Abstract
Mitral annular disjunction (MAD) is a rare and under-recognized entity in the pediatric population. We present 2 cases of MAD in previously healthy pediatric patients and highlight clinical scenarios where MAD should be suspected.
Key Words: mitral annular disjunction, mitral valve prolapse, pediatrics, sudden cardiac arrest, ventricular arrhythmia
Graphical abstract
Case 1
A previously healthy 16-year-old female patient experienced sudden cardiac arrest while walking at school. Immediate cardiopulmonary resuscitation was initiated, and automated external defibrillator tracings demonstrated ventricular fibrillation. She underwent multiple rounds of defibrillation and had return of spontaneous circulation within approximately 40 minutes. She was admitted to an external facility where a baseline electrocardiogram was abnormal (Figure 1), and demonstrated sinus rhythm, mild first-degree atrioventricular block, nonspecific intraventricular conduction delay, nonspecific ST-segment changes, and borderline QTc prolongation (455 ms). Echocardiogram revealed moderate-severe left ventricular (LV) systolic dysfunction and a thickened mitral valve with trace mitral regurgitation (MR). Cardiac magnetic resonance (CMR) performed 2 days after the arrest reported biventricular dysfunction, right ventricular (RV) wall motion abnormalities, and RV dilation prompting concern for arrhythmogenic cardiomyopathy; she was referred to our center for further evaluation. Upon admission, physical examination noted several features suspicious for a connective tissue disorder (CTD), including elongated limbs and digits, hypermobility, horizontal skin striae, and a mild pectus excavatum. Otherwise, the patient had no significant medical history, including syncope, palpitations, or chest pain. Additionally, there was no history of neurological impairment, seizures/epilepsy, or headache. The family history was remarkable for paternal mitral valve endocarditis but was negative for arrhythmia or sudden cardiac death.
Learning Objectives
-
•
To identify and recognize MAD in the pediatric population.
-
•
To understand the role of MAD in cardiac arrest and ventricular arrhythmia in pediatric patients.
-
•
To understand the diagnostic work-up for MAD in the pediatric population.
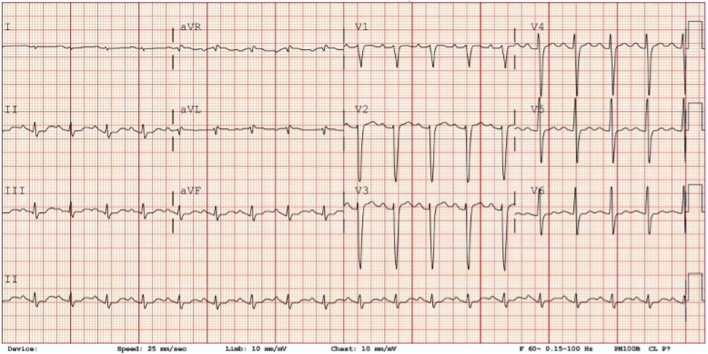
Figure 1.
Baseline Electrocardiogram, Case 1
Sinus rhythm, borderline first-degree atrioventricular block (PR 180 ms), mild nonspecific intraventricular conduction delay, nonspecific ST changes, and borderline prolonged QTc (455 ms).
Differential diagnosis included arrhythmogenic cardiomyopathy, undiagnosed channelopathy, unrecognized structural heart disease, primary neurologic disease, or drug intoxication. Given prolonged out-of-hospital resuscitation, she underwent head computed tomography, which was negative for primary intracranial pathology or bleeding. Due to clinical suspicion for a CTD and concerns for arrhythmogenic cardiomyopathy, a repeat CMR was performed 2 weeks after her cardiac arrest, which demonstrated normalization of the RV and LV end-diastolic volumes (82 and 90 mL/m2, respectively) with improved LV (EF 51%) and RV function (EF 46%). Additionally, a clear separation between the posterior leaflet of the mitral valve and the basal portion of the LV posterolateral wall was noted with a maximum distance of 18 mm, consistent with mitral annular disjunction (MAD). These findings are best noted on the horizontal long-axis and vertical long-axis cines in end-systole (Figure 2). There was bileaflet mitral valve prolapse (MVP) without MR or late gadolinium enhancement (LGE) of the LV myocardium or papillary muscles to suggest myocardial fibrosis or scarring (Figure 2). Retrospective review of her echocardiogram corroborated these findings, with a 15-mm separation of the posterior mitral leaflet from the LV free wall (Figure 3). Genetic testing for comprehensive cardiomyopathy, arrhythmia, and aortopathy genes resulted in a pathogenic exon deletion (exons 25-26) in fibrillin-1 (FBN1), confirming a diagnosis of Marfan syndrome. Given her unprovoked cardiac arrest, she underwent uncomplicated placement of a secondary-prevention dual-chamber transvenous implantable cardioverter-defibrillator. Despite her significant resuscitation, she currently has no clinical sequelae from her arrest and no additional arrhythmias have been detected.
