Abstract
Due to the complicated metabolic and regulatory networks of l-serine biosynthesis and degradation, microbial cell factories for l-serine production using non-model microorganisms have not been reported. In this study, a combination of synthetic biology and process optimization were applied in an ethanologenic bacterium Zymomonas mobilis for l-serine production. By blocking the degradation pathway while introducing an exporter EceamA from E. coli, l-serine titer in recombinant Z. mobilis was increased from 15.30 mg/L to 62.67 mg/L. It was further increased to 260.33 mg/L after enhancing the l-serine biosynthesis pathway. Then, 536.70 mg/L l-serine was achieved by removing feedback inhibition with a SerA mutant, and an elevated titer of 687.67 mg/L was further obtained through increasing serB copies while enhancing the precursors. Finally, 855.66 mg/L l-serine can be accumulated with the supplementation of the glutamate precursor. This work thus not only constructed an l-serine producer to help understand the bottlenecks limiting l-serine production in Z. mobilis for further improvement, but also provides guidance on engineering non-model microbes to produce biochemicals with complicated pathways such as amino acids or terpenoids.
Keywords: Zymomonas mobilis, Metabolic engineering, l-serine, Feedback inhibition, Anaerobic fermentation
Graphical abstract
Highlights
-
•
l-serine producer using the anaerobic bacterium Zymomonas mobilis constructed.
-
•
l-serine titer elevated 55.6 folds with fermentation optimization.
-
•
Anaerobic flask fermentation of l-serine at ca. 1 g/L achieved.
1. Introduction
Serine has broad applications in food, cosmetic, as a nutritional additive, and in pharmaceutical industries with fast-growing market demands [1,2]. It plays a crucial role in various biological processes, including one-carbon unit (C-1) metabolism, protein synthesis, purine and pyrimidine synthesis, as well as cellular membrane production and processing [3]. Direct fermentative production of l-serine, rather than extraction from protein hydrolysates, chemical synthesis, and enzyme or expensive precursor glycine has indeed garnered significant attentions in recent years [2,[4], [5], [6], [7]]. It offers a promising alternative to traditional methods as a more cost-effective, sustainable, and scalable approach for meeting the increasing demand for this valuable amino acid in various industries.
However, l-serine production with high titers still faces several challenges even in well-studied model species such as Escherichia coli and Corynebacterium glutamicum, since l-serine plays critical roles in many biochemical reactions as an important intermediate in the central metabolic pathway [2]. Furthermore, the serine cycle with its unique characteristic of naturally evolved oxygen-insensitive pathway can synthesize acetyl-CoA (the C2 building block) from multiple groups of C1 compound without carbon loss [[8], [9], [10]]. Therefore, the production and application of l-serine still face several challenges, especially in non-model microorganisms that are not naturally amino acid producers, due to its complicated regulatory network and significance in numerous cellular reactions.
There are two major l-serine degradation pathways in both E. coli and C. glutamicum. Serine hydroxymethyl transferase (SHMT, encoded by glyA) catalyzes the conversion of serine to glycine while transferring one carbon unit to tetrahydrofolate (THF), which is an important cofactor required for C1-metabolism. After blocking its degradation pathway by deleting sdaA, sdaB, tdcG and glyA, and overexpressing l-serine biosynthesis pathway genes along with the cysteine/homoserine transporter EamA, a recombinant strain of E. coli MG1655 produced 11.7 g/L l-serine [11], and l-serine production was further promoted to 37 g/L after 52 h of fermentation with the application of metabolic engineering and adaptive laboratory evolution strategies [12]. Then, through translation initiation optimization, an industrialized E. coli ALE-5 (DE3) reached 50 g/L serine in fed batch fermentations, which was the highest titer reported thus far [13].
The gene of glyA for glycine biosynthesis can be deleted in E. coli with two alternative threonine degradation pathways, while it was essential in C. glutamicum [2,7]. Although wild-type C. glutamicum ATCC13032 cannot accumulate l-serine, C. glutamicum SYPS-062 screened naturally can generate up to 6.65 g/L l-serine from sugar [14]. Subsequently, various genetic manipulations and fermentation strategies were applied alone or together to improve l-serine production in C. glutamicum [[15], [16], [17], [18]]. The highest l-serine titer of 43.9 g/L with a yield of 0.44 g/g sucrose was achieved in C. glutamicum A36 by overexpressing serE gene encoding a novel exporter and l-serine synthetic pathway key genes of serAΔ197, serC, and serB [19].
Despite of significant progress achieved on l-serine production in model microorganisms of E. coli and C. glutamicum, there are still many challenges that must be addressed in other microbial cell factories, such as aeration requirements, dissolved oxygen level, fermentation efficiency and cost of production. To date, the fermentation processes for the l-serine production in model microorganisms require continuous aeration to maintain the metabolic activity, which are energy-intensive and will increase operational costs.
Zymomonas mobilis is a non-model generally regarded as safe (GRAS) strain, which is the only known microorganisms possessing an anaerobic Enter-Doudoroff (ED) pathway with many excellent characteristics, such as high sugar utilization efficiency at broad pH ranges (3.5–7.5) [20]. With the rapid technology advancement in systems and synthetic biology, native and exogenous CRISPR-Cas genome editing toolkits [21,22] as well as systems for biological parts identification and characterization [23] have been established in Z. mobilis. Although various recombinant strains have been constructed to produce platform biochemicals such as cellulosic ethanol, lactate, acetoin, isobutanol, 2,3-butanediol (2,3-BDO), and poly-3-hydroxybutyrate (PHB) [21,[24], [25], [26], [27], [28]], there are limited reports on engineering Z. mobilis for the production of biochemicals involved in sophisticated regulations such as amino acids.
Z. mobilis possesses the unique anaerobic ED pathway, which can only gain one net ATP molecule per consumed glucose. In addition, the formation of l-serine by consuming 3-phosphoglycerate leads to decreased ATP formation, thereby limiting the maximal l-serine productivity. In this study, Z. mobilis was utilized in this study to explore the bottleneck for producing high titer of l-serine in a non-model microorganism that is not naturally suitable for amino acid production due to its low ATP generation, which can help explore the pathway compatibilities among different microbial hosts for future rational design of synthetic microbial cell factories. l-serine tolerance of Z. mobilis was evaluated. Subsequently, various metabolic engineering and synthetic biology strategies were applied for enhanced l-serine production to address the aforementioned challenges (Fig. 1). These efforts aim not only to help generate recombinant strains for anaerobic l-serine production, but also to establish a foundation for further engineering of Z. mobilis for higher l-serine production and other biochemicals with sophisticated metabolic and regulatory pathways.
Fig. 1.
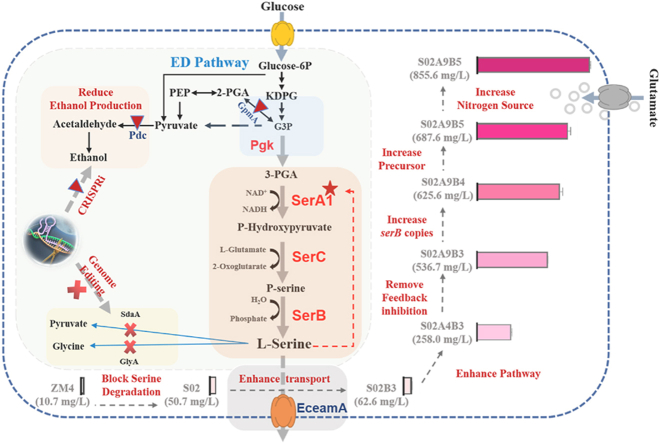
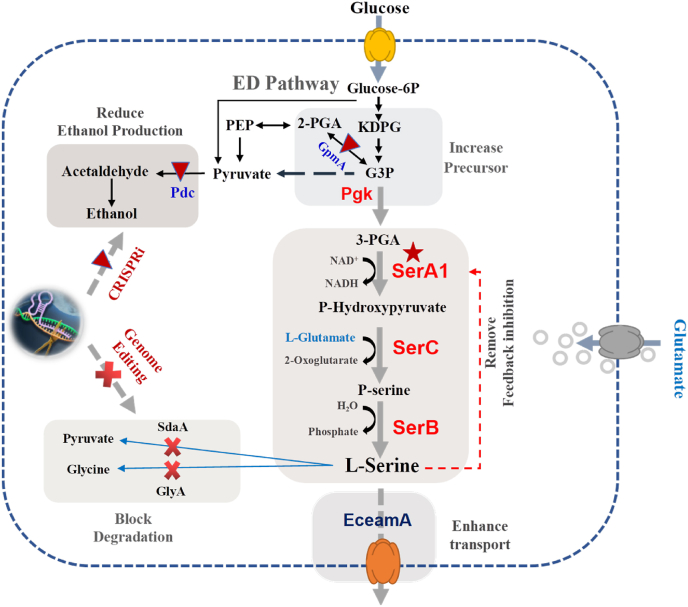
Schematic diagram of l-serine biosynthesis from glucose in Z. mobilis, and metabolic engineering strategies for efficient production of l-serine employed in this study. Relevant reactions are represented by the proteins and genes. Red forks mean gene deletion, and red triangle denote repression of genes with CRISPRi. The red star represents enzyme modification to remove feedback inhibition. G3P: Glycerate-1,3P; KDPG: 2-Keto-3-deoxy-6-phosphogluconate; EceamA: l-serine transporter; GpmA: glycerate 3-phosphate mutase; GlyA: serine hydroxymethyl transferase; PGA: 3-P-d-Glycerate; 2-PGA: 2-phosphoglycerate; PEP: Phosphoenolpyruvate; P-Serine: 3-Phosphoserine; Pdc: pyruvate decarboxylase; Pgk: phosphoglycerate kinase; SdaA: l-serine deaminase; SerA1: 3-phosphoglycerate dehydrogenase; SerB: phosphoserine phosphatase; SerC: phosphoserine aminotransferase.
