Abstract
Objectives
This study aims to elucidate the expression of circulating exosomal miRNAs miRNA 21, miRNA 184, and miRNA 145 in the studied groups, including patients with (i) leukoplakia; (ii) oral submucous fibrosis; (iii) oral submucous fibrosis with leukoplakia; (iv) oral squamous cell carcinoma; and (v) healthy individuals.
Study Design
An observational study was conducted among 54 patients who reported to the outpatient department of Saveetha Dental College and Hospitals. The patients were divided into three groups: Group I healthy individuals (n = 18), Group II: case group (leukoplakia, OSMF, and leukoplakia and OSMF) (n = 18), and Group III: OSCC (n = 18). Real-time polymerase chain reaction analysis was carried out to assess the expression profiles of miRNA 21, miRNA 184, and miRNA 145. The statistical analysis was calculated using SPSS software version 23.
Results
All three miRNAs showed a statistically significant difference in the one-way ANOVA test between the case group (leukoplakia, OSMF, and leukoplakia and OSMF), healthy group, and OSCC group (p < 0.005). The case group (leukoplakia, OSMF, leukoplakia and OSMF) showed upregulated expression of miRNA 21 and miRNA 184 with threefold change and fourfold change and downregulated expression of miRNA 145 with 1.5-fold change when compared to apparently healthy individuals.
Conclusion
Plasma circulating exosomal miRNAs miRNA 21, miRNA 145, and miRNA 184 expression could be a novel panel of plasma biomarkers to categorise case group (leukoplakia, OSMF, leukoplakia and OSMF) patients with a high risk of malignant transformation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12105-024-01627-4.
Keywords: microRNA, Plasma, Exosomes, Leukoplakia, OSMF, OSCC, miRNA 21, miRNA 184, miRNA 145
Introduction
Oral potentially malignant disorders (OPMDs) are conditions that include a variety of oral mucosal conditions that are associated with an increased risk of developing oral cancer. Several risk factors for OPMDs are associated with the consumption of betel quid, areca nut, forms of tobacco, alcohol, low socio-economic status, and a diet poor of antioxidants. The most common ailment in India is oral submucous fibrosis, with a prevalence rate of 8.06%. This is followed by leukoplakia at 4.02% and erythroplakia at 0.24%. Overall, the frequency of these conditions is 13.7% [1]. The oral leukoplakia prevalence study by Iocca et al. showed an annual malignant transformation rate (MTR) of 3.57% [2]. The literature shows a varied rate of malignant transformation among different lesions in OPMDs. In 2022, the World Health Organization (WHO) revised and established the histologic criteria for evaluating cellular and tissue dysplasia. Oral submucous fibrosis (OSMF) is a disorder of collagen metabolism characterised by excessive collagen formation and decreased collagen degradation. The OSMF prevalence rate in Southeast Asian and Indian populations ranges between 0.2 and 0.5%. This is owing to the increased use and prominence of commercially prepared areca nut and tobacco products, such as gutkha, pan masala, flavoured supari, etc. Between 2 and 8% of OSMF cases undergo malignant transformation into oral squamous cell carcinoma (OSCC) through malignant transformation [3–5].
A novel, non-invasive approach called liquid biopsy is used to extract circulating biomarkers from bodily fluids [6]. The term liquid biopsy refers to a variety of components, including circulating tumour cells (CTCs), cell-free DNA (cfDNA) and circulating tumour DNA (ctDNA), exosomes, and circulating cell-free nucleic acids. Exosomes are naturally occurring nanocarriers and intercellular messengers. They are extracellular vesicles with a lipid bilayer and a diameter of 30 to 150 nm that form when multivesicular bodies fuse with the plasma membrane [7, 8]. The quantity of exosome concentrations in liquid biopsies is approximately ≥ 10 [9] vesicles/mL [9, 10]. Large amounts of exosomes are secreted by tumour cells, which aid in tumour progression [11]. Exosomes contain nucleic acids and proteins, which can be dysregulated in disease conditions. Tumour-derived exosomes regulate cellular metabolism through angiogenesis, immune suppression, metastasis, cancer-associated fibroblast activation, and extracellular remodelling [12, 13]. Recent research indicates that exosomes are crucial in the diagnosis, prognosis, and treatment of OSCC [14]. The exosomal miRNA is preferred as a stable source of miRNA over cell-free circulating miRNA because of its stability inside the bilipid exosome membrane, and the circulating tumour cells are low in quantity when compared to circulating exosomes [15]. The circulating exosomes are preferred over salivary exosomes because of their abundance of tumour cell-derived exosomes in circulation [16].
miRNAs are non-coding RNAs composed of 18–25 nucleotides that bind to the complementary binding site 3′ untranslated regions (3′UTR) of target mRNA control post-transcriptional gene expression [17]. Emerging evidence has shown aberrant expression of miR-21, miR-184, and miR-145 in OPMDs such as leukoplakia and OSMF [18]. On chromosome 17q23.2, the human miRNA 21 gene comprises 3433 nucleotides and is situated in an intergenic region [19]. miRNA 21 bioregulation in tumour cells may vary as a result of modifications to the processes governing its production, transcription, transport, binding with the RNA-induced silencing complex (RISC), and destruction [20]. The activation of caspases and subsequent increase in apoptotic cell death were caused by miR-21 knockdown in cultivated glioblastoma cells, indicating that miR-21 functions as an anti-apoptotic agent [21]. Additionally, miRNA 184’s role as an oncogene in some cancers has been shown. For instance, miRNA 184 promoted cell proliferation and prevented apoptosis in squamous cell carcinoma of the tongue [22]. Furthermore, surgically removing the primary squamous cell carcinoma of the tongue significantly reduced the expression of miRNA 184 in plasma [23]. Researchers studying tongue SCC have shown that miR-184 manages the sex-determining region Y-box 7 (SOX7) gene, which controls cell death and growth [24]. The expression of miRNA 145, which is situated in the 5p32 chromosomal region, is controlled by p53 and other transcriptional factors [25]. Michael et al.’s initial research on miRNA 145’s low expression in colon adenocarcinomas was published in 2003 [26]. The major tumour suppressor miR-145 has been shown to express at low levels in a variety of epithelial tumours [27]. This research is also important for identification of potential targeted therapy for these premalignant lesions. The present study aimed to elucidate the expression of circulating exosomal miRNAs miRNA 21, miRNA 184, and miRNA 145 in the case group of patients with leukoplakia, OSMF, and leukoplakia and OSMF when compared to healthy controls and OSCC and their correlations with the clinicopathological parameters.
