Abstract
Introduction
Encephalocraniocutaneous lipomatosis (ECCL) is a rare congenital syndrome with complex skin, eye, and central nervous system (CNS) symptoms. Diagnosis and treatment are challenging due to its rarity and diverse manifestations. It often involves issues like porencephalic cysts, cortical atrophy, and low-grade gliomas in the CNS, resulting in developmental delays. The spinal cord is frequently affected, leading to problems like medullary compression and radiculopathy, causing back pain and sensory/motor deficits. Surgical interventions are reserved for symptomatic cases to address hydrocephalus or alleviate spinal lipomas. This article reviews a case series to assess surgical risks and neurological outcomes.
Case series
We present a case series ECCL, focusing on the diffuse lipomatosis of the spinal cord and the intricate surgical procedures involved. A multi-stage surgical approach was adopted, with continuous neuromonitoring employed to safeguard motor pathways. We discuss clinical characteristics, imaging studies, and indications for neurosurgical interventions.
Discussion
ECCL is a complex syndrome. Diagnosis is challenging and includes clinical evaluation, neuroimaging, and genetic testing. Treatment targets specific symptoms, often requiring surgery for issues like lipomas or cerebral cysts. Surgery involves laminectomies, spinal fusion, and motor pathway monitoring. Thorough follow-up is crucial due to potential CNS complications like low-grade gliomas. Hydrocephalus occurs in some cases, with endoscopic third ventriculostomy (ETV) preferred over ventriculoperitoneal shunt placement.
Conclusion
Neurosurgery for ECCL is for symptomatic cases. ETV is preferred for hydrocephalus, while the treatment for lipoma is based on the presence of symptoms; the follow-up should assess growth and prevent deformities.
Keywords: Encephalocraniocutaneous lipomatosis, Surgical decompression, Hydrocephalus, Case series
Introduction
Encephalocraniocutaneous lipomatosis (ECCL) is a rare congenital syndrome characterized by signs and symptoms involving the skin, eyes, and central nervous system (CNS) [1]. This disease, of unknown origin, poses a challenge both for diagnosis and treatment due to its rarity and the complexity of its manifestations [2]. Reported CNS manifestations include porencephalic cysts, cortical atrophy, and low-grade gliomas (LGG), which are consistently associated with developmental delay and mental retardation. Most problems focus on the spinal cord with diffuse lipomatosis. Specifically, clinical presentations can be compressive medullary or radiculopathy, resulting in consequences such as back pain, radiculopathy, or motor or sensory deficits. Neurosurgical interventions are complex and reserved for symptomatic cases. In this article, we analyze our series and reviews to evaluate the risk of surgery and neurological outcomes.
Case series
We present a series of three patients with ECCL treated at our institute between 2008 and 2023 (Table 1).
Table 1.
We report the main clinical, radiological, and neurosurgical data of patients affected by ECCL described in our case series
| Patient | Sex | Age at diagnosis (months) | Sing and symptoms | Level of lipoma | Hydrocephalus | First surgery | Second surgery | Third surgery | Complications | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| A | M | 36 | Spastic tetraparesis, scoliosis, neurogenic bladder, ocular lipoma, restrictive ventilatory deficit | Cervico-dorsal | Yes | Ventriculocisternostomy | Debulking lipoma | Debulking lipoma + vertebral arthrodesis | No | Low-grade glioneuronal neoplasm with deceased |
| B | M | 2 | Alopecia, scoliosis, psychomotor delay, hydrobulbia hydromyelia | Bulb-dorsal | Yes | Debulking lipoma | DVE | Debulking lipoma + vertebral arthrodesis | No | Deceased |
| C | M | 60 | Lower back pain, motor disturbances, pigmented skin lesions, attention deficit | Cervico-lumbar | No | Debulking lipoma | Debulking lipoma | Debulking lipoma + vertebral arthrodesis | Intraoperative loss of motor potentials | In progress |
This table shows the age at diagnosis of ECCL expressed in months and describes the clinical characteristics of the patients
We evaluated the extent of the spinal cord lipoma and the development of hydrocephalus, highlighting the neurosurgical strategies, complications, and outcome
M male, DVE external ventricular drainage
Patient A had scoliosis and a congenital lipoma in the right eye. Magnetic resonance imaging (MRI) revealed obstructive hydrocephalus and a spinal lipoma extending into the cervical-dorsal region (Fig. 1A), leading to the development of spastic tetraparesis and neurogenic bladder. Consequently, the patient underwent ventriculocisternostomy and later had two surgical procedures to remove the spinal lipoma. Following the surgical removal of the spinal lipoma, the patient developed kyphosis, requiring stabilization with spinal fusion. During follow-up, the patient developed a diffuse low-grade glioneuronal neoplasm throughout the brain, ultimately resulting in death.
