Abstract
In compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses, we conducted this systemic review on the prevalence, mechanism, and therapy of sleep disorder in patients with cardiovascular disease (CVD). After searching PubMed and Embase, 78 articles were selected for this review. This review discusses the bidirectional relationship between CVD and sleep disorders. Sleep impairment is highly prevalent in patients with CVD and mainly involves insomnia and sleep-breathing disorders. Several valuable biomarkers could be implicated in predicting sleep disorders in CVD patients, such as placental growth factor, vascular endothelial growth factor family, high sensitivity C-reactive protein, endoglin, fms-like tyrosine kinase-1, plasminogen activator inhibitor-1, erythropoietin. Moreover, non-drug therapies, namely physical exercise, cognitive behavioral therapy for insomnia (CBT-I), and continuous positive airway pressure benefit the prognosis of patients with CVD. In conclusion, this study highlights the importance of sleep quality, which is responsible for long- and short-term cardiac outcomes in patients with CVD.
Keywords: Sleep disorders, Cardiovascular disease, Depression, Anxiety
Graphical abstract
The outline of this systematic review. CVD, cardiovascular disease; CAD, coronary artery disease; SBD, sleep breathing disorders; CBT, cognitive behavioral therapy; CPAP, continuous positive airway pressure; IMT, inspiratory muscle training; OSA, obstructive sleep apnea; HF, heart failure; HTN, hypertension.
1. Introduction
Sleep accounts for one-third of our lives and plays a crucial role in maintaining public health. However, many individuals experience sleep disorders throughout their lifetime. According to the International Classification of Sleep Disorders-3 (ICSD-3) diagnostic criteria, sleep disorders are classified into seven major categories: insomnia disorders, sleep-related breathing disorders (SBD), central disorders of hypersomnolence, circadian rhythm sleep-wake disorders, sleep-related movement disorders, parasomnias, and other sleep disorders [1]. Approximately 10–30% of the general population is affected by insomnia [2]. Moreover, approximately one billion adults (aged 30–69 years) are affected by obstructive sleep apnea (OSA), and half of these have moderate to severe OSA [3]. One-third of the patients with OSA also have insomnia [4].
There is a bidirectional correlation between sleep disorders and cardiovascular disease (CVD) [5,6]. Accumulating evidence has shown a high prevalence of sleep disorders among patients with CVD [7]. Pengo et al. [8] have highlighted this global problem and urged cardiologists to focus on sleep health. As cardiologists, we must recognize sleep impairments in patients in clinical practice. In cardiology departments, many patients report sleep disorders. A high prevalence (40–80%) of OSA is observed in patients with CVD, including those with coronary artery disease (CAD), atrial fibrillation (AF), heart failure (HF), and hypertension, leading to adverse cardiac outcomes [9]. Moreover, the combination of insomnia and OSA was associated with a higher CVD risk after adjustment for confounding factors. The sleep-breathing impairment index (SBII) is considered a new metric for evaluating the severity of OSA. Cao et al. showed that a higher SBII score was strongly associated with a higher 10-year Framingham CVD risk score [10]. Poor sleep increases risk and mortality of CVD (hazard ratio (HR):1.67, 95% confidence interval (CI): 1.27–2.19) [-[11], [12]]. Similarly, Yan et al. [13] also identified that poor objective sleep efficiency could significantly predict CVD mortality (HR: 1.887, 95% CI: 1.224–2.909, P < 0.05). Sleep quality can be evaluated not only by subjective feelings but also by polysomnography.
The potential mechanisms underlying sleep disorders and CVD include parasympathetic and sympathetic nerve imbalance, immune inflammation, genetics, interactions in the microbiome-gut-brain axis, blood lipid abnormalities, and altered hematopoiesis. Sleep deficiency increases the expression of interleukin −6 (IL-6) and tumor necrosis factor-alpha (TNF-α) via the nuclear factor-kappa B (NF-κB) signaling pathway [14]. Moreover, the inflammation affects small peripheral arteries and sympathetic and sensory-motor nerves, leading to blood elevation, which exacerbates the CVD prognosis. Inflammation is closely associated with blood lipid abnormalities. Insomnia disturbs the blood lipid metabolism, The elevated LDL cholesterol is a risk factor for carotid artery intima-media thickness (CIMT) and plaque incidence, indicating the relationship between insomnia, higher CIMT values, plaque incidence, and CVD [15].
Improving sleep disorders benefits cardiovascular recovery and vice versa. Despite the sedative-hypnotics for treating sleep disorders, we recommend some non-drug therapies. Exercise, cognitive behavioral therapy for insomnia (CBT-I), and continuous positive airway pressure (CPAP) are the most commonly used non-drug treatments. Exercise is an easy and convenient method for most patients. Both the American College of Cardiology and the American Heart Association recommend exercise and physical activity to prevent CVD primarily [16]. Furthermore, traditional Chinese exercises such as Tai Chi help improve sleep quality by reducing waking time [17]. Exercise can also benefit patients with OSA and CVD by reducing inflammation and sympathetic activation [18]. Strong evidence has shown that CBT is an effective method for treating CVD and insomnia. Conventionally, CBT is conducted face-to-face by physicians, although internet technology has also been implicated in CBT. Internet-based CBT (ICBT) helps alleviate depressive symptoms in patients with CVD; hence, ICBT is beneficial for CVD treatment [19]. CPAP can improve physical activity, relieve depressive mood, and reduce adverse cardiac events in patients with co-occurring CVD and OSA [20]. CPAP is a valuable therapy for reducing the risk of CVD by controlling risk factors, such as lowering blood pressure or blood glucose [21].
Therefore, to improve sleep health and reduce cardiac events in patients with CVD, the interrelationship between sleep disorders and CVD should be explored further. In this systematic review, we illustrate the epidemiology, mechanisms, drug therapy, and non-drug therapy of sleep disorders in patients with CVD.
2. Method
2.1. Design
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol was registered in PROSPSPERO (CRD42023451234).
2.2. Search strategy
We searched the related literature with the Pubmed and Embase databases using the key words:((sleep) OR (insomnia)) AND (((((((((coronary artery disease) OR (cardiac arrhythmia)) OR (hypertension)) OR (valvular heart disease)) OR (dilated cardiomyopathy)) OR (hypertrophic cardiomyopathy)) OR (myocarditis)) OR (heart failure)) OR (cardiovascular disease)).
2.3. Inclusion and exclusion criteria
Inclusion criteria: 1) patients with cardiovascular disease and sleep disorders; 2) age ≥18 years; 3) both women and men; 4) clinical research.
Exclusion criteria: 1) pregnancy; 2) no full text; 3) text not in English.
2.4. Study screening and data extraction
Two authors independently reviewed the papers by extracting the following data: author, publication date, study type, sleep disorders, methods of sleep assessment, type of CVD, and the main outcomes. If there were different opinions, a third author was consulted to reach an agreement.
2.5. Outcomes
This review highlights the prevalence, mechanisms, and therapies of sleep disorders in patients with CVD.
2.6. Study quality and risk of bias assessment
The Cochrane Risk of Bias (CoB) tool was used to assess the risk of bias/quality of randomized controlled trials (RCT) [22], the Newcastle-Ottawa (cohort) scale (NOS) was used for cohort studies [23], Combie was used for cross-sectional studies [24], and methodological index for non-randomized studies (MINORS) was used for non-randomized studies.
3. Results
3.1. Search results
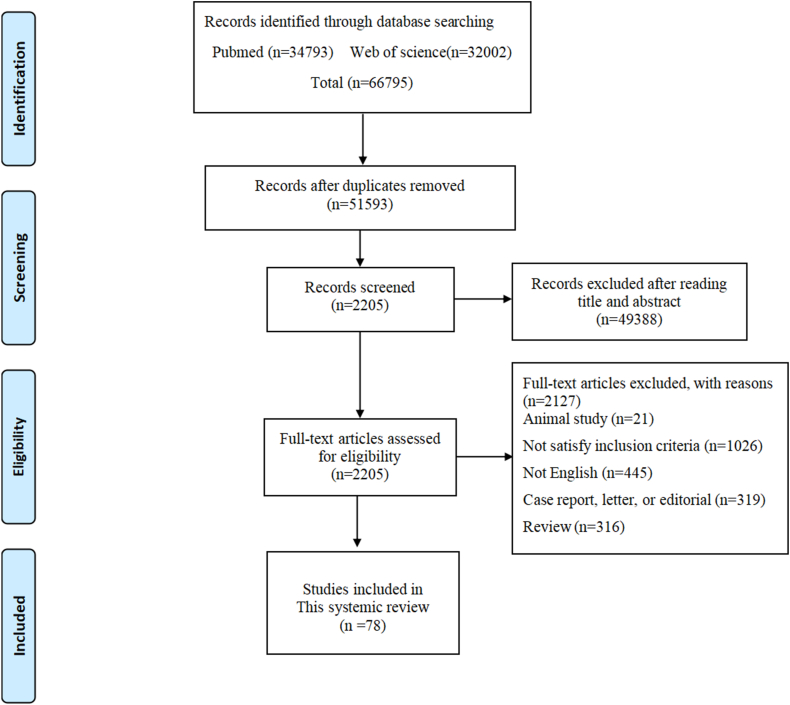
A total of 66795 references were identified in the two databases. After careful reading, 78 articles were included in this systemic review [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102]]. The selection flowchart was shown in Fig. 1.
Fig. 1.
Flow chart of article selection.
3.2. Characteristics of the studies
Table 1 summarized the basic characteristics of the included studies. There were 22 articles on sleep disorders in patients with CAD [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]], 19 on hypertension [[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]], 16 on HF [[66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]], 10 on cardiac arrhythmia [[82], [83], [84], [85], [86], [87], [88], [89], [90], [91]], 11 on other CVD [[92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102]]. Except for one study including 36 countries [29], the other studies covered 19 countries, comprising China (n = 12) [33,41,44,50,57,59,63,64,86,89,94,101], USA (n = 14) [56,61,62,68,[72], [73], [74],77,81,83,87,88,90,102], Lithuania (n = 3) [25,32,37], Brazil (n = 4) [26,30,31,54], Israel (n = 1) [27], Iran (n = 2) [35,100], Spain (n = 3) [39,58,65], Germany (n = 11) [28,38,49,67,69,75,78,84,[97], [98], [99]], Sweden (n = 5) [36,40,46,55,95], Egypt (n = 1) [79], Turkey (n = 2) [70,85], Japan (n = 3) [42,92,96], France (n = 3) [45,76,93], Nigeria (n = 2) [53,66], India (n = 2) [51,71], Malaysia (n = 1) [52], Canada (n = 4) [43,48,82,91], Pakistan (n = 1) [34], Poland (n = 1) [60], Romania (n = 1) [80], and Italy (n = 1) [47]. These articles included several types of study designs: cross-sectional studies (n = 33), prospective cohort studies (n = 26), RCT (n = 7), retrospective cohort studies (n = 7), case-control studies (n = 3), pilot study (n = 1), longitudinal study (n = 1). These studies investigated the sleep conditions of patients with CVD, including SDB, insomnia disorders, and sleep quality. Polysomnography and the Berlin Questionnaire were used to assess SBD, and the Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality.
Table 1.
The study characteristics of sleep disorders in patients with CVD.