Materials and Methods
An observational study was done with the patients consistent with the inclusion criteria. The sample size calculation was done using G power by keeping the alpha error probability at 0.05, the allocation ratio at 1, critical z as − 1.9599640, and power of the study (1-beta error probability) as 0.90; the total sample size was calculated as n = 54. G power was used to calculate the sample size. The total sample size of n = 54 participants was included in the study based on G-Power sample size calculation of which healthy individuals (n = 18), case group (leukoplakia, OSMF, leukoplakia and OSMF) (n = 18), and OSCC (n = 18) were recruited for the study and intravenous blood samples were collected for isolation of plasma and exosomes are extracted using Invitrogen Exosome Isolation kit. The Institutional Review Board of Saveetha Institute of Medical and Technical Sciences, Chennai, India, approved the initiative. [SRB Number: SRB/SDC/OPATH-2003/21/TH-045]. This investigation adhered to the ethical principles outlined in the Helsinki Declaration. Participants were recruited from the newly diagnosed patient cohort at the Department of Oral Pathology at Saveetha Dental College, Saveetha Institute of Medical and Technical Sciences, Chennai, India, between May 2021 and December 2022. The study participants were selected based on criteria which includes patients with age 18–70 years diagnosed with OSCC, case group (leukoplakia, OSMF, and leukoplakia and OSMF), and healthy controls with no history of any chronic illness. The patients who had undergone or were currently receiving intervention for OSCC, as well as those in the case group with leukoplakia, OSMF, or both leukoplakia and OSMF, were eliminated. Additionally, patients who had received chemotherapy or radiation treatment were also removed.
The clinical parameters of type and duration of smoking and smokeless tobacco (chewing arecanut and tobacco) and alcohol habits were assessed. The histopathological prognostic parameters assessed include histopathological diagnosis of case group (leukoplakia, OSMF, leukoplakia and OSMF) with grading and pTNM staging of OSCC. The pTNM staging was done as per the AJCC Cancer Staging Manual, 8th edition (2017). The tumours were graded histologically as Well-Differentiated Squamous Cell Carcinoma (WDSCC) (n = 12), Moderately Differentiated Squamous Cell Carcinoma (MDSCC) (n = 5), and Poorly Differentiated Squamous Cell Carcinoma (PDSCC) (n = 1) according to their degree of differentiation. The case group included leukoplakia, OSMF, and a combination of leukoplakia and OSMF. WHO dysplasia grading criteria were used for epithelial dysplasia. The epithelial dysplasia was graded histologically as mild ED (n = 4), moderate ED (n = 7), and severe ED (n = 3). Pindborg grading criteria were used for epithelial dysplasia. The OSMF was graded histologically as very early (n = 0), early (n = 0), moderately advanced (n = 5), and advanced oral submucous fibrosis (n = 6). The combination of leukoplakia with OSMF (n = 7) was also considered as a subgroup of the case group. The reagents used in polymerase chain reaction are 10 μL of Emerald Master mix (Catalogue number RR310A, Takara, Japan), 2 μL of hsa-miR- reverse primer, 2 μL of hsa-miR-forward primer, 1 μL of internal control forward primer U6 (0.2 μM), 1 μL of internal control reverse primer U6 (0.2 μM), 1 μL of DNA (10 nanograms), 3 μL of deionised water. The table of primers is represented in Table 1. Using the Exosome Isolation kit, plasma exosomes were isolated from healthy, the case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC patients samples. Total RNA was obtained from healthy, the case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC samples using a TRIR kit (Total RNA Isolation Reagent Invitrogen). cDNA conversion, RT-PCR, and mRNA markers assessed using RT PCR were miRNA 21, miRNA 145, miRNA 184, and the normalisation was done in comparison with (Universal housekeeping) U6. Melt curve and amplification curve studies calculated the relative amounts.
Table 1.
Design of allele-specific primers
| S. no | Primer | Sequence |
|---|---|---|
| 1 | miR-21-3p Forward primer | 5’-CGGCGGAACACCAGTCGATG-3’ |
| 2 | miR-21-3p Reverse primer | 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGACAGCCCA-3’ |
| 3 | hsa-miR-184 Forward primer | 5’-TGGACGGAGAACTGATAAGGGT-3’ |
| 4 | hsa-miR-184 Reverse primer | 5’‐CCTTATCAGTTCTCCGTCCATT‐3’ |
| 5 | hsa-miR-145 Forward primer | 5′-GTCCAGTTTTCCCAGGAATC-3′ |
| 6 | hsa-miR-145 Reverse primer | 5′-AGAACAGTATTTCCAGGAAT-3′ |
| 7 | U6 Forward primer | 5’-CTCGCTTCGGCAGCACA-3’ |
| 8 | U6 Reverse primer | 5’-AACGCTTCACGAATTTGCGT-3’ |
The formula for calculating 2^-ΔΔCT is
Amplification by Agarose Gel Electrophoresis
Aliquots of amplified PCR products measuring 10 μL each were subjected to analysis by running them in an agarose gel containing 1.5% TAE (tris acetate EDTA) buffer at 100 V for 15 min. The DNA bands were seen by staining the gel with ethidium bromide, which is a DNA intercalating chemical that fluoresces when stimulated by UV light in the range of 302 to 364 nm. Following this, gel electrophoresis was done.
Data were analysed using SPSS 25.0 (IBM, Chicago). All data are expressed as means ± SD. Comparison of mean CT of miRNAs was made using one-way ANOVA and Turkey HSD post hoc test. Correlation of tobacco habit, tobacco use in years, and association of leukoplakia, OSMF, staging, and grading of OSCC with CT values and fold increase was done using Kendall’s tau correlation. P < 0.05 was considered to be statistically significant.
Results
Demographic data of the study population included a total of n = 54 patients who were divided into three groups. Group I consists of apparently healthy individuals (n = 18), Group II case group consists of patients with leukoplakia, OSMF, leukoplakia and OSMF (n = 18), and Group III consists of OSCC patients at various grades and stages (n = 18). The mean age among the case group was 50.22, the healthy group was 33.83, and the OSCC group was 54.50. There was a male preponderance seen among the two groups the case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC with 72.2 and 94.4%, respectively (Table 2).
Table 2.