2. Materials and methods
2.1. Plasmids, strains and culture medium
All bacterial strains and plasmids used in this study are listed in Table S1 and Table S2, respectively. Wild-type Z. mobilis ZM4 was used as the parent strain for strain engineering. E. coli DH5α was used for plasmid construction, and E. coli Trans 110 was utilized as host for plasmid demethylation. Shuttle vectors pEZ15Asp [27] and pEZ39p [25] were used for gene over-expression, and pL2R [21] was used for gene deletion in Z. mobilis. E. coli strains were grown in Luria-Bertani medium (LB, 10 g/L NaCl, 10 g/L tryptone, 5 g/L yeast extract) at 37 °C, 250 rpm. Z. mobilis ZM4 and its derivative mutants were cultured in RMG5 (50 g/L glucose, 10 g/L yeast extract, 2 g/L KH2PO4) at 30 °C, 100 rpm. Minimal medium (MMG2, 20 g/L glucose, 1 g/L KH2PO4, 1 g/L K2HPO4, 1 g/L (NH4)2SO4, 0.5 g/L NaCl, 0.42 g/L MgCl2⋅6H2O, 0.001 g/L calcium pantothenate) was also used in l-serine tolerance testing. When required, antibiotics of tetracycline (1.5 μg/mL), spectinomycin (100 μg/mL), and kanamycin (50 μg/mL) were used for E. coli or Z. mobilis.
2.2. DNA manipulation techniques and recombinant strain construction
The genome of ZM4 has been deposited into the Genbank with the accession number of CP 023715-9 [29]. All plasmid constructions were performed using the Gibson assembly method [25]. Designed primers were ordered from Tsingke Biotechnology Co., Ltd. (Tsingke, Beijing, China), with 15–20 nucleotides overlapping adjacent DNA fragments. The DNA polymerases used were Primer STAR (Takara, Kyoto, Japan) or Taq DNA polymerases (Tsingke, Beijing, China). The PCR products were separated by agarose gel electrophoresis, purified by gel purification kit (TsingKe, Beijing, China), and quantified using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). The vector fragment and gene were ligated through the T5 exonuclease (NEB, WA, USA). After validation through colony PCR and Sanger sequencing (Tsingke, Beijing, China), the correct recombinant plasmids were transformed into Z. mobilis competent cells via electroporation using Bio-Rad Gene Pulser (Bio-Rad, CA, USA). Recombinants were confirmed by colony PCR and Sanger sequencing (Tsingke, Beijing, China).
Gene editing was performed with the endogenous type I-F CRISPR-Cas system of Z. mobilis [30]. The spacer was designed to bear the entire 32 bp sequence containing a 5′-CCC-3′ PAM [22]. The plasmids pL2R-sdaA and pL2R-glyA were constructed to knock out sdaA and glyA in the Z. mobilis ZM4 genome sequentially, resulting in the recombinant strains S01 (ΔsdaA) and S02 (ΔsdaA, ΔglyA).
CRISPR interference (CRISPRi) was employed to suppress the transcription of target genes with the mutation of chromosome Cas3 into dCas3 (His221Ala, His222Ala) in Z. mobilis, which lost its DNA cleavage ability but retained its capacity to bind specific DNA sequences guided by gRNA [22,31]. The plasmid pL2R-dCas3 was utilized to replace Cas3 with dCas3 (His221Ala, His222Ala) on the genome of strain S02, resulting in the recombinant strain S03. Sequences of the primers used in genome editing in this study are listed in Table S3.
For gene over-expression, the derivative plasmids from pEZ15Asp were designated as the A series, while the plasmids from pEZ39p were designated as the B series, such as plasmid pEZ-A4 and 39p-B3, respectively. All strains were named on the base of strain S01, S02, and S03. For instance, strain S02B3 was obtained with plasmid 39p-B3 transformed into strain S02. Similarly, strain S02A4B3 was constructed using strain S02B3 by introducing plasmid pEZ-A4 into it by electroporation.
2.3. Sequence alignment and modeling analysis
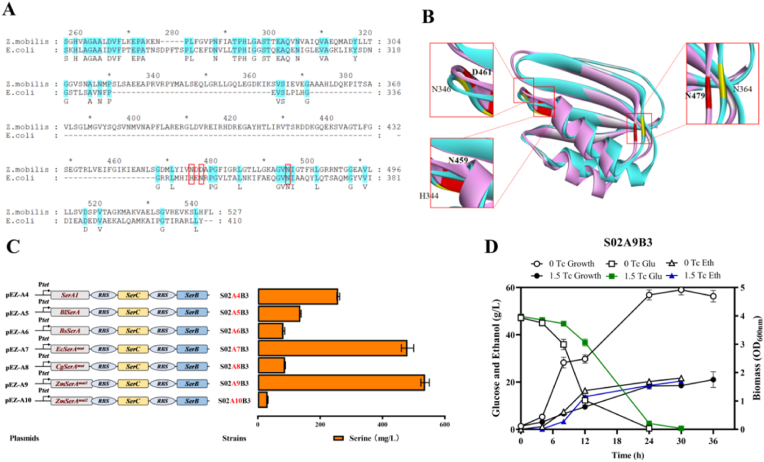
The serA genes from Z. mobilis (NC_006526), E. coli (NC_000913), and C. glutamicum (GCA_000404185.1) were download from the NCBI database (https://www.ncbi.nlm.nih.gov/), and then aligned using MEGA 11 for sequence comparison. The mutation sites H344, N346, and N364 in serAmut gene from E. coli were corresponding to the positions N459, D461, and N479 in serA1 gene in Z. mobilis [11]. The serA gene in C. glutamicum was modified with 197 amino acids deletion at the C-terminus [32], which was also applied to serA1 in Z. mobilis. Accordingly, two reconstructed serA genes were generated and named as ZmserAmut1 and ZmserAmut2, respectively. Based on the sequence alignment result, the structure of SerA1 from Z. mobilis was predicted by AlphaFold2. The mutation sites scheme was further confirmed by structural alignment using Discovery Studio 2021 (Dassault Systèmes, Paris, France).
2.4. Shake-flask fermentation
The fermentation experiments were conducted with an initial OD600nm of 0.1 in 50-mL shake flasks with 40 mL RMG5 or MMG2 at 30 °C, 100 rpm. For l-serine tolerance test, 10, 20, 30, and 40 g/L as well as 4, 8, 12, and 16 g/L l-serine were added into RMG5 and MMG2, respectively. To assess the impact of nitrogen sources supplementation on l-serine production, 5 g/L glutamate hydrochloride or (NH4)2SO4 was added in RMG5. Three or more technical replicates were used for each condition. Samples were taken at different time points for further analysis.
2.5. Analytical methods
During the fermentation, cell growth was determined by measuring the optical density at OD600nm using a UV-visible spectrophotometer UV-1800 (AOE Instrument Co., Ltd, Shanghai, China). Glucose, ethanol, and acetate were analyzed by high-performance liquid chromatography (HPLC) (LC-20AD, Shimadzu, Tokyo, Japan) equipped with a refractive index detector (RID-10A) and a column of Bio-Rad Aminex HPX-87H (300 × 7.8 mm) (Bio-Rad, CA, United States). The mobile phase was 5 mM H2SO4, with a flow rate of 0.5 mL/min, and the temperature of the detector and column oven was maintained at 40 °C and 60 °C, respectively.
