Abstract
Background
Severe traumatic brain injuries (TBIs) are an important health issue worldwide, which are associated with harmful side effects. This meta-analysis investigates the cognitive and functional outcomes in severe brain trauma cases. It assesses the impact on memory, verbal and visual abilities, attention, learning, and the presence of depression. The study provides a comprehensive overview of the consequences of severe brain trauma injury on cognitive and functional domains.
Objective
The main objective of the current comprehensive meta-analysis study is to assess and analyze the impact of severe TBI on functional and cognitive outcomes, including verbal, visual, attention, learning, memory, and emotional stability.
Methods
We collected data from three online databases, including PubMed, Cochrane Library, and Embase. Case–control trials related to severe TBI association with cognitive and functional outcomes were included. Verbal strength, visual functions, learning abilities, attention, memory, and depression were considered primary outcomes.
Results
We have included 13 case–control studies with 1,442 subjects in this meta-analysis, which provide adequate data to determine the pooled effect size for targeted outcomes. The effect of severe TBI on the inducement of depression and impairment of memory, verbal, visual, attention, and learning abilities compared to the control group showed statistically significant outcomes (p < 0.05).
Conclusion
Severe TBI is strongly associated with impaired cognitive and functional abilities, including visual and verbal disabilities, impaired memory, depression inducement, attention deficits, and learning disabilities.
Keywords: brain trauma, severe TBI, depression, verbal and visual disabilities, learning and memory impairment, cognitive disabilities
1. Introduction
Severe traumatic brain injury (TBI) represents a major global health concern, with profound implications for individuals, families, and healthcare systems. Survivors of severe brain trauma often face long and arduous journeys toward recovery, marked by a spectrum of cognitive and functional challenges. Understanding the factors that influence cognitive and functional outcomes in this population is not only crucial for optimizing patient care but also for informing healthcare policies and interventions (Robinson, 2021).
The pathophysiology of severe brain trauma is complex and multifaceted. The initial mechanical insult, often resulting from accidents, falls, or violence, sets in motion a cascade of events, including primary and secondary injury mechanisms that can lead to structural damage, inflammation, and neurochemical imbalances (Orr and Gensel, 2017; Ng and Lee, 2019). These processes, occurring within the highly delicate and intricately interconnected neural networks of the brain, can result in a wide range of neurological deficits (Ng and Lee, 2019). While advances in neuroimaging and neurocritical care have expanded our understanding of TBI, there remains a pressing need to comprehensively assess the cognitive and functional consequences of severe brain trauma (Pavlovic et al., 2019; Olsen et al., 2021; Howlett et al., 2022). Various case–control research studies have concluded that the incidence of severe TBI is strongly linked to altered cognitive functionality. As a consequence of the brain injury, TBI patients experienced impaired memory and altered verbal, visual, and learning functions. Moreover, the prevalence of depression is significantly higher in severe TBI cases (Hellawell et al., 1999; Ashman et al., 2008).
Our objective is to provide a comprehensive and evidence-based understanding of the cognitive and functional outcomes in severe TBI cases. This study represents an extensive exploration of cognitive and functional outcomes in severe brain trauma cases through a comprehensive meta-analysis. Our analysis synthesizes data on a range of cognitive domains, including memory, attention, vision, language, and executive functions. By synthesizing data on functional outcomes, we aim to highlight the challenges faced by TBI survivors and the target areas where intervention strategies may be most effective.
2. Materials and methods
2.1. Search methodology
Various search engines were employed to gather data, including the Cochrane Library,1 PubMed,2 and Embase3 databases. We used Medical Subject Headings (MeSH) terms as keywords, including “traumatic brain injury,” “severe TBI,” “cognitive impairments associated with TBI,” “severe TBI related physical impairments,” and “TBI induced depression.” Moreover, aside from conducting a methodical search on PubMed, we expanded our search scope by utilizing the “related articles” feature to precisely evaluate the abstracts, studies, and citations. Our database search was confined to records available exclusively in the English language.