Distribution of demographic variables among healthy individuals, case group (OED, OSMF, OED + OSMF), and OSCC with mean ± SD minimum and maximum values for age and sex
| Characteristics | Healthy individuals | Case group (OED, OSMF, OED + OSMF) | OSCC |
|---|---|---|---|
| Age (Mean ± SD) | 33.83 ± 8.522 | 50.22 ± 12.98 | 54.50 ± 10.63 |
| Min–Max (in years) | 25–58 | 22–78 | 32–70 |
| Male (%) | 6 (33.3) | 13 (72.2) | 17 (94.4) |
| Female (%) | 12 (66.7) | 5 (27.8) | 1 (5.6) |
Site occurrence of case group (leukoplakia, OSMF, leukoplakia and OSMF) among the study patients showed most of the lesions presented on the right buccal mucosa (55.5%). The habits of smoking, smokeless tobacco, and alcohol were found to be more prevalent among the case group. 44.4% of the patients were having both (tobacco and alcohol) and tobacco. Mean years of habit distribution among the study patients showed the mean years for use of tobacco (smoke) among the patients with the case group (leukoplakia, OSMF, leukoplakia and OSMF) (5.940) (Table 3).
Table 3.
Demographic data
| Clinical parameters | Category | N (%) |
|---|---|---|
| Site of lesion: OPMD | Left buccal mucosa | 5 (27.8) |
| Right buccal mucosa | 10 (55.5) | |
| Left and right buccal mucosa | 3 (16.7) | |
| Site of lesion: OSCC | Left buccal mucosa | 3 (16.7) |
| Right buccal mucosa | 2 (11.1) | |
| Left lateral border of the tongue | 2 (11.1) | |
| Right lateral border of the tongue | 6 (33.3) | |
| Left buccal mucosa + left GBS | 4 (22.3) | |
| Right retromolar trigone | 1 (5.6) | |
| Habits: OPMD | Both (tobacco (smoking and smokeless) + alcohol) | 8 (44.4) |
| Tobacco (smoking and smokeless) | 8 (44.4) | |
| Habits: OSCC | Nil | 1 (5.6) |
| Both (tobacco (smoking and smokeless) + alcohol) | 7 (38.9) | |
| Tobacco (smoking and smokeless) | 7 (38.9) | |
| Alcohol | 3 (16.7) | |
| OPMD | Hyperkeratosis with Epithelial Dysplasia and OSMF | 7 (38.8) |
| Dysplasia alone | 7 (38.8) | |
| OSMF alone | 4 (22.2) | |
| Epithelial dysplasia | Mild | 4 (28.7) |
| Moderate | 7 (50) | |
| Severe | 3 (21.3) | |
| OSMF | Moderately advanced OSMF | 5 (45.4) |
| Advanced OSMF | 6 (54.5) | |
| Histopathological type | WDSCC | 12 (66.7) |
| MDSCC | 5 (27.8) | |
| PDSCC | 1 (5.6) | |
| Stage | I | 1 (5.6) |
| II | 4 (22.2) | |
| III | 1 (11.1) | |
| IV | 11 (61.1) |
Expression level of miRNA 21, miRNA 184, and miRNA 145 with healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC was done using RT PCR analysis. To compare the mean CT values of miRNAs in all three groups: healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC, one-way ANOVA was done. miRNA 21 showed less CT value (1.425) for the case group (leukoplakia, OSMF, leukoplakia and OSMF), indicating over-expression of the gene when compared to the healthy group (1.499). There was a significant difference in the mean CT of miRNA 21 (p = 0.000). Similarly, mean CT values for miRNA 184 among the study group showed low CT values (1.389) for the case group (leukoplakia, OSMF, leukoplakia and OSMF), indicating over-expression of the gene when compared to the healthy group (1.525) with a p-value of (0.000). The mean CT values for miRNA 145 among the study group showed high CT values (1.397) for the case group (leukoplakia, OSMF, leukoplakia and OSMF), indicating over-expression of the gene when compared to healthy (p = 0.000) (Table 4).
Table 4.
Comparison of mean CT values of microRNAs
| microRNAs | Mean ± SD | p value | ||
|---|---|---|---|---|
| Healthy | Case group (leukoplakia, OSMF, OED + OSMF) | OSCC | ||
| miRNA 21 | 1.499 ± 0.000 | 1.425 ± 0.021 | 1.359 ± 0.000 | 0.000* |
| miRNA 184 | 1.587 ± 0.014 | 1.389 ± 0.026 | 1.334 ± 0.030 | 0.000* |
| miRNA 145 | 1.367 ± 0.023 | 1.397 ± 0.012 | 1.416 ± 0.030 | 0.000* |
Tukey HSD post hoc test showed a significant mean difference between patients of healthy and case group (leukoplakia, OSMF, leukoplakia and OSMF) group (p = 0.000), patients of healthy and OSCC group (p = 0.000), and patients of case group (leukoplakia, OSMF, leukoplakia and OSMF) and oral cancer (p = 0.001) for miRNA 21. Similarly, there was a significant mean difference in CT values of miRNA 184 between the healthy and case group (leukoplakia, OSMF, leukoplakia and OSMF) (p = 0.000); healthy; and OSCC (p = 0.000). There was a significant difference in mean difference in CT values of miRNA 145 between the healthy and case group (leukoplakia, OSMF, leukoplakia and OSMF) (p = 0.000); healthy; and OSCC (p = 0.000). There was no significant mean difference in CT values between the case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC (p = 0.55) for miRNA 184 and (p = 0.064) for miRNA 145 (Table 5).
Table 5.
Tukey HSD post hoc test of mean CT of microRNAs: miRNA 21, miRNA 184, and miRNA 145 in groups: healthy, case group (OED, OSMF, OED + OSMF), and OSCC
| microRNAs | Pair | Mean difference | p value |
|---|---|---|---|
| miRNA 21 | Healthy vs case group (OED, OSMF, OED + OSMF) | 0.112 | 0.000* |
| Healthy vs OSCC | 0.132 | 0.000* | |
| Case group (OED, OSMF, OED + OSMF) vs OSCC | 0.020 | 0.001* | |
| miRNA 184 | Healthy vs case group (OED, OSMF, OED + OSMF) | 0.158 | 0.000* |
| Healthy vs OSCC | 0.135 | 0.000* | |
| Case group (OED, OSMF, OED + OSMF) vs OSCC | -0.022 | 0.055 | |
| miRNA 145 | Healthy vs case group (OED, OSMF, OED + OSMF) | 0.143 | 0.000* |
| Healthy vs OSCC | 0.124 | 0.000* | |
| Case group (OED, OSMF, OED + OSMF) vs OSCC | − 0.019 | 0.064 |
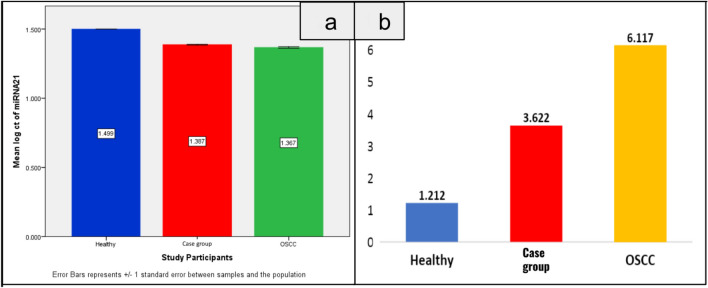
Fold changes were calculated based on the CT values of the miRNA and U6. The mean fold change of 2^-ΔΔCT values of miRNA 21 showed there was a 3.62-fold increase in the CT values for patients with case group (leukoplakia, OSMF, leukoplakia and OSMF) and a 6.11-fold increase among the patients with OSCC (Fig. 1). Also, the mean fold change of 2^-ΔΔCT values of miRNA 184 showed there was a fourfold increase in the 2^-ΔΔCT values for patients with case group (leukoplakia, OSMF, leukoplakia and OSMF) and 6.72-fold increase among the patients with OSCC (Fig. 2). The mean fold change of 2^-ΔΔCT values of miRNA 145 showed there was a twofold increase in the 2^-ΔΔCT values for healthy patients compared to the case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC group patients (Fig. 3). The comparative data of miRNAs 21, 184, and 145 in the healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC are represented in Fig. 4.