Fig. 1.
Patient A: (A1) Pre-operative X-ray of the spinal column demonstrates scoliosis in the thoracic and lumbar spine. (A2–A3) Sagittal T1WI image shows a high signal intensity intradural lesion on the dorsal surface of the cervico-thoracic cord. (A4–A5) Sagittal and axial T2W show a diffuse thalamo-mesencephalic glioma with microcysts and a cerebrospinal fluid-like density in FLAIR sequences. (A6) Sagittal T2W image shows signal flux in ETV (endoscopic third ventriculostomy). (A7–A8) Posterior arthrodesis and instrumentation using DePuy Synthes EXPEDIUM 5.5 Titanium with polyaxial screws and hooks completed with a cross-connector system. (A9) Progression of gliomas involving the temporo-parietal lobes. Patient B: (B1) T2WI sagittal MR at presentation shows an extensive medullary bulbo-cervico-dorsal lipoma with compressive effects. (B2) T2WI sagittal MR results after decompressive suboccipital craniotomy with cervical laminectomy and debulking of the lipoma at the bulbo-medullary junction, associated with a patch graft. (B3) T2WI sagittal MR image shows 7 months after the second step postoperative decompression of the dorsal parts of the lipoma. (B4–B5) Surgical views of the lipoma pre- and post-debulking. Patient C: (C1) Pre-operative X-ray (lateral view) shows cervico-dorsal kyphosis. (C2) Sagittal T2W reveals polilobulated lipomas extending between D1 and L1, adhering to the posterior surface of the spinal cord. The spinal cord appears displaced anteriorly in the dorsal tract and markedly thinned due to compression at multiple points, especially at the C7 level and in the D3-D10 tract. The spinal canal exhibits a bone remodeling phenomenon of the posterior dorsal neural arches. (C3) Sagittal T2W displays post-operative partial removal of intradural cervical and dorsal lipomas, demonstrating slightly reduced thickness. (C4) Partial cervico-dorsal fixation with growing rods connected only to lumbar pedicle screws on the left
Patient B presented with scoliosis and psychomotor delay. MRI revealed a spinal lipoma extending into the bulbo-dorsal region, along with hydrobulbia and hydromyelia (Fig. 1B). Surgical treatment was performed in two stages. After the first debulking surgery, the patient developed obstructive hydrocephalus, necessitating external ventricular drainage. The follow-up was concluded due to the patient’s death.
Patient C presented with lower back pain, motor disturbances, pigmented skin lesions, and attention deficits. MRI showed a cervical-lumbar spinal lipoma associated with kyphosis (Fig. 1C). The patient underwent three-stage surgical removal of the spinal lipoma, which included laminotomies and spinal stabilization. The patient’s follow-up is ongoing and has revealed an increase in kyphosis, requiring the use of a brace and rehabilitative therapy.
