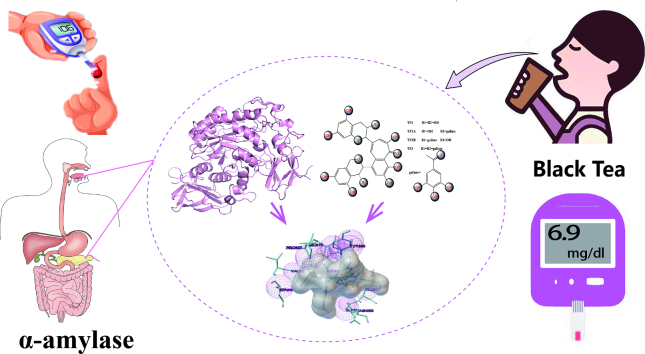
Abstract
Hyperglycemia can cause early damage to human bady and develop into diabates that will severely threaten human healthy. The effectively clinical treatment of hyperglycemiais is by inhibiting the activity of α-amylase. Black tea has been reported to show inhibitory effect on α-amylase and can be used for hyperglycemia treatment. However, the mechanism underlying is unclear. In this study, in vivo experiment showed that black tea theaflavins extract (BTE) effectively alleviated hyperglycemia. In vitro experiment showed that the effects may be caused by the interation between theaflavins and α-amylase. While TF1 and TF3 were mixed type inhibitors of α-amylase, TF2A and TF2B were competitive inhibitors of α-amylase. Molecular docking analysis showed that theaflavins monomers interacted with the hydrophobic region of α-amylase. Further study verified that monomer-α-amylase complex was spontaneously formed depending on hydrophobic interactions. Taken together, theaflavins showed potential anti-hyperglycemia effect via inhibiting α-amylase activity. Our results suggested that theaflavins might be utilized as a new type of α-amylase inhibitor to prevent and cure hyperglycemia.
Keywords: Black tea, Theaflavins, α-amylase, Interaction mechanism, Hypoglycemic activity
Graphical abstract
Highlights
-
•
Black tea theaflavins show significant anti-hyperglycemic effect on diabetic mice.
-
•
Theaflavins monomers bind to the hydrophobic regionso that show high inhibition activity against α-amylase.
-
•
This paper will helpful for the application oftheaflavins into novel low-glycemic index functional foods or medicines.
1. Introduction
As the International Diabetes Federation 2023 reported, 10.5% of adults that aged 20 to 79 are suffering from diabetes and is estimated to reach 11.3% by 2030 and 12.2% by 2045. Type II diabetes has been linked mostly to postprandial hyperglycemia, therefore the prevention of high blood glucose is of great importance in prevention of diabetes. α-amylase is an important digestive enzyme by converting carbohydrates into oligosaccharides through cleavaging internal α-D-(1–4) glyosidic bonds (Fettach et al., 2019; Sales, Souza, Simeoni, Magalhães, & Silveira, 2012), finally contribute to the increase peaks of postprandial glucose(Lin et al., 2023). Usually, treatment for hyperglycemia mainly focuses on stimulating insulin secretion from the β-cells of pancreatic islets, inhibiting the insulin degradation process, repairing or regenerating pancreatic beta cells, and inhibiting the activity of starch hydrolases, α-amylase or α-glucosidase(Jarald, Joshi, & Jain, 2008). Therefore, α-amylase inhibitors that can effectively reduce glucose level has been suggested as a potential treatment for hyperglycemia(Magaji, Sacan, & Yanardag, 2020). Some α-amylase inhibitors have been developed into anti-hyperglycemia drugs, such acarbose and miglitol(Jung et al., 2020). However, there are many side effects caused by the current use of these drugs, such as weight gain, gastrointestinal disorders, and allergic reactions(Das et al., 2021). Interestingly, plants are rich in phenolic substances that can be used as α-amylase inhibitors to reduce glucose level without any side effects(Gandhi et al., 2020) (Lim, Yu, Lee, Choi, & Kim, 2021) (Tan, Chang, & Zhang, 2017) and are believed to have good prospects in the field of hyperglycemia treatment.
Theaflavins are important bioactive compounds in black tea, even though they only account for 2–20 g/kg(dry weight)(Sang et al., 2004), they contribute a lot to the distinctive flavor along with the health advantages offered by black tea (Bhuyan, Borah, Sabhapondit, Gogoi and Bhattacharyya, 2015) (Sharma & Rao, 2009). >20 theaflavins have been identified, of which theaflavin (TF1), theaflavin-3-gallate (TF2A), theaflavin-3-gallate (TF2B) and theaflavin-3,3′-digallate (TF3) are the four characteristic structures(J. Teng et al., 2017). Apparently, there have been reported that black tea showed antidiabetic effects and contributed to the regeneration of Diabetes-induced mice's pancreatic cells were treated with streptozotocin (STZ) (J. Teng et al., 2017) (Gomes, Vedasiromoni, Das, Sharma, & Ganguly, 1995). Further results verified that oral administration of theaflavins from black tea significantly inhibited body weight gain, lowered blood sugar and enhanced tolerance to insulin (Cai et al., 2021). However, further in-depth studies are necessary for the elucidation of the mechanism underlying theaflavins' anti-hyperglycemic activity.
In recent years, in-silico techniques have been favored by new drug development workers. Molecular docking is an exemplary tool to identify the intermolecular framework of ligand–protein, protein–nucleic acid, and protein–protein complexes (Singh, Bhardwaj, Sharma, Purohit, & Kumar, 2022). Some researchers have sought inhibitors of the SARS-CoV-2 from tea by molecular docking to treat COVID-19 (Bhardwaj et al., 2021) (Singh et al., 2022) (J. Sharma et al., 2021) (Singh, Bhardwaj, & Purohit, 2021) (Chauhan et al., 2022). An successful in-silico model will help to obtain better understand of theaflavins' inhibitory activity against α-amylase and provide new explaination on the anti-hyperglycemic effects of black tea.
This study fully explored the potential α-amylase inhibitors of theaflavins and further elucidated the underlying mechanisms. Here we evaluated the anti-hyperglycemic effect of black tea theaflavins extract (BTE) on diabetic mice. The inhibition effect of BTE and four theaflavins monomers on α-amylase was further investigated. The interaction mechanism between theaflavins and α-amylase was explained by molecular docking, Ultraviolet-Visible Absorption Spectroscopy, and Fluorescence Spectroscopy. Theaflavins in black tea were found to have the potential to successfully lower blood glucose levels by blocking the activity of α-amylase. As such, they can be utilized as natural α-amylase inhibitors in treating hyperglycemia.
2. Materials and methods
2.1. Chemical and reagents
BTE (total theaflavins>68% including 7.41% TF1, 17.4% TF2A, 6.63% TF2B and 36.57% TF3) was extracted from black tea and provided by Hunan Agricultural University (Changsha, China). TF1 (HPLC ≥98%), TF2A (HPLC ≥98%), TF2B (HPLC ≥98%), TF3 (HPLC ≥98%), STZ (HPLC ≥98.5%) and α-Amylase (A109181, 2100 U/mL) were purchased from Aladdin Biotechnology Co., Ltd. (Shanghai, China). 3,5-Dinitrosalicylic acid (DNS) and soluble starch were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Animal treatment
The animal study was carried out according to a previous study (Nakatsuru et al., 2018). All operations were carried out in compliance with Hunan Agricultural University's Guidelines for Care and Use of Laboratory Animals and were authorized by the Animal Ethics Committee of Hunan Agricultural University. SPF-grade male 4-week-old ICR mice (18 ± 2 g) purchased from Hunan SJA Laboratory animal Co., Ltd. (Changsha, China) were housed under standard conditions with a strict 12 h light/dark cycle specific pathogen-free animal laboratory (humidity at 50 ± 15%, temperature 22 ± 2 °C). All mice were provided with basal diet and pure water.
