of cannabinoid receptors. Am. J. Physiol. Endocrinol. Metab. 2017;313:E359–E366. doi: 10.1152/ajpendo.00080.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bagger J.I., Knop F.K., Lund A., Vestergaard H., Holst J.J., Vilsbøll T. Impaired regulation of the incretin effect in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2011;96(3):737–745. doi: 10.1210/jc.2010-2435. [DOI] [PubMed] [Google Scholar]
- 54.McCall A.L., Lieb D.C., Gianchandani R., MacMaster H., Maynard G.A., Murad M.H., et al. Management of individuals with diabetes at high risk for hypoglycemia: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2023;108(3):529–562. doi: 10.1210/clinem/dgac596. [DOI] [PubMed] [Google Scholar]
- 55.Ruetten H., Gebauer M., Raymond R.H., Calle R.A., Cobelli C., Ghosh A., et al. Mixed meal and intravenous l-arginine tests both stimulate incretin release across glucose tolerance in man: lack of correlation with β cell function. Metab. Syndr. Relat. Disord. 2018;16(8):406–415. doi: 10.1089/met.2018.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vilsbøll T., Krarup T., Madsbad S., Holst J.J. Both GLP-1 and GIP are insulinotropic at basal and postprandial glucose levels and contribute nearly equally to the incretin effect of a meal in healthy subjects. Regul. Pept. 2003;114(2–3):115–121. doi: 10.1016/s0167-0115(03)00111-3. [DOI] [PubMed] [Google Scholar]
- 57.Rask E., Olsson T., Söderberg S., Johnson O., Seckl J., Holst J.J., et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes. Care. 2001;24:1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]
- 58.Ryskjær J., Deacon C.F., Carr R.D., Krarup T., Madsbad S., Holst J., et al. Plasma dipeptidyl peptidase-IV activity in patients with type-2 diabetes mellitus correlates positively with HbAlc levels, but is not acutely affected by food intake. Eur. J. Endocrinol. 2006;155(3):485–493. doi: 10.1530/eje.1.02221. [DOI] [PubMed] [Google Scholar]
- 59.Lamiquiz-Moneo I., Pérez-Calahorra S., Gracia-Rubio I., Cebollada A., Bea A.M., Fumanal A., et al. Effect of the consumption of alcohol-free beers with different carbohydrate composition on postprandial metabolic response. Nutrients. 2022;14(5) doi: 10.3390/nu14051046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schönknecht Y.B., Crommen S., Stoffel-Wagner B., Coenen M., Fimmers R., Holst J.J., et al. Acute effects of three different meal patterns on postprandial metabolism in older individuals with a risk phenotype for cardiometabolic diseases: a randomized controlled crossover trial. Mol. Nutr. Food. Res. 2020;64(9) doi: 10.1002/mnfr.201901035. [DOI] [PubMed] [Google Scholar]
- 61.Carnevale R., Loffredo L., Del Ben M., Angelico F., Nocella C., Petruccioli A., et al. Extra virgin olive oil improves post-prandial glycemic and lipid profile in patients with impaired fasting glucose. Clin. Nutr. 2017;36(3):782–787. doi: 10.1016/j.clnu.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 62.Muangchan N., Khiewvan B., Chatree S., Pongwattanapakin K., Kunlaket N., Dokmai T., et al. Riceberry rice (Oryza sativa L.) slows gastric emptying and improves the postprandial glycaemic response. Br. J. Nutr. 2022;128:424–432. doi: 10.1017/S0007114521003494. [DOI] [PubMed] [Google Scholar]
- 63.Törrönen R., Sarkkinen E., Niskanen T., Tapola N., Kilpi K., Niskanen L. Postprandial glucose, insulin and glucagon-like peptide 1 responses to sucrose ingested with berries in healthy subjects. Br. J. Nutr. 2012;107(10):1445–1451. doi: 10.1017/S0007114511004557. [DOI] [PubMed] [Google Scholar]
- 64.Dicks L., Jakobs L., Sari M., Hambitzer R., Ludwig N., Simon M.-C., et al. Fortifying a meal with oyster mushroom powder beneficially affects postprandial glucagon-like peptide-1, non-esterified free fatty acids and hunger sensation in adults with impaired glucose tolerance: a double-blind randomized controlled crossover trial. Eur. J. Nutr. 2022;61(2):687–701. doi: 10.1007/s00394-021-02674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belinova L., Kahleova H., Malinska H., Topolcan O., Vrzalova J., Oliyarnyk O., et al. Differential acute postprandial effects of processed meat and isocaloric vegan meals on the gastrointestinal hormone response in subjects suffering from type 2 diabetes and healthy controls: a randomized crossover study. PLoS. One. 2014;9(9) doi: 10.1371/journal.pone.0107561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Alyami J., Whitehouse E., Yakubov G.E., Pritchard S.E., Hoad C.L., Blackshaw E., et al. Glycaemic, gastrointestinal, hormonal and appetitive responses to pearl millet or oats porridge breakfasts: a randomised, crossover trial in healthy humans. Br. J. Nutr. 2019;122(10):1142–1154. doi: 10.1017/S0007114519001880. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura Y., Takemoto A., Oyanagi T., Tsunemi S., Kubo Y., Nakagawa T., et al. Effects of cooked rice containing high resistant starch on postprandial plasma glucose, insulin, and incretin in patients with type 2 diabetes. Asia. Pac. J. Clin. Nutr. 2023;32(1):48–56. doi: 10.6133/apjcn.202303_32(1).0008. [DOI] [PubMed] [Google Scholar]
- 68.Di Mauro A., Tuccinardi D., Watanabe M., Del Toro R., Monte L., Giorgino R., et al. The Mediterranean diet increases glucagon-like peptide 1 and oxyntomodulin compared with a vegetarian diet in patients with type 2 diabetes: a randomized controlled cross-over trial. Diabetes. Metab. Res. Rev. 2021;37(6) doi: 10.1002/dmrr.3406. [DOI] [PubMed] [Google Scholar]
- 69.Rijkelijkhuizen J.M., McQuarrie K., Girman C.J., Stein P.P., Mari A., Holst J.J., et al. Effects of meal size and composition on incretin, alpha-cell, and beta-cell responses. Metabolism. 2021;59(4):502–511. doi: 10.1016/j.metabol.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 70.Smith K., Taylor G.S., Walker M., Brunsgaard L.H., Bowden Davies K.A., Stevenson E.J., et al. Pre-meal whey protein alters postprandial insulinemia by enhancing β-cell function and reducing insulin clearance in T2D. J. Clin. Endocrinol. Metab. 2023;108(8):e603–e612. doi: 10.1210/clinem/dgad069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bajka B.H., Pinto A.M., Perez-Moral N., Saha S., Ryden P., Ahn-Jarvis J., et al. Enhanced secretion of satiety-promoting gut hormones in healthy humans after consumption of white bread enriched with cellular chickpea flour: a randomized crossover study. Am. J. Clin. Nutr. 2023;117(3):477–489. doi: 10.1016/j.ajcnut.2022.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Otten J., Ryberg M., Mellberg C., Andersson T., Chorell E., Lindahl B., et al. Postprandial levels of GLP-1, GIP and glucagon after 2 years of weight loss with a Paleolithic diet: a randomised controlled trial in healthy obese women. Eur. J. Endocrinol. 2019;180(6):417–427. doi: 10.1530/EJE-19-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arjmand G., Abbas-Zadeh M., Fardaei M., Eftekhari M.H. The effect of short-term Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diet on hunger hormones, anthropometric parameters, and brain structures in middle-aged overweight and obese women: a randomized controlled trial. Iran. J. Med. Sci. 2022;47(5):422–432. doi: 10.30476/IJMS.2021.90829.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stentz F.B., Mikhael A., Kineish O., Christman J., Sands C. High protein diet leads to prediabetes remission and positive changes in incretins and cardiovascular risk factors. Nutr. Metab. Cardiovasc. Dis. 2021;31(4):1227–1237. doi: 10.1016/j.numecd.2020.11.027. [DOI] [PubMed] [Google Scholar]
- 75.Oliveira C.L.P., Boulé N.G., Elliott S.A., Sharma A.M., Siervo M., Berg A., et al. A high-protein total diet replacement alters the regulation of food intake and energy homeostasis in healthy, normal-weight adults. Eur. J. Nutr. 2022;61(4):1849–1861. doi: 10.1007/s00394-021-02747-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuglsang-Nielsen R., Rakvaag E., Langdahl B., Knudsen K.E.B., Hartmann B., Holst J.J., et al. Effects of whey protein and dietary fiber intake on insulin sensitivity, body composition, energy expenditure, blood pressure, and appetite in subjects with abdominal obesity. Eur. J. Clin. Nutr. 2021;75(4):611–619. doi: 10.1038/s41430-020-00759-4. [DOI] [PubMed] [Google Scholar]
