Abstract
In China, Russia, Mongolia, Japan, North Korea, and Mexico, Sedum aizoon L. (S. aizoon) is used as an edible plant. Up to now, over 234 metabolites, including phenolic acids, flavonoids, triterpenes, phytosterols, and alkaloids, among others, have been identified. In addition to its antioxidant, anti-inflammatory, anti-fatigue, antimicrobial, anti-cancer, and hemostatic activities, S. aizoon is used for the treatment of cardiovascular disease. This paper provides an overview of the history, botany, nutritional value, traditional use, phytochemistry, pharmacology, toxicology, and quality control of S. aizoon.
Keywords: Sedum aizoon L., pharmacological activities, quality control, hemostatic activity, active metabolites
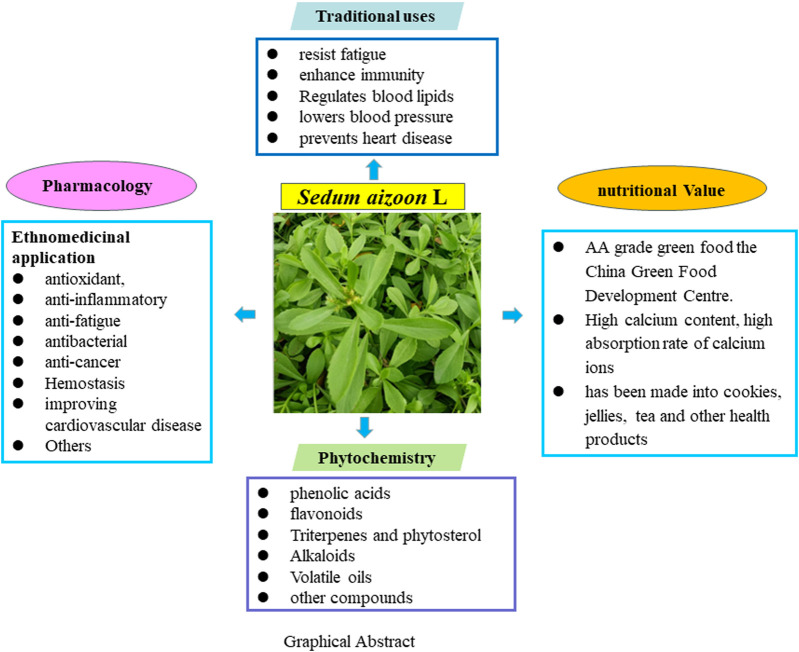
Graphical Abstract
Highlights
• S. aizoon L. is frequently prescribed in both China and other countries as a traditional folk herbal remedy for various diseases
• This review contributes to updating the herbalogical textual research, traditional use, botany, phytochemistry, pharmacology, toxicity, and nutritional value and quality control of S. aizoon L.
• In earlier literature, there was no systematic review of S. aizoon L
1 Introduction
Sedum aizoon L. (Chinese name:景天三七) is a perennial herbaceous plant that is widely distributed in China, Russia, Mongolia, Japan, North Korea, and Mexico. It is a member of the Sedum genus in the Sedum family (Crassulaceae) (Guo and Lin, 2007). Its name is also consistent with the plant name recorded in “The Plant List” (http://www.theplantlist.org), which is now incorporated into the requirement for traditional medicine in the provinces of Jiangsu and Fujian (Jia et al., 2014). It is one of the renowned “Taibai seven medicine (太白七药)” in the Qinling Mountains, which has the effects of dispersing blood stasis, stopping bleeding, tranquilizing the mind, detoxifying, and analgesia, and is used in the treatment of various kinds of bleeding, palpitations, and insomnia. Growing in the natural environment, S. aizoon is a unique pest-free plant that does not require pesticides during its whole phenological cycle and has been designated as AA grade green food by the China Green Food Development Center. Its fresh stems and leaves are consumed as vegetables (Xue, 2015).
Despite the fact that the phytochemistry and ethnopharmacology of S. aizoon have been previously reviewed, a comprehensive study linking its bioactive metabolites with its pharmacological properties is lacking. Therefore, this paper provides an overview of the history, botany, nutritional value, traditional use, phytochemistry, pharmacology, toxicology, and quality control of S. aizoon.
2 Materials and methods
Information about S. aizoon was gathered from scientific literature sources, including PubMed, Baidu Scholar, Google Scholar, Web of Science, SciFinder, CNKI, Wanfang, the Plant List (www.theplantlist.org), and books. The history, nutritional value, traditional uses, botany, phytochemistry, pharmacology, toxicology, and quality control or a combination between them was used as keywords to search for data up to July 2023. Approximately, 767 research studies of S. aizoon were gathered from various databases. With the removal of duplicate literatures, 300 literatures were selected according to research purpose, relevance, and article type. The articles which contained information apart from that mentioned above or written in languages rather than English were also excluded. ChemBioDraw Ultra version 14.0 was used to draw chemical structures.
3 History and traditional uses
3.1 History
S. aizoon was first recorded in “Jiu Huang Ben cao” (救荒本草) (Ming Dynasty), which is the earliest book with agronomy and botany as its monograph on the history of China. Later, it was also included in many other famous works on Chinese herbal medicine, including “Wild Vegetables Bo lu” (野菜博录) (Ming Dynasty), “Plants Ming Shi Tu Kao” (植物名实图考), and “Discussion on varieties of Chinese medicinal materials” (中药材品种论述).
The medicinal parts of S. aizoon were roots and grass in S. aizoon, and S. kamshaticum. S. aizoon has more than 60 synonyms and is distributed in more than 20 provinces or autonomous regions. In addition, the herb and the syrup were included in the Pharmacopoeia of the People’s Republic of China (Part I) (1977 edition) (Chinese Pharmacopoeia Committee, 2005).
3.2 Traditional uses
In folk medicine, the flat and sweet whole herb and the roots of S. aizoon are widely used for dispersing blood stasis and stopping blood bleeding. For instance, daily administration of 60–90 g of S. aizoon decoction can treat bleeding symptoms, including hemoptysis, bleeding gums, epistaxis, gingival bleeding, and internal bleeding. The fresh juice was effectively used for the treatment of leukemia, aplastic anemia, thrombocytopenic purpura, hemoptysis, and different forms of bleeding (i.e., gingival, digestive tract, and hematuria) (Chinese herbal medicine research group, 1971). In addition, ancient medical classics, such as Li Shizhen’s “Compendium of Materia Medica” (本草纲目), Chen Shiduo’s “New Compilation of Materia Medica” (本草新编), and Zhang Xichun’s “Intergrating Chinese And Western Medicine” (医学衷中参西录), explicitly stated that S. aizoon had good hemostasis and analgesic function, which was known as “the god medicine for hemostasis” (止血神药). It is also used as a heart and mind tranquillizing agent with an excellent effect on hysteria palpitation, restlessness, hypertension, and rheumatic heart disease (Chen, 2003). Likewise, the detoxifying and clearing heat effects have also been reported.
Of note, S. aizoon has a long history as both an edible and medicinal herb. For example, vegetables with S. aizoon’s stems and leaves as metabolites have good nutritional value. “Jiu Huang Ben Cao” (救荒本草) in the Ming Dynasty stated that the regular consumption of the fresh, tender stems and leaves of S. aizoon can promote blood circulation and calm the heart.
4 Nutritional value
The tender stems and leaves contain moisture (87 g), protein (2.1 g), fat (0.7 g), carbohydrate (8.0 g), crude fiber (1.5 g), ash (1.2 g), energy (196.65 KJ), Ca (315 mg), P (39 mg), Fe (3.2 mg), carotene (2.54 mg), vitamin B1 (0.05 mg), vitamin B2 (0.07 mg), vitamin PP (90 mg), and vitamin C (90 mg) (Yi, 2000; Liu et al., 2012). Owing to its unique aroma and taste, S. aizoon is used for the preparation of cookies, jellies, and tea (Wang, 2013).
5 Botany
5.1 Geographical repartition
S. aizoon belongs to the genus Sedum of the Crassulaceae family. There are approximately 600 species widely distributed in the temperate and subtropical regions of the northern hemisphere with Mexico being the largest center of origin and diversity of Sedum species.
5.2 Morphology
S. aizoon is an annual or perennial, succulent herb, growing in clusters and has a strong ability to bifurcate. S. aizoon has coarse, woody rhizomes that resemble ginseng in form. The stems are erect, cylindrical, and glabrous, which can reach heights of 15–50 cm. At each node, the stems carry just one leaf, which is nearly opposite on both sides. The leaves are 2.5–5 cm long, 5–12 mm wide, obovate or long oval in shape, and broad and thick with more juice. Additionally, they feature a cuneate base, a serrated border toward the apex, a moderately rounded top, and few sessile leaves. The loose, terminal verticillaster contains ten stamens that are around the same length as the petals, five distinct pistils that are slightly longer than the stamens, five orange–yellow petals with lancolate, sharp tips, and five sepals with blunt ends. Follicles are either reddish or brown in color and are grouped in a star pattern. Seeds are obovate, smooth, have wings along the edge, and have a wider apical. Flowers usually bloom in summer. The photos of S. aizoon are pictured and shown in Figure 1.
FIGURE 1.

Morphological characteristics of S. aizoon: (A) leaves, (B) roots, (C) dry drug, (D) buds, and (E) whole plant.
6 Phytochemistry
Up to now, more than 234 metabolites, including flavonoids (1–48), phenolic acids (49–78), triterpenes and phytosterols (79–90), alkaloids (91–98), volatile constituents (99–216), and others (217–234), have been preliminarily isolated or identified from S. aizoon. Among these, flavonoids are the main metabolites of S. aizoon. The main metabolites and their structure are given in Table 1 and Figures 2–5.
TABLE 1.