2.2. Selection standards for included studies
This comprehensive meta-analysis has included case–control studies aimed at assessing the association between severe TBI and the development of cognitive disorders and physical impairment. The study included published data spanning various time periods from 1997 to the present, to ensure thorough coverage of the existing literature. In order to maintain the integrity of the exclusion criteria, the following aspects were considered in study selection: (1) the exclusion of animal studies, (2) only limited to studies with published data, (3) the exclusion of articles with inappropriate methodology, data collection, statistical analysis, and overall outcome quality, (4) avoidance of derivative data sources, such as review articles, (5) the exclusion of participants with severe TBI who also had concurrent life-threatening conditions such as cancer, cardiac disorders, neurodegenerative disorders, and other neurological conditions., which ensured a focused analysis of the specific impact of TBI on health outcomes, and (6) the exclusion of participants with mild and moderate TBI.
The characteristics of all included studies are concisely summarized in Table 1. For data retrieval and inclusion, the following parameters were taken into account: (1) case–control studies were selected to gather data on the association between severe TBI and cognitive disorders and physical impairments, (2) the focus was exclusively on patients with severe TBI, (3) studies with sufficient sample size, (4) inclusion of studies with verified measurement methods and appropriate statistical analyses, (5) population inclusion of both males and females, and (6) the inclusion of research studies with well-conducted analyses and outcomes.
Table 1.
Characteristics and findings of the included studies.
| Sr. no. | Author/country | Year | Study design | Study participants | Sex | Age | Follow-up period | Outcome measures | Evaluation | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ashman et al.,/USA | 2008 | Case–control study | 54 TBI patients and 40 control | Both males and females | > 55 | 6 months to 1 year | CVLT, WMS-LM, WMS-VR, WMS-LM, WMS-LM, COWAT (FAS), WCST CS | TBI patients have impaired attention and verbal memory | Ashman et al. (2008) |
| 2 | Peretz et al.,/Israel | 1997 | Case–control study | 54 TBI patients and 10 control | Both males and females | > 60 | - | WMS-VR | In older TBI patients, impaired visual activity has been found | Aharon-Peretz et al. (1997) |
| 3 | Breed et al.,/USA | 2008 | Case–control study | 56 TBI patients and 50 control | Both males and females | > 65 | - | WMS-VR, WMS-LM | In older TBI patients, cognitive impairment has been found | Breed et al. (2008) |
| 4 | Rapoport et al.,/Canada | 2006 | Case–control study | 49 TBI patients and 69 control | Both males and females | > 50 | 1 year | COWAT, CVLT | With aging, in TBI patients, cognitive functions significantly declined | Rapoport et al. (2006) |
| 5 | Rao et al.,/USA | 2010 | Case–control study | The total number of participants was 43 | Both males and females | > 45 | 1 year | MMSE | In the early-aged TBI patients, mild depression has been evident | Rao et al. (2010) |
| 6 | Ping ma et al.,/Taiwan | 2019 | Case–control study | The total number of participants was 440 | Both males and females | > 45 | 2 year | PSQI, BDI, BAI | In the TBI patients, sleep disorder, anxiety, and depression have been evident | Ma et al. (2019) |
| 7 | Richard et al.,/Canada | 2000 | Case–control study | The total number of participants was 80 | Both males and females | > 26 | - | BDI, CVLT | Anxiety and learning difficulties have been found to be more significant in elderly TBI patients | Richards (2000) |