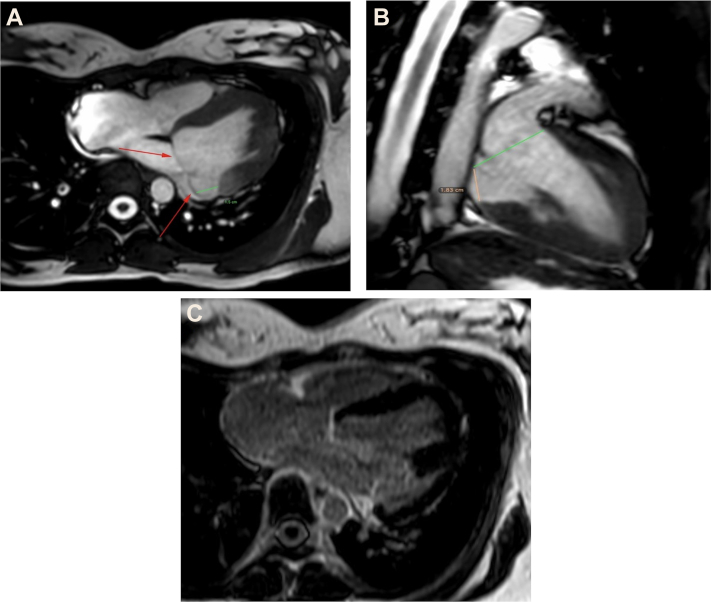
Figure 2.
CMR, Case 1
Cardiac magnetic resonance in horizontal long-axis view (A), showing mitral annual disjunction distance of 15 mm; the red arrows indicate the mitral annular plane. (B) Vertical long-axis view with mitral annual disjunction, distance of 18 mm. (C) No evidence of late gadolinium enhancement of the left ventricle.
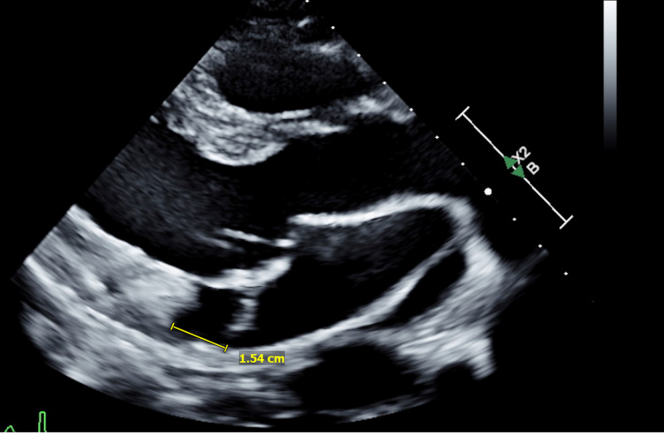
Figure 3.
Echocardiogram, Case 1
Transthoracic echocardiogram in parasternal long-axis view showing mitral annular disjunction with disjunction distance of (15 mm) in end systole.