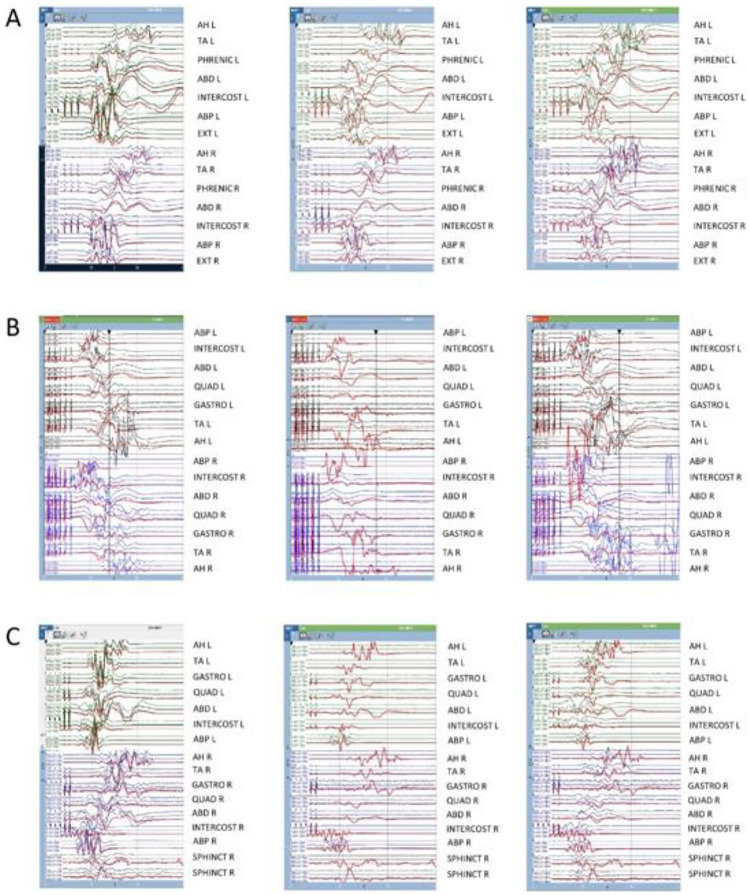
In all patients, surgical treatment was performed using a multi-step approach to better control the sequelae. Continuous neuromonitoring was applied during surgical procedures, including electroencephalogram (EEG), electromyogram (EMG), somatosensory evoked potentials (SSEPs), and motor evoked potentials (MEPs). Neuronal integrity was assessed through wake-up tests during surgery. This strategy was particularly crucial in the case of Patient C, as a loss of motor potentials in the lower limbs was detected during surgery (Fig. 2), allowing for immediate resolution of the complication.
Fig. 2.
Intraoperative neurophysiology of Patient C: The figure illustrates transcranial motor evoked potentials (TcMEPs) recorded before, during, and at the end (panels from left to right) of the three surgeries (A, B, C) for Patient C. The thick red lines represent the baseline signal. A Debulking of the rostral part of the lipoma resulted in a transient reduction in the amplitude of TcMEPs recorded at the left TA muscle (middle panel). TcMEPs returned to baseline by the end of the surgery (right panel). B Debulking of the caudal part of the lipoma caused a widespread loss of TcMEPs following durectomy (middle panel). TcMEPs gradually recovered over time, and no significant changes were observed compared to baseline at the end of the surgery (right panel). C Placement of growing rods resulted in a widespread loss of TcMEPs (except for bilateral ABPs) following the rods correction procedure (middle panel). TcMEPs improved after the release of the correction (right panel), with the rods ultimately connected only to lumbar pedicle screws on the left. Abbreviations: AH abductor halluces, TA tibialis anterior, ABD abdominal, INTERCOST intercostal, ABP abductor pollicis brevis, EXT extensor digitorum communis brevis, QUAD quadriceps, GASTRO gastrocnemius, SPHINCT anal sphincter
Discussion
ECCL is a multisystemic syndrome that involves three main components: cutaneous involvement is often one of the most apparent signs with various skin defects, including superficial lipomas, pigmented skin lesions, and facial angiofibromas [3, 4]. Central nervous system (CNS) involvement is the most severe component and the most important prognostic factor [5]. The distribution of lipomas is often widespread along the spinal cord, typically remaining stable throughout a patient’s life. Diagnosing ECCL can be challenging due to its clinical variability and similarity to other neurocutaneous diseases. Typically, it is made through a detailed clinical evaluation, neuroimaging studies, and sometimes genetic tests to identify specific genetic mutations associated [6]. ECCL is primarily associated with mosaic mutations in the FGFR1 gene as revealed by exome sequencing. The connection between mosaic mutations and developmental pathologies, with potential overlap in tumorigenesis-related signaling pathways like RAS-MAPK, is highlighted. However, not all ECCL cases are linked to identifiable FGFR1 mutations, suggesting the possibility of other genetic factors or mechanisms at play [4, 7, 8]. The treatment of ECCL is complex and aimed at managing the specific manifestations of the disease. In symptomatic cases, surgical removal may be necessary [9]. In particular, in patient with joint cervicomedullary involvement, posterior fossa craniectomy with cervical laminectomies is reserved for sleep apnea and upper deficits. Extensive laminectomy can lead to progressive kyphosis, necessitating consideration of surgical atlantoaxial transarticular screw fixation. In pre-operative scoliosis cases where debulking occurs in multiple steps, a complete posterior arthrodesis is indicated. Authors recommend subpial excision of lipomas, although intramedullary invasiveness may prevent complete removal [10]. Motor evoked potentials monitoring provides real-time feedback on the integrity of motor pathways, reducing the risk of neurological injury. Another reason for rigorous follow-up is that CNS malformations may increase morbidity and mortality [11]. We analyzed clinical cases of ECCL with spinal cord lipoma described in the literature (Table 2), and we found descriptions of 7 cases of ECCL with spinal cord lipoma. The extent of the lipoma ranged from involvement of only the dorsal portion to the entire spinal cord. Four patients underwent a surgical laminectomy with resection of the spinal lipoma [5, 10, 12, 13], but only one case was the procedure performed in two stages [10]. Of the cases described in the literature, only two presented obstructive hydrocephalus [10, 14], and in both cases, the patients underwent surgical treatment. In one case, an endoscopic third ventriculostomy (ETV) was performed [10], while in the other, a ventriculo-peritoneal shunt (VPS) was placed [14]. In fact, ventriculomegaly and macrocephaly are common findings in ECCL patients, but not all of them develop hydrocephalus. Hydrocephalus was diagnosed in approximately one-third of patients with ECCL, and ETV should be considered as the first option instead of VPS placement.