- 77.Samkani A., Skytte M.J., Thomsen M.N., Astrup A., Deacon C.F., Holst J.J., et al. Acute effects of dietary carbohydrate restriction on glycemia, lipemia and appetite regulating hormones in normal-weight to obese subjects. Nutrients. 2018;10(9) doi: 10.3390/nu10091285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samkani A., Skytte M.J., Kandel D., Kjaer S., Astrup A., Deacon C.F., et al. A carbohydrate-reduced high-protein diet acutely decreases postprandial and diurnal glucose excursions in type 2 diabetes patients. Br. J. Nutr. 2018;119(8):910–917. doi: 10.1017/S0007114518000521. [DOI] [PubMed] [Google Scholar]
- 79.Ohlsson B., Darwiche G., Roth B., Höglund P. Alignments of endocrine, anthropometric, and metabolic parameters in type 2 diabetes after intervention with an Okinawa-based Nordic diet. Food. Nutr. Res. 2018;62 doi: 10.29219/fnr.v62.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rondanelli M., Miraglia N., Putignano P., Castagliuolo I., Brun P., Dall'Acqua S., et al. Effects of 60-day Saccharomyces boulardii and superoxide dismutase supplementation on body composition, hunger sensation, pro/antioxidant ratio, inflammation and hormonal lipo-metabolic biomarkers in obese adults: a double-blind, placebo-controlled trial. Nutrients. 2021;13(8) doi: 10.3390/nu13082512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Müller M., Hermes G.D.A., Emanuel E.C., Holst J.J., Zoetendal E.G., Smidt H., et al. Effect of wheat bran derived prebiotic supplementation on gastrointestinal transit, gut microbiota, and metabolic health: a randomized controlled trial in healthy adults with a slow gut transit. Gut. Microbes. 2020;12(1) doi: 10.1080/19490976.2019.1704141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Birkeland E., Gharagozlian S., Gulseth H.L., Birkeland K.I., Hartmann B., Holst J.J., et al. Effects of prebiotics on postprandial GLP-1, GLP-2 and glucose regulation in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled crossover trial. Diabet. Med. 2021;38(10) doi: 10.1111/dme.14657. [DOI] [PubMed] [Google Scholar]
- 83.Rabiei S., Hedayati M., Rashidkhani B., Saadat N., Shakerhossini R. The effects of synbiotic supplementation on body mass index, metabolic and inflammatory biomarkers, and appetite in patients with metabolic syndrome: a triple-blind randomized controlled trial. J. Diet. Suppl. 2019;16(3):294–306. doi: 10.1080/19390211.2018.1455788. [DOI] [PubMed] [Google Scholar]
- 84.Atabaki-Pasdar N., Ohlsson M., Viñuela A., Frau F., Pomares-Millan H., Haid M., et al. Predicting and elucidating the etiology of fatty liver disease: a machine learning modeling and validation study in the IMI DIRECT cohorts. PLoS. Med. 2020;17(6) doi: 10.1371/journal.pmed.1003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stinson S.E., Jonsson A.E., Lund M.A.V., Frithioff-Bøjsøe C., Aas Holm L., Pedersen O., et al. Fasting plasma GLP-1 is associated with overweight/obesity and cardiometabolic risk factors in children and adolescents. J. Clin. Endocrinol. Metab. 2021;106:1718–1727. doi: 10.1210/clinem/dgab098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kreymann B., Williams G., Ghatei M.A., Bloom S.R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 88.Caruso I., Di Gioia L., Di Molfetta S., Cignarelli A., Palmer S.C., Natale P., et al. Glucometabolic outcomes of GLP-1 receptor agonist-based therapies in patients with type 2 diabetes: a systematic review and network meta-analysis. EClinicalMedicine. 2023;64 doi: 10.1016/j.eclinm.2023.102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosenstock J., Frias J., Jastreboff A.M., Du Y., Lou J., Gurbuz S., et al. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023;402(10401):529–544. doi: 10.1016/S0140-6736(23)01053-X. [DOI] [PubMed] [Google Scholar]
- 90.Davies M.J., Aroda V.R., Collins B.S., Gabbay R.A., Green J., Maruthur N.M., et al. Management of hyperglycemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes. Care. 2022;45(11):2753–2786. doi: 10.2337/dci22-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bak M.J., Wewer Albrechtsen N.J., Pedersen J., Knop F.K., Vilsbøll T., Jørgensen N.B., et al. Specificity and sensitivity of commercially available assays for glucagon-like peptide-1 (GLP-1): implications for GLP-1 measurements in clinical studies. Diabetes. Obes. Metab. 2014;16(11):1155–1164. doi: 10.1111/dom.12352. [DOI] [PubMed] [Google Scholar]
- 92.A.C. Heijboer, A. Frans, M. Lomecky, M.A. Blankenstein, Analysis of glucagon-like peptide 1; what to measure? Clin. Chim. Acta. 412(13–14) 1191–1194, 10.1016/j.cca.2011.03.010. [DOI] [PubMed]