Fig. 1.
Bar graph represents CT values a and 2^-ΔΔCT b in mean ± SD. Mean comparison of CT value and 2^-ΔΔCT of miRNA 21 in the healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC. Based on miRNA 21, the case group (leukoplakia, OSMF, leukoplakia and OSMF) group showed threefold increase when compared to the healthy with 3.672-fold change and the OSCC group showed a 6.117-fold change
Fig. 2.
Bar graph represents CT values a and 2^-ΔΔCT b in mean ± SD. Mean comparison of CT value and 2^-ΔΔCT of miRNA 184 in the healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC. Based on the miRNA 184, the case group (leukoplakia, OSMF, leukoplakia and OSMF) group showed a fourfold increase when compared to a healthy 4.007-fold change and the OSCC group showed a 6.719-fold change
Fig. 3.
Bar graph represents CT values a and 2^-ΔΔCT b in mean ± SD. Mean comparison of CT value and 2^-ΔΔCT of miRNA 145 in the healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC. Based on the miRNA 145, the healthy group showed twofold increase when compared to the case group and OSCC
Fig. 4.
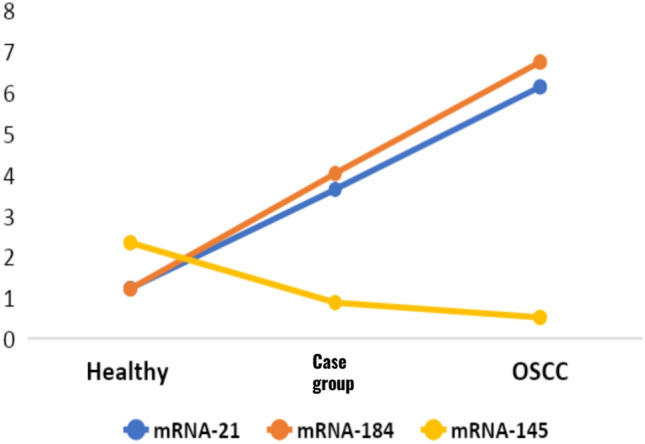
Line graph represents 2^-ΔΔCT in mean ± SD. mean comparison of 2^-ΔΔCT value (fold change) of miRNA 21, miRNA 184, and miRNA 145 in the healthy, case group (leukoplakia, OSMF, leukoplakia and OSMF), and OSCC. The miRNA 21 and miRNA 184 showed a significant upregulation in the case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC group when compared to the healthy group. The miRNA 145 showed a significant downregulation in the case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC group
Alterations in the expression level in case group (leukoplakia, OSMF, leukoplakia and OSMF) samples were analysed by RT PCR followed by melt curve analysis. The amplification curve of the miRNA 21, miRNA 145, and miRNA 184 is shown in Supplementary Figs. 5a, 5a and 5a. The melt curve analysis is shown in Supplementary Figs. 5b, 5b and 5b. The melt peaks of the miRNAs were found at 70- 85 °C.
The correlation of the expression level of miRNAs with tobacco habits and years of smoking and smokeless tobacco (both chewing and pusage were done using Kendall’s tau correlation test. There is no significant correlation between the type of tobacco habit and the log CT value of miRNA 21, miRNA 184, and miRNA 145 (p > 0.05) (Table 6). There is no significant correlation using Spearman’s correlation test between years of tobacco use with CT values for miRNA 21, miRNA 184, and miRNA 145 (p > 0.05) (Table 7).
Table 6.
Association of type of tobacco habit with CT values of miRNAs (miRNA 21, miRNA 184, and miRNA 145) in case group (OED, OSMF, OED + OSMF) and OSCC groups
| miRNAs | Type of tobacco habit | Mean ± SD CT values | Correlation coefficient (r); p value |
|---|---|---|---|
| miRNA 21 | Both | 1.379 ± 0.026 | 0.044; 0.732 |
| Smokeless | 1.376 ± 0.021 | ||
| Smoking | 1.378 ± 0.018 | ||
| miRNA 184 | Both | 1.379 ± 0.016 | − 0.017; 0.898 |
| Smokeless | 1.377 ± 0.027 | ||
| Smoking | 1.375 ± 0.019 | ||
| miRNA 145 | Both | 1.436 ± 0.016 | 0.097; 0.450 |
| Smokeless | 1.422 ± 0.033 | ||
| Smoking | 1.419 ± 0.033 |
Table 7.
Association of years of any form of tobacco (both smoking and smokeless) use with 2^-ΔΔct values of miRNAs (miRNA 21, miRNA 184 and miRNA 145) in case group (OED, OSMF, OED + OSMF) and OSCC groups
| miRNAs | Correlation variables | Correlation coefficient (r) | p value |
|---|---|---|---|
| miRNA 21 | Years of tobacco use * CT values | 0.118 | 0.495 |
| miRNA 184 | Years of tobacco use * CT values | − 0.127 | 0.459 |
| miRNA 145 | Years of tobacco use * CT values | 0.301 | 0.074 |
Expression levels of circulating exosomal miRNAs—miRNA 21, miRNA 184, miRNA 145 with the histological type of case group (leukoplakia, OSMF, leukoplakia and OSMF)—were accessed using Kendall’s tau correlation test. There is no significant correlation between the grade of OSMF with the 2^-ΔΔCT value of miRNA 21, miRNA 184, and miRNA 145 (p > 0.05). There was no significant correlation using Kendall’s tau correlation test between the grade of dysplasia with the 2^-ΔΔCT value of miRNA 21, miRNA 184, miRNA 145 (p > 0.05) (Table 8). A significant correlation was found using Kendall’s tau between leukoplakia and OSMF with the 2^-ΔΔCT value of miRNA 21 (p < 0.005) (Table 9).