Table 2.
We report the main clinical, radiological, and neurosurgical data of patients affected by ECCL described in the literature
| Case | Sex | Age at diagnosis (months) | Sing and symptoms | Level of lipoma | Hydrocephalus | First surgery | Second surgery | Third surgery | Fourth surgery | Complications | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A12 | M | 108 | Neck pain, alopecia, scalp lipoma, coloboma, chorioretinitis, mental retardation, cerebral lipoma | Dorsal | No | Lipoma resection | No | No | No | Paraparesis, neurogenic bladder, and bowel | Worsed |
| B13 | M | 108 | Paraplegia, alopecia, chorioretinitis, mental retardation, sphenocavernosal lipoma | Dorsal | No | No | No | No | No | No | N/A |
| C5 | M | 1 | Multiple lipoma in the cranio-facial region, limbal dermoid, glaucoma, alopecia, epibulbar dermoid | Cervical and lumbar | No | Epibulbar dermoid resection | Spinal cord lipoma resection | No | No | No | Improvement |
| D10 | M | 3 | Lipomatous mass in the temporal limbus, coloboma, alopecia, scoliosis, cerebral lipoma, tethering spinal cord | Cervico-sacral | Yes | ETV | Debulking of the cervicomedullary junction lipoma | VPS | Resection lumbar lipoma + spinal cord untethering | No | Progressive scoliosis |
| E14 | F | N/A | Multiple lipomatous mass, mild liver dysfunction, choroidal coloboma, dermolipoma of the right limbus, unilateral hearing loss, lipomatous lesion in APC | Cervico-dorsal | No | Lipoma debulking | No | No | No | No | Improvement |
| F15 | F | 0 | Alopecia, nodules on the right side of the face, epibulbar dermoid, aniridia, low-set ears, coloboma, multiple soft subcutaneous masses over the back, lipoma at right APC, tethered spinal cord | Cervico-lumbar | Yes | VPS | No | No | No | No | N/A |
This table shows the age at diagnosis of ECCL expressed in months and describes the clinical characteristics of the patients
We evaluated the extent of the spinal cord lipoma and the development of hydrocephalus, highlighting the neurosurgical strategies, complications, and outcome
ETV endoscopic third ventriculostomy, VPS ventriculoperitoneal shunt, APC cerebellopontine angle
Conclusion
Early identification of ECCL is crucial for accurate diagnosis, effective symptomatic management, and better patient care, ultimately improving the quality of life for affected individuals [4]. In terms of treatment, the approach varies. In cases of associated hydrocephalus, ETV is usually preferred to alleviate intracranial pressure. The surgical approach for spinal cord lipomas is recommended in symptomatic cases and involves the removal of the lipoma, sometimes in multiple stages, to preserve nerve function. In our experience, we believe it is appropriate to perform surgical procedures in symptomatic cases, and we consider follow-up crucial not only to assess the growth of lipomatous lesions but also to prevent vertebral deformities; specifically, we anticipate cervical stabilization to prevent the development of kyphosis.
Author contributions
All authors contributed equally to the writing of the manuscript.
Funding
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors have no relevant financial or no-financial interests to disclose.