- 93.Näslund E., Gutniak M., Skogar S., Rössner S., Hellström P.M. Glucagon-like peptide 1 increases the period of postprandial satiety and slows gastric emptying in obese men. Am. J. Clin. Nutr. 1998;68(3):525–530. doi: 10.1093/ajcn/68.3.525. [DOI] [PubMed] [Google Scholar]
- 94.Malin S.K., Kullman E.L., Scelsi A.R., Godin J.-P., Ross A.B., Kirwan J.P. A whole-grain diet increases glucose-stimulated insulin secretion independent of gut hormones in adults at risk for type 2 diabetes. Mol. Nutr. Food. Res. 2019;63(7) doi: 10.1002/mnfr.201800967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iepsen E.W., Lundgren J., Holst J.J., Madsbad S., Torekov S.S. Successful weight loss maintenance includes long-term increased meal responses of GLP-1 and PYY3-36. Eur. J. Endocrinol. 2016;174:775–784. doi: 10.1530/EJE-15-1116. [DOI] [PubMed] [Google Scholar]
- 96.Vaag A.A., Holst J.J., Vølund A., Beck-Nielsen H.B. Gut incretin hormones in identical twins discordant for non-insulin-dependent diabetes mellitus (NIDDM)--evidence for decreased glucagon-like peptide 1 secretion during oral glucose ingestion in NIDDM twins. Eur J Endocrinol. 1996;135(4):425–432. doi: 10.1530/eje.0.1350425. [DOI] [PubMed] [Google Scholar]
- 97.Vilsbøll T., Holst J.J. Incretins, insulin secretion and type 2 diabetes mellitus. Diabetologia. 2004;47(3):357–366. doi: 10.1007/s00125-004-1342-6. [DOI] [PubMed] [Google Scholar]
- 98.Creutzfeldt W. The entero-insular axis in type 2 diabetes--incretins as therapeutic agents. Exp. Clin. Endocrinol. Diabetes. 2001;109(Suppl 2):S288–S303. doi: 10.1055/s-2001-18589. [DOI] [PubMed] [Google Scholar]
- 99.Zander M., Madsbad S., Madsen J.L., Holst J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359(9309):824–830. doi: 10.1016/S0140-6736(02)07952-7. [DOI] [PubMed] [Google Scholar]
- 100.Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y., et al. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proc. Natl. Acad. Sci. U S A. 1999;96(26):14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Scrocchi L.A., Brown T.J., MaClusky N., Brubaker P.L., Auerbach A.B., Joyner A.L., et al. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nat. Med. 1996;2(11):1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 102.Gasbjerg L.S., Helsted M.M., Hartmann B., Jensen M.H., Gabe M.B.N., Sparre-Ulrich A.H., et al. Separate and combined glucometabolic effects of endogenous glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 in healthy individuals. Diabetes. 2019;68(5):906–917. doi: 10.2337/db18-1123. [DOI] [PubMed] [Google Scholar]
- 103.Matikainen N., Bogl L.H., Hakkarainen A., Lundbom J., Lundbom N., Kaprio J., et al. GLP-1 responses are heritable and blunted in acquired obesity with high liver fat and insulin resistance. Diabetes. Care. 2014;37(1):242–251. doi: 10.2337/dc13-1283. [DOI] [PubMed] [Google Scholar]
- 104.Mortensen K., Petersen L.L., Ørskov C. Colocalization of GLP-1 and GIP in human and porcine intestine. Ann. N. Y. Acad. Sci. 2000;921:469–472. doi: 10.1111/j.1749-6632.2000.tb07017.x. [DOI] [PubMed] [Google Scholar]
- 105.Mortensen K., Christensen L.L., Holst J.J., Orskov C. GLP-1 and GIP are colocalized in a subset of endocrine cells in the small intestine. Regul. Pept. 2003;114(2–3):189–196. doi: 10.1016/s0167-0115(03)00125-3. [DOI] [PubMed] [Google Scholar]
- 106.Bahne E., Sun E.W.L., Young R.L., Hansen M., Sonne D.P., Hansen J.S., et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI. Insight. 2018;3(23) doi: 10.1172/jci.insight.93936. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Migoya E.M., Bergeron R., Miller J.L., Snyder R.N.K., Tanen M., Hilliard D., et al. Dipeptidyl peptidase-4 inhibitors administered in combination with metformin result in an additive increase in the plasma concentration of active GLP-1. Clin. Pharmacol. Ther. 2010;88(6):801–808. doi: 10.1038/clpt.2010.184. [DOI] [PubMed] [Google Scholar]
- 108.Wu T., Thazhath S.S., Bound M.J., Jones K.L., Horowitz M., Rayner C.K. Mechanism of increase in plasma intact GLP-1 by metformin in type 2 diabetes: stimulation of GLP-1 secretion or reduction in plasma DPP-4 activity? Diabetes. Res. Clin. Pract. 2014;106(1) doi: 10.1016/j.diabres.2014.08.004. e3–6. [DOI] [PubMed] [Google Scholar]