Main active metabolites identified in S. aizoon.
| Metabolite | Plant part | Molecular formula | Reference |
|---|---|---|---|
| Flavonoid | |||
| Trifolin | Leaves and stems | C21H20O11 | Xu et al. (2019) |
| Rutin | C27H30O16 | ||
| Isoquercitrin | C21H20O12 | ||
| Isorhamnetin | C16H12O7 | ||
| Astragalin | C21H20O11 | ||
| Genistein | C15H10O5 | ||
| Lonicerin | C27H30O15 | ||
| Scutellarein | C15H10O6 | ||
| Catechin | C15H14O6 | ||
| Rhamnetin-3-O-β-D-glucopyranoside | Rhizome | C22H22O12 | Li et al. (2020a) |
| Isorhamnetin-3-O-β-D-xylopyranoside | C21H20O11 | ||
| Isorhamnetin-3-O-α-L-arabinopyranoside | C21H20O11 | ||
| Rhamnazin-3-O-β-D-glucopyranoside | Aerial parts | C23H26O12 | Xiong et al. (2019) |
| Quercetin | Aerial parts, rhizome, and leaves and stems | C15H10O7 | |
| Myricetin | Aerial parts and leaves and stems | C15H10O8 | |
| Luteoloside | N/A | C21H20O11 | |
| Quercitrin | Aerial parts and leaves and stems | C21H20O11 | Wolbi and Olszewska (1996), Li et al. (2007) |
| Myricitrin | Aerial parts | C21H20O12 | |
| Quercetin-3-o-(2′-galloyl) rhamnoside | N/A | C28H30O9 | Wolbi and Olszewska (1996) |
| Quercetin-3-O-α-L-arabinopyranoside | Leaves and stems and rhizome | C20H18O11 | Han et al. (2017) |
| Myricetin-3-O-α-L-arabinopyranoside | Aerial parts | C20H18O12 | |
| Kaempferol-7-O-glucoside | Leaves and stems | C21H20O11 | |
| Kaempferol-3-O-β-D-glucopyranoside | C21H20O11 | ||
| Herbacetin-3-O-α-L-arabinopyranoside | C20H18O10 | ||
| Myricetin-3-β-D-glucopyranoside | Aerial parts and leaves and stems | C21H20O13 | Li et al. (2008) |
| Myricetin-3-β-D-(6″-o-galloyl)-glucopyranoside | Whole grass | C28H24O17 | |
| Myricetin-3-o-β-D-(6″-o-galloyl)-galactopyranoside | C28H24O17 | ||
| Myricetin-3′-o-β-D-glucopyranoside | Leaves and stems | C21H20O13 | Jia et al. (2014) |
| Kaempferol | Leaves and stems and rhizome | C15H10O6 | Lin et al. (2014), Xiong et al. (2019) |
| Kaempferol-3-O-α-L-rhamnoside | Leaves and stems | C21H20O10 | Zhang et al. (2010) |
| Herbacetin-8-O-α-D-lyxoside | C20H18O11 | ||
| Herbacetin-8-O-β-D-xylopyranoside | C20H18O11 | ||
| Luteolin | C15H10O6 | ||
| Herbacetin-8-O-β-D-glucopyranoside | Aerial parts | C25H23O7D3 | Xu et al. (2015) |
| Herbacetin-3-O-β-D-glucopyranosyl-8-O-α-L-arabinopyranoside | C74H105O32 | ||
| Herbacetin-3-O-α-L-rhamnopyranosyl-8-O-α-D-lyxopyranoside | C26H28O14 | ||
| Herbacetin-3-O-α-L-arabinopyranosyl-8-O-β-D-xylopyranoside | C25H26O14 | ||
| Gossypetin-3-O-β-D-glucopyranosyl-8-O-β-D-xylopyranoside | C73H106O34 | ||
| 3′-Methoxyl-gossypetin-3-O-β-D-glucopyranosyl-8-O-β-D-xylopyranosie | C27H30O17 | ||
| 6″-O-(E)-feruloyl isorhamnetin | Whole plant | C32H30O15 | (Li J. X. et al., 2011) |
| 6″-O-(E)-feruloyl quercetin | C31H28O15 | ||
| 3,4′,5,7-Tetrahydroxyflavone-7-O-α-D-xylopyranoside | Whole grass | C20H18O10 | Han et al. (2021) |
| Sedacin A | Whole plant | C28H32O7 | Li J. X. et al. (2011) |
| Sedacin B | C29H34O7 | ||
| 1,3,8,10,10b-Pentahydroxy-5a-(4-hydroxy-3-methoxyphenyl)-9-(4-hydroxybenzoyl)-5a,10b-dihydro-11H-benzofuro[2,3-b]chromen-11-one | Roots | C29H21O12 | Li et al. (2017) |
| 1,3,8,10,10b-Pentahydroxy-9-(4-hydroxybenzoyl)-5a-(4-hydroxyphenyl)-5a,10b-dihydro-11H-benzofurochromen-11-one | C28H19O11 | ||
| 5a-(3,4-Dihydroxyphenyl)-1,3,8,10,10b-pentahydroxy-9-(4-hydroxybenzoyl)-5a,10b-dihydro-11H-benzofurochromen-11-one | C28H19O12 | ||
| 1,8,10,10b-Tetrahydroxy-5a-(4-hydroxy-3-methoxyphenyl)-9-(4-hydroxybenzoyl)-3-methoxy-5a,10b-dihydro-11H-benzofuro[2,3-b]chromen-11-one | C30H23O12 | ||
| Phenolic acids | |||
| Sedumol | Whole grass | C12H16O8 | Han et al. (2021) |
| Vanillic acid | Aerial parts | C8H8O4 | Lin (2014) |
| Protocatechuic acid | Aerial parts and leaves and stems | C7H6O4 | |
| Caffeic acid | N/A | C9H8O4 | |
| P-hydroxybenzoic acid | Aerial parts and leaves and stems | C7H6O3 | Lin et al. (2014) |
| Pyrogallol | Aerial parts | C6H6O3 | |
| 5,7-Dihydroxychromone | N/A | C9H6O4 | |
| Glucosyringic acid | Leaves and stems | C15H20O10 | Jia et al. (2014) |
| P-hydroxybenzoyl arbutin | C19H20O9 | ||
| Pyroside | C14H18O8 | ||
| Arbutin | Roots and leaves and stems | C12H16O7 | |
| 4-Methoxy-3,5-dihydroxybenzoic acid | Whole grass | C8H8O5 | Han et al. (2021) |
| 4-Hydroxybenzeneethanol | C8H10O2 | ||
| 4-Hydroxybenzaldehyde | C7H6O2 | ||
| cis-4-Coumaric acid | Aerial parts | C9H8O3 | Xiong et al. (2019) |
| 2-O-(trans-caffeoyl) malic acid | C13H12O8 | ||
| 2-O-(trans-caffeoyl)-malic acid 1-methyl-ester | C14H14O8 | ||
| 2-O-(trans-caffeoyl)-malic acid 1,4-dimethyl ester | C15H16O8 | ||
| Isolariciresinol-9-O-β-D-glucopyranoside | C26H34O11 | ||
| Iriflophenone-2-O-β-D-glucopyranoside | C19H20O10 | ||
| Ethyl gallate | Aerial parts and leaves and stems | C9H10O5 | |
| Gallic acid | Aerial parts, whole plant, and leaves and stems | C7H6O5 | Zhang et al. (2010) |
| Methyl gallate | Aerial parts and leaves and stems | C8H8O5 | |
| Echinochlorin A | Rhizome | C26H40O8 | Li et al. (2020a) |
| 1-O-sinapoyl glucopyranoside | Aerial parts | C17H22O10 | Xu et al. (2015) |
| Chrysophanol-8-O-β-D-glucoside | Whole grass | C21H20O9 | Li et al. (2008) |
| Hydroquinone | Roots and whole grass | C6H6O2 | |
| Vanilloloside | Leaves and stems | C14H20O8 | Han et al. (2017) |
| Woodorien | C13H9N3O2 | ||
| Iriflophene | Aerial parts and rhizome | C13H10O5 | Xiong et al. (2019), Li et al. (2020a) |
| Triterpenes | |||
| Ginsenoside Re | Roots | C48H82O18 | Gong (2020) |
| α-Amyrin | N/A | C30H50O | |
| Ursolic acid | Roots | C30H48O3 | Li et al. (2008) |
| Glutin-5-en-3-one | Leaves and stems | C30H48O | |
| Isomoliol-3β-acetate | C32H52O2 | ||
| Taraxerone | Rhizome | C30H48O | Li et al. (2020a) |
| Isomotiol | C30H50O | ||
| Oleanolic acid | Roots | C30H48O3 | Lin (2014) |
| Phytosterols | |||
| β-Sitosteryl linoleate | Rhizome | C47H80O2 | Li et al. (2020a) |
| Daucosterol | Rhizome and whole grass | C35H60O6 | |
| β-Sitosterol | Rhizome, leaves and stems, and roots | C29H50O | Zhang et al. (2010) |
| Stigmasterol | N/A | C29H48O | Cao (2011) |
| Alkaloids | |||
| Sedinine | N/A | C17H25NO2 | Kim et al. (1996) |
| Despun methylisopelletierine | C9H17NO | ||
| Sedamine | Roots | C14H21NO | Li et al. (2008) |
| Aizoonoside A | Aerial parts | C18H19NO8 | Xu et al. (2015) |
| Thymine | Aerial parts | C5H6N2O2 | Lin et al. (2014) |
| Senecionine | Roots | C18H25NO5 | Wu et al. (2008) |
| Seneciphylline | C18H23NO5 | ||
| Integerrimine | C18H25NO5 | ||
| Volatile oils | |||
| 2,6-Di(tbutyl)-4-hydroxy-4-methyl-2,5-cyclohexadien-1-one | Whole plant | C15H24O2 | Qian et al. (2018) |
| β-Ionone | C13H20O | ||
| Epiglobulol | C15H26O | ||
| α-Guaiene | C15H24 | ||
| Isophytol | C20H40O | ||
| Squalene | C30H50 | ||
| Tritriacontane | C33H68 | ||
| Hexadecane | C16H34 | ||
| Pristane | C19H40 | ||
| Octadecane | C18H38 | ||
| Tricosane | C23H48 | ||
| Tetracosane | C24H50 | ||
| Pentacosane | C25H52 | ||
| Hexacosane | C26H54 | ||
| Heptacosane | C27H56 | ||
| Octacosane | C28H58 | ||
| Nonacosane | C29H60 | ||
| Hentriacontane | C31H64 | ||
| Cetyl palmitate | C32H64O2 | ||
| 4, 8, 12, 16-Tetramethyl heptadecan-4-olide | C21H40O2 | ||
| Cyclohexyl benzoate | C13H16O2 | ||
| Methyl palmitoleate | C17H32O2 | ||
| Methyl palmitate | C17H34O2 | ||
| Ethyl palmitate | C18H36O2 | ||
| Methyl linolelaidate | C19H34O2 | ||
| Methyl oleate | C19H36O2 | ||
| Methyl stearate | C19H38O2 | ||
| Ethyl linoleate | C20H36O2 | ||
| Ethyl oleate | C20H38O2 | ||
| 1-Hexacosanol | C26H52O | ||
| Hexahydrofarnesyl acetone | Whole plant and fresh herbs | C18H36O | Guo et al. (2006), Qian et al. (2018) |
| 2-Undecanone | Fresh herbs | C11H22O | Guo et al. (2006) |
| 2-Tridecanone | C13H26O | ||
| Nerolidol | C15H26O | ||
| (−)-Spathulenol | C15H24O | ||
| Cedrol | C15H26O | ||
| Globulol | C15H26O | ||
| 1-Nonene | C9H18 | ||
| (十)-Aromadendrene | C15H24 | ||
| Calamenene | C15H22 | ||
| Caryophyllene epoxide | C15H24 | ||
| Bornyl acetate | C12H20O2 | ||
| Geraniol acetate | C12H20O2 | ||