The l-serine in the fermentation solution was determined by o-phthalaldehyde (OPA)/3-mercaptopropionic acid as the precolumn derivatization reagent, using HPLC (LC-20AD, Shimadzu, Tokyo, Japan) equipped with an ultraviolet detector (SPD-20A) and a column of Agilent AdvanceBio Amino Acid Analysis (3.0 mm × 100 mm) (Agilent, DE, USA). Mobile phase A was 10 mM Na2HPO4 and 10 mM Na2B4O7 (pH 8.2). Mobile phase B was acetonitrile:methanol:water (45:45:10, v:v:v). The column was set at 40 °C and the flow rate was 1.5 mL/min. The UV detector was used at 340 nm. The detailed procedure was started with A:B (98:2, v/v), 0.35 min with A:B (98:2, v/v), 13.4 min with A:B (43:57, v/v), 13.5 min with A:B (0:100, v/v), 15.7 min with A:B (0:100, v/v), 15.8 min with A:B (98:2, v/v), 18 min ended.
The data presented in the graphs were analyzed using GraphPad Prism statistical software (version 8.3.0) to calculate the mean standard deviation and perform T-tests or One-way ANOVA. P < 0.05 was considered as statistically significant difference.
3. Results
3.1. Investigation of serine tolerance of Z. mobilis
Although l-serine serves as easily assimilable carbon and nitrogen sources for cell growth, it can also have detrimental effects on microorganisms since it interferes with branched-chain amino acid biosynthesis, which may result in the accumulation of reactive by-products such as hydroxypyruvate and acrylates in high concentrations [11]. For example, E. coli was very sensitive to even low concentrations of serine. When all three l-serine dehydratases of major serine degradation pathways from E. coli K-12 were deleted, the resultant strain had only minimal growth with the supplementation of serine as low as 1.5 g/L [33].
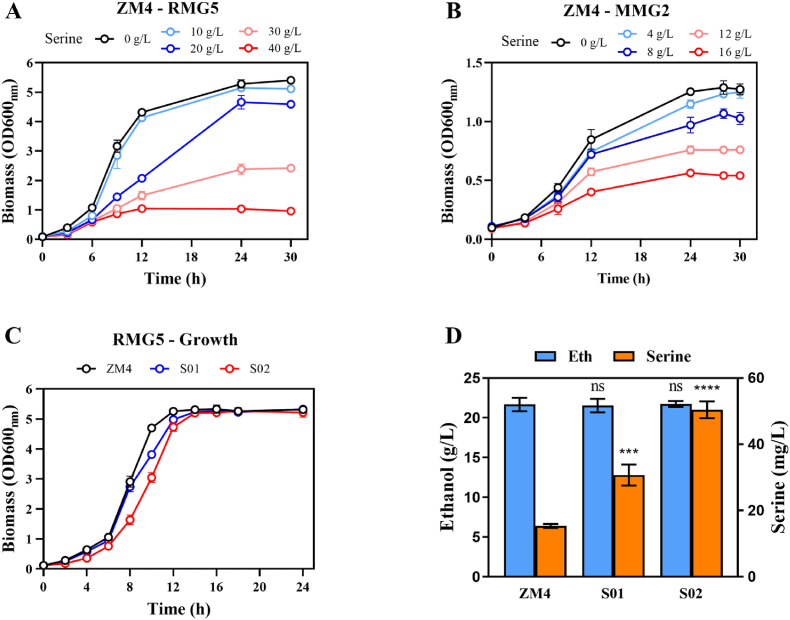
Considering the cytotoxicity and inhibition of cell growth with high l-serine concentrations, the tolerance of wild-type Z. mobilis ZM4 to 10, 20, 30, 40 g/L l-serine in RMG5 and 4, 8, 12, 16 g/L in MMG2 were investigated in this study. As shown in Fig. 2A, wild-type Z. mobilis ZM4 exhibited tolerance to 10 g/L l-serine with growth unaffected when compared with the control without l-serine supplementation in RMG5. The OD600nm of ZM4 with the addition of 30 g/L l-serine decreased to 2.42, which was less than half of the biomass in the presence of 0 or 10 g/L l-serine. Severe impact on cell growth was observed in ZM4 with the biomass of OD600nm no more than 1.00 when 40 g/L l-serine was supplemented in RMG5. Similarly, the growth rate was also significantly declined from 0.39 in control to 0.07 with 40 g/L l-serine supplementation (Table S4). Under conditions in minimal medium of MMG2, ZM4 demonstrated tolerance to 16 g/L l-serine with an OD600nm value of 0.54 (Fig. 2B) and a minimal growth rate of 0.07 (Table S4). Overall, the ability of Z. mobilis ZM4 to maintain growth in the presence of high l-serine concentrations suggests that Z. mobilis possesses relatively robust serine tolerance capability for efficient serine production.
Fig. 2.
Cell growth of strain ZM4 with the supplementation of 10, 20, 30, and 40 g/L l-serine in RMG5 (A), 4, 8, 12, and 16 g/L l-serine in MMG2 (B), and the fermentation performance of OD600nm (C), ethanol and serine production (D) of strain ZM4, S01, and S02 in RMG5. Three replicates were performed, and T-test was conducted for data analysis. ns represents no significant difference (p-value >0.05), * represents a significant difference with p-value <0.05. ** represents a significant difference with p-value <0.01. *** represents a significant difference with p-value <0.001. **** represents a significant difference with p-value <0.0001.
3.2. Blockage of l-serine degradation pathway
l-serine is indeed a crucial metabolite in cellular metabolism, playing multiple roles in biosynthetic pathways. Its role in central carbon metabolism is significant because of its ability to interconvert with glycine and pyruvate, two key metabolic intermediates. This interconversion supplies hydroxymethyl groups for cellular anabolism or to serve as a precursor for other amino acids, phospholipids, purines, and protein synthesis [2]. The metabolic engineering strategy of blocking l-serine degradation with l-serine deaminase deletions to push carbon flux towards l-serine accumulation in E. coli and C. glutamicum was proven to be effective (Peters-Wendisch et al., 2005; [7,11,32]).
Two genes of ZMO1201 (glyA) and ZMO0189 (sdaA) responsible for l-serine degradation were identified in Z. mobilis. To accumulate more l-serine, the degradation pathways for glycine or pyruvate in Z. mobilis were knocked out by deleting sdaA and glyA, resulting in the recombinant strains of S01 (ΔsdaA) and S02 (ΔsdaAΔglyA). Then, the growth and production of the recombinant strains were analyzed. Although a 3–4 h lag phase existed in strain S02, no significant difference was found among three strains after 24 h fermentation in RMG5 (Fig. 2C). The l-serine titers in S01 (ΔsdaA) and S02 (ΔsdaAΔglyA) were 30.70 ± 3.20 mg/L and 50.40 ± 2.50 mg/L, which were 2.01 and 3.29 folds of that in the wild-type strain ZM4 (15.30 ± 0.60 mg/L), respectively (Fig. 2D).
Although l-serine titer was enhanced in strain S02, it is still relatively low. In a previous study, by deleting three l-serine deaminase genes sdaA, sdaB, and tdcG in E. coli, 530 mg/L l-serine was accumulated in 24 h fermentation; this was further increased to 890 mg/L after glyA deletion [11]. Subsequently, production of 36 g/L l-serine was achieved by controlling SHMT activity with a folate supply during a 60-h fed-batch fermentation process in C. glutamicum [34]. In the present experiments, the ethanol yield of 21.74 ± 0.37 g/L (Fig. 2D) was not affected by the marginal promotion of l-serine production in strain S02. Hence, to overcome the limitation of l-serine accumulation in Z. mobilis, more metabolic engineering strategies were applied in strain S02 subsequently.
3.3. Increase of l-serine tolerance and production through transporter engineering
Export of amino acids from the cell could not only help the removal of feedback inhibition by reducing intracellular concentrations, but it also supports reducing toxicity [35]. A dedicated serine exporter of ThrE with the function of l-serine/l-threonine co-transporter has been reported in C. glutamicum in 2001 [36]. So far, no well-characterized serine exporters have been described in E. coli. Considering the structural similarity between serine and cysteine, the l-cysteine efflux pump EamA have previously been identified in E. coli to have the favorable transport capabilities for l-serine [11]. The homologous gene NCgl0580 (designated as serE) of eamA was also identified in C. glutamicum, which exhibited specific transport function of l-serine [19]. A recent study also indicated that NCgl0254 and NCgl0255 are l-serine exporters in C. glutamicum; however, their transportation capabilities were lower than that of Ncgl0850 [37].
