Abstract
Purpose
Brain stem tumors in children < 3 months at diagnosis are extremely rare. Our aim is to study a retrospective cohort to improve the understanding of the disease course and guide patient management.
Methods
This is a multicenter retrospective analysis across the European Society for Pediatric Oncology SIOP-E HGG/DIPG Working Group linked centers, including patients with a brainstem tumor diagnosed between 2009 and 2020 and aged < 3 months at diagnosis. Clinical data were collected, and imaging characteristics were analyzed blindly and independently by two neuroradiologists.
Results
Five cases were identified. No patient received any therapy. The epicenter of two tumors was in the medulla oblongata alone and in the medulla oblongata and the pons in three. For patients with tumor in equal parts in the medulla oblongata and the pons (n = 3), the extension at diagnosis involved the spinal cord; for the two patients with the tumor epicenter in the medulla oblongata alone (n = 2), the extension at diagnosis included the pons (n = 2) and the spinal cord (n = 1). Biopsy was performed in one patient identifying a pilocytic astrocytoma. Two patients died. In one patient, autopsy revealed a high-grade glioma (case 3). Three survivors showed either spontaneous tumor regression (n = 2) or stable disease (n = 1). Survivors were followed up for 10, 7, and 0.6 years, respectively. One case had the typical imaging characteristics of a dorsal exophytic low-grade glioma.
Conclusions
No patient fulfilled the radiologic criteria defining a high-grade glioma. Central neuroradiological review and biopsy may provide useful information regarding the patient management.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00381-023-06272-w.
Keywords: Brain stem tumors, Diffuse midline glioma (DMG), Neonatal, Congenital
Introduction
Pediatric brainstem tumors including brain stem gliomas account for 10–20% of all central nervous system pediatric neoplasms [1]. The mean age at diagnosis is 7–9 years [2] but may occur at any age. Approximately 80% of pediatric brainstem gliomas arise within the pons, while the remaining 20% arise in the medulla, midbrain, or cervicomedullary junction [3]. Approximately 80% of those arising in the pons are diffuse intrinsic pontine gliomas (DIPGs). In these cases, with typical imaging characteristics and the classic clinical triad of long tract signs with motor weakness, cranial neuropathies, and cerebellar signs, therapy is often initiated without histological and/or molecular confirmation. In all other cases of brainstem tumors, a biopsy is mandatory for diagnosis [4]. Biopsy has now emerged as a routine standard of care in many institutions in DIPG at presentation [5–7]. Up to 90% of DIPGs harbor a point mutation in H3F3A (65% of tumors) or HIST1H3B (25% of tumors), the remaining 10% of patients have a histone 3 wild-type tumor [6, 8–10].
Brain tumors in infants differ in clinical presentation, anatomical distribution, histopathological diagnosis, therapy, and prognosis from those occurring in older children. The diagnosis of brain tumors in patients less than 12 months is usually delayed by the lack of symptoms and verbal complaints [11]. Macrocephaly, changes of behavior, and delayed developmental milestones are usual clinical findings [12]. The most common tumors in children aged less than 3 months are high-grade glioma (19.3%), teratoma (17.5%), and ungraded glioma (14.6%), while in the 3 to 6 months age group, low-grade glioma (18.9%), high-grade glioma (14.4%), and central nervous system (CNS) atypical teratoid/rhabdoid tumor (AT/RT) (13.9%) [13].
Brain stem tumors occurring in children aged less than 3 months at diagnosis are extremely rare. Primary brain tumors detected in utero or during early postnatal life are estimated to affect 1.1–3.4 per million live births [14, 15]. Given the rarity and absence of clinical trial data in children aged less than 6–12 months, the knowledge about diagnosis and management of tumors in this age group is limited. We report five cases of infants aged < 3 months at diagnosis with a brainstem tumor who were diagnosed between 2009 and 2020. We wish to improve the understanding of presentation and patient management regarding diagnosis and treatment of brain stem tumors in this age group.
Methods and patients
Study design and inclusion criteria
This is a multicenter retrospective analysis across the European Society for Pediatric Oncology (SIOP-E) HGG/DIPG Working Group linked centers. Inclusion criteria were defined as children aged less than three months at diagnosis with brainstem tumor, diagnosed between 2009 and 2020, and with informed consent signed by the legal guardians of the patients. Between 2009 and 2020, five eligible children aged less than 3 months at diagnosis with a brainstem tumor fulfilled the inclusion criteria and were included in this study.