| 15-ene-heptadecanal | C17H48O | ||
| Hexadecanoic acid | C16H32O2 | ||
| Phytol | Leaves, stems, fruits, and fresh herbs | C20H40O | |
| 4-hepten-2-one | Aerial parts | C7H12O | Chen et al. (2014) |
| Elsholtzia ketone | C10H14O2 | ||
| 3-Methyl-2-butanol | C5H12O | ||
| 2,3-Butanediol | C4H10O2 | ||
| 1-Octanol | C8H18O | ||
| 4-Terpineol | C10H18O | ||
| 3-Hexen-1-ol | C6H12O | ||
| Pentylfuran | C9H14O | ||
| β-Phellandrene | C10H16 | ||
| 4-Carene | C10H16 | ||
| β-Terpinene | C10H16 | ||
| Isoterpinolene | C10H16 | ||
| α-Thujene | C10H16 | ||
| β-Farnesene | C15H24 | ||
| π-Muurolene | C15H24 | ||
| Heptanal | C7H14O | ||
| Benzaldehyde | C7H6O | ||
| Hexanal | C6H12O | ||
| Furfural | C5H4O2 | ||
| Octanal | C8H16O | ||
| Benzeneacetaldehyde | C8H8O | ||
| Nonanal | C9H18O | ||
| Decanal | C10H20O | ||
| 1-Octadecanol | Roots and leaves | C18H38O | Chen and Qiang (2017) |
| (Z) 9-Octadecenoic acid, methyl ester | Roots and stems | C19H36O2 | |
| 2,2′-Methylenebis(6-tert-butyl-4-methylphenol | Leaves, stems, and fruits | C23H32O2 | |
| Dimethyl phthalate | C10H10O4 | ||
| Methyl tetradecanoate | C15H30O2 | ||
| Heptadecanoic acid methyl ester | C18H36O2 | ||
| Pentatriacontane | Leaves, stems, and roots | C35H72 | |
| Heptadecane | Leaves, and whole plant | C17H36 | |
| 3-Ethyl-2,4-dimethyl-pentane | Leaves | C9H20 | |
| 2,6-Dimethyl-octane | C10H22 | ||
| 6,10,14-Trimethyl2 pentadecanone | C18H36O | ||
| 1-Pentadecanol | C15H32O | ||
| Oxacycloheptadec-8-en-2-one | C16H28O2 | ||
| Tridecanoic acid, methyl ester | C14H28O2 | ||
| 2,6,11-Trimethylodlodecane | C15H32 | ||
| 3-Methyl-undecane | C12H26 | ||
| Octadecane | Fruits | C18H38 | |
| 2,6,10,14-Tetramethyl-hexadecane | C20H42 | ||
| Icosane | Stems | C20H42 | |
| Nonadecane | C19H40 | ||
| 3,8-Dimethyl-decane | C12H26 | ||
| 4-Methyl-pentadecane | C16H34 | ||
| 1-Octadecene | C18H36 | ||
| 2-Methyl-tridecane | C14H30 | ||
| Tetratetracontane | C44H90 | ||
| Tetradecane | C14H30 | ||
| Pentadecane | Leaves and stems | C15H32 | |
| 2,4,4-Trimethylhexane | C9H20 | ||
| 2,4-Dimethylhexane | C8H18 | ||
| 4,6-Dimethyl-dodecane | C14H30 | ||
| Heneicosanoic acid-methyl ester | C22H44O2 | ||
| Tricosanoic acid, methyl ester | C24H48O2 | ||
| 2,4-bis(1,1-Dimethylethyl)-phenol | C14H22O | ||
| Hexadecyl-oxirane | C18H36O | ||
| 3,3- Dimethylhexane | C8H18 | ||
| 3, 3-Dimethyl-heptane | Roots | C9H20 | |
| Tetratriacontane | C34H70 | ||
| 1-Heptadecanol | C17H36O | ||
| Octacosanoic acid, methyl ester | C29H58O2 | ||
| Octadecanal | C18H36O | ||
| 2-Hexadecyl-1,1′-bi-cyclopentyl | C26H50 | ||
| P-Cymene | Aerial parts | C10H14 | |
| Pentadecanoic acid, methyl ester | Roots, leaves, stems, and fruits | C16H32O2 | |
| Dibutyl phthalate | C16H22O4 | ||
| (Z,Z,Z)-9, 12, 15-octadecatrienoic acid, methyl ester | C19H32O2 | ||
| Eicosanoic acid, methyl ester | C21H42O2 | ||
| Docosanoic acid, methyl ester | C23H46O2 | ||
| Tetracosanoic acid, methyl ester | C25H50O2 | ||
| Hexacosanoic acid, methyl ester | C27H54O2 | ||
| Others | |||
| Glucose | Whole grass | C6H12O6 | Zheng (1975) |
| Fructose | C6H12O6 | ||
| Sedoheptulose | C7H14O7 | ||
| Sucrose | C12H22O11 | ||
| (3S,5R,6R,7E,9S)-megastigman-7-ene-3,5,6,9-tetrol 9-O-β-D-glucopyranoside | Aerial parts | C28H35O4D | Xu et al. (2015) |
| (3S,5R,6R,7E,9S)-megastigman-7-ene-3,5,6,9-tetrol 3-O-β-D-glucopyranoside | C28H35O4D | ||
| Picein | Leaves and stems | C14H18O7 | Jia et al. (2014) |
| Koaburaside | C14H20O9 | ||
| Hexacosoic acid | Whole grass | C26H52O2 | Li et al. (2008) |
| Salidroside | C14H20O7 | ||
| Malic acid | N/A | C4H6O5 | Xuan (2014) |
| N-triacontanoic acid | Roots and stem | C33H66O2 | Li et al. (2020a) |
| 1-Hexadecanol | C16H34O | ||
| Dioctadecylsulfide | C36H74S | ||
| 1-Naphthalen-2-yl-ethanone | Whole grass | C12H10O | Lin et al. (2011) |
| Lotaustralin | Aerial parts | C11H19NO6 | Xiong et al. (2019) |
| Butanedioic acid | C4H6O4 | ||
| 9(Z)-octadecenamide | C18H35NO | ||
N/A: not applicable or not explicitly stated.
FIGURE 2.
Structures of flavonoids from S. aizoon (1–48).
FIGURE 5.
Structures of alkaloids (91–98) from S. aizoon.
6.1 Flavonoids
So far, 48 flavonoid metabolites (1–48) with definite structure have been isolated and identified from S. aizoon, which are grouped into flavonols (1–36), isoflavones (37–39), flavones (40–43), flavanonols (44–47), and flavan-3-ol (48). Among flavonols, rhamnazin-3-O-β-D-glucopyranoside (4), myricitrin (10), myricetin-3-O-α-L-arabinopyranoside (14) (Xiong et al., 2019), herbacetin-8-O-β-D-glucopyranoside (26), herbacetin-3-O-β-D-glucopyranosyl-8-O-α-L-arabinopyranoside (27), herbacetin-3-O-α-L-rhamnopyranosyl-8-O-α-D-lyxopyranoside (28), herbacetin-3-O-α-L-arabinopyranosyl-8-O-β-D-xylopyran-oside (29), gossypetin-3-O-β-D-glucopyranosyl-8-O-β-D-xylopyranoside (31), and 3′-methoxyl-gossypetin-3-O-β-D-glucopyranosyl-8-O-β-D-xylopyranosie (35) (Xu et al., 2015) were obtained mainly from the aerial part of S. aizoon. Later, Xu et al. (2019) successfully identified four flavonols [i.e., trifolin (1), rutin (2), astragalin (32), and isoquercitrin (5)], two flavones [i.e., lonicerin (43) and scutellarein (46)], and one isoflavone [i.e., genistein (39)] in the leaves and stems of S. aizoon. Two new prenylated isoflavones, sedacin A (37) sedacin B (38), and two flavonols, sedacin C (6″-O-(E)-feruloyl quercetin) (33) and sedacin D (6″-O-(E)-feruloyl isorhamnetin) (34), were isolated from the whole plant of S. aizoon (Li W. L. et al., 2011). Among them, sedacin A and sedacin B had the function of scavenging DPPH and ABTS+ free radicals (Li J. X. et al., 2011). Rhamnetin-3-O-β-D-glucopyranoside (3), quercetin-3-O-α-L-arabinopyranoside (8), isorhamnetin-3-O-β-D-xylopyranoside (22), and isorhamnetin-3-O-α-L-arabinopyranoside (23) have also been detected in rhizomes (Li et al., 2020a). Four flavanonols (44–47) with rare dimeric structures, with the character of an iriflophene unit and a flavonoid unit connecting via a furan ring, were isolated from the roots and identified using NMR, IR, UV, HRESIM, DEPT, HSQC, HMBC, and CD methods. In addition, studies were conducted to assess the activity of these four substances, and they revealed that 5a-(3,4-dihydroxyphenyl)-1,3,8,10,10b-pentahydroxy-9-(4-hydroxybenzoyl)-5a,10b-dihydro-11H-benzofurochromen-11-one (46) and 1,8,10,10b-tetrahydroxy-5a-(4-hydroxy-3-methoxyphenyl)-9-(4-hydroxybenzoyl)-3-methoxy-5a,10b-dihydro-11H-benzofuro[2,3-b]chromen-11-one (47) had good anti-proliferative activities in vitro against the tumor cell lines BXPC-3, A549, and MCF-7 (Li et al., 2017). The structures of flavonoids from S. aizoon are displayed in Figure 2.
6.2 Phenolic acids
Phenolic acids are the most important chemical derivatives of plant secondary metabolites. Currently, 31 phenolics (49–78) have been found from S. aizoon, including phenolic acids (49–58, 60), lignans (61), phenylpropanoids (59, 62–63), and other phenolics (64–78). Two phenolic acids, namely, sedumol (49) and 4-methoxy-3,5-dihydroxybenzoic acid (56) (Han et al., 2021), were obtained from the 95% ethanol extract of S. aizoon ’s whole grass. Other phenolic acids, including vanillic acid (50) (Lin, 2014), protocatechuic acid (51), cis-4-coumaric acid (52), p-hydroxybenzoic acid (54) (Xiong et al., 2019), and caffeic acid (53) (Lin et al., 2014), were isolated from the aerial part of S. aizoon. Isolariciresinol-9-O-β-D-glucopyranoside (61) is classified as cyclolignans, which was obtained from the 70% ethanol extract via silica gel column chromatography (300–400 mesh). 2-O-(trans-caffeoyl)-malic acid 1,4-dimethyl ester (59) (Xiong et al., 2019), echinochlorin A (62) (Li et al., 2020a), 1-O-sinapoyl glucopyranoside (63) (Xu et al., 2015), and chrysophanol-8-O-β-D-glucoside (64) (Li et al., 2008) have been identified in S. aizoon. The structures of phenolic acids from S. aizoon are displayed in Figure 3.
FIGURE 3.
Structures of phenolic acids from S. aizoon (49–78).
