Abstract
Pregnancy complicated with pulmonary arterial hypertension (PAH) is a severe and dangerous condition for both the mother and the fetus. Pregnancy-specific alterations in the maternal cardiovascular system suggest that PAH in pregnancy may manifest more severe symptoms compared with those in non-pregnant patients. Although most societal guidelines recommend early termination in the case of PAH, some recent data suggests that maternal mortality among patients with PAH is lower than previously observed and suggests if a woman decides to proceed with the pregnancy, she should be counseled about the potential risks of continuing with the pregnancy.
This review paper starts with a real clinical case of PAH complicating with pregnancy, then summarizes the clinical features, diagnosis, and risk stratification. Effective treatments were also clarified, including pre-conception counseling and monitoring, general and supportive care, medication and immune therapy, delivery and postpartum care, counseling on contraception and breastfeeding, maternal and fetal outcomes, and cardiac surgery. The article summarizes points of uncertainty in both laboratory and clinical practices, as well as current guidelines and clinical recommendations.
Keywords: Pulmonary arterial hypertension (PAH), Pregnancy, Risk assessment, Management
A 30-year-old primigravida, at 24 weeks and 6 days of gestation, was admitted in stable condition to the Department of Obstetrics following the diagnosis of oligohydramnios and pulmonary arterial hypertension (PAH) for 6 days. She received regular antenatal examinations, and important examinations such as thyroid function, TORCH, NIPT, and nuchal translucency (1.5 mm) all showed normal results. During admission, she had yet to finish OGTT, fetal system ultrasonography, or fetal cardiac ultrasonography. Two weeks before the admission, she felt bilateral leg edema, which could be relieved after rest. 6 days before the admission, a regular antenatal examination found oligohydramnios (AFI = 4.3 cm), which could not be alleviated by transfusion treatment. On the day of admission, the fetal ultrasonography found oligohydramnios (AFI = 2.0 cm, AFV = 1.2 cm), thickened placenta (thickness = 4.7 cm), and bilateral smaller-than-average fetal kidneys (left:1.9 × 1.4 cm, right:2.0 × 1.1 cm). A physical examination revealed that she had a grade 2/6 systolic murmur on the left sternal border. There was pitting edema in the lower extremities, and no clubbing or other positive signs were found. The echocardiography detected moderate pulmonary arterial hypertension (mPAP = 62 mmHg) and mild-medium tricuspid regurgitation. How should this patient be further evaluated and treated?
1. The clinical problem
Pulmonary hypertension (PH) is a heterogeneous disease that could be primary or secondary to other conditions, defined as a resting mean pulmonary artery pressure (mPAP) of 20 mm Hg or above [1]. The elevations in pulmonary pressures lead to a constellation of abnormal cellular, prothrombotic, and vascular constriction effects, which further result in right ventricular (RV) stress and eventually RV failure and death [2,3].
Primary pulmonary arterial hypertension (PAH) is the most prevalent type of PH among women of reproductive age, and it is 4 times more common among women compared with men [4]. According to modern studies, the estimated maternal mortality for pregnant PAH patients is around 9–17% [[5], [6], [7], [8], [9]]. Due to the high risks, most societal guidelines recommend against pregnancy in patients with PAH and even recommend early termination [10].But for patients with mild disease, there are still promising signs of improving outcomes.
Most patients are referred for evaluation of pulmonary hypertension after the detection of increased right ventricular systolic pressure (RVSP) by cardiac ultrasonography. For pregnant women with suspicion of PAH, it is recommended to undergo a complete diagnostic evaluation and risk stratification (Fig. 1). According to the updated World Symposium on Pulmonary Hypertension (WSPH), causes include idiopathic, heritable, drug and toxin-induced, other disease-associated, and calcium channel blockers responsive, etc [1]. Multidisciplinary management from pre-conception monitoring to medication treatment, delivery care, postpartum care, and complication management should be undertaken in an experienced center. It is important to deliver counseling on contraception and breastfeeding to patients with PAH.
Fig. 1.
Diagnostic Approach in a Patient with Possible Pulmonary Arterial Hypertension in Pregnancy.
Abbreviations: PFT- Pulmonary function test, V/Q scan – Ventilation-Perfusion scan, ANA – Antinuclear antibodies, CFT – Complement fixation test, HIV - Human immunodeficiency virus, mPAP – mean pulmonary arterial pressure, PVR – pulmonary vascular resistance, PAWP – Pulmonary artery wedge pressure, PAH – Pulmonary artery hypertension, PVOD/PCH – Pulmonary venous-occlusive disease/Pulmonary capillary hemangiomatosis, PH- Pulmonary hypertension, ESC - European Society of Cardiology, ERS – European Respiratory Society.
1.1. Key clinical points
1.1.1. Pregnancy with pulmonary arterial hypertension
-
1.
Common presentations include fatigue, dyspnea, syncope, hepatomegaly, ascites, and ankle edema, all of which are signs of increased RV stress or RV failure, but also might occur with normal pregnancy.
-
2.
Mean pulmonary arterial pressure (mPAP) ⩾20 mm Hg at rest, pulmonary arterial wedge pressure (PAWP) ⩽15 mmHg, and pulmonary vascular resistance ⩾2 wood units measured by right heart catheterization are diagnostic.