Data collections and analysis
Coded health-related personal and clinical data of patients aged less than 3 months with brainstem tumors treated at SIOP-E (Société Internationale d’Oncologie Pédiatrique) linked centers were collected by each co-investigator. All patients/legal representatives signed consent to have their child’s data collected and used for this study. Five children were included in this report (Spain, n = 2; Germany, n = 2; UK, n = 1). Each co-investigator filled out an excel spreadsheet with 17 variables (the complete list of variables is available in Supplementary Table 1), after investigating the medical file of every patient. Original clinical, laboratory, and imaging data were included. Data were recorded on gender, medical history, presenting symptoms, cerebrospinal fluid analysis, biopsy/autopsy findings, treatment details, clinical evolution, survival, and follow-up time. These coded data were then verified by two authors. Coded imaging data (magnetic resonance imaging) were further analyzed by two experienced neuroradiologists (blind to clinical information) in an independent manner. The following radiologic variables were recorded: epicenter, extension at diagnosis, contact with the surface of the brain, brainstem enlargement, brain stem segment involved, tumor margin, enhancement characteristics, the presence of necrosis/hemorrhage/edema, the presence of metastases, and the presence/absence of hydrocephalus.
Statistical analyses
Overall survival (OS) (days) was defined as the time from date of diagnosis to death (or date of last follow-up). Descriptive data were presented as frequencies and percentages.
Ethical considerations
Ethical approval was obtained from the institutional board of the Geneva Research Ethics Committee (Commission cantonale d’éthique de la recherche). The assigned protocol number is 2022-01599.
Results
Baseline characteristics
Patients’ key characteristics are displayed in Table 1 (complete patients’ characteristics are illustrated in Supplementary Table 1). The median age at diagnosis was 18 days (range 0–42 days) of life; with prenatal imaging providing the diagnosis in one case. The symptoms were evident in all but one child at birth. Case 3 developed symptoms during the first month of life. All patients presented with hypotonia and frequently (n = 3) with respiratory distress. The median interval between onset of symptoms and diagnosis was 18 days.
Table 1.
Patients’ characteristics
| Patients | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Gender | Female | Female | Male | Female | Female |
| Age at diagnosis | 18 days | 1st day | 42 days | Prenatal | 19 days |
| Symptoms | Poor feeding, hypotonia | Respiratory distress, hypotonia | Respiratory distress, hypotonia | Respiratory distress, hypotonia | Poor feeding, nystagmus, hypotonia |
| Date of onset symptoms | At birth | At birth | 1st month of life | At birth | At birth |
MRI characteristics
Initial cranial MRI studies were available for all five patients and centrally reviewed by two experienced neuroradiologists (Figs. 1, 2, 3 and 4), summarized in Table 2. At diagnosis, a cerebral MRI was performed for every patient with additional spinal MRI available for cases 1 and 4. The epicenter of two tumors was in the medulla oblongata alone and in the medulla oblongata and the pons in three tumors. For all patients with equal parts of the tumor in the medulla oblongata and the pons (n = 3), the extension at diagnosis included the spinal cord; for the two patients with epicenter in the medulla oblongata alone (n = 2), the extension at diagnosis involved the pons (n = 2) and the spinal cord (n = 1). Brainstem enlargement was marked in all patients. Tumor margins were able to be delineated in three patients. There was no evidence of metastatic spread in any patient. Only one patient’s tumor was dorsal exophytic (case 5). Hydrocephalus was documented in four patients (severe hydrocephalus; case 2). No peritumoral edema nor necrosis was seen in any of the initial MRI studies. There were no obvious residues reacted to bleeding, although this was unclear for one patient (case 4). All five tumors showed a predominantly low signal on T1-weighted images, three of them with a heterogeneous signal. Most of the tumors (n = 4) showed a bright signal on T2-weighted images, although it was heterogeneous in four cases. Macroscopic leptomeningeal dissemination could be excluded in three patients, only cranial dissemination could be excluded in cases 2 and 3, as no spinal MRI was performed. No scan demonstrated the definite imaging diagnosis of a pontine diffuse midline glioma (DIPG). Case 3 had an isointense signal on T2-weighted images, suggestive of high cellularity, although this could not be confirmed radiologically, as diffusion-weighted images were not provided. All cases were radiologically compatible with low-grade glioma. The tumor type in case 5 was confirmed by histology. Two patients died with a rapidly expanding tumor.
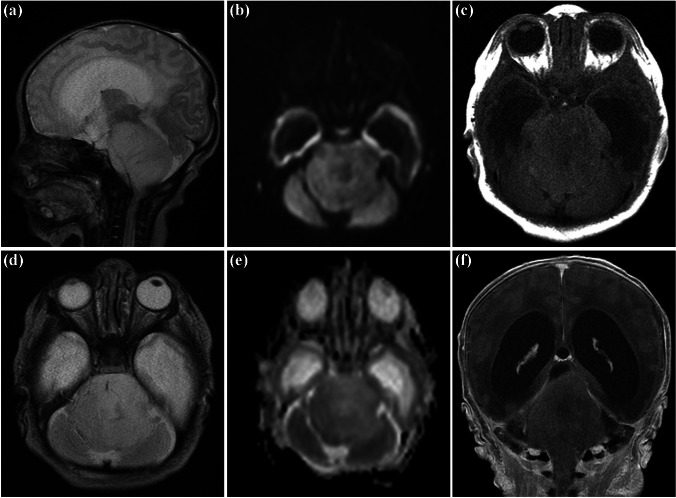
Fig. 1.