6.3 Triterpenes and phytosterol
6.3.1 Triterpenes
A type of terpenoids known as triterpenoids has a parent nucleus that contains 30 carbon atoms. Triterpenoids exist in plants in free form or as glycosides or esters and have various biochemical activities. Up to now, eight triterpenes (79–86) were separated from S. aizoon, including one tetracyclic triterpenes (79) and seven pentacyclic triterpenes (80–86). The only tetracyclic triterpene, ginsenoside Re (79), is a dammarane-type triterpene. Seven pentacyclic triterpenes are divided into four groups: ursane type (80), oleanane type (81–83), friedelane type (84), and fernane type (85–86). In the studies of Li et al. (2008, 2020a), glutin-5-en-3-one (84), isomoliol-3β-acetate (86), taraxerone (82), and isomotiol (85) were isolated from S. aizoon for the first time. The structures of triterpenoids from S. aizoon are displayed in Figure 4.
FIGURE 4.
Structures of triterpenoids (79–86) and phytosterol (87–90) from S. aizoon.
6.3.2 Phytosterols
Up to now, a total of four phytosterols (87–90) have been identified in S. aizoon. These include β-sitosteryl linoleate (87) (Li et al., 2020a), daucosterol (89) (Guo et al., 2010; Lin et al., 2011), β-sitosterol (88), and stigmasterol (90) (Cao, 2011). The structures of phytosterol from S. aizoon are displayed in Figure 4.
6.4 Alkaloids
Eight alkaloids (91–98) have been isolated and identified from S. aizoon. In 1996, Kim et al. (1996) examined the alkaloids in Sedum species and discovered the presence of three alkaloids, namely, sedinine (91), sedamine (92), and despun methylisopelletierine (93) in S. aizoon. Thymine (95) was obtained from the ethyl acetate fraction of aqueous extracts of Sedum aizoon L. In the study of Gao et al. (2006), three pyrrolizidine alkaloids (PAs), namely, senecionine (96), seneciphylline (97), and integerrimine (98) were identified in the extracts of S. aizoon’s root, which had strong hepatotoxicity. The structures of alkaloids from S. aizoon are displayed in Figure 5.
7 Pharmacological activities
According to pharmacological studies, S. aizoon has a wide range of pharmacological activities, including antioxidant, anti-fatigue, and anti-inflammatory activities, improving cardiovascular disease, and other activities. The related biological activities and main effects are listed in Table 2.
TABLE 2.
Biological activities of the S. aizoon extracts and bioactive metabolites.
| Tested substance | Model | Key result | Reference |
|---|---|---|---|
| Ethanol extract | In vitro, total antioxidant capacity, superoxide anion, OH radical scavenging assay, and blood antioxidant | Obvious antioxidant activity | Ma et al. (2019), Qi et al. (2022) |
| Stomach bleeding model in mice, clean grade healthy ICR Mice | Reduced gastric mucosal injury and shortened the bleeding time and clotting time in mice | Zhong et al. (2014) | |
| In vitro, aeromonas, Rhizopus nigricans, Botrytis cinerea, Penicillium italicum, Pseudomonas fragi, and Shewanella putrefaciens isolated from sea food | Exhibited antibacterial activity, caused membrane damage, disruption of mycelial morphology, the bacterial surface, and internal ultrastructure, resulted in the leakage of sugars and proteins, retarded the microbial growth, and delayed meat spoilage | Xu et al. (2019), Luo et al. (2020), Wang et al. (2020), Wang et al. (2022a), Wang et al. (2022b), Wang et al. (2023c), Ge et al. (2023) | |
| Human liver cancer cell line | The inhibitory rate of liver cancer cells was as high as 52.04% with 200 μg/mL ethanol extract | Wang et al. (2013) | |
| ICR mice weigh 18∼20 g | Reduced the weight gain of mice and TC and TG levels increased HDL-C levels | Wang et al. (2013) | |
| Type 1 diabetes mellitus mice | Significantly restored body weight gain, improved food utilization, decreased the coefficients for both the liver and kidney, the levels of TC and TG, and the MDA content, increased the levels of HO-1 and NQO1 in the livers of mice, activated the Nrf2 pathway, thereby regulating the expression of downstream proteins, and regulated glucose metabolism in T1DM mice | Qi et al. (2022) | |
| In vitro, MDRPA, Staphylococcus aureus, Staphylococcus epidermidis, Micrococcaceae, Escherichia coli, Salmonella paratyphi B, bacillary dysentery, Proteus mirabilis, Clostridium perfringens, Bacillus subtilis, Bacillus anthracis, Candida parapsilosis, Candida tropicalis, and Candida albicans | The MIC50 for pseudomonas aeruginosa was 0.125 g/mL, which exerted definite bacteriostatic effects on bacteria and weak effect on fungus | Zhang et al. (2011), Zhang et al. (2012) | |
| Sap | In vivo, the liver in Cyprinus carpio Linnaeus | Increased SOD, POD activities, and MDA content | Zhang and Wang (2012) |
| College students who have completed exhaustive exercise | Prolonged the time of extreme exercise in mice, decreased BUN and MDA levels and LDH, increased SOD, muscle glycogen content, and liver glycogen content, play an anti-fatigue role, increased the amount of blood return and the content of hemoglobin in the blood, reduced the blood flow at the end of the limb and the concentration of cortisol and serum creatine kinase in the blood, improved the ability of metabolic regulation and response speed, accelerated fatigue recovery, and prevented and relieved fatigue | Ding, 2019; Ren (2020) | |
| In vivo, rats with gastrointestinal tract hemorrhage induced by aspirin | Turned positive rat fecal occult blood into negative, increased PC, GPⅡb/Ⅲa, P selectin, PLT, IL8, ET-1, and platelet number and aggregation, decreased PAF, significantly shortened TT and APTT, and significantly increased FIB | Liu et al. (2011), Liu et al. (2015), Bai et al. (2016) | |
| Senile stroke patients | Promoted blood circulation, removed blood stasis, and reduced blood pressure | Chen (2000) | |
| Ethyl acetate extracts | LPS-stimulated RAW 264.7 cells | Inhibited LPS-induced NO, TNF-α, and IL-6 production | Lin et al. (2015a) |
| α-Glucosidase activity assay | Inhibit α-glucosidase activity | Cao (2011) | |
| N-Butanol extracts | α-Glucosidase activity assay | Inhibit α-glucosidase activity | Cao (2011) |
| Methanol extracts | α-Glucosidase activity assay | Inhibit α-glucosidase activity | Cao (2011) |
| In vivo , male ICR mouse croton oil-induced ear edema, rat CGN-induced paw edema, TPA-induced ear edema assay of sub-chronic inflammation, mouse acetic acid-induced writhing, and LPS-stimulated RAW 264.7 cells | Inhibited PGE2 production by the downregulation of COX-2 expression and COX-2 induction and inhibited acute as well as sub-chronic inflammation dose-dependently | Kim et al. (2004) | |
| In vivo, H/R model in neonatal rat cardiomyocytes | Decreased the LDH, apoptosis, and caspase-3 activity, activated P13K/Akt, increased eNOS phosphorylation, NO, and the Bcl-2/Bax ratio, reduced H/R-induced cardiomyocyte damage, and protected cardiomyocytes | Qiang (2013) | |
| S. aizoon tablet | 244 cases with peptic ulcer bleeding | Increased the PC and shortened bleeding time | Xu (2012) |
| Aqueous extracting—ethanol precipitating extract | Stomach bleeding model in mice | Exerted the strongest protective effects on gastric mucosa | Zhong et al. (2014) |
| Petroleum ether | Stomach bleeding model in mice | Reduced gastric mucosal injury and shortened the bleeding time and clotting time in mice | Chen et al. (2012) |
| Ethyl acetate of water extraction | Clean grade healthy ICR mice | Good hemostatic effect | Chen et al. (2012) |
| Aqueous extracts | Stomach bleeding model in mice | Reduced gastric mucosal injury and shortened the bleeding time and clotting time in mice | Chen et al. (2012) |
| In vitro, MDRPA, Staphylococcus aureus, and Pseudomonas aeruginosa | Have certain bacteriostasis, and the MIC50 for pseudomonas aeruginosa was 0.5 g/mL | Tan et al. (2001) | |
| In vivo, male Kunming mice | Increased the amount of sleeping mice and decreased the autonomic activities in mice | Guo et al. (2009) | |
| Esophageal carcinoma cells | Destroyed the structure of phospholipid and resulted in the damage of the ultrastructure of esophageal carcinoma cells | Fu et al. (2008) | |
| In vivo, patients with cardiovascular and cerebrovascular diseases | Protected blood vessels, removed blood stasis, and prevented blood clots | Xuan (2015) | |
| Herbacetin-3-O-α-L-rhamnopyranosyl-8-O-α-D-lyxopyranoside | Escherichia coli; Staphylococcus aureus | Showed certain growth inhibition, and it showed more potency against Gram-positive than against Gram-negative bacteria | Xu et al. (2015) |
| Rosenbach and Bacillus subtilis | |||
| Myricetin-3-O-β-D-glucopyranoside | Escherichia coli, Staphylococcus aureus Rosenbach, and Bacillus subtilis | Showed more potency against Gram-positive than against Gram-negative bacteria | Xu et al. (2015) |
| Gossypetin-3-O-β-D-glucopyranosyl-8-O-β-D-xylopyranoside | Escherichia coli, Staphylococcus aureus Rosenbach, and Bacillus subtilis | Showed more potency against Gram-positive than against Gram-negative bacteria | Xu et al. (2015) |
| Ethyl acetate from alcohol extract | In vivo, male Kunming mice | Obviously decreased the autonomic activities in mice, prolonged the sleeping time, and increased the amount of sleeping mice | Guo et al. (2010) |
| N-butanol extracted from alcohol extract | In vivo, the male Kunming mice | Obviously decreased the autonomic activities in mice, prolonged the sleeping time, and increased the amount of sleeping mice | Guo et al. (2010) |
| Yangxincao Anshen Granule | In vivo, Kunming mice | Significantly decreased spontaneous activity, prolonged sleep time, and increased rates of sleeping in mice on the high (12 g/kg/d) and medium dosages (6 g/kg/d) | Zhang et al. (2015b) |
| S. aizoon (30 g) and Semen Ziziphus Spinosa (15 g) | In vivo, Kunming mice | Extented the sleep time significantly and increased the sleep rate | Zhang et al. (2015a) |
| S. aizoon (22.5 g) and Semen Ziziphus Spinosa (22.5 g) | In vivo, Kunming mice | Extended the sleep time and increased the sleep rate | Zhang et al. (2015a) |
| Myricetin-3-O-β-D-glucopyranoside | In vitro, human hepatoma cell line (HepG2), human breast cancer (MCF-7), and human lung carcinoma (A549) tumor cell lines | Had anti-proliferative activities on cell proliferation with IC50 values of 46.30, 75.27, and 49.76 μmol/L, respectively | Xu et al. (2015) |
| 5a-(3,4-Dihydroxyphenyl)-1,3,8,10,10b-pentahydroxy-9-(4-hydroxybenzoyl)-5a,10b-dihydro-11H-benzofuro chromen-11-one, an iriflophene unit, and a quercetin unit connecting via a furan ring | In vitro , in situ pancreatic adenocarcinoma cell (BXPC-3), A549, and human breast cancer (MCF-7) tumor cell lines | Exhibited moderate cytotoxic activities against BXPC-3, A549, and MCF-7 tumor cell lines with IC50 ranging from 24.84 to 37.22 μmol/L | Li et al. (2017) |
| 1,8,10,10b-Tetrahydroxy-5a-(4-hydroxy-3-methoxyphenyl)-9-(4-hydroxybenzoyl)-3-methoxy-5a,10b-dihydro-11H-benzofuro [2,3-b]chromen-11-one, an iriflophene unit and a rhamnazin unit connecting via a furan ring | In vitro, anti-proliferative activities against BXPC-3, A549, and MCF-7 tumor cell lines | Exhibited moderate cytotoxic activities against BXPC-3, A549, and MCF-7 tumor cell lines with IC50 ranging from 24.84 to 37.22 μmol/L | Li et al. (2017) |
| EtOAc fraction of aqueous extract | In vitro, LPS-stimulated RAW 264.7 macrophages | Inhibited the release of NO from inflammatory cells | Lin (2014) |
| 3′,4′,5,7-Tetrahydroxy | In vitro, LPS-stimulated RAW 264.7 macrophages | Inhibited the release of TNF-α | Lin (2014) |
| Galuteolin | In vitro, LPS-stimulated RAW 264.7 macrophages | Inhibited the release of NO and TNF-α | Lin (2014) |
| Protocatechuic acid | In vitro, LPS-stimulated RAW 264.7 macrophages | Inhibited the release of TNF-α, IL-6, NO, and IL-1β | Huang, 2014; Lin (2014) |
| Caffeic acid | In vitro, LPS-stimulated RAW 264.7 macrophages | Inhibited the release of TNF-α, IL-6, NO, and IL-1β | Huang, 2014; Lin (2014) |
| 6% S. aizoon | Renal hypertensive male rat model | Lowered SBP and MAP, thereby lowering blood pressure | Han et al. (2022) |
| 10% S. aizoon | Renal hypertensive male rat model | Decreased SBP, MAP, blood pressure, serum creatine kinase CK activity, left ventricular stroke index LVWI (LW/BW) and HWI (HW/BW), and the expression of AT1 protein, increased the expression of AT2 and catalase protein, reversed myocardial remodeling, and protected the heart | Han et al. (2022) |
| Yangxincao capsules | Hyperlipidemia rat model | Significantly decreased the levels of serum TC, TG, and LDL-C, decreased the level of apoB, and increased the levels of HDL-C and its subcomponents HDL2-C, HDL3-C, and the ratio of HDL-C/TC; significantly increased the activities of LCAT and LPL and the level of apoA in the serum | Liu et al. (2005) |
| Leaching solution | Rabbit and frog | Stimulated the action of the heart and reduced the toxicity of amphetamine | Zheng (1975) |
| Polysaccharide | Mice | Significantly improved thymus index and spleen index, T- and B-lymphocyte transformation and proliferation, and NK cell activity; increased the percentage values of CD3+, CD4+, CD19+, and CD4+/CD8+ in the peripheral blood | Huang (2019) |
N/A, not applicable or not explicitly stated.