-
3.
The cause (idiopathic, heritable, drug and toxin-induced, other disease-associated, and calcium channel blockers responsive) should be sought; in developing countries, the main causes include congenital heart disease associated, idiopathic, and connective tissue diseases associated.
-
4.
Considering the high mortality, pregnancy is strongly discouraged in patients with PAH and, if pregnancy occurs, early termination is recommended. Once the diagnosis is confirmed, the patient should promptly be referred to a PAH experienced center and receive multidisciplinary management.
-
5.
Counseling on contraception and breastfeeding should also be delivered to patients with PAH. As for options for contraceptives, progesterone-only methods can be employed, whilst estrogen should be avoided given the prothrombotic effects.
-
6.
For those who want to continue with pregnancy, a risk assessment and stratification is essential. Currently a series of PAH risk assessment tools are available, which help to determine the treatment as well as assess the prognosis [[11], [12], [13], [14]].
2. Strategies and evidence
2.1. Clinical presentation
The maternal cardiovascular system undergoes significant changes during pregnancy, beginning in the first trimester and the magnitude of change increases as gestation progresses. For instance, maternal cardiac output and blood volume can increase by 30–50% in the third trimester [15]. Also, hormonal changes during pregnancy can lead to accumulation of a significant amount of fluid in the interstitial space, which may further shift to the maternal circulation system during delivery, causing an increase in cardiac preload and hypertension. Prostacyclin production also increases in pregnancy, which may lead to an up to 30% decrease in pulmonary vascular resistance (PVR) [16]. These pregnancy-specific alterations all suggest that PAH in pregnancy may manifest more severe symptoms compared with those in non-pregnant patients. Non-specific symptoms such as fatigue and dyspnea, which can also be seen in normal pregnancy, are common presentations among PAH patients, contributing to delayed diagnosis and treatment. Syncope might also manifest as a sign of low cardiac output. Signs of RV failure, including hepatomegaly, ascites, and lower limb edema, might also be obscured by pregnancy.
2.2. Diagnosis and risk stratification
The initial diagnostic evaluation of PAH includes a careful history and physical examination (Fig. 1). A thorough family history should be obtained, including ages of onset and specific disease type and severity among family members. It is reported up to 80% of familial cases of PAH have been linked to germ-line mutations in the gene coding for the bone morphogenetic protein receptor type II (BMPR2), a member of the transforming growth factor (TGF)-β signaling family [17]. BMPR2 mutation is also seen in idiopathic cases without a family history of PAH(18). Recent studies also identified other PAH associated mutations of genes, including ACVRL1, Caveolin 1, and KCNK3, etc. [18]. According to the latest version of PAH classification, PAH causes include idiopathic PAH, heritable PAH, drug- and toxin-induced PAH, PAH associated with connective tissue diseases (CTD), PAH associated with HIV infection, PAH associated with portal hypertension, PAH associated with congenital heart diseases (CHD), PAH associated with schistosomiasis, PAH long-term responders to calcium channel blockers, PAH with overt features of venous/capillaries (PVOD/PCH) involvement, and persistent PH of the newborn syndrome [1]. Based on the latest classification, it is clear that PAH risk factors, such as a family history of PAH, exposure to drugs/toxins known to induce PAH (e.g., anorexigens), CTD, CHD, HIV, and portal hypertension, should be carefully checked and excluded. Since the symptoms are not definitive, doppler echocardiography is strongly recommended for those suspicious of PAH. In normal subjects, mPAP at rest is 14±3 mm Hg (1 mm Hg = 0.133 kPa). An mPAP of ≥20 mm Hg measured by right cardiac catheterization, a pulmonary artery wedge pressure of ≤15 mm Hg, and a pulmonary vascular resistance of >2 wood units is diagnosed clinically as PAH [19]. Alternative imaging methods like chest computed tomography and pulmonary ventilation/pulmonary perfusion imaging (V/Q) should be carefully considered before implementation, given their invasive nature and the potential risk to the fetus. Right heart catheterization is considered the sole gold standard for PAH diagnosis, albeit being an invasive hemodynamic test. However, the procedure carries potential complications including fatal outcomes such as malignant ventricular arrhythmia and venous thrombosis (Fig. 1).
Risk stratification tools include the WHO functional class and the REVEAL risk score, both are helpful in guiding the prognosis of patients with PAH, not only at the time of diagnosis but also during follow-up [20]. However, none of the tools have been validated among pregnant women [21,22]. Most probably, pregnant women of functional class I and II, if well-controlled during pregnancy, could have favorable outcomes (Table 1). One recent study reported a maternal mortality rate of around 4%, and all deaths were in functional class IV. It is thus recommended that symptoms and functional status should be monitored monthly in the first and second trimesters, and then weekly during the third trimester [23]. If the woman is at higher risk by assessment, a closer supervision should be applied.
Table 1.