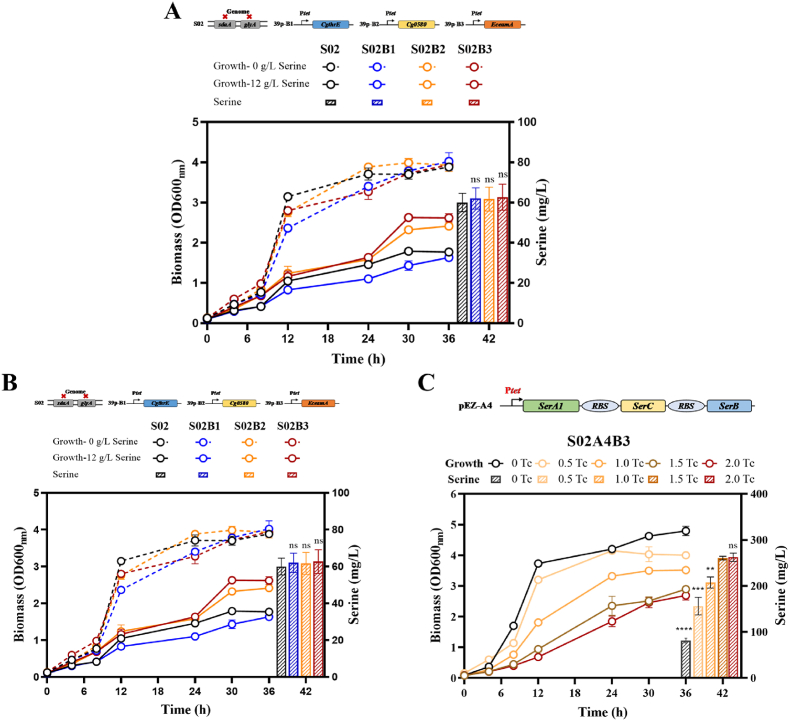
The homologous genes of eamA, NCgl0580, and thrE in Z. mobilis were then analyzed. Since there were no serine transport homologous genes identified in Z. mobilis, thrE and NCgl0580 genes from C. glutamicum (designated as CgthrE and Cg0580) as well as eamA from E. coli (designated as EceamA) were introduced into strain S02. These three genes were first cloned into the shuttle vector pEZ39p to generate the plasmids of 39p-B1, 39p-B2, and 39p-B3 under the control of tetracycline-inducible promoter Ptet, respectively (Table S1). Then, recombinant strains S02B1, S02B2, and S02B3 were constructed and further tested for l-serine production and tolerance. In RMG5, strain S02B1 and S02B3 grew slightly slower than the control strain S02 without l-serine supplementation (Fig. 3A). No significant difference of l-serine production was found in the control strain, S02B1, S02B2, and S02B3, which were 60.00 ± 4.58, 62.00 ± 5.29, 61.67 ± 6.03, and 62.67 ± 6.51 mg/L, respectively (Fig. 3A). This can be explained by the fact that l-serine is the precursor of many cellular metabolites, and therefore only a small percentage could be accumulated and secreted into the medium.
Fig. 3.
The screening of transporter of l-serine, as well as the strains tolerance with growth of OD600nm under 12 g/L serine in RMG5 (A). Fermentation performance of OD600nm and serine production in recombinant strain S02A1B3, S02A2B3, and S02A3B3 under strong promoters of Pgap, Peno, and P_ZMO1098 (B), and S02A4B3 under inducible promoter of Ptet (C). The concentrations of the tetracycline inducer were 0, 0.5, 1.0, 1.5, and 2.0 μg/mL. Three replicates were performed for the experiment, and T-test was conducted for data analysis. ns represents no significant difference (p-value >0.05).
However, overexpression of both eamA and thrE in the model microorganism of E. coli can increase the l-serine production [11,38]. Overexpression of NCgl0580 in C. glutamicum also increased l-serine production by 15.8%; this can be further enhanced by overexpressing both NCgl0580 and the l-serine biosynthetic pathway genes [19]. Therefore, our subsequent effort to increase l-serine production was conducted by overexpressing the key enzymes of its biosynthesis pathway.
The l-serine tolerance was analyzed first in recombinant strains with 1.5 μg/mL tetracycline induction. With 12 g/L l-serine in RMG5, strain S02B2 (OD600nm of 2.41) and S02B3 (OD600nm of 2.61) showed 36.93% and 48.30% increase in growth compared with the control strain S02 (OD600nm of 1.76), respectively (Fig. 3A). It suggested that EceamA exhibited superior l-serine transportation ability and its introduction into Z. mobilis could also promote strains tolerance towards l-serine. Consequently, strain S02B3 with the transporter of EceamA was utilized in subsequent experiments.
3.4. Enhancement of l-serine biosynthesis pathway
l-serine synthesis begins from 3-phosphoglycerate with three steps, the first step involves 3-phosphoglycerate dehydrogenase (PGDH), which is NAD-dependent and encoded by serA1, to produce 3-phosphohydroxypyruvate. Subsequently, 3-phosphoserine is generated by transamination catalyzed by phosphoserine aminotransferase (PSAT, encoded by serC). Lastly, the l-serine was generated through the catalysis of phosphoserine phosphatase (PSP, encoded by serB). The genes of serA1, serC, and serB in an operon for serine biosynthesis were overexpressed using constitutively strong promoters of Pgap, Peno, and P_ZMO1980, as well as the tetracycline-inducible promoter Ptet into the shuttle plasmid pEZ15Asp, respectively. Then, recombinant strains S02A1B3, S02A2B3, S02A3B3, and S02A4B3 were constructed and tested for l-serine production.
Cell growth and l-serine production of above three recombinant strains were compared, and the results demonstrated that the highest titer of 157.00 ± 1.63 mg/L was achieved in S02A1B3 using the strong promoter of Pgap with OD600nm of 4.00 (Fig. 3B). Different concentrations of 0, 0.5, 1, and 2 μg/mL tetracycline were then applied to S02A4B3. With the tetracycline concentration increased from 0 to 1.5 μg/mL, l-serine titer of S02A4B3 was also promoted from 81.33 ± 4.50 to 260.33 ± 4.04 mg/L (Fig. 3C). No significant increase was observed with the further elevation of tetracycline to 2 μg/mL. These results demonstrated that overexpression of l-serine biosynthetic pathway in combination with its export can facilitate l-serine production in Z. mobilis, which was similar with the results in C. glutamicum [19].
In contrast to our previous study for desired products accumulation, such as isobutanol, PHB, lactate, acetoin [[24], [25], [26], [27],39,40], the application of a constitutive strong promoter failed to increase l-serine production in the current study. It was speculated that the accumulation of l-serine in the medium, coupled with the additional burden on the cells overexpressing the exporters, may impose excessive metabolic stress–especially under a strong promoter. The previous study also demonstrated that overexpression of membrane proteins can lead to increased potential toxicity and stress responses [41]. Furthermore, the complicated cellular regulation of Z. mobilis with polyploidic genome may also hinder the precise modification for efficient production [42].
3.5. Selection and engineering of PGDH with high efficiency
Rate-limiting enzyme PGDH encoded by serA plays a pivotal role in l-serine biosynthesis. Its activity is also affected by the feedback inhibition of l-serine accumulation. Overexpression of serA to remove the feedback inhibition in C. glutamicum elevated the l-serine titer from 11.0 g/L to 21.6 g/L [32], and the overexpression of serAfr, serC, serB was also effective in E. coli for l-serine production [43,44]. In addition, various studies have been conducted to alleviate serine feedback inhibition of PGDH through enzyme modification including the mutations of His 344, Asn 346, and Asn 364 to alanine [45]. In addition, SerAΔ197 lacking the 197 C-terminal amino acid residues exhibited released serine feedback inhibition, and subsequently demonstrated high activity in C. glutamicum ([46]; Peters-Wendisch et al., 2005).
Correspondingly, modification sites of endogenous PGDH in Z. mobilis were determined with bioinformatics tools (Fig. 4A). ZmserA1mut1 was mutated with Asn 459, Asp 461, and Asn 479 to alanine in SerA, while ZmserA1mut2 was modified with the deletion of 197 amino acids at the C-terminus. Structural prediction from AlphaFold2 further verified the successful modification of ZmserA1mut1 and ZmserA1mut2 that were well-aligned with mutations in reference literatures (Fig. 4B).
Fig. 4.
Sequence analysis (A) and structure comparison (B) of SerA in Z. mobilis, with serine production in recombinant strains with different SerA (C) as well as glucose, ethanol, and cell growth of OD600nm in strain S02A9B3 (D) with RMG5. The modification sites of SerA in sequence analysis and AlphaFold-2 structure were indicated by red box. Seven strains were constructed and evaluated for serine production with a native serA1 from Z. mobilis (S02A4B3), BlserA from B. licheniformis (S02A5B3), BsserA from B. subtilis (S02A6B3), EcserAmut from E. coli (S02A7B3), CgserAmut from C. glutamicum (S02A8B3), and two modified ZmserAmut1(S02A9B3) and ZmserAmut2 (S02A10B3) from Z. mobilis. Strain S02A9B3 with the highest serine production was further analyzed for its fermentation performance. Three replicates were performed for the experiment. The concentrations of the tetracycline inducer were 0 and 1.5 μg/mL.