| No. | First author Publication year |
Country | Study design | CVD | Sleep disorders | Sample /Age (years) |
Sleep measurement | Main findings | Study quality assessment |
|---|---|---|---|---|---|---|---|---|---|
| Coronary artery disease | |||||||||
| 1 | Alonderis2020 [25] | Lithuania | Cross-sectional study | CAD with LVEF≥ 50% | SA | N = 450 | Polysomnography | Up to 35% of coronary artery disease patients were likely to have undiagnosed sleep apnoea. | Combie high quality |
| AMI+SA+ | |||||||||
| N = 156/59.4 ± 9.2yrs | |||||||||
| AMI+SA- | |||||||||
| N = 294/56.1 ± 9.1yrs | |||||||||
| 2 | Andrechuk2015 [26] | Brazil | Cross-sectional study | AMI | OSA | N = 113/59.7 ± 12.3 yrs | Berlin Questionnaire | The high prevalence of obstructive sleep apnoea was 60.2% | Combie high quality |
| 3 | Araújo2009 [31] | Brazil | Cross-sectional study | CAD | OSA | N = 53 | Polysomnography | OSA was not related to myocardial ischemia, heart rate variability or arrhythmias in patients with SCAD. | Combie high quality |
| Control | |||||||||
| n = 23/57.47 ± 10.59yrs | |||||||||
| Apnea | |||||||||
| n = 30/59.00 ± 10.42yrs | |||||||||
| 4 | Aronson2014 [27] | Israel | Prospective cohort study | AMI | SDB | N = 180 | Watch-PAT 100 | A high prevalence of previously undiagnosed SDB among patients with AMI. SDB in the setting of AMI is associated with higher pulmonary artery systolic pressure. SDB was not associated with adverse clinical outcomes. | NOS high quality |
| AMI + SDB | |||||||||
| N = 116/59±9yrs | |||||||||
| AMI non-SDB | |||||||||
| N = 64/56 ± 11yrs | |||||||||
| 5 | Assari2013 [35] | Iran | Cross-sectional study | CAD | Sleep quality | N = 717/57.7 ± 11.7yrs | PSQI | Among female patients with CAD, low education and income were associated with poor sleep quality. | Combie high quality |
| 6 | Barcelo2016 [39] | Spain | RCT | ACS | OSA | N = 312/61.2 ± 10.3yrs | Polygraph | In patients with ACS, elevated plasma levels of PlGF are associated with the presence of OSA and with adverse outcomes during short-term follow up. | CoB |
| Controls | |||||||||
| N = 226/56.5 ± 11.5yrs | |||||||||
| OSA | |||||||||
| N = 312/61.2 ± 10.3yrs | |||||||||
| 7 | Barger2017 [29] | 36 countries | Prospective cohort study | ACS | Short sleep duration OSA |
N = 12924/64years | Berlin questionnaire a sleep survey | Short sleep duration, and OSA, are under-recognized as predictors of adverse outcomes after acute coronary syndrome. | NOS high quality |
| No OSA | |||||||||
| N = 8084/65 (60,71) | |||||||||
| OSA | |||||||||
| N = 4840/63 (57, 69) | |||||||||
| 8 | Buchner2015 [28] | Germany | Prospective cohort study | AMI | SDB | N = 54 | Polysomnography | SDB may contribute to enlargement of the right heart after AMI. | NOS high quality |
| AMI + SDB+ | |||||||||
| N = 29/55 ± 10yrs | |||||||||
| AMI + SDB- | |||||||||
| N = 25/53 ± 10yrs | |||||||||
| 9 | Cai 2022 [33] | China | Cross-sectional study | CAD | Sleep quality | N = 84 | PSQI | MDD may be responsible for poor sleep quality, in patients with CHD, treatment for depressive symptoms may also improve CHD prognosis. | Combie high quality |
| CHD MDD (+) | |||||||||
| 58.46 ± 6.12yrs | |||||||||
| CHD MDD (−) | |||||||||
| 57.68 ± 5.50yrs | |||||||||
| 10 | Clark2014 [36] | Sweden | Prospective cohort study | AMI | Sleep impairment | N = 1588/60±7yrs | Karolina Sleep Questionnaire | In women, disturbed sleep showed a consistently higher risk of long-term cardiovascular events; In men, a strong effect on case fatality was observed in regard to impaired awakening | NOS high quality |
| Women | |||||||||
| No disturbed sleep | |||||||||
| N = 418/62±7yrs | |||||||||
| Disturbed sleep | |||||||||
| N = 78/61±7yrs | |||||||||
| Men | |||||||||
| No disturbed sleep | |||||||||
| N = 1008/59±7yrs | |||||||||
| Disturbed sleep | |||||||||
| N = 77/59±7yrs | |||||||||
| 11 | Correia2012 [30] | Brazil | Prospective cohort study | UA NSTEMI |
OSA | N = 168/70 ± 12yrs | Berlin Questionnaire | During a median hospitalization of 8 days, the incidence of cardiovascular events was 13%. Incidence of the primary endpoint was 18% in individuals with high probability of OSA. | NOS high quality |
| Low OSA probability | |||||||||
| N = 45/69 ± 14yrs | |||||||||
| High OSA probability | |||||||||
| N = 123/71 ± 12yrs | |||||||||
| 12 | Feng2022 [43] | Canada | Prospective cohort study | CAD | Sleep quality | N = 113/63.7 ± 6.4yrs | PSQI | A marker of late-stage lipid peroxidation is elevated in CAD patients with poor sleep and associated with daily disturbances, but not with other factors or with sleep quality and its factors after exercise intervention. | NOS high quality |
| Normal sleep quality | |||||||||
| N = 54/64.8 ± 6.6yrs | |||||||||
| Poor sleep quality | |||||||||
| N = 59/62.6 ± 6.1yrs | |||||||||
| 13 | Huang2020 [41] | China | Cross-sectional study | CAD | OSA | N = 1243 | Overnight portable respiratory monitoring | Elevated levels of MHR were independently associated with a higher likelihood of OSA in patients with CAD. | Combie high quality |
| MHR Quartile 1 | |||||||||
| N = 311/63.3 ± 9.1yrs | |||||||||
| MHR Quartile 2 | |||||||||
| N = 311/62.6 ± 9.5yrs | |||||||||
| MHR Quartile 3 | |||||||||
| N = 311/62.6 ± 13.0yrs | |||||||||
| MHR Quartile 4 | |||||||||
| N = 310/60.2 ± 10.8yrs | |||||||||
| 14 | Juskiene2018 [32] | Lithuania | Cross-sectional study | CAD | Sleep quality OSA |
N = 879/58 ± 9 yrs | Polysomnography PSQI |
In CAD patients, type D personality and NA are associated with worse subjective sleep quality and this association is mediated by depression and anxiety symptoms irrespective of OSA presence. | Combie high quality |
| Men | |||||||||
| No OSA | |||||||||
| N = 374/55.0 ± 9.2yrs | |||||||||
| OSA | |||||||||
| N = 286/59.0 ± 8.8yrs | |||||||||
| Women | |||||||||
| No OSA | |||||||||
| N = 156/60.5 ± 7.9yrs | |||||||||
| OSA | |||||||||
| N = 63/63 ± 6.9yrs | |||||||||
| 15 | Katsumata2020 [42] | Japan | Cross-sectional study | CAD | OSA | N = 178 | Polysomnography | Elevated SNX16-Ab level associated with the history of CAD. | Combie high quality |
| Healthy adults | |||||||||
| N = 64/42.5yrs | |||||||||
| OSA N = 82/59yrs | |||||||||
| ACS N = 96/67yrs | |||||||||
| 16 | Kazukauskiene2022 [37] | Lithuania | Cross-sectional study | CAD | OSA | N = 328/57 ± 10yrs | Polysomnography | CAD males with OSA and clinically elevated NT-proBNP levels experienced inferior psychomotor performance | Combie high quality |
| OSA | |||||||||
| N = 75/59.7 ± 8.1yrs | |||||||||
| No OSA | |||||||||
| N = 253/56.1 ± 10.1yrs | |||||||||
| 17 | Khan2014 [34] | Pakistan | Cross-sectional study | CAD | OSA | N = 400 | Berlin questionnaire | A significant proportion of CAD patients are at high risk of OSA in Pakistan. Moreover, OSA is also associated with greater levels of anxiety in CAD patients | Combie high quality |
| CAD | |||||||||
| N = 200/55.07 ± 6.88yrs | |||||||||
| Healthy | |||||||||
| N = 200/55.19 ± 6.69yrs | |||||||||
| 18 | Labeix2022 [46] | Sweden | RCT | CAD | OSA | N = 45 | PSQI | A specific IMT during cardiac rehabilitation contributes to reduce significantly AHI in CAD patients with moderate OSA. | CoB |
| Control | |||||||||
| N = 23/59.3 ± 10.3yrs | |||||||||
| IMT | |||||||||
| N = 22/61.0 ± 8.4yrs | |||||||||
| 19 | Li2020 [44] | China | Cross-sectional study | CAD | OSA | N = 154/54.9 ± 9.4yrs | Portable cardiorespiratory monitoring device | Plasma CTRP9 levels were independently related to the prevalence of moderate/severe OSA in patients with CAD. | Combie high quality |
| Moderate/severe OSA | |||||||||
| N = 89/54.4 ± 8.8yrs | |||||||||
| No/mild OSA | |||||||||
| N = 65/55.1 ± 10.3yrs | |||||||||
| 20 | Milleron2004 [45] | France | Long-term prospective cohort study | CAD | OSA | N = 54/57.3 ± 10.1yrs | Polysomnography | The treatment of OSA in CAD patients is associated with a decrease in the occurrence of new cardiovascular events, and an increase in the time to such events. | NOS high quality |
| OSA-treated | |||||||||
| N = 25/57.7 ± 10.1yrs | |||||||||
| OSA-untreated | |||||||||
| N = 29/57.0 ± 10.2yrs | |||||||||
| 21 | Strehmel2016 [38] | Germany | Cross-sectional study | CAD | OSA | N = 41 | Polysomnography | CPAP has the potential to normalize elevated NT-proBNP serum levels in patients with severe OSA and coexisting CAD. Levels of NT-proBNP and hs-TropT correlated with AHI and oxygen desaturation | Combie high quality |
| OSA + CAD | |||||||||
| N = 21/61 ± 11yrs | |||||||||
| OSA | |||||||||
| N = 20/54 ± 12yrs | |||||||||
| 22 | Thunström2015 [40] | Sweden | Cross-sectional study | CAD | OSA | N = 439 | Cardiorespiratory Polygraphy | Obstructive sleep apnea with oxygen desaturation index ≥5 was independently associated with increased inflammatory activity in this nonobese coronary artery disease cohort. | Combie high quality |
| CAD + nonobese + OSA | |||||||||
| N = 234/65.3 ± 7.1yrs | |||||||||
| CAD + nonobese + nonOSA | |||||||||
| N = 95/61.4 ± 9.5yrs | |||||||||
| CAD + obese + OSA | |||||||||
| N = 110/62.9 ± 8.6yrs | |||||||||
| Hypertension | |||||||||
| 1 | Akintunde2014 [53] | Nigeria | Cross-sectional study | HTN | OSA | N = 104 | Berlin questionnaire | OSA is associated with significant additional left ventricular changes in hypertensive subjects. | Combie high quality |
| Low risk of OSA | |||||||||
| N = 49/58.8 ± 12.6 | |||||||||
| High risk of OSA | |||||||||
| N = 55/58.6 ± 11.2 | |||||||||
| 2 | Ayanaw2022 [47] | Italy | Cross-sectional study | HTN | Sleep quality | N = 563/65yrs | PSQI | More than one-third of the study participants had poor sleep quality. | Combie high quality |
| 3 | Bacci2017 [54] | Brazil | Cross-sectional study | HTN | OSA Sleep quality |
N = 43/52.9 ± 14.5yrs | PSQI ESS BQ | Patients at high risk for OSAHS had poor sleep quality and high levels of DBP. | Combie high quality |
| 4 | Bengtsson Boström2010 [55] | Sweden | Cross-sectional study | HTN | OSA | N = 170 | Polysomnography | Hypertensive men carrying the Arg389Arg genotype had higher crude and age-adjusted AHI than carriers of the Arg389Gly/Gly389Gly genotypes | Combie mid quality |
| Normotension | |||||||||
| N = 96/60 ± 6.3yrs | |||||||||
| HTN | |||||||||
| N = 74/62 ± 6.2yrs | |||||||||
| 5 | Cai2017 [50] | China | Cross-sectional study | HTN | OSA | N = 971 | Polysomnography | In a Chinese hypertensive population, OSA prevalence is strikingly high. Hypertensive subjects with the most severe OSA are at greater cardiovascular risk. | Combie high quality |
| Without OSA | |||||||||
| N = 286/56.5 ± 13.3yrs | |||||||||
| With OSA | |||||||||
| N = 685/59.3 ± 11.7yrs | |||||||||
| 6 | Cai2022 [57] | China | Single-center, observational, Retrospective cohort study |
HTN | OSA | N = 2067/49.51 ± 10.73 yrs | Polysomnography | There was a positive association between CMI levels and the risk of new-onset CVD in patients with hypertension and OSA. | NOS high quality |
| CMI˂0.73 | |||||||||
| 52.13 ± 11.41yrs | |||||||||
| 0.73–1.21 | |||||||||
| 49.05 ± 10.87yrs | |||||||||
| ≥1.21 | |||||||||
| 47.52 ± 9.43yrs | |||||||||
| 7 | Chang2013 [59] | China | Retrospective cohort study | HTN | Insomnia | N = 4063 | ICD-9-CM | The use of bisoprolol and atenolol was associated with the lowest risk of insomnia in elderly patients, as compared to propranolol. β-blockers with high selectivity in β1-receptors and/or low lipophilicity were associated with a lower risk of insomnia | NOS high quality |
| Propranolol user | |||||||||
| N = 760/73.3 ± 6.3yrs | |||||||||
| Non-propranolol user | |||||||||
| N = 3303/72.3 ± 6.1yrs | |||||||||
| 8 | Chaudhary2023 [51] | India | Cross-sectional study | HTN | OSA | N = 179/52.07 ± 11.40yrs | Polysomnography | More than half (53.1%) of the patients enrolled in the study had OSA. More than half of our hypertensive patients had OSA. These two conditions often co-exist and are known as a dangerous pair. | Combie high quality |
| 9 | Chen2020 [64] | China | RCT | HTN | OSA | N = 60/18–75yrs | Polysomnography | The CPAP treatment did not show significant ambulatory BP lowering effect in patients with moderate-severe OSAS and nocturnal hypertension. However, it may be effective in lowering daytime BP in patients with a faster pulse rate. | CoB |
| 10 | Ching2023 [52] | Malaysia | Cross-sectional study | HTN | OSA | N = 410/56.4 ± 11.3yrs | Polysomnography | The prevalence of probable OSA among patients with hypertension was 54.4%. | Combie high quality |
| Probable OSA | |||||||||
| N = 223/58.3 ± 10.6yrs | |||||||||
| Non-probable OSA | |||||||||
| N = 187/54.2 ± 11.7yrs | |||||||||