| 8 | Hellawell et al.,/UK | 1999 | Retrospective case–control study | The total number of participants was 96 | Both males and females | > 25 | 1 year | NART, TMT, GOS, HISC | Patients with moderate to severe brain injuries have experienced altered physical and cognitive functions | Hellawell et al. (1999) |
| 9 | Sigurdardottir et al.,/Norway | 2009 | Case–control study | The total number of participants was 115, where 41 cases had severe brain injuries | Both males and females | > 25 | 1 year | GOSE, COWAT | In severe TBI patients, cognitive recovery after 1 year of follow-up was found insignificant | Sigurdardottir et al. (2009) |
| 10 | Salmond et al.,/UK | 2005 | Case–control study | The total number of participants was 63, where 31 cases had severe brain injuries and 32 were control | Both males and females | > 30 | - | BDI | The results revealed that TBI patients experienced severe depression | Salmond et al. (2005) |
| 11 | Anderson et al.,/Australia | 2004 | Case–control study | The total number of participants was 117, where 84 cases and 33 control | Both males and females | 2–7 | - | EOWPVT, NMI, | Learning and memory disabilities have been significantly found in TBI patients compared to healthy individuals | Anderson et al. (2004) |
| 12 | Hawley et al.,/UK | 2004 | Case–control study | The total number of participants was 81, where 67 cases and 14 control | Both males and females | 6–18 | - | WISC-III | In the TBI patients, intellectual and verbal strengths were found compromised | Hawley (2004) |
| 13 | Mangels et al.,/USA | 2002 | Case–control study | The total number of participants was 25, where 15 cases and 10 control | Both males and females | > 25 | - | WMS-R | Significant evidence of impaired memory has been observed in severe TBI patients | Mangels et al. (2002) |
2.3. Extraction of data
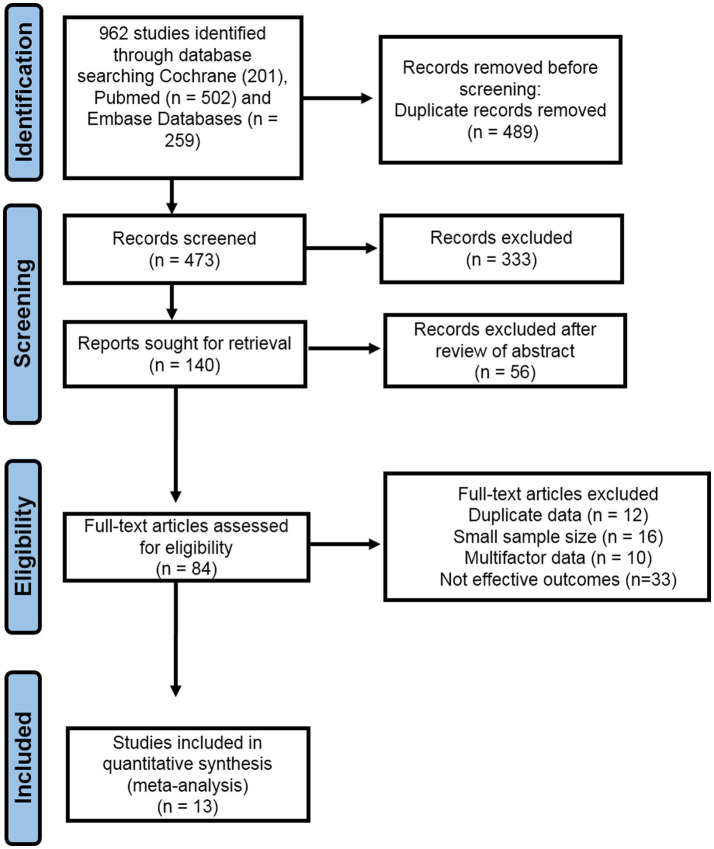
Two investigators independently conducted data extraction. Data from the studies included in the analysis were systematically collected and organized within a standardized Excel spreadsheet. The collected data from these studies have included various information, including author names, study locations, publication years, sample sizes, sex distribution, age demographics, follow-up periods, outcome measurements, and research findings. The study selection process is shown in Figure 1, using the PRISMA flow chart. We extracted mean values, standard deviations, and sample sizes from the research studies to evaluate the potential risk of cognitive disorders and physical impairments associated with severe TBI.
Figure 1.
A visual representation of the included studies via systemic PRISMA flow chart.
To create a comprehensive database for our meta-analysis, a Microsoft Excel spreadsheet was established, including all the pertinent data for the analysis, and was thoroughly reviewed by the third author to resolve any inconsistencies. The third author precisely addressed discrepancies through comprehensive data examination, consultations with co-authors, and utilization of statistical methodologies, ensuring a robust and reliable meta-analysis outcome.
2.4. Description of included studies
We have included 13 case–control studies in this meta-analysis. In total, 6 studies out of 13 have a population age of ≥45 years and 7 studies include patient populations of age ≥ 25 years. In all included studies, the risk of cognitive disorders, including depression, impaired memory, and poor attention, was assessed along with the physical impairments such as visual disabilities and impaired verbal and learning functions in severely traumatic brain-injured patients. In Table 1, the characteristics and findings of the included studies are summarized.