Risk assessment guidelines for pulmonary arterial hypertension.
| Determinants of prognosis (estimated I-year mortality) | High risk >10% | Intermediate risk 5–10% | Low risk <5% |
|---|---|---|---|
| Clinical signs of right heart failure | Present | Absent | Absent |
| Progression of symptoms | Rapid | Slow | No |
| Syncope | Repeated syncopec | Occasional syncope | No |
| WHO functional class | Ⅳ | Ⅲ | Ⅰ Ⅱ |
| 6MWD | <165 m | 165–440 m | >440 m |
| Cardiopulmonary exercise testing | Peak VO2 < 11 ml/min/kg (<35% pred.) VE/VCO2, slope ≥45 |
Peak VO2 1l-15 ml/min/kg (35–65% pred.) VE/VCO2, slope 36–44.9 |
Peak VO2 > 15 ml/min/kg (>65% pred.) VE/VCO2, slope <36 |
| NT-proBNP plasma levels | BNP >300 ng/l NT-proBNP >1400 ng/l |
BNP 50–300 ng/l NT-proBNP 300–1400 ng/l |
BNP <50 ng/l NT-proBNP <300 ng/l |
| Imaging (echocardiography, CMR imaging) | RA area >26 cm2 Pericardial effusion |
RA area 18–26 cm2 No or minimal, pericardial effusion |
RA area <18 cm2 No pericardial effusion |
| Haemodynamics | RAP >14 mmHg CI < 2.0 l/min/m2 SvO2 <60% |
RAP 8–14 mmHg CI 2.0–2.4 l/min/m2 SvO260-65% |
RAP <8 mmHg CI ≥ 2.5 l/min/m2 SvO2 >65% |
WHO: World Health Organization; 6MWD: 6-min walking distance; BNP: brain natriuretic peptide; NT-proBNP: N-terminal pro-brain natriuretic peptide; CMR:cardiac magnetic resonance; VO2: oxygen consumption VE/VCO2: ventilatory equivalents for carbon dioxide; RA: right atrial; RAP: Right Atrial Pressure; CI: Cardiac Index.
Echocardiography is a non-invasive tool during pregnancy to monitor the RV ejection fraction, which is a crucial marker of RV function among patients of PAH. The index of tricuspid annular plane systolic excursion (TAPSE) assesses the longitudinal component of RV contraction, and the RV fractional area change assesses both the longitudinal and transversal components and a lower TAPSE level indicates an impaired right ventricular function in most patients [24]. Besides, RV tissue Doppler imaging is a non-invasive tool that measures the peak systolic velocity and the RV myocardial performance index, facilitating early clinical evaluation of RV dysfunction in PAH with pregnancy [25]. In a most recently published study, the researchers found a higher proportion of preterm births and low birth weight babies in pregnant women with moderate to severe pulmonary hypertension detected by echocardiography. Thus, a stricter-than-normal obstetric follow-up and more careful assessment of continuing pregnancy should be given to these women, though no cut-off criteria on echocardiography for pregnant women with PAH is currently available in clinical practice.
Right Heart Catheter (RHC) is considered the diagnostic gold standard for PAH, the parameters of which help classify PAH into several subgroups, assess the risk of adverse events or mortality, and make therapeutic decisions [26]. However, since it is an invasive procedure with a risk of radiation to the fetus, it is not generally recommended during pregnancy. Recent studies have investigated the roles of lab markers such as BNP and NT-proBNP in PHA. In regular follow-ups, monitoring indexes for disease progression include evaluation of WHO functional class, 6-min walk test (6MWT), cardiopulmonary exercise testing, BNP/plasma NT-proBNP, and echocardiograms [27].
3. Management
A comprehensive treatment from an experienced multidisciplinary team should be part of the routine management of women with PAH of reproductive age. Effective treatments include pre-conception counseling and monitoring, general and supportive care, medication and immune therapy, delivery and postpartum care, counseling on contraception and breastfeeding, and cardiac surgery. Besides, psychological counseling should be initiated promptly, since sharp hormone fluctuation in pregnancy accompanied by with severe cardiac complication can precipitate symptoms of anxiety and depression.
3.1. Pre-conception counseling and monitoring
Those PAH patients who wish to become pregnant should have their condition as reasonably controlled as possible, and the monitoring plan should be established at a tertiary care center with a multidisciplinary team. It is also important to inform patient of the potential teratogenicity of some of the medications during preconception counseling. Some experts recommended echocardiography and cardiopulmonary exercise stress tests as a routine part of pre-pregnancy examination so as to establish a baseline anatomical and functional status for those PAH patients who plan to get pregnant [28].
For patients with confirmed or suspected hereditary PAH as well as PAH associated with CHD, a genetic counseling should be considered, since these conditions have been linked with genetic disorders [[17], [18], [29]]. Despite societal guidelines advocating for early termination, recent data indicates that maternal mortality in patients with pulmonary hypertension (PH) may be lower than initially observed. Pregnant women classified as functional class I and II, and well-controlled during pregnancy, likely have favorable outcomes. However, if a woman chooses to continue with the pregnancy, she should receive counseling regarding the potential risks of continuing with the pregnancy.
3.2. General and supportive care
For women opting to proceed with the pregnancy, they should receive regular outpatient counseling and monitoring with a cardio-obstetric team. Consensus pulmonary guidelines recommend monthly appointments during the first and second trimesters and weekly appointments during third trimester [23]. The overall treatment strategy is to optimize therapy, close monitoring, avoid teratogenic drugs, and incorporate a multidisciplinary team approach. Treatment is generally tailored to the individual patient, with an emphasis on preserving euvolemia and preventing systemic hypotension, hypoxemia, and acidosis [30]. Women are advised to lie on their side whenever feasible to minimize compression on the inferior vena cava(supine hypotensive syndrome).
