Abstract
Introduction
Primary cutaneous apocrine carcinomas of the axilla represents an extremely rare entity, with <200 cases reported in the literature. It can be challenging, even almost impossible, to distinguish histologically from metastases of breast origin. We herein present the first case of an axillary cutaneous apocrine adenocarcinoma followed and treated in our institute.
Case presentation
A 58-year-old man with a history of myopathy, presented for a right axillary swelling. Physical examination revealed the presence of a 10 cm right axillary mass, no palpable adenopathy, and bilateral gynecomastia. A biopsy of the mass was performed, showing a pattern consistent with a secondary localization of mammary neoplasia. Breast and distant radiological examinations were negative. The tumor markers' levels were not raised. Therefore, the patient underwent surgery with a large excision, a right axillary lymph node dissection, covered with a pedicled pectoralis major flap. Histological and immunohistochemical examinations showed a high expression of CK7 with a negative expression of TTF1, RH, PSA, and CK20. The diagnosis of an apocrine adenocarcinoma from cutaneous origin was confirmed.
Clinical discussion
Primary cutaneous apocrine carcinomas are a group of uncommon malignant adnexal tumors, whose diagnosis is almost impossible to confirm preoperatively. Wide, local excision with clear margins, with or without lymph node dissection is the standard treatment.
Conclusion
This case illustrates the importance of clinico-pathological correlation of skin cancers, especially apocrine ones. Clinical particularity and careful histological analysis are used to guide the diagnostic approach.
Keywords: Apocrine adenocarcinoma, Sweat glands, Axillary mass, Adnexal neoplasm
Highlights
-
•
Cutaneous apocrine carcinoma is an extremely rare entity.
-
•
this type of neoplasm occurs mainly in areas where apocrine gland density is high, particularly in the axilla.
-
•
It is challenging to differentiate primary cutaneous apocrine carcinoma from other tumor types.
1. Introduction
Cutaneous primary apocrine carcinoma is an exceptional neoplasm of the epidermal adnexa accounting for <1 % of all primary skin malignancies [1]. It mainly occurs in axillary region where apocrine sweat gland are abundant [2]. Its histological diagnosis represents a real challenge since they are difficult to differentiate from metastases from other organs neoplasms, including breast cancer [3,4]. The prognosis of these tumors is related to both local and distant recurrence. With the aim to gain a better understanding of the clinicopathological characteristics and therapeutic options of this entity, we report according to the SCARE checklist, the first case of an axillary cutaneous apocrine adenocarcinoma followed and treated in our institute [5].
2. Case presentation
A 58-year-old Caucasian man, suffering from myopathy, with no family history of cancer, presented with a right axillary swelling, evolving for eight months, without any improvement following antibiotic treatment. Physical examination showed a bilateral gynecomastia, with a voluminous, ulcerated, right axillary mass, of about 10 cm (Fig. 1). No associated palpable adenopathy was found. A full body CT scan was done and revealed no suspicious lesions except for right axillary adenopathies with necrotic centers, the largest of which was 7 cm.
Fig. 1.

Clinical appearance of the right axillary mass.
The axillary mass was biopsied and the anatomopathological examination concluded to an apocrine adenocarcinoma of which the cutaneous origin was the most likely, but a metastatic localization of another neoplasm had to be excluded. All tumoral markers were negative. An oeso-gastro-duodenal endoscopy and a colonoscopy were performed and concluded to be without abnormalities. We completed a bilateral mammogram which also turned out to be negative.
The case was discussed in a multidisciplinary consultation meeting and the decision was to have surgery. The patient was operated on and had a large excision, an axillary lymph node dissection and coverage by a pedicled pectoralis major flap (Fig. 2). Histological and immunohistochemical examination confirmed the diagnosis of a cutaneous apocrine adenocarcinoma. TTF1, Hormonal receptors, PSA and CK20 were negative, only the CK7 was positive (Fig. 3, Fig. 4). The axillary lymph node dissection yielded 27 lymph nodes, 21 of which were metastatic.
Fig. 2.
