Abstract
Metabolic engineering and synthetic biology endeavors benefit from promoters that perform consistently (or robustly) with respect to cellular growth phase (exponential and stationary) and fermentation scale (microtiter plates, tubes, flasks, and bioreactors). However, nearly all endogenous promoters (especially in Saccharomyces cerevisiae) do not perform in this manner. In this work, a hybrid promoter engineering strategy is leveraged to create novel synthetic promoters with robustness across these conditions. Using a multi-dimensional RNA-seq dataset, promoters with specific phase dependencies were identified. Fragments enriched with functional transcription factors were identified using MEME suite. These motif-containing fragments could impart activity dependence in the opposing condition. Specifically, we obtain two new promoters with high and consistent expression across both phases by increasing the exponential phase activity of the starting stationary-phase scaffold by 38 and 23-fold respectively. Further, we show that these promoters function consistently across various laboratory growth scales over time in a microtiter plate and in flasks. Overall, this work presents and validates a new strategy for engineering promoters in S. cerevisiae with high levels of expression that are robust to cellular growth phase and the scale of the culture.
1. Introduction
Common metabolic engineering and synthetic biology goals of replacing unsustainable industrial processes with greener alternatives [[1], [2], [3]], developing novel cellular circuitry [4], and enabling biosensing applications all rely upon robust cellular performance. Robustness of gene expression can be defined in many ways. Broadly, we refer to robustness as a stability in production in the face of various process perturbations. Well-documented challenges in achieving this robustness can be attributed to microbial metabolism shifts as a result of cellular growth phase [[5], [6], [7]], increasing volumes of fermentation during scale-up [8], varying carbon sources [9], and changes in fermentation temperature [10].
Achieving cell growth phase-invariant expression is one form of robustness that would benefit batch reactor processing, improve scale-up success when screening at the lab bench scale, and improve expression of heterologous pathway enzymes [11]. In contrast to 2-phase production systems in which growth and production are decoupled [[12], [13], [14]], there are certain cases for which a consistent expression level across both exponential and stationary growth phase is desired. While truly orthogonal, inducible promoter systems can enable consistent expression [[15], [16], [17], [18]], there are very few constitutive promoters that display this behavior. Among the options available, engineering promoter elements provides the most direct opportunity to establish consistent, growth-phase invariant promoters. Previous instances of hybrid promoter engineering have been successful in identifying and combining promoter element fragments for a variety of functions such as inducibility [[19], [20], [21], [22]], identification and substitution of core promoter regions [23,24], and tandem insertions of motifs to increase constitutive expression levels [21,25,26].
As a complement to these experimental design approaches, many computational approaches have been used to identify key regulatory regions of sequences such as promoters. For example, the MEME suite [27] can be used to identify sequence motifs enriched in a population of user-defined input sequences, and has been successfully leveraged in the past for motif discovery applications in promoter sequences specifically [28]. In other cases, this information has been leveraged for the construction of synthetic promoters with desired properties such as stress-induction [29], sexual state-dependent gene expression [30], and creation of non-native promoters with high levels of constitutive expression [31].
In this work, we focus on attaining consistent and high levels of promoter expression across both exponential and stationary growth-phase and across multiple scales of production (micro-titer plates, tubes, and flasks) by establishing using a bioinformatics-driven hybrid promoter engineering approach. Specifically, we leverage six different RNA-seq datasets from S. cerevisiae cultures grown in either tubes, flasks, or bioreactor, and each harvested at either exponential or stationary growth phase. Failing to identify any singular transcript (and corresponding promoter) capable of consistent and high-level expression under all six of these conditions, we hypothesized that promoters that are strongly expressed in just one growth phase can be synthetically engineered with transcription factor elements regulating high-level expression of other promoters in the alternate growth phase to establish a consistent hybrid promoter. Ultimately, we identify hybrid promoters for S. cerevisiae that not only achieve consistent promoter activity at different growth phases and production scale, but also result in higher production compared with only using individual and dual phase-dependent promoters.
2. Materials and methods
2.1. Media and strain construction
Escherichia coli strain NEB10β was used for all cloning and plasmid propagation. NEB10β was cultivated at 37 °C in lysogeny broth (LB) supplemented with 50 μg/mL of ampicillin. Saccharomyces cerevisiae strain BY4741 (Mat a; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0) was routinely cultivated at 30 °C in yeast extracted peptone dextrose (YPD) medium, or yeast synthetic complete medium (YSC). YPD is composed of 10 g/L yeast extract, 20 g/L peptone, and 20 g/L glucose. YSC dropout medium is composed of 6.7 g/L yeast nitrogen base, 20 g/L glucose or galactose, and CSM dropout supplement (MP Biomedicals, Solon, OH).
Plasmids were transformed to E. coli by electroporation in 2 mm cuvettes at 2.5 kV and 25 μF using a Gene Pulser Xcell (Bio-Rad). Plasmids were subsequently isolated from E. coli using the GeneJET Plasmid Miniprep kit (Thermo). All S. cerevisiae integrations were done by the EZ Yeast Transformation II Kit (Zymo) according to manufacturer's instruction. Correct construct integrations in biological triplicate in S. cerevisiae were confirmed through colony PCR and subsequent Sanger sequencing.
2.2. Plasmid construction and transformation
PCRs were carried out using Q5 Hot Start (New England Biolabs) according to the manufacturer's instructions. PCR purifications were done with the Qiagen PCR Purification Kit. Gel extractions were done using the Thermo Gel Extraction kit. Plasmids were constructed via Gibson assembly. All plasmids in this study were constructed based on the Mumberg et al. series of yeast shuttle vectors [32]. Promoters were defined as the 1000 base pairs upstream of the relevant gene ORF from RNA-seq. All correct plasmid assemblies were verified using Sanger sequencing.