Following outcome measures from the case–control studies were involved in the pooled estimation of the analysis, such as CVLT, WMS-III, WMS-LM, ES, WMS-VR, COWAT (FAS), WCST CS, MMSE, PSQI, BAI, BDI, GOS, NART, TMT, HISC, GOSE, EOWPVT, NMI, and WMS-R (Table 2). The current comprehensive meta-analysis was performed, involving a meticulous literature search that identified and included 13 research studies. In the first step, a total of 962 studies were identified via database search. After removing duplicates and conducting a preliminary screening of full-text articles, a total of 84 articles were assessed for eligibility. Out of these, 71 studies were excluded based on criteria such as duplicate data, limited sample size, unreliable and complex data, poor assessment methodology, and sub-optimal outcomes. The study selection is illustrated in a PRISMA flow diagram (Figure 1).
Table 2.
List of abbreviations with full forms.
| Sr. no. | Abbreviations | Full form |
|---|---|---|
| 1 | CVLT | California verbal learning test |
| 2 | WMS-III | Wechsler memory scale—third edition |
| 3 | WMS-LM | Wechsler memory scale—logical memory |
| 4 | WMS-ES | Wechsler memory scale—effect size |
| 5 | VR | Visual reproduction |
| 6 | COWAT (FAS) | Controlled oral word association test (FAS) |
| 7 | WCST CS | Wisconsin card sorting test—categories solved |
| 8 | MMSE | Mini-mental state examination |
| 9 | PSQI | Pittsburgh sleep quality index |
| 10 | BAI | Beck anxiety inventory |
| 11 | BDI | Beck depression inventory |
| 12 | GOS | Glasgow outcome scale |
| 13 | NART | National adult reading test |
| 14 | TMT | Trail making test |
| 15 | HISC | Hopkins verbal learning test—immediate recall |
| 16 | GOSE | Glasgow outcome scale—extended |
| 17 | EOWPVT | Expressive one-word picture vocabulary test |
| 18 | NMI | Neuropsychological memory index |
| 19 | WMS-R | Wechsler memory scale—revised |
| 20 | SMD | Standardized mean difference |
2.5. Statistical analyses
In this meta-analysis, all statistical analyses were performed using Review Manager, Version 5.3 (Cochrane Collaboration in Oxford, England). The study adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The means and standard deviations of included research studies were extracted to determine the association between severe TBI and the risk of cognitive disorders and physical impairments by a random-effects model. The estimated pooled mean difference and the corresponding 95% confidence interval (CI) were calculated to estimate the incidence of cognitive disorders in severe TBI patients. We assessed heterogeneity among the studies via the chi-square (χ2) test and the I2 statistics. Cochran’s Q-test yielded a p-value below 0.10, indicating significant statistical heterogeneity.
3. Results
3.1. Association between severe TBI and verbal, visual, and learning abilities
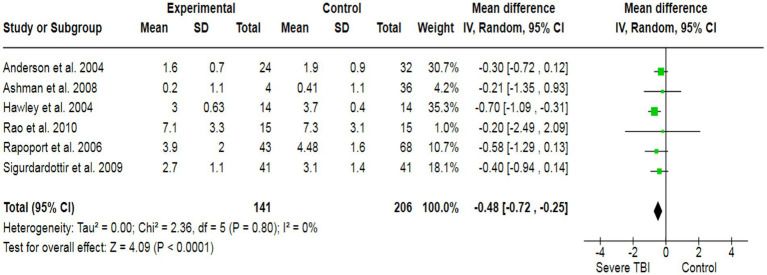
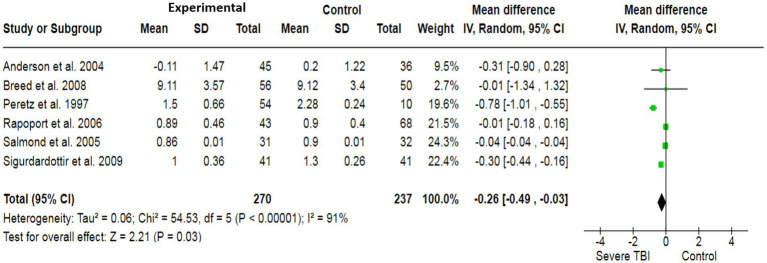
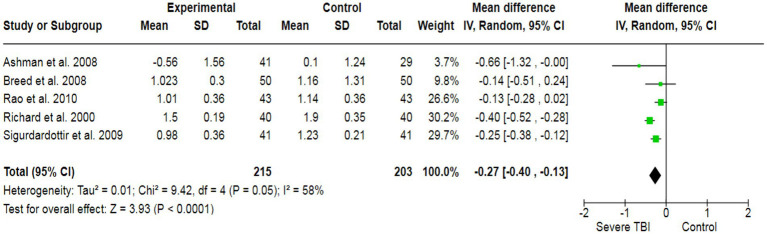
To assess the association between severe TBI and verbal functionality, a total of 6 case–control studies with a sample size of 568 have generated a pooled estimate of the study’s effect size (Figure 2). The outcomes have reported an SMD of −0.48, which suggests a substantial effect size, indicating that individuals with severe TBI possess lower verbal performance compared to healthy individuals. For visual quality, a total of 6 studies (507 participants) have produced a pooled SMD of −0.26 using a random-effect model, showing a strong association of severe TBI with poor visual outcomes (Figure 3). Moreover, 5 case–control trials with 418 subjects have produced a pooled SMD of −0.27, indicating altered learning abilities in severe TBI patients (Figure 4).