| 11 | Friedman2010 [48] | Canada | Case-control study | Drug-resistant hypertension | Sleep quality | N = 156 | Polysomnography | Compared to subjects with CH or normotension, those with RH have shorter total and REM sleep times and lower sleep efficiency independently of OSA. These data suggest that reduced sleep time may contribute to the severity of hypertension | NOS high quality |
| Normotension | |||||||||
| N = 40/52.2 ± 9.9yrs | |||||||||
| Control HTN | |||||||||
| N = 54/58.5 ± 11.3yrs | |||||||||
| Resistant HTN | |||||||||
| N = 62/58.9 ± 10.8yrs | |||||||||
| 12 | Gaddam2010 [62] | USA | Prospective cohort study | Resistant hypertension | OSA | N = 12/56.5 ± 6.5yrs | Polysomnography | This study provides preliminary evidence that treatment with a mineralocorticoid receptor antagonist substantially reduces the severity of OSA. |
NOS mid quality |
| 13 | Gonzaga2010 [61] | USA | Retrospective study | HTN | OSA | N = 109/55.9 ± 9.1yrs | Polysomnography | Severity of OSA was greater in those patients with hyperaldosteronism and related to the degree of aldosterone excess. | NOS mid quality |
| High aldosterone level | |||||||||
| N = 31/56.4 ± 7.8 | |||||||||
| Normal aldosterone level | |||||||||
| N = 78/55.7 ± 9.7 | |||||||||
| 14 | Jafari2013 [56] | USA | Cross-sectional study | HTN | OSA | N = 95 | Polysomnography | These data show that patients with OSA and hypertension have marked impairment of FMD, independent of hypoxia exposure, which is associated with increased sEng. | Combie mid quality |
| Non-OSA | |||||||||
| Normotension | |||||||||
| N = 19/47.5 ± 2.1yrs | |||||||||
| HTN | |||||||||
| N = 13/45.7 ± 2.3yrs | |||||||||
| OSA | |||||||||
| Normotension | |||||||||
| N = 27/47.9 ± 2.2yrs | |||||||||
| HTN | |||||||||
| N = 36/56.1 ± 1.4yrs | |||||||||
| 15 | Li2021 [49] | Germany | Prospective cohort study | HTN | Sleep quality | N = 1959/25–65 yrs | A three-point Likert response scale | Our findings add a new piece of evidence that work stress together with impaired sleep increase risk of coronary and cardiovascular mortality in hypertensive workers. | NOS high quality |
| 16 | Lui2021 [63] | China | RCT | HTN | OSA | N = 92/53.2 ± 8.7yrs | Polysomnography | In a cohort with OSA and multiple cardiovascular risk factors including difficult-to-control hypertension, short-term CPAP treatment improved ambulatory BP, and alleviated subclinical myocardial injury and strain. | CoB |
| CPAP | |||||||||
| N = 46/52.5 ± 9.0yrs | |||||||||
| Control | |||||||||
| N = 46/53.9 ± 8.4yrs | |||||||||
| 17 | Martínez-García 2013 [65] |
Spain | RCT | Resistant hypertension | OSA | N = 194/56.0 ± 9.5yrs | Respiratory polygraphy | Among patients with OSA and resistant hypertension, CPAP treatment for 12 weeks compared with control resulted in a decrease in 24-h mean and diastolic blood pressure and an improvement in the nocturnal blood pressure pattern. | CoB |
| Control | |||||||||
| N = 96/58.2 ± 9.6yrs | |||||||||
| CPAP | |||||||||
| N = 98/57.8 ± 9.5yrs | |||||||||
| 18 | Wolf2016 [60] | Poland | Cross-sectional study | HTN | OSA | N = 88 | Polysomnography | Beta-blockers do not potentiate apnea-induced HR decelerations, attenuate apnea-induced increases in heart rate and do not influence incidence of ectopies and conduction abnormalities in patients with hypertension and moderate-to-severe, untreated OSA | Combie high quality |
| BB− | |||||||||
| N = 32/55 (46–63)yrs | |||||||||
| BB+ | |||||||||
| N = 56/57.5 (54–62)yrs | |||||||||
| 19 | Zamarrón2008 [58] | Spain | Cross-sectional study | HTN | OSA | N = 96/53.3 ± 8.2yrs | Polysomnography | OSAS patients presented higher circulating levels of PAI than the control group, which was even greater when patients had associated hypertension. | Combie high quality |
| Control | |||||||||
| N = 32/48.2(43.6, 52.9)yrs | |||||||||
| OSAS | |||||||||
| N = 32/52.7(49.9,55.4)yrs | |||||||||
| OSAS + HT | |||||||||
| N = 32/54.1 (50.6, 57.6)yrs | |||||||||
| Heart failure | |||||||||
| 1 | Abdelbasset2020 [79] | Egypt | Pilot study | HF | Sleep disturbance | N = 8/69.4 ± 4.2 yrs | PSQI | Low-intensity exercise program five sessions weekly for four weeks. low-intensity aerobic exercise may improve the quality of sleep and ventilator efficiency in elderly HF patients. | MINORS mid quality |
| 2 | Alosco2013 [68] | USA | Cross-sectional study | HF | Sleep quality | N = 53/69.81 ± 8.79yrs | PSQI | 75.5% of HF patients reported impaired sleep. Decreased cerebral perfusion and greater WMH may contribute to sleep difficulties in HF. | Combie high quality |
| 3 | Arzt2017 [69] | Germany | Cross-sectional study | HFrEF | SDB | N = 1557 | Polysomnography | Prevalence of SDB in HFpEF, HFmrEF and HFrEF (36%, 41% and 48%, respectively). The prevalence of coexisting OSA-CSA, OSA and CSA were 40%, 29% and 31% in patients with HFrEF respectively. |
Combie high quality |
| OSA | |||||||||
| N = 452/66 ± 11yrs | |||||||||
| OSA-CSA | |||||||||
| N = 624/69 ± 10yrs | |||||||||
| CSA | |||||||||
| N = 481/69 ± 10yrs | |||||||||
| 4 | Avci2021 [70] | Turkey | Cross-sectional study | HF | Sleep quality | N = 95/75.44 ± 6.36yrs | PSQI | Elderly patients with HF experienced significant sleep problems and that their sleep quality decreased as the depression symptom levels increased. | Combie high quality |
| 5 | Awotidebe2017 [66] | Nigeria | Case-control study | CHF | Sleep quality | N = 100 | Pittsburgh Sleep | Patients with heart failure demonstrated lower functional capacity and poorer sleep quality. | NOS high quality |
| Patient | |||||||||
| N = 50/57.8 ± 8.9yrs | |||||||||
| Control | |||||||||
| N = 50/54.9 ± 7.9yrs | |||||||||
| 6 | Beres2022 [80] | Romania | Prospective, mono-center, cohort study | HF | CSA | N = 36/65.7 ± 10.8yrs | Polysomnography | The association of PAP therapy with drug therapy in patients with HFrEF and CSAS improves hemodynamic parameters and quality of life. | NOS mid quality |
| 7 | Bhalla2020 [71] | India | Prospective cohort study | CHF | OSA | N = 77/30–80 yrs | Polysomnography | The prevalence of OSA in CHF was 50.6%. Predictors of OSA in CHF were left ventricular ejection fraction (LVEF) 20%–30% and NYHA class 2. | NOS high quality |
| 8 | Bitter2011 [67] | Germany | Prospective cohort study | CHF | OSA | N = 255 | Cardiorespiratory polygraphy | In patients with CHF, CSA and OSA are independently associated with an increased risk for ventricular arrhythmias and appropriate cardioverter-defibrillator therapies. | NOS high quality |
| AHI≥5h-1 | |||||||||
| OSA | |||||||||
| N = 82/67.9 (63.9,73.7)yrs | |||||||||
| CSA | |||||||||
| N = 87/68.4 (62.1,72.4)yrs | |||||||||
| noSDB | |||||||||
| N = 86/65.7 (56.5,71.6)yrs | |||||||||
| 9 | Bughin2021 [76] | France | A longitudinal study | HF | Sleep patterns | N = 119/69yrs | PSQI ESS ISI BQ |
CNS drugs intake and decreased total sleep time were independently associated with an increased risk of MACE in patients with HF. | MINORS high quality |
| 10 | Calvin2014 [72] | USA | Prospective cohort study | HF LVEF≤35% | CSA | N = 46 | Polysomnography | Increased LAVI is associated with heightened CO2 chemosensitivity and greater frequency of CSA. | NOS mid quality |
| HF without CSA | |||||||||
| N = 21/59.3 ± 9.9yrs | |||||||||
| HF with CSA | |||||||||
| N = 25/68.5 ± 8.1yrs | |||||||||
| 11 | Calvin2010 [73] | USA | Prospective cohort study | HF | CSA | N = 51 | Polysomnography | In non-anaemic HF patients, advanced HF and hypoxaemia due to CSA may each be independently associated with increased serum EPO concentration. | NOS mid quality |
| Healthy controls | |||||||||
| N = 18/54.7 ± 16.8yrs | |||||||||
| HF without CSA | |||||||||
| N = 15/59.9 ± 12.0yrs | |||||||||
| HF with CSA | |||||||||
| N = 14/65.7 ± 10.7yrs | |||||||||
| 12 | Calvin2011 [74] | USA | Prospective cohort study | HF | CSA | N = 33 | Polysomnography | ANP and BNP concentrations performed similarly for detection of CSA; low concentrations appear associated with low risk for CSA in men. | NOS mid quality |
| HF without CSA | |||||||||
| N = 9/61.1 ± 12.4yrs | |||||||||
| HF with CSA | |||||||||
| N = 24/66.7 ± 9.9yrs | |||||||||
| 13 | Cundrle2018 [77] | USA | Cross-sectional study | HF | CSA | N = 56/65 ± 10yrs | Polysomnography | Low leptin concentration may have utility for the screening of heart failure patients for central sleep apnea. | Combie mid quality |
| CSA | |||||||||
| N = 18/67 ± 10yrs | |||||||||
| Mixed apnea | |||||||||
| N = 15/64 ± 10yrs | |||||||||
| No apnea | |||||||||
| N = 11/59 ± 10yrs | |||||||||
| OSA | |||||||||
| N = 12/68±8yrs | |||||||||
| 14 | Ferreira2020 [75] | Germany | RCT | HF | OSA | N = 749/69 ± 10 yrs | Not mentioned | Three biomarkers added significant prognostic information on top of the best clinical model: soluble suppression of tumorigenicity 2 (primary outcome), Notch-3 (CV and all-cause death), and GDF-15 (all-cause death). | CoB |
| Control | |||||||||
| N = 368/69.1 ± 10.2yrs | |||||||||
| ASV | |||||||||
| N = 381/69.3 ± 9.4yrs | |||||||||
| 15 | Gerçek2022 [78] | Germany | Retrospective study | HF | SDB | N = 146 | Polysomnography | SDB treatment in HF patients with ICD leads to significant improvements in VT burden, ATP and shock therapy, and may even affect survival. | NOS high quality |
| Control | |||||||||
| N = 73/67.67 ± 10.78 yrs | |||||||||
| SDB-treated | |||||||||
| N = 73/67.2 ± 10.10 yrs | |||||||||
| 16 | Redeker2022 [81] | USA | RCT | HF | Insomnia | N = 175/63 ± 12.9 yrs | PSQI | CBT-I produced sustained improvements in insomnia, fatigue, daytime sleepiness, and objectively measured physical function among adults with chronic HF, compared with a robust HF self-management program that included sleep hygiene education. | CoB |
| Healthy sleep | |||||||||
| N = 91/62.0 ± 13.1yrs | |||||||||
| Healthy heart | |||||||||
| N = 84/64.1 ± 12.6yrs | |||||||||
| Cardiac arrhythmia | |||||||||
| 1 | Abumuamar2018 [82] | Canada | Prospective cohort study | AF | OSA | N = 123/63.6 ± 13.3yrs | Polysomnography | OSA was detected in 85% of these patients. | NOS high quality |
| 2 | Abumuamar2019 [91] | Canada | Prospective cohort study | AF | OSA | N = 100/63 ± 13yrs | Polysomnography | There is a significant decrease in atrial and ventricular ectopy count/24 h in patients with AF and OSA at 3 and 6 months of CPAP treatment compared to baseline. | NOS high quality |
| OSA | |||||||||
| N = 85/65 ± 13yrs | |||||||||
| Non-OSA | |||||||||
| N = 15/55 ± 14yrs | |||||||||
| 3 | Albuquerque2012 [83] | USA | Prospective cohort study | AF | SDB | N = 151/69.1 ± 11.7yrs | Polysomnography | The prevalence of SDB in this population was 81.4%. | NOS mid quality |
| No EDS | |||||||||
| N = 98/70.1 ± 1.2yrs | |||||||||
| EDS | |||||||||
| N = 53/67.1 ± 1.6yrs | |||||||||
| 4 | Anter2017 [87] | USA | Cross-sectional study | PAF | OSA | N = 184 | polysomnography or a home sleep apnea testing device | In patients with paroxysmal AF, OSA is associated with structural and functional atrial remodeling and increased incidence of extra-PV triggers. | Combie mid quality |
| (+)OSA(+)PVI(+) Triggers | |||||||||
| N = 43/49 ± 12yrs | |||||||||
| (−)OSA(+)PVI(+) Triggers | |||||||||
| N = 43/54 ± 14yrs | |||||||||
| (−)OSA(+)PVI(−) Triggers | |||||||||
| N = 48/59 ± 12yrs | |||||||||
| (+)OSA(+)PVI(−) Triggers | |||||||||
| N = 50/51 ± 15yrs | |||||||||
| 5 | Bitter2009 [84] | Germany | Cross-sectional study | AF | SDB | N = 150/66.1 ± 1.7yrs | Cardiorespiratory polygraphy | Patients with AFib were found to have not only a high prevalence of obstructive sleep apnea, as has been described previously, but also a high prevalence of CSA/CSR. | Combie mid quality |
| CSA/CSR | |||||||||
| N = 47/64.1 ± 5.0yrs | |||||||||
| OSA | |||||||||
| N = 64/67.4 ± 2.1yrs | |||||||||
| No SDB | |||||||||
| N = 39/65.4 ± 4.8yrs | |||||||||
| 6 | Brgdar2021 [90] | USA | Retrospective study | AF | OSA | N = 156,521 | ICD-10 | Although OSA is highly prevalent in AF patients, inpatient mortality and cardiovascular outcomes such as cardiac arrest, stroke, or major bleeding were similar in AF patients with or without concomitant OSA with no significant differences in length of stay. | NOS high quality |
| Pre-match | |||||||||
| OSA | |||||||||
| N = 23,678/65.18 ± 10yrs | |||||||||
| Non-OSA | |||||||||
| N = 132,843/71.4 ± 13yrs | |||||||||
| Post-match | |||||||||
| OSA | |||||||||
| N = 23,678/65.18 ± 12.7yrs | |||||||||
| Non-OSA | |||||||||
| N = 23,678/65.09 ± 12.7yrs | |||||||||
| 7 | Cang2023 [86] | China | Retrospective cohort study | AF | Sleep quality | N = 416 | Questionnaire Self-reported sleep pattern |