- 109.Thondam S.K., Cross A., Cuthbertson D.J., Wilding J.P., Daousi C. Effects of chronic treatment with metformin on dipeptidyl peptidase-4 activity, glucagon-like peptide 1 and ghrelin in obese patients with type 2 diabetes mellitus. Diabet. Med. 2012;29(8):e205–210. doi: 10.1111/j.1464-5491.2012.03675.x. [DOI] [PubMed] [Google Scholar]
- 110.Buse J.B., DeFronzo R.A., Rosenstock J., Kim T., Burns C., Skare S., et al. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and 12-week dose-ranging studies. Diabetes. Care. 2016;39(2):198–205. doi: 10.2337/dc15-0488. [DOI] [PubMed] [Google Scholar]
- 111.DeFronzo R.A., Buse J.B., Kim T., Burns C., Skare S., Baron A., et al. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: results from two randomised trials. Diabetologia. 2016;59(8):1645–1654. doi: 10.1007/s00125-016-3992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mannucci E., Tesi F., Bardini G., Ognibene A., Petracca M.G., Ciani S., et al. Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without type 2 diabetes. Diabetes. Nutr. Metab. 2004;17(6):336–342. [PubMed] [Google Scholar]
- 113.Beysen C., Murphy E.J., Deines K., Chan M., Tsang E., Glass A., et al. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55(2):432–442. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- 114.Watson L.E., Xie C., Wang X., Li Z., Phillips L.K., Sun Z., et al. Gastric emptying in patients with well-controlled type 2 diabetes compared with young and older control subjects without diabetes. J. Clin. Endocrinol. Metab. 2019;104(8):3311–3319. doi: 10.1210/jc.2018-02736. [DOI] [PubMed] [Google Scholar]
- 115.Borg M.J., Jones K.L., Sun Z., Horowitz M., Rayner C.K., Wu T. Metformin attenuates the postprandial fall in blood pressure in type 2 diabetes. Diabetes. Obes. Metab. 2019;21(5):1251–1254. doi: 10.1111/dom.13632. [DOI] [PubMed] [Google Scholar]
- 116.Schirra J., Katschinski M., Weidmann C., Schäfer T., Wank U., Arnold R., et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J. Clin. Invest. 1996;97(1):92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hjerpsted J.B., Flint A., Brooks A., Axelsen M.B., Kvist T., Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes. Obes. Metab. 2018;20(3):610–619. doi: 10.1111/dom.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jensterle M., Ferjan S., Ležaič L., Sočan A., Goričar K., Zaletel K., et al. Semaglutide delays 4-hour gastric emptying in women with polycystic ovary syndrome and obesity. Diabetes. Obes. Metab. 2023;25(4):975–984. doi: 10.1111/dom.14944. [DOI] [PubMed] [Google Scholar]
- 119.Drucker D.J., Buse J.B., Taylor K., Kendall D.M., Trautmann M., Zhuang D., et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240–1250. doi: 10.1016/S0140-6736(08)61206-4. [DOI] [PubMed] [Google Scholar]
- 120.Stevens J.E., Horowitz M., Deacon C.F., Nauck M., Rayner C.K., Jones K.L. The effects of sitagliptin on gastric emptying in healthy humans - a randomised, controlled study. Aliment. Pharmacol. Ther. 2012;36(4):379–390. doi: 10.1111/j.1365-2036.2012.05198.x. [DOI] [PubMed] [Google Scholar]
- 121.Vella A., Bock G., Giesler P.D., Burton D.B., Serra D.B., Saylan M.L., et al. The effect of dipeptidyl peptidase-4 inhibition on gastric volume, satiation and enteroendocrine secretion in type 2 diabetes: a double-blind, placebo-controlled crossover study. Clin. Endocrinol. (Oxf). 2008;69(5):737–744. doi: 10.1111/j.1365-2265.2008.03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yunker A.G., Luo S., Jones S., Dorton H.M., Alves J.M., Angelo B., et al. Appetite-regulating hormones are reduced after oral sucrose vs glucose: influence of obesity, insulin resistance, and sex. J. Clin. Endocrinol. Metab. 2021;106(3):654–664. doi: 10.1210/clinem/dgaa865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hjørne A.P., Modvig I.M., Holst J.J. The sensory mechanisms of nutrient-induced GLP-1 secretion. Metabolites. 2022;12(5) doi: 10.3390/metabo12050420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roberge J.N., Brubaker P.L. Regulation of intestinal proglucagon-derived peptide secretion by glucose-dependent insulinotropic peptide in a novel enteroendocrine loop. Endocrinology. 1993;133(1):233–240. doi: 10.1210/endo.133.1.8319572. [DOI] [PubMed] [Google Scholar]