Figure 2.
The forest plot of severe TBI and the risk of verbal impairment; diamond (pooled estimate), squares (individual study effects), and horizontal lines (confidence intervals).
Figure 3.
The forest plot of severe TBI and visual impairment risk; diamond (pooled estimate), squares (individual study effects), and horizontal lines (confidence intervals).
Figure 4.
The forest plot of severe TBI and the risk of impairment of learning abilities; diamond (pooled estimate), squares (individual study effects), and horizontal lines (confidence intervals).
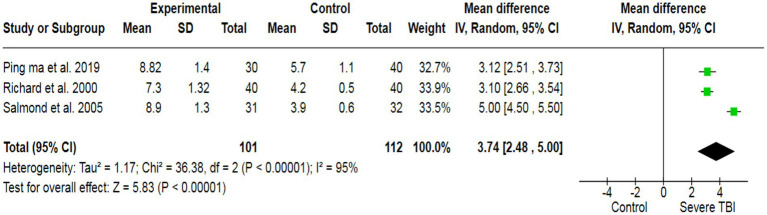
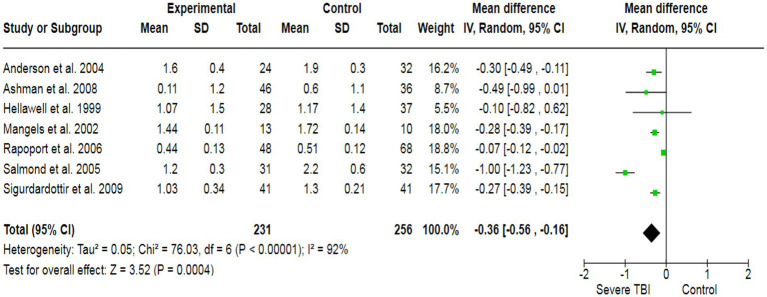
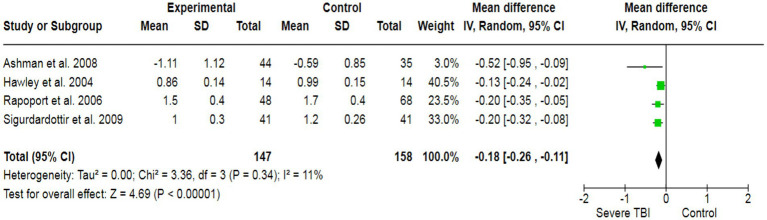
3.2. Association between severe TBI and risk depression, altered memory, and impaired attention
To assess the potential inducement of depression in severe TBI patients, we analyzed data from three studies (213 participants), which collectively yielded a pooled effect size of SMD = 3.74 (Figure 5). This indicated a significant association between severe TBI and an increased likelihood of experiencing depression compared to healthy individuals. Additionally, our analysis revealed a strong association between severe TBI and the risk of altered memory (pooled SMD: −0.36) (Figure 6), as well as a significant association between severe TBI and impaired attention (pooled SMD: −0.18) (Figure 7).
Figure 5.
The forest plot of severe TBI and depression risk; diamond (pooled estimate), squares (individual study effects), and horizontal lines (confidence intervals).
Figure 6.
The forest plot of severe TBI and the risk of altered memory; diamond (pooled estimate), squares (individual study effects), and horizontal lines (confidence intervals).
Figure 7.
The forest plot of severe TBI and the risk of altered attention; diamond (pooled estimate), squares (individual study effects), and horizontal lines (confidence intervals).