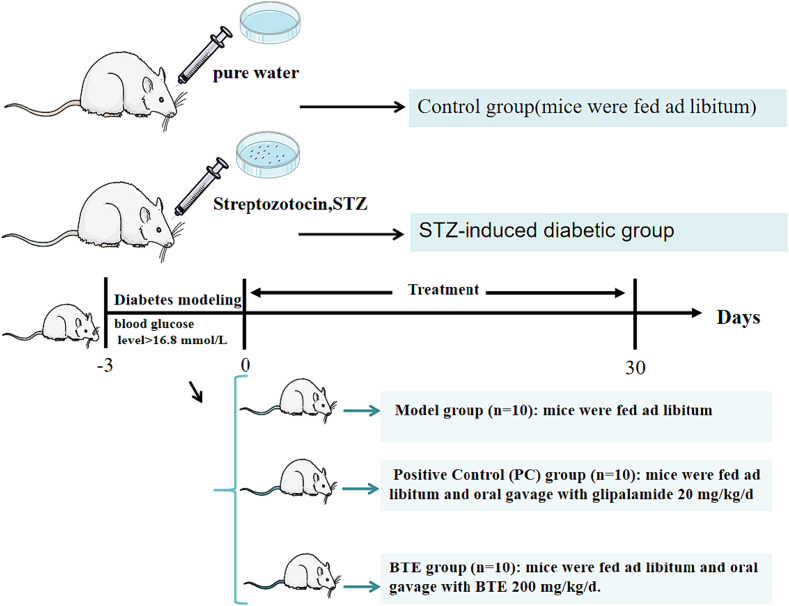
STZ is a broad-spectrum antibiotic with antitumor, carcinogenic, and diabetogenic properties. It was isolated from Streptomyces achromogenes in the 1960s and has since been shown to have diabetogenic properties(Gomes, Vedasiromoni, Das, Sharma, & Ganguly, 1995b). Therefore, STZ was selected to establish diabetes mice model. Following a week of accommodation, the mice were divided into two groups randomly (Fig. 1). Control group (n = 10): mice were fed ad libitum. STZ-induced diabetic mice(Furman, 2021): >30 health mice were fed ad libitum, provided pure water and injected with STZ 150 mg/kg/d. Fasting blood glucose test was performed after 72 h. Once blood glucose level exceeded 16.8 mmol/L, the mice were selected as STZ-induced diabetic mice for the next stage of experiments.
Fig. 1.
Timeline depicting the diet of BTE or ad libitum in each group.
Next, STZ-induced diabetes mice were randomly assigned to three groups. Model group (n = 10): mice were fed ad libitum. Positive Control (PC) group (n = 10): mice were fed ad libitum and oral gavage with glipalamide 20 mg/kg/d. BTE group (n = 10): mice were fed ad libitum and oral gavage with BTE 200 mg/kg/d. Control groups were fed ad libitum at the same time. 30 days later, mice in all of the four groups fasted for 12 h and then were weighed and executed by cervical dislocation. The blood and liver tissues were collected and stored at −80 °C.
2.3. α-amylase activity test
The described approach was followed in determining the α-amylase activity (Little et al., 2022). Before the experiment was conducted, BTE (0.75, 1, 1.25, 2, 2.5 mg/mL), TF1, TF2A, TF2B, TF3 (1, 10, 50, 100, 250 μg/mL respectively), and acarbose (1, 10, 100, 250, 1000 μg/mL) solutions were prepared in phosphate buffered saline (PBS). In the first step, 1.0 mL BTE, theaflavins monomers and acarbose were added to 0.35 mL of α-amylase solution (10 U/mL) and incubated at 37 °C for 5 min, respectively. Then, 0.4 mL soluble starch solution (1%) was added and incubated at 37 °C for 15 min. Finally, 0.5 mL DNS was added and maintained at 100 °C for 5 min. The absorbance was measured at 540 nm by microplate reader (Thermo Scientific, Multiskan FC, USA). Deionized water was used as sample negative control and control blank, acarbose was used as positive control. Inhibition rate of α-amylase was calculated following Eq. 1.
| (1) |
A1: the absorbance of sample reaction, A2: the absorbance of sample negative control, A3: the absorbance of control reaction, A4: the absorbance of control blank.
2.4. Inhibitory kinetic assay
The Michaelis-Menten kinetics analysis was determined based on earlier research (Li et al., 2021). 0.35 mL α-amylase solution (10 U/mL), 0.35 mL α-amylase solution (10 U/mL) with theaflavins or TF1, TF2A, TF2B, TF3 were incubated at 37 °C for 5 min, respectively. Then, 0.5%, 1.0%, 1.5% and 2.0% soluble starch solution was added and the mixed solutions were maintained at 37 °C for 15 min. Finally, 0.5 mL of DNS reagent was added and maintained at 100 °C for 5 min. The absorbance was measured at 540 nm by microplate reader. The inhibition kinetics of α-amylase was calculated by Eq. 2.
| (2) |
V: the starting reaction velocity (mg/L/min), [S]: the starch solution concentration (mg/mL), Vmax: the maximum reaction velocity (mg/L/min), Km: the α-amylase Michaelis–Menten constant (mg/mL).
2.5. Molecular docking analysis
The molecular docking analysis was carried out according to a previous study(Wang et al., 2012). α-Amylase (PDB: 1BVN)(Deng, 2021) structure was obtained from the Protein Data Bank (PDB: https://www.rcsb.org/). The molecule structures of inhibitors (TF1, TF2A, TF2B and TF3) were drawn using ChemDraw 20.0 (PerkinElmer Informatics, Boston, MA, USA). Autodock 1.5.7 (Scripps Research Institute, San Diego, CA, USA) and PyMOL 2.2.0 (DeLano Scientific LLC, South SanFrancisco, CA, USA) were used to predict the interaction sites between α-amylase and inhibitors. Before the formal molecular docking operation began, the α-amylase molecule was treated with PyMOL to remove solvents and small molecules and then together with the inhibitors were subjected to hydrogenation and charge calculations using Autodock. As for TF1, docking simulations was carried out in a computation grid box at the active site of the enzyme with x, y and z dimensions of 78.0, 66.0 and 68.0. The x, y and z center dimensions were 18.205, 10.849 and 41.797. As for TF2A, docking simulations was carried out in a computation grid box at the active site of the enzyme with x, y and z dimensions of 79.333, 79.333 and 79.333. The x, y and z center dimensions were 17.282, 11.599 and 39.260. As for TF2B, docking simulations was carried out in a computation grid box at the active site of the enzyme with x, y and z dimensions of 82.0, 60.0 and 62.0. The x, y and z center dimensions were 17.282,11.599 and 39.26. As for TF3, docking simulations was carried out in a computation grid box at the active site of the enzyme with x, y and z dimensions of 116.0, 86.0 and 82.0. The x, y and z center dimensions were 0.185, 0.212 and 38.002. The results were visualized by Discovery Studio 2021 (Dassault Systèmes, Concord, MA, USA).
2.6. Ultraviolet-visible absorption spectroscopy analysis
The Ultraviolet-Visible Absorption Spectroscopy was carried out using PerkinElmer LAMBDA 365 (Waltham, MA, USA) according to a previous study(Shi, Pan, Jiang, Liu, & Wang, 2016). α-amylase (10 U/mL) and TF1, TF2A, TF2B or TF3 (0.3 mg/mL) were mixed to form the monomer-α-amylase system, respectively. The UV–Vis absorption spectra of the monomer-α-amylase system was scanned in the range of 190–800 nm.
2.7. Fluorescence spectroscopy analysis
Fluorescence Spectroscopy was carried out using a fluorescence spectrophotometer (Vaikhan LUX, Thermo Fisher Scientific, Waltham, MA) according to a previous study(Lv et al., 2022). 50 μL α-amylase (10 U/mL) was mixed with TF1, TF2A, TF2B or TF3 (10 μg/mL-200 μg/mL) at 298 K, 303 K and 310 K to form the monomer-α-amylase system, respectively. The fluorescence spectra of the monomer-α-amylase system was determined at 278 nm wavelength with a scanning range of 290-450 nm. The thermodynamic parameters (ΔH and ΔS), quenching rate constant (Ksv), binding constant (Ka) and the number of binding sites (n) were calculated by Eq. 3- Eq. 6.
| (3) |
| (4) |
| (5) |
| (6) |
F0 or F: the fluorescence intensities in the presence or absence of TF1, TF2A, TF2B and TF3, [Q]: the concentration of TF1, TF2A, TF2B and TF3, τ0: the constant of the lifetime of the fluorophore (10−8 s), ΔH: changes in enthalpy of the system, ΔS: changes in entropy of the system, R: the gas constant of 8.31 J (mol K) −1.
2.8. Statistical analysis
All the data analysis was performed with the SPSS 20.0 (Chicago, IL, USA). Statistical significance between groups employed the one-way ANOVA coupled with the Tukey and LSD multiple comparison tests. Figures were generated using GraphPad Prism 8.0.1 (San Diego, CA, USA). Results were expressed as Mean ± SD (standard deviation).