2.3. RNA-seq analysis
Wild-type S228C was inoculated from glycerol stock into a 75 mL culture in a 500 mL flask with YSC as described above, and grown 24 h as a seed culture. Then, eight 4 mL tubes of culture and two 35 mL flasks were inoculated at OD600 = 0.01 using YSC. A 1.5 L bioreactor was inoculated at OD600 = 0.05 with sugar rich minimal media (80 g/L glucose, 6.7 g/L YNB). The tubes had a loose cap to allow air transfer, and the flasks had foam stoppers. The Bioreactor was pH controlled to pH 5.00 with 2.5 M sodium hydroxide, and oxygen was controlled to 50% saturation by a DO probe and agitation rate (250 rpm, never reached <50% saturation). RNA was extracted using an Ambion RiboPure Yeast RNA extraction kit. RNA was extracted in exponential phase at a target OD600/ml = 0.7. For the tubes and flasks, this was at 14–14.5 hrs, and for the bioreactor this was 15–15.5 hrs. (12 mL of culture was extracted for tubes and flasks, 15 mL for bioreactor). RNA was extracted in stationary phase at 60 h for all three (4 mL of culture for each). RNA samples were sent to the GSAF at UT Austin for library preparation and sequencing. All library preparation and high throughput RNA sequencing was performed by the University of Texas at Austin Genomic Sequencing and Analysis Facility (GSAF). An Illumina platform was used. RNA integrity was measured using an Agilent Bioanalyzer 2100. PolyA enrichment was used to remove non-coding RNA prior to sequencing.
All computational processing of RNA-seq data was performed on the Texas Advanced Computing Center's Lonestar6. Adapter sequences of FASTA files were trimmed with cutadapt [33]. Alignments were carried out using hisat2 [34] and the reference genome as S. cerevisiae (S288c) [35]. Transcript counts were transformed to transcripts per million (TPM) before use in subsequent analyses.
2.4. Flow cytometry characterization
Fluorescence of yECitrine was measured with LSRFortessa (BD Biosciences). All strains were measured at an excitation wavelength of 488 nm and a detection wavelength of 530 nm, and 20,000 events were collected from each sample. Flow cytometry data was analyzed using FlowJo software (Tree Star Inc.). Specifically, yeast populations were gated around the center of the heat map in the logarithmic plot of side scatter (SSC) and forward scatter (FSC) containing singlet cells. The average and standard deviation of the geometric mean fluorescence of three individual biological replicates were reported. Flow data from each individual graph was collected on the same day to mitigate possible variations in day-to-day fluorescence values.
2.5. Tube growth experiments
All yeast cultures were started from glycerol stock and were grown for 48 h to stationary phase in CSM -Uracil selective medium. All cultures were inoculated at OD600 = 0.01 in 2 mL fresh selective media in 15 mL polystyrene tubes with loose caps to allow for oxygen transfer. Cells were harvested for flow cytometry at 14 h for exponential phase measurements, and 60 h for stationary phase measurements.
2.6. Plate reader experiment
All yeast cultures were started from glycerol stock and grown for 48 h to stationary phase in CSM -Uracil selective media for single-plasmid strains, and in CSM -Uracil, Leucine selective media for dual-plasmid strains. All cultures were inoculated at OD600 = 0.01 in 200 μL fresh selective media in a Costar 96-well flat-bottom black polystyrene plate, and were grown in a Tecan infinite 200Pro plate reader. Every 15 min, orbital shaking (1.5 mm amplitude) was performed for 30 s, and absorbance at 600 nm, and fluorescence at 502 nm excitation and 523 nm emission was recorded. This was repeated for 60 h of total fermentation time.
2.7. Flask growth experiment
All yeast cultures were started from glycerol stock and grown for 48 h to stationary phase in CSM -Uracil selective media for single-plasmid strains, and in CSM -Uracil, Leucine selective media for dual-plasmid strains. All cultures were inoculated at OD600 = 0.01 in 35 mL media in 225 mL flasks at 30 °C. Cells were harvested for flow cytometry at 12, 18, 24, 30, 36, and 60 h into fermentation.
2.8. RNA extraction and cDNA synthesis
Fermentation samples in biological triplicate were spun down in a microcentrifuge at maximum speed, the supernatant decanted, and the pellet was flash frozen in liquid nitrogen and stored at −20 °C until RNA extraction. RNA extraction was performed with the Invitrogen™ RiboPure™ RNA Purification Kit, yeast (catalog # AM1926). After the extraction, RNA samples were kept at −80 °C until subsequent cDNA synthesis. For the 20 μL cDNA synthesis reaction, 1 μg of each RNA extraction was used with the NEB ProtoScript® II First Strand cDNA Synthesis Kit (catalog #E6560S) and the Oligo d(T)23 VN primer. No enzyme and no RNA controls were also run.
2.9. RT-qPCR and analysis
RT-qPCR was conducted in technical triplicate using 1 μL of the cDNA synthesis reaction in 10 μL total volume and the Applied Biosystems™ PowerUp™ SYBR™ Green Master Mix for qPCR (catalog # A25742). The reaction was run in a single 384 well plate (Applied Biosystems™ catalog #4309849) on the ViiA 7 Real-Time PCR System. For each biological replicate, ΔCT was calculated by subtracting the average CT of technical replicates of the YFP primer set relative to the housekeeping primer set. ΔΔCT's of all samples were normalized by subtracting the ΔCT of the sample from the average ΔCT of biological replicates of the ENO + ENO samples at the 20-h time point. Relative transcript abundance is then defined as 2ΔΔCT.
3. Results
3.1. Commonly utilized promoters exhibit differential growth-phase activity
As an initial baseline for this work, we sought to gauge the variability in performance of several commonly used promoters as a function of growth phase at tube scale. To this end, we chose 6 different promoters and cloned them directly upstream of a YFP reporter gene on a replicative plasmid. Strains were grown as 2 mL culture with flow cytometry sampling taken at 14 h (exponential phase) and 60 h (stationary phase). From this analysis, we observe significant variations in YFP signal between exponential and stationary phases for each of these promoters (Fig. S1a). While each of these show a higher degree of exponential phase signal, the extent of the difference between these expression levels is variable across promoters (Fig. S1b). The use of these promoters can lead to challenges in later phases of development and scale-up as production phenotypes in shorter, exponential phase-dominant conditions would not necessarily guarantee production in longer, stationary-phase dominant conditions. Overall, these results showcase the need for new promoters that are both high-performing and expression-phase robust.