Footnotes
The corresponding author accepts responsibility for the integrity of the submitted work and attests that no undisclosed authors contributed to the manuscript.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Biesecker LG. The challenges of Proteus syndrome: diagnosis and management. Eur J Hum Genet. 2006;14(11):1151–1157. doi: 10.1038/sj.ejhg.5201638. [DOI] [PubMed] [Google Scholar]
- 2.Klein S, Hamerschmidt R, Waissmann W. Encephalocraniocutaneous lipomatosis. Revista brasileira de neurologia (Rev Bras Neurol) 2009;45(3):69–72. [Google Scholar]
- 3.Herrera-Gómez Á, Carrasco-Rozas A, Sánchez-Romero F, Herrera-Gómez FJ (2019) Encephalocraniocutaneous lipomatosis: clinical, neuroimaging, and genetic features. A review of 32 cases. Neurologia 34(1):46–54
- 4.Karaman ZF, Özüdoğru ŞE. Encephalocraniocutaneous lipomatosıs (Haberland syndrome) in a newborn baby: a case report with review of literature. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2021;37(12):3951–3955. doi: 10.1007/s00381-021-05099-7. [DOI] [PubMed] [Google Scholar]
- 5.Moog U, Jones MC, Bird LM et al (2005) Encephalocraniocutaneous lipomatosis plus: a syndrome including intracranial lipomas, uveal colobomas, and complex vascular anomalies. Report of two patients and review of the literature. Neuropediatrics 36(6):347–352
- 6.Slavotinek AM, Garcia ST, Chandratillake G, Bardakjian T, Ullah E, Wu D, et al. Exome sequencing in 32 patients with anophthalmia/microphthalmia and developmental eye defects. Clin Genet. 2015;88(5):468–473. doi: 10.1111/cge.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett JT, Tan TY, Alcantara D, Tétrault M, Timms AE, Jensen D, Collins S, Nowaczyk MJ, Lindhurst MJ, Christensen KM, Braddock SR, Brandling-Bennett H, Hennekam RCM, Chung B, Lehman A, Su J, Ng S, Amor DJ, University of Washington Center for Mendelian Genomics. Care4Rare Canada Consortium, ... . McDonell LM. Mosaic activating mutations in fgfr1 cause encephalocraniocutaneous lipomatosis. Am J Hum Genet. 2016;98(3):579–587. doi: 10.1016/j.ajhg.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kordacka J, Zakrzewski K, Gruszka R, Witusik-Perkowska M, Taha J, Sikorska B, Liberski PP, Zakrzewska M. Sensitive detection of FGFR1 N546K mosaic mutation in patient with encephalocraniocutaneous lipomatosis and pilocytic astrocytoma. Am J Med Genet A. 2019;179(8):1622–1627. doi: 10.1002/ajmg.a.61256. [DOI] [PubMed] [Google Scholar]
- 9.Caro-Domínguez P, Esteban-Sanchis A, Gomis-Couto A, Mínguez-Martínez I, Mata-Gregori J, Sanahuja-Martínez A, et al. Encephalocraniocutaneous lipomatosis: a case report and review of literature. Case Rep Med. 2020;2020:9056079. [Google Scholar]
- 10.Ayer REZA (2011) Encephalocraniocutaneous lipomatosis: a review of its clinical pathology and neurosurgical indications. J Neurosurg Pediatr 8(3):316–320 [DOI] [PubMed]
- 11.Brassesco MS, Valera ET, Becker AP, Castro-Gamero AM, de Aboim MA, Santos AC, et al. Low-grade astrocytoma in a child with encephalocraniocutaneous lipomatosis. J Neurooncol. 2010;96:437–441. doi: 10.1007/s11060-009-9978-1. [DOI] [PubMed] [Google Scholar]
- 12.Deda G, Caksen H, Yavuzer G, Arasil T. Encephalocraniocutaneous lipomatosis associated with iris coloboma, chorioretinitis and spinal cord involvement: a case report. Brain Dev. 2001;23(5):355–358. doi: 10.1016/s0387-7604(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 13.Chiang CC, Lin SC, Wu HM, Wang JC, Yang TF, Chen HH, Ho DM, Wong TT. Clinical manifestation and neurosurgical intervention of encephalocraniocutaneous lipomatosis–a case report and review of the literature. Childs Nerv Syst. 2014;30(1):13–17. doi: 10.1007/s00381-013-2252-z. [DOI] [PubMed] [Google Scholar]
- 14.Naous A, Shatila AR, Naja Z, Naja AS, Rajab M (2015) Encephalocraniocutaneous lipomatosis: a rare association with tethered spinal cord syndrome with review of literature. Child Neurol Open 2(1):2329048X14553297. 10.1177/2329048X14553297. PMID: 28503585; PMCID: PMC5417016 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.