Table 8.
Correlation of grade of OSMF lesion with 2^-ΔΔct value of miRNA 21, miRNA 184, miRNA 145
| Type of OSMF | miRNA 21 | Correlation coefficient (r); p value | miRNA 184 | Correlation coefficient (r); p value | miRNA 145 | Correlation coefficient (r); p value |
|---|---|---|---|---|---|---|
| Advanced OSMF (6) | 1.387 ± 0.013 | 0.098; 0.715 | 1.367 ± 0.112 | 0.050; 0.855 | 1.419 ± 0.031 | 0.000; 1.000 |
| Moderately advanced OSMF (5) | 1.383 ± 0.019 | 1.371 ± 0.003 | 1.420 ± 0.035 | |||
| Hyperkeratosis with Mild Epithelial Dysplasia (4) | 1.397 ± 0.007 | 0.407; 0.071 | 1.371 ± 0.013 | 0.135; 0.547 | 1.416 ± 0.008 | 0.067; 0.764 |
| Hyperkeratosis with Moderate Epithelial Dysplasia (7) | 1.388 ± 0.009 | 1.364 ± 0.014 | 1.417 ± 0.031 | |||
| Hyperkeratosis with Severe Epithelial Dysplasia (3) | 1.367 ± 0.016 | 1.353 ± 0.002 | 1.427 ± 0.043 |
Table 9.
Correlation of hyperkeratosis with epithelial dysplasia + OSMF, OSMF alone, epithelial dysplasia alone with 2^-ΔΔct value of miRNA 21, miRNA 184, miRNA 145
| Dysplasia + OSMF | miRNA21 | Correlation coefficient (r); p value | miRNA184 | Correlation coefficient (r); p value | miRNA145 | Correlation coefficient (r); p value |
|---|---|---|---|---|---|---|
| Hyperkeratosis with Epithelial Dysplasia + OSMF (7) | 1.361 ± 0.106 | 0.524; 0.049* | 1.351 ± 0.005 | 0.429; 0.176 | 1.423 ± 0.024 | 0.143; 0.652 |
| OSMF alone (4) | 1.354 ± 0.028 | 0.183; 0.718 | 1.365 ± 0.012 | 0.667; 0.174 | 1.401 ± 0.046 | 1.000; 0.627 |
| Epithelial dysplasia alone (7) | 1.377 ± 0.043 | 0.232; 0.629 | 1.418 ± 0.024 | 0.134; 0.356 | 1.386 ± 0.035 | 0.235; 0.106 |
Expression levels of circulating exosomal miRNAs—miRNA 21, miRNA 184, and miRNA 145 with OSCC grading and staging—were compared using the Kendall’s tau correlation test. There is a significant positive correlation between tumour grade with the 2^-ΔΔCT value of miRNA 21 (p = 0.007), but there was no significant correlation of tumour grade with the 2^-ΔΔCT value of miRNA 184, miRNA 145 (p > 0.05) (Table 10). There was no significant correlation using Kendall’s tau correlation test between tumour staging with the 2^-ΔΔCT value of miRNA 21, miRNA 184, and miRNA 145 (p > 0.05) (Table 11).
Table 10.
Correlation of tumour grade with 2^-ΔΔct value of miRNA 21, miRNA 184, miRNA 145
| Tumour Grading | miRNA21 | Correlation coefficient (r); p value | miRNA184 | Correlation coefficient (r); p value | miRNA145 | Correlation coefficient (r); p value |
|---|---|---|---|---|---|---|
| PDSCC (1) | 1.384 | 0.059; 0.07* | 1.369 | 0.117; 0.558 | 1.436 | − 0.049; 0.807 |
| MDSCC (5) | 1.369 ± 0.021 | 1.406 ± 0.021 | 1.444 ± 0.035 | |||
| WDSCC (15) | 1.367 ± 0.024 | 1.386 ± 0.026 | 1.432 ± 0.031 |
Table 11.
Correlation of tumour staging with 2^-ΔΔct value of miRNA 21, miRNA 184, miRNA 145
| Tumour staging | miRNA21 | Correlation coefficient (r); p value | miRNA184 | Correlation coefficient (r); p value | miRNA145 | Correlation coefficient (r); p value |
|---|---|---|---|---|---|---|
| Stage 1 (1) | 1.382 | 0.178; 0.258 | 1.386 | 0.119; 0.540 | 1.443 | 0.059; 0.759 |
| Stage 2 (4) | 1.361 ± 0.028 | 1.398 ± 0.020 | 1.428 ± 0.047 | |||
| Stage 3 (1) | 1.346 ± 0.028 | 1.381 ± 0.015 | 1.445 ± 0.028 | |||
| Stage 4 (11) | 1.352 ± 0.020 | 1.368 ± 0.031 | 1.449 ± 0.028 |
Discussion
Oral potentially malignant disorders are defined as “any oral mucosal abnormality that is statistically associated with increased risk of developing oral cancer” [28]. Oral submucous fibrosis, leukoplakia, and erythroplakia are the most prevalent in the Indian population. Oral squamous cell carcinoma is one of the most common cancers in South Asian countries, especially India [29]. MTR across all OPMD groups is reported to be 7.9% by Iocca 2020. Proliferative verrucous leukoplakia has been reported to have the highest MTR of 49.5%, followed by oral erythroplakia at 33.1%, oral leukoplakia at 5.7–9.5%, OSMF at 5.2–6%, oral lichenoid lesions at 3.8%, and oral lichen planus 1.4% reported by Iocca 2020 [2, 30]. A 5.5-year follow-up study by Nevanpaa et al. showed 10.9% of leukoplakia patients underwent a malignant transformation to OSCC. Oral leukoplakia patients had a 44.7-fold higher risk of developing OSCC [31, 32]. Considering the prevalence and malignant transformation rate, leukoplakia, OSMF, and leukoplakia and OSMF were only included in the case group. The prevalence of the OSMF with leukoplakia is 7 to 43%, and the malignant transformation rate of leukoplakia and OSMF varies from 3 to 19% by Hande et al. [33]. The present study showed the distribution of lesions in the case group showed 38.8% of patients with a combination of leukoplakia and OSMF and leukoplakia group, this is inconcordance with the similar results by Ho et al. [34]. This may be because of the combination of habits of smokeless tobacco and smoking since only the South Asian population have a high frequency of this combination of habits. The atrophic epithelium becomes progressively transformed into cancerous tissue as the potential carcinogens increase [35]. This suggests that more attention and research on the leukoplakia and OSMF are required to understand the underlying pathogenesis.