7.1 Antioxidant activity
S. aizoon has excellent antioxidant activity, as demonstrated by several pharmacological studies in vitro and in vivo. An in-depth in vivo study showed that the juice from the stems and leaves of S. aizoon increased the peroxidase (POD) and superoxide dismutase (SOD) of the liver in Cyprinus carpio Linnaeus as well as reduced the content of malondialdehyde (MDA), thus preventing the peroxidation damage of the liver cell membrane (Zhang and Wang, 2012). Experimental tests in vivo showed that ethanol extracts of S. aizoon were able to enhance antioxidant enzymes in T1DM mice and successfully alter the Nrf2/Keap1/ARE signaling pathway (Qi et al., 2022). Additionally, 95% ethanol extract of S. aizoon increased the activity of SOD, CAT, and GSH-Px and reduced the contents of MDA and ROS on the rat adrenal pheochromocytoma cell line (PC12) induced by H2O2, showing a protective effect on the cell (Zhao, 2015).
7.2 Anti-fatigue effects
As national fitness activities expand, more individuals participate in sports, and the negative consequences of exercise fatigue on the body become more obvious. The effective recuperation of the body and the rapid removal of exercise exhaustion are becoming increasingly vital to society. The animal experiments (mice) demonstrated that the extracts of S. aizoon (3.6 and 0.9 mL/kg, 30 days) prolonged the time of extreme exercise in mice, reduced the contents of blood urea nitrogen (BUN), lactic acid (LAC), MDA, and lactate dehydrogenase (LDH) in the serum of mice, improved the activity of SOD and GSH-Px, and increased the contents of liver and muscle glycogen of mice (Ding, 2019). In a human clinical trial, it has been found that the administration of the sap (0.225 mL/kg.d, 0.9 mL/kg.d, and 3.6 mL/kg.d, 28 days) of the aerial part from S. aizoon [5 mL/(60 kg.d), 14 days] reduced the response time of male college students to the stimulus signal, improved fatigue resistance, and accelerated fatigue recovery by decreasing the content of blood perfusion index, cortisol, and creatine kinase in the serum and increasing hemoglobin and the load of final exercise (Ren, 2020). The above studies showed that S. aizoon improved exercise endurance, affected their metabolic activity, and produced anti-fatigue effect. S. aizoon’s probable anti-fatigue effects of action are shown in Figure 6.
FIGURE 6.
Schematic diagram of anti-fatigue effects of S. aizoon.
7.3 Hemostatic activity
S. aizoon has an effect comparable to that of Notoginseng Radix in terms of reducing bleeding without causing stasis and nourishing blood. A series of experiments in vivo and in vitro revealed that extracts and preparations of S. aizoon exhibited good hemostatic activities. Previous studies showed that alcohol and aqueous extracts (6, 12 g/kg b.w) of S. aizoon could significantly shorten the bleeding time and clotting time of mice (Chen et al., 2012). The juice of the whole herb from S. aizoon could increase the levels of GP Ⅱb/Ⅲa, P selectin, and ET-1 and the number of platelets and enhance the platelet aggregation and release function of the rats with aspirin-induced gastric hemorrhage, thus achieving hemostasis. Since S. aizoon could increase the level of IL-8, it was used in patients with bleeding accompanied by inflammation (Huang, 2014).
S. aizoon combined with other drugs can also be used for the treatment of bleeding diseases. Patients with bleeding peptic ulcers was treated upon treatments with herbs S. aizoon in conjunction with omeprazole (Xu, 2012). After intravenous injection in rabbits and intraperitoneal injection in mice of S. aizoon syrup, the blood coagulation time and bleeding time were decreased (Chinese Academy of Medical Sciences, 1972). The probable hemostatic mechanism is shown in Figure 7.
FIGURE 7.
S. aizoon’s probable hemostatic activity.
7.4 Antimicrobial activity
The crude extracts from S. aizoon have antimicrobial activity. According to transcriptome and RNA sequencing analyses, the ethanol extracts extracted from S. aizoon had significant antimicrobial activities against B. cinerea (Wang K. et al., 2022), Aeromonas (Xu et al., 2019), postharvest citrus blue mold (Luo et al., 2020), Shewanella putrefaciens (Wang et al., 2020), and Pseudomonas fragi (Wang H. X. et al., 2022). Studies revealed that alcohol extracts had a good inhibitory ability against 20 strains of multidrug-resistant Pseudomonas aeruginosa (MIC50 value = 0.125 g/mL) (Zhang et al., 2012; Wang H. et al., 2023), Staphylococcus aureus, Staphylococcus epidermidis, and Micrococcus (MIC value = 0.125 g/mL). However, the inhibitory impact on three types of fungus, including Candida tropicalis, Candida parapsilosis, and Candida albicans, was very poor, with MIC values above 0.5 g/mL (Zhang et al., 2011).
Furthermore, monomer metabolites isolated from S. aizoon also have antimicrobial activity. Xu et al. (2015) revealed that herbacetin-3-O-α-L-rhamnopyranosyl-8-O-α-D-lyxopyranoside (28), myricetin-3-O-β-D-glucopyranoside (12), and gossypetin-3-O-β-D-glucopyranosyl-8-O-β-D-xylopyranoside (31) exhibited more potency against Gram-positive than against Gram-negative bacteria. S. aizoon’s probable antimicrobial actions are shown in Figure 8.
FIGURE 8.
S. aizoon’s probable antimicrobial action.
7.5 Sedative and hypnotic effects
Traditional Chinese medicine and its preparations are commonly used to treat sleeplessness, agitation, and other symptoms. They offer the benefits of safety and dependability, as well as fewer toxicity and side effects, as compared to Western medication with sedative and hypnotic properties. Using the mouse model, Guo et al. (2009) showed that the water and alcohol extracts have tranquilizing mind and the calming effects. Later, they also found that the ethyl acetate and butanol extracts could effectively lower the autonomic activity in mice, lengthen sleeping duration in mice, and increase the number of sleeping mice (Guo et al., 2010).
Additionally, the S. aizoon’s prescription or in combination with other drugs also possess sedative and hypnotic properties, which are often used to treat sleeplessness, restlessness, and other disorders. For instance, Yangxincao Anshen Granules made with S. aizoon (12, 6 g/kg/d) significantly reduced the spontaneous movements of mice, and the granules, in conjunction with pentobarbital, extended the duration of their sleep, providing good sedative and hypnotic effects without negative side effects (Zhang R. Z. et al., 2015). Similar results have been recorded for the combination between S. aizoon and Semen ziziphus spinosa (Zhang L. et al., 2015).
7.6 Anti-cancer activity
S. aizoon’s active metabolites and crude extracts with anti-cancer potential have piqued the interests of researchers in recent years. The ethanol extracts isolated from S. aizoon (50, 100, and 200 μg/mL) could lower the survival rate of human liver cancer cells HepG2 and inhibit human hepatocarcinoma proliferation by 11.15%, 41.96%, and 52.04%, respectively. With the increase in concentration, the inhibition rate of liver cancer cells increased, showing a certain dose–effect relationship (Wang et al., 2013). The aqueous extracts of S. aizoon [equivalent to adding 15.9 mg raw drug, containing 31.7 μg gallic acid (60)] could destroy the phospholipid-dominated structures and block nucleic acid synthesis and metabolism, which caused the death of cancer cells, and the killing effect was improved when the drug treatment period was extended (Fu et al., 2008).
Among the active metabolites tested, myricetin-3-O-D-glucopyranoside (12) obtained from the aerial portion of S. aizoon exhibited an effect on cell proliferation against HepG2, MCF-7, and A549 tumor cells, with IC50 values of 46.30, 75.27, and 49.76 mol/L, respectively (Xu et al., 2015). Li et al. (2017) found that 5a-(3,4-dihydroxyphenyl)-1,3,8,10,10b-pentahydroxy-9-(4-hydroxybenzoyl)-5a,10b-dihydro-11H-benzofuro chromen-11-one, an iriflophene unit, and a quercetin unit connecting via a furan ring (44) and 1,8,10,10b-tetrahydroxy-5a-(4-hydroxy-3-methoxyphenyl)-9-(4-hydroxybenzoyl)-3-methoxy-5a,10b-dihydro-11H-benzofuro[2,3-b]chromen-11-one, an iriflophene unit, and a rhamnazin unit connecting via a furan ring (47) isolated from the roots of S. aizoon exhibited cytotoxic activities against BXPC-3, A549, and MCF-7 tumor cell lines, with IC50 ranging from 24.84 to 37.22 μmol/L. S. aizoon’s probable anti-cancer actions are shown in Figure 9.