Sleep disorders such as inadequate sleep time (time <7 h or >8 h), insomnia and excessive sleepiness during daytime were associated with a higher risk of recurrence. | NOS high quality |
| Healthy sleep score 0-1 | |||||||||
| N = 20/63.50 ± 7.99yrs | |||||||||
| Healthy sleep score 2-3 | |||||||||
| N = 188/63.65 ± 9.55yrs | |||||||||
| Healthy sleep score 4-5 | |||||||||
| N = 208/63.15 ± 9.72yrs | |||||||||
| 8 | Dalgaard2020 [88] | USA | Retrospective cohort study | AF | OSA | N = 22,760/73.0 (65.0–80.0)yrs | A medical history and prior diagnosis. | Among patients with AF, OSA is an independent risk factor for MACNE and, more specifically, stroke/SE. | NOS high quality |
| OSA | |||||||||
| N = 4045/68.0 (61.0–75.0)yrs | |||||||||
| No OSA | |||||||||
| N = 18,715/74.0 (66.0–81.0)yrs | |||||||||
| 9 | Kayrak2013 [85] | Turkey | Case-control study | AF | Sleep quality | N = 303 | PSQI | Patients with AF have shorter sleep duration and poor SQ. Maintenance of sinus rhythm after DCC may have a favorable effect on the SQ of patients with AF. Nevertheless, AF is an independent predictor of poor SQ. | NOS high quality |
| AF | |||||||||
| N = 153/63 ± 12yrs | |||||||||
| Control | |||||||||
| N = 150/61 ± 14yrs | |||||||||
| 10 | Tang2009 [89] | China | Prospective cohort study | paroxysmal AF | OSA | N = 178 | Berlin questionnaire | The recurrence rate and incidence of complications did not differ in patients with different risk profiles for OSA. | NOS high quality |
| Low risk of OSA | |||||||||
| N = 74/56 ± 12yrs | |||||||||
| High risk of OSA | |||||||||
| N = 104 58 ± 11yrs | |||||||||
| Other CVD | |||||||||
| 1 | Abe2009 [96] | Japan | Cross-sectional study | HF with MV and/or AV Valve repair surgery |
SAS (OSA, CSA) | N = 150 | Polysomnography | The treatment led to a significant improvement in PCWP and mean PAP, and CSA-AI, improvement of cardiac function with valvular surgery reduces the severity of CSA in HF patients with valvular heart diseases. | Combie high quality |
| mild-to-no SA | |||||||||
| N = 47/66.0 ± 11.4yrs | |||||||||
| SAS | |||||||||
| N = 103/69.5 ± 8.8yrs | |||||||||
| 2 | Amofah2016 [95] | Sweden | Prospective cohort study | SAVR or TAVI surgery | Sleep quality | N = 143/83 ± 2.7yrs | Self-reports actigraphy MISS |
In patients undergoing SAVR or TAVI, sleep evolves differently during the in-hospital postoperative phase. | NOS high quality |
| SAVR | |||||||||
| N = 78/82 ± 2.0yrs | |||||||||
| TAVI | |||||||||
| N = 65/85 ± 2.8yrs | |||||||||
| 3 | Banno2004 [92] | Japan | Cross-sectional study | Idiopathic cardiomyopathy (DCM, HCM) | SDB | N = 35 | PSG | Of these 35, 16 (80%) of the DCM patients and 7 (47%) of the HCM patients had sleep-disordered breathing. | Combie mid quality |
| DCM | |||||||||
| CSAHS | |||||||||
| N = 10/48.4 ± 14.2yrs | |||||||||
| OSAHS | |||||||||
| N = 6/43.3 ± 9.5yrs | |||||||||
| non-SAHS | |||||||||
| N = 4/47.0 ± 14.0yrs | |||||||||
| HCM | |||||||||
| OSAHS | |||||||||
| N = 7/48.4 ± 8.3yrs | |||||||||
| non-SAHS | |||||||||
| N = 8/47.8 ± 15.7yrs | |||||||||
| 4 | Biener2023 [97] | Germany | Prospective cohort study | Mitral regurgitation Mitral valve repair surgery | SDB | N = 53/76.0 ± 8.5yrs | polygraphy | TMVR may be a suitable therapy not only for MR but also for the accompanying CSA. LAVI may be a useful indicator for CSA in patients with MR. | NOS high quality |
| SDB | |||||||||
| N = 36/75.8 ± 8.1yrs | |||||||||
| No SDB | |||||||||
| N = 17/76.5 ± 9.6yrs | |||||||||
| 5 | Bodez2016 [93] | France | Cross-sectional study | Cardiac Amyloidosis | SDB | N = 70/71 ± 12 yrs | nocturnal polygraphy | In CA population, prevalence of SDB is high (90%) and dominated by the obstructive pattern. | Combie high quality |
| AL | |||||||||
| N = 31/65 ± 12yrs | |||||||||
| m-TTR | |||||||||
| N = 22/71 ± 12yrs | |||||||||
| WT-TTR | |||||||||
| N = 17/81±7yrs | |||||||||
| 6 | Roggenbach2014 [98] | Germany | Prospective cohort study | Elective cardiac surgery | SDB | N = 92/67.5 ± 8.9yrs | Polygraphic recordings | Preoperative SDB were strongly associated with postoperative delirium, and may be a risk factor for postoperative delirium. | NOS mid quality |
| No postoperative delirium | |||||||||
| N = 48/64.5±9yrs | |||||||||
| Postoperative delirium | |||||||||
| N = 44/70.8 ± 7.8yrs | |||||||||
| 7 | Javaherforooshzadeh 2022 [100] |
Iran | Prospective cohort study | cardiac surgery | OSA | N = 306 | STOP-Bang questionnaire | OSA is common in patients undergoing cardiac surgery. Our findings indicate that these patients manifest a higher incidence of postoperative complications compared to those with a lower risk of OSA. | NOS high quality |
| Low risk | |||||||||
| N = 33/54.5 ± 12.9yrs | |||||||||
| Intermediate risk | |||||||||
| N = 100/59.1 ± 10.2yrs | |||||||||
| High risk | |||||||||
| N = 173/60.1 ± 8.6yrs | |||||||||
| 8 | Tafelmeier2019 [99] | Germany | Prospective cohort study | cardiac surgery | SDB | N = 141/68±9yrs | Polysomnography | Among the established risk factors for delirium, central sleep apnoea was independently associated with delirium. Our findings contribute to identifying patients at high risk of developing post-operative delirium who may benefit from intensified delirium prevention strategies. | NOS high quality |
| 9 | Xu2021 [94] | China | Cross-sectional study | hypertrophic cardiomyopathy | OSA | N = 589/50.5 ± 12.8yrs | Polysomnography | Data from clinical characteristics and polysomnography studies were recorded. OSA was present in 346 patients (58.7%). Patients who had OSA were older, more likely to be male and had more clinical comorbidities such as hypertension, atrial fibrillation and cardiac remodeling | Combie high quality |
| No OSA | |||||||||
| N = 243/45.7 ± 13.7yrs | |||||||||
| OSA | |||||||||
| N = 346/53.8 ± 11.1yrs | |||||||||
| 10 | Foldvary-Schaefer 2015 [102] |
USA | Prospective cohort study | Cardiovascular Surgery | OSA | N = 107/67.3 ± 13.3yrs | Polysomnography | OSA is highly prevalent in patients undergoing cardiovascular surgery. It could not be shown that OSA was significantly associated with adverse postoperative outcomes, but this may have been due to an insufficient number of subjects. | NOS mid quality |
| AHI˂15 | |||||||||
| N = 56/65.1 ± 13.8yrs | |||||||||
| AHI≥15 | |||||||||
| N = 51/69.7 ± 12.5yrs | |||||||||
| 11 | Ding2016 [101] | China | A Prospective Single-Center Study | Cardiac Valve Replacement Surgery |
OSA | N = 290/51.4 ± 10.4yrs | polysomnography | RVHD patients with OSA have an increased incidence of perioperative adverse events. OSA was independently associated with overall postoperative recovery, respiratory insufficiency, and higher rate of postoperative pacemaker use, while CSA was not associated with postoperative events. | NOS mid quality |
| No SDB | |||||||||
| N = 175/49.13 ± 10.51yrs | |||||||||
| OSA | |||||||||
| N = 54/54.43 ± 9.49yrs | |||||||||
| CSA | |||||||||
| N = 61/55.20 ± 9.05yrs | |||||||||
ACS: acute coronary syndrome; AF: atrial fibrillation; AL: light-chain amyloidosis; AMI: acute myocardial infarction; AV: aortic valvular; ASV: adaptive servo-ventilation.
BQ: Berlin Questionnaire.
CHF: chronic heart failure; CoB: Cochrane Risk of Bias; CRT-D:cardioverter-defibrillator; CSA:central sleep apnea.
DCC: direct current cardioversion; DCM: dilated cardiomyopathy; ESS: Epworth Sleepiness Scale; EDS: excessive daytime sleepiness.
HCM: hypertrophic cardiomyopathy; HFrEF: HF with reduced ejection fraction; HTN: hypertension; HTN: hypertension; IMT: inspiratory muscle training; ISI: Insomnia Severity Scale.
LAVI: left atrial volume index; LVEF: left ventricular ejection fraction.
MDD: major depressive disorder; MISS: Minimal Insomnia Symptom Scale; MHR: monocyte to high-density lipoprotein ratio; MV: mitral valvular.
MINORS: methodological index for non-randomized studies; m-TTR: hereditary transthyretin amyloidosis.
NOS: Newcastle-Ottawa (cohort) scale; NSTEMI: non-ST elevation acute myocardial infarction.
OSA: obstructive sleep apnea.
PAF: paroxysmal AF; PAP: Positive respiratory pressure therapy.
PSQI: Pittsburgh Sleep Quality Index.
RCT: randomized controlled trial; RVHD: rheumatic valvular heart disease.
SAVR: surgical aortic valve replacement; SAS: sleep apnea syndrome; SA: sleep apnea; SR: sinus rhythm; SSD: Short sleep duration.
TAVI: transcatheter aortic valve implantation; TMVR: transcatheter mitral valve repair.
UA: unstable angina; WT-TTR: wild-type transthyretin amyloidosis.
3.3. Sleep disorders in patients with CAD
In this review, 22 studies [[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] focused on sleep disorders in patients with CAD. Three studies [[25], [26], [27]] reported a high prevalence of sleep disorders in patients with CAD. Patients with acute myocardial infarction (AMI) had a higher prevalence of OSA than those with stable CAD (64% vs. 35%). Four studies [[28], [29], [30], [31]] elucidated the adverse effects of sleep disorders on cardiac function and reported increased adverse cardiac outcomes in patients with CAD and OSA. Seven studies [[32], [33], [34], [35], [36], [37], [38]] uncovered an association between psychological conditions and sleep disorders in patients with CAD. The personality disorders included type D personality, depression, anxiety, and cognition. Six studies [[39], [40], [41], [42], [43], [44]] reported several biomarkers that were implicated in predicting sleep disorders in patients with CAD, such as placental growth factor (PIGF), vascular endothelial growth factor family (VEGF), high sensitivity C-reactive protein (hs-CRP) et al. Three studies [38,45,46] reported that patients with CAD benefited from sleep disorder therapies, namely CPAP and inspiratory muscle training (IMT).
3.4. Sleep disorders in patients with hypertension
In this review, 19 studies [[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65]] focused on sleep disorders in patients with hypertension. Eight studies [[47], [48], [49], [50], [51], [52], [53], [54]] reported the prevalence (>30%) and adverse cardiac outcomes of sleep disorders in hypertensive patients. Four studies [[55], [56], [57], [58]] uncovered the potential mechanisms of hypertension combined with OSA and listed several biomarkers implicated in predicting sleep disorders in hypertensive patients, such as endoglin (sEng), fms-like tyrosine kinase-1 (sFlt-1), and plasminogen activator inhibitor-1 (PAI-1). Four studies [[59], [60], [61], [62]] illustrated the effects of antihypertensive drugs on poor sleep. Three studies [[63], [64], [65]] highlighted the beneficial effects of CPAP in the treatment of hypertensive patients with OSA.
3.5. Sleep disorders in patients with HF
In this review, 16 studies [[66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81]] focused on sleep disorders in patients with HF were included. Four studies [[66], [67], [68], [69]] showed that sleep disorders were highly prevalent (31–75.5%) in patients with HF. One study [70] revealed an association between depression and sleep quality in patients with HF. Five [[71], [72], [73], [74], [75]] studies explored several potential predictors of sleep disorders in patients with HF, including left ventricular ejection fraction (LVEF), left atrial volume index (LAVI), and erythropoietin (EPO). Two studies [76,77] showed that sedative-hypnotics for treating sleep disorders presented inconsistent effects in patients with HF, either positive or negative. Four studies [[78], [79], [80], [81]] reported several non-drug therapies, including CPAP and CBT-I, for treating sleep disorders in patients with HF.
3.6. Sleep disorders in patients with cardiac arrhythmia
In this review, ten studies [[82], [83], [84], [85], [86], [87], [88], [89], [90], [91]] focused on sleep disorders in patients with cardiac arrhythmias. Nine studies [[82], [83], [84], [85], [86], [87], [88], [89], [90]] reported the prevalence and cardiac outcomes of sleep disorders in patients with arrhythmias. One study [91] demonstrated the important role of CPAP in treating patients with AF and OSA.
3.7. Sleep disorders in patients with other CVD
In this review, 11 studies [[92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102]] focused on sleep disorders in patients with other CVDs such as cardiomyopathy, mitral regurgitation, and cardiac surgery. Three studies [[92], [93], [94]] reported the prevalence of sleep disorders in patients with cardiomyopathy. Other studies [[95], [96], [97], [98], [99], [100], [101], [102]] have elucidated the prevalence and adverse effects of sleep disorders on cardiac function after cardiac surgery.