Case 2
A 12-year-old healthy female patient was referred to cardiology clinic for evaluation of a murmur. She was asymptomatic and her family history was negative for cardiac disease or arrythmia. An echocardiogram was performed that demonstrated bileaflet MVP with mild to moderate MR (Figure 4); the electrocardiogram was normal for age. CMR confirmed the findings and noted a regurgitant fraction of 29%, mild LV dilatation, and normal biventricular systolic function. MAD was noted with a maximum distance of 11 mm, without evidence of LV scarring or fibrosis (Figure 5). She underwent exercise stress testing that showed normal exercise capacity and heart rate response, without evidence of arrhythmia. Genetic testing for CTDs revealed an intronic variant of uncertain significance in Mothers Against Decapentaplegic homolog 3 (SMAD3), a gene associated with Loeys-Dietz syndrome. Given the patient was asymptomatic, without LGE, arrhythmia, or clinical manifestations of Loeys-Dietz syndrome, no intervention was performed. She will continue with yearly clinical evaluation, including ambulatory rhythm monitoring and CMR.
Figure 4.
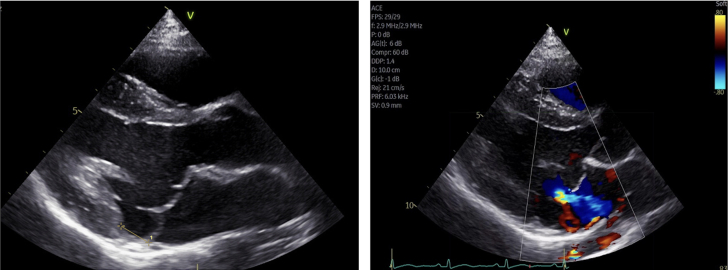
Echocardiogram, Case 2
Transthoracic echocardiogram in parasternal long-axis view showing mitral valve prolapse along with mitral annular disjunction with disjunction distance of 11 mm in end systole. Color Doppler image shows mitral regurgitation.
Figure 5.
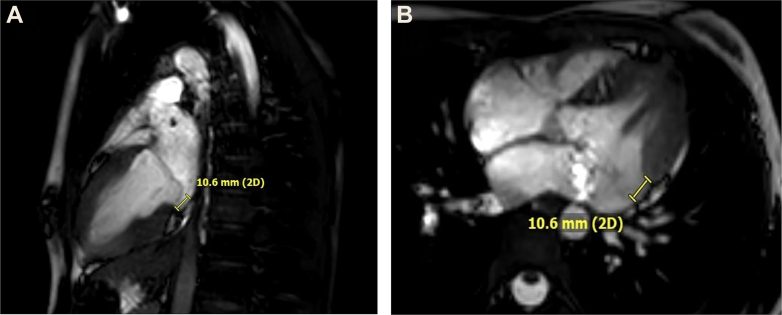
CMR, Case 2
Cardiac magnetic resonance in vertical long-axis view (A), showing mitral annual disjunction distance of 10.6 mm, and (B) in horizontal long-axis view with same distance of 10.6 mm.
Discussion
Mitral annular disjunction is defined as separation of the myocardium between the left atrial wall and LV free wall at the posterior mitral valve insertion point. To date, MAD is more commonly described in adults and has been linked to ventricular arrhythmias and other serious cardiac events, but is rarely reported in pediatric patients1,2 (Supplemental Table 1). MAD is often associated with MVP and increases the risk of arrhythmic events.3 It has also been reported in patients with CTDs such as Marfan and Loeys-Dietz syndrome, and usually indicates a more severe phenotype.4 The prevalence of MAD varies across studies, mainly attributed to differences in the definition of MAD distance. A systematic review of 3,925 patients found an 8.7% prevalence, which increases in patients with MVP.5 Additionally, the degree of MAD appears to influence clinical outcomes, and longer distances (>8.5 mm) have been associated with a higher incidence of adverse events.6 MAD can be readily diagnosed by transthoracic echocardiography or CMR. CMR imaging has shown a higher detection rate, especially in patients with a shorter disjunction distance,7 because of its superior resolution and volumetric imaging of the heart. CMR is also helpful in detecting myocardial fibrosis, which can be observed in patients with MAD, providing critical information for risk stratification.8 Importantly, fibrosis is not necessary for a patient to experience serious cardiac events,9 as illustrated in Case 1, highlighting the complex pathophysiology of arrhythmia generation in this population.