3.2. RNA-seq as a discovery tool to identify candidate scaffold promoters for further engineering
To help identify and then establish promoters with desirable traits, we sought to use a multi-dimensional RNA-seq analysis to guide promoter selection. Specifically, S. cerevisiae cultures were grown at three different scales: tubes (2 mL culture), flasks (35 mL culture), and bioreactor (1.5 L culture with pH and oxygenation control). For each of these scales, samples were harvested during both exponential and stationary growth phases (corresponding to 14 and 60 h for tubes and flasks, and at 15 and 60 h for bioreactor) (Fig. S2). Using these six datasets, we examined the resultant transcript per million (TPM) counts emerging from each transcript and noted that no single promoter had consistent and also ‘high’ activity, which we defined as having a TPM count over 1000, across all six conditions.
Failing to identify a singular scaffold that immediately possessed all desired traits, we embarked on a synthetic promoter approach. To do so, we sought to identify promoters that had a large differential expression between exponential and stationary phase while still possessing modest to high net expression levels with the hypothesis that elements from these promoters could be hybridized to create a consistent promoter. The following selection criteria was established to choose candidate promoter scaffolds: for phase-dependent promoters, TPM count in the intended condition must be greater than 3000 and promoters were then ranked by decreasing log2fold change from the intended condition over the opposing condition (i.e. in selecting exponential phase-dependent promoters, these were ranked by decreasing log2fold(exponential TPM/stationary TPM)). The TPM filter threshold of 3000 was chosen to balance choosing candidate promoters with predicted high expression level (through a high TPM value) while still maintaining promoters with high log-fold change values (as we sought to identify phase-dependent traits).
Using this selection criteria, we chose three exponential phase-dependent (ENO2, TDH2, and RPS12) and three stationary phase-dependent (HSP26, DDR2, and SIP18) candidate promoters for initial screening. To test the performance of the promoters corresponding to these identified transcripts with desired traits, we defined the promoter sequence generously as the 1000 base pairs upstream of the start codon for the corresponding transcript. As with the well-characterized promoters above, these promoters were cloned immediately upstream of a YFP reporter gene and fluorescence was measured at 14 h and 60 h into tube-scale fermentation. Strong correlations were identified between fluorescence and TPM counts for these promoters thus validating our RNA-seq informed promoter selection process (Fig. 1). Ultimately, we chose the two promoters with the highest phase dependence in each respective phase, ENO2 and RPS12 for exponential phase, and HSP26 and SIP18 for stationary phase, for additional promoter engineering efforts moving forward.
Fig. 1.
Initial promoter screening performance. a Each selected promoter was placed upstream of a YFP reporter gene on a replicative plasmid, and YFP fluorescence was measured at 14 h for exponential phase and 60 h for stationary phase using flow cytometry. b Correlations between YFP signal and RNAseq TPM counts for each promoter show good agreement between the two values (R2 = 0.857). Error bars represent standard deviation of biological triplicate.
3.3. Use of MEME suite to identify regional clusters of enriched motifs
Hybrid promoter design requires identifying the key transcription factors and/or regions within promoters responsible for imparting desired traits (such as high phase-dependent activity in this case). For the four promoters identified above, we conjectured that particular transcription factor binding sites within these elements (denoted as TFBS*) are responsible for the high phase-dependent activity and that these elements would be clustered into regions of high densities. If this hypothesis is true, these elements could easily be portable for hybrid promoter construction. To evaluate this hypothesis, we implemented a computational pipeline utilizing the AME [36] and DREME [37] components of the MEME suite [27] to identify enriched sequence motifs for a given growth phase more generically and then mapped these to the candidate promoters described above (Fig. 2).
Fig. 2.
Schematic of MEME suite computational pipeline. First, our RNAseq dataset is used to identify two populations of promoters, exponential-dominant and stationary-dominant to use as inputs to MEME tools. AME uses a pre-defined list of TFBS* and identifies which of these motifs are enriched in a given population compared to the control population. DREME uses only the two promoter populations to discover novel enriched motifs. The motifs identified by both of these tools are then combined and used as an input to FIMO. FIMO annotates where these enriched motifs occur along the length of input promoters, ENO2 and RPS12 in the case of exponential, and HSP26 and SIP18 in the case of stationary.
To define promoter sequence populations to use as inputs to AME and DREME, we altered the filtering used in the RNA-seq dataset. To remove biases from very low expression transcripts, we ignored promoters whose genes had a TPM count less than 200 in the intended growth phase condition. All remaining promoters were evaluated based on their phase-specific behavior by rank ordering each promoter by log-fold difference in signal across growth phase. As a threshold, the top 150 promoters for each growth phase were used as inputs to AME and DEME to extract a set of phase-specific transcription factors (TFBS*). These motif discovery tools were selected based on their function. Specifically, AME utilizes a list of user-provided motifs and scans a population of input DNA sequences to determine which motifs, if any, occur more often in this population compared to a provided negative control population. For our list of user-provided motifs as inputs to AME, we used the previously reported complete list of known TFBS in S. cerevisiae, as listed in the JASPAR [38] database. The other approach, DREME, also compares two populations of DNA sequences to identify enriched motifs, but is a de novo pattern discovery tool that can uncover novel motifs. After running the MEME analysis, we found 6 known TFBS* from AME, and 3 novel motifs from DREME that were enriched in our exponential promoter population and 28 stationary TFBS* and 3 novel motifs that were enriched in our stationary promoter population (Table S1).