3.8. Study quality assessment
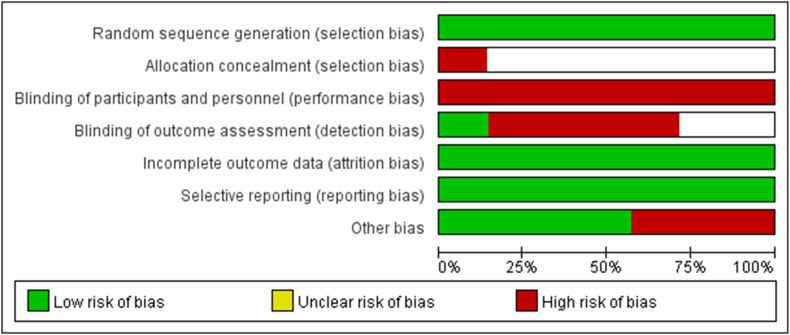
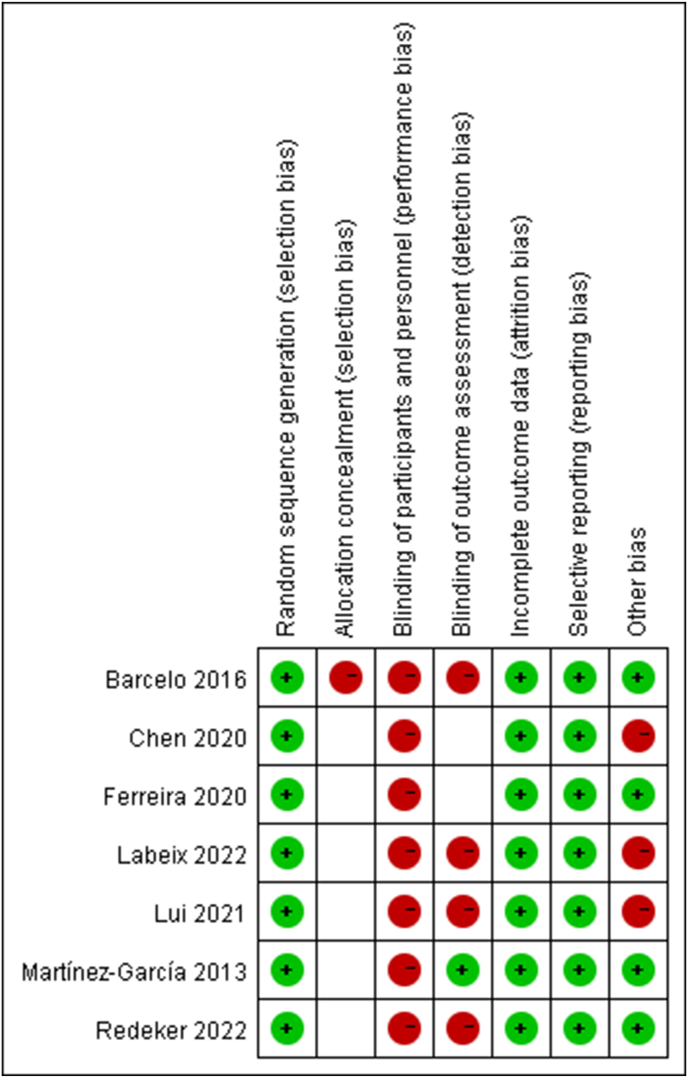
Seven studies were evaluated by CoB, and the risk of bias was shown in Fig. 2, Fig. 3. Thirty-six studies were assessed by NOS, including 26 studies of high quality and 10 studies of mid quality. Thirty-three studies were assessed by Combie, including 28 studies of high quality and 5 studies of mid quality. Two studies were evaluated by MINORS, including 1 study of high quality and 1 study of mid quality (Table 1).
Fig. 2.
Risk of bias graph.
Fig. 3.
Risk of bias summary.
4. Discussion
This review comprised data on the prevalence, mechanisms, and therapies for sleep disorders in patients with CVD, including CAD, hypertension, HF, cardiac arrhythmia, and other CVD.
4.1. Sleep disorders in patients with CAD
Sleep disorders are considered risk factors for worsening cardiac function and increasing cardiac events in patients with CAD. Buchner et al. [28] found that patients with AMI and OSA had larger right atria and ventricles than those without OSA, implying that OSA may damage right heart function. A multinational, double-blind, placebo-controlled trial including data from 36 countries revealed that short sleep duration and OSA could predict adverse outcomes (major cardiac events and major adverse cardiac events) in patients with ACS after a median follow-up of 2.5 years [29]. In patients with unstable angina (UA) or non-ST-elevation acute myocardial infarction (NSTEMI), there is a high prevalence of cardiovascular events in the hospital, and this was a primary endpoint in those with a high probability of OSA [30]. In contrast, Araújo et al. [31] reported that OSA had no significant effect on myocardial ischemia or cardiac arrhythmia in patients with stable CAD. However, the conclusion may be biased due to the small sample size (CAD + OSA n = 30) and short study period. In a prospective Israeli study with a follow-up period of 68 months, Aronson et al. [27] showed that SDB did not affect clinical outcomes (involving HF, MI, UA, and death); however, they found a relationship between SDB and pulmonary artery systolic pressure.
Personality and psychosocial state may influence sleep quality in patients with CAD. Regardless of OSA, patients with CAD with type D personality and negative affectivity have worse sleep quality, which may be related to depression and anxiety [32]. Patients with CAD and major depressive disorder (MDD) have higher PSQI scores than those without [33]. An investigation in Pakistan showed a high prevalence of anxiety in patients with CAD and OSA, indicating a close relationship between anxiety and sleep disorders, especially in patients with CAD [34]. Sex differences in social status were associated with poor sleep quality and poor sleep quality-related adverse outcomes. Low education and income are related to poor sleep quality in women with CAD, but not in men [35]. Therefore, this highlights the health benefits of improving the social status of women. Another study [36] focused on sex specificity and cardiac prognosis in patients with AMI and poor sleep quality and showed that women with sleep disturbances had a higher risk of long-term cardiac events, while men with impaired awakening had short-term case fatality (death within 28 days of initial AMI). Sleep disorders also affect cognitive function in patients with CAD. Kazukauskiene et al. [37] evaluated the inflammatory factors and NT-proBNP levels in men with CAD and OSA. They found that OSA and NT-proBNP levels were associated with cognitive dysfunction. Patients with CAD, OSA, and higher NT-proBNP levels had lower digit span and digit symbol test scores, which were used to measure cognition [38].
Certain biomarkers may be effective for evaluating sleep disorders in patients with CAD. PlGF is an angiogenic protein that belongs to the VEGF family. PlGF plays a vital role in pathological angiogenesis, especially in cardiac tissues. According to a multicenter randomized controlled trial, higher PIGF levels can predict OSA and cardiovascular complications in patients with acute coronary syndrome (ACS) [39]. Inflammation and lipid metabolism are associated with CAD and OSA prognoses. Patients with CAD and OSA have higher high-sensitivity C-reactive protein (hs-CRP) and IL-6 levels [40]. The monocyte-to-high-density lipoprotein ratio (MHR), which reflects the inflammatory response during CVD, is considered a new predictor of CVD. Huang et al. [41] conducted a study on patients with CAD, uncovering the association between an elevated MHR and a high risk of OSA. Sorting nexins, which act on sorting membrane proteins, have been implicated in arteriosclerosis. SNX16-Ab levels are elevated in patients with CAD and OSA. Thus, SNX16-Ab may serve as a potential biomarker for predicting CAD in patients with OSA [42]. There is a high level of late-stage (8-isoprostane, 8-ISO) lipid peroxidation products in patients with CAD and poor sleep quality [43]. C1q tumor necrosis factor-related protein 9 (CTRP9) protects cardiac function. Lower CTRP9 levels increase the risk of OSA and cardiac dysfunction in patients with CAD [44].
Accumulating evidence has shown that the treatment of OSA benefits cardiac function and reduces adverse cardiac outcomes [45]. CPAP is regarded as the first-line treatment for OSA. CPAP therapy reduced the NT-proBNP concentration in patients with OSA [38]. A resistive loading device was used to perform IMT, which helps improve the function of specific upper airway dilator muscles. After receiving 6-week IMT, patients with CAD and moderate OSA showed improved inspiratory pressure (MIP) and reduced AHI [46].
4.2. Sleep disorders in patients with hypertension
A history of hypertension predicts future insomnia in older but not middle-aged adults [103]. More than one-third of hypertensive patients report poor sleep quality [47]. In particular, those with resistant hypertension have reduced sleep time and poor sleep quality [48]. The MONICA/KORA study, which included 1959 hypertensive workers, indicated that work stress, impaired sleep, and work stress combined with impaired sleep increased the risk of CVD mortality in hypertensive workers compared with that of those with low work stress and non-impaired sleep [49]. Patients with hypertension have a high prevalence of OSA [50]. Chaudhary et al. [51] reported that more than half of hypertensive patients suffer from OSA. This prevalence was similar to that reported in Ching's study [52]. OSA may influence the prognosis of hypertensive patients, by increasing the left ventricular mass, posterior wall, and interventricular septum [53]. Moreover, hypertensive patients with OSA have poorer sleep quality and higher diastolic blood pressure [54].
Based on potential pathophysiological mechanisms, there are some special markers that assist in the evaluation of OSA in hypertensive patients. β1-adrenergic receptor gene polymorphisms are correlated with OSA severity. Bengtsson et al. [55] found that the Arg389Arg genotype is associated with the apnea-hypopnea index (AHI) in men with hypertension, but not in women. Compared with those carrying the Arg389Gly/Gly389Gly genotypes, the AHI was higher in hypertensive men with the Arg389Arg genotype. Moreover, the inflammatory response and hypercoagulability can impair the endothelial function of blood vessels. Endothelial dysfunction is associated with hypertension and OSA [56]. Certain angiogenesis inhibitors, including sEng and fms-like tyrosine kinase-1 (sFlt-1), could reflect the inflammatory response. Patients with OSA have higher serum sEng and sFlt-1 levels, indicating impaired flow-mediated vasodilation (FMD). In addition, the cardiometabolic index (CMI) plays an important role in evaluating metabolic prognosis. This index is calculated using the waist-to-height ratio and triglyceride/high-density lipoprotein cholesterol ratio. Thus, the CMI could be used as an indicator to assess CVD risk in hypertensive patients [57]. Moreover, the coagulation system was activated by OSA. Furthermore, the levels of plasminogen activator inhibitor-1 (PAI-1), which is a procoagulant molecule that inhibits fibrinolysis, are much higher in patients with hypertension and OSA [58].
Nondrug therapy is vital for treating OSA in hypertensive patients. CPAP also reduces serum troponin I and brain natriuretic peptide levels in patients with OSA and hypertension [63]. Moreover, CPAP effectively lowered daytime blood pressure in patients with nocturnal hypertension and OSA [64]. Similarly, in a randomized clinical trial that included patients with resistant hypertension and OSA, Martínez-García et al. [65] showed that a 12-week CPAP treatment reduced the 24-h mean and diastolic blood pressure and improved the nocturnal blood pressure pattern. In addition, regular physical exercise is an excellent way to lower blood pressure in patients with hypertension or pre-hypertension [104].
4.3. Sleep disorders in patients with HF
Patients with HF frequently experience sleep problems [66], which may be due to insufficient cerebral perfusion. Sleep disorders increase the risk of ventricular arrhythmias and require appropriate cardioverter-defibrillator (CRT-D) implantation in patients with HF [67]. Alosco et al. [68] reported that 75.5% of patients with HF exhibit impaired sleep. The incidence of SDB was 36%, 41%, and 48% in patients with HF and preserved ejection fraction (HFpEF), HF and mid-range ejection fraction (HFmrEF), and HF and reduced ejection fraction (HFrEF), respectively. Meanwhile, there were differences in the SDB subtypes. Among patients with HFrEF, the incidence of central sleep apnea (CSA), OSA, and OSA-CSA was 31%, 29%, and 40%, respectively [69]. Poor sleep quality is also associated with depression, and sleep quality worsens as depressive symptoms become more severe [70].
Owing to the important role of SDB in cardiac function, suitable predictors of SDB in patients with HF are necessary. Indicators of heart function, such as LVEF and LAVI, are potential predictors. Bhalla et al. [71] found that LVEF (20–30%) and NYHA class 2 were predictors of OSA in patients with HF. Calvin et al. [72] showed that an increase in LAVI was associated with an increase in CSA incidence. Therefore, LAVI was used as an indicator of CSA in patients with HF. Serum biomarkers can also be used as predictors. EPO is a glycoprotein hormone involved in the pathogenesis of CVD and OSA. Elevated serum EPO levels are linked to the development of CSA-induced HF in patients with non-anemic HF [73]. Furthermore, among the many circulating protein biomarkers involved in the cardiovascular pathways, there are representative biomarkers of HF and sleep disorders. Atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) are important cardiac markers for heart function. ANP and BNP concentrations are related to the risk of CSA. Thus, lower ANP and BNP levels present a lower risk of CSA [74]. In a randomized clinical study involving patients with HFrEF and CSA, soluble suppression of tumorigenicity 2, neurogenic locus notch homolog protein 3 (Notch-3), and growth differentiation factor 15 (GDF-15) were suitable for predicting prognostic outcomes [75].