Exosomal miRNAs are epigenetic biomarkers that show promising stable tools for the diagnosis of oral lesions [36]. This study was done to understand the expression level of plasma circulating exosomal miRNA in the case group (leukoplakia, OSMF, leukoplakia and OSMF). Our previous systematic review by Uma Maheswari and Pratibha Ramani suggested salivary miRNA 184 and miRNA 21 were upregulated in the case group and miRNA 145 was downregulated, proving that these miRNAs were significant as a diagnostic biomarker for leukoplakia and OSMF [18]. Considering the quantity and stability of circulating exosomes, we aimed to quantify the expression level of circulating exosomal miRNAs miRNA 21, miRNA 184, and miRNA 145 in patients with the case group (leukoplakia, OSMF, leukoplakia and OSMF) when compared to healthy controls and OSCC.
A variety of cancers, including OSCC and head and neck cancers, were found to be associated with dysregulated expression of miRNA 21. In our study, miRNA 21 showed significant upregulation of the mean CT value for the case group (leukoplakia, OSMF, leukoplakia and OSMF) when compared to the healthy group (p = 0.000). This is consistent with the previous study on miR 21 and miR 184 by Zahran et al., which showed the highest levels in OSCC, followed by OPMD without dysplasia, OPMD with dysplasia, recurrent aphthous ulcers, and healthy controls [37]. The correlation of miRNA 21 was found to be statistically significant in the histopathological grading of the tumour; this was similar to a meta-analysis by Dioguardi et al. [38]. It is also hypothesised that miRNA 21 overexpression contributes to OSCC carcinogenesis and invasiveness by targeting DKK2 through the Wnt/b-catenin pathway. Programmed cell death 4 (PDCD4), phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a matrix metalloproteinase inhibitor RECK, and PTEN have all been identified as targets of miRNA 21 in studies [39, 40]. Through the AKT (‘protein kinase B’, PKB) and ERK1/2 (extracellular signal-regulating kinase) pathways, PTEN can affect cell invasiveness when it is targeted. PDCD4 (programmed cell death 4) could be downregulated by miRNA 21 at the posttranscriptional level leading to inhibition in the production of tumour suppressor proteins [41]. Hence, this study is not only for identification of diagnostic markers and it is also important for potential targeted therapy for these premalignant lesions.
Numerous studies have revealed that miR 184 overexpression may function as an onco-miRNA in the anti-apoptotic and proliferative processes [22]. Our study demonstrated significant upregulation of mean CT values of miRNA 184 for the case group (leukoplakia, OSMF, leukoplakia and OSMF), when compared to the healthy group (p = 0.000). A recent study on Tongue OSCC with miRNA 184 showed anti-apoptosis and tumour cell proliferation on tongue OSCC by suppressing SOX7 and c-Myc expression [24]. Consistent with previous studies, OSCC showed a 59-fold increase in miRNA 184 expression in OSCC cells when compared to matched normal cells [22]. Similar findings were made by Yu et al. about the impact of miRNA 184 on cancer cell lines, particularly Cal27, and epithelial cells through AKT signalling. The dysregulated expression of miRNA 184 suppressed the AKT pathway, which is linked with increased cell apoptosis and mortality [42]. Thus, correlating the previous studies and our results miRNA 184 could be a prognostic biomarker in leukoplakia and OSMF, but elaborate research needs to be done in order to establish malignant transformation of leukoplakia and OSMF.
Tumour suppression by miRNA 145 is commonly due to the silencing of specific genes during the chromosome rearrangements that accompany malignant transformation; miRNA 145 showed significant downregulation of mean CT values among the case group (leukoplakia, OSMF, leukoplakia and OSMF) group which showed high CT values, indicating upregulation of the gene expression when compared to healthy (p = 0.000). This downregulation of miRNA 145 might be due to the dysregulation of MDM2, a p53 target and an oncogene that is overexpressed in many human cancers and promotes cellular transformation [43, 44]. As a result of miRNA 145, p53, p21, and BAX are upregulated when MDM2 is downregulated leading to tumour cell proliferation. A previous study on miRNA 145 suppresses MUC1 can lead to upregulation in B-catenin, targeting miRNA 21 leading to upregulation [25, 45]. Thus, b-catenin pathway controlled miRNA 21 production via STAT3, increasing cell invasion and proliferation [46, 47]. miRNA 21 and miRNA 184, on the other hand, appear to work synergistically on several common pathways, such as the AKT/mTOR pathway, increasing cell proliferation and preventing apoptosis [39, 42]. miRNA 145 shows promising novel therapeutic targets because of the regulation of MDM2-p53 loop and B-Catenin by miRNA 145 in leukoplakia and OSMFs. A similar study on tongue SCC showed miR-145 was consistently downregulated in TSCC tissues leading to dysregulation of apoptosis and oxidative stress via PI3K, AKT, and phosphorylated-AKT pathways [48].
Our study showed circulating exosomal miRNA 21 and miRNA 184 with increased fold change in the case group (leukoplakia, OSMF, leukoplakia and OSMF). Similar to this, a study found a substantial relationship between salivary miRNA 21 and severe dysplasia, with a 3.6-fold rise, and salivary miRNA 31 and severe dysplasia, with a 2.5-fold increase [49]. Previous research was based on salivary cell-free miRNA, whereas in our study the source of miRNA was circulating exosomal miRNA. The correlation of miRNA 21 with leukoplakia and OSMF was statistically significant (p = 0.049); this may be because of the synergistic effect of the leukoplakia and oral submucous fibrosis leading to malignant transformation. This is in concordance with the study on miRNA 21 in patients with OSMF, and leukoplakia showed significant association. They also suggested that it might be because of the varied combination of tobacco chewing and smoking habits [50].
Correlating the association type of case group (leukoplakia, OSMF, leukoplakia and OSMF) with expression CT value of plasma circulating exosomal miRNAs, miRNA 21 showed a significant correlation with the leukoplakia and OSMF group (p = 0.049), whereas no significant correlation was evident in OSMF group (p > 0.005) and leukoplakia group (p > 0.005). No significant correlation between miRNA21, miRNA 184, and miRNA 145 was evident with the grades of dysplasia (p > 0.005) and OSMF (p > 0.005). A previous study by Kaneuin et al. showed 73 dysregulated miRNAs with miRNA 21 and miRNA 31 were most frequently examined and expressed in leukoplakia [51]. Previous research on the miRNA profile linked to the development of oral cancer from leukoplakia was evaluated, and upregulation in miRNA 21, miR-181b, and miR-345 expression was linked to increasing degrees of dysplasia [52]. Expression of miRNA 21 has been consistently upregulated in the OSMF group with chewing habits when compared to the healthy group. Studies on miRNA in leukoplakia and OSMF groups have been done individually, but there are no studies on accessing the miRNA expression level in leukoplakia and OSMF [50].