3.3. Medication and immune therapy
The primary goal of initial medication is to eliminate pulmonary vasoconstriction and promote pulmonary vasodilation. Specific therapies target the four known mechanisms of vasoconstriction and vasodilation—calcium channels, nitric oxide/cyclic guanosine monophosphate (cGMP), endothelin, and the prostacyclin pathway [31]. Noticeably, two classes among the above four medications, the endothelin receptor antagonist (ERA) and Guanylyl Cyclase stimulator (sGC), both of which are used commonly to treat PAH, are teratogenic and contraindicated in pregnancy [31]. Other medications such as diuretics, digoxin, and coumadin should also be applied in case of signs of edema, right ventricular dysfunction, and histological findings of the thrombotic phenomenon in the lungs. Serum potassium levels should be carefully monitored and controlled throughout the medication. Modern studies observed the roles of altered cellular and humoral immunity in PAH development, and come up with recommendations for the development of new, safe and effective immune-targeted therapies for PAH [32,33].
3.4. Delivery and post-partum care
Upon the hospitalization of a patient with PAH for delivery, it is advised to promptly assemble an expert multidisciplinary team. This team should include specialists in pulmonary hypertension, obstetricians, critical care professionals, anesthesiologists, and neonatologists. The goal is to ensure favorable outcomes for both the mother and the baby. In lower-risk patients, recommended medications include a combination of oral phosphodiesterase inhibitors and inhaled prostacyclin analog. For higher-risk patients, intravenous medication should be considered.
Right heart catheterization is not routinely recommended, but may be considered in higher risk cases. Arrhythmias associated with hemodynamical instability should be given additional consideration, since they are probably poorly tolerated by PAH patients [34]. Inotropes, pressors, and even extracorporeal membranous oxygenation (ECMO) are also possible medication options in high-risk situations.
There is no perfect time for delivery. Generally, deterioration in the first two trimesters is associated with a poor outcome and these patients require termination or delivery to save the life of the mother [35]. If the patient remains stable during pregnancy, the optimal time for delivery should be a balance of the needs of both the mother and the fetus. Compared with term delivery, there is evidence that there may be a delay in cognitive and behavioral development in infants delivered at 32–35 weeks gestation [36]. Early delivery is also associated with more neonatal complications calling for the necessity of a special care unit for the infant. An expert experience recommends delivering at around 34 weeks if the patient is stable and a later delivery during the pregnancy may also be considered when justified in specific cases [37].
Selection of delivery mode is based on a case-by-case basis. However, many centers advocate elective delivery using cesarean section for PAH patients for the following strong points. Firstly, an elective procedure guarantees the presence of all members of an expert multidisciplinary team. Secondly, cesarean section minimizes the impact of delivery on the cardiovascular system, compared with vaginal delivery during which the cardiac output increases by over 30% at the end of the first stage of labor and significantly reduces cardiac output by reducing venous return to the right ventricle with potentially deleterious consequences [37]. Additionally, the frequent use of the Valsalva maneuver during vaginal delivery increases intrathoracic pressures and decreases pre-load, leading to risks to a sensitive RV. Oxytocin, a hysterotonics most commonly used in both caesarean section and vaginal delivery should be cautiously used as it can also increase pulmonary vascular resistance.
As for the selection of anesthesia mode, epidural anesthesia and combined spinal-epidural
Anesthesia is usually advocated. Among the two anesthesia modes, the spinal-epidural approach provides the advantages of a low spinal block with a denser sensory block than the epidural approach, and also avoids the risk of hypotension with a spinal [37]. Regional anesthesia also has the advantage of providing postoperative analgesia and has the capacity for top-up anesthesia and ease of return to theatre. General anesthesia has also been used in many medical centers. However, tracheal intubation may lead to a rise in pulmonary artery pressure, and positive pressure ventilation can have negative effects on venous return [38]. Some experts recommend that regional anesthesia should be applied first but general anesthesia needs to be made to proceed to in selected cases [37].