Nondrug therapy for sleep disorders is common in patients with HF. Gerçek et al. [78] conducted a retrospective study investigating the effect of SDB treatment on ventricular tachycardia (VT) burden in patients with HF and an implantable cardioverter-defibrillator (ICD). The study included several types of SDB treatments such as CPAP, Assisted Spontaneous Breathing, phrenic nerve stimulation, and Bilevel Positive Airway Pressure. They demonstrated that SDB treatment significantly reduced the VT burden, lowered the rate of anti-tachycardia pacing, and decreased the number of shocks, thereby improving the quality of life for these patients. Abdelbasset [79] reported that 4-week low-intensity exercise program was beneficial for improving sleep quality and increasing maximum oxygen uptake (VO2peak) in older adult patients with HF. In addition, a prospective cohort study showed that positive respiratory pressure therapy at home for 3 months increased LVEF and decreased AHI in patients with HFrEF and CSA [80]. Furthermore, CBT-Is are commonly used to treat insomnia. CBT-I improves sleep quality in patients with HF and insomnia by relieving insomnia severity, fatigue, excessive daytime sleepiness, and ameliorating the 6-min walk distance [81].
4.4. Sleep disorders in patients with cardiac arrhythmia
Patients with cardiac arrhythmias have a high prevalence of sleep disorders. In a prospective cohort study including 100 consecutive patients with AF from two major arrhythmia clinics in Canada, the prevalence of OSA was 85%. This study also found that during rapid eye movement sleep, 27% of patients had a higher AHI, even though they had a normal AHI overall during sleep [82]. In another prospective study, 81.4% of patients with AF also had SDB [83]. Patients with AF also have a high prevalence of CSA [84]. In a controlled clinical trial including 150 patients with nonvalvular AF and 150 age-matched individuals with sinus rhythm, stepwise multivariate logistic regression was used to analyze the association between AF and sleep disorders. After controlling for confounders, including age, sex, body mass index, SBP, and heart rate, patients with nonvalvular atrial fibrillation exhibited shorter sleep duration and poorer sleep quality than those with a normal rhythm [85]. OSA affects the structure and remodeling of the atrium. The association between OSA and arrhythmia recurrence in patients with AF remains controversial. Sleep disorders increase the risk of AF recurrence after catheter ablation [86]. Anter et al. [87] reported a higher incidence of extraPV triggers and arrhythmia recurrence in patients with paroxysmal AF and OSA. Moreover, OSA was associated with an elevated risk of major adverse cardiac and neurologic events in patients with AF (HR: 1.16, 95% CI: 1.03–1.31, P = 0.011) [88]. Conversely, Tang et al. showed that OSA might not affect the recurrence of paroxysmal AF after catheter ablation [89]. Similarly, Brgdar et al. [90] showed no differences in inpatient cardiac outcomes between patients with AF with or without OSA.
Non-drug therapy plays an important role in the treatment of patients with cardiac arrhythmia and OAS. For patients with both AF and OSA, the combined treatment exerts a better effect than treatment for AF alone [105]. A meta-analysis of 17 studies and 6523 patients, showed that CPAP treatment could reduce the risk of AF (OR: 0.51, 95% CI: 0.27–0.97, P = 0.04) [106]. In a prospective non-randomized interventional study, CPAP was beneficial in reducing atrial and ventricular ectopy count/24 h in patients with AF and OSA. After 3 months of CPAP treatment, the atrial premature beat count/24 h reduced significantly in patients with paroxysmal AF, whereas the ventricular premature beat count/24 h reduced significantly in patients with permanent AF [91].
4.5. Sleep disorders in patients with other CVD
Sleep disorders are highly prevalent among patients with cardiomyopathy. In a cross-sectional study, Banno et al. [92] investigated 35 patients with idiopathic cardiomyopathy and found that the incidence of SDB was 80% among 20 patients with dilated cardiomyopathy, and 47% among 15 patients with hypertrophic cardiomyopathy (HCM). The prevalence of SDB was 90% in patients with cardiac amyloidosis [93]. Xu et al. [94] reported that the incidence of OSA was 58.7% (346/589) in patients with HCM, particularly in those who were older or had other complications (hypertension, AF, etc.). Patients with HCM and OSA exhibit specific characteristics, including a higher body mass index (BMI), higher prevalence of AF, and larger waist and neck circumference [107]. In addition, Konecny et al. [108] identified that exercise tolerance was impaired and the prevalence of SDB was 32% among patients with HCM.
Cardiac surgery is stressful to trigger sleep disorders in CVD patients. In hospitals, patients undergoing surgical aortic valve replacement or transcatheter aortic valve implantation reported insomnia and disturbed sleep-wake patterns during the postsurgical phase [95]. However, the improvement of heart function would benefit sleep health. Abe et al. [96] showed that OSA symptoms were relieved in patients who underwent valve repair surgery because of improved cardiac function. Biener et al. [97] reported that transcatheter mitral valve repair surgery improved heart function and reduced the AHI and central events in patients with mitral regurgitation and CSA with 3.9 ± 4.4 months follow-up. The different results of these studies may be explained by their specific follow-up periods. Moreover, cardiac surgery and sleep disorders are associated with postoperative cognitive dysfunction in patients with CVD post-surgery. OSA increases the risk of post-surgery delirium in patients undergoing cardiac surgery (OR: 6.4, 95% CI: 2.6–15.4, P < 0.001). Therefore, OSA could be regarded as a risk factor for post cardiac surgery delirium [98]. Patients taking zolpidem are more likely to develop delirium after cardiac surgery [109]. Tafelmeier et al. [99] demonstrate that central sleep apnea is associated with the risk of post-surgery delirium in patients undergoing cardiac surgery (OR 4.99, 95% CI 1.41–17.69; p = 0.013). Javaherforooshzadeh et al. [100] reported that patients with a higher risk of OSA had a higher prevalence of postcardiac surgery complications. OSA increases the risk of perioperative adverse events in patients with rheumatic valvular heart disease undergoing cardiac valve replacement surgery [101]. In contrast, although there is a high incidence of OSA after cardiac surgery, Foldvary-Schaefer et al. [102] identified no association between OAS and adverse cardiac outcomes.
Certain indicators may be useful for predicting CAS in patients with mitral regurgitation (MR). LAVI was used to evaluate the structure of left atrial and cardiac diastolic functions. Biener et al. [97] found that LAVI is a valuable indicator of CSA in patients with MR.
5. Limitation
First, this review focused on patients with CVD only, excluding those without, which hindered further examinations of the interrelationship between sleep disorders and CVD. Therefore, the study lacks evidence to prove that sleep disorders would lead to CVD. Second, we did not include animal studies, which could help elucidate the biological mechanisms of sleep disorders in patients with CVD. Therefore, this review is limited in that it only illustrates the underlying pathophysiological mechanisms.
6. Conclusion
This systematic review highlighted the significant prevalence and adverse cardiac outcomes of sleep disorders which hinder treatment among patients with CVD. In conclusion, clinical physicians should carefully consider the sleep quality of patients with CVD to improve their prognosis.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All the authors agree to publish this manuscript.
Availability of data and materials
Not applicable.
Funding
It is supported by China Women's Development Foundation (No.2021573), National Academy of Innovation Strategy (Grant No. 2022-pgs-11), China International Medical Foundation (z-20114-03-2205).
CRediT authorship contribution statement
Lijun Zhang: Data curation, Methodology, Software, Writing – original draft. Guo Li: Data curation, Methodology. Yanping Bao: Writing – review & editing. Meiyan Liu: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Declaration of competing interest
All the authors declared no competing interests of this manuscript.
Acknowledgements
Sincere thanks to China Women's Development Foundation, National Academy of Innovation Strategy and China International Medical Foundation for supporting our study.
Handling editor: D Levy
Abbreviations
- ACh
acetylcholine
- ACS
acute coronary syndrome
- AF
atrial fibrillation
- AHI
apnea-hypopnea index
- AMI
acute myocardial infarction
- ANP
Atrial natriuretic peptide
- BDZs
benzodiazepines
- BMI
body mass index
- BNP
brain natriuretic peptide
- CAD
coronary artery disease
- CBT-I
cognitive behavioral therapy for insomnia
- CIMT
carotid artery intima-media thickness
- CMI
cardiometabolic index
- CNS
central nervous system
- CoB
Cochrane Risk of Bias
- CPAP
continuous positive airway pressure
- CRT-D
cardioverter-defibrillator
- CSA
central sleep apnoea
- CTRP9
C1q tumor necrosis factor related protein 9
- CVD
cardiovascular disease
- PO
erythropoietin
- FMD
flow mediated vasodilation
- GDF-15
growth differentiation factor 15
- HCM
hypertrophic cardiomyopathy
- HF
heart failure
- HFmrEF
heart failure with mid-range ejection fraction
- HFpEF
heart failure and preserved ejection fraction
- HFrEF
heart failure and reduced ejection fraction
- HR
hazard ratio
- hs-CRP
high sensitivity C-reactive protein
- ICBT
Internet-based CBT
- ICD
implantable cardioverter-defibrillator
- ICSD-3
International Classification of Sleep Disorders-3
- IL-6
interleukin (IL)-6
- IMT
inspiratory muscle training
- LAVI
left atrial volume index
- LVEF
left ventricular ejection fraction
- MACEs
major adverse cardiovascular events
- MDD
major depressive disorder
- MIP
inspiratory pressure
- MR
mitral regurgitation
- NF-κB
nuclear factor-kappa B
- NOS
Newcastle-Ottawa (cohort) scale
- Notch-3
neurogenic locus notch homolog protein 3
- NREM
nonrapid eye movements
- NSTEMI
non-ST elevation acute myocardial infarction
- OSA
obstructive sleep apnea
- PAI-1
plasminogen activator inhibitor-1
- PIGF
placental growth factor
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Anlyses
- PSQI
Pittsburgh Sleep Quality Index
- RCTs
randomized controlled trials
- sEng
endoglin
- sFlt-1
fms-like tyrosine kinase-1
- SNXs
Sorting nexins
- TAVI
transcatheter aortic valve implantation
- TMVR
transcatheter mitral valve repair
- TNF-α
tumor necrosis factor-alpha
- UA
unstable angina
- VEGF
vascular endothelial growth factor family
- VO2peak
maximum oxygen uptake
- VT
ventricular tachycardia
References
- 1.Gauld C., Lopez R., Geoffroy P.A., Morin C.M., Guichard K., Giroux É., et al. A systematic analysis of ICSD-3 diagnostic criteria and proposal for further structured iteration. Sleep Med. Rev. 2021;58 doi: 10.1016/j.smrv.2021.101439. [DOI] [PubMed] [Google Scholar]
- 2.Jansen P.R., Watanabe K., Stringer S., Skene N., Bryois J., Hammerschlag A.R., et al. Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat. Genet. 2019;51(3):394–403. doi: 10.1038/s41588-018-0333-3. [DOI] [PubMed] [Google Scholar]
- 3.Yeghiazarians Y., Jneid H., Tietjens J.R., Redline S., Brown D.L., El-Sherif N., et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;144(3):e56–e67. doi: 10.1161/cir.0000000000000988. [DOI] [PubMed] [Google Scholar]
- 4.Rissling M.B., Gray K.E., Ulmer C.S., Martin J.L., Zaslavsky O., Gray S.L., et al. Sleep disturbance, diabetes, and cardiovascular disease in postmenopausal veteran women. Gerontol. 2016;56(Suppl 1):S54–S66. doi: 10.1093/geront/gnv668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sum-Ping Oliver, Geng Yong-Jian1. Impact of sleep on cardiovascular health: a narrative review. Heart and Mind. Jul–Sep 2022;6(3):120–126. doi: 10.4103/hm.hm_29_22. [DOI] [Google Scholar]
- 6.Lu L., Geng Q., Wang J., Bai C., Cheng G., Cui Y., et al. Expert consensus on diagnosis and treatment of adult mental stress induced hypertension in China (2022 revision): Part A. Heart Mind. 2022;6:45–51. [Google Scholar]
- 7.Siebmanns S., Johansson L., Sandberg J., Johansson P., Broström A. Experiences and management of incidents that influence sleep in patients with cardiovascular disease and insomnia. J. Cardiovasc. Nurs. 2020;35(4):364–374. doi: 10.1097/jcn.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 8.Pengo M.F., Javaheri S., Sanchez-de-la-Torre M., Schwarz E.I. What cardiologists should know about sleep. Eur. Heart J. 2022;43(31):2911–2913. doi: 10.1093/eurheartj/ehac349. [DOI] [PubMed] [Google Scholar]
- 9.Chirinos J.A., Gurubhagavatula I., Teff K., Rader D.J., Wadden T.A., Townsend R., et al. CPAP, weight loss, or both for obstructive sleep apnea. N. Engl. J. Med. 2014;370(24):2265–2275. doi: 10.1056/NEJMoa1306187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakatsuka Y., Murase K., Matsumoto T., Tabara Y., Nakamoto I., Minami T., et al. Markers of cardiovascular disease risk in sleep-disordered breathing with or without comorbidities: the Nagahama study. J. Clin. Sleep Med. 2021;17(12):2467–2475. doi: 10.5664/jcsm.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertisch S.M., Pollock B.D., Mittleman M.A., Buysse D.J., Bazzano L.A., Gottlieb D.J., et al. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: sleep Heart Health Study. Sleep. 2018;41(6) doi: 10.1093/sleep/zsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang B.H., Duncan M.J., Cistulli P.A., Nassar N., Hamer M., Stamatakis E. Sleep and physical activity in relation to all-cause, cardiovascular disease and cancer mortality risk. Br. J. Sports Med. 2022;56(13):718–724. doi: 10.1136/bjsports-2021-104046. [DOI] [PubMed] [Google Scholar]