3.3. Heterogeneity and a risk of bias
The heterogeneity test was statistically analyzed via the Cochran Q-test. A large heterogeneity was found among studies concerning the relationship between severe TBI and the risk of visual impairment (I2 = 91%), learning abilities (I2 = 58%), the risk of depression (I2 = 95%), altered memory (I2 = 92%), and impaired attention (I2 = 58%), which may have influenced the validity and generalizability of the results.
The potential risk of bias for each included study was independently evaluated by the Cochrane Collaboration to assess methodological quality (Figure 8). Several research trials showed high risk, with major sources of bias associated with performance bias, allocation concealment, self-reporting outcomes, random sequence generation, and protection from other potential biases. Due to the challenge of unsegregated participants and the age factor, along with multiple outcome measures, the majority of RCTs included here are at greater risk of bias.
Figure 8.
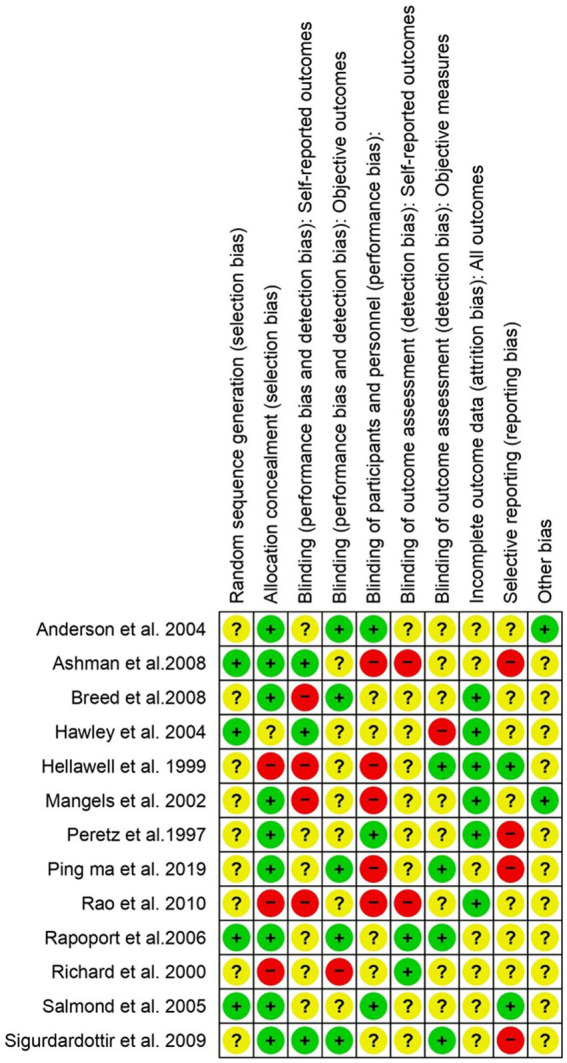
The risk of bias assessment for each included study in accordance with “Cochrane RoB 2”. Low risk (+), unclear (?), and high risk (−).
4. Discussion
Severe TBI significantly affects various dimensions, including cognitive functions, emotional wellbeing, and physical abilities. The complex interplay of neural damage, disrupted neurochemical balance, and associated complications often leads to critical challenges in memory and executive functions, emotional regulation, and motor skills. Additionally, it can lead to behavioral changes and impaired learning abilities. These alterations disturb a person’s social and psychological wellbeing (Arciniegas et al., 2002). The primary aim of this comprehensive meta-analysis is to determine the altered functional cognitive impairments, including verbal, visual, learning, attention, and memory disabilities, and the inducement of depression in severe traumatic brain-injured patients. This comprehensive meta-analysis review included 13 studies with 1,442 study participants, which provided data for analysis. The estimated pooled analysis outcomes of our study revealed that severe traumatic brain injuries have a significantly strong association with impaired memory, visual functionality, learning abilities, verbal functions, attention deficits, and the inducement of depression.
Numerous research studies corroborate our findings. An investigative study has shown that patients who have experienced severe TBI exhibit highly significant alterations in verbal and visual functionalities, as well as impaired attention and memory. This study was conducted by measuring various outcome measures, including CVLT, COWAT, WMS-LM, and WMS-VR (Ashman et al., 2008). In this comprehensive meta-analysis, 13 case–control studies were included with 1,442 participants to determine the association between severe TBI and cognitive and functional disabilities, including memory, depression, attention deficit, learning disabilities, and verbal and visual impairments. The pooled outcomes of the current analysis revealed that severe TBI has a significant association with altered cognitive and functional abilities. The outcome measuring instruments for the analysis are good in construct validity and reliability, elaborating that these are high-quality measures of primary and secondary outcomes, including depression, verbal, visual, learning, memory, and attention.