Next, we sought to test the hypothesis of TFBS* clustering within selected promoters. To do so, we utilized the FIMO tool [39] to annotates where the phase-enriched motifs occurred in the DNA sequence for the above selected exponential (ENO2, RPS12) and stationary (HSP26, SIP18) phase promoter candidates. As each motif is multiple base pairs in length, the center basepair location of the respective TFBS was used to plot these locations onto a heat-map across each promoter to visualize potential motif clustering (Fig. S3a). To quantify the degree of motif clustering, we compared the nearest neighbor distribution of each motif annotated on promoters to the nearest neighbor distance distribution of an equal number of points randomly placed across the length of the promoters (Fig. S4). Given the lower density of motifs in ENO2 and SIP18, this analysis was only conducted with RPS12 and HSP26. Overall, the mean of the nearest neighbor distance for each annotation on both RPS12 and HSP26 is significantly less than those of randomly generated positions thus indicating a clustering of transcription factor binding sites.
To further characterize the importance of this motif clustering, we performed a cross-analysis in which TFBS* from the opposite growth phase were mapped onto the promoter and evaluated for clustering (Fig. S3b). Specifically, we found that fewer motifs occurred on promoters when annotated with the opposing phase-dependence subset, and that these motifs less clustered.
3.4. Combinatorial construction of hybrid promoters
Encouraged by the emergence of enriched clusters within these phase-dependent promoters, we wanted to see if synthetically combining these clusters into a single promoter would result in consistent behavior across growth phases. Fragments were defined as the TFBS* cluster buffered by 30-bp on either side. These fragments were amplified and placed within the sequence of the “scaffold” promoter of the opposing growth condition either proximally or distally to that promoter's own enrichment cluster. Negative control fragments from each promoter were selected as the either proximal or distal sequence to the enrichment fragment in regions that contained no annotated TFBS. We note that in the case of SIP18, the stationary enrichment motif annotations spanned nearly 600 bp total. To reduce the length of these fragments, we decided to only amplify the three enrichment motif clusters that were closest to the transcription start site. Through this process, a total of 26 synthetic promoter constructs were constructed (Fig. 3) and cloned as per constructs above.
Fig. 3.
Enrichment and control fragment definitions and insertion sites. a For each candidate promoter, enrichment fragments were defined as the sequence span containing annotated TFBS*, plus a 30 bp buffer region on either side of this fragment. Control fragments started where enrichment fragments ended, and were made to be the same length as the enrichment fragment, unless the start of the TATA box or the end of the promoter region occurred first. b For each promoter used as the scaffold, fragments of differing promoters were inserted either distally or proximally to the scaffold promoter's motif cluster.
3.5. Stationary enrichment fragments inserted proximally in exponential promoters abolish function
First, we focus on the use of exponential-phase dominant ENO2 promoter as a scaffold for insertion of the various fragments from the stationary-phase dominant HSP26 promoter. In this case, we found that stationary enrichment fragments inserted at the proximal position (construct 1.1) reduced exponential phase promoter activity by a remarkable 99% compared to that of the ENO2 scaffold and the stationary phase signal likewise decreased by 47% compared (Fig. 4a). We also note that when the distal control fragment was inserted at the proximal position (construct 1.2), the resultant signals were reduced across both growth phases (58% and 51% reductions in exponential and stationary phase respectively), but there was no significant difference in the log2fold change in signal compared to that of the HSP26 scaffold (Fig. 4a). Finally, when any HSP26 fragments were inserted at distal positions (constructs 1.4–1.6), no significant changes in activity in either growth phase were seen. Together, these results suggest that this stationary enrichment fragment contains DNA elements responsible for stationary-phase specific expression, and points to the function of our computational pipeline in identifying the identity and position of these elements.
Fig. 4.
Exponential promoters as scaffolds for stationary fragment insertions. a ENO2 as a scaffold for HSP26 insertions. When the HSP26 stationary enrichment fragment was inserted at the proximal position of the ENO2 scaffold (construct 1.1), the exponential phase signal of the resultant promoter is significantly abolished, and reverses the phase-dependence of the resultant promoter compared to the ENO2 scaffold, as seen in the log-fold change metric. When the distal control fragment of HSP26 was inserted at the proximal position (construct 1.2), exponential and stationary phase activities decreased to similar degrees. When the proximal control fragment was inserted at the proximal position (construct 1.3), or when any of the three fragments were inserted at the distal position (constructs 1.4–1.6), no significant changes in signal were observed. b RPS12 as a scaffold for HSP26 insertions. When the HSP26 stationary enrichment fragment was inserted at the proximal position of the RPS12 scaffold (construct 2.1), the exponential phase signal of the resultant promoter is significantly abolished. When the enrichment fragment is inserted at the distal position (construct 2.2), no significant changes in signal were observed. We note the pattern of the log-fold change metric mirrors those in the case of ENO2 and HSP26. c ENO2 as a scaffold for SIP18 stationary fragment insertions. Effects mirror those of panels a and b. Error bars represent standard deviation of biological triplicate.
Next, we wanted to see if these overall effects on expression level could be repeated using our second exponential-dominant RPS12 promoter as a scaffold in which to insert HSP26 fragments. In general, the results of this study parallel those seen in the ENO2 + HSP26 case (Fig. 4b). When the stationary enrichment fragment is inserted at the proximal position (construct 2.1), we observe a 97% reduction in exponential phase signal, and only an 8% reduction in stationary phase signal. These changes in signal were unique to the insertion of the enrichment fragment at the proximal position. Additionally, we again observe no significant changes in signal when any fragments, control or enrichment, were inserted at the distal position of the RPS12 scaffold (Fig. S5).
Finally, we wanted to see if these overall effects on expression level could be repeated using the stationary-dominant SIP18 promoter as the source for TFBS* stationary enrichment fragments. Mirroring the effects of our HSP26 study, when the SIP18 enrichment fragment is inserted into the ENO2 construct in the proximal position (construct 3.1), we likewise see significant reductions in signal across both growth phases, and a significant shift from positive to negative in the log2fold change metric (Fig. 4c). We also note that similar to the HSP26 case, insertions of SIP18 fragments in the distal position have no effects on promoter signal (Fig. 4c and Fig. S6), and shifts in the log2fold metric are unique to the SIP18 enrichment fragment, and are not seen in control fragments.