Six serA genes including BlserA from Bacillus licheniformis, BsserA from Bacillus subtilis, EcserAmut from E. coli [11], CgserAmut from C. glutamicum (Zhang et al., 2020a), as well as ZmserA1mut1 and ZmserA1mut2, along with serC and serB from Z. mobilis, were then constructed into strain S02B3 to generate the corresponding recombinant strains. The highest l-serine of 536.70 ± 13.50 mg/L was produced in S02A9B3, followed by 480.30 ± 19.90 mg/L in S02A7B3 under 1.5 μg/mL tetracycline induction in shake flasks (Fig. 4C). These results were 2.08 folds and 1.86 folds of the control strain S02A4B3 (258.00 ± 4.60 mg/L) using native serine biosynthesis genes. These results indicated that the application of ZmserA1mut1 and EcserAmut can release feedback inhibition of PGDH effectively in Z. mobilis. In contrast, deletion of 197 amino acids of PGDH in Z. mobilis was ineffective, which may be ascribed to crucial structural changes disrupting its function.
Glucose consumption and ethanol production was further investigated in S02A9B3. Cell growth was inhibited dramatically in S02A9B3 with OD600nm of 1.75 under 1.5 μg/mL tetracycline induction reducing about 62.64% than the control group of 4.69 without tetracycline induction (Fig. 4D). Similar results were also reported in model microorganisms of E. coli and C. glutamicum [11,19]. Although l-serine accumulation reduced the cell growth of S02A9B3, the ethanol production was nearly unaffected (Fig. 4D).
3.6. Increase of l-serine titer by modifying promoter strength and gene copy number
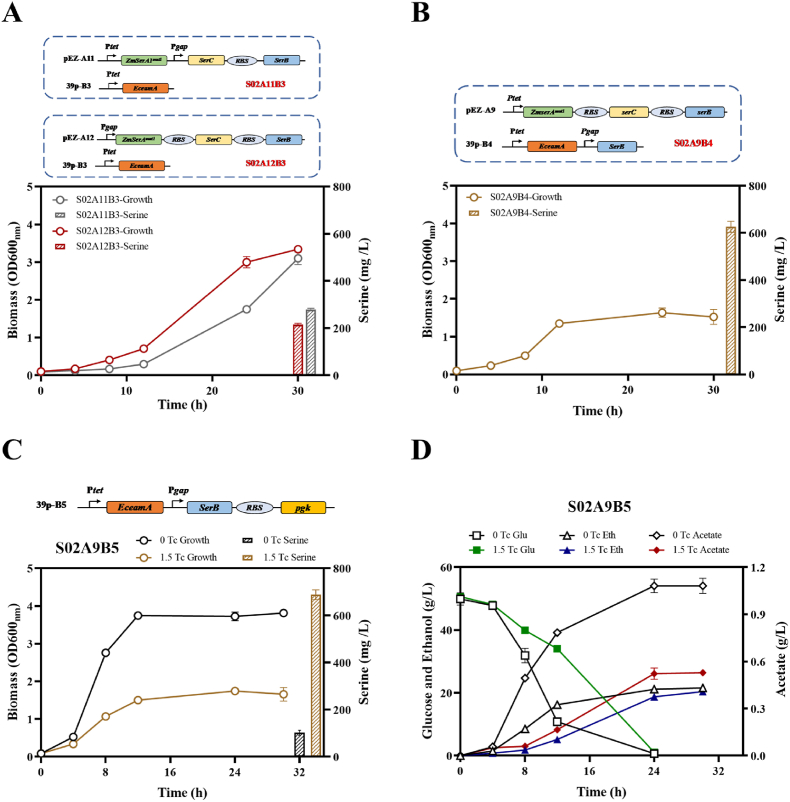
A previous study found that genes of serAfbr, serB, and serC in l-serine producer E. coli SSW-10/SP-09 were upregulated through transcriptomic study [38], and the accelerated expression of biosynthesis genes was beneficial for l-serine accumulation [2,7]. Therefore, the inducible promoter Ptet for ZmserAmut1, serB, and serC was replaced with constitutive strong promoter of Pgap in Z. mobilis. Recombinant strain S02A11B3 was constructed with ZmserA1mut1 driven by Ptet, and the operon of serB and serC driven by Pgap. While S02A12B3 was obtained with the operon of ZmserA1mut1, serB, and serC driven by the strong promoter Pgap (Fig. 5A). Interestingly, the production of l-serine significantly decreased to 214.66 ± 6.02 mg/L in S02A12B3 and 277.64 ± 7.97 mg/L in S02A11B3 (Fig. 5A). It was reduced to nearly one half of that of its parental strain S02A9B3 (536.70 ± 13.50 mg/L); this indicates that overexpression of serA, serB, and serC may be detrimental for serine production.
Fig. 5.
Fermentation performance of OD600nm and serine production in recombinant strain S02A11B3, S02A12B3 (A), S02A9B4 (B) and S02A9B5 (C), glucose consumption, ethanol and acetate production (D) in recombinant strain S02A9B5 with RMG5. Three replicates were performed for the experiment. The concentrations of the tetracycline inducer were 0 and 1.5 μg/mL.
The transcriptomic result of E. coli SSW-10/SP-09 showed that serB expression was significantly higher than those of serC and serA with log2RPKM values of 11.2, 5.33, and 4.0, respectively [38], which suggested that overexpression of serB alone was crucial. Subsequently, recombinant strain S02A9B4 was constructed with another plasmid 39p-B4 electroporated into S02A9B3, which possesses serB driven by Pgap and EceamA transporter driven by Ptet (Fig. 5B). The production of l-serine was increased to 625.66 ± 23.45 mg/L in S02A9B4, which was 16.58% more than that of S02A9B3 (Fig. 4D). No significant change of growth was observed in S02A9B4 with OD600nm of 1.53 when compared with S02A9B3 (OD600nm of 1.75). The results suggested that phosphoserine phosphatase encoded by serB, the last enzyme in l-serine biosynthesis, affected the overall efficiency of l-serine production, and overexpression of serB combined with exporter was crucial for serine production in Z. mobilis.
3.7. Direction of carbon flux to l-serine biosynthesis
In E. coli and C. glutamicum, diverting the metabolic flux to the precursor of l-serine (glycerate-3-phosphate) played a vital role in its accumulation [2,7]. Blocking or reducing the carbon flux toward undesirable by-products in competing reactions, or improving the intracellular precursor availability can be pursued for desired purpose. In previous studies, redirection of the carbon flux from l-alanine and l-valine to l-serine increased its titer from 21.27 ± 0.52 to 26.23 ± 0.70 g/L in C. glutamicum [18], and carbon flux of central metabolic pathways redirected towards the glycerate-3-phosphate resulted in a 42.8% increase [15]. Synthetic protein scaffolds utilized in E. coli also support the effective redirection of carbon flux to l-serine [47].
Since nearly 95% of the carbon source was directed towards ethanol production in Z. mobilis [48]. It could be an efficient approach to reduce the carbon flux from pyruvate to ethanol by inhibiting pyruvate decarboxylase (encoded by pdc) for l-serine biosynthesis. In addition, the precursor of glycerate-3-phosphate diverted into 2-phosphoglyceric acid with glycerate 3-phosphate mutase (encoded by gpmA) could also be a target for inhibition. The strategy of CRISPRi was then employed to downregulate the expression of pdc and gpmA. The recombinant strains of S03B4-pdc and S03B4-gpmA were obtained, respectively. Its ethanol production exhibited varying degrees of reduction, but no expected increase of l-serine was observed (Fig. S1). Despite our previous research demonstrated that suppressing pdc expression can increase acetoin synthesis and reduce ethanol metabolic flux [24], the results in the current study imply that there are still challenges to attenuate the metabolic flow of the ED pathway from ethanol biosynthesis to l-serine production in Z. mobilis.
Subsequently, the strategy of increasing the carbon flux towards the precursor glycerate-3-phosphate by overexpressing phosphoglycerate kinase (encoded by pgk) was conducted to generate recombinant strain S02A9B5 (Fig. 5C). A slightly improvement of cell growth (OD600nm of 1.78) and l-serine production (687.67 ± 20.65 mg/L) was achieved in S02A9B5 (Fig. 5C) compared to its parental strain S02A9B4. It thus demonstrated that pushing glycerate-2-phosphate to glycerate-3-phosphate in Z. mobilis benefits for l-serine accumulation. However, no significant difference (p < 0.05) of ethanol production (20.40 ± 0.35 g/L) was observed.