MAD is rarely reported in the pediatric population and mostly consists of isolated case reports and small numbers of pediatric patients in larger adult cohorts2,10 (Supplemental Table 1). This report highlights the importance of considering MAD in pediatric patients with ostensibly isolated MVP, and/or those who have a history of sudden cardiac arrest or malignant ventricular arrhythmias. CTDs, if not already suspected, should be considered, and genetic testing should be discussed given the clinical implications. Additionally, Case 1 draws attention to profound MAD with mild valve prolapse, but no regurgitation or LGE, as a risk factor for sudden cardiac arrest in pediatric patients. This challenges the hypothesis that chronic papillary stress from MAD contributes to scar and arrhythmic substrate formation over time. Whether there is subclinical fibrosis below the current level of detection, inherent predisposition, or a different mechanism altogether, further work is needed to understand the relationship between MAD and arrhythmogenesis.
Although this report highlights the importance of recognizing MAD in susceptible pediatric patients, the generalizability is limited by the rare nature of the presentation. In our experience, a high level of suspicion is required to identify pediatric MAD, and a thorough history with comprehensive diagnostic work-up, including CMR and genetic testing, should be considered. Early identification provides an opportunity for intervention before the onset of significant arrhythmic events, although long-term follow-up is required to better understand the natural history in pediatric patients.
Conclusions
MAD is a known risk factor for malignant arrhythmias and sudden cardiac death in adults and can be associated with MVP and CTDs. These cases suggest that MAD should also be considered in pediatric patients with MR and/or unexplained sudden cardiac arrest for which standard evaluation has been unrevealing. Concentrated expertise in advanced cardiac imaging and cardiovascular genetics were critical in the evaluation and diagnosis of these patients. Therapy should be directed toward managing coexisting mitral valve disease and preventing future arrhythmic events, including sudden cardiac death, as well as addressing comorbidities associated with a CTD diagnosis, if established.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Bennett S., Thamman R., Griffiths T., et al. Mitral annular disjunction: a systematic review of the literature. Echocardiography. 2019;36(8):1549–1558. doi: 10.1111/echo.14437. [DOI] [PubMed] [Google Scholar]
- 2.Carr K., Yetman A., Garg R. Mitral annular disjunction associated with fatal ventricular arrhythmia in an adolescent with Marfan syndrome. J Am Coll Cardiol Case Rep. 2021;3(13):1551–1556. doi: 10.1016/j.jaccas.2021.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Essayagh B., Sabbag A., Antoine C., et al. The mitral annular disjunction of mitral valve prolapse: presentation and outcome. J Am Coll Cardiol Img. 2021;14(11):2073–2087. doi: 10.1016/j.jcmg.2021.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Chivulescu M., Krohg-Sørensen K., Scheirlynck E., et al. Mitral annulus disjunction is associated with adverse outcome in Marfan and Loeys-Dietz syndromes. Eur Heart J Cardiovasc Imaging. 2021;22(9):1035–1044. doi: 10.1093/ehjci/jeaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett S., Tafuro J., Duckett S., et al. Definition, prevalence, and clinical significance of mitral annular disjunction in different patient cohorts: a systematic review. Echocardiography. 2022;39(3):514–523. doi: 10.1111/echo.15299. [DOI] [PubMed] [Google Scholar]
- 6.Carmo P., Andrade M.J., Aguiar C., Rodrigues R., Gouveia R., Silva J.A. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 2010;8:53. doi: 10.1186/1476-7120-8-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantegazza V., Volpato V., Gripari P., et al. Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart. 2021;107(1):25–32. doi: 10.1136/heartjnl-2020-317330. [DOI] [PubMed] [Google Scholar]
- 8.Dejgaard L.A., Skjølsvik E.T., Lie Ø.H., et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 2018;72(14):1600–1609. doi: 10.1016/j.jacc.2018.07.070. [DOI] [PubMed] [Google Scholar]
- 9.Ezzeddine F.M., Siontis K.C., Giudicessi J., et al. Substrate characterization and outcomes of catheter ablation of ventricular arrhythmias in patients with mitral annular disjunction. Circ Arrhythm Electrophysiol. 2022;15(9) doi: 10.1161/CIRCEP.122.011088. [DOI] [PubMed] [Google Scholar]
- 10.Demolder A., Timmermans F., Duytschaeyer M., et al. Association of mitral annular disjunction with cardiovascular outcomes among patients with Marfan syndrome. JAMA Cardiol. 2021;6(10):1–10. doi: 10.1001/jamacardio.2021.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.