Looking at this trend in both the case of the ENO2 and RPS12 scaffold, and in the HSP26 and SIP18 enrichment fragment, it would appear that these fragments contain critical sequence motifs that potentially recruit cellular machinery that blocks transcription during exponential growth phase specifically, and allows it only in stationary growth phase.
3.6. Exponential enrichment fragments inserted at the proximal position of stationary phase promoters can create a robust promoter
Next, we used our stationary-dominant HSP26 and SIP18 promoters as scaffolds into which to insert fragments of our two exponential-dominant promoters, ENO2 or RPS12. Focusing first on the ENO2 insertion fragments and the HSP26 scaffold, we show when the exponential enrichment fragment was placed the proximal position of the stationary scaffold (construct 4.1), the exponential phase signal of the resultant promoters increased by a remarkable 38-fold, while the stationary phase signal was increased by 2.8-fold (Fig. 5a). These changes were not observed in control fragments, or when fragments were inserted at the distal position (constructs 4.2–4.4).
Fig. 5.
Stationary promoters as scaffolds for exponential fragment insertions. a HSP26 as a scaffold for ENO2 insertions. When the exponential enrichment fragment of ENO2 was placed in the proximal position of the HSP26 scaffold (construct 3.1), the exponential phase signal of the resultant promoters increased by 38-fold. Insertion of the ENO2 enrichment fragment at the proximal position (construct 3.1) significantly reduces the absolute value of log-fold change in signal, thus resulting in a promoter with more consistent and therefore robust performance across growth phases. These effects were limited to the case of insertion of the enrichment fragment at the proximal position. b HSP26 as a scaffold for RPS12 insertions. When the exponential enrichment fragment of RPS12 was placed in the proximal position of the HSP26 scaffold (construct 4.1), the exponential phase signal of the resultant promoters increased by 23-fold. This effect was not realized when the enrichment fragment was inserted at the distal position (construct 4.2). We note the log-fold change in signal from construct 5.1 is significantly reduced, thus resulting in a promoter with more consistent and therefore robust performance across growth phases. Error bars represent standard deviation of biological triplicate. c SIP18 as a scaffold for ENO2 insertions. Effects mirror those of panels a and b. Error bars represent standard deviation of biological triplicate.
Focusing next on the RPS12 insertion fragments, we overall observed effects similar to the ENO2 case. We found that when the RPS12 exponential enrichment fragment was placed in the proximal position of the stationary scaffold (construct 5.1), the exponential phase signal of the resultant promoter increased by 23-fold, and the stationary signal was not significantly impacted (Fig. 5b). Again, these changes were not seen in the case of control fragments, or insertions at the distal position (Fig. S7).
Finally, we repeated the above experiment using SIP18 as the scaffold promoter and the ENO2 enrichment fragment. As in our HSP26 study, when the ENO2 enrichment fragment is inserted in the proximal position (construct 6.1), we see a 35-fold activation in exponential phase signal, and a 6.2-fold activation in stationary phase signal (Fig. 5c). When the ENO2 enrichment fragment is placed in this distal position (construct 6.2), we see no significant changes in signal in either growth phase. These results recapitulate the trends seen when inserting ENO2 enrichment fragments into the HSP26 scaffold, suggesting the properties of the ENO2 enrichment fragment are transferrable across multiple scaffold backgrounds.
Based on the totality of these results, two key observations emerge. First, when the exponential enrichment fragment is placed in front of the stationary enrichment motifs of the scaffold, it is possible to obtain higher promoter activity across both growth phases. Second, these changes occur such that the final activity is overall more consistent, or robust, across both phases.
3.7. Robust promoters can outperform the use of multiple phase-specific promoters
To further test the utility of these novel synthetic promoters, we wanted to see if the YFP production emerging from our newly engineered robust promoters (constructs 4.1 and 5.1) was higher and more consistent across both growth phases compared to strains simply harboring one unmodified exponential-dominant scaffold promoter and one unmodified stationary-dominant scaffold promoter. To accomplish this, we created new strains containing two different promoters driving production of YFP on two separate plasmids (Fig. S8). To create a fair comparison to strains containing one exponential-dominant promoter driving YFP, and one stationary-dominant promoter driving YFP, we created one strain containing two copies of our robust promoters, and two additional strains containing two copies of each phase-dependent promoter (Fig. S8).
Initially, we looked at the YFP signal emerging from these strains over time in a 200 μL microplate. Strains were grown for a total of 48 h and total fluorescence signal from each well was normalized by absorbance. Broadly, we see agreement between growth-phase specific production trends at the 200 μL plate reader scale, and those at the 2 mL tube scale previously measured by flow cytometry (Fig. 6). Specifically, we see a peak in the YFP signal emerging from our exponential-dominant ENO2 promoter at approximately 16 h, corresponding to mid-exponential growth phase (Fig. 6a). We see a peak in the YFP signal emerging from our stationary-dominant HSP26 promoter at approximately 32 h, corresponding to stationary growth phase. Interestingly, in the strains containing our robust promoters, we see a plateau behavior in YFP signal at spanning both growth phases from 16 to 32 h. We note these results are mirrored in the case of strains containing RPS12 native promoters, and RPS12-derived robust promoters (Fig. S9a).
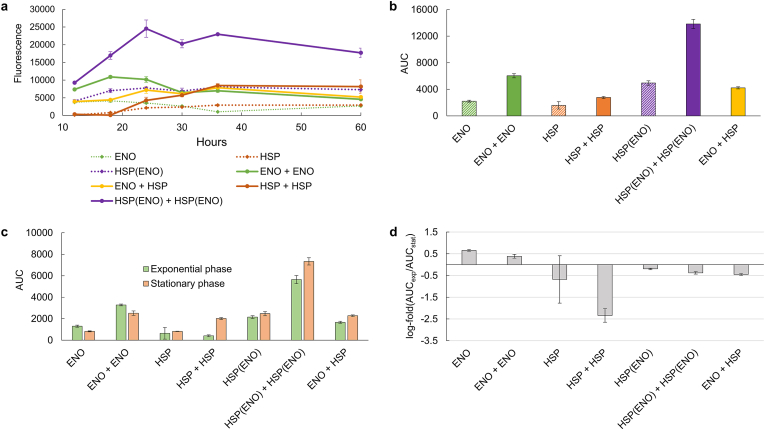
Fig. 6.