The enzymes from the Z. mobilis ED and glycolysis pathway constitute approximately 50% of the total cellular proteins, and each of them are highly expressed [49,50]. Therefore, it was speculated that there may exist other carbon flux conversion to supply for l-serine biosynthesis. Acetate, the main byproduct under aerobic fermentation in Z. mobilis, was analyzed subsequently. Interestingly, acetate production dropped from 1.08 g/L without tetracycline induction to 0.53 g/L under 1.5 μg/mL tetracycline induction (Fig. 5D). Although the primary carbon flux towards ethanol is challenging in Z. mobilis, there is a high likelihood of redirecting carbon from the byproduct acetate towards l-serine accumulation. Redirection of the carbon flux from ethanol production towards l-serine can be further carried out by knocking out or knocking down key genes of gpmA and pdc in the future.
The strategy of increasing the copy number of targeting genes is commonly proven to be beneficial in overcoming limitations imposed by enzyme catalytic efficiency and substrate availability, which ultimately leads to improved productivity of the products. Therefore, two editing plasmids of pL2R-PtACB and pL2R-PgACB, targeting the location of ZMO0038 gene in Z. mobils genome with increased l-serine biosynthesis genes ZmserAmut1, serC, serB were constructed and electroporated into the S02 strain. Subsequently, plasmids pEZ-A9 and 39p-B5 were further transformed to generate strains of S04A9B5 and S05A9B5, respectively. Compared to the production of 687.6 mg/L in S02A9B5 (Fig. 5C), no significant promotion of l-serine production was found in S04A9B5 and S05A9B5 (Fig. S2). Instead, the production of 598.3 mg/L l-serine in S05A9B5 even decreased by 12.9%, and the biomass of both S04A9B5 and S05A9B5 was also significantly affected with OD600nm no more than 3.00.
Presumably, the cofactor and energy imbalance of NAD+ and ATP may be responsible for the decreased growth and l-serine production in Z. mobilis. The synthesis of l-serine requires the conversion of glucose into 3-phosphoglycerate (3 PG), which is then further converted into serine through a series of enzymatic reactions with the consumption of NAD+. Moreover, in the enhanced l-serine biosynthesis pathway, 3 PG is consumed without phosphoenolpyruvate (PEP) formation in the ED pathway, thereby hindering ATP molecule production. Previous anaerobic l-alanine fermentation in Z. mobilis reached a production level of 7.5 g/L with equilibrium of ATP and NAD+, ensuring no ATP loss and the recycling of NAD+ [51]. Furthermore, a thiamine auxotrophy mutant of Z. mobilis was used to inhibit PDC activity. The absence of PDC cofactor thiamine PPi in the culture medium allows the carbon source to flow more towards alanine rather than ethanol.
To alleviate energy and NADH imbalance for l-serine production, the native gene of ndh (ZMO1113) and heterogenous gene of noxE from Saccharomyces cerevisiae were overexpressed in Z. mobilis. However, l-serine production was not improved either (date not show).
There are various strategies to adjust ATP supply, including regulating ATP synthase activity, adjusting metabolic pathways, increasing ADP supply, and modulating the electron transport chain [52,53]. Unlike the ED pathway utilized in Z. mobilis, most microorganisms tend to metabolize glucose through EMP pathway. The EMP pathway can generate 2 mol ATP per 1 mol glucose while only 1 mol ATP in Z. mobilis with the ED pathway. Previous study also indicated that the EMP pathway appears to evolve from the ED pathway, with slightly higher energy efficiency [54]. Thus, reconstructing the EMP pathway in Z. mobilis may potentially solve the energy balance issues associated with anaerobic production of l-serine in Z. mobilis. Moreover, recently study provided a strategy of introducing a polyphosphate kinase-mediated ATP regeneration system in Streptomyces albulus to increase intracellular ATP levels by 71.56% [55]. The ATP regeneration system has also been applied in many other microorganisms, such as C. glutamicum [56] and E. coli [57] to increase intracellular ATP levels. The application of polyphosphate kinase (PPK) performs remarkably well to re-phosphorylate ADP for ATP production with polyP as phosphate donor, such as additional phosphate salts. More work in fine-tunning the balance of the energy for l-serine production will be conducted in the future for efficient l-serine production in Z. mobilis.
3.8. Supplementation of nitrogen sources to enhance l-serine production
The deficiency of nitrogen sources limits amino acid production, and supplementation of nitrogen sources in a medium is widely used for l-serine production. This includes inorganic nitrogen sources such as NH3·H2O, NH4Cl and (NH4)2SO4, and organic nitrogen sources like corn steep liquor. The former is capable of regulating pH during l-serine production [12,18,34,58].
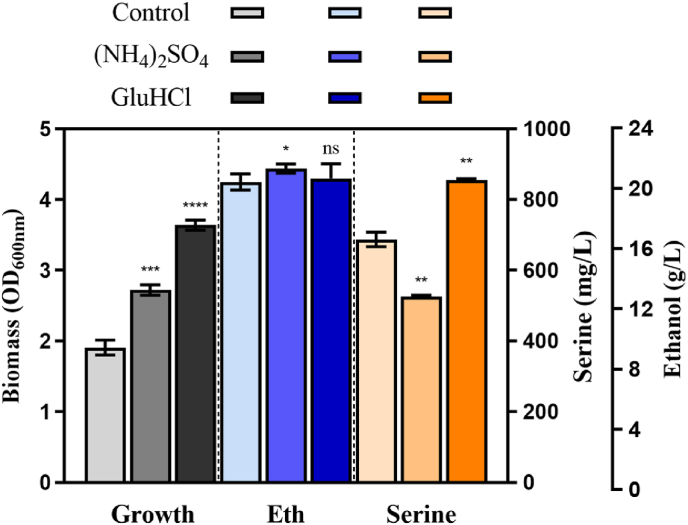
In this study, (NH4)2SO4 glutamate and hydrochloride were added for fermentation using the recombinant strain S02A9B5. l-serine production was observed to have a significant decrease from 687.67 ± 20.65 (control) to 525.78 ± 4.10 mg/L with the addition of (NH4)2SO4 in S02A9B5 (Fig. 6). However, the growth and ethanol production were both enhanced. The supplementation of NH4+ is beneficial for cell metabolism as it accelerates the conversion of intracellular l-serine to many other metabolites. In contrast, in the group of glutamate hydrochloride, l-serine increased to 855.66 ± 4.04 mg/L in strain S02A9B5 with no significant ethanol difference (Fig. 6). Glutamate is usually involved in the conversion of 3-phosphohydroxypyruvate to 3-phosphoserine by transamination catalyzed by phosphoserine aminotransferase (encoded by serC). Therefore, supplementation of glutamate promoted the accumulation of l-serine. Furthermore, cell growth of S02A9B5 was also improved with the final OD600nm increased from 1.91 to 3.64.
Fig. 6.
Cell growth of OD600nm as well as serine and ethanol production in recombinant strain S02A9B5 with the addition of 5 g/L (NH4)2SO4 or glutamate hydrochloride (GluHCl). Black refers to cell growth, blue represents ethanol production, and yellow denotes serine production. The color gradient represents the control, addition of (NH4)2SO4, and addition of GluHCl, respectively.
4. Conclusion
In this work, the l-serine tolerance of Z. mobilis was investigated by constructing various recombinant strains for l-serine production. Z. mobilis can generally tolerate up to 40 g/L of l-serine. Blockage of l-serine degradation pathways and the introduction of exporter EceamA were effective in improving l-serine production. Additionally, metabolic engineering strategies to enhance the biosynthesis pathway, remove feedback inhibition, and increase serB copies and l-serine precursors were combined to promote l-serine production in Z. mobilis. Lastly, glutamate supplementation was found to further elevate l-serine production of S02A9B5 to 855.66 mg/L; this indicated a 55.6-fold increase compared to the parental strain. This work thus not only lays a solid foundation for constructing an l-serine producer of Z. mobilis in the future, but also provides guidance on engineering non-model microbes to produce biochemicals with complicated pathways.
Funding and acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFA0911800), National Natural Science Foundation of China (CN) (21978071), the Key Science and Technology Innovation Project of Hubei Province (2021BAD001), and the Innovation Base for Introducing Talents of Discipline of Hubei Province (2019BJH021). We also acknowledge the support from the State Key Laboratory of Biocatalysis and Enzyme Engineering, and appreciate the assistance of Jessey Yang at University of Pennsylvania for manuscript editing.
CRediT authorship contribution statement
Zhen Wang: performed the experiments, Methodology, Writing – original draft. Xia Wang: Methodology, writing editing, Funding acquisition, Supervision. Xiongying Yan: Methodology, and, data analysis. Haixia Yi: performed the fermentations and HPLC. Shuche He: bioinformatics analysis and data analysis. Haoyu Zhang: performed the fermentations and HPLC. Xinli Zhou: and, data analysis. Qiaoning He: Funding acquisition, Formal analysis, Writing – review & editing. Shihui Yang: Conceptualization, Funding acquisition, Formal analysis, Writing – review & editing, and, manuscript submission.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
A patent application is associated with this study.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2024.03.008.
Contributor Information
Zhen Wang, Email: zhen.wang@stu.hubu.edu.cn.