- 13.Yan B., Yang J., Zhao B., Fan Y., Wang W., Ma X. Objective sleep efficiency predicts cardiovascular disease in a community population: the sleep heart health study. J. Am. Heart Assoc. 2021;10(7) doi: 10.1161/jaha.120.016201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin M.R., Wang M., Ribeiro D., Cho H.J., Olmstead R., Breen E.C., et al. Sleep loss activates cellular inflammatory signaling. Biol. Psychiatr. 2008;64(6):538–540. doi: 10.1016/j.biopsych.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan X.L., Nie L., Zhao S.Y., Zhang X.B., Zhang S., Su Z.F. The association between insomnia and atherosclerosis: a brief report. Nat. Sci. Sleep. 2022;14:443–448. doi: 10.2147/nss.S336318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;140(11):e563–e595. doi: 10.1161/cir.0000000000000677. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siu P.M., Yu A.P., Tam B.T., Chin E.C., Yu D.S., Chung K.F., et al. Effects of Tai Chi or exercise on sleep in older adults with insomnia: a randomized clinical trial. JAMA Netw. Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.37199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrosielski D.A., Papandreou C., Patil S.P., Salas-Salvadó J. Diet and exercise in the management of obstructive sleep apnoea and cardiovascular disease risk. Eur. Respir. Rev. 2017;26(144) doi: 10.1183/16000617.0110-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neher M., Nygårdh A., Nilsen P., Broström A., Johansson P. Implementing internet-delivered cognitive behavioural therapy for patients with cardiovascular disease and psychological distress: a scoping review. Eur. J. Cardiovasc. Nurs. 2019;18(5):346–357. doi: 10.1177/1474515119833251. [DOI] [PubMed] [Google Scholar]
- 20.Teckchandani P.H., Truong K.K., Zezoff D., Healy W.J., Khayat R.N. Transvenous phrenic nerve stimulation for central sleep apnea: clinical and billing review. Chest. 2022;161(5):1330–1337. doi: 10.1016/j.chest.2021.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celik Y., Yapici-Eser H., Balcan B., Peker Y. Association of excessive daytime sleepiness with the zung self-rated depression subscales in adults with coronary artery disease and obstructive sleep apnea. Diagnostics. 2021;11(7) doi: 10.3390/diagnostics11071176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X., Zhang Y., Dong Z., Fan J., Nie S., Wei Y. Effect of continuous positive airway pressure on long-term cardiovascular outcomes in patients with coronary artery disease and obstructive sleep apnea: a systematic review and meta-analysis. Respir. Res. 2018;19(1):61. doi: 10.1186/s12931-018-0761-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wells G., Shea B., O'Connell D., Peterson j., Welch V., Losos M., et al. 2000. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-randomized Studies in Meta-Analysis. [Google Scholar]
- 24.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25.Alonderis A., Raskauskiene N., Gelziniene V., Zaliunaite V., Brozaitiene J. Undiagnosed sleep apnoea in cardiac rehabilitation: age-dependent effect on diastolic function in coronary artery disease patients with preserved ejection fraction. Eur. J. Cardiovasc. Nurs. 2021;20(3):202–211. doi: 10.1177/1474515120941373. [DOI] [PubMed] [Google Scholar]
- 26.Andrechuk C.R., Ceolim M.F. High risk for obstructive sleep apnea in patients with acute myocardial infarction. Rev Lat Am Enfermagem. 2015;23(5):797–805. doi: 10.1590/0104-1169.0511.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aronson D., Nakhleh M., Zeidan-Shwiri T., Mutlak M., Lavie P., Lavie L. Clinical implications of sleep disordered breathing in acute myocardial infarction. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0088878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchner S., Eglseer M., Debl K., Hetzenecker A., Luchner A., Husser O., et al. Sleep disordered breathing and enlargement of the right heart after myocardial infarction. Eur. Respir. J. 2015;45(3):680–690. doi: 10.1183/09031936.00057014. [DOI] [PubMed] [Google Scholar]
- 29.Barger L.K., Rajaratnam S.M.W., Cannon C.P., Lukas M.A., Im K., Goodrich E.L., et al. Short sleep duration, obstructive sleep apnea, shiftwork, and the risk of adverse cardiovascular events in patients after an acute coronary syndrome. J. Am. Heart Assoc. 2017;6(10) doi: 10.1161/jaha.117.006959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correia L.C., Souza A.C., Garcia G., Sabino M., Brito M., Maraux M., et al. Obstructive sleep apnea affects hospital outcomes of patients with non-ST-elevation acute coronary syndromes. Sleep. 2012;35(9):1241–1245a. doi: 10.5665/sleep.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Araújo C.M., Solimene M.C., Grupi C.J., Genta P.R., Lorenzi-Filho G., Da Luz P.L. Evidence that the degree of obstructive sleep apnea may not increase myocardial ischemia and arrhythmias in patients with stable coronary artery disease. Clinics. 2009;64(3):223–230. doi: 10.1590/s1807-59322009000300013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juskiene A., Podlipskyte A., Bunevicius A., Varoneckas G. Type D personality and sleep quality in coronary artery disease patients with and without obstructive sleep apnea: mediating effects of anxiety and depression. Int. J. Behav. Med. 2018;25(2):171–182. doi: 10.1007/s12529-017-9708-6. [DOI] [PubMed] [Google Scholar]
- 33.Cai L., Wei L., Yao J., Qin Y., You Y., Xu L., et al. Impact of depression on the quality of sleep and immune functions in patients with coronary artery disease. Gen Psychiatr. 2022;35(6) doi: 10.1136/gpsych-2022-100918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan M.S., Bawany F.I., Khan A., Hussain M., Ali S.S., Shah S.R., et al. Risk assessment for obstructive sleep apnea and anxiety in a Pakistani population with coronary artery disease. Sleep Breath. 2015;19(1):291–296. doi: 10.1007/s11325-014-1018-5. [DOI] [PubMed] [Google Scholar]
- 35.Assari S., Moghani Lankarani M., Kazemi Saleh D., Ahmadi K. Gender modifies the effects of education and income on sleep quality of the patients with coronary artery disease. Int Cardiovasc Res J. 2013;7(4):141–146. [PMC free article] [PubMed] [Google Scholar]
- 36.Clark A., Lange T., Hallqvist J., Jennum P., Rod N.H. Sleep impairment and prognosis of acute myocardial infarction: a prospective cohort study. Sleep. 2014;37(5):851–858. doi: 10.5665/sleep.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kazukauskiene N., Fineberg N.A., Podlipskyte A., Bunevicius A., Linares N.F.N., Poitras M., et al. Contribution of obstructive sleep apnoea to cognitive functioning of males with coronary artery disease: a relationship with endocrine and inflammatory biomarkers. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.899597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strehmel R., Valo M., Teupe C. Natriuretic peptide and high-sensitive troponin T concentrations correlate with effectiveness of short-term CPAP in patients with obstructive sleep apnea and coronary artery disease. Clin Med Insights Circ Respir Pulm Med. 2016;10:33–39. doi: 10.4137/ccrpm.S40939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barcelo A., Bauça J.M., Yañez A., Fueyo L., Gomez C., de la Peña M., et al. Impact of obstructive sleep apnea on the levels of placental growth factor (PlGF) and their value for predicting short-term adverse outcomes in patients with acute coronary syndrome. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thunström E., Glantz H., Fu M., Yucel-Lindberg T., Petzold M., Lindberg K., et al. Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apnea. Sleep. 2015;38(3):463–471. doi: 10.5665/sleep.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Z., Liu Y., Wu Y., Chen P., Li G., Wang L., et al. A risk biomarker for obstructive sleep apnea in patients with coronary artery disease: monocyte to high-density lipoprotein ratio. Sleep Breath. 2021;25(3):1519–1526. doi: 10.1007/s11325-020-02262-3. [DOI] [PubMed] [Google Scholar]
- 42.Katsumata Y., Terada J., Matsumura T., Koshikawa K., Sakao S., Tomiyoshi G., et al. Circulating anti-sorting nexins 16 antibodies as an emerging biomarker of coronary artery disease in patients with obstructive sleep apnea. Diagnostics. 2020;10(2) doi: 10.3390/diagnostics10020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng V., Tumati S., Wang R., Bawa K.K., Gallagher D., Herrmann N., et al. The relationship between oxidative stress and subjective sleep quality in people with coronary artery disease. Brain Sci. 2022;12(8) doi: 10.3390/brainsci12081070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Z.X., Du Y.H., Jia L.X., Fan J.Y., Guo R.F., Ma X.L., et al. Association of C1q/TNF-related protein-9 (CTRP9) level with obstructive sleep apnea in patients with coronary artery disease. Mediat. Inflamm. 2020;2020 doi: 10.1155/2020/7281391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Milleron O., Pillière R., Foucher A., de Roquefeuil F., Aegerter P., Jondeau G., et al. Benefits of obstructive sleep apnoea treatment in coronary artery disease: a long-term follow-up study. Eur. Heart J. 2004;25(9):728–734. doi: 10.1016/j.ehj.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Labeix P., Berger M., Zellag A., Garcin A., Barthelemy J.C., Roche F., et al. Resistance training of inspiratory muscles after coronary artery disease may improve obstructive sleep apnea in outpatient cardiac rehabilitation: RICAOS study. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.846532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ayanaw T., Temesgen M., Azagew A.W., Ferede Y.M. Sleep quality and associated factors among adult hypertensive patients attending a chronic follow up care clinic in northwest Amhara regional state referral hospitals, Northwest Ethiopia. PLoS One. 2022;17(7) doi: 10.1371/journal.pone.0271072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Friedman O., Bradley T.D., Ruttanaumpawan P., Logan A.G. Independent association of drug-resistant hypertension to reduced sleep duration and efficiency. Am. J. Hypertens. 2010;23(2):174–179. doi: 10.1038/ajh.2009.220. [DOI] [PubMed] [Google Scholar]
- 49.Li J., Atasoy S., Fang X., Angerer P., Ladwig K.H. Combined effect of work stress and impaired sleep on coronary and cardiovascular mortality in hypertensive workers: the MONICA/KORA cohort study. Eur J Prev Cardiol. 2021;28(2):220–226. doi: 10.1177/2047487319839183. [DOI] [PubMed] [Google Scholar]
- 50.Cai A.P., Zhou Y.L., Zhang J.W., Zhong Q., Wang R., Wang L. Epidemiological characteristics and gender-specific differences of obstructive sleep apnea in a Chinese hypertensive population: a cross-sectional study. BMC Cardiovasc. Disord. 2017;17 doi: 10.1186/s12872-016-0447-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chaudhary S.C., Gupta P., Sawlani K.K., Gupta K.K., Singh A., Usman K., et al. Obstructive sleep apnea in hypertension. Cureus. 2023;15(4) doi: 10.7759/cureus.38229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ching S.M., Singh R., Azmi F.S.B., Chong K.L., Ong C.R.S., Ayob N.A.B., et al. Prevalence and factors associated with probable obstructive sleep apnea among patients with hypertension in two primary care clinics in Miri, Sarawak, Malaysia. Ir. J. Med. Sci. 2023 doi: 10.1007/s11845-023-03397-4. [DOI] [PubMed] [Google Scholar]
- 53.Akintunde A., Kareem L., Bakare A., Audu M. Impact of obstructive sleep apnea and snoring on left ventricular mass and diastolic function in hypertensive nigerians. Ann. Med. Health Sci. Res. 2014;4(3):350–354. doi: 10.4103/2141-9248.133458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bacci M.R., Emboz J.N.M., Alves B., Veiga G.L.D., Murad N., Meneghini A., et al. Obstructive sleep apnea syndrome and sleep quality in hypertensive patients. Rev. Assoc. Med. Bras. 2017;63(12):1055–1060. doi: 10.1590/1806-9282.63.12.1055. 1992. [DOI] [PubMed] [Google Scholar]
- 55.Bengtsson Boström K., Hedner J., Grote L., Melander O., von Wowern F., Råstam L., et al. Polymorphisms in α- and β-adrenergic receptor genes, hypertension, and obstructive sleep apnea: the skaraborg sleep study. Int. J. Hypertens. 2010;2010 doi: 10.4061/2010/458410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jafari B., Mohsenin V. Endothelial dysfunction and hypertension in obstructive sleep apnea - is it due to intermittent hypoxia? J. Cardiovasc. Dis. Res. 2013;4(2):87–91. doi: 10.1016/j.jcdr.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai X., Hu J., Wen W., Wang J., Wang M., Liu S., et al. Associations of the cardiometabolic index with the risk of cardiovascular disease in patients with hypertension and obstructive sleep apnea: results of a longitudinal cohort study. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/4914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zamarrón C., Ricoy J., Riveiro A., Gude F. Plasminogen activator inhibitor-1 in obstructive sleep apnea patients with and without hypertension. Lung. 2008;186(3):151–156. doi: 10.1007/s00408-008-9076-8. [DOI] [PubMed] [Google Scholar]
- 59.Chang C.H., Yang Y.H., Lin S.J., Su J.J., Cheng C.L., Lin L.J. Risk of insomnia attributable to β-blockers in elderly patients with newly diagnosed hypertension. Drug Metabol. Pharmacokinet. 2013;28(1):53–58. doi: 10.2133/dmpk.dmpk-12-rg-004. [DOI] [PubMed] [Google Scholar]
- 60.Wolf J., Drozdowski J., Czechowicz K., Winklewski P.J., Jassem E., Kara T., et al. Effect of beta-blocker therapy on heart rate response in patients with hypertension and newly diagnosed untreated obstructive sleep apnea syndrome. Int. J. Cardiol. 2016;202:67–72. doi: 10.1016/j.ijcard.2015.08.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzaga C.C., Gaddam K.K., Ahmed M.I., Pimenta E., Thomas S.J., Harding S.M., et al. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J. Clin. Sleep Med. 2010;6(4):363–368. [PMC free article] [PubMed] [Google Scholar]