3.5. Maternal and fetal outcomes
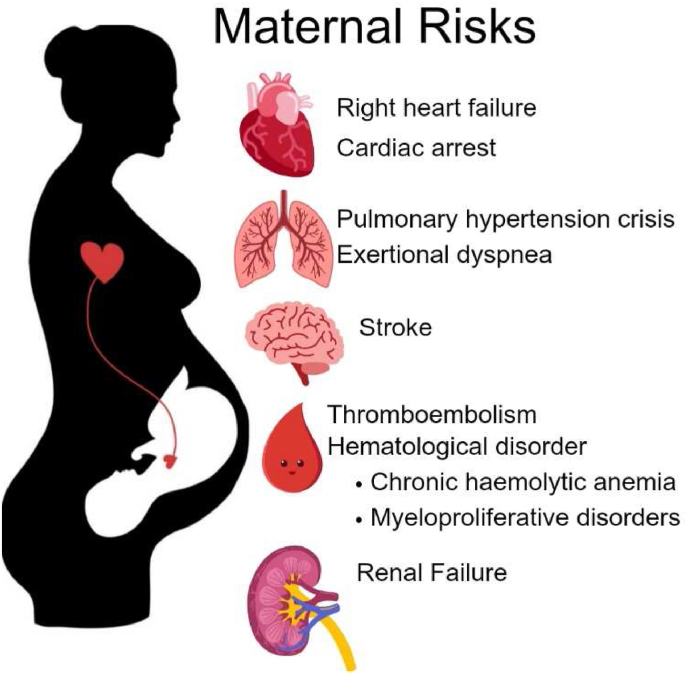
Severe maternal complications include right heart failure, cardiac arrest pulmonary hypertension crisis, stroke, premature labor or birth of the child etc (Fig. 2). Fetal outcomes include low birth weight, week of delivery, neonatal preterm birth, and small for gestational age infants, low birth weight infants, neonatal asphyxia, and higher neonatal mortality [39]. According to the etiology, idiopathic pulmonary arterial hypertension (iPAH), pulmonary arterial hypertension associated with congenital heart disease (CHD-PAH), pulmonary hypertension associated with left heart disease (LDH-PH), and pulmonary arterial hypertension caused by other diseases (oPAH) constitute the majority of fetal complications. Further, The sPAP levels of iPAH and CHD-PAH were significantly higher than those of LDH-PH and oPAH (p < 0.05) [40].
Fig. 2.
Maternal risks of pregnant patients with PAH.
3.6. Counseling on contraception and breastfeeding
As mentioned above, pregnancy is generally not recommended among women with PAH due to the high mortality, and therapeutic abortion should be offered to high-risk cases. Women with PAH should be counseled to adopt appropriate means of contraception. For women with PAH, long-acting reversible contraceptives, such as intrauterine devices and progestin-only subcutaneous implants, are safe and effective, and generally carry a low risk of thromboembolism [41]. Tubal ligation is a permanent procedure for contraception, and modern studies have shown it also could protect against ovarian cancer [42]. There is no consensus on whether breastfeeding is allowed among PAH patients, since no data is available on drugs used to treat PH and whether they cross into the breast milk. Generally, breastfeeding is not recommended among PAH mothers due to the potential risk to the neonate, as well as lack of rest and sleep may cause adverse effects on the heart function of the mother.
4. Areas of uncertainty
Whether PAH more likely coexists with preeclampsia or gestational PAH is a prodromal sign of preeclampsia remains unexplored. We and other researchers both observed an interesting phenomenon that a great proportion of PAH patients further develop into gestational hypertension and preeclampsia. In our case, the woman's PAH remained relatively stable, but further developed into preeclampsia at 34 weeks and 4 days of gestation and accompanied by rapid deterioration of liver/kidney function and called for an emergency termination of pregnancy. Other researchers observed the same phenomenon and suggested that pulmonary arterial hypertension and preeclampsia have shared disease mechanisms and translational opportunities [43]. Studies found impaired immune system is the key step for the progression of both PAH and preeclampsia, including an imbalance in CD4+ helper T cell populations, as well as the excessive activation or impairment of additional immune cell types, including macrophages, dendritic cells, CD8+ T cells, B cells and Natural Killer cells [43]. Identification of common immune mechanisms raises the possibility of new therapeutic strategies that target the immune component of gestational PAH and preeclampsia. Future trials are necessary to confirm the correlation of preeclampsia, oligohydramnios and PAH.
5. Guidelines from professional societies
Few clinical practice guidelines focus on PAH in pregnancy (Table 2) [[44], [45], [46], [47], [48], [49]]. Current guidelines clarify the classification, pathophysiology, and therapy of PH.
Table 2.
Representative guideline recommendations for pregnant women with PAH.
| Organization (Year) | pregnancy avoidance Recommendation | Contraception approach recommendation | Delivery approach recommendation | disease-targeted therapies recommendation | Post-menopausal HRT recommendation | Genetic counseling recommendation |
|---|---|---|---|---|---|---|
| ESC/ERS(2022) | Yes | Barrier contraceptive methods; Progesterone-only contraceptives; | Planned elective delivery (epidural anesthesia preferred) | CCBs; Phosphodiesterase type 5 inhibitors; Prostacyclin analogues and prostacyclin receptor agonists |
Unclear | BMPR2 mutation carriers suggested to remain childless |
| 6th WSPH(2018) | Not mentioned | Not mentioned | Not mentioned | Endothelin receptor antagonists; Prostacyclin pathway agents; Endothelin receptor antagonists; Nitric oxide pathway agents; New drugs(FK506,olaparib,Imatinib,Elafin and so on) |
Not mentioned | Inform relevant gene mutation population about the possibility of a genetic condition and that family members could carry a mutation that increases the risk of PAH (55) |
| Chinese (2021) | Yes | Not addressed | Planned elective delivery (local anesthesia or epidural anesthesia preferred) | CCBs; Endothelin receptor antagonist; Phosphodiesterase type 5 inhibitors; Soluable guanylate cyclase; Prostacyclin analogues and prostacyclin receptor agonists |
Unclear | Pathogenic gene for PAH:BMPR2、BMP9、ALK1、Endoglin、SMAD9、 BMPR1B、TBX4、CAV1 and KCNK3 |
| CCS/CTS(2019) | Yes | Not addressed | Not addressed | Warfarin; CCBs; Prostacyclin pathway agents; Endothelin receptor antagonists; Nitric oxide pathway agents |
Not mentioned | Not mentioned |
| TSOC(2018) | Not mentioned | Not mentioned | Not mentioned | Prostacyclin analogues and prostacyclin receptor agonists; Endothelin receptor antagonists; SGC stimulator; Phosphodiesterase type 5 inhibitors |
Not mentioned | Not mentioned |
| TSOC(2018) | Not mentioned | Not mentioned | Not mentioned | Prostacyclin analogues and prostacyclin receptor agonists; Endothelin receptor antagonists; SGC stimulator; Phosphodiesterase type 5 inhibitors |
Not mentioned | Not mentioned |
Abbreviation:ESC/ERS: ESC: European Society of Cardiology, and ERS: European Respiratory Society; 6th WSPH:the 6th world symposia on pulmonary hypertension; CCS/CTS: CCS: Canadian Cardiovascular Society, and CTS: Canadian Thoracic Society; TSOC: Taiwan Society of Cardiology; CCBs: Calcium channel blockers; SGC: Soluble guanylate cyclase.