A case–control study involving TBI patients has demonstrated that several cognitive dysfunctions were highly significant in patients over the age of 65 years who had experienced severe TBI. This study assessed learning and visual abilities using outcome measures such as WMS-VR and WMS-LM. Outcomes from the study revealed that in older adult patients, significant alterations in visual and learning abilities were found (Breed et al., 2008). Similarly, in younger severe TBI patients aged 6 to 40 years, statistically significant alterations in intellectual, verbal, and memory strengths were found, which were measured using MS-III and WMS-R outcome measures (Mangels et al., 2002; Hawley, 2004). Similarly, a case–control study was conducted in 2004, including 117 participants, consisting of 33 control subjects and 84 severe TBI patients aged 2–7 years. The outcomes revealed that severe TBI patients in this group exhibited impaired learning and memory functionality, which was assessed using the EOWPVT and NMI outcome measures (Anderson et al., 2004).
To prevent cognitive and functional disabilities in severe TBI patients, it is important to get immediate and specialized medical care, with a focus on reducing injury and alleviating cognitive dysfunctionalities. Offering cognitive therapy and emotional support can help to improve their long-term recovery. Further advanced research studies are required in this field to provide a more comprehensive understanding of the association between severe TBI and functional and cognitive disabilities, including depression, attention deficit, verbal and visual impairment, and learning abilities. Novel research studies are needed to explore the intricate mechanisms underlying the association between severe TBI and cognitive disabilities, utilizing sophisticated neuroimaging techniques, longitudinal assessments, and detailed neurobehavioral analyses. Moreover, intervention-focused studies aimed at evaluating personalized therapeutic approaches and their impact on long-term functional outcomes will significantly contribute to improving patient care.
Our findings indicated a strong association between severe TBI and the risk of cognitive and functional disabilities. However, there are some study limitations in this meta-analysis, including randomized controlled studies that are not evaluated with reference to some essential factors, including patients’ age, body mass index, ethnicity, social and demographic statuses, sample size, and co-morbidities.
5. Conclusion
Our findings demonstrate a strong association between severe TBI and impaired cognitive and functional abilities. The results indicate significant impairments in memory, verbal and visual disabilities, depression inducement, attention deficits, and learning disabilities among individuals with severe TBI compared to the control group. These outcomes highlight the importance of understanding the long-term effects of TBI on various cognitive and functional domains.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZS: Conceptualization, Methodology, Writing – original draft. YJ: Software, Writing – original draft. NM: Software, Writing – original draft. WZ: Formal analysis, Writing – original draft. ZY: Formal analysis, Writing – original draft. SW: Data curation, Writing – original draft. QL: Data curation, Writing – original draft. JR: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Footnotes
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Aharon-Peretz J., Kliot D., Amyel-Zvi E., Tomer R., Rakier A., Feinsod M. (1997). Neurobehavioral consequences of closed head injury in the elderly. Brain Inj. 11, 871–876. doi: 10.1080/026990597122945, PMID: [DOI] [PubMed] [Google Scholar]
- Anderson V. A., Morse S. A., Catroppa C., Haritou F., Rosenfeld J. V. (2004). Thirty month outcome from early childhood head injury: a prospective analysis of neurobehavioural recovery. Brain 127, 2608–2620. doi: 10.1093/brain/awh320, PMID: [DOI] [PubMed] [Google Scholar]
- Arciniegas D. B., Held K., Wagner P. (2002). Cognitive impairment following traumatic brain injury. Curr. Treat. Options Neurol. 4, 43–57. doi: 10.1007/s11940-002-0004-6 [DOI] [PubMed] [Google Scholar]