Case 2. First MRI at the second day of life. Sagittal (a) and axial (d) T2-weighted images showing a confluent mass expanding pons and medulla oblongata and occluding the foramen magnum. Mildly increased diffusion on diffusion-weighted imaging (iso- to hypointense signal on b1000 (b) and bright signal on apparent diffusion coefficient map (e), no contrast enhancement, axial T1-weighted image before (c), and coronal T1-weighted image after application of gadolinium-based contrast agent (f). Dilated supratentorial ventricles and no signs of dissemination
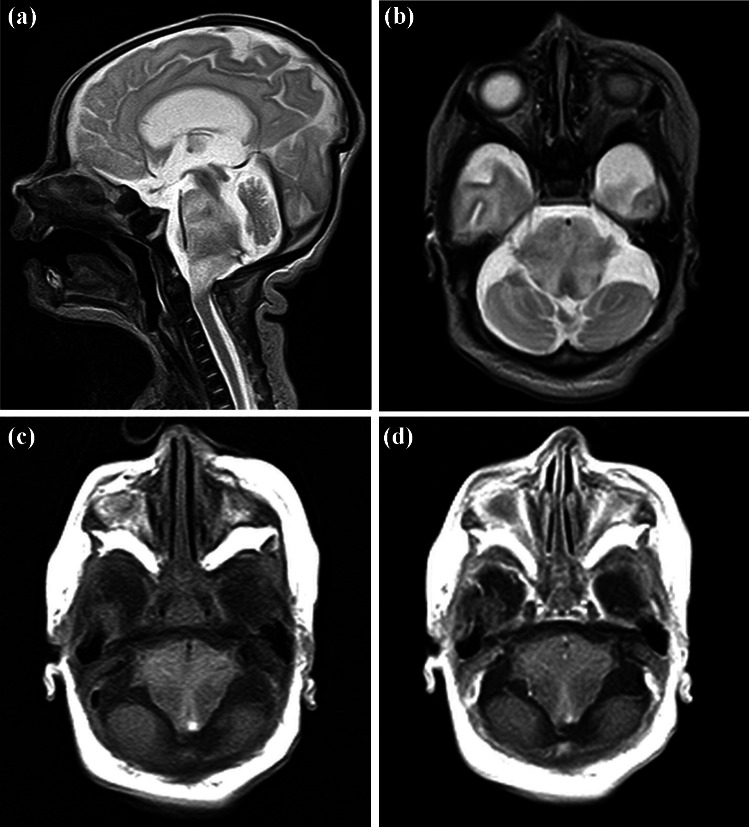
Fig. 2.
Case 4. First postnatal MRI at the twelfth day of life with sagittal (a) and axial (b) T2-weighted images demonstrating the diffuse infiltrating tumor with moderate expansion in equal parts in pons and medulla oblongata, no enhancement after application of gadolinium-based contrast agent (T1-weighted images without (c) and with contrast agent (d), both axial planes)
Fig. 3.
Case 4. Follow-up. All T2-weighted images, sagittal plane; intrauterine MRI obtained 24 days before birth (a), at the 12th day of life (b), at the age of 5 months (c), and 2 years old (d). After detecting the tumor with ultrasound, the brainstem glioma is already visible prenatally on MRI, demonstrating an increased volume at day 12 of life and a spontaneous and continues regression documented until the second year of life
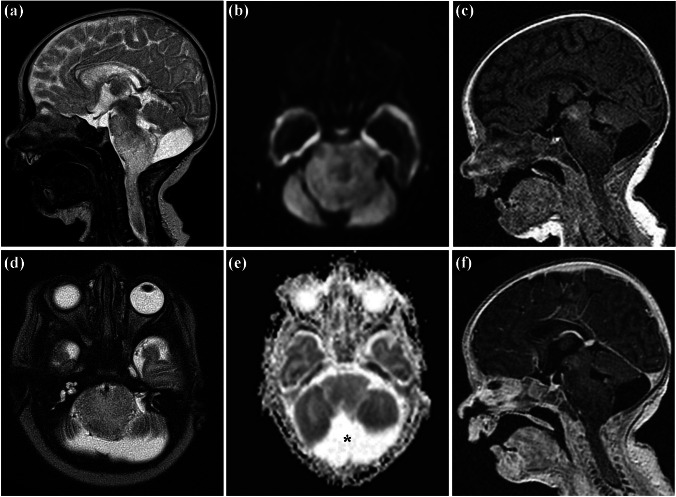
Fig. 4.