Xia Wang, Email: xxwang@hubu.edu.cn.
Xiongying Yan, Email: xiongying.Yan@stu.hubu.edu.cn.
Haixia Yi, Email: haixiayi@stu.hubu.edu.cn.
Shuche He, Email: shuchehe@stu.hubu.edu.cn.
Haoyu Zhang, Email: Haoyu.Zhang@stu.hubu.edu.cn.
Xinli Zhou, Email: Zhouxl@stu.hubu.edu.cn.
Qiaoning He, Email: qiaoninghe@hubu.edu.cn.
Shihui Yang, Email: Shihui.Yang@hubu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Maugard M., Vigneron P.-A., Bolaños J.P., Bonvento G. L-serine links metabolism with neurotransmission. Prog. Neurobiol. 2021;197 doi: 10.1016/j.pneurobio.2020.101896. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X., Xu G., Shi J., Koffas M.A.G., Xu Z. Microbial production of L-serine from renewable feedstocks. Trends Biotechnol. 2018;36(7):700–712. doi: 10.1016/j.tibtech.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 3.De Koning T.J., Snell K., Duran M., Berger R., Poll-The B.-T., Surtees R. L-serine in disease and development. Biochem J. 2003;371(3):653–661. doi: 10.1042/BJ20021785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng Z., Pan X., Liu Y., You J., Zhang H., Investigatio Z.Z., Qiao Z., Rao Z. Engineering serine hydroxymethyltransferases for efficient synthesis of L-serine in Escherichia coli. Bioresour Technol. 2023 doi: 10.1016/j.biortech.2023.130153. [DOI] [PubMed] [Google Scholar]
- 5.Guo S., Tan X., Zhu J., Lü C., Yang C., Wang Q., Gao C., Xu P., Ma C. Enhanced L-serine production from glycerol by integration with thermodynamically favorable d-Dlycerate oxidation. ACS Sustainable Chem Eng. 2022;10(8):2587–2592. [Google Scholar]
- 6.Chen Z., Li Q., Zhou P., Li B., Zhao Z. Transcriptome sequencing reveals key metabolic pathways for the synthesis of L-serine from glycerol and glucose in Escherichia coli. Biochem Eng J. 2023;191 [Google Scholar]
- 7.Xu G., Zhang X., Xiao W., Shi J., Xu Z. Production of L-serine and its derivative L-cysteine from renewable feedstocks using Corynebacterium glutamicum: advances and perspectives. Crit Rev Biotechnol. 2023:1–14. doi: 10.1080/07388551.2023.2170863. [DOI] [PubMed] [Google Scholar]
- 8.Bang J., Lee S.Y. Assimilation of formic acid and CO2 by engineered Escherichia coli equipped with reconstructed one-carbon assimilation pathways. Proc Natl Acad Sci USA. 2018;115(40):E9271–E9279. doi: 10.1073/pnas.1810386115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian J., Deng W., Zhang Z., Xu J., Yang G., Zhao G., Yang S., Jiang W., Gu Y. Discovery and remodeling of Vibrio natriegens as a microbial platform for efficient formic acid biorefinery. Nat Commun. 2023;14(1):7758. doi: 10.1038/s41467-023-43631-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu H., Liao J.C. A modified serine cycle in Escherichia coli coverts methanol and CO2 to two-carbon compounds. Nat Commun. 2018;9(1):3992. doi: 10.1038/s41467-018-06496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundhada H., Schneider K., Christensen H.B., Nielsen A.T. Engineering of high yield production of L-serine in Escherichia coli. Biotechnol Bioeng. 2016;113(4):807–816. doi: 10.1002/bit.25844. [DOI] [PubMed] [Google Scholar]
- 12.Mundhada H., Seoane J.M., Schneider K., Koza A., Christensen H.B., Klein T., Phaneuf P.V., Herrgard M., Feist A.M., Nielsen A.T. Increased production of L-serine in Escherichia coli through adaptive laboratory evolution. Metab Eng. 2017;39:141–150. doi: 10.1016/j.ymben.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Rennig M., Mundhada H., Wordofa G.G., Gerngross D., Wulff T., Worberg A., Nielsen A.T., Nørholm M.H. Industrializing a bacterial strain for l-serine production through translation initiation optimization. ACS Synth Biol. 2019;8(10):2347–2358. doi: 10.1021/acssynbio.9b00169. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X., Xu G., Li H., Dou W., Xu Z. Effect of cofactor folate on the growth of Corynebacterium glutamicum SYPS-062 and L-serine accumulation. Appl Biochem Biotechnol. 2014;173:1607–1617. doi: 10.1007/s12010-014-0945-8. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X., Lai L., Xu G., Zhang X., Shi J., Koffas M.A., Xu Z. Rewiring the central metabolic pathway for high‐yield L‐serine production in Corynebacterium glutamicum by using glucose. Biotechnol J. 2019;14(6) doi: 10.1002/biot.201800497. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X., Lai L., Xu G., Zhang X., Shi J., Xu Z. Effects of pyruvate kinase on the growth of Corynebacterium glutamicum and L-serine accumulation. Process Biochem. 2017;55:32–40. [Google Scholar]
- 17.Zhang X., Zhang X., Xu G., Zhang X., Shi J., Xu Z. Integration of ARTP mutagenesis with biosensor-mediated high-throughput screening to improve L-serine yield in Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2018;102:5939–5951. doi: 10.1007/s00253-018-9025-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhu Q., Zhang X., Luo Y., Guo W., Xu G., Shi J., Xu Z. L-serine overproduction with minimization of by-product synthesis by engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2015;99:1665–1673. doi: 10.1007/s00253-014-6243-0. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X., Gao Y., Chen Z., Xu G., Zhang X., Li H., Shi J., Koffas M.A.G., Xu Z. High-yield production of L-serine through a novel identified exporter combined with synthetic pathway in Corynebacterium glutamicum. Microb Cell Factories. 2020;19(1):115. doi: 10.1186/s12934-020-01374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y., Geng B., Song H., Hu M., He Q., Chen S., Bai F., Yang S. Progress and perspective on development of non-model industrial bacteria as chassis cells for biochemical production in the synthetic biology era. Chin J Biotechnol. 2021;37(3):874–910. doi: 10.13345/j.cjb.200626. [DOI] [PubMed] [Google Scholar]
- 21.Shen W., Zhang J., Geng B., Qiu M., Hu M., Yang Q., Bao W., Xiao Y., Zheng Y., Peng W. Establishment and application of a CRISPR–Cas12a assisted genome-editing system in Zymomonas mobilis. Microb Cell Factories. 2019;18:1–11. doi: 10.1186/s12934-019-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y., Han J., Wang B., Hu X., Li R., Shen W., Ma X., Ma L., Yi L., Yang S. Characterization and repurposing of the endogenous type IF CRISPR–Cas system of Zymomonas mobilis for genome engineering. Nucleic Acids Res. 2019;47(21):11461–11475. doi: 10.1093/nar/gkz940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Y., Shen W., Huang J., Li R., Xiao Y., Wei H., Chou Y.-C., Zhang M., Himmel M.E., Chen S. Prediction and characterization of promoters and ribosomal binding sites of Zymomonas mobilis in system biology era. Biotechnol Biofuels. 2019;12(1):1–13. doi: 10.1186/s13068-019-1399-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bao W., Shen W., Peng Q., Du J., Yang S. Metabolic engineering of Zymomonas mobilis for acetoin production by carbon redistribution and cofactor balance. Fermentation. 2023;9(2):113. [Google Scholar]
- 25.Li Y., Wang Y., Wang R., Yan X., Wang J., Wang X., Chen S., Bai F., He Q., Yang S. Metabolic engineering of Zymomonas mobilis for continuous co-production of bioethanol and poly-3-hydroxybutyrate (PHB) Green Chem. 2022;24(6):2588–2601. [Google Scholar]