Performance of ENO2-based strains at plater-reader scale.a Fluorescence signal curves for ENO2-related strains. Strains containing only ENO2 promoters show a peak at approximately 16 h, corresponding to exponential phase. Strains containing only HSP26 show a peak at approximately 32 h, corresponding to stationary phase. Strains containing robust promoters (HSP(ENO)), exhibit a plateau in YFP signal at spanning both growth phases from 16 to 32 h b AUC's for YFP signal from plate reader time-course. A 3.2 fold higher AUC in our strain containing two robust promoters (HSP(ENO) + HSP(ENO)) is seen compared to our strain containing one of each phase-dependent promoter (ENO + HSP). c phase-specific contributions of AUC for each strain, where exponential phase was defined as hours 10–22, and stationary phase as hours 22–34. d Log-fold change in signal of exponential AUC over stationary AUC. We note the phase-specific signals mirror those seen in the case of 2 mL tubes. Error bars represent standard deviation of biological triplicate.
To better evaluate the overall production capacity and phase-consistency of these dual-plasmid strains, we conducted multiple area under curve (AUC) analyses of these YFP signals over time, as this is a good surrogate for a final product titer for each strain. First, we looked at the AUC's of our YFP signals across the entire fermentation (Fig. 6b). Here, we see a 3.2-fold higher AUC in our strain containing two robust promoters (HSP(ENO) + HSP(ENO)) compared to our strain containing one of each phase-dependent promoter (ENO + HSP).). Next, we examined phase-specific contributions of AUC for each strain. For this analysis, we defined exponential phase as hours 10–22, and stationary phase as hours 22–34. We see similar phase-contributions emerging from this analysis when compared to our single time-point flow cytometry studies in 2 mL tubes (Fig. 6c–d). We note that these results are again mirrored in the case of RPS12 promoters (Figs. S9c–d).
3.8. Evaluation of strain behavior at flask scale shows agreement with plate reader scale
Encouraged by the results of our plate-reader test, we next sought to evaluate if these trends could be repeated at larger scale. Once again, similar trends were seen in the flask scale as seen in the plate reader scale (Fig. 7 and Fig. S10), with the robust promoters showing a more consistent fluorescent level and the individual growth-phase promoters exhibiting peaks at their respective conditions. A similar AUC analysis was performed in these flasks, demonstrating that two copies of our robust promoters out-performs the ENO + HSP strain, with a 3.3-fold increase in AUC (Fig. 7b). The results of our phase-specific AUC analysis mirror those observed in micro-titer plates (Fig. 7c–d).
Fig. 7.
Performance of ENO2-based strains at flask level. a Strains containing only ENO2 show peaks during exponential phase. Strains containing only HSP26 show peaks during stationary phase. Strains containing robust promoters show a plateau spanning both phases. b AUC's for YFP signal from flask time-course. Our strain containing two robust promoters out-performs all other strains. c Phase-specific contributions of AUC for each strain, where exponential phase was defined as hours 10–22, and stationary phase as hours 22–34. d Log-fold change in signal of exponential AUC over stationary AUC. We note the phase-specific signals mirror those seen in the case of 2 mL tubes and plate-reader scale. Error bars represent standard deviation of biological triplicate.
To further confirm that changes seen in expression level in our strains were indeed occurring at the transcriptional level, we conducted qPCR measurements on these multi-promoter strains at the flask scale at 20, 30, and 40 h post-inoculation. These results (Fig. 8) show that relative YFP transcript abundance significantly increases during stationary phase in our HSP26-containing strain, decreases in stationary phase in our ENO2-containing strain, and remains most consistent in our robust promoter-containing strain. We note that these qPCR measurements were highly correlative with our same-day flow cytometry measurements (Fig. S11).
Fig. 8.
Relative transcript abundance from qPCR study. Relative YFP transcript abundance increases during stationary phase in the HSP26-containing strain, decreases in stationary phase in the ENO2-containing strain, and remains most consistent in the strain with the robust hybrid promoter. Error bars represent standard deviation of biological triplicate.
4. Discussion
Through this work, we sought to develop promoters that achieved growth-phase robustness. By exploring a multi-parameter RNA-seq data and incorporating this data into a novel computational pipeline, new potential scaffolds were identified possessing high phase-specific activity. We found that transcription factors responsible for imparting phase-specific behavior were indeed clustered in the sequence space of a promoter. Moreover, we found that motifs associated with stationary phase-specific activity tended to repress exponential activity of native exponential-dominant promoters if they were placed distal to the exponential phase-dependent motifs. We also found that exponential phase-dependent motifs could be placed proximal to stationary phase-dependent motifs within native promoters and result in strong activation of exponential phase signal of the resultant promoter. Through this approach, we increased the exponential phase activity of a stationary phase-dependent native HSP26 promoter scaffold by 23- and 38- fold when inserting fragments from RPS12 and ENO2 promoters, respectively. The resultant robust promoters also express much more consistently across both growth phases.
In several comparisons, we show that the robust promoters function also function reliably across various laboratory growth scales from microtiter plates to flasks. We compare the quantity and consistency of YFP production in strains containing one exponential-dominant promoter and one stationary-dominant promoter to strains containing two copies of synthetic phase-consistent promoters and find that our robust promoters produce between 2- and 3-fold higher cumulative YFP both in the plate reader and in flasks.
Overall, this work perhaps suggest that the exponential phase enrichment sequence fragments from our ENO2 and RPS12 promoter contain TFBS’ responsible for recruitment of transcriptional machinery during exponential phase. In the case of the stationary enrichment fragments of HSP26 and SIP18, these findings suggest that perhaps during stationary phase, these sequences recruit TF's that serve as roadblocks, disrupting transcription only when they are placed in the proximal insertion site with respect to the exponential enrichment fragments of the scaffold promoters. Further studies out of the scope of the current work would be necessary to further investigate these potential mechanisms.