- Ashman T. A., Cantor J. B., Gordon W. A., Sacks A., Spielman L., Egan M., et al. (2008). A comparison of cognitive functioning in older adults with and without traumatic brain injury. J. Head Trauma Rehabil. 23, 139–148. doi: 10.1097/01.HTR.0000319930.69343.64 [DOI] [PubMed] [Google Scholar]
- Breed S., Sacks A., Ashman T. A., Gordon W. A., Dahlman K., Spielman L. (2008). Cognitive functioning among individuals with traumatic brain injury, Alzheimer's disease, and no cognitive impairments. J. Head Trauma Rehabil. 23, 149–157. doi: 10.1097/01.HTR.0000319931.76966.ff [DOI] [PubMed] [Google Scholar]
- Hawley C. A. (2004). Behaviour and school performance after brain injury. Brain Inj. 18, 645–659. doi: 10.1080/02699050310001646189 [DOI] [PubMed] [Google Scholar]
- Hellawell D. J., Taylor R. T., Pentland B. (1999). Cognitive and psychosocial outcome following moderate or severe traumatic brain injury. Brain Inj. 13, 489–504. doi: 10.1080/026990599121403 [DOI] [PubMed] [Google Scholar]
- Howlett J. R., Nelson L. D., Stein M. B. (2022). Mental health consequences of traumatic brain injury. Biol. Psychiatry 91, 413–420. doi: 10.1016/j.biopsych.2021.09.024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. P., Chen P. S., Wong C. S., Chang C. F., Ou J. C., Tsai Y. R., et al. (2019). Psychometric evaluation of anxiety, Depression, and Sleep Quality after a Mild Traumatic Brain Injury: a Longitudinal Study. Behav. Neurol. 2019:4364592. doi: 10.1155/2019/4364592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangels J. A., Craik F. I., Levine B., Schwartz M. L., Stuss D. T. (2002). Effects of divided attention on episodic memory in chronic traumatic brain injury: a function of severity and strategy. Neuropsychologia 40, 2369–2385. doi: 10.1016/S0028-3932(02)00084-2, PMID: [DOI] [PubMed] [Google Scholar]
- Ng S. Y., Lee A. Y. W. (2019). Traumatic brain injuries: pathophysiology and potential therapeutic targets. Front. Cell. Neurosci. 13:528. doi: 10.3389/fncel.2019.00528, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen A., Babikian T., Bigler E. D., Caeyenberghs K., Conde V., Dams-O’Connor K., et al. (2021). Toward a global and reproducible science for brain imaging in neurotrauma: the ENIGMA adult moderate/severe traumatic brain injury working group. Brain Imaging Behav. 15, 526–554. doi: 10.1007/s11682-020-00313-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M. B., Gensel J. C. (2017). Interactions of primary insult biomechanics and secondary cascades in spinal cord injury: implications for therapy. Neural Regen. Res. 12, 1618–1619. doi: 10.4103/1673-5374.217332, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic D., Pekic S., Stojanovic M., Popovic V. (2019). Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary 22, 270–282. doi: 10.1007/s11102-019-00957-9, PMID: [DOI] [PubMed] [Google Scholar]
- Rao V., Bertrand M., Rosenberg P., Makley M., Schretlen D. J., Brandt J., et al. (2010). Predictors of new-onset depression after mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 22, 100–104. doi: 10.1176/jnp.2010.22.1.100, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M. J., Herrmann N., Shammi P., Kiss A., Phillips A., Feinstein A. (2006). Outcome after traumatic brain injury sustained in older adulthood: a one-year longitudinal study. Am. J. Geriatr. Psychiatry 14, 456–465. doi: 10.1097/01.JGP.0000199339.79689.8a, PMID: [DOI] [PubMed] [Google Scholar]
- Richards B. (2000). The effects of aging and mild traumatic brain injury on neuropsychological performance. York University, Ontario. Canada: Citeseer. [Google Scholar]
- Robinson C. P. (2021). Moderate and severe traumatic brain injury. Continuum (Minneap Minn) 27, 1278–1300. doi: 10.1212/CON.0000000000001036, PMID: [DOI] [PubMed] [Google Scholar]
- Salmond C. H., Chatfield D. A., Menon D. K., Pickard J. D., Sahakian B. J. (2005). Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain 128, 189–200. doi: 10.1093/brain/awh352, PMID: [DOI] [PubMed] [Google Scholar]
- Sigurdardottir S., Andelic N., Roe C., Schanke A. K. (2009). Cognitive recovery and predictors of functional outcome 1 year after traumatic brain injury. J. Int. Neuropsychol. Soc. 15, 740–750. doi: 10.1017/S1355617709990452, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.