FIGURE 9.
S. aizoon’s probable anti-cancer action.
7.7 Anti-inflammatory effect
In Northeast Asia, S. aizoon has been used as a traditional medicine to treat inflammatory illnesses. Several extracts (PE, EtOAc, and H2O) of S. aizoon were administered to LPS-stimulated RAW 264.7 cells to investigate anti-inflammatory activities. The phenolic and flavonoid-rich EtOAc extracts reduced NO, TNF-α, and IL-6 production induced by LPS (Lin et al., 2015a). In a study by Kim et al. (2004), methanol extracts of S. kamtschaticum Fischer showed a significant inhibitory effect in the inflammation models of mouse ear edema (50–400 mg/kg for 3 days) and rat paw edema (400–800 mg/kg for 3 days) induced by croton oil and multiple phorbol ester. The cyclooxygenase-2 expression was downregulated. Possible mechanisms of action are given in Figure 10.
FIGURE 10.
S. aizoon’s probable anti-inflammatory mechanism of action.
7.8 Cardioprotective effects
S. aizoon lowered blood pressure, serum CK activity, and AT1 protein expression, reversed myocardial remodeling, and increased AT2 and catalase protein expression (Han et al., 2022). Chen (2000) showed that fresh S. aizoon grass could help stroke victims regain consciousness. It is thought that this herb has evident effects in improving blood circulation, reducing blood stasis, and decreasing blood pressure. Using the method of network pharmacology and molecular docking, studies found that S. aizoon had the effect of treating atherosclerosis and coronary heart disease (Zhu et al., 2022, 2023).
Interestingly, the extract of S. aizoon increased cardiac activity and decreased amphetamine toxicity (Zheng, 1975). According to the study of Wang et al. (2013), S. aizoon had the ability to regulating blood lipid levels and could dramatically lower the mice’s liver index and fat coefficient. Additionally, when hyperlipidemia rats were treated with Yangxincao capsules (derived from whole grass extract), the serum levels of TC, TG, and LDL-C were decreased, while HDL-c and its subcomponents (HDL-c, HDL-3-C, and HDL-C/TC) were increased, implying that the mechanism of lipid regulation of S. aizoon was related to the enhancement of the activities of LPL, LCAT, and HDL2-C (Wu et al., 2006).
7.9 Other activities
In T1MD mice, it has been shown that S. aizoon extract has the ability to enhance glucolipid metabolism and organ coefficient and decrease liver tissue damage (Qi et al., 2022). In addition, polysaccharides from S. aizoon have an immune-stimulating effect by increasing the thymus index, spleen index, T- and B-lymphocyte transformation proliferation, and NK cell activity of mice, as well as enhancing the percentage values of CD3+, CD4+, and CD19+ and the percentage values of CD4+/CD8+ in the peripheral blood. Such effect was associated with the increased secretion of IL-2 and IFN-γ(Huang, 2019).
8 Acute toxicity
A previous study showed that excessive consumption may cause small hepatic vein occlusion disease with upper quadrant abdominal pain, hepatomegaly, liver dysfunction, and ascites as the main symptoms (Wu et al., 2008; Shao et al., 2015).
9 Quality control
The quality of traditional Chinese medicine is the basis for ensuring the stability of its efficacy and the safety of its application, and its standardization and modernization are the important prerequisites for promoting Chinese medicine toward internationalization. In order to better identify the plant, Scholars (Han, 2008) have controlled the quality of S. aizoon from four aspects: morphology, microscopy, TLC, and RAPD. It is required that the water content shall not exceed 10.53%, the ash content shall not exceed 14.70%, and the leaching content shall not be less than 32.57% (Wei et al., 2020). The linear ranges of quercitroside, quercetin, and kaempferol were 0.0029 ∼ 0.183, 0.0016 ∼ 0.1020, and 0.0045 ∼ 0.260 μg/μL, respectively (He and Du, 2016), and those of luteolin and isorhamnetin were 1.12 ∼ 112.00 and 0.98 ∼ 97.60 μg/mL (Lin et al., 2013), respectively. However, these methods may not be sufficient to evaluate the quality of S. aizoon.
Traditional Chinese medicine (TCM) fingerprints can comprehensively and quantitatively reflect the chemical information contained in TCM and is an effective means of quality control of TCM. Lin et al. (2015b) used 11 standards to analyze the phytochemical profiles of the active extracts by HPLC fingerprints. Yang et al. (2023) established the HPLC-ECD fingerprint spectra of S. aizoon from different origins and identified 12 metabolites.
10 Conclusion and future perspectives
This review provides comprehensive and detailed information about the history, traditional uses, botany, phytochemistry, pharmacological activities, and acute toxicity of S. aizoon. So far, more than 200 metabolites have been identified with a variety of pharmacological activities. These modern pharmacological studies supported most traditional uses of S. aizoon as folk medicine. However, gaps still exist in the systematic study of S. aizoon.
First, S. aizoon has many nicknames, which results in being mixed with other herbs. Therefore, molecular biological studies are required to screen out the reference genes for better identification of S. aizoon.
Second, the pharmacological potential of S. aizoon has not yet been fully discovered, which may be further investigated by a combination of in vitro and in vivo bioactivity assays, metabolomics, network pharmacology, and in silico bioactivity prediction methods. In addition, the therapeutic potential of S. aizoon and its bioactive metabolites, safety, efficacy, and potential mechanism of action require further preclinical and clinical studies to validate for future clinical applications.
Third, S. aizoon is widely popular in herbal healthcare as a commonly used medicinal and edible substance and is especially used in immunomodulation and blood lipid regulation. Nevertheless, the use of S. aizoon in combination with other herbs in healthcare products should be strengthened, and studies on improving memory and promoting digestion may be conducted.
Fourth, the spectrum–efficacy relationship of S. aizoon in immunomodulation and anti-inflammatory therapy should be further investigated in order to better uncover its active metabolites.
Funding Statement
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This research was carried out with the support of the Natural Science Foundation of Hubei Provincial Department of Education, grant number B2020104, the Innovation and Entrepreneurship Training Program for college students of Hubei University of Medicine, grant number X202110929016, and Hubei Key Laboratory of Wudang Local Chinese Medicine Research (Hubei University of Medicine), grant number WDCM2023025.
Author contributions
B-LW: conceptualization, funding acquisition, methodology, and writing–original draft. Z-KG: writing–review and editing, formal analysis and validation. J-RQ: writing–review and editing, formal analysis and validation. S-QL: data curation, investigation, visualization, and writing–original draft. X-CH: funding acquisition and writing–review and editing. Y-HZ: writing–review and editing and visualization.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| ABTS | 2, 2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) |
| AGG | Abrus agglutinin |
| APTT | Activated partial thromboplastin time |
| BXPC-3 | Human pancreatic adenocarcinoma cells |
| CAT | catalase |
| CNKI | China National Knowledge Infrastructure |
| CT | Coagulation time |
| DPPH | 2, 2-Diphenyl-1-picrylhydrazyl |
| EtOAc | Ethyl acetate |
| FBG | Fasting blood glucose |
| GP Ⅱb/Ⅲa | Platelet membrane glycoprotein |
| HepG2 | Human hepatoma cell line |
| H/R | Hypoxia/reoxygenation |
| HSQC | Heteronuclear singular quantum correlation |
| IL-1β | Interleukin 1β |
| IR | Infrared spectroscopy |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malondialdehyde |
| MTD | Maximum tolerance dose |
| NMR | Nuclear magnetic resonance |
| PC12 | Adrenal pheochromocytoma cell line |
| Pseud. aeruginosa | Pseudomonas aeruginosa |
| RAPD | Random amplified polymorphic DNA |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| STz | Streptozotocin |
| TCM | Traditional Chinese medicine |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-α |
| T-SOD | Total superoxide dismutase |
| UV | Ultraviolet and visible spectrum |
| Ac | Acetate |
| Ara | Arabinopyranoside |
| A549 | Human lung carcinoma |
| BUN | Blood urea nitrogen |
| CD | Circular dichroic |
| CGN | λ-Carrageenan |
| DEGS | Differentially expressed genes |
| E.coli | Escherichia coli |
| ET-1 | Endothelin 1 |
| Glu | Glucopyranoside |
| GSH-Px | Glutathione peroxidase |
| HMBC | 1H-detected heteronuclear multiple-bond correlation |
| HRESIMS | High-resolution electrospray ionization mass spectroscopy |
| IC50 | 50% inhibitory concentration |
| IL-6 | Interleukin 6 |
| LAC | Lactic acid |
| LD50 | Semi-lethal dosage |
| MAP | Mean arterial pressure |
| MCF-7 | Human breast cancer |
| MDRPA | Multidrug-resistant pseudomonas aeruginosa |
| MTT | 3-(4,5-Dimethylthiazol-2yl) −2,5-diphenyltetrazolium bromide |
| OGTT | Oral glucose tolerance test |
| POD | Peroxidase |
| PT | Prothrombin time |
| Rha | Rhamnopyranosyl |
| SBP | Systolic blood pressure |
| Staphy.Auren | Staphylococcus aureus |
| TC | Total cholesterol |
| T1DM | Type 1 diabetes mellitus |
| TLC | Thin-layer chromatography |
| TPA | 12-O-tetradecanoylphorbol 13-acetate |
| TT | Thrombin time |
| Xyl | Xylopyranoside |
References
- Bai Y. L., Sun T., Wang Y. X., Liu Z. Y., Zheng W. Z. (2016). Effect of sedum aizoon L. On platelet and vessel wall function in rats with gastrointestinal tract hemorrhage induced by aspirin. Lab. Med. 31, 131–134 10.3969/j.issn.1673-8640.2016.02.013 [DOI] [Google Scholar]
- Cao N. F. (2011). Studies on bioactive constituents of sedum Aizoon and Euphorbia humifusa . Master: Henan University. [Google Scholar]
- Chen E. D., Wang X. M., Gong L. L., Li H. F., Rong R., Jiang H. Q. (2014). Analysis of volatile components of above-ground parts of sedum aizoon by gas chromatography-mass spectrometry. Shandong J. Traditional Chin. Med. 33, 491–492. 10.16295/j.cnki.0257-358x.2014.06.001 [DOI] [Google Scholar]
- Chen K. K., Qiang Y. (2017). GC-MS analysis of fat soluble components from different parts of sedum aizoon . China J. Exp. Traditional Med. Formulae 23, 77–81. 10.13422/j.cnki.syfjx.2017200077 [DOI] [Google Scholar]
- Chen L. C. (2003). Sedum aizoon L. Treat insomnia. China's naturopathy , 60. [Google Scholar]
- Chen W. C. (2000). Fresh Sedum aizoon L for stroke, 46. China's Naturopathy. [Google Scholar]
- Chen W. L., Qiu Q., Lin Z. C., Guo S. H. (2012). Screening of hemostasis effective fraction from sedum aizoon L. J. Zhejiang Chin. Med. Univ. 36, 182–183. 10.16466/j.issn1005-5509.2012.02.032 [DOI] [Google Scholar]
- Chinese Academy of Medical Sciences, I.O.M.M (1972). Study on Chinese pharmaceutical active component (volume one). Beijing: People's Medical Publishing House, 424. [Google Scholar]
- Chinese Herbal Medicine Research Group (1971). Clinical observation on hemostatic effect of herbal sedum aizoon . J. New Med., 14–15. [Google Scholar]
- Chinese Pharmacopoeia Committee (2005). Chinese Pharmacopoeia. Beijing: Chinese Medical Science and Technology Press, 590–591. [Google Scholar]
- Ding Q. (2019). Experimental study on anti-exercise-induced fatigue of jingtian panax Notoginseng. Beijing: Capital University of Physical Education and Sports. [Google Scholar]
- Fu Y. R., Bo A. H., Sun L., Guo C. Y., Bai X. M., Li H. F. (2008). The effect of tusanqi on ultrastructure of esophageal carcinama cells. Lishizhen Med. Mater. Med. Res. 150, 368–369. [Google Scholar]
- Gao X. S., Xiao S. S., He J. F. (2006). Analysis of alkaloids in sedum aizoon and establishment of hepatic veno-occlusive model in mice. Chin. J. Integr. T Rad. West Med. Dig., 311–313. [Google Scholar]
- Ge Q., Wang K., Shao X., Wei Y., Zhang X., Liu Y., et al. (2023). Inhibitory mechanism of flavonoids from sedum aizoon L. On rhizopus nigricans. Foodborne Pathog. Dis. 20, 197–208. 10.1089/fpd.2022.0083 [DOI] [PubMed] [Google Scholar]
- Gong P. J. (2020). Analysis of cutting propagation technique and principal nutrient components of excellent sedum aizoon variety. J. Shandong For. Sci. Technol. 50, 56–58. [Google Scholar]
- Guo S. H., Che S. R., Zhu Y. Q., Zhang N., Wang Y. H. (2006). Analysis of the chemical composition of the volatile oil of sedum aizoon by gas phase - mass spectrometry. China J. Traditional Chin. Med. Pharm., 689–690. [Google Scholar]
- Guo S. H., Huang H. H., Lin D., Xu F. (2010). Sedative and hypnotic effects of different extracted parts of sedum aizoon L. In mice. J. Fujian Univ. Tradit. Chin. Med. 20, 22–23. 10.13261/j.cnki.jfutcm.002315 [DOI] [Google Scholar]
- Guo S. H., Huang H. H., Xu F., Lin D. (2009). Pharmacodynamic comparison of tranquilizing mind effect between water extract and alcohol extract of sedum aizoon L. J. Fujian Univ. Tradit. Chin. Med. 19, 28–29. 10.13261/j.cnki.jfutcm.002205 [DOI] [Google Scholar]
- Guo S. H., Lin Z. C. (2007). Studies on quality standard of sedum aizoon L. China J. Traditional Chin. Med. Pharm., 761–763. [Google Scholar]
- Han R. C. (2008). Study on the pharmacognosy of four species plants of sedum L. Master. Liaoning: Liaoning University Of Traditional Chinese Medicine. [Google Scholar]
- Han R. J., Zhao C., Chen H. J., Sun Y. J., Chen H., Li M., et al. (2021). Study on chemical constituents from sedum aizoon L. Chin. J. Tradit. Chin. Med. Pharm. 36, 4223–4226. [Google Scholar]
- Han W., Li M. X., Lv C. N., Hao Y. M., Lu J. C. (2017). Study on chemical constituents from stems and leaves of sedum aizoon L. Chin. Med. Her. 14, 33–36. [Google Scholar]
- Han W. D., Xue S., Chen J. Y., He Z. L., Zhao X. M., Tan R., et al. (2022). Analysis of the therapeutic effect of sedum aizoon L. On hypertension and its active ingredients. Renowned Dr. No 130, 42–44. [Google Scholar]
- He J. J., Du J. (2016). Simultaneous determinate the content of quercetin, quercitroside and kaempferol in sedum aizoon L. By HPLC. Lishizhen Med. Materia Medica Res. 27, 524–526. 10.3969/j.issn.1008-0805.2016.03.005 [DOI] [Google Scholar]
- Huang A. Y. (2014). “Study on ethyl acetate parts of hemostatic and anti-inflammatory effect of sedum aizoon L,” in Activity screening. Master (Fujian: Fujian University of Traditional Chinese Medicine; ). [Google Scholar]
- Huang R. (2019). Study on the immunological activity of feicai polysaccharide. Master. Jilin: Yanbian University. [Google Scholar]
- Jia L. Y., Xu T. Y., Wang J., Lv C. N., Lu J. C. (2014). Chemical constituents from the stems and leaves of sedum aizoon L (Ⅱ), 31. Liaoning: Journal of Shenyang Pharmaceutical University, 673–676. [Google Scholar]
- Kim D. W., Son K. H., Chang H. W., Bae K., Kang S. S., Kim H. P. (2004). Anti-inflammatory activity of Sedum kamtschaticum . J. Ethnopharmacol. 90, 409–414. 10.1016/j.jep.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Thart H., Stevens J. F. (1996). Alkaloids of some asian sedum species. Phytochemistry 41, 1319–1324. 10.1016/0031-9422(95)00562-5 [DOI] [Google Scholar]
- Li M., Qi Z., Hao Y., Lv C., Jia L., Wang J., et al. (2017). New adducts of iriflophene and flavonoids isolated from sedum aizoon L. With potential antitumor activity. Molecules 22, 1859. 10.3390/molecules22111859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Yu Y., Li M. X., Xu T. Y., Lu J. C., Lv C. N. (2020a). Isolation and ldentification of the chemical constituents from the rhizome of sedum aizoon L. Mod. Chin. Med. 22, 353–357. 10.13313/j.issn.1673-4890.20191203001 [DOI] [Google Scholar]
- Li Q. Y., Wu L. Q. (2011). Two new prenylated isoflavones from sedum aizoon L. Fitoterapia 82, 405–407. 10.1016/j.fitote.2010.11.021 [DOI] [PubMed] [Google Scholar]
- Li J. X., Cao N. F., Kang W. Y. (2011). Antioxidant activity of sedum aizoon L. From henan. Nat. Prod. Res. Dev. 23, 337–340. 10.16333/j.1001-6880.2011.02.033 [DOI] [Google Scholar]
- Li W. L., Jing Y., Luo Q. Y., Bai S. P., Yan F. L. (2008). Study of chemical constituents on sedum aizoon L. J. Xinxiang Med. Univ. 25, 558–561. [Google Scholar]
- Li W. L., Luo Q. Y., Wu L. Q., Xiao L. (2011). Two new flavonol glycosides from. Sedum Aizoon L. Heterocycles. 83, 135–141. [Google Scholar]
- Li Z. H., Hu H. B., Chen J., Wang M. Q., Du G. H. (2007). Analysis of the constituents in sedum aizoon L. By HPLC ESI-MS/MS. Chin. J. Nat. Med., 431–434. [Google Scholar]
- Lin Z., Fang Y., Huang A., Chen L., Guo S., Chen J. (2014). Chemical constituents from sedum aizoon and their hemostatic activity. Pharm. Biol. 52, 1429–1434. 10.3109/13880209.2014.895019 [DOI] [PubMed] [Google Scholar]
- Lin Z. C. (2014). Studies on hemostatic and anti-inflammatory effective substances of sedum aizoon L. And its quality evaluation. Nanjing, China: Nanjing University of Chinese Medicine. [Google Scholar]
- Lin Z. C., Fang Y. J., Huang A. Y., Chen L. Y., Guo S. H. (2013). Simultaneous determination of four flavonoids in sedum aizoon L. From different origin and medicinal parts by high performance liquid chromatography. J. Anal. Sci. 29, 819–822. [Google Scholar]
- Lin Z. C., Huang H. H., Chen D. W., Chen J. H., Guo S. H. (2011). Study on chemical constituents of active parts of calming the heart and tranquilizing the mind from sedum aizoon L. China J. Traditional Chin. Med. Pharm. 26, 3000–3002. [Google Scholar]
- Lin Z. C., Zhang L., Zhang R. Z., Huang A. Y., Guo S. H. (2015a). Anti-inflammatory effect of ethyl acetate extract of sedum aizoon L. LPS-stimulated RAW 264. 7 macrophages and its HPLC fingerprint. J. Chin. Pharm. Sci. 24, 647–653. [Google Scholar]
- Lin Z. C., Zhang L., Zhang R. Z., Huang A. Y., Guo S. H. (2015b). Antiinflammatory effect of ethyl acetate extract of sedum aizoon L. In LPS stimulated RAW 264.7 macrophages and its HPLC fingerprint. J. Chin. Pharm. Sci. 24, 647–653. 10.5246/jcps.2015.10.082 [DOI] [Google Scholar]
- Liu K. Q., Yin W. D., Zheng H. Z., Shi Y. W. (2011). The influence of jingtiansanqi on platelet and coagulation function in rat treated with aspirin. Labeled Immunoassays Clin. Med. 18, 407–410. [Google Scholar]
- Liu X. F., Ma Z. R., Wu X. Q., Lai M. Q., Zhang Y. (2012). Research progress of active principle and functional compounds of sedum aizoon L. China J. Traditional Chin. Med. Pharm. 27, 2135–2138. [Google Scholar]
- Liu X. M., Wu F. H., Huang Q. F. (2005). Pharmacodynamic study on regulating lipid of Yangxincao capsule. Chin. J. Pathophysiol. 21, 1628. [Google Scholar]
- Liu Z. K., Min C., Dong H., Zhang Z. S. (2021). Improvement of adventitious root formation in sedum aizoon L. And the production of flavonoids. South Afr. J. Bot. 137, 483–491. 10.1016/j.sajb.2020.10.024 [DOI] [Google Scholar]
- Liu Z. Y., Wang Y. X., Sun T., Bai Y. L., Zheng W. Z. (2015). Observation the effect of jingtian sanqi on hamostatic function and coagulation in rats with gastric hemorrhage. Chin. Med. Mod. Distance Educ. China 13, 142–144. 10.3969/j.issn.1672-2779.2015.13.072 [DOI] [Google Scholar]
- Luo J., Xu F., Zhang X., Shao X., Wei Y., Wang H. (2020). Transcriptome analysis of penicillium italicum in response to the flavonoids from sedum aizoon L. World J. Microbiol. Biotechnol. 36, 62. 10.1007/s11274-020-02836-z [DOI] [PubMed] [Google Scholar]
- Ma J., Gao L., Shi Y., Cai D. B., Xiong S. L. (2019). Purification, free radical scavenging activity and preliminary identification of ethanol extract s from sedum aizoon L. Sci. Technol. Food Ind. 40, 207–213+219. 10.13386/j.issn1002-0306.2019.04.034 [DOI] [Google Scholar]
- Qi X., Lu X. T., Sun X. H., Lin C. Q., Cui C. B. (2022). The regulatory effect of total flavonoids of sedum aizoon L. On oxidative stress in type 1 diabetic mice. Curr. Res. Food Sci. 5, 1140–1147. 10.1016/j.crfs.2022.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y. X., Li Y., Guo D. G. (2018). An analysis of chemical constituents of volatile oil of sedum aizoon L. J. Guiyang Coll. Tradit. Chin. Med. 40, 47–49. 10.16588/j.cnki.issn1002-1108.2018.01.012 [DOI] [Google Scholar]
- Qiang Y. (2013). Study on germplasm resources and bioactive components of sedum aizoon L. Doctor Shaanxi: Shaanxi Normal University. [Google Scholar]
- Ren M. J. (2020). Experimental study on the preventive effect of sedum aizoon L. On exercise fatigue in mice and humans. Master. Beijing: Capital University of Physical Education and Sports. [Google Scholar]
- Shao H., Chen H. Z., Zhu J. S., Ruan B., Zhang Z. Q., Lin X., et al. (2015). Computed tomography findings of hepatic veno-occlusive disease caused by sedum aizoon with histopathological correlation. Braz J. Med. Biol. Res. 48, 1145–1150. 10.1590/1414-431X20154563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., He X. C., Li T., Du J. (2018). Research progress on chemical constituents and medical functions of phedimus aizoon. Chin. J. Ethnomedicine Ethnopharmacy 27, 49–52. [Google Scholar]
- Tan P., Yao L. F., Peng C. X. (2001). MIC test on 40 species of ''Qi" herbs from Mt▪Qinling. Hubei: Lishizhen Medicine and Materia Medica Research, 484–485. [Google Scholar]
- Wang H., Ge Q., Shao X., Wei Y., Zhang X., Wang H., et al. (2023a). Influences of flavonoids from sedum aizoon L. On biofilm formation of Pseudomonas fragi. Appl. Microbiol. Biotechnol. 107, 3687–3697. 10.1007/s00253-023-12526-z [DOI] [PubMed] [Google Scholar]
- Wang H. F., Liu F., Xu C., Lin Y., Li H. S., Shao X. F., et al. (2013). Study on blood lipid regulation and hepatocarcinoma proliferation of total flavonoid from sedum aizoon L. J. Chin. Inst. Food Sci. Tech. 13, 23–27. 10.16429/j.1009-7848.2013.04.002 [DOI] [Google Scholar]
- Wang H. X., Xu F., Zhang X., Shao X. F., Wei Y. Y., Wang H. F. (2022a). Transcriptomic analysis reveals antibacterial mechanism of flavonoids from sedum aizoon L. Against Pseudomonas fragi . Food control. 134, 108755. 10.1016/j.foodcont.2021.108755 [DOI] [Google Scholar]
- Wang J., Chi Z., Zhao K., Wang H., Zhang X., Xu F., et al. (2020). A transcriptome analysis of the antibacterial mechanism of flavonoids from sedum aizoon L. Against Shewanella putrefaciens . World J. Microbiol. Biotechnol. 36, 94. 10.1007/s11274-020-02871-w [DOI] [PubMed] [Google Scholar]
- Wang K., Zhang X., Shao X., Wei Y., Xu F., Wang H. (2022b). Flavonoids from sedum aizoon L. Inhibit botrytis cinerea by negatively affecting cell membrane lipid metabolism. Appl. Microbiol. Biotechnol. 106, 7139–7151. 10.1007/s00253-022-12196-3 [DOI] [PubMed] [Google Scholar]
- Wang K. Y., Ge Q. Q., Shao X. F., Wei Y. Y., Zhang X., Xu F., et al. (2023c). Influences of flavonoids from sedum aizoon L. On the cell membrane of botrytis cinerea . Food Biosci. 52, 102386. 10.1016/j.fbio.2023.102386 [DOI] [Google Scholar]
- Wang X. H. (2013). Processing technology of leaf tea and its beverage of sedum aizoon L. Master Shandong: Shandong Agricultural University. [Google Scholar]
- Wei X., Liu C., Yao Y. M., Liu X. L., Peng L. J. (2020). Stduy on quality standard of sedum aizoon . Stud. Trace Elem. Health 37, 39–40+43. [Google Scholar]
- Wolbi M., Olszewska M. (1996). Polyphenolic compounds from sedum aizoon L. [Google Scholar]
- Wu F. H., Liu X. M., Guo S. H. (2006). Study on mechanism of Yangxincao capsule in regulating lipid metabolism. Chin. J. Integr. Tradit. West. Med. 26, 131–134. [PubMed] [Google Scholar]
- Wu G. L., Yu G. Y., Chen J. (2008). Clinical analysis of hepatic veno-occlusive disease induced by sedum aizoon . China J. Chin. materia medica 33, 2402–2404. [PubMed] [Google Scholar]
- Xiong Y., Du C. X., Duan Y. S., Yuan C. M., Huang L. J., Gu W., et al. (2019). Chemical constituents and pharmacological activities of sedum aizoon form guizhou province. Chin. Tradit. Herb. Drugs. 50, 5404–5410. [Google Scholar]
- Xu F., Cao S., Wang C., Wang K., Wei Y., Shao X., et al. (2019). Antimicrobial activity of flavonoids from sedum aizoon L. Against Aeromonas in culture medium and in frozen pork. Food Sci. Nutr. 7, 3224–3232. 10.1002/fsn3.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L. (2012). Evaluation of herb sedi aizoon in the treatment of peptic ulcer bleeding. Hainan Med. J. 23, 32–33. [Google Scholar]
- Xu T., Wang Z., Lei T., Lv C., Wang J., Lu J. (2015). New flavonoid glycosides from sedum aizoon L. Fitoterapia 101, 125–132. 10.1016/j.fitote.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Xu Z. C., Wen Y. H., Li M. J., Liang S., Wei Y., Zheng W. Z. (2016). Study on hemostatic and activating blood effect and mechanism of jingtiansanqi in rat treated with aspirin. Lishizhen Med. Mater. Med. Res. 27, 84–85. [Google Scholar]
- Xuan X. M. (2014). Experimental study on anti-atherosclerotic effect of total phenolic acid in the sedum aizoon L. Clin. J. Chin. Med. 6, 139–141. [Google Scholar]
- Xuan X. M. (2015). The observation of the clinical efficacy of gynurajaponica in the treatment of cardiovascular diseases. J. North Pharm. 12, 28–29. [Google Scholar]
- Xue G. X. (2015). Research of fermented Sedum kamtschaticum tea beverage. China Brew. 34, 151–155. 10.11882/j.issn.0254-5071.2015.10.034 [DOI] [Google Scholar]
- Yang X. J., Wang Y., Chen Z. H., Liu M. Y., Wu J., Xiang Y., et al. (2023). Study on the spectrum effect relationship of antioxidant activity of sedum aizoon L. Based on HPLC-ECD. Sci. Technol. Food Industry 44, 15–24. 10.13386/j.issn1002-0306.2022100158 [DOI] [Google Scholar]
- Yi Y. J. (2000). Utilization value and planting of sedum aizoon . Special Econ. Animal Plant 3 (4), 38. [Google Scholar]
- Zhang G. S., Wang G. P. (2012). Effect of sedum aizoon L. On the antioxidase activity and MDA content of the liver in Cyprinus carpio Linnaeus. Sichuan J. Zoology 31, 909–911. 10.3969/j.issn.1000-7083.2012.06.013 [DOI] [Google Scholar]
- Zhang J. J., Wang J., Xue J., Cao B. Y., Lu J. C. (2010). Chemical constituents from the stems and leaves of sedum aizoon L, 27. Journal of Shenyang Pharmaceutical University, 635–638. [Google Scholar]
- Zhang L., Lin Z. C., Zhang R. Z., Guo S. H. (2015a). Experimental study of the hypnotic effect of the compatibility of sedum aizoon L. And semen ziziphus spinosa by increase-decrease baseline geometric proportion design method. Strait Pharm. J. 27, 17–18. [Google Scholar]
- Zhang R. Z., Lin Z. C., Qiu Q., Zhang L., Guo S. H. (2015b). Study on the sedative-hypnotic effect and acute toxicity of Yangxincao anshen granule. J. Liaoning Univ. Tradit. Chin. Med. 17, 31–33. 10.13194/j.issn.1673-842x.2015.11.010 [DOI] [Google Scholar]
- Zhang Y. X., Qiao H. Y., Shan Y. Q., Liu W. J., Liu Z. X., Kang M. (2011). Experimental study on the anti-bacteria effects of sedum aizoon L. in vitro . J. Hebei North Univ.:Nat. Sci. 27, 78–80. [Google Scholar]
- Zhang Y. X., Xu X. H., Qiao H. X., Zhang Y. T. (2012). Study on antibacterial activity of Sedum Aizoon L. against multi - drug resistant Pseudomonas aeruginosa . J. Hebei North Univ.:Nat. Sci. 28, 76–78. 10.3969/j.issn.1673-1492.2012.04.022 [DOI] [Google Scholar]
- Zhao C. S. (2015). Study on protective effect of total flavonoids of sedum aizoon L. On oxidative damage of rat pheochromocytoma cells induced by hydrogen peroxide. Chin. Remed. Clin. 15, 1268–1269. 10.11655/zgywylc2015.09.017 [DOI] [Google Scholar]
- Zheng H. C. (1975). Research of phytochemistry and biological action of sedum . J. Int. Pharm. Res., 32–34. 10.13220/j.cnki.jipr.1975.01.006 [DOI] [Google Scholar]
- Zhong L. M., Xia X. H., Jiang D. J., Zeng G. R., Gao S. (2014). Study on gastric- mucosal protective effect of different extracting-parts of stonecrop Notoginseng in mice. Chin. J. Clin. Pharmacol. 30, 208–211. 10.13699/j.cnki.1001-6821.2014.03.001 [DOI] [Google Scholar]
- Zhu B. J., Nai G. Y., Pan T. X., Ma Z. F., Huang Z. D., Shi Z. Z., et al. (2022). To explore the active constituents of Sedum aizoon L in the treatment of coronary heart disease based on network pharmacology and molecular docking methodology. Ann. Transl. Med. 10, 1327. 10.21037/atm-22-5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu B. J., Nai G. Y., Pan T. X., Ma Z. F., Zhou W. J. (2023). Combining network pharmacology and bioinformatics to identify bioactive compounds and potential mechanisms of action of sedum aizoon L. In the treatment of atherosclerosis. Qual. Assur. Saf. Crops Foods 15, 104–116. 10.15586/qas.v15i3.1333 [DOI] [Google Scholar]