We do recognize that in this work, we were unable to impart high stationary phase activity onto an existent exponential-dominant native promoter scaffold. Using this pipeline thus far in a more generic sense, a promoter with a high basal level of stationary phase activity was necessary for engineering purposes. However, as we only tested our engineering approach with two exponential-dominant and stationary-dominant promoters each, it is possible that alternative mechanisms for transcription factor activation and/or repression could be uncovered. Likewise, it is also possible that the repression established by stationary-phase specific TFBS is a more dominant factor in promoter activity. Further promoter construction would be necessary to make firm conclusions. Despite these limitations, we have elucidated a design strategy for increasing the production capacity and consistency of S. cerevisiae strains across growth phases for the first time, and expect this work to have important implications for future metabolic engineering endeavors.
5. Conclusions
In this work, we designed synthetic promoter sequences in S. cerevisiae capable of driving consistent, strong expression across cell growth phases and scale of production. This was accomplished by mining RNA-seq datasets for phase-specific motifs and creating hybrid promoter elements. The resulting phase-consistent promoters retained activity across various laboratory growth scales through use of microtiter plates and flasks. The utility of phase-robust promoters was demonstrated when compared with using only phase dependent, common promoters. These tools can be useful for both synthetic biology and metabolic engineering applications.
CRediT authorship contribution statement
Kristin V. Presnell: conceived the study, designed and performed experiments including promoter designs and testing, wrote the manuscript. Omar Melhem: designed and performed experiments including promoter designs and testing. Sarah M. Coleman: assisted with qPCR and analysis. Nicholas J. Morse: designed and performed experiments related to RNA-seq. Hal S. Alper: conceived the study, wrote the manuscript.
Declaration of competing interest
HA is an Associate Editor for Synthetic and Systems Biotechnology and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Acknowledgements
We acknowledge support from the Air Force Office of Scientific Research under Award No. FA9550-14-1-0089. Sequencing was conducted at the Genomic Sequencing and Analysis Facility (RRID no. SCR_021713) and flow cytometry as conducted at the Microscopy and Imaging Facility (RRID no. SCR_021756) at the UT Austin Center for Biomedical Research Support.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.synbio.2024.03.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Ko Y.S., Kim J.W., Lee J.A., Han T., Kim G.B., Park J.E., et al. Tools and strategies of systems metabolic engineering for the development of microbial cell factories for chemical production. Chem Soc Rev. 2020;49:4615–4636. doi: 10.1039/d0cs00155d. [DOI] [PubMed] [Google Scholar]
- 2.Yang D., Park S.Y., Park Y.S., Eun H., Lee S.Y. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020;38:745–765. doi: 10.1016/j.tibtech.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Intasian P., Prakinee K., Phintha A., Trisrivirat D., Weeranoppanant N., Wongnate T., et al. Enzymes, in vivo biocatalysis, and metabolic engineering for enabling a circular economy and sustainability. Chem Rev. 2021;121:10367–10451. doi: 10.1021/acs.chemrev.1c00121. [DOI] [PubMed] [Google Scholar]
- 4.Xia P.F., Ling H., Foo J.L., Chang M.W. Synthetic genetic circuits for programmable biological functionalities. Biotechnol Adv. 2019;37 doi: 10.1016/j.biotechadv.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 5.den Ridder M., van den Brandeler W., Altiner M., Daran-Lapujade P., Pabst M. Proteome dynamics during transition from exponential to stationary phase under aerobic and anaerobic conditions in yeast. Mol Cell Proteomics. 2023;22 doi: 10.1016/j.mcpro.2023.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahn W.S., Antoniewicz M.R. Metabolic flux analysis of CHO cells at growth and non-growth phases using isotopic tracers and mass spectrometry. Metab Eng. 2011;13:598–609. doi: 10.1016/j.ymben.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Alreshidi M., Dunstan H., MacDonald M., Saeed M., Elkahoui S., Roberts T. Significant changes in cytoplasmic amino acid composition occur in the transition between mid-exponential and stationary phases of growth of Staphylococcus aureus: an example of adaptive homeostasis in response to nutrient limitations. Microorganisms. 2023;11:147. doi: 10.3390/microorganisms11010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollinshead W., He L., Tang Y.J. Biofuel production: an odyssey from metabolic engineering to fermentation scale-up. Front Microbiol. 2014;5 doi: 10.3389/FMICB.2014.00344/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waschina S., D'Souza G., Kost C., Kaleta C. Metabolic network architecture and carbon source determine metabolite production costs. FEBS J. 2016;283:2149–2163. doi: 10.1111/febs.13727. [DOI] [PubMed] [Google Scholar]
- 10.Thorwall S., Schwartz C., Chartron J.W., Wheeldon I. Stress-tolerant non-conventional microbes enable next-generation chemical biosynthesis. Nat Chem Biol. 2020;16:113–121. doi: 10.1038/s41589-019-0452-x. [DOI] [PubMed] [Google Scholar]
- 11.Madzak C. Yarrowia lipolytica: recent achievements in heterologous protein expression and pathway engineering. Appl Microbiol Biotechnol. 2015;99:4559–4577. doi: 10.1007/s00253-015-6624-z. [DOI] [PubMed] [Google Scholar]
- 12.Su B., Song D., Yang F., Zhu H. Engineering a growth-phase-dependent biosynthetic pathway for carotenoid production in Saccharomyces cerevisiae. J Ind Microbiol Biotechnol. 2020;47:383–393. doi: 10.1007/s10295-020-02271-x. [DOI] [PubMed] [Google Scholar]