3. Results and discussion
3.1. Anti-hyperglycemic effect of BTE on STZ-induced diabetic mice
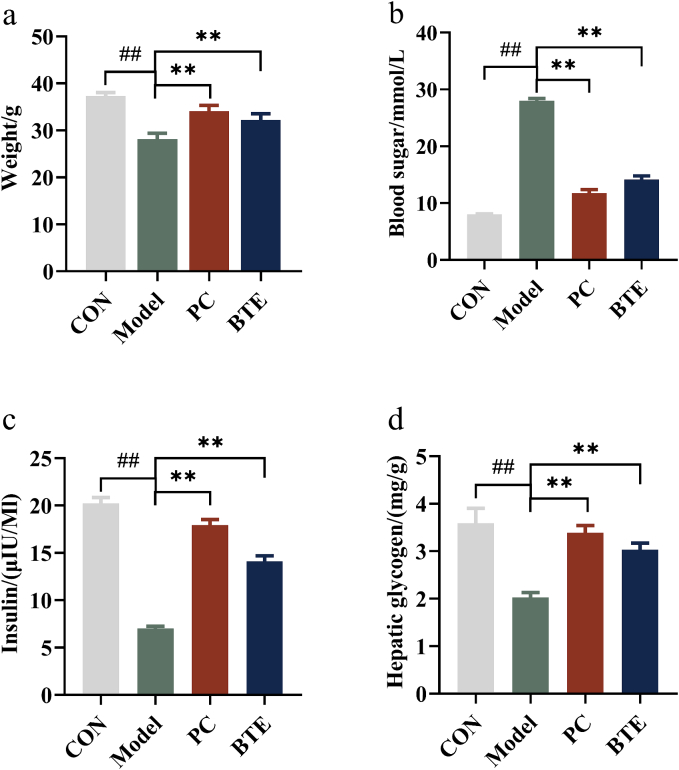
As can be seen from Fig. 2, the blood glucose level in the model group was 28.001 mmol/L (>16.8 mmol/L). This indicated that the diabetic mice model was established successfully. The body weight, blood glucose, liver glycogen content and plasma insulin levels of different groups were recorded. Compared with normal mice, the blood glucose of diabetic mice increased (p < 0.01) while the body weight, plasma insulin levels and liver glycogen content of them decreased (p < 0.01). The administration of BTE caused a significant change in body weight, plasma insulin levels, liver glycogen content and blood sugar, indicating that BTE showed effective anti-diabetic activity and that effect was comparable to that of glipalamide. These findings were consistent with previous study(Li et al., 2021). Gomes et al. reported that STZ treated animals showed rapid normalisation in blood glucose level after receiving tea extract indicating the tea treated animals overcome the toxic effect of STZ(Gomes et al., 1995b). Similarly, Matsui et al. (Matsui et al., 2007) demonstrated that theaflavin-3-gallate had a stronger inhibitory effect on the elevation of blood glucose in rats fed maltose. Reported data also provided some explanations for the above results. Theaflavins were believed to show anti-hyperglycemic activity by inducing insulin secretion (Abeywickrama, Ratnasooriya, & Amarakoon, 2011), increasing the activity of insulin-degrading enzymes(Abeywickrama et al., 2011), stimulating insulin receptor correlated signaling pathways(Shoji & Nakashima, 2006) (Wootton-Beard & Ryan, 2011). However, none of the above studies has fully clarified the underlying mechanisms of theaflavins' hypoglycemic effect.
Fig. 2.
Effect of the BTE on STZ-induced diabetic mice. Control: normal control group were fed ad libitum. Model: The diabetic mice induced by STZ were fed ad libitum. PC: STZ-induced diabetic mice were fed ad libitum and oral gavage with glipalamide 20 mg/kg/d. BTE: STZ-induced diabetic mice were fed ad libitum and oral gavage with BTE 200 mg/kg/d. ##: p < 0.01 versus Control. **; p < 0.01 versus Model.
3.2. Inhibition kinetics of α-amylase against BTE and the theaflavins monomers
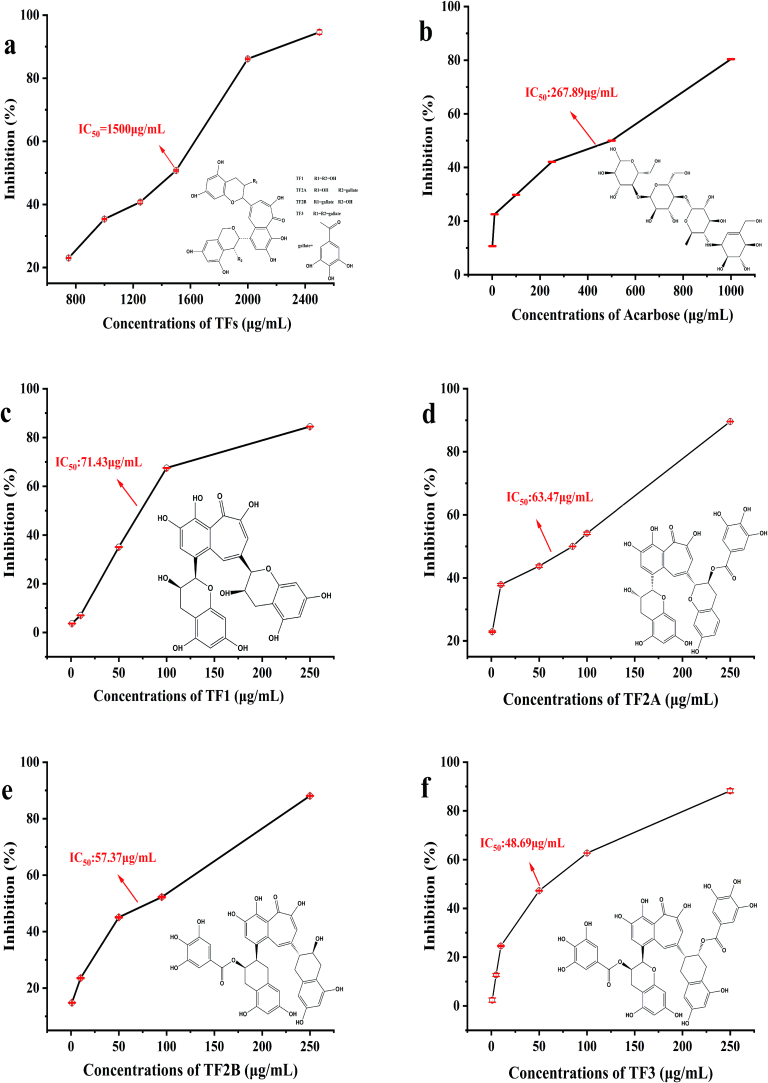
The inhibitory activity on α-amylase of the five compounds decreased as follows: TF3 (IC50 = 48.69 μg/mL) > TF2B (IC50 = 57.37 μg/mL) > TF2A (IC50 = 63.47 μg/mL) > TF1 (IC50 = 71.43 μg/mL) > acarbose (IC50 = 267.89 μg/mL)(See Fig. 3). This finding was basically consistent with previous report that the inhibition of α-amylase activity by tea polyphenols with gallate groups was higher than that of tea polyphenols without gallate groups(Sun, Warren, Netzel, & Gidley, 2016). Compared with TF1, which has (E)C as the structural unit, the other theaflavins monomers have one additional hydroxyl group on the B ring and galactoacyl groups at C3. We speculated that this can partly explain the order of theaflavins monomers' inhibitory activity on α-amylase. Besides, previous reports also have found that the inhibitory effect of polyphenolic compounds on α-amylase are closely affiliated the hydrogen bonds that formed between the -OH groups in the compounds and the amino acids in the α-amylase side chain(Kawamura-Konishi et al., 2012). The inhibitory activity of a polyphenols also depended on 3 and/or 3’galloyl (GM) in their molecular structures(Sun, Gidley, & Warren, 2017). Tea polyphenols that consist GM in the C-ring enhanced their binding to α-amylase, thereby increased their inhibitory activity against the enzyme(Sun, Warren, et al., 2016). The role of GM in the binding of tea polyphenols to α-amylase attributed to form the hydrogen binds and π stacks (Cao et al., 2020).
Fig. 3.
The inhibition effects of BTE and theaflavins monomers on α-amylase.
a: BTE; b: Acarbose; c:TF1; d:TF2A; e:TF2B; f:TF3.
In a word, BTE and the theaflavins monomers could effectively inhibit the activity of α-amylase and are promising α-amylase inhibitors. Their effects are closely related with gallate groups in the inhibitors' molecular structure.