- 62.Gaddam K., Pimenta E., Thomas S.J., Cofield S.S., Oparil S., Harding S.M., et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. J. Hum. Hypertens. 2010;24(8):532–537. doi: 10.1038/jhh.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lui M.M., Tse H.F., Lam D.C., Lau K.K., Chan C.W., Ip M.S. Continuous positive airway pressure improves blood pressure and serum cardiovascular biomarkers in obstructive sleep apnoea and hypertension. Eur. Respir. J. 2021;58(5) doi: 10.1183/13993003.03687-2020. [DOI] [PubMed] [Google Scholar]
- 64.Chen Q., Cheng Y.B., Shen M., Yin B., Yi H.H., Feng J., et al. A randomized controlled trial on ambulatory blood pressure lowering effect of CPAP in patients with obstructive sleep apnea and nocturnal hypertension. Blood Pres. 2020;29(1):21–30. doi: 10.1080/08037051.2019.1686343. [DOI] [PubMed] [Google Scholar]
- 65.Martínez-García M.A., Capote F., Campos-Rodríguez F., Lloberes P., Díaz de Atauri M.J., Somoza M., et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–2415. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 66.Awotidebe T.O., Adeyeye V.O., Adedoyin R.A., Ogunyemi S.A., Oke K.I., Ativie R.N., et al. Assessment of functional capacity and sleep quality of patients with chronic heart failure. Hong Kong Physiother. J. 2017;36:17–24. doi: 10.1016/j.hkpj.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bitter T., Westerheide N., Prinz C., Hossain M.S., Vogt J., Langer C., et al. Cheyne-Stokes respiration and obstructive sleep apnoea are independent risk factors for malignant ventricular arrhythmias requiring appropriate cardioverter-defibrillator therapies in patients with congestive heart failure. Eur. Heart J. 2011;32(1):61–74. doi: 10.1093/eurheartj/ehq327. [DOI] [PubMed] [Google Scholar]
- 68.Alosco M.L., Brickman A.M., Spitznagel M.B., Griffith E.Y., Narkhede A., Cohen R., et al. Reduced cerebral blood flow and white matter hyperintensities predict poor sleep in heart failure. Behav. Brain Funct. 2013;9:42. doi: 10.1186/1744-9081-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arzt M., Oldenburg O., Graml A., Erdmann E., Teschler H., Wegscheider K., et al. Phenotyping of sleep-disordered breathing in patients with chronic heart failure with reduced ejection fraction-the SchlaHF registry. J. Am. Heart Assoc. 2017;6(12) doi: 10.1161/jaha.116.005899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avci M. Gun. The effect of activities of dialy living and depression symptom level of sleep quality in the elderly with heart failure. Pakistan Heart J. 2021;54(1):64–72. doi: 10.47144/phj.v54i1.2067. [DOI] [Google Scholar]
- 71.Bhalla S., Sharma K., Yadave R.D., Desai H.D., Vora T., Khan E., et al. Prevalence and patterns of obstructive sleep apnea in Asian Indians with congestive heart failure. Cureus. 2020;12(11) doi: 10.7759/cureus.11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Calvin A.D., Somers V.K., Johnson B.D., Scott C.G., Olson L.J. Left atrial size, chemosensitivity, and central sleep apnea in heart failure. Chest. 2014;146(1):96–103. doi: 10.1378/chest.13-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Calvin A.D., Somers V.K., Steensma D.P., Rio Perez J.A., van der Walt C., Fitz-Gibbon J.M., et al. Advanced heart failure and nocturnal hypoxaemia due to central sleep apnoea are associated with increased serum erythropoietin. Eur. J. Heart Fail. 2010;12(4):354–359. doi: 10.1093/eurjhf/hfq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calvin A.D., Somers V.K., van der Walt C., Scott C.G., Olson L.J. Relation of natriuretic peptide concentrations to central sleep apnea in patients with heart failure. Chest. 2011;140(6):1517–1523. doi: 10.1378/chest.10-2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreira J.P., Duarte K., Woehrle H., Cowie M.R., Wegscheider K., Angermann C., et al. Biomarkers in patients with heart failure and central sleep apnoea: findings from the SERVE-HF trial. ESC Heart Fail. 2020;7(2):503–511. doi: 10.1002/ehf2.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bughin F., Jaussent I., Ayoub B., Aguilhon S., Chapet N., Soltani S., et al. Prognostic impact of sleep patterns and related-drugs in patients with heart failure. J. Clin. Med. 2021;10(22) doi: 10.3390/jcm10225387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cundrle, Somers V.K., Singh P., Johnson B.D., Scott C.G., Olson L.J. Low leptin concentration may identify heart failure patients with central sleep apnea. J. Sleep Res. 2018;27(2):240–243. doi: 10.1111/jsr.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerçek M., Gerçek M., Alzein K., Sciacca V., Sohns C., Sommer P., et al. Impact of sleep-disordered breathing treatment on ventricular tachycardia in patients with heart failure. J. Clin. Med. 2022;11(15) doi: 10.3390/jcm11154567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdelbasset W.K., Osailan A. Sleep quality and ventilatory efficiency in elderly heart failure patients: a pilot study on the short-term effect of 4-week low-intensity aerobic exercise. Kardiologiia. 2020;60(6):938. doi: 10.18087/cardio.2020.6.n938. [DOI] [PubMed] [Google Scholar]
- 80.Beres E., Babes K., Beres Z.L., Botea M., Davidescu L. Effect of home non-invasive ventilation on left ventricular function and quality of life in patients with heart failure and central sleep apnea syndrome. Balneo and Prm Research Journal. 2022;13(4) doi: 10.12680/balneo.2022.520. [DOI] [Google Scholar]
- 81.Redeker N.S., Yaggi H.K., Jacoby D., Hollenbeak C.S., Breazeale S., Conley S., et al. Cognitive behavioral therapy for insomnia has sustained effects on insomnia, fatigue, and function among people with chronic heart failure and insomnia: the HeartSleep Study. Sleep. 2022;45(1) doi: 10.1093/sleep/zsab252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abumuamar A.M., Dorian P., Newman D., Shapiro C.M. The prevalence of obstructive sleep apnea in patients with atrial fibrillation. Clin. Cardiol. 2018;41(5):601–607. doi: 10.1002/clc.22933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Albuquerque F.N., Calvin A.D., Kuniyoshi F.H.S., Konecny T., Lopez-Jimenez F., Pressman G.S., et al. Sleep-Disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest. 2012;141(4):967–973. doi: 10.1378/chest.11-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bitter T., Langer C., Vogt J., Lange M., Horstkotte D., Oldenburg O. Sleep-disordered breathing in patients with atrial fibrillation and normal systolic left ventricular function. Dtsch Arztebl Int. 2009;106(10):164–170. doi: 10.3238/arztebl.2009.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kayrak M., Gul E.E., Aribas A., Akilli H., Alibasiç H., Abdulhalikov T., et al. Self-reported sleep quality of patients with atrial fibrillation and the effects of cardioversion on sleep quality. Pacing Clin. Electrophysiol. 2013;36(7):823–829. doi: 10.1111/pace.12115. [DOI] [PubMed] [Google Scholar]
- 86.Cang J., Shi N., Zhu D., Liu Y., Zhou Q., Chen L. Self-reported sleep pattern and recurrence of atrial fibrillation after catheter ablation. Clin. Cardiol. 2023;46(3):336–344. doi: 10.1002/clc.23975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anter E., Di Biase L., Contreras-Valdes F.M., Gianni C., Mohanty S., Tschabrunn C.M., et al. Atrial substrate and triggers of paroxysmal atrial fibrillation in patients with obstructive sleep apnea. Circ Arrhythm Electrophysiol. 2017;10(11) doi: 10.1161/circep.117.005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dalgaard F., North R., Pieper K., Fonarow G.C., Kowey P.R., Gersh B.J., et al. Risk of major cardiovascular and neurologic events with obstructive sleep apnea among patients with atrial fibrillation. Am. Heart J. 2020;223:65–71. doi: 10.1016/j.ahj.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang R.B., Dong J.Z., Liu X.P., Kang J.P., Ding S.F., Wang L., et al. Obstructive sleep apnoea risk profile and the risk of recurrence of atrial fibrillation after catheter ablation. Europace. 2009;11(1):100–105. doi: 10.1093/europace/eun315. [DOI] [PubMed] [Google Scholar]
- 90.Brgdar A., Yi J., Awan A., Taha M., Ogunti R., Gharbin J., et al. Impact of obstructive sleep apnea on in-hospital outcomes in patients with atrial fibrillation: a retrospective analysis of the national inpatient sample. Cureus. 2021;13(12) doi: 10.7759/cureus.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abumuamar A.M., Newman D., Dorian P., Shapiro C.M. Cardiac effects of CPAP treatment in patients with obstructive sleep apnea and atrial fibrillation. J. Intervent. Card Electrophysiol. 2019;54(3):289–297. doi: 10.1007/s10840-018-0482-4. [DOI] [PubMed] [Google Scholar]
- 92.Banno K., Shiomi T., Sasanabe R., Otake K., Hasegawa R., Maekawa M., et al. Sleep-disordered breathing in patients with idiopathic cardiomyopathy. Circ. J. 2004;68(4):338–342. doi: 10.1253/circj.68.338. [DOI] [PubMed] [Google Scholar]
- 93.Bodez D., Guellich A., Kharoubi M., Covali-Noroc A., Tissot C.M., Guendouz S., et al. Prevalence, severity, and prognostic value of sleep apnea syndromes in cardiac amyloidosis. Sleep. 2016;39(7):1333–1341. doi: 10.5665/sleep.5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu H., Wang J., Yuan J., Guo C., Hu F., Yang W., et al. Clinical predictors of the presence of obstructive sleep apnea in patients with hypertrophic cardiomyopathy. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-93039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Amofah H.A., Broström A., Fridlund B., Bjorvatn B., Haaverstad R., Hufthammer K.O., et al. Sleep in octogenarians during the postoperative phase after transcatheter or surgical aortic valve replacement. Eur. J. Cardiovasc. Nurs. 2016;15(2):168–177. doi: 10.1177/1474515115620992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abe H., Takahashi M., Yaegashi H., Eda S., Kitahara H., Tsunemoto H., et al. Valve repair improves central sleep apnea in heart failure patients with valvular heart diseases. Circ. J. 2009;73(11):2148–2153. doi: 10.1253/circj.cj-09-0307. [DOI] [PubMed] [Google Scholar]
- 97.Biener L., Vogelhuber J., Alboany H., Tiyerili V., Weber M., Linhart M., et al. Prevalence of sleep-disordered breathing in patients with mitral regurgitation and the effect of mitral valve repair. Sleep Breath. 2023;27(2):599–610. doi: 10.1007/s11325-022-02667-2. [DOI] [PubMed] [Google Scholar]
- 98.Roggenbach J., Klamann M., von Haken R., Bruckner T., Karck M., Hofer S. Sleep-disordered breathing is a risk factor for delirium after cardiac surgery: a prospective cohort study. Crit. Care. 2014;18(5) doi: 10.1186/s13054-014-0477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tafelmeier M., Knapp M., Lebek S., Floerchinger B., Camboni D., Creutzenberg M., et al. Predictors of delirium after cardiac surgery in patients with sleep disordered breathing. Eur. Respir. J. 2019;54(2) doi: 10.1183/13993003.00354-2019. [DOI] [PubMed] [Google Scholar]
- 100.Javaherforooshzadeh F., Amjadzadeh M., Haybar H., Sharafkhaneh A. Impact of obstructive sleep apnea diagnosed using the STOP-bang Questionnaire scale on postoperative complications following major cardiac surgery: a prospective observational cohort study. Cureus. 2022;14(6) doi: 10.7759/cureus.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding N., Ni B.Q., Wang H., Ding W.X., Xue R., Lin W., et al. Obstructive sleep apnea increases the perioperative risk of cardiac valve replacement surgery: a prospective single-center study. J. Clin. Sleep Med. 2016;12(10):1331–1337. doi: 10.5664/jcsm.6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Foldvary-Schaefer N., Kaw R., Collop N., Andrews N.D., Bena J., Wang L., et al. Prevalence of undetected sleep apnea in patients undergoing cardiovascular surgery and impact on postoperative outcomes. J. Clin. Sleep Med. 2015;11(10):1083–1089. doi: 10.5664/jcsm.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jarrin D.C., Ivers H., Lamy M., Chen I.Y., Harvey A.G., Morin C.M. Cardiovascular autonomic dysfunction in insomnia patients with objective short sleep duration. J. Sleep Res. 2018;27(3) doi: 10.1111/jsr.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Valenzuela P.L., Carrera-Bastos P., Gálvez B.G., Ruiz-Hurtado G., Ordovas J.M., Ruilope L.M., et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 2021;18(4):251–275. doi: 10.1038/s41569-020-00437-9. [DOI] [PubMed] [Google Scholar]
- 105.Faulx M.D., Mehra R., Reis Geovanini G., Ando S.I., Arzt M., Drager L., et al. Obstructive sleep apnea and its management in patients with atrial fibrillation: an international collaboration of sleep apnea cardiovascular trialists (INCOSACT) global survey of practicing cardiologists. Int J Cardiol Heart Vasc. 2022;42 doi: 10.1016/j.ijcha.2022.101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Affas Z., Affas S., Tabbaa K. Continuous positive airway pressure reduces the incidence of atrial fibrillation in patients with obstructive sleep apnea: a meta-analysis and systematic review. Spartan Med Res J. 2022;7(2) doi: 10.51894/001c.34521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nerbass F.B., Pedrosa R.P., Genta P.R., Antunes M.O., Arteaga-Fernandez E., Drager L.F., et al. Lack of reliable clinical predictors to identify obstructive sleep apnea in patients with hypertrophic cardiomyopathy. Clinics. 2013;68(7):992–996. doi: 10.6061/clinics/2013(07)17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Konecny T., Geske J.B., Ludka O., Orban M., Brady P.A., Abudiab M.M., et al. Decreased exercise capacity and sleep-disordered breathing in patients with hypertrophic cardiomyopathy. Chest. 2015;147(6):1574–1581. doi: 10.1378/chest.14-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Louzada L.L., Machado F.V., Nóbrega O.T., Camargos E.F. Zopiclone to treat insomnia in older adults: a systematic review. Eur. Neuropsychopharmacol. 2021;50:75–92. doi: 10.1016/j.euroneuro.2021.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.