- 13.Li S., Jendresen C.B., Grünberger A., Ronda C., Jensen S.I., Noack S., et al. Enhanced protein and biochemical production using CRISPRi-based growth switches. Metab Eng. 2016;38:274–284. doi: 10.1016/j.ymben.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Lo T.M., Chng S.H., Teo W.S., Cho H.S., Chang M.W. A two-layer gene circuit for decoupling cell growth from metabolite production. Cell Syst. 2016;3:133–143. doi: 10.1016/j.cels.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Soma Y., Hanai T. Self-induced metabolic state switching by a tunable cell density sensor for microbial isopropanol production. Metab Eng. 2015;30:7–15. doi: 10.1016/j.ymben.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A., Reizman I.M.B., Reisch C.R., Prather K.L.J. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit. Nat Biotechnol. 2017;35:273–279. doi: 10.1038/nbt.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang T., Li C., Teng Y., Zhang R., Yan Y. Recent advances in improving metabolic robustness of microbial cell factories. Curr Opin Biotechnol. 2020;66:69–77. doi: 10.1016/J.COPBIO.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gong Z., Nielsen J., Zhou Y.J. Engineering robustness of microbial cell factories. Biotechnol J. 2017;12 doi: 10.1002/BIOT.201700014. [DOI] [PubMed] [Google Scholar]
- 19.Jung Y.K., Kim T.Y., Park S.J., Lee S.Y. Metabolic engineering of Escherichia coli for the production of polylactic acid and its copolymers. Biotechnol Bioeng. 2010;105:161–171. doi: 10.1002/bit.22548. [DOI] [PubMed] [Google Scholar]
- 20.Blazeck J., Liu L., Redden H., Alper H. Tuning gene expression in Yarrowia lipolytica by a hybrid promoter approach. Appl Environ Microbiol. 2011;77:7905–7914. doi: 10.1128/AEM.05763-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blazeck J., Garg R., Reed B., Alper H.S. Controlling promoter strength and regulation in Saccharomyces cerevisiae using synthetic hybrid promoters. Biotechnol Bioeng. 2012;109:2884–2895. doi: 10.1002/bit.24552. [DOI] [PubMed] [Google Scholar]
- 22.Jiao S., Li X., Yu H., Yang H., Li X., Shen Z. In situ enhancement of surfactin biosynthesis in Bacillus subtilis using novel artificial inducible promoters. Biotechnol Bioeng. 2017;114:832–842. doi: 10.1002/bit.26197. [DOI] [PubMed] [Google Scholar]
- 23.Madzak C., Tréton B., Blanchin-Roland S. Strong hybrid promoters and integrative expression/secretion vectors for quasi-constitutive expression of heterologous proteins in the yeast Yarrowia lipolytica. J Mol Microbiol Biotechnol. 2000;2:207–216. [PubMed] [Google Scholar]
- 24.Guan C., Cui W., Cheng J., Zhou L., Guo J., Hu X., et al. Construction and development of an auto-regulatory gene expression system in Bacillus subtilis. Microb Cell Factories. 2015;14:1–15. doi: 10.1186/s12934-015-0341-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blazeck J., Miller J., Pan A., Gengler J., Holden C., Jamoussi M., et al. Metabolic engineering of Saccharomyces cerevisiae for itaconic acid production. Appl Microbiol Biotechnol. 2014;98:8155–8164. doi: 10.1007/s00253-014-5895-0. [DOI] [PubMed] [Google Scholar]
- 26.Shabbir Hussain M., Wheeldon I., Blenner M.A. A strong hybrid fatty acid inducible transcriptional sensor built from Yarrowia lipolytica upstream activating and regulatory sequences. Biotechnol J. 2017;12 doi: 10.1002/biot.201700248. [DOI] [PubMed] [Google Scholar]
- 27.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME suite. Nucleic Acids Res. 2015;43:W39–W49. doi: 10.1093/NAR/GKV416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Mazarei M., Peng Y., Fethe M.H., Rudis M.R., Lin J., et al. Computational discovery of soybean promoter cis-regulatory elements for the construction of soybean cyst nematode-inducible synthetic promoters. Plant Biotechnol J. 2014;12:1015–1026. doi: 10.1111/pbi.12206. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y., Lee J.H., Poindexter M.R., Shao Y., Liu W., Lenaghan S.C., et al. Rational design and testing of abiotic stress-inducible synthetic promoters from poplar cis-regulatory elements. Plant Biotechnol J. 2021;19:1354–1369. doi: 10.1111/pbi.13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raborn R.T., Spitze K., Brendel V.P., Lynch M. Promoter architecture and sex-specific gene expression in Daphnia pulex. Genetics. 2016;204:593–612. doi: 10.1534/genetics.116.193334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McQuillan J.L., Berndt A.J., Sproles A.E., Mayfield S.P., Pandhal J. Novel cis-regulatory elements as synthetic promoters to drive recombinant protein expression from the Chlamydomonas reinhardtii nuclear genome. N Biotechnol. 2022;68:9–18. doi: 10.1016/j.nbt.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 32.Mumberg D., Müller R., Funk M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 1995;156:119–122. doi: 10.1016/0378-1119(95)00037-7. [DOI] [PubMed] [Google Scholar]
- 33.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetJournal. 2011;17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 34.Kim D., Paggi J.M., Park C., Bennett C., Salzberg S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol. 2019;37:907–915. doi: 10.1038/s41587-019-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engel S.R., Wong E.D., Nash R.S., Aleksander S., Alexander M., Douglass E., et al. New data and collaborations at the Saccharomyces Genome Database: updated reference genome, alleles, and the Alliance of Genome Resources. Genetics. 2022;220 doi: 10.1093/genetics/iyab224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McLeay R.C., Bailey T.L. Motif enrichment analysis: a unified framework and an evaluation on ChIP data. BMC Bioinf. 2010;11:1–11. doi: 10.1186/1471-2105-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey T.L. DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics. 2011;27:1653–1659. doi: 10.1093/bioinformatics/btr261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro-Mondragon J.A., Riudavets-Puig R., Rauluseviciute I., Berhanu Lemma R., Turchi L., Blanc-Mathieu R., et al. JASPAR 2022: the 9th release of the open-access database of transcription factor binding profiles. Nucleic Acids Res. 2022;50:D165–D173. doi: 10.1093/nar/gkab1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grant C.E., Bailey T.L., Noble W.S. FIMO: scanning for occurrences of a given motif. Bioinformatics. 2011;27:1017–1018. doi: 10.1093/bioinformatics/btr064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.