- 125.Rocca A.S., Brubaker P.L. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology. 1999;140(4):1687–1694. doi: 10.1210/endo.140.4.6643. [DOI] [PubMed] [Google Scholar]
- 126.Miguéns-Gómez A., Casanova-Martí À., Blay M.T., Terra X., Beltrán-Debón R., Rodríguez-Gallego E., et al. Glucagon-like peptide-1 regulation by food proteins and protein hydrolysates. Nutr. Res. Rev. 2021;34(2):259–275. doi: 10.1017/S0954422421000019. [DOI] [PubMed] [Google Scholar]
- 127.Herrmann C., Göke R., Richter G., Fehmann H.C., Arnold R., Göke B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion. 1995;56(2):117–126. doi: 10.1159/000201231. [DOI] [PubMed] [Google Scholar]
- 128.Qualmann C., Nauck M.A., Holst J.J., Orskov C., Creutzfeldt W. Glucagon-like peptide 1 (7-36 amide) secretion in response to luminal sucrose from the upper and lower gut. A study using alpha-glucosidase inhibition (acarbose) Scand. J. Gastroenterol. 1995;30(9):892–896. doi: 10.3109/00365529509101597. [DOI] [PubMed] [Google Scholar]
- 129.Belza A., Ritz C., Sørensen M.Q., Holst J.J., Rehfeld J.F., Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am. J. Clin. Nutr. 2013;97(5):980–989. doi: 10.3945/ajcn.112.047563. [DOI] [PubMed] [Google Scholar]
- 130.van der Klaauw A.A., Keogh J.M., Henning E., Trowse V.M., Dhillo W.S., Ghatei M.A., et al. High protein intake stimulates postprandial GLP1 and PYY release. Obesity. (Silver Spring). 2013;21(8):1602–1607. doi: 10.1002/oby.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shah M., Franklin B., Adams-Huet B., Mitchell J., Bouza B., Dart L., et al. Effect of meal composition on postprandial glucagon-like peptide-1, insulin, glucagon, C-peptide, and glucose responses in overweight/obese subjects. Eur. J. Nutr. 2017;56(3):1053–1062. doi: 10.1007/s00394-016-1154-8. [DOI] [PubMed] [Google Scholar]
- 132.Chen Q., Reimer R.A. Dairy protein and leucine alter GLP-1 release and mRNA of genes involved in intestinal lipid metabolism in vitro. Nutrition. 2009;25(3):340–349. doi: 10.1016/j.nut.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Modvig I.M., Kuhre R.E., Jepsen S.L., Xu S.F.S., Engelstoft M.S., Egerod K.L., et al. Amino acids differ in their capacity to stimulate GLP-1 release from the perfused rat small intestine and stimulate secretion by different sensing mechanisms. Am. J. Physiol. Endocrinol. Metab. 2021;320(5):E874–E885. doi: 10.1152/ajpendo.00026.2021. [DOI] [PubMed] [Google Scholar]
- 134.Modvig I.M., Kuhre R.E., Holst J.J. Peptone-mediated glucagon-like peptide-1 secretion depends on intestinal absorption and activation of basolaterally located calcium-sensing receptors. Physiol. Rep. 2019;7(8) doi: 10.14814/phy2.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Svendsen B., Pedersen J., Albrechtsen N.J.W., Hartmann B., Toräng S., Rehfeld J.F., et al. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156(3):847–857. doi: 10.1210/en.2014-1710. [DOI] [PubMed] [Google Scholar]
- 136.Eissele R., Göke R., Willemer S., Harthus H.P., Vermeer H., Arnold R., et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Invest. 1992;22(4):283–291. doi: 10.1111/j.1365-2362.1992.tb01464.x. [DOI] [PubMed] [Google Scholar]
- 137.Hansen C.F., Vrang N., Sangild P.T., Jelsing J. Novel insight into the distribution of L-cells in the rat intestinal tract. Am. J. Transl. Res. 2013;5:347–358. [PMC free article] [PubMed] [Google Scholar]
- 138.Nandhra G.K., Chaichanavichkij P., Birch M., Scott S.M. Gastrointestinal transit times in health as determined using ingestible capsule systems: a systematic review. J. Clin. Med. 2023;12(16) doi: 10.3390/jcm12165272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Holst J.J. Glucagon-like peptide-1: are its roles as endogenous hormone and therapeutic wizard congruent? J. Intern. Med. 2022;291(5):557–573. doi: 10.1111/joim.13433. [DOI] [PubMed] [Google Scholar]