We correlated the fold change of plasma circulating exosomal miRNAs: miRNA 21, miRNA 184, and miRNA 145 with histological grading of OSCC; a positive correlation with statistically significant was evident with miRNA 21 (p = 0.007). Based on the pTNM staging of OSCC, no statistically significant differences were evident between the groups (p > 0.005). Significantly more miR-21 were found in the blood and primary OSCC cells of OSCC patients. According to evidence, downregulated PTEN and BCL2-6 tumour suppressors may work in conjunction with miRNA 21-rich exosomes to increase cell proliferation and invasion [53]. miRNA panel was strongly associated with pTNM stage IV and PDSCC histologic grading. The high-risk group had a 23-fold higher mortality risk than the low-risk group, with a median time to recurrence of 6 months and time to death of 11 months [54]. A recent study on miRNA 21 in OSCC samples also showed a significant correlation with the histological grading of tumour [55]. MiRNA 21 shows a strong correlation with histopathological grading of OSCC suggesting that miRNA 21 can be potential prognostic markers for OSCC to predict the overall survival and disease-free survival of OSCC patients.
The limitations of our study include the time period of follow-up details taken in order to analyse the malignant transformation of the patients. The maximum follow-up was noted to be 6 months. Varied malignant transformation rate among different OPMDs makes it biased when compiled together as OPMDs; further studies on between OPMDs need to be done. Another limitation is its applicability to other patient populations in other areas of the world. Hence, further research, in a large sample size and increased follow-up time period would provide a better insight regarding correlation of and survival. Molecular docking of miRNA protein can also be done in order to emphasise the role on miRNA as a possible therapeutic target for the case group (leukoplakia, OSMF, leukoplakia and OSMF) and OSCC. Future research is needed on identifying the underlying signalling pathways which can lead to a better understanding of the molecular pathogenesis of leukoplakia and OSMF.
Conclusion
Plasma circulating exosomal miRNAs, miRNA 21, and miRNA 184 were found to have a definite upregulated expression in the case group (leukoplakia, OSMF, leukoplakia and OSMF) group than the apparently healthy individuals and downregulated expression when compared to the OSCC group in the Indian population. The plasma exosomal expression of miRNA 145 was found to be downregulated in the case group (leukoplakia, OSMF, leukoplakia and OSMF) group when compared to the apparently healthy individuals and upregulated when compared to OSCC in the Indian population. This could be because of the synergistic role and of miRNA 21, miRNA 184, miRNA 145 via common signalling pathways such as AKT/mTOR/PI3K pathway, increasing tumour cell proliferation and preventing apoptosis. Thus, these three circulating exosomal miRNAs, miRNA 21, miRNA 184, and miRNA 145 could be a novel panel of biomarkers to predict the biological behaviour and malignant transformation of leukoplakia and OSMF. Altered expression levels of plasma circulating exosomal miRNA levels indicate that these can be a potential prognostic biomarker in the malignant transformation of oral potentially malignant disorders.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- 3′UTR
3′ Untranslated binding region
- AKT
Protein kinase B
- cDNA
Complementary DNA
- CDK6
Cyclin-dependent kinase 6
- CTC
Circulating tumour cells
- cfDNA
Cell-free tumour DNA
- ctDNA
Circulating tumour DNA
- ERK
Extracellular signal-regulated kinase
- miRNA
MicroRNA
- MDM2
Murine double minute 2
- MUC1
Metastasis gene mucin 1
- OED
Oral epithelial dysplasia
- OPMDs
Oral potentially malignant disorders
- OSCC
Oral squamous cell carcinoma
- OSMF
Oral submucous fibrosis
- PBS
Phosphate buffer saline
- PCR
Polymerase chain reaction
- PDCD
Programmed cell death 4
- PI3K
Phosphoinositide 3-kinase
- PTEN
Phosphatase and tensin homolog deleted on chromosome 10
- pTNM
Pathological tumour (T), nodal (N), metastasis (M)
- RISC
RNA-induced silencing complex
- WHO
World Health Organisation
Author contribution
Dr. Dinesh Yasothkumar involved in investigation, resources, writing—original draft, Dr. Pratibha Ramani took part in conceptualisation, validation, writing—review and editing, supervision, project administration, Dr. Selvaraj Jayaraman took part in methodology, software, formal analysis, Dr. Karthikeyan Ramalingam involved in validation, data curation, visualisation, Dr. W. M. Tilakaratne involved in study guidance and manuscript review.
Funding
This study was not supported by any funding.
Data Availability
The data supporting the findings are not available openly and are available with corresponding author upon reasonable request.