Case 5. First MRI at the age of 19 days. Sagittal (a) and axial (d) T2-weighted images showing a diffuse infiltrating tumor centered in the medulla oblongata, having a dorsal exophytic component, extending into the pons and the upper spinal cord. The foramen magnum is occluded. No change of diffusion on diffusion-weighted image (b) or apparent diffusion coefficient-map (e), no contrast enhancement visible on T1-weighted images before (c) and after application of a gadolinium-based contrast agent (f), both sagittal planes. Regular width of the ventricles, no dissemination detected. Mega cisterna magna as an incidental finding (asterisk)
Table 2.
Review of brain MRI studies obtained at diagnosis
| Case no. | MRI | Epicenter | Extension at diagnosis | Brainstem enlargement | Involved brainstem segment | Tumor margin | Signal T1 | Signal T2 | Contrast enhancement (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Brain and spinal | Med obl | Pons | Marked | Med obl > pons | Blurred | Hypo/hetero | Hyper/hetero | Multifocal (≤ 25%) | |
| 2 | Brain | Med obl and pons | Spinal cord | Marked | Med obl = pons | Mostly delineated | Hypo/hetero | Ηyper/hetero | Focal (≤ 10%) | |
| 3 | Brain | Med obl and pons | Spinal cord | Marked | Med obl = pons | Mostly delineated | Hypo/homo | Iso/homo | None | |
| 4 | Brain and spinal | Med obl and pons | Spinal cord | Marked | Med obl = pons | Mostly delineated | Hypo/hetero | Hyper/hetero | Focal (≤ 10%) | |
| 5a | Brain | Med obl | Pons & Spinal cord | Marked | Med obl > pons | Blurred | Hypo/homo | Hyper/hetero | Focal (≤ 10%) | |
MRI magnetic resonance imaging, Med obl medulla oblongata, Hypo hypointense, Homo homogeneous, Hetero heterogeneous
aDorsal exophytic
Survival and further clinical course of the patients
All five patients underwent observation and best supportive care (Table 3). No patient underwent tumor resection, and no patient received any tumor directed therapy. Two patients died (overall survival; 9 days, case 2; 66 days, case 3). Biopsy was performed in one patient; this analysis revealed a pilocytic astrocytoma (case 5) with strongly positive staining for GFAP. There was an absence of IDH1 p.R132H expression, and H3 p.K28M (K27M) immunohistochemistry was negative. The Ki-67 index was approximatively 3%. Autopsy was performed in one patient (case 3) showing a high-grade glioma not otherwise specified (NOS), with necrotic elements that made immunohistochemistry results difficult to interpret. We noticed spontaneous regression (n = 1, case 1), partial regression of the tumor (n = 1, case 4), and stability of the disease (n = 1, case 5). The surviving patients were followed up for 10, 7, and 0.6 years, respectively.
Table 3.
Patients’ further clinical course
| Patients | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Biopsy | No | No | No | No | Pilocytic astrocytoma (grade I) |
| Autopsy | No | No | High-grade gliomaa | No | No |
| Treatment | Observation | Observation | Observation | Observation | Observation |
| Progression of disease | Complete remission | Progressive disease | Progressive disease | Partial remission | Stable disease |
| Vital status | Alive | Dead | Dead | Alive | Alive |
| Follow-up | 10 years | 9 days | 2 months | 7 years | 7 months |
aGlial infiltration of pons and medulla. Necrotic elements made immunohistochemistry results inconclusive
Discussion
Pediatric brain stem gliomas are a heterogeneous group of entities and may be classified based on their clinical and MRI appearances [16, 17]. Brain stem tumors in children less than three months remain extremely rare and result in a challenging situation. Little is known in the literature about brain stem tumors appearing in this age group. Our aim was to study a retrospective cohort of this limited age group to improve the understanding of these cases and subsequent patient management.
In our multicenter retrospective study, we identified five cases of brain stem tumors in children aged < 3 months. After MRI review by two experienced neuroradiologists, no case fulfilled the diagnostic imaging criteria of a DIPG. One demonstrated the typical appearance of a dorsal exophytic brainstem low-grade glioma and the rest compatible with low-grade gliomas of the brain stem. One case had an intermediate signal on T2-weighted images, suggesting higher cellularity (no diffusion-weighted images available). To aid diagnosis, a comprehensive MRI according to guidelines is required with advanced MRI techniques such as MR-spectroscopy and ASL-perfusion desirable [18]. The radiologic diagnosis was confirmed by histopathology in case 5, for case 1 and 4 the clinical course was in keeping with the radiologic diagnosis. However, this is less clear for cases 2 and 3. The patient in case 2 had a swollen pontomedullary tumor occluding the foramen magnum and compressed the brain at the level of the craniospinal junction. This patient had a rapid and fatal clinical course corresponding to an unusual evolution for a low-grade glioma patient. The histologic evaluation of the autopsy of case 3 suggested evidence for a high-grade glioma, although analysis was limited due to relatively low tissue and imaging quality.
There are few previous reports regarding imaging characteristics and clinical evolution in brainstem tumors during the first months of life. Pontine gliomas in neonates and young infants may include a variety of different entities with different biological behavior [19–22]. There have been a small number of case reports of neonates with brain stem tumors (Table 4). However, the patients included had very different outcomes [23–30].
Table 4.
Published cases of brain stem tumors in children less than 3 months identified in the literature
| Cases | Age at Dx* | Symptoms | Imaging features | Biopsy | Autopsy | Treatment | Vital status | Follow-up |
|---|---|---|---|---|---|---|---|---|
|
Thomson and Kosnic et al. Case 1 |
2 days | Inspiratory stridor, hypotonia, facial palsy, vertical nystagmus, and disconjugate gaze | Brain MRI on day 2: large expansive mass involving the brainstem, predominantly the pons and extending into the medulla. Hypointense on T1-weighted and hyperintense on T2-weighted images | No | No | Supportive care | Alive | 4 years: brainstem improved but still abnormal contour |
|
Thomson and Kosnic et al. Case 2 |
1 week | Facial palsy, hemiparesis, weak cough/gag, disconjugate gaze, and hypertonicity | Brain MRI at 1st week: large mass centered within the pons extending from the inferior medulla oblongata up to the midbrain. Infiltrative and heterogenous in signal | No | No | Supportive care | Alive |
10 years: remarkable regression of the lesion with many areas of cystic degeneration Slight facial asymmetry, subtle dysmetria on the right side during finger-to-nose testing |
| Schomerus et al. | 7 weeks | Severe postnatal asphyxia, hypotonia, and horizontal nystagmus |
Cranial US scan at 7 weeks: enlarged and very echogenic pons Brain MRI: hydrocephalus with enlarged third and lateral ventricles and swollen pons Large areas of the pons hypointense on T1-weighted and hyperintense on T2 Small area of old hemorrhage within the left basal pons and signs of old periventricular hemorrhage in the left occipitoparietal region |
No | No | VPS at 2 months | Alive | MRI at 27 months: pons no longer swollen, signs of bilateral periventricular leukomalacia. 38 months: motor development of a 3-month-old |
| Airewele et al. | 11 weeks | 6-week history of vomiting, progressive respiratory stridor, horizontal nystagmus, right facial nerve palsy, tongue fasciculation | Brain MRI: poorly enhancing mass arising from medulla and extending posteriorly to compress right side of 4th ventricle, mild hydrocephalus | No | No | No treatment | Alive | 12 years: mass remained stable on MRI. Normal neurological examination |
|
Shah et al. Case 1 |
Prenatal | Mild respiratory distress | Brain MRI on day 2: hydrocephalus and large intrinsic pontine mass extending to the midbrain, upper cervical cord, and posterior fossa. Low T1 and high T2 signals and lacked contrast enhancement. Spinal MRI: normal features | No | Yes: PNET | Dexamethasone | Dead | 12 days |
|
Shah et al. Case 2 |
2 days | Hypotonia, right facial palsy, stridor, abnormal eye movements, pooling of secretions and abnormal gag reflex | Brain CT: mass originating in the posterior fossa. Brain MRI on day 2: intrinsic pontine mass 3.5 cm in diameter with involvement of the medulla oblongata and the middle cerebellar peduncle | No | No | Dexamethasone | Dead | 11 days |
| Swenson et al. | Prenatal | Severe respiratory distress, with clinical signs of increased intracranial pressure | Fetal MRI: expansile, poorly marginated brainstem lesion with abnormal T1 hypointensity and T2 hyperintensity. Centered in the pons and extended cephalad into the midbrain, caudally into the medulla and posteriorly into the cerebellar peduncles. Severe hydrocephalus | No | Yes: anaplastic oligodendroglioma, with focal areas of grade IV astrocytoma or ganglioglioma (low grade) | EVD and intravenous dexamethasone | Dead | 3 days |
| Gabel et al. | 4 days | Axial hypotonia, right esotropia, facial weakness, poor feeding and abnormal respirations | Brain MRI: large tumor centered in the pons without reduced diffusivity on diffusion-weighed sequences and absent gadolinium enhancement | No | No | Palliative care | Dead | 7 days |
| Suo-Palosaari et al. | Prenatal | No abnormal neurological signs or symptoms | Fetal MRI: expansive brainstem tumor, dislocating the cerebellum posteriorly. Homogeneous and hyperintense on T2-weighted images. Lateral ventricles were marginally dilated | No | No | Supportive care | Alive | 4 years: diameter of the tumor has not diminished, but the size of the tumor has clearly decreased. No neurological signs or symptoms |
| Satrom et al. | 5 days | Inspiratory stridor, respiratory distress with sternal retractions, and a grade II/VI systolic murmur, normal neurologic examination | Brain MRI: abnormal, mass-like expansion with T2 signal abnormality of the pons and medulla | No | Yes: WHO grade III astrocytoma | Palliative care | Dead | First days of life |
Dx diagnosis, MRI magnetic resonance imaging, CT computed tomography scanning, US ultrasound, PNET brainstem primitive neuroectodermal tumor, VPS ventriculoperitoneal shunt, EVD external ventricular drain
In the patients presented in this study, we observed spontaneous tumor regression without receiving any treatment in most cases (n = 2 and stable disease n = 1). This observation is well described in the literature as case reports [23, 26, 28, 30]; Thomson and Kosnik reported in 2005 two full-term newborns with cranial nerve palsies and limb weakness and with MRI features compatible with diffuse brainstem lesions. Surprisingly in both patients a spontaneous partial tumor regression without any treatment was observed [30]. Schomerus et al. reported in 2006 a full-term male neonate who presented with severe post-natal asphyxia, low muscle tone and horizontal nystagmus at birth. The MRI at 7 weeks of age showed hydrocephalus with enlarged third and lateral ventricles and a swollen pons. Spontaneous partial tumor regression was observed. At the age of 10 years, his clinical condition appears to be normal [26]. Airewele et al. in 2007 reported an 11-week-old female who presented with vomiting and respiratory stridor and whose MRI features demonstrated a relatively non-enhancing mass arising from the medulla oblongata and extending posteriorly to compress the right lateral aspect of the fourth ventricle. The girl received no treatment, and a follow-up MRI at 5 years showed reduction of the mass; this radiologic situation remained stable at a follow-up MRI 7 years later [23]. Suo-Palosaari et al. in 2016 presented a female newborn with a diffuse brainstem lesion diagnosed by fetal MRI and without biopsy confirmation. Follow-up MRI demonstrated regression of the mass extension without any treatment, the patient was well with no neurological deficit on last follow-up at the age of 4 years [28]. In our retrospective study, biopsy was performed in one patient (n = 1) with the histological diagnosis of pilocytic astrocytoma, in contrast with previously published cases where no biopsy was performed in any patient [23–30]. Most of our patients (n = 3) are alive at the time of this retrospective review. The autopsy of case 3 revealed a high-grade glioma (Table 3). Of the cases described in Table 4, autopsy was performed in three patients out of the five who died (n = 3) and these revealed the diagnosis of brainstem primitive neuroectodermal tumor (PNET), anaplastic oligodendroglioma with focal areas of grade IV astrocytoma or ganglioglioma (low-grade), and WHO grade III astrocytoma [25, 27, 29].
Our study has several limitations. We have incomplete staging as spinal MRI was performed in only two patients (cases 1 and 4). The reason for incomplete staging might be explicable by the aggressive clinical behavior with rapid lethal evolution in two patients (cases 2 and 3); thus, spinal MRI would not have changed the management of these two patients. A biopsy was performed only in case 5 revealing a pilocytic astrocytoma, and an autopsy in one of two patients died, without any additional molecular analysis available. More recently, biopsy has emerged as a routine consideration in DIPG at presentation, not only in the context of an approved clinical study, but also as part of routine care at many institutions. However, in this context, it appears not to be surprising that only one patient was biopsied. In our study, we could not use the fifth edition of the WHO Classification of Tumors of the Central Nervous System (WHO CNS5) published in 2021, incorporating numerous molecular changes with clinicopathologic utility [31], due to several reasons. The retrospective study was conducted between 2009 and 2020; the tumors were not all biopsied, and comprehensive molecular analyses were not assessed.
Surgery to obtain tissue for histological and molecular analysis in this population often leads to a difficult decision; surgery presents an increased risk of morbidity and mortality, although postmortem tumor donations are a valuable opportunity to collect tissue to investigate underlying tumor biology. The main reason for parents to refuse autopsy is their wish to avoid actions the child disliked while alive even after their death. Parental psychological distress represents the second most common cause for refusal, and religious reasons appeared to play only a secondary role in the parents’ decision making [32]. A qualitative study conducted in Australia by Robertson et al. showed that parents felt comforted knowing that the donation would help better understand their child’s tumor [33].
Our retrospective study is small, and we are unable to draw any definitive conclusions. For future patients, we recommend transferring these rare cases to specialized centers. Biopsy might be considered depending on the clinical situation. By investigating the tissue of such rare tumors, we may learn more about molecular features, treatment possibilities, and prognosis. Complete staging at diagnosis, central radiologic review of the imaging, and biopsy to perform pathologic and molecular analyses would help to better characterize and diagnose the disease and thus might be helpful for the patient management [34].
Conclusions
In our retrospective analysis, no child fulfilled the radiologic criteria for diffuse intrinsic pontine gliomas. Spontaneous regression may be the case, so close clinical and radiologic follow-up is highly recommended especially in the absence of histologic/molecular confirmation of the aggressiveness of the lesion. Biopsy of brain stem tumors with complete molecular assessment combined with detailed radiologic evaluation might be helpful in the decision concerning patient management. A prudent approach in this age group with this symptom and radiologic findings should be considered carefully. A multidisciplinary approach for these challenging cases, including palliative care teams with end-of-life discussions and adequacy of interventions is critical in such fragile cases.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
A.O.v.B. and A.M.l.M.: conceived the idea of the study and the principal study design D.P., A.O.v.B., B.B., S.B., K.E., C.M.K., A.M.l.M, O.C.M.: collection and assembly of data B.B., L.B.: images analyses and interpretation B.B.: designed the figures D.P., A.O.v.B.: manuscript writing. All authors: data analysis and interpretation. All authors: manuscript editing. All authors approved the final version of the manuscript. All authors agree to the submission of the manuscript.
Funding
Open access funding provided by University of Geneva This project was part of a fund granted by CANSEARCH Foundation. Open access funding was provided by the University of Geneva.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choux M, Lena G, Do L. Pediatric Neurosurgery. In: Choux M, Di Rocco C, Hockley A, editors. Brainstem tumors. New York: Churchill Livingstone; 2000. pp. 471–491. [Google Scholar]
- 2.Jallo G, Biser-Rohrbaugh A, Freed D. Brainstem gliomas. Child’s Nervous. System. 2004;20:143–153. doi: 10.1007/s00381-003-0870-6. [DOI] [PubMed] [Google Scholar]
- 3.Freeman C, Farmer J (1998) Pediatric brain stem gliomas: a review. JP Int J Radiat Oncol Biol Phys 40(2):265–271 [DOI] [PubMed]
- 4.Vanan M, Eisenstat D (2015) DIPG in children – what can we learn from the past? Front Oncol 5. 10.3389/fonc.2015.00237 [DOI] [PMC free article] [PubMed]
- 5.Walker DA, Liu J, Kieran M, et al. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris) using the Delphi method. Neuro Oncol. 2011;15:462–468. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams J, Young C, Vitanza N, et al. Progress in diffuse intrinsic pontine glioma: advocating for stereotactic biopsy in the standard of care. Neurosurg Focus. 2020;48(1):E4. doi: 10.3171/2019.9.FOCUS19745. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Zhang L. Correlation between MRI findings and histological diagnosis of brainstem glioma. Can J Neurol Sci. 2013;40:348–354. doi: 10.1017/S0317167100014293. [DOI] [PubMed] [Google Scholar]
- 8.Castel D, Cathy Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130:815–827. doi: 10.1007/s00401-015-1478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janjua M, Ban V, Ahmadieh T, et al. Diffuse intrinsic pontine gliomas: diagnostic approach and treatment strategies. J Clin Neurosci. 2020;72:15–19. doi: 10.1016/j.jocn.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Von Bueren A, Karremann M, Gielen G et al (2018) A suggestion to introduce the diagnosis of diffuse midline glioma of the pons, H3 K27 wildtype (WHO grade IV). Acta Neuropathol 136(1):171–173. 10.1007/s00401-018-1863-6 [DOI] [PubMed]
- 11.Bognár L. Brain tumors during the first year of life. Ann N Y Acad Sci. 1997;824:148–155. doi: 10.1111/j.1749-6632.1997.tb46217.x. [DOI] [PubMed] [Google Scholar]
- 12.Tomita T, McLone DG. Brain tumors during the first twenty-four months of life. Neurosurgery. 1985;17:913–919. doi: 10.1227/00006123-198512000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hart M, Anderson-Mellies A, Beltrami A, Gilani A, Green AL (2022) Population-based analysis of CNS tumor diagnoses, treatment, and survival in congenital and infant age groups. J Neurooncol 157(2):333–344. 10.1007/s11060-022-03967-z [DOI] [PMC free article] [PubMed]
- 14.Isaacs H. Perinatal brain tumors: a review of 250 cases. Pediatr Neurol. 2002;27:249–261. doi: 10.1016/S0887-8994(02)00472-1. [DOI] [PubMed] [Google Scholar]
- 15.Jänish W, Hass J, Schreiber D, Gerlach H. Primary central nervous system tumors in stillborns and infants. 1984 doi: 10.1007/BF00177895. [DOI] [PubMed] [Google Scholar]
- 16.Albright AL, Price RA, Guthkelch AN. Brain stem gliomas of children. A clinicopathological study Cancer. 1983;52:2313–2319. doi: 10.1002/1097-0142(19831215)52:12<2313::aid-cncr2820521226>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Green AL. Kieran M W (2015) Pediatric brainstem gliomas: new understanding leads to potential new treatments for two very different tumors. Curr Oncol Rep. 2015;17:12. doi: 10.1007/s11912-014-0436-7. [DOI] [PubMed] [Google Scholar]
- 18.Avula S, Peet A, Morana G, et al. (2021) European Society for Paediatric Oncology (SIOPE) MRI guidelines for imaging patients with central nervous system tumours. Child’s Nervous System. 2021;37:2497–2508. doi: 10.1007/s00381-021-05199-4. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca A, Bouffet E (2021) Brainstem gliomas… the devil is in the details. Neuro-Oncology 86923(6):869–871. 10.1093/neuonc/noab064 [DOI] [PMC free article] [PubMed]
- 20.Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG registries. J Clin Oncol. 2018;36(19):1963–1972. doi: 10.1200/JCO.2017.75.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwartzentruber J, Korshunov A, Liu XY et al (2012) Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482(7384):226–231 [DOI] [PubMed]
- 22.Wagner S, Warmuth-Metz M, Emser A, et al. Treatment options in childhood pontine gliomas. J Neuro-Oncol. 2006;79:281–287. doi: 10.1007/s11060-006-9133-1. [DOI] [PubMed] [Google Scholar]
- 23.Airewele G, Miller G, McCluggage C et al (2007) Lesion regression. J Neurosurg 107:80–81 author replies 81 [DOI] [PubMed]
- 24.Gabel BC, Yoon J, Levy ML, Crawford JR. A diffuse intrinsic pontine glioma in a neonate diagnosed by MRI. BMJ Case Rep. 2014 doi: 10.1136/bcr-2013-202270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satrom KM, Phelan RA, Moertel CL, et al. Neonatal respiratory failure caused by congenital diffuse intrinsic pontine glioma. J Child Neurol. 2016;32(6):533–536. doi: 10.1177/0883073816689640. [DOI] [PubMed] [Google Scholar]
- 26.Schomerus L, Merkenschlager A, Kahn T, et al. Spontaneous remission or a diffuse brainstem lesion in a neonate. Pediatr Radiol. 2007;37:399–402. doi: 10.1007/s00247-007-0424-2. [DOI] [PubMed] [Google Scholar]
- 27.Shah NC, Ray A, Bartels U, et al. Diffuse intrinsic brainstem tumors in neonates. report of two cases. J Neurosurg Pediatr. 2008;1:382–385. doi: 10.3171/PED/2008/1/5/382. [DOI] [PubMed] [Google Scholar]
- 28.Suo-Palosaari M, Rantala H, Lehtinen S, Kumpulainen T, Salokorpi N. Long-term survival of an infant with diffuse brainstem lesion diagnosed by prenatal MRI: a case report and review of literature. Childs Nerv Syst. 2016;32:1163–1168. doi: 10.1007/s00381-016-3045-y. [DOI] [PubMed] [Google Scholar]
- 29.Swenson DW, Nickel BJ, Boxerman JL, et al. Prenatal MRI characterization of brainstem glioma. Pediatr Radiol. 2013;43:1404–1407. doi: 10.1007/s00247-013-2706-1. [DOI] [PubMed] [Google Scholar]
- 30.Thompson WD, Jr, Kosnik EJ. Spontaneous regression of a diffuse brainstem lesion in the neonate. Report of two cases and review of the literature. J Neurosurg. 2005;102:65–71. doi: 10.3171/ped.2005.102.1.0065. [DOI] [PubMed] [Google Scholar]
- 31.Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncology 23(8):1231–1251. 10.1093/neuonc/noab106 [DOI] [PMC free article] [PubMed]
- 32.Angelini P, Hawkins C, Laperriere N, Bouffet E, Bartels U. Postmortem examinations in diffuse intrinsic pontine glioma: challenges and chances. J Neurooncol. 2011;101:75–81. doi: 10.1007/s11060-010-0224-7. [DOI] [PubMed] [Google Scholar]
- 33.Robertson EG, Wakefield CE, Tsoli M et al (2021) Parents’ experiences of postmortem tumor donation for high-grade gliomas: benefits and suggested improvements. Neuro-Oncol Adv 3(1):1–9. 10.1093/noajnl/vdab087 [DOI] [PMC free article] [PubMed]
- 34.Buus-Gehrig C, Lehrnbecher T, Porto L, et al. Pontine tumor in neonate: case report and analysis of the current literature. J Neurosurg Pediatr. 2019 doi: 10.3171/2018.10.PEDS18215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.