- 140.Jang H.-J., Kokrashvili Z., Theodorakis M.J., Carlson O.D., Kim B.-J., Zhou J., et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl. Acad. Sci. U S A. 2007;104(38):15069–15074. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee A.A., Owyang C. Sugars, sweet taste receptors, and brain responses. Nutrients. 2017;9(7) doi: 10.3390/nu9070653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Takai S., Yasumatsu K., Inoue M., Iwata S., Yoshida R., Shigemura N., et al. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB. J. 2015;29(6):2268–2280. doi: 10.1096/fj.14-265355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carreiro A.L., Dhillon J., Gordon S., Higgins K.A., Jacobs A.G., McArthur B.M., et al. The macronutrients, appetite, and energy intake. Annu. Rev. Nutr. 2016;36:73–103. doi: 10.1146/annurev-nutr-121415-112624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Layer P., Holst J.J., Grandt D., Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig. Dis. Sci. 1995;40(5):1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 145.Maljaars P.W.J., Peters H.P.F., Mela D.J., Masclee A.A.M. Ileal brake: a sensible food target for appetite control. A review. Physiol. Behav. 2008;95(3):271–281. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 146.Holst J.J., Albrechtsen N.J.W., Rosenkilde M.M., Deacon C.F. Physiology of the Incretin hormones, GIP and GLP-1-Regulation of Release and Posttranslational Modifications. Compr Physiol. 2019;9:1339–1381. doi: 10.1002/cphy.c180013. [DOI] [PubMed] [Google Scholar]
- 147.Mansour A., Hosseini S., Larijani B., Pajouhi M., Mohajeri-Tehrani M.R. Nutrients related to GLP1 secretory responses. Nutrition. 2013;29(6):813–820. doi: 10.1016/j.nut.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 148.Bodnaruc A.M., Prud'homme D., Blanchet R., Giroux I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: a review. Nutr. Metab. (Lond). 2016;13:92. doi: 10.1186/s12986-016-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Y. Fujiwara, S. Eguchi, H. Murayama, Y. Takahashi, M. Toda, K. Imai, et al, Relationship between diet/exercise and pharmacotherapy to enhance the GLP-1 levels in type 2 diabetes. Endocrinol. Diabetes. Metab. 2(3) e00068, 10.1002/edm2.68. [DOI] [PMC free article] [PubMed]
- 150.Mattes R.D., Hunter S.R., Higgins K.A. Sensory, gastric, and enteroendocrine effects of carbohydrates, fat, and protein on appetite. Curr. Opin. Endocr. Metab. Res. 2019;4:14–20. doi: 10.1016/j.coemr.2018.09.002. [DOI] [Google Scholar]
- 151.Hutch C.R., Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158(12):4139–4151. doi: 10.1210/en.2017-00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Le Roux C.W., Welbourn R., Werling M., Osborne A., Kokkinos A., Laurenius A., et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann. Surg. 2007;246(5):780–785. doi: 10.1097/SLA.0b013e3180caa3e3. [DOI] [PubMed] [Google Scholar]
- 153.Dirksen C., Jørgensen N.B., Bojsen-Møller K.N., Kielgast U., Jacobsen S.H., Clausen T.R., et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int. J. Obes. (Lond). 2013;37(11):1452–1459. doi: 10.1038/ijo.2013.15. [DOI] [PubMed] [Google Scholar]
- 154.Falkén Y., Hellström P.M., Holst J.J., Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J. Clin. Endocrinol. Metab. 2011;96(7):2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- 155.Violi F., Loffredo L., Pignatelli P., Angelico F., Bartimoccia S., Nocella C., et al. Extra virgin olive oil use is associated with improved post-prandial blood glucose and LDL cholesterol in healthy subjects. Nutr. Diabetes. 2015;5(7):e172. doi: 10.1038/nutd.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kawakami Y., Watanabe Y., Mazuka M., Yagi N., Sawazaki A., Koganei M., et al. Effect of cacao polyphenol-rich chocolate on postprandial glycemia, insulin, and incretin secretion in healthy participants. Nutrition. 2021;85 doi: 10.1016/j.nut.2020.111128. [DOI] [PubMed] [Google Scholar]
- 157.Hall K.D., Ayuketah A., Brychta R., Cai H., Cassimatis T., Chen K.Y., et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell. Metab. 2019;30:67–77.e3. doi: 10.1016/j.cmet.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shannon M., Green B., Willars G., Wilson J., Matthews N., Lamb J., et al. The endocrine disrupting potential of monosodium glutamate (MSG) on secretion of the glucagon-like peptide-1 (GLP-1) gut hormone and GLP-1 receptor interaction. Toxicol. Lett. 2017;265:97–105. doi: 10.1016/j.toxlet.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 159.Li P., Li M., Wu T., Song Y., Li Y., Huang X., et al. Systematic evaluation of antimicrobial food preservatives on glucose metabolism and gut microbiota in healthy mice. NPJ. Sci. Food. 2022;6(1):42. doi: 10.1038/s41538-022-00158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hosaka H., Kusano M., Zai H., Kawada A., Kuribayashi S., Shimoyama Y., et al. Monosodium glutamate stimulates secretion of glucagon-like peptide-1 and reduces postprandial glucose after a lipid-containing meal. Aliment. Pharmacol. Ther. 2012;36(9):895–903. doi: 10.1111/apt.12050. [DOI] [PubMed] [Google Scholar]