Declarations
Conflict of interest
There are no financial or non-financial interests that are directly or indirectly related to the work submitted for publication.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Consent for Publication
Consent for publication was obtained for every individual person’s data included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar S et al (2015) Prevalence and risk factors for oral potentially malignant disorders in Indian population. Adv Prev Med 2015:208519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iocca O et al (2020) Potentially malignant disorders of the oral cavity and oral dysplasia: a systematic review and meta-analysis of malignant transformation rate by subtype. Head Neck 42:539–555 [DOI] [PubMed] [Google Scholar]
- 3.Sabarinath B, Sriram G, Saraswathi TR, Sivapathasundharam B (2011) Immunohistochemical evaluation of mast cells and vascular endothelial proliferation in oral submucous fibrosis. Indian J Dent Res 22:116–121 [DOI] [PubMed] [Google Scholar]
- 4.Shih Y-H, Wang T-H, Shieh T-M, Tseng Y-H (2019) Oral submucous fibrosis: a review on etiopathogenesis, diagnosis, and therapy. Int J Mol Sci 20:2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitiyage G, Tilakaratne WM, Tavassoli M, Warnakulasuriya S (2009) Molecular markers in oral epithelial dysplasia: review. J Oral Pathol Med 38:737–752 [DOI] [PubMed] [Google Scholar]
- 6.Lauritano D et al (2019) Liquid biopsy in head and neck squamous cell carcinoma: prognostic significance of circulating tumor cells and circulating tumor DNA. A systematic review. Oral Oncol 97:7–17 [DOI] [PubMed] [Google Scholar]
- 7.Yang W-Y et al (2020) Liquid biopsy in head and neck squamous cell carcinoma: circulating tumor cells, circulating tumor DNA, and exosomes. Expert Rev Mol Diagn 20:1213–1227 [DOI] [PubMed] [Google Scholar]
- 8.Yap T et al (2020) Extracellular vesicles in oral squamous cell carcinoma and oral potentially malignant disorders: a systematic review. Int J Mol Sci 21:1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munson PB, Shukla A (2018) Introduction to exosomes and cancer. Diagnostic and Therapeutic Applications of Exosomes in Cancer. Academic Press, London, pp 1–10 [Google Scholar]
- 10.Lima LG, Möller A (2018) Biodistribution of cancer-derived exosomes. Diagnostic and Therapeutic Applications of Exosomes in Cancer. Academic Press, London, pp 175–186 [Google Scholar]
- 11.Hochendoner P, Zhao Z, He M (2018) Diagnostic potential of tumor exosomes. Diagnostic and Therapeutic Applications of Exosomes in Cancer. Academic Press, London, pp 161–1735 [Google Scholar]
- 12.Lu Y et al (2021) The emerging role of exosomes in oral squamous cell carcinoma. Front Cell Dev Biol 9:628103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakha S, Muramatsu T, Ueda K, Inazawa J (2016) Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci Rep 6:38750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann L et al (2020) The emerging role of exosomes in diagnosis, prognosis, and therapy in head and neck cancer. Int J Mol Sci 21:4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilsiz N (2020) Role of exosomes and exosomal microRNAs in cancer. Future Sci OA 6:FSO465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallo A, Tandon M, Alevizos I, Illei GG (2012) The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS ONE 7:e30679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sempere LF (2019) MicroRNA as biomarkers in cancer diagnostics and therapy
- 18.Maheswari TNU, Venugopal A, Sureshbabu NM, Ramani P (2018) Salivary micro RNA as a potential biomarker in oral potentially malignant disorders: a systematic review. Tzu Chi Med J 30:55–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas J et al (2012) A novel source for miR-21 expression through the alternative polyadenylation of VMP1 gene transcripts. Nucleic Acids Res 40:6821–6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surina S et al (2021) miR-21 in human cardiomyopathies. Front Cardiovasc Med 8:767064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chan JA, Krichevsky AM, Kosik KS (2005) MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65:6029–6033 [DOI] [PubMed] [Google Scholar]
- 22.Wong T-S et al (2008) Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res 14:2588–2592 [DOI] [PubMed] [Google Scholar]
- 23.Wong T-S, Ho W-K, Chan JY-W, Ng RW-M, Wei WI (2009) Mature miR-184 and squamous cell carcinoma of the tongue. SciWorldJ 9:130–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen D et al (2018) miR-184 promotes cell proliferation in tongue squamous cell carcinoma by targeting SOX7. Oncol Lett 16:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sachdeva M et al (2009) p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A 106:3207–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ (2003) Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res 1:882–891 [PubMed] [Google Scholar]
- 27.Pagliuca A et al (2013) Analysis of the combined action of miR-143 and miR-145 on oncogenic pathways in colorectal cancer cells reveals a coordinate program of gene repression. Oncogene 32:4806–4813 [DOI] [PubMed] [Google Scholar]
- 28.Warnakulasuriya S et al (2020) Oral potentially malignant disorders: a consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis 27:1862–1880 [DOI] [PubMed] [Google Scholar]
- 29.Petti S (2009) Lifestyle risk factors for oral cancer. Oral Oncol 45:340–350 [DOI] [PubMed] [Google Scholar]
- 30.Mello FW et al (2018) Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med 47:633–640 [DOI] [PubMed] [Google Scholar]
- 31.Nevanpää TT, Terävä AE, Laine HK, Rautava J (2022) Malignant transformation of oral epithelial dysplasia in Southwest Finland. Sci Rep 12:8261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murthy V et al (2022) Malignant transformation rate of oral submucous fibrosis: a systematic review and meta-analysis. J Clin Med Res 11:1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hande A et al (2018) Role of hypoxia in malignant transformation of oral submucous fibrosis. J Datta Meghe Inst Med Sci Univ 13:38 [Google Scholar]
- 34.Ho MWS (2013) Patients with both oral leukoplakia and oral submucous fibrosis had a higher rate of malignant transformation compared to patients with oral leukoplakia or oral submucous fibrosis alone. J Evid Based Dent Pract 13:183–184 [DOI] [PubMed] [Google Scholar]
- 35.Jayasooriya PR, Nadeeka Jayasinghe KA, Mudiyanselage Tilakaratne W (2011) Relationship between thickness of fibrosis and epithelial dysplasia in oral submucous fibrosis. J Investig Clin Dent 2:171–175 [DOI] [PubMed] [Google Scholar]
- 36.Raisch J, Darfeuille-Michaud A, Nguyen HTT (2013) Role of microRNAs in the immune system, inflammation and cancer. World J Gastroenterol 19:2985–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zahran F, Ghalwash D, Shaker O, Al-Johani K, Scully C (2015) Salivary microRNAs in oral cancer. Oral Dis 21:739–747 [DOI] [PubMed] [Google Scholar]
- 38.Dioguardi M et al (2022) MicroRNA-21 expression as a prognostic biomarker in oral cancer: systematic review and meta-analysis. Int J Environ Res Public Health 19:3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han M et al (2012) Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS ONE 7:e39520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Z-L, Wang H, Liu J, Wang Z-X (2013) MicroRNA-21 (miR-21) expression promotes growth, metastasis, and chemo- or radioresistance in non-small cell lung cancer cells by targeting PTEN. Mol Cell Biochem 372:35–45 [DOI] [PubMed] [Google Scholar]
- 41.Asangani IA et al (2008) MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27:2128–2136 [DOI] [PubMed] [Google Scholar]
- 42.Yu J et al (2008) MicroRNA-184 antagonizes microRNA-205 to maintain SHIP2 levels in epithelia. Proc Natl Acad Sci U S A 105:19300–19305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J et al (2013) Loss of microRNA-143/145 disturbs cellular growth and apoptosis of human epithelial cancers by impairing the MDM2-p53 feedback loop. Oncogene 32:61–69 [DOI] [PubMed] [Google Scholar]
- 44.Zhang BG et al (2012) microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep 27:1019–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawakita A et al (2014) MicroRNA-21 promotes oral cancer invasion via the Wnt/β-catenin pathway by targeting DKK2. Pathol Oncol Res 20:253–261 [DOI] [PubMed] [Google Scholar]
- 46.Han L et al (2012) MicroRNA-21 expression is regulated by β-catenin/STAT3 pathway and promotes glioma cell invasion by direct targeting RECK. CNS Neurosci Ther 18:573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.González-Moles MA, Ruiz-Ávila I, Gil-Montoya JA, Plaza-Campillo J, Scully C (2014) β-catenin in oