In this issue of RPTH journal, Authi et al. [1] proposed an inhibitory function of inositol 1,3,4,5-tetrakisphosphate (IP4) in platelet activation using a selective inositol (1,4,5)-trisphosphate (IP3)-kinase inhibitor, GNF362.
Phospholipid metabolism plays a crucial role in platelet activation. Within a few seconds after platelet receptor stimuli, various forms of phosphatidylinositols (PIs) are produced and released from the plasma membrane into the cytoplasm, transmitting diverse signaling in the dense tubular system, mitochondria, secretory granules, and actin cytoskeleton. These metabolic changes are regulated by PI kinases and PI phosphatases, which phosphorylate or dephosphorylate hydroxyl groups of the inositol ring at different positions, resulting in 7 phosphorylated derivatives. Among these PIs, PI (4,5)-bisphosphate (PIP2) plays a central role in platelet signaling.
Platelet receptor–induced phospholipase C activation induces PIP2 hydrolysis, tightly regulating Ca2+ responses by mobilizing intracellular Ca2+ stores in the dense tubular system and activating Ca2+ channels in the plasma membrane. PIP2 hydrolysis serves as an important precursor of second messengers such as diacylglycerol and inositol (1,4,5) trisphosphate (IP3) [2]. These second messengers enhance platelet Ca2+ responses through the activation of the diacylglycerol-sensitive TRPC6 channel and IP3-receptor (IP3R)-mediated Ca2+ store depletion, respectively. The decreased Ca2+ level in the store activates stromal interaction molecule 1, which translocates to the plasma membrane, initiating store-operated Ca2+ entry (SOCE) by activating the ORAI1 channel [2]. Additionally, PIP2 is converted to PI (3,4,5)-trisphosphate (PIP3) by PI3-kinase (PI3K) during platelet activation. PIP3 recruits pleckstrin-homology (PH) domain–containing proteins from the cytoplasm to the plasma membrane, regulating downstream effectors such as protein kinase B (PKB)/AKT, Bruton’s tyrosine kinase (BTK), and the Ras and Rap GTPase activating protein 3 (RASA3), which was originally identified as an IP4-binding protein (GAP1IP4BP) in platelets [3,4].
In activated platelets, IP3 undergoes rapid phosphorylation to form inositol IP4 or dephosphorylation to form inositol 1,4-bisphosphate, thereby deactivating IP3R function and subsequent Ca2+ store depletion. The regulation of this process involves IP3 kinases (ITPK-A, ITPK-B, and ITPK-C), which phosphorylate IP3 to produce IP4 (Figure). Although the enzymatic activity of ITPKs and their metabolic products have been studied upon platelet activation [5,6], the role of IP4-mediated platelet signaling, particularly its involvement in thrombosis and hemostasis, has not been investigated.
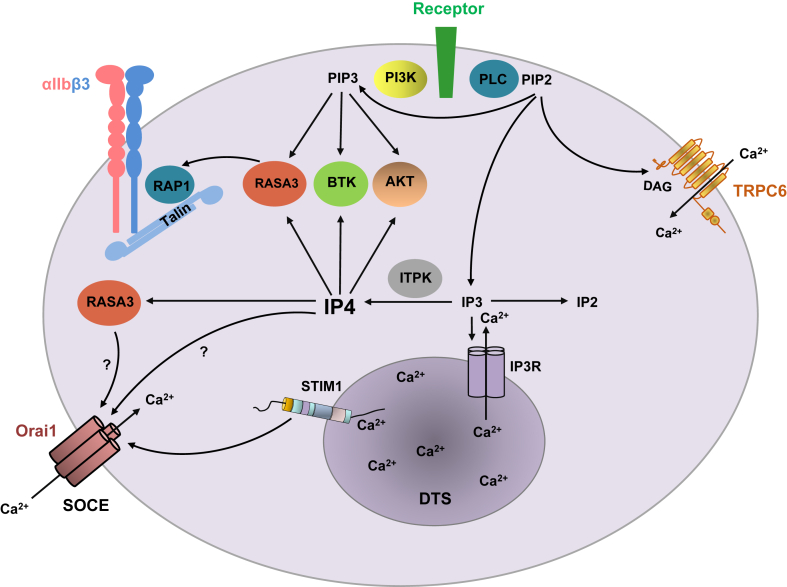
Figure.
Proposed model of inositol 1,3,4,5-tetrakisphosphate (IP4)-mediated signaling in platelets. AKT, protein kinase B; BTK, Bruton’s tyrosine kinase; DAG, diacylglycerol; DTS, dense tubular system; IP2, inositol 1,4-bisphosphate; IP3R, (1,4,5)-trisphosphate-receptor; ITPK, IP3-kinases; PI, phosphatidylinositol; PIP2, PI (4,5)-bisphosphate; PI3K, PI3-kinase, PIP3, PI (3,4,5)-trisphosphate; PLC, platelet receptor–induced phospholipase C; RASA3, Ras and Rap GTPase activating protein 3; SOCE, store-operated Ca2+ entry; STIM1, stromal interaction molecule 1.
In mammalian cells, ITPK function is modulated by protein kinase C, protein kinase A, and Ca2+/calmodulin-activated protein kinase II [7]. The overexpression of ITPKs can rapidly convert IP3 to IP4, reducing the lifetime of IP3 and attenuating IP3-mediated Ca2+ responses [7]. Conversely, ITPK deficiency does not alter IP3 levels in many cell types [8], suggesting an alternative metabolic route for IP3. To date, only a limited number of IP4-binding proteins or downstream effectors have been identified, including ORAI1 channel and RASA3/GAP1IP4BP. Using patch clamp assay, it has been observed that increased levels of IP4 in the cytoplasm can rapidly block ORAI1 channel function [9]. Thapsigargin-induced SOCE was also inhibited by IP4 [10]. However, the exact molecular mechanism of channel desensitization remains elusive. It is still an open question whether IP4 might directly bind to the channel pore of ORAI1 or indirectly regulate the channel function through RASA3/GAP1IP4BP signaling (Figure).
The tissue-specific distribution and functional roles of ITPKs have been studied in mammals. ITPK-A exhibits high expression in the central nervous system. In ITPK-A–deficient mice, enhanced long-term potentiation was observed in neurons [11]. ITPK-B is detected in hematopoietic cells, T and B cells, as well as neutrophils [12]. Deficiency of ITPK-B significantly reduces IP4 production in mouse thymocytes, also resulting in impairment in T-cell maturation and B-cell survival [8,13]. Abnormally increased SOCE was observed in ITPK-B–deficient B cells, probably due to the absence of ORAI1 channel desensitization through IP4-mediated signaling [9,14]. ITPK-B–deficient mice exhibit hyperactivated neutrophils, resulting from dysregulated phospholipid metabolism and consequent PIP3-mediated PKB/AKT response [15]. In various ITPK-B–deficient cells, enhanced PIP3-dependent membrane translocation of PKB/AKT and RASA3/GAP1IP4BP was observed, suggesting that IP4 appears to compete with PIP3 by binding similar PH-domain–containing proteins, thereby blocking PIP3-induced protein trafficking. The expression profile of ITPK-C closely resembles that of ITPK-B, and ITPK-C–deficient mice are healthy; therefore, it was proposed that ITPK-B can compensate for the absence of ITPK-C functions in vivo [8,16]. Despite the generation of several mouse models emphasizing ITPK-dependent pathomechanisms, limited and controversial studies have linked human ITPK gene polymorphism or dysfunction to disorders such as Kawasaki syndrome [17], and no platelet results have been published.
In this issue of RPTH journal, Authi et al. [1] demonstrated that both ITPK-A and ITPK-B isoforms are expressed in platelets. The novel ITPK inhibitor GNF362 effectively inhibits both ITPK isoforms in platelets. At the threshold concentration of platelet agonists, including adenosine diphosphate, collagen, thrombin, and thromboxane A2 analogue U46619, aggregation responses were enhanced in the presence of GNF362 [1]. Using GNF362-treated whole blood under arterial shear stress conditions, the flow chamber assay showed increased thrombus formation on the collagen-coated surface ex vivo, suggesting an antithrombotic potential of ITPK-mediated signaling [1].
Platelets loaded with Ca2+ indicator Fura-2 displayed elevated Ca2+ responses after agonist stimuli in the presence of GNF362, correlating enhanced Ca2+ responses with reduced IP4 and slightly increased IP3 levels in the cytoplasm [1]. As IP4 is not a membrane-permeable compound, extracellular IP4 cannot penetrate into platelets. To overcome this technical difficulty, the authors used saponin-permeabilized platelets to further investigate IP4-mediated signaling [1]. Saponin-treated platelets were activated with a nonhydrolyzable analogue of guanosine triphosphate (GTPγS) or IP3 to accelerate platelet aggregation, and this response was diminished in IP4-treated platelets. Interestingly, GTPγS treatment induced Ser473 phosphorylation of AKT in platelets, and this effect was inhibited by IP4 or the PI3K inhibitor LY294002, indicating a functional crosstalk between IP4- and PI3K-mediated signaling [1].
RASA3/GAP1IP4BP signaling inhibits Rap1 function and consequent αIIbβ3 integrin-mediated platelet aggregation [18,19]. The authors proved this concept by demonstrating that GTPγS can induce Rap1 activation in saponin-permeabilized platelets, and this process was impaired in the presence of IP4. Interestingly, LY294002 did not affect GTPγS-induced Rap1 activation, indicating that the IP4/RASA3/GAP1IP4BP signaling route regulates Rap1 activity independently of PI3K function.
Abolished ITPK function increases PIP3-mediated signaling in many cell types. The authors performed a PIP3 pull-down assay with platelet lysates and showed that RASA3/GAP1IP4BP could effectively interact with PIP3. This interaction was inhibited with IP4 inclusion but not with IP3, highlighting the crucial role of IP4 in the regulation of RASA3/GAP1IP4BP function. In agreement with previous findings in mammalian cells, the interaction between PIP3 and BTK was also inhibited in the presence of IP4 in the platelet lysates. Altogether, these results suggest that IP4 acts as a strong competitor of PIP3 by binding to similar PH-domain–containing proteins and modulating their functions in platelets.
The study by Authi et al. [1] has provided new insights into the regulatory function of IP4 in platelet signaling [1]. ITPK activity controls phospholipid metabolism in resting platelets, maintaining an equilibrium between phospholipid pools of IP3, PIP3, and IP4. Upon platelet activation, ITPK rapidly increases IP4 levels, thereby triggering antagonistic effects on IP3- and PIP3-mediated signaling, which could influence IP3R, RASA3/GAP1IP4BP, BTK, and AKT pathways (Figure). The development of cell-permeable mimetics of IP4 and the identification of novel ITPK agonists are crucial for elucidating the antithrombotic potential of IP4 and ITPK activity in vivo. Understanding the molecular mechanism of IP4-mediated regulation of ORAI1 function is an essential step in this research, given that the blockade of ORAI channel activity has therapeutic potential in arterial thrombosis and stroke [2,20]. Further studies in ITPK mouse models and human patients are necessary to investigate the link between dysregulated ITPK functions and abnormal IP4 levels in platelet-related diseases.
Acknowledgments
Author contributions
A.B. and E.M.-B. wrote the manuscript.
Relationship Disclosure
A.B. and E.M.-B. have no competing interests to disclose.
Footnotes
Handling Editor: Carsten Deppermann
References
- 1.Authi K.S., Khan S., Gibbins J.M., Brain S.D. Evidence that inositol-1,4,5-trisphosphate-3-kinase and inositol-1,3,4,5- tetrakisphosphate are negative regulators of platelet function. Res Pract Thromb Haemost. 2024;8 doi: 10.1016/j.rpth.2024.102326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mammadova-Bach E., Nagy M., Heemskerk J.W.M., Nieswandt B., Braun A. Store-operated calcium entry in thrombosis and thrombo-inflammation. Cell Calcium. 2019;77:39–48. doi: 10.1016/j.ceca.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Cullen P.J., Dawson A.P., Irvine R.F. Purification and characterization of an Ins(1,3,4,5)P4 binding protein from pig platelets: possible identification of a novel non-neuronal Ins(1,3,4,5)P4 receptor. Biochem J. 1995;305:139–143. doi: 10.1042/bj3050139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Rourke F., Matthews E., Feinstein M.B. Isolation of InsP4 and InsP6 binding proteins from human platelets: InsP4 promotes Ca2+ efflux from inside-out plasma membrane vesicles containing 104 kDa GAP1IP4BP protein. Biochem J. 1996;315:1027–1034. doi: 10.1042/bj3151027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Communi D., Vanweyenberg V., Erneux C. Purification and biochemical properties of a high-molecular-mass inositol 1,4,5-trisphosphate 3-kinase isoenzyme in human platelets. Biochem J. 1994;298:669–673. doi: 10.1042/bj2980669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raha S., Jones G.D., Gear A.R. Sub-second oscillations of inositol 1,4,5-trisphosphate and inositol 1,3,4,5-tetrakisphosphate during platelet activation by ADP and thrombin: lack of correlation with calcium kinetics. Biochem J. 1993;292:643–646. doi: 10.1042/bj2920643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell M.J. Inositol trisphosphate 3-kinases: focus on immune and neuronal signaling. Cell Mol Life Sci. 2010;67:1755–1778. doi: 10.1007/s00018-009-0238-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pouillon V., Hascakova-Bartova R., Pajak B., Adam E., Bex F., Dewaste V., et al. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nat Immunol. 2003;4:1136–1143. doi: 10.1038/ni980. [DOI] [PubMed] [Google Scholar]
- 9.Miller A.T., Dahlberg C., Sandberg M.L., Wen B.G., Beisner D.R., Hoerter J.A., et al. Inhibition of the inositol kinase Itpkb augments calcium signaling in lymphocytes and reveals a novel strategy to treat autoimmune disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller A.T., Sandberg M., Huang Y.H., Young M., Sutton S., Sauer K., et al. Production of Ins(1,3,4,5)P4 mediated by the kinase Itpkb inhibits store-operated calcium channels and regulates B cell selection and activation. Nat Immunol. 2007;8:514–521. doi: 10.1038/ni1458. [DOI] [PubMed] [Google Scholar]
- 11.Jun K., Choi G., Yang S.G., Choi K.Y., Kim H., Chan G.C., et al. Enhanced hippocampal CA1 LTP but normal spatial learning in inositol 1,4,5-trisphosphate 3-kinase(A)-deficient mice. Learn Mem. 1998;5:317–330. [PMC free article] [PubMed] [Google Scholar]
- 12.Dewaste V., Roymans D., Moreau C., Erneux C. Cloning and expression of a full-length cDNA encoding human inositol 1,4,5-trisphosphate 3-kinase B. Biochem Biophys Res Commun. 2002;291:400–405. doi: 10.1006/bbrc.2002.6456. [DOI] [PubMed] [Google Scholar]
- 13.Schurmans S., Pouillon V., Marechal Y. Regulation of B cell survival, development and function by inositol 1,4,5-trisphosphate 3-kinase B (Itpkb) Adv Enzyme Regul. 2011;51:66–73. doi: 10.1016/j.advenzreg.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Elich M., Sauer K. Regulation of hematopoietic cell development and function through phosphoinositides. Front Immunol. 2018;9:931. doi: 10.3389/fimmu.2018.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia Y., Schurmans S., Luo H.R. Regulation of innate immunity by inositol 1,3,4,5-tetrakisphosphate. Cell Cycle. 2008;7:2803–2808. doi: 10.4161/cc.7.18.6688. [DOI] [PubMed] [Google Scholar]
- 16.Scoumanne A., Molina-Ortiz P., Monteyne D., Perez-Morga D., Erneux C., Schurmans S. Specific expression and function of inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) in wild type and knock-out mice. Adv Biol Regul. 2016;62:1–10. doi: 10.1016/j.jbior.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Onouchi Y., Gunji T., Burns J.C., Shimizu C., Newburger J.W., Yashiro M., et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battram A.M., Durrant T.N., Agbani E.O., Heesom K.J., Paul D.S., Piatt R., et al. The phosphatidylinositol 3,4,5-trisphosphate (PI(3,4,5)P3) binder Rasa3 regulates phosphoinositide 3-kinase (PI3K)-dependent integrin αIIbβ3 outside-in signaling. J Biol Chem. 2017;292:1691–1704. doi: 10.1074/jbc.M116.746867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefanini L., Paul D.S., Robledo R.F., Chan E.R., Getz T.M., Campbell R.A., et al. RASA3 is a critical inhibitor of RAP1-dependent platelet activation. J Clin Invest. 2015;125:1419–1432. doi: 10.1172/JCI77993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun A., Varga-Szabo D., Kleinschnitz C., Pleines I., Bender M., Austinat M., et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2009;113:2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]