6. Conclusions and recommendations
The vignette at the start of the review outlines the case of a 30-year-old woman who exhibited oligohydramnios, bilateral leg edema, and an elevated mPAP level indicative of pulmonary hypertension. After confirmation of the PAH diagnosis, prompt evaluation to establish the cause is a priority. Family history, infection, immunity test, and primary heart diseases should be excluded to precise the classification and risk stratification; additional testing might be justified depending on the clinical presentation. Pregnancy is generally not recommended among women with PAH due to the high mortality, and therapeutic abortion should be offered to high-risk cases. For those who are determined to continue with the pregnancy, a close monitoring throughout the pregnancy and delivery period by a multidisciplinary team is necessary for both the mother and the fetus. There is no current consensus on medication and dosage selection for PAH women. Timing of pregnancy termination, delivery and anesthesia mode are selected based on individual needs and risk assessment. All female PAH patients should avoid pregnancy. As for options for contraceptives, progesterone-only methods can be employed, whilst estrogen should be avoided given the prothrombotic effects. For those who have completed delivery, the permanent procedure for contraception of tubal ligation is recommended. Generally, breastfeeding is not recommended among PAH mothers due to the potential risk to the neonate, as well as lack of rest and sleep may cause adverse effects on the heart function of the mother. The potential link between PAH, oligohydramnios, and preeclampsia is worth further exploration.
CRediT authorship contribution statement
Yue Teng: Writing – original draft. Lu Zong: Project administration. Jie Ding: Writing – review & editing. Mengmin Wu: Validation. Xuelan Li: Supervision.
Acknowledgments
No acknowledgements.
Handling editor: Dr D Levy
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcrp.2024.200252.
Contributor Information
Yue Teng, Email: navimoon@126.com.
Lu Zong, Email: catherazong@163.com.
Jie Ding, Email: 17792173806@163.com.
Mengmin Wu, Email: wumengmin@163.com.
Xuelan Li, Email: lixuelan1225@126.com.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Simonneau G., Montani D., Celermajer D.S., Denton C.P., Gatzoulis M.A., Krowka M., et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019;53(1) doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuster V., Steele P.M., Edwards W.D., Gersh B.J., McGoon M.D., Frye R.L. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70(4):580–587. doi: 10.1161/01.cir.70.4.580. [DOI] [PubMed] [Google Scholar]
- 3.Bédard E., Dimopoulos K., Gatzoulis M.A. Has there been any progress made on pregnancy outcomes among women with pulmonary arterial hypertension? Eur. Heart J. 2009;30(3):256–265. doi: 10.1093/eurheartj/ehn597. [DOI] [PubMed] [Google Scholar]
- 4.Frost A.E., Badesch D.B., Barst R.J., Benza R.L., Elliott C.G., Farber H.W., et al. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest. 2011;139(1):128–137. doi: 10.1378/chest.10-0075. [DOI] [PubMed] [Google Scholar]
- 5.Luo J., Shi H., Xu L., Su W., Li J. Pregnancy outcomes in patients with pulmonary arterial hypertension: a retrospective study. Medicine. 2020;99(23) doi: 10.1097/MD.0000000000020285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duarte A.G., Thomas S., Safdar Z., Torres F., Pacheco L.D., Feldman J., et al. Management of pulmonary arterial hypertension during pregnancy: a retrospective, multicenter experience. Chest. 2013;143(5):1330–1336. doi: 10.1378/chest.12-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng M.-L., Landau R., Viktorsdottir O., Banayan J., Grant T., Bateman B., et al. Pulmonary hypertension in pregnancy: a report of 49 cases at four tertiary north American sites. Obstet. Gynecol. 2017;129(3):511–520. doi: 10.1097/AOG.0000000000001896. [DOI] [PubMed] [Google Scholar]
- 8.Roos-Hesselink J., Baris L., Johnson M., De Backer J., Otto C., Marelli A., et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry of Pregnancy and Cardiac disease (ROPAC) Eur. Heart J. 2019;40(47):3848–3855. doi: 10.1093/eurheartj/ehz136. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Q., Peng P., Liu X., Liu J., Gao J., Chen W. Evaluation of maternal and fetal outcomes in pregnancy complicated with pulmonary arterial hypertension. Ann. Palliat. Med. 2021;10(2):1404–1410. doi: 10.21037/apm-20-551. [DOI] [PubMed] [Google Scholar]
- 10.Galiè N., Humbert M., Vachiery J.-L., Gibbs S., Lang I., Torbicki A., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of Cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital Cardiology (AEPC), international society for heart and lung transplantation (ISHLT) Eur. Respir. J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 11.Humbert M., Sitbon O., Chaouat A., Bertocchi M., Habib G., Gressin V., et al. Pulmonary arterial hypertension in France: results from a national registry. Am. J. Respir. Crit. Care Med. 2006;173(9):1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 12.Galiè N., Humbert M., Vachiery J.L., Gibbs S., Lang I., Torbicki A., et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of Cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital Cardiology (AEPC), international society for heart and lung transplantation (ISHLT) Eur. Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 13.Benza R.L., Gomberg-Maitland M., Elliott C.G., Farber H.W., Foreman A.J., Frost A.E., et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-Based risk assessment strategies. Chest. 2019;156(2):323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Siu S.C., Sermer M., Colman J.M., Alvarez A.N., Mercier L.A., Morton B.C., et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 15.Emmanuel Y., Thorne S.A. Heart disease in pregnancy. Best Pract. Res. Clin. Obstet. Gynaecol. 2015;29(5):579–597. doi: 10.1016/j.bpobgyn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Rubin L.J. Primary pulmonary hypertension. N. Engl. J. Med. 1997;336(2):111–117. doi: 10.1056/NEJM199701093360207. [DOI] [PubMed] [Google Scholar]
- 17.Cogan J.D., Pauciulo M.W., Batchman A.P., Prince M.A., Robbins I.M., Hedges L.K., et al. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2006;174(5):590–598. doi: 10.1164/rccm.200602-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin V.V., Shah S.J., Souza R., Humbert M. Management of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2015;65(18):1976–1997. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 19.Humbert M., Kovacs G., Hoeper M.M., Badagliacca R., Berger R.M.F., Brida M., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart J. 2022;43(38):3618–3731. doi: 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 20.Afify H., Kong A., Bernal J., Elgendy I.Y. Pulmonary hypertension in pregnancy: challenges and solutions. Integrated Blood Pres. Control. 2022;15:33–41. doi: 10.2147/IBPC.S242242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farber H.W., Miller D.P., Poms A.D., Badesch D.B., Frost A.E., Muros-Le Rouzic E., et al. Five-Year outcomes of patients enrolled in the REVEAL Registry. Chest. 2015;148(4):1043–1054. doi: 10.1378/chest.15-0300. [DOI] [PubMed] [Google Scholar]
- 22.McLaughlin V.V., McGoon M.D. Pulmonary arterial hypertension. Circulation. 2006;114(13):1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 23.Hemnes A.R., Kiely D.G., Cockrill B.A., Safdar Z., Wilson V.J., Al Hazmi M., et al. Statement on pregnancy in pulmonary hypertension from the pulmonary vascular research institute. Pulm. Circ. 2015;5(3):435–465. doi: 10.1086/682230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostermann J., Pott J., Hennigs J.K., Roedl K., Sinning C., Harbaum L., et al. Residual risk identified in routine noninvasive follow-up assessments in pulmonary arterial hypertension. ERJ Open Res. 2023;9(3) doi: 10.1183/23120541.00072-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H., Bae Lee J., Park J.-H., Yoo B.-S., Son J.-W., Yang D.H., et al. A comparison of echocardiographic variables of right ventricular function with exercise capacity after bosentan treatment in patients with pulmonary arterial hypertension: results from a multicenter, prospective, cohort study. J. Clin. Ultrasound. 2017;45(1):28–34. doi: 10.1002/jcu.22396. [DOI] [PubMed] [Google Scholar]
- 26.Barańska-Pawełczak K., Wojciechowska C., Jacheć W. Diagnostic and predictive value of right heart catheterization-derived measurements in pulmonary hypertension. Wiad. Lek. 2021;74(3 cz 1):546–553. [PubMed] [Google Scholar]
- 27.Vachiéry J.L., Yerly P., Huez S. How to detect disease progression in pulmonary arterial hypertension. Eur. Respir. Rev. 2012;21(123):40–47. doi: 10.1183/09059180.00009011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clapp M.A., Bernstein S.N. Preconception counseling for women with cardiac disease. Curr. Treat. Options Cardiovasc. Med. 2017;19(9):67. doi: 10.1007/s11936-017-0565-z. [DOI] [PubMed] [Google Scholar]
- 29.Thomson J.R., Machado R.D., Pauciulo M.W., Morgan N.V., Humbert M., Elliott G.C., et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J. Med. Genet. 2000;37(10):741–745. doi: 10.1136/jmg.37.10.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., Blomström-Lundqvist C., Cífková R., De Bonis M., et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 31.Daraz Y., Murthy S., Wolfe D. Pregnancy in pulmonary arterial hypertension: a multidisciplinary approach. J Cardiovasc Dev Dis. 2022;9(6) doi: 10.3390/jcdd9060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M.Q., Wang C.C., Pang X.B., Shi J.Z., Li H.R., Xie X.M., et al. Role of macrophages in pulmonary arterial hypertension. Front. Immunol. 2023;14 doi: 10.3389/fimmu.2023.1152881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Funk-Hilsdorf T.C., Behrens F., Grune J., Simmons S. Dysregulated immunity in pulmonary hypertension: from companion to composer. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.819145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krishnathasan K., Constantine A., Rafiq I., Barradas Pires A., Douglas H., Price L.C., et al. Management of pulmonary arterial hypertension during pregnancy. Expet Rev. Respir. Med. 2023;17(5):413–423. doi: 10.1080/17476348.2023.2210838. [DOI] [PubMed] [Google Scholar]
- 35.Bonnin M., Mercier F.J., Sitbon O., Roger-Christoph S., Jaïs X., Humbert M., et al. Severe pulmonary hypertension during pregnancy: mode of delivery and anesthetic management of 15 consecutive cases. Anesthesiology. 2005;102(6):1133–1137. doi: 10.1097/00000542-200506000-00012. ; discussion 5A-6A. [DOI] [PubMed] [Google Scholar]
- 36.Huddy C.L., Johnson A., Hope P.L. Educational and behavioural problems in babies of 32-35 weeks gestation. Arch. Dis. Child. Fetal Neonatal Ed. 2001;85(1):F23–F28. doi: 10.1136/fn.85.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiely D.G., Condliffe R., Wilson V.J., Gandhi S.V., Elliot C.A. Pregnancy and pulmonary hypertension: a practical approach to management. Obstet. Med. 2013;6(4):144–154. doi: 10.1177/1753495X13495193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaise G., Langleben D., Hubert B. Pulmonary arterial hypertension: pathophysiology and anesthetic approach. Anesthesiology. 2003;99(6):1415–1432. doi: 10.1097/00000542-200312000-00027. [DOI] [PubMed] [Google Scholar]
- 39.Ma R., Gao H., Cui J., Shi H., Yang Z., Jin Z., et al. Pregnancy feasibility in women with mild pulmonary arterial hypertension: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2023;23(1):427. doi: 10.1186/s12884-023-05752-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai Q., Shang M., Zhou Y., Wei Q. Evaluation of maternal-fetal outcomes in pregnancy complicated with severe pulmonary hypertension and its influencing factors: a single-center retrospective study in China. J Matern Fetal Neona. 2023;36(2) doi: 10.1080/14767058.2023.2290923. [DOI] [PubMed] [Google Scholar]
- 41.Apter D., Briggs P., Tuppurainen M., Grunert J., Lukkari-Lax E., Rybowski S., et al. A 12-month multicenter, randomized study comparing the levonorgestrel intrauterine system with the etonogestrel subdermal implant. Fertil. Steril. 2016;106(1):151. doi: 10.1016/j.fertnstert.2016.02.036. 7.e5. [DOI] [PubMed] [Google Scholar]
- 42.Russo A., Cain B.P., Jackson-Bey T., Lopez Carrero A., Miglo J., MacLaughlan S., et al. Increased local testosterone levels alter human fallopian tube mRNA profile and signaling. Cancers. 2023;15(7) doi: 10.3390/cancers15072062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jafri S., Ormiston M.L. Immune regulation of systemic hypertension, pulmonary arterial hypertension, and preeclampsia: shared disease mechanisms and translational opportunities. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017;313(6):R693–r705. doi: 10.1152/ajpregu.00259.2017. [DOI] [PubMed] [Google Scholar]
- 44.Humbert M., Kovacs G., Hoeper M.M., Badagliacca R., Berger R.M.F., Brida M., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2023;61(1) doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 45.Galiè N., Channick R.N., Frantz R.P., Grünig E., Jing Z.C., Moiseeva O., et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur. Respir. J. 2019;53(1) doi: 10.1183/13993003.01889-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sitbon O., Gomberg-Maitland M., Granton J., Lewis M.I., Mathai S.C., Rainisio M., et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur. Respir. J. 2019;53(1) doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrell N.W., Aldred M.A., Chung W.K., Elliott C.G., Nichols W.C., Soubrier F., et al. Genetics and genomics of pulmonary arterial hypertension. Eur. Respir. J. 2019;53(1) doi: 10.1183/13993003.01899-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirani N., Brunner N.W., Kapasi A., Chandy G., Rudski L., Paterson I., et al. Canadian cardiovascular society/Canadian thoracic society position statement on pulmonary hypertension. Can. J. Cardiol. 2020;36(7):977–992. doi: 10.1016/j.cjca.2019.11.041. [DOI] [PubMed] [Google Scholar]
- 49.Huang W.C., Hsu C.H., Sung S.H., Ho W.J., Chu C.Y., Chang C.P., et al. 2018 TSOC guideline focused update on diagnosis and treatment of pulmonary arterial hypertension. J. Formos. Med. Assoc. 2019;118(12):1584–1609. doi: 10.1016/j.jfma.2018.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.