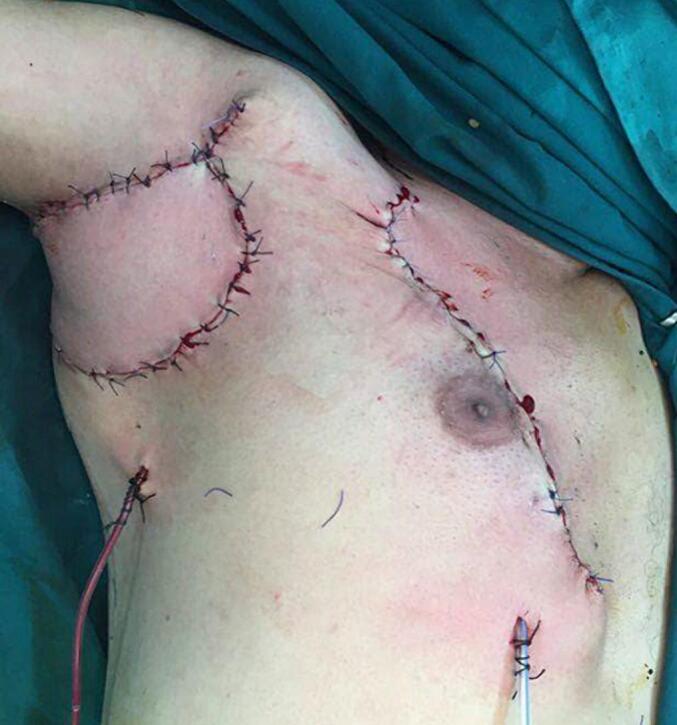
Postoperative appearance.
Fig. 3.

Scanning view of the specimen showing a mixture of cystic and papillary elements.
Fig. 4.
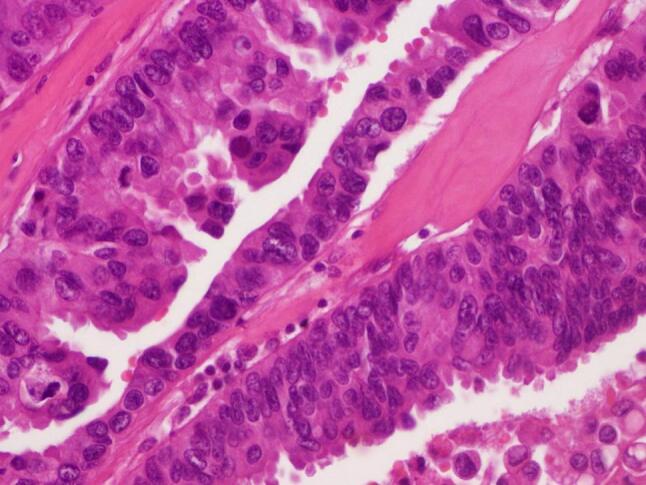
Large epithelioid cells with abundant eosinophilic cytoplasm containing vesicular nuclei and prominent eosinophilic nucleoli. Mitotic activity is identified.
The patient subsequently underwent 4 cycles of adjuvant anthracycline- and cyclophosphamide-based chemotherapy followed by adjuvant axillary and supra-clavicular radiotherapy at a dose of 50Gy with a 12Gy axillary boost. After a 4-year follow-up, the patient has not developed locoregional relapse or distant metastases. The planned follow-up is a six-monthly clinical examination and an annual CT scan for at least 5 years.
3. Discussion
Primary cutaneous apocrine carcinomas (CAC) are a group of uncommon malignant adnexal tumors, with approximately 200 cases described in the literature, arising in skin areas containing a large number of apocrine glands [3]. The described cases were mostly located in the axilla, due to their high concentration in sweat glands, but other localizations were reported, notably; scalp, forehead, eyelids, upper lip, cheeks, pubic area, nipple and the fingers [[6], [7], [8], [9], [10]].
They manifest clinically as asymptomatic, often confluent, nippled patches or nodules. The evolution is mostly painless, slowly progressing, but may become more aggressive, with a risk of local recurrence or even metastatic evolution, particularly in the lungs, brain and bones [11].
Histologically, primary cutaneous apocrine carcinomas are invasive tumors, spreading from the reticular dermis to the subcutaneous fat tissue, forming clusters of tubular structures in different sizes and shapes [12]. Microscopically, they show considerable variability in their aspects; complex, glandular, papillary, tubular, cribriform, solid, and cord-like appearances have been reported. The difficulty of histological differential diagnosis between axillary CAC and other adenocarcinoma's metastasis has been highlighted [6,13]. In general, adnexal carcinomas should always raise the possibility of cutaneous metastases of an adenocarcinoma of other location, especially lobular breast adenocarcinoma, and therefore require full complementary examinations. It's above all the atypical “Indian-file” architectural organization that leads to this confusion [14]. Immunostaining with anti-herceptin-2 (Her-2) can be used, as it is sometimes positive in cutaneous metastases of breast adenocarcinoma, whereas primary apocrine cutaneous carcinomas do not bind anti-Her-2 [15]. Hormone receptors, on the other hand, do not help distinguish between CAC and lobular breast adenocarcinoma. Regarding the immunohistochemical investigations, the characteristic of CAC has a debatable aspect. The expression of CK7 and the absence of expression of CK20 are strongly suggestive and may even confirm the diagnosis of cutaneous apocrine carcinoma [3]. Also, the tumors being negative for hormonal receptors and for all other markers is supportive of the diagnosis.
The referral treatment is wide surgical excision with 2–3 cm margins in surrounding healthy tissue, with or without axillary lymph node dissection, which is indicated in the presence of suspicious adenopathies [8,16]. CACs are chemo resistant, but chemotherapy using adriablastin/cyclophosphamide or Taxanes and palliative treatment are indicated for metastatic apocrine adenocarcinoma of the axillary region [17,18]. Also, adjuvant radiotherapy may be used in locally or regionally advanced CAC [17]. There is no consensus on the indications for adjuvant radiotherapy. It may be proposed for large tumors (>5 cm) or moderately to poorly differentiated tumors with lymphovascular emboli, those with positive margins (<1 cm), or in cases of massive lymph node invasion [8,17].
There are still conflicting opinions regarding local and distant recurrence rates [4,19]. Further studies on larger populations are required to evaluate the recurrence potential of this type of tumor.
4. Conclusion
In conclusion, this case illustrates the importance of the clinicopathological correlation of skin cancers, especially apocrine ones. Clinical particularity and careful histologic analyses may help guide the diagnostic approach. Further studies will help determine a consensus on treatment.
Ethics approval
Case reports do not require ethical approval in our institution.
Consent to participate
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Consent for publication
Not applicable.
Funding
No source of funding.
CRediT authorship contribution statement
NK, YF: Data acquisition, literature review, conception, drafting and preparing the manuscript.
SS, IZ: Data acquisition, preparing and editing the manuscript.
IA: Data Acquisition and preparing figs.
TD: Editing and revising the manuscript critically.
Registration of research studies
Not applicable.
Guarantor
Nayssem Khessairi.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
Not applicable.
Data availability
Data supporting our findings were taken from the patient's folders.
References
- 1.Hollowell K.L., Agle S.C., Zervos E.E., et al. Cutaneous apocrine adenocarcinoma: defining epidemiology, outcomes, and optimal therapy for a rare neoplasm. J. Surg. Oncol. 2012;105:415–419. doi: 10.1002/jso.22023. [DOI] [PubMed] [Google Scholar]
- 2.Kathrotiya P.R., Bridge A.T., Warren S.J., et al. Primary apocrine adenocarcinoma of the axilla. Cutis. 2015;95(271–274):281. [PubMed] [Google Scholar]
- 3.Maury G., Guillot B., Bessis D., et al. Unusual axillary apocrine carcinoma of the skin: histological diagnostic difficulties. Ann. Dermatol. Venereol. 2010;137:555–559. doi: 10.1016/j.annder.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Pai R.R., Kini J.R., Achar C., et al. Apocrine (cutaneous) sweat gland carcinoma of axilla with signet ring cells: a diagnostic dilemma on fine-needle aspiration cytology. Diagn. Cytopathol. 2008;36:739–741. doi: 10.1002/dc.20889. [DOI] [PubMed] [Google Scholar]
- 5.Sohrabi C., Mathew G., Maria N., et al. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. Lond. Engl. 2023;109:1136–1140. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasad V., Kao W.H., Kao G.F. Cutaneous adnexal carcinoma with apocrine differentiation. Cutis. 2016;98:E16–E19. [PubMed] [Google Scholar]
- 7.Guo J., Wu G., Xu J., et al. Cutaneous apocrine carcinoma in groin with bilateral lymph node metastasis: a case report and review of the literature. Int. J. Clin. Exp. Pathol. 2014;7:8266–8270. [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J.H., Oh H.M., Kim K.S., et al. Primary cutaneous apocrine carcinoma of the scalp: two case reports and literature review. Medicine (Baltimore) 2022;101 doi: 10.1097/MD.0000000000028808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chadha S., Kumar R., Singhal S., et al. Primary cutaneous apocrine carcinoma: case report and literature review. Indian J. Pathol. Microbiol. 2021;64:183–185. doi: 10.4103/IJPM.IJPM_341_19. [DOI] [PubMed] [Google Scholar]
- 10.Griffith M., Singh R., Alabkaa A., et al. Primary cutaneous apocrine carcinoma, arising in tubular apocrine adenoma. J. Cutan. Pathol. 2023;50:1042–1047. doi: 10.1111/cup.14509. [DOI] [PubMed] [Google Scholar]
- 11.Loh S.-H., Oh Y.-J., Lew B.-L., et al. Primary cutaneous apocrine carcinoma. Ann. Dermatol. 2016;28:669–670. doi: 10.5021/ad.2016.28.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos-Juanes J., Bernaldo de Quirós J.F., Galache Osuna C., et al. Apocrine carcinoma, adenopathies, and raised TAG-72 serum tumor marker. Dermatol. Surg. Off. Publ. Am. Soc. Dermatol. Surg. Al. 2004;30:566–569. doi: 10.1111/j.1524-4725.2004.30180.x. [DOI] [PubMed] [Google Scholar]
- 13.Katsuo K., Otani T., Sano H. Apocrine adenocarcinoma of the axilla encapsulated by a fibrous capsule: a resemblance to encapsulated papillary carcinoma of the breast. J. Dermatol. 2023;50:e102–e103. doi: 10.1111/1346-8138.16630. [DOI] [PubMed] [Google Scholar]
- 14.Toledo-Pastrana T., Llombart-Cussac B., Traves-Zapata V., et al. Case report: differential diagnosis between primary cutaneous apocrine adenocarcinoma versus extramammary or metastatic breast adenocarcinoma. Am. J. Dermatopathol. 2014;36:e175–e178. doi: 10.1097/DAD.0b013e318288cdd2. [DOI] [PubMed] [Google Scholar]
- 15.Hiatt K.M., Pillow J.L., Smoller B.R. Her-2 expression in cutaneous eccrine and apocrine neoplasms. Mod. Pathol. Off. J. U S Can. Acad. Pathol. Inc. 2004;17:28–32. doi: 10.1038/modpathol.3800007. [DOI] [PubMed] [Google Scholar]
- 16.Wu J.-D., Changchien C.-H., Liao K.-S. Primary cutaneous cribriform apocrine carcinoma: case report and literature review. Indian J. Dermatol. Venereol. Leprol. 2018;84:569–572. doi: 10.4103/ijdvl.IJDVL_830_16. [DOI] [PubMed] [Google Scholar]
- 17.Ogata D., Kiyohara Y., Yoshikawa S., et al. Treatment strategy for cutaneous apocrine carcinoma. Int. J. Clin. Oncol. 2014;19:712–715. doi: 10.1007/s10147-013-0594-x. [DOI] [PubMed] [Google Scholar]
- 18.Collette F., Hamoir M., Van Eeckhout P., et al. Metastatic cutaneous apocrine adenocarcinoma successfully treated with systemic anti-androgen therapy—a case report. Clin. Case Rep. 2020;8:3472–3478. doi: 10.1002/ccr3.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Somasundaram S.K., Balasubramanian R.K., Aref F., et al. A rare and unusual case of bilateral, axillary, metachronous apocrine carcinoma. Int. Surg. 2007;92:335–338. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting our findings were taken from the patient's folders.