- 161.Forde C.G. Beyond ultra-processed: considering the future role of food processing in human health. Proc. Nutr. Soc. 2023;82(3):406–418. doi: 10.1017/S0029665123003014. [DOI] [PubMed] [Google Scholar]
- 162.Handgraaf S., Dusaulcy R., Visentin F., Philippe J., Gosmain Y. 17-β Estradiol regulates proglucagon-derived peptide secretion in mouse and human α- and L cells. JCI. Insight. 2018;3(7) doi: 10.1172/jci.insight.98569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brennan I.M., Feltrin K.L., Nair N.S., Hausken T., Little T.J., Gentilcore D., et al. Effects of the phases of the menstrual cycle on gastric emptying, glycemia, plasma GLP-1 and insulin, and energy intake in healthy lean women. Am. J. Physiol. Gastrointest. Liver. Physiol. 2009;297(3):G602–G610. doi: 10.1152/ajpgi.00051.2009. [DOI] [PubMed] [Google Scholar]
- 164.Hur K.Y., Lee M.-S. Gut microbiota and metabolic disorders. Diabetes. Metab. J. 2015;39(3):198–203. doi: 10.4093/dmj.2015.39.3.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Karlsson F.H., Tremaroli V., Nookaew I., Bergström G., Behre C.J., Fagerberg B., et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498(7452):99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 166.Tang W.H.W., Bäckhed F., Landmesser U., Hazen S.L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73(16):2089–2105. doi: 10.1016/j.jacc.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 168.Vallianou N.G., Stratigou T., Tsagarakis S. Metformin and gut microbiota: their interactions and their impact on diabetes. Hormones. (Athens). 2019;18(2):141–144. doi: 10.1007/s42000-019-00093-w. [DOI] [PubMed] [Google Scholar]
- 169.Sonnenburg J.L., Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Everard A., Cani P.D. Gut microbiota and GLP-1. Rev. Endocr. Metab. Disord. 2014;15(3):189–196. doi: 10.1007/s11154-014-9288-6. [DOI] [PubMed] [Google Scholar]
- 171.Müller T.D., Finan B., Bloom S.R., D'Alessio D., Drucker D.J., Flatt P.R., et al. Glucagon-like peptide 1 (GLP-1) Mol. Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Grieco M., Giorgi A., Gentile M.C., d'Erme M., Morano S., Maras B., et al. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front. Neurosci. 2019;13:1112. doi: 10.3389/fnins.2019.01112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Holst J.J., Jepsen S.L., Modvig I. GLP-1-Incretin and pleiotropic hormone with pharmacotherapy potential. Increasing secretion of endogenous GLP-1 for diabetes and obesity therapy. Curr. Opin. Pharmacol. 2022;63 doi: 10.1016/j.coph.2022.102189. [DOI] [PubMed] [Google Scholar]
- 174.Abdalqadir N., Adeli K. GLP-1 and GLP-2 orchestrate intestine integrity, gut microbiota, and immune system crosstalk. Microorganisms. 2022;10(10) doi: 10.3390/microorganisms10102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Suez J., Zmora N., Segal E., Elinav E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019;25(5):716–729. doi: 10.1038/s41591-019-0439-x. [DOI] [PubMed] [Google Scholar]
- 176.Hernando-Redondo J., Toloba A., Benaiges D., Salas-Salvadó J., Martínez-Gonzalez M.A., Corella D., et al. Mid- and long-term changes in satiety-related hormones, lipid and glucose metabolism, and inflammation after a Mediterranean diet intervention with the goal of losing weight: a randomized, clinical trial. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.950900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Meslier V., Laiola M., Roager H.M., Filippis de F., Roume H., Quinquis B., et al. Mediterranean diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut. 2020;69(7):1258–1268. doi: 10.1136/gutjnl-2019-320438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.