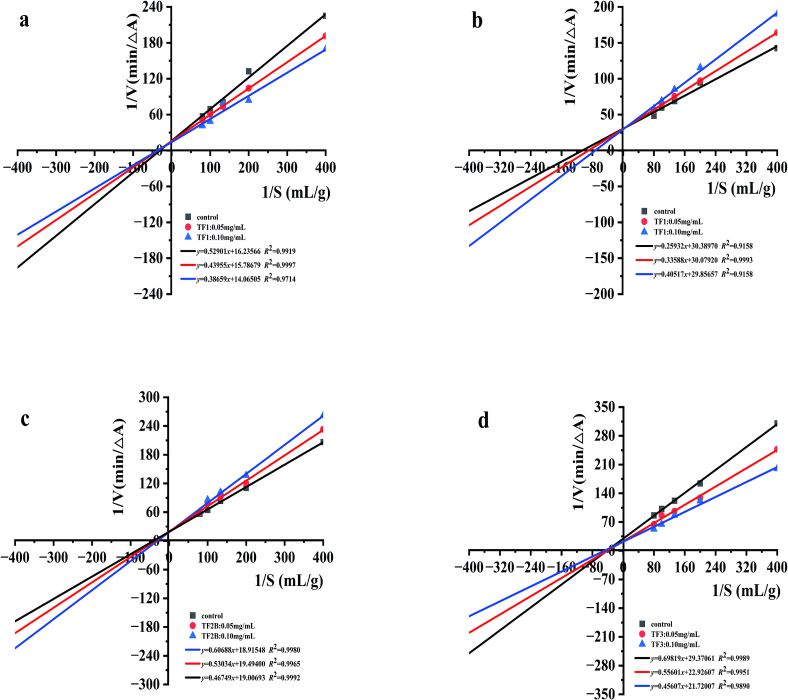
3.3. Michaelis-Menten kinetics analysis of α-amylase against TF1, TF2A, TF2B, TF3
The activity of enzyme is influenced not only by their own spatial structure, but also by the structure of the inhibitor molecule(Kroll, Rawel, & Rohn, 2003). In enzymatic reactions, when the substrate and inhibitor compete for the same enzyme binding site, the inhibitor can dramatically alter the binding between the enzyme and the substrate and show a competitive inhibition effect. When the inhibitor and the substrate can bind to the enzyme at the same time, which indicate there is no competition between them, the inhibitor show a non-competitive inhibition effect. The inhibition type of compound against α-amylase was determined by using Lineweaver-Burk double reciprocal plots. When the Lineweaver-Burk plots of compound with different concentrations had an intersection at y axis, indicating that there was no change of Vmax. Meanwhile, the Km increased as inhibitor concentrations increased. The compound was a competitive inhibitor against α-amylase (Yaqin et al., 2018). When the Lineweaver-Burk plots of compound with different concentrations had an intersection at x axis,indicating that there was no change of Km. Meanwhile, the Vmax decreased as inhibitor concentrations increased. The compound was a non-competitive inhibitor against α-amylase (Martinez-Gonzalez, Díaz-Sánchez, de la Rosa, Bustos-Jaimes, & Alvarez-Parrilla, 2018). When the curves met at the third quadrant of the figure, indicating that the Vmax and Km values decreased with the increasing inhibitor concentration, the compound exhibited a mixed-type non-competitive inhibition of α-amylase(L. Wang et al., 2018).
As can be seen from Fig. 4, the lines of the inhibition plot were not intercepted with the concentration of TF1 on either the vertical or horizontal axis. When the concentration of TF1(Fig. 4a) and TF3 (Fig. 4d) increased, Vmax and Km decreased, indicating that both TF1 and TF3 inhibit activity of α-amylase in a mixed manner. Meanwhile, Vmax remained constant and Km increased when the concentration of TF2A (Fig. 4b) and TF2B (Fig. 4c) increased, suggesting that TF2A and TF2B are both competitive inhibitors of α-amylase. These results are identical to previous studies(Sun et al., 2016). When TF2A or TF2B binded to α-amylase, the EI-complex formed due to the competitive inhibition. While, TF1 or TF3 formed the ES-complex with α-amylase and further formed the ESI-complex due to the uncompetitive inhibition. α-Amylase activity was inhibited by those mixed effects. Previous studies also have reported similar results(Gong et al., 2020). Different theaflavins (TF1, TF2A, TF2B, TF3) exhibited different inhibition modes, which attributed to a decrease in the substrate binding affinity of α-amylase after the inhibitor bound to this site(Narita & Inouye, 2011).
Fig. 4.
The double-reciprocal Lineweaver-Burk plot analysis for the inhibition of α-amylase against theaflavins monomers.
a:TF1; b:TF2A; c:TF2B; d:TF3.
To clarify the inhibitory mechanism of theaflavins monomers on α-amylase, further studies were conducted by molecular docking analysis, UV–Vis spectroscopy, and fluorescence spectroscopy.
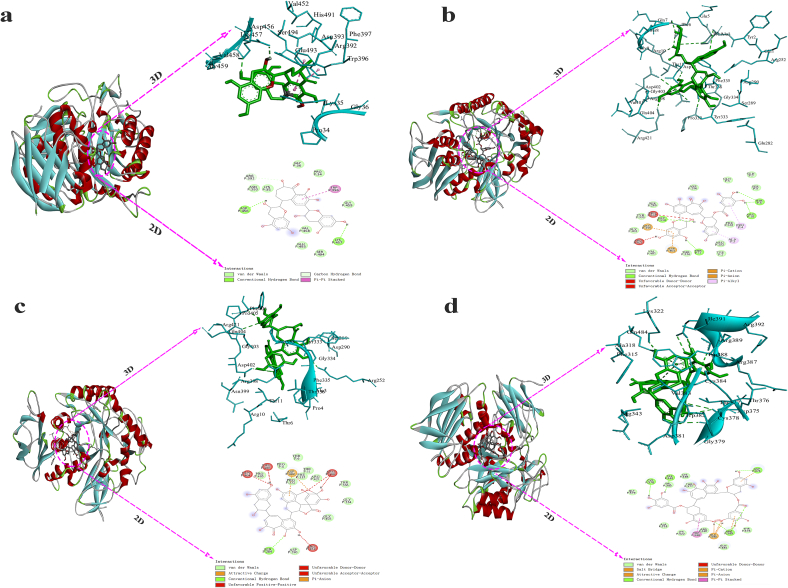
3.4. Molecular docking of α-amylase with TF1, TF2A, TF2B, and TF3
Molecular docking has been extensively employed to look into the connections bridging organic substances and macromolecular compounds(Miao, Jiang, Jiang, Zhang, & Li, 2015). Results showed that TF1, TF2A, TF2B and TF3 had low binding energy to bind with α-amylase: TF2B (−9.4 kcal/mol) < TF3 = TF2A (−9.0 kcal/mol) < TF1(−8.6 kcal/mol). These results indicated that TF1, TF2A, TF2B and TF3 had high α-amylase inhibitory activity. It has been suggested that polyphenols with high molecular weights possess a considerable number of hydroxyl groups, which might promote hydrogen bonding and hydrophobic interactions between polyphenols and α-amylase, thereby reducing enzyme’ activity(Bandyopadhyay, Ghosh, & Ghosh, 2012). In this study, some theaflavin molecules were embedded in the hydrophobic pocket of α-amylase, the larger the molecular weight are, the more parts were involved in the interaction. This help explained the finding that TF3 showed the strongest inhibitory effect on α-amylase.
Molecular docking also revealed the binding sites and binding forces between theaflavins monomers and α-amylase. As Fig. 5a showed, TF1 formed hydrogen bonds with α-amylase at Asp456 and Lys457, hydrogen carbon bonds with Agr392, π - π stacking interaction with Trp396, van der Waals force with Gly36, Val458, Pro34, Gly459 and Asn393. Similarly, TF2A formed hydrogen bonds with α-amylase at Thr11, Thr6, Arg10 and Gly334, van der Waals force with Gl403, Tyr333, Ser289, Asp290, Gln5, Gln7, Ser8, Phe335, Arg252, Tyr2, Thr336 and Val401, pi-Alkyl interaction with Pro4 and Ala3, pi-cation and pi-anion electrostatic interaction with Arg398 and Asp402(Fig. 5b). TF2B formed hydrogen bonds with α-amylase at Ser289, pi-anion and electrostatic interaction with Asp402, van der Waals force with Thr6, Pro4, Pro405, Thr11, Pro332, Arg421, Thr336, Gly334, Gky403 and Asp290 (Fig. 5c). TF3 formed hydrogen bonds with α-amylase at Cys378, Trp382, Asp375 and Arg389, a salt bridge, pi-cationic, pi-anion and electrostatic interaction with Glu390, van der Waals force with Gly379, Val383, Cys384, Thr377, Ala318, Cys322, Arg387, Thr376 and Gln484(Fig. 5d). These interactions caused the formation of the monomer- α-amylase complex and changed the catalytic activity of α-amylase and ultimately induced the anti-hyperglycemic activity of the theaflavins monomers(Xiang et al., 2020). Similar behaviour of theaflavins was also reported in studies of natural inhibitors of α-glucosidase(Zeng, Ding, Hu, Zhang, & Gong, 2019) (Zhang et al., 2019).
Fig. 5.
The image of the interaction obtained after the docking of TF1(a), TF2A(b), TF2B(c), TF3(d) with α-amylase.
It should be noted that TF3 showed relatively higher binding affinity to α-amylase than TF2B, the Eb values were on the opposite (TF3 < TF2B). This may result from the higher steric hindrance of TF3-enzyme than that of TF2B-enzyme due to higher molecular weights of TF3(Cao et al., 2020). In addition, molecular docking analysis, although very effective, provided static poses of protein-ligand interactions. Acctually, these interactions were highly dynamic.Therefore, molecular docking analysis were only used in the preliminary screening procedure especilly when the number of compounds was large, more validation tests need to confirm the results(Durrant & Mccammon, 2011) (Salmaso & Moro, 2018).
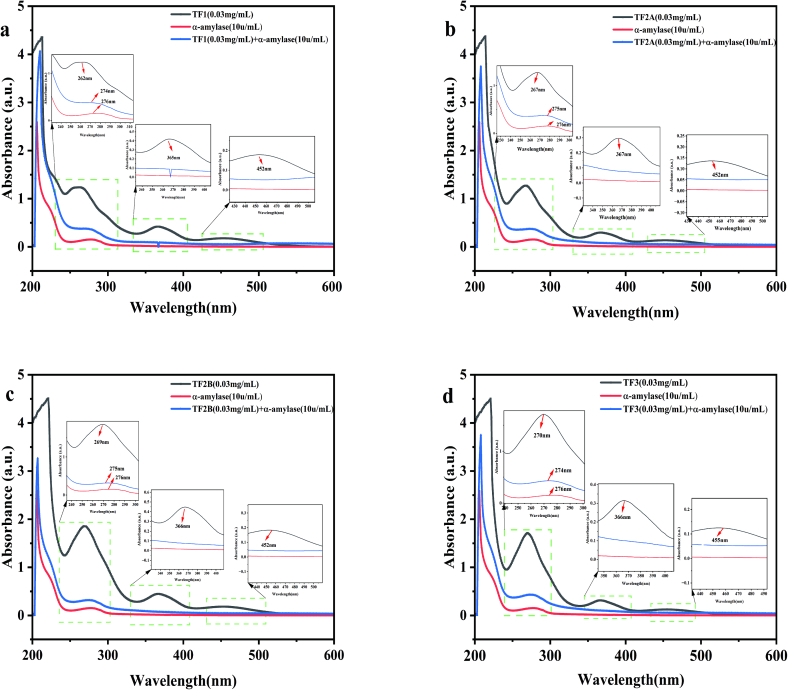
3.5. Investigation of the interaction between theaflavins monomers and α-amylase by ultraviolet-visible absorption spectroscopy
Ultraviolet-Visible Absorption Spectroscopy is a method that irradiate protein molecules and collect the Ultraviolet-Visible absorption spectra (Nienhaus & Nienhaus, 2005) (Yu et al., 2020). The spectra can be used to determine whether the microenvironment or the structural conformation of proteins has been changed (Abbasi, Benvidi, Gharaghani, & Rezaeinasab, 2018).
In this study, the spectra were used to determine whether the interaction occurred between theaflavins monomers and α-amylase. As Fig. 6 showed, the theaflavins monomers showed three absorption peaks in the wavelength of 200–600 nm (TF1: 262 nm, 365 nm, 452 nm; TF2A: 267 nm, 367 nm, 452 nm; TF2B: 269 nm, 366 nm, 452 nm; TF3: 270 nm, 366 nm, 455 nm), and the tryptophan and tyrosine amino acid residues in α-amylase produced the greatest adsorption peak, which was measured at 276 nm(Abbasi et al., 2018). Strong interactions between α-amylase and theaflavins monomers could change the maximum adsorption peak(Abdollahi, Ince, Condict, Hung, & Kasapis, 2020). As we can see from Fig. 6a-d, the addition of theaflavins monomers steadily raised the absorbance peak of α-amylase. Meanwhile, the maximum absorption spectra of theaflavins monomers had a slight red shift when α-amylase existed. These findings verified that changes in the framework conformation of α-amylase were caused due to the formation of monomer-α-amylase complex(Yue, Zhao, Liu, Yan, & Sun, 2017) and that there existed strong interactions between α-amylase and theaflavins monomers.
Fig. 6.
Ultraviolet-Visible absorption spectra of theaflavins monomer-α-amylase system.
a:TF1; b:TF2A; c:TF2B; d: TF3.
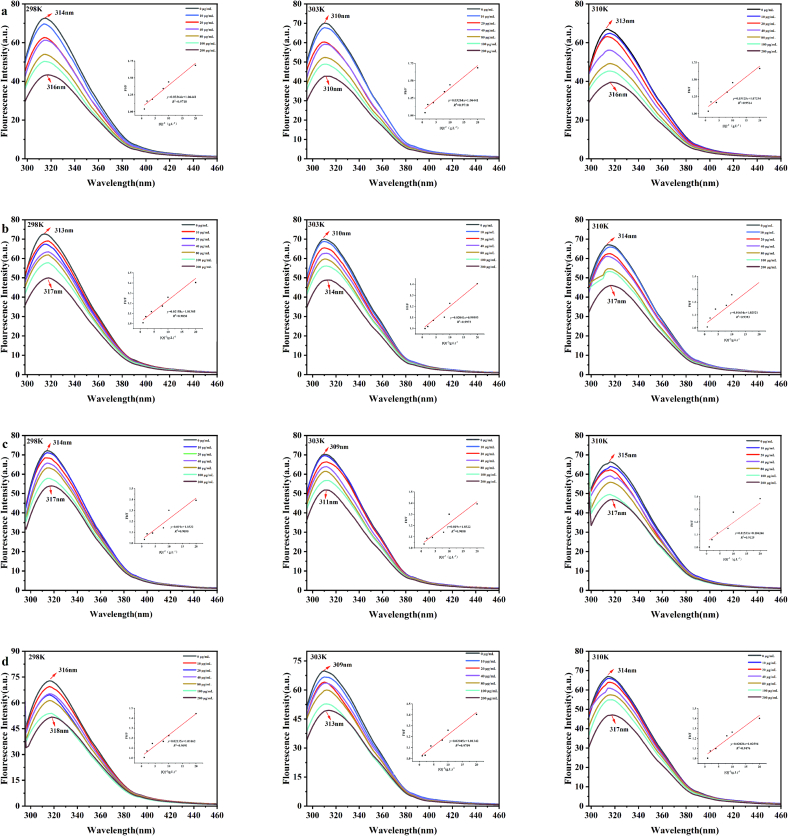
3.6. Investigation of the interaction between four theaflavins monomers and α-amylase by fluorescence spectroscopy
Fluorescence spectroscopy has long been used to investigate how ligands and proteins interact with each other(Y. Teng, Zhang, & Liu, 2011). Tryptophan, tyrosine and phenylalanine are the main contributors to endogenous protein fluorescence (M. Li & Hagerman, 2014).
Results showed that the characteristic emission peak of α-amylase (314 nm) quenched when theaflavins monomers added in the system (Fig. 7). The α-enzyme's fluorescence intensity consistently decreased when the concentrations of theaflavins monomers increased, demonstrating the presence of interactions between the α-amylase and the theaflavins monomers. Meanwhile, the Stern-Volmer plot of theaflavins monomers was linear with the concentrations range of theaflavins monomers, which further verified the quenching effect of theaflavins monomers on fluorescence in α-amylase(M. Li & Hagerman, 2014). Besides, when the concentrations of theaflavins monomers increased, the characteristic peak of α-amylase were slightly red-shifted, indicating that the hydrophobicity of the amino acid residues of α-amylase was reduced and the hydrophilicity of the environment was increased. This may be attributed to existence of many hydrophilic hydroxyl groups in theaflavins monomers(Liu, Han, Zhang, Liu, & Kong, 2019).
Fig. 7.
Fluorescence spectrum of α-amylase (10u/mL) with different concentrations of theaflavins monomer at 298 K, 303 K, 310 K.
a:TF1; b:TF2A; c:TF2B; d: TF3.
Besides, Further analysis of the fluorescence quenching data was conducted using Eq.(3)(Ashwar, Gani, Shah, Wani, & Masoodi, 2016) and Eq.(4) to clarify the mechanism underlying the formation of monomer-α-amylase complex (C. Li, Yu, Wu, & Chen, 2020). Table 1 showed that Stern-Volmer curved lines of α-amylase were approximate linear with the intercept is 1. The higher the temperature was, the smaller the quenching constant (Ksv) slope represent is. These findings were according with the characteristics of the static quenching mechanism(Svihus & Hervik, 2016), suggesting that theaflavins monomers and α-amylase formed fluorescent compounds(Raeessi-babaheydari, Farhadian, & Shareghi, 2021). The results mentioned above indicated that static quenching rather than dynamic collision could be the mechanism underlying the fluorescence quenching of α-amylase induced by theaflavins monomers(Abdollahi et al., 2020).
Table 1.
Binding Constants of theaflavins monomers with α-Amylase.
| Samples | T(K) | Ksv(L/g) | n | Ka(L/g) | ΔH0(KJ/g) | ΔS0(g/KJ) | ΔG0(KJ/g) |
|---|---|---|---|---|---|---|---|
| TF1 | 298 | 4.7890 | 0.9855 | 4.4955 | 0.0048 | 0.0351 | −10.4550 |
| 303 | 4.6468 | 1.0400 | 5.1038 | −10.6305 | |||
| 310 | 4.2950 | 1.0488 | 5.6151 | 10.87622 | |||
| TF2A | 298 | 2.5203 | 1.0486 | 2.9090 | 0.0003 | 0.0027 | −0.8163 |
| 303 | 2.3127 | 0.9782 | 2.0702 | −0.8299 | |||
| 310 | 1.3875 | 1.0573 | 2.8982 | −0.8491 | |||
| TF2B | 298 | 1.9936 | 1.0015 | 2.0248 | 0.0005 | 0.2769 | −82.4859 |
| 303 | 2.5579 | 0.7198 | 1.0433 | −83.8699 | |||
| 310 | 1.8134 | 1.0573 | 3.0371 | −85.8075 | |||
| TF3 | 298 | 3.0635 | 0.8870 | 2.0494 | 0.0071 | 0.0278 | −8.2773 |
| 303 | 2.8108 | 0.7728 | 1.3423 | −8.4163 | |||
| 310 | 2.5895 | 1.1765 | 2.6581 | −8.6109 |
The values of n were approximate near to 1, indicating that there existed a single high-affinity bonding point between theaflavins monomers and α-amylase(Zhao, Huang, Sun, Zhao, & Tang, 2020). The enzyme-ligand binding forces including hydrogen bonds, hydrophobic interactions, electrostatic force and van der Waals force four(Wu et al., 2018). The associated thermodynamic parameters (enthalpy change ΔH°, entropy ΔS°, and gibbs free energy ΔG°) were calculated to determine the interaction type of theaflavins monomers and α-enzyme. The binding reaction values of ΔH° and ΔS° are positive, demonstrating that hydrophobic interactions is the primary binding force(Qie et al., 2020). The binding reaction's ΔG values are negative, suggesting that the reaction is spontaneous.
Previous study reported that the backbone of phenolic acid displayed π-π T-shaped, π-π stacking interactions with His 201, Try 3, and Try 151 in α-amylase, which contributed to the stability of the complex and the inhibition of α-amylase (Aditi et al., 2018). In addition, enhanced interactions altered the secondary structure of α-amylase through a hydrogen bonding network, which blocked substrate accessing into the catalytic site and led to α-amylase inactivation (Xu, Xie, Xie, Liu, & Chen, 2018). Therefore, we speculate that the interaction between theaflavins and α-amylase showed influence on enzyme function and substrate binding in the following two aspects. First, theaflavins entered the hydrophobic pocket of α-amylase and formed a complex under electrostatic attraction and hydrophobicity spontaneously, which led to static fluorescence quenching. Second, the complex stability were enhanced by interaction forces (e.g., hydrogen bonding), which caused secondary structure changes in α-amylase and loss of enzyme activity. Ultimitely, the starch digestion was reduced and the BTE showed alleviation effect on postprandial hyperglycemia.
In this paper, the in vitro and in vivo studies as well as the mechanism underlying the hypoglycemic effects of theaflavins was performed. These findings will lay the theoretical basis for the use of black tea theaflavins into novel low-glycemic index functional foods or medicines targeting α-amylase. However, whether their exists side effecs in the application of theaflavins remains unknown. Besides, theaflavins are phenolic compounds that has low bioavailability in the gastrointestinal tract which may limit their health benefits and hinder their further application(Karaś, Jakubczyk, Szymanowska, Złotek, & Zielińska, 2017). Study also found that theaflavins affected the human health via the microbiota-gut-brain axis instead of being absorbed(Li et al., 2023). Therefore, more work need to be conducted to fully understand the hypoglycemic effects of theaflavins. Finally, the interaction between theaflavins and α-amylase was not confirmed in the in vivo study and there was a lack of direct proof for the connection between the hypoglycemic effects of theaflavins and their interaction with α-amylase. Further animal studies and human studies will be done in the future. We hope that theaflavins can be deveioped into safe and effective α-amylase inhibitors and can be used in hyperglycemia prevention and treatment.
4. Conclusions
In this research, we revealed the mechanism underlying the anti-hyperglycemic effect of BTE, our data suggest that: (1) BTE show significant anti-hyperglycemic effect by suppressing hyperglycemia, and restoring normal liver in the diabetic mice. (2) Theaflavins monomers show high inhibition activity against α-amylase, while TF2A and TF2B in a competitive mode TF1 and TF3 in a reversible mixed mode. (3) Theaflavins monomers bind to the hydrophobic area surrounding the active site of α-amylase by hydrophobic interactions. These findings will help clarify the mechanism of black tea's anti-hyperglycemic effects and lay the theoretical foundation for the applacation of black tea theaflavins into novel low-glycemic index functional foods or medicines.
Funding
This research was funded by National Natural Science Foundation of China (NO. 32001680), the Scientific Research Foundation of Hunan Provincial Education Department (NO. 23A0187), China Postdoctoral Science Foundation (NO. 2020M682568) and the Graduate Innovation Project by Postgraduate Innovation Project of Hunan Province (NO. CX20230721).
CRediT authorship contribution statement
Maiquan Li: Writing – review & editing, Writing – original draft, Validation, Supervision, Investigation, Funding acquisition. Yunxia Dong: Writing – original draft, Methodology, Data curation. Mangjun Kang: Writing – review & editing. Tiantian Tao: Writing – review & editing. Wenlan Li: Writing – review & editing. Sheng Zhang: Writing – review & editing. Wei Quan: Writing – review & editing, Writing – original draft. Zhonghua Liu: Writing – review & editing, Writing – original draft, Supervision.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product service and/or company that could be construed as influencing the position presented in the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2024.101296.
Contributor Information
Wei Quan, Email: reus_quan@hunau.edu.cn.
Zhonghua Liu, Email: zhonghua-liu@hunau.edu.cn.
Appendix A. Supplementary data
Supplementary material
Data availability
I have shared the link to my data at the Attach File Step
References
- Abbasi S., Benvidi A., Gharaghani S., Rezaeinasab M. Chemometric studies of thymol binding with bovine serum albumin: A developing strategy for the successful investigation of pharmacological activity. Bioelectrochemistry. 2018;124:172–184. doi: 10.1016/j.bioelechem.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Abdollahi K., Ince C., Condict L., Hung A., Kasapis S. Combined spectroscopic and molecular docking study on the pH dependence of molecular interactions between β-lactoglobulin and ferulic acid. Food Hydrocolloids. 2020;101 doi: 10.1016/j.foodhyd.2019.105461. [DOI] [Google Scholar]
- Abeywickrama K.R.W., Ratnasooriya W.D., Amarakoon A.M.T. Oral hypoglycaemic, antihyperglycaemic and antidiabetic activities of Sri Lankan broken Orange pekoe Fannings (BOPF) grade black tea (Camellia sinensis L.) in rats. Journal of Ethnopharmacology. 2011;135(2):278–286. doi: 10.1016/j.jep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Aditi W., Su H., Seong H., Ah J., Jae, Sue Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chemistry. 2018;276:383–389. doi: 10.1016/j.foodchem.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Ashwar B.A., Gani A., Shah A., Wani I.A., Masoodi F.A. Preparation, health benefits and applications of resistant starch—A review. Starch-Stärke. 2016;68(3–4):287–301. doi: 10.1016/j.jff.2022.105094. [DOI] [Google Scholar]
- Bandyopadhyay P., Ghosh A.K., Ghosh C. Recent developments on polyphenol–protein interactions: Effects on tea and coffee taste, antioxidant properties and the digestive system. Food & Function. 2012;3(6):592–605. doi: 10.1039/c2fo00006g. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V.K., Singh R., Sharma J., Rajendran V., Purohit R., Kumar S. Bioactive molecules of tea as potential inhibitors for Rna-dependent Rna polymerase of Sars-Cov-2. Frontiers in Medicine. 2021;8 doi: 10.3389/fmed.2021.684020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan L.P., Borah P., Sabhapondit S., Gogoi R., Bhattacharyya P. Spatial variability of theaflavins and thearubigins fractions and their impact on black tea quality. Journal of Food Science and Technology. 2015;52:7984–7993. doi: 10.1007/s13197-015-1968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Liu Z., Dong X., Wang Y., Zhu L., Li M., Xu Y. Hypoglycemic and lipid lowering effects of theaflavins in high-fat diet-induced obese mice. Food & Function. 2021;12(20):9922–9931. doi: 10.1039/d1fo01966j. [DOI] [PubMed] [Google Scholar]
- Cao J., Zhang Y., Han L., Zhang S., Duan X., Sun L., Wang M. Number of galloyl moieties and molecular flexibility are both important in alpha-amylase inhibition by galloyl-based polyphenols. Food & Function. 2020;11(5):3838–3850. doi: 10.1039/C9FO02735A. [DOI] [PubMed] [Google Scholar]
- Chauhan M., Bhardwaj V.K., Kumar A., Kumar V., Kumar P., Enayathullah M.G.…Kumar S. Theaflavin 3-gallate inhibits the main protease (Mpro) of SARS-CoV-2 and reduces its count in vitro. Scientific Reports. 2022;2(1):13146. doi: 10.1038/s41598-022-17558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das R.R., Rahman M.A., Al-Araby S.Q., Islam M.S., Rashid M.M., Babteen N.A.…Rafi M.K.J. The antioxidative role of natural compounds from a green coconut mesocarp undeniably contributes to control diabetic complications as evidenced by the associated genes and biochemical indexes. Oxidative Medicine and Cellular Longevity. 2021;2021:1–22. doi: 10.1155/2021/9711176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng K.Y. Expression analysis and active cyclic peptide design of Chuanxiong ligustici α-amylase inhibitor[D] Southwest Jiaotong University. 2021 doi: 10.27414/d.cnki.gxnju.2021.000996. [DOI] [Google Scholar]
- Durrant J.D., Mccammon J.A. Molecular dynamics simulations and drug discovery. BMC Biology. 2011;9:71. doi: 10.1080/17460441.2018.1403419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettach S., Mrabti H.N., Sayah K., Bouyahya A., Salhi N., Cherrah Y., El Abbes F.M. Phenolic content, acute toxicity of Ajuga iva extracts and assessment of their antioxidant and carbohydrate digestive enzyme inhibitory effects. South African Journal of Botany. 2019;125:381–385. doi: 10.1016/j.sajb.2019.08.010. [DOI] [Google Scholar]
- Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Current Protocols. 2021;1(4) doi: 10.1002/cpz1.78. [DOI] [PubMed] [Google Scholar]
- Gandhi G.R., Vasconcelos A.B.S., Wu D.-T., Li H.-B., Antony P.J., Li H.…Gan R.-Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients. 2020;12(10):2907. doi: 10.3390/nu12102907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A., Vedasiromoni J., Das M., Sharma R., Ganguly D. Anti-hyperglycemic effect of black tea (Camellia sinensis) in rat. Journal of Ethnopharmacology. 1995;45(3):223–226. doi: 10.1016/0378-8741(95)01223-Z. [DOI] [PubMed] [Google Scholar]
- Gomes A., Vedasiromoni J.R., Das M., Sharma R.M., Ganguly D.K. Anti-hyperglycemic effect of black tea (Camellia sinensis) in rat. Journal of Ethnopharmacology. 1995;45(3):223–226. doi: 10.1016/0378-8741(95)01223-z. [DOI] [PubMed] [Google Scholar]
- Gong T., Yang X., Bai F., Li D., Zhao T., Zhang J., Sun L., Guo Y. Young apple polyphenols as natural α-glucosidase inhibitors: In vitro and in silico studies. Bioorganic Chemistry. 2020;96 doi: 10.1016/j.bioorg.2020.103625. [DOI] [PubMed] [Google Scholar]
- Jarald E., Joshi S.B., Jain D.C. Diabetes VS Herbal Medicines. Iranian Journal of Pharmacology and Therapeutics. 2008;7(1) [Google Scholar]
- Jung D.-H., Seo D.-H., Kim Y.-J., Chung W.-H., Nam Y.-D., Park C.-S. The presence of resistant starch-degrading amylases in Bifidobacterium adolescentis of the human gut. International Journal of Biological Macromolecules. 2020;161:389–397. doi: 10.1016/j.ijbiomac.2020.05.235. [DOI] [PubMed] [Google Scholar]
- Karaś M., Jakubczyk A., Szymanowska U., Złotek U., Zielińska E. Digestion and bioavailability of bioactive phytochemicals. International Journal of Food Science and Technology. 2017;52(2) doi: 10.1111/ijfs.13323. [DOI] [Google Scholar]
- Kawamura-Konishi Y., Watanabe N., Saito M., Nakajima N., Sakaki T., Katayama T., Enomoto T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. Journal of Agricultural and Food Chemistry. 2012;60(22):5565–5570. doi: 10.1021/jf300165j. [DOI] [PubMed] [Google Scholar]
- Kroll J., Rawel H.M., Rohn S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Science and Technology Research. 2003;9(3):205–218. doi: 10.3136/fstr.9.205. [DOI] [Google Scholar]
- Li B., Fu L., Kojima R., Yamamoto A., Ueno T., Matsui T. Theaflavins prevent the onset of diabetes through ameliorating glucose tolerance mediated by promoted incretin secretion in spontaneous diabetic Torii rats. Journal of Functional Foods. 2021;86 doi: 10.1016/j.jff.2021.104702. [DOI] [Google Scholar]
- Li C., Yu W., Wu P., Chen X.D. Current in vitro digestion systems for understanding food digestion in human upper gastrointestinal tract. Trends in Food Science & Technology. 2020;96:114–126. doi: 10.1016/j.tifs.2019.12.015. [DOI] [Google Scholar]
- Li L., Xu H., Zhou J., Yu J., Copeland L., Wang S. Mechanisms underlying the effect of tea extracts on in vitro digestion of wheat starch. Journal of Agricultural and Food Chemistry. 2021;69(29):8227–8235. doi: 10.1021/acs.jafc.1c02526. [DOI] [PubMed] [Google Scholar]
- Li M., Hagerman A.E. Role of the flavan-3-ol and galloyl moieties in the interaction of (−)-epigallocatechin gallate with serum albumin. Journal of Agricultural and Food Chemistry. 2014;62(17):3768–3775. doi: 10.1021/jf500246m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Zhang C., Xiao X., Zhu M., Quan W., Liu X., Zhang S., Liu Z. Theaflavins in black tea mitigate aging-associated cognitive dysfunction via the microbiota-gut-brain axis. Journal of Agricultural and Food Chemistry. 2023;71(5):2356–2369. doi: 10.1021/acs.jafc.2c06679. [DOI] [PubMed] [Google Scholar]
- Lim S.H., Yu J.S., Lee H.S., Choi C.I., Kim K.H. Antidiabetic flavonoids from fruits of Morus alba promoting insulin-stimulated glucose uptake via Akt and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Pharmaceutics. 2021;13(4):526. doi: 10.3390/pharmaceutics13040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q.Z., Qiu C., Li X.J., Sang S.Y., McClements D.J., Chen L.…Jin Z.Y. The inhibitory mechanism of amylase inhibitors and research progress in nanoparticle-based inhibitors. Critical Reviews in Food Science and Nutrition. 2023;63(33):12126–12135. doi: 10.1080/10408398.2022.2098687. [DOI] [PubMed] [Google Scholar]
- Little K., Llorián-Salvador M., Scullion S., Hernández C., Simó-Servat O., Del Marco A.…Van Bergen T. Common pathways in dementia and diabetic retinopathy: Understanding the mechanisms of diabetes-related cognitive decline. Trends in Endocrinology and Metabolism. 2022;33(1):50–71. doi: 10.1016/j.tem.2021.10.008. [DOI] [PubMed] [Google Scholar]
- Liu H., Han G., Zhang H., Liu Q., Kong B. Improving the physical and oxidative stability of emulsions based on the interfacial electrostatic effects between porcine bone protein hydrolysates and porcine bone protein hydrolysate-rutin conjugates. Food Hydrocolloids. 2019;94:418–427. [Google Scholar]
- Lv Y., Liang Q., Li Y., Liu X., Zhang D., Li X. Study of the binding mechanism between hydroxytyrosol and bovine serum albumin using multispectral and molecular docking. Food Hydrocolloids. 2022;122 doi: 10.1016/j.foodhyd.2021.107072. [DOI] [Google Scholar]
- Magaji U.F., Sacan O., Yanardag R. Alpha amylase, alpha glucosidase and glycation inhibitory activity of Moringa oleifera extracts. South African Journal of Botany. 2020;128:225–230. doi: 10.1016/j.sajb.2019.11.024. [DOI] [Google Scholar]
- Martinez-Gonzalez A.I., Díaz-Sánchez Á.G., de la Rosa L.A., Bustos-Jaimes I., Alvarez-Parrilla E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR) Spectrochimica Acta Part A Molecular and Biomolecular Spectroscopy. 2018;206:437–447. doi: 10.1016/j.saa.2018.08.057. [DOI] [PubMed] [Google Scholar]
- Matsui T., Tanaka T., Tamura S., Toshima A., Tamaya K., Miyata Y., Tanaka K., Matsumoto K. Alpha-Glucosidase inhibitory profile of catechins and theaflavins. Journal of Agricultural and Food Chemistry. 2007;55(1):99. doi: 10.1021/jf0627672. [DOI] [PubMed] [Google Scholar]
- Miao M., Jiang B., Jiang H., Zhang T., Li X. Interaction mechanism between green tea extract and human α-amylase for reducing starch digestion. Food Chemistry. 2015;186:20–25. doi: 10.1016/j.foodchem.2015.02.049. [DOI] [PubMed] [Google Scholar]
- Nakatsuru Y., Murase-Mishiba Y., Bessho-Tachibana M., Terasaki J., Hanafusa T., Imagawa A. Taurine improves glucose tolerance in STZ-induced insulin-deficient diabetic mice. Diabetology International. 2018;9(4):234–242. doi: 10.1007/s13340-018-0353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita Y., Inouye K. Inhibitory effects of chlorogenic acids from green coffee beans and cinnamate derivatives on the activity of porcine pancreas a-amylase isozyme I. Food Chemistry. 2011;11(23):3902. doi: 10.1007/s13340-018-0353-3. [DOI] [Google Scholar]
- Nienhaus K., Nienhaus G.U. Probing heme protein-ligand interactions by UV/visible absorption spectroscopy. Protein-Ligand Interactions: Methods and Applications. 2005;215–241 doi: 10.1385/1-59259-912-5:215. [DOI] [PubMed] [Google Scholar]
- Qie X., Chen Y., Quan W., Wang Z., Zeng M., Qin F., Chen J., He Z. Analysis of β-lactoglobulin–epigallocatechin gallate interactions: The antioxidant capacity and effects of polyphenols under different heating conditions in polyphenolic–protein interactions. Food & Function. 2020;11(5):3867–3878. doi: 10.1039/d0fo00627k. 2020. [DOI] [PubMed] [Google Scholar]
- Raeessi-babaheydari E., Farhadian S., Shareghi B. The interaction of the green tea polyphenol (catechin) with pepsin: Insights from spectroscopic to molecular dynamics studies. Journal of Molecular Liquids. 2021;326 doi: 10.1016/j.molliq.2020.115196. [DOI] [Google Scholar]
- Sales P.M., Souza P.M., Simeoni L.A., Magalhães P.O., Silveira D. α-Amylase inhibitors: A review of raw material and isolated compounds from plant source. Journal of Pharmacy & Pharmaceutical Sciences. 2012;15(1):141–183. doi: 10.18433/J35S3K. [DOI] [PubMed] [Google Scholar]
- Salmaso V., Moro S. Bridging molecular docking to molecular dynamics in exploring ligand-protein recognition process: An overview. Frontiers in Pharmacology. 2018;9:923. doi: 10.3389/fphar.2018.00923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang S., Lambert J.D., Tian S., Hong J., Hou Z., Ryu J.-H.…Yang C.S. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorganic & Medicinal Chemistry. 2004;12(2):459–467. doi: 10.1016/j.bmc.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Sharma J., Bhardwaj V.K., Singh R., Rajendran V., Purohit R., Kumar S. An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chemistry. 2021;346 doi: 10.1080/10408390802068066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V., Rao L.J.M. A thought on the biological activities of black tea. Critical Reviews in Food Science and Nutrition. 2009;49(5):379–404. doi: 10.1080/10408390802068066. [DOI] [PubMed] [Google Scholar]
- Shi J., Pan D., Jiang M., Liu T.-T., Wang Q. Binding interaction of ramipril with bovine serum albumin (BSA): Insights from multi-spectroscopy and molecular docking methods. Journal of Photochemistry and Photobiology B: Biology. 2016;164:103–111. doi: 10.1016/j.jphotobiol.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Shoji Y., Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-ay/TaJcl diabetic mouse. Archives of Pharmacal Research. 2006;29(9):786–794. doi: 10.1007/BF02974080. [DOI] [PubMed] [Google Scholar]
- Singh R., Bhardwaj V.K., Purohit R. Potential of turmeric-derived compounds against RNA-dependent RNA polymerase of SARS-CoV-2: An in-silico approach. Computers in Biology and Medicine. 2021;139 doi: 10.1016/j.compbiomed.2021.104965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Bhardwaj V.K., Sharma J., Purohit R., Kumar S. In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors. Journal of Traditional and Complementary Medicine. 2022;12(1):35–43. doi: 10.1016/j.jtcme.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Chen W., Meng Y., Yang X., Yuan L., Guo Y. Interactions between polyphenols in thinned young apples and porcine pancreatic α-amylase: Inhibition, detailed kinetics and fluorescence quenching. Food Chemistry. 2016;208:51–60. doi: 10.1016/j.jff.2016.07.012. [DOI] [PubMed] [Google Scholar]
- Sun L., Gidley M., Warren F. The mechanism of interactions between tea polyphenols and porcine pancreatic alpha-amylase: Analysis by inhibition kinetics, fluorescence quenching, differential scanning calorimetry and isothermal titration calorimetry. Molecular Nutrition & Food Research. 2017;61(10):1700324. doi: 10.1002/mnfr.201700324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Warren F.J., Netzel G., Gidley M.J. 3 or 3′-Galloyl substitution plays an important role in association of catechins and theaflavins with porcine pancreatic α-amylase: The kinetics of inhibition of α-amylase by tea polyphenols. Journal of Functional Foods. 2016;26:144–156. doi: 10.1016/j.jff.2016.07.012. [DOI] [Google Scholar]
- Svihus B., Hervik A.K. Digestion and metabolic fates of starch, and its relation to major nutrition-related health problems: A review. Starch-Stärke. 2016;68(3–4):302–313. doi: 10.1002/star.201500295. [DOI] [Google Scholar]
- Tan Y., Chang S.K., Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chemistry. 2017;214:259–268. doi: 10.1016/j.foodchem.2016.06.100. [DOI] [PubMed] [Google Scholar]
- Teng J., Gong Z., Deng Y., Chen L., Li Q., Shao Y., Lin L., Xiao W. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis) Lwt. 2017;84:263–270. doi: 10.1016/j.lwt.2017.05.065. [DOI] [Google Scholar]
- Teng Y., Zhang H., Liu R. Molecular interaction between 4-aminoantipyrine and catalase reveals a potentially toxic mechanism of the drug. Molecular BioSystems. 2011;7(11):3157–3163. doi: 10.1016/j.foodchem.2021.131334. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang B., Xiao J., Huang Q., Li C., Fu X. Physicochemical, functional, and biological properties of water-soluble polysaccharides from Rosa roxburghii Tratt fruit. Food Chemistry. 2018;249:127–135. doi: 10.1016/j.foodchem.2018.01.011. [DOI] [PubMed] [Google Scholar]
- Wang Z.J., Si Y.X., Oh S., Yang J.M., Yin S.J., Park Y.D.…Qian G.Y. The effect of fucoidan on tyrosinase: Computational molecular dynamics integrating inhibition kinetics. Journal of Biomolecular Structure and Dynamics. 2012;30(4):460–473. doi: 10.1080/07391102.2012.682211. [DOI] [PubMed] [Google Scholar]
- Wootton-Beard P.C., Ryan L. Improving public health?: The role of antioxidant-rich fruit and vegetable beverages. Food Research International. 2011;44(10):3135–3148. doi: 10.1016/j.foodres.2011.09.015. [DOI] [Google Scholar]
- Wu X., Ding H., Hu X., Pan J., Liao Y., Gong D., Zhang G. Exploring inhibitory mechanism of gallocatechin gallate on a-amylase and a-glucosidase relevant to postprandial hyperglycemia. Journal of Functional Foods. 2018;48:200–209. doi: 10.1016/j.jff.2018.07.022. [DOI] [Google Scholar]
- Xiang H., Waterhouse D.-S., Liu P., Waterhouse G.I., Li J., Cui C. Pancreatic lipase-inhibiting protein hydrolysate and peptides from seabuckthorn seed meal: Preparation optimization and inhibitory mechanism. LWT. 2020;134 doi: 10.1016/j.lwt.2020.109870. [DOI] [Google Scholar]
- Xu Y., Xie L., Xie J., Liu Y., Chen W. Pelargonidin-3-O-rutinoside as a novel α-glucosidase inhibitor for improving postprandial hyperglycemia. Chemical Communications. 2018;55:39–42. doi: 10.1039/c8cc07985d. [DOI] [PubMed] [Google Scholar]
- Yaqin X., Yingying G., Suyang D., Hong W., Yusong L., Libo W., Xin H., Yu Y. Effects of ultrasound irradiation on the characterization and bioactivities of the polysaccharide from blackcurrant fruits. Ultrasonics Sonochemistry. 2018;49:206–214. doi: 10.1016/j.ultsonch.2018.08.005. [DOI] [PubMed] [Google Scholar]
- Yu X., Cai X., Luo L., Wang J., Ma M., Wang M., Zeng L. Influence of tea polyphenol and bovine serum albumin on tea cream formation by multiple spectroscopy methods and molecular docking. Food Chemistry. 2020;333 doi: 10.1016/j.foodchem.2020.127432. [DOI] [PubMed] [Google Scholar]
- Yue Y., Zhao S., Liu J., Yan X., Sun Y. Probing the binding properties of dicyandiamide with pepsin by spectroscopy and docking methods. Chemosphere. 2017;185:1056–1062. doi: 10.1016/j.chemosphere.2017.07.115. [DOI] [PubMed] [Google Scholar]
- Zeng L., Ding H., Hu X., Zhang G., Gong D. Galangin inhibits α-glucosidase activity and formation of non-enzymatic glycation products. Food Chemistry. 2019;271:70–79. doi: 10.1016/j.foodchem.2018.07.148. [DOI] [PubMed] [Google Scholar]
- Zhang J., Sun L., Dong Y., Fang Z., Nisar T., Zhao T., Wang Z.-C., Guo Y. Chemical compositions and α-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chemistry. 2019;299 doi: 10.1016/j.foodchem.2019.125102. [DOI] [PubMed] [Google Scholar]
- Zhao J., Huang L., Sun C., Zhao D., Tang H. Studies on the structure-activity relationship and interaction mechanism of flavonoids and xanthine oxidase through enzyme kinetics, spectroscopy methods and molecular simulations. Food Chemistry. 2020;323(126807) doi: 10.1016/j.foodchem.2020.126807. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
I have shared the link to my data at the Attach File Step