- 26.Qiu M., Shen W., Yan X., He Q., Cai D., Chen S., Wei H., Knoshaug E.P., Zhang M., Himmel M.E. Metabolic engineering of Zymomonas mobilis for anaerobic isobutanol production. Biotechnol Biofuels. 2020;13(1):1–14. doi: 10.1186/s13068-020-1654-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang S., Fei Q., Zhang Y., Contreras L.M., Utturkar S.M., Brown S.D., Himmel M.E., Zhang M. Zymomonas mobilis as a model system for production of biofuels and biochemicals. Microb Biotechnol. 2016;9(6):699–717. doi: 10.1111/1751-7915.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu M., Bao W., Peng Q., Hu W., Yang X., Xiang Y., Yan X., Li M., Xu P., He Q. Metabolic engineering of Zymomonas mobilis for co-production of D-lactic acid and ethanol using waste feedstocks of molasses and corncob residue hydrolysate. Front Bioeng Biotechnol. 2023;11 doi: 10.3389/fbioe.2023.1135484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang S., Vera J.M., Grass J., Savvakis G., Moskvin O.V., Yang Y., McIlwain S.J., Lyu Y., Zinonos I., Hebert A.S. Complete genome sequence and the expression pattern of plasmids of the model ethanologen Zymomonas mobilis ZM4 and its xylose-utilizing derivatives 8b and 2032. Biotechnol Biofuels. 2018;11:1–20. doi: 10.1186/s13068-018-1116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geng B., Liu S., Chen Y., Wu Y., Wang Y., Zhou X., Li H., Li M., Yang S. A plasmid-free Zymomonas mobilis mutant strain reducing reactive oxygen species for efficient bioethanol production using industrial effluent of xylose mother liquor. Front Bioeng Biotechnol. 2022;10 doi: 10.3389/fbioe.2022.1110513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y., Li J., Wang B., Han J., Hao Y., Wang S., Ma X., Yang S., Ma L., Yi L. Endogenous type I CRISPR-Cas: from foreign DNA defense to prokaryotic engineering. Front Bioeng Biotechnol. 2020;8:62. doi: 10.3389/fbioe.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu G., Zhu Q., Luo Y., Zhang X., Guo W., Dou W., Li H., Xu H., Zhang X., Xu Z. Enhanced production of l-serine by deleting sdaA combined with modifying and overexpressing serA in a mutant of Corynebacterium glutamicum SYPS-062 from sucrose. Biochem Eng J. 2015;103:60–67. [Google Scholar]
- 33.Zhang X., Newman E. Deficiency in L-serine deaminase results in abnormal growth and cell division of Escherichia coli K‐12. Mol Microbiol. 2008;69(4):870–881. doi: 10.1111/j.1365-2958.2008.06315.x. [DOI] [PubMed] [Google Scholar]
- 34.Stolz M., Peters-Wendisch P., Etterich H., Gerharz T., Faurie R., Sahm H., Fersterra H., Eggeling L. Reduced folate supply as a key to enhanced L-serine production by Corynebacterium glutamicum. Appl Environ Microbiol. 2007;73(3):750–755. doi: 10.1128/AEM.02208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv X., Xue H., Qin L., Li C. Transporter engineering in microbial cell factory boosts biomanufacturing capacity. BioDes Res. 2022;2022 doi: 10.34133/2022/9871087. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simic P., Sahm H., Eggeling L. L-threonine export: use of peptides to identify a new translocator from Corynebacterium glutamicum. J Bacteriol. 2001;183(18):5317–5324. doi: 10.1128/JB.183.18.5317-5324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y., Zhang X., Xu G., Zhang X., Li H., Shi J., Xu Z. Enhanced L-serine production by Corynebacterium glutamicum based on novel insights into L-serine exporters. Biotechnol J. 2023 doi: 10.1002/biot.202300136. [DOI] [PubMed] [Google Scholar]
- 38.Wang C., Li Q., Zhou P., Chen X., Shi J., Zhao Z. Bioprocess engineering, transcriptome, and intermediate metabolite analysis of L-serine high-yielding Escherichia coli W3110. Microorganisms. 2022;10(10):1927. doi: 10.3390/microorganisms10101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., He Q., Yang Y., Wang J., Haning K., Hu Y., Wu B., He M., Zhang Y., Bao J. Advances and prospects in metabolic engineering of Zymomonas mobilis. Metab Eng. 2018;50:57–73. doi: 10.1016/j.ymben.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Yan X., Wang X., Yang Y., Wang Z., Zhang H., Li Y., He Q., Li M., Yang S. Cysteine supplementation enhanced inhibitor tolerance of Zymomonas mobilis for economic lignocellulosic bioethanol production. Bioresour Technol. 2022;349 doi: 10.1016/j.biortech.2022.126878. [DOI] [PubMed] [Google Scholar]
- 41.Wagner S., Bader M.L., Drew D., de Gier J.-W. Rationalizing membrane protein overexpression. Trends Biotechnol. 2006;24(8):364–371. doi: 10.1016/j.tibtech.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 42.Fuchino K., Wasser D., Soppa J. Genome copy number quantification revealed that the ethanologenic alpha-Proteobacterium Zymomonas mobilis is polyploid. Front Microbiol. 2021;12 doi: 10.3389/fmicb.2021.705895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu P., Yang F., Su T., Li F., Li Y., Qi Q. Construction of an L-serine producing Escherichia coli via metabolic engineering. J Ind Microbiol Biotechnol. 2014;41(9):1443–1450. doi: 10.1007/s10295-014-1476-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhang X., Lai L., Xu G., Zhang X., Xu Z. Rewiring the central metabolic pathway for high-yield L-Serine production in Corynebacterium Glutamicum by using glucose. Biotechnol J. 2019;14(6) doi: 10.1002/biot.201800497. [DOI] [PubMed] [Google Scholar]
- 45.Grant G.A., Hu Z., Xu X.L. Identification of amino acid residues contributing to the mechanism of cooperativity in Escherichia coli D-3-phosphoglycerate dehydrogenase. Biochemistry. 2005;44(51):16844–16852. doi: 10.1021/bi051681j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peters-Wendisch P., Netzer R., Eggeling L., Sahm H. 3-Phosphoglycerate dehydrogenase from Corynebacterium glutamicum: the C-terminal domain is not essential for activity but is required for inhibition by L-serine. Appl Microbiol Biotechnol. 2002;60:437–441. doi: 10.1007/s00253-002-1161-y. [DOI] [PubMed] [Google Scholar]
- 47.Tran K.-N.T., Kumaravel A., Jeong J., Hong S.H. High yield fermentation of L-serine in recombinant Escherichia coli via co-localization of SerB and EamA through protein scaffold. Biotechnol Bioproc Eng. 2022;27(2):262–267. [Google Scholar]
- 48.Rogers P., Lee K., Skotnicki M., Tribe D. Microbial reactions. Springer; 1982. Ethanol production by Zymomonas mobilis; pp. 37–84. [Google Scholar]
- 49.Algar E.M., Scopes R.K. Studies on cell-free metabolism: ethanol production by extracts of Zymomonas mobilis. J Biotechnol. 1985;2(5):275–287. [Google Scholar]
- 50.An H., Scopes R., Rodriguez M., Keshav K., Ingram L. Gel electrophoretic analysis of Zymomonas mobilis glycolytic and fermentative enzymes: identification of alcohol dehydrogenase II as a stress protein. J Bacteriol. 1991;173(19):5975–5982. doi: 10.1128/jb.173.19.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uhlenbusch I., Sahm H., Sprenger G.A. Expression of an L-alanine dehydrogenase gene in Zymomonas mobilis and excretion of L-alanine. Appl Environ Microbiol. 1991;57(5):1360–1366. doi: 10.1128/aem.57.5.1360-1366.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hara K.Y., Kondo A. ATP regulation in bioproduction. Microb Cell Factories. 2015;14:1–9. doi: 10.1186/s12934-015-0390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Man Z., Guo J., Zhang Y., Cai Z. Regulation of intracellular ATP supply and its application in industrial biotechnology. Crit Rev Biotechnol. 2020;40(8):1151–1162. doi: 10.1080/07388551.2020.1813071. [DOI] [PubMed] [Google Scholar]
- 54.Ronimus R.S., Morgan H.W. Distribution and phylogenies of enzymes of the Embden-Meyerhof-Parnas pathway from archaea and hyperthermophilic bacteria support a gluconeogenic origin of metabolism. Archaea. 2003;1(3):199–221. doi: 10.1155/2003/162593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H., Zhu D., Kai L., Wang L., Zhang H., Zhang J., Chen X. Engineering Streptomyces albulus to enhance ε-poly-L-lysine production by introducing a polyphosphate kinase-mediated ATP regeneration system. Microb Cell Factories. 2023;22(1):1–14. doi: 10.1186/s12934-023-02057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lv Q., Hu M., Tian L., Liu F., Wang Q., Xu M., Rao Z. Enhancing l-glutamine production in Corynebacterium glutamicum by rational metabolic engineering combined with a two-stage pH control strategy. Bioresour Technol. 2021;341 doi: 10.1016/j.biortech.2021.125799. [DOI] [PubMed] [Google Scholar]
- 57.Krauser S., Hoffmann T., Heinzle E. Directed multistep biocatalysis for the synthesis of the polyketide oxytetracycline in permeabilized cells of escherichia coli. ACS Catal. 2015;5(3):1407–1413. [Google Scholar]
- 58.Chen Z., Chen X., Li Q., Zhou P., Zhao Z., Li B. Transcriptome analysis reveals potential mechanisms of L-serine production by Escherichia coli fermentation in different carbon–nitrogen ratio medium. Foods. 2022;11(14):2092. doi: 10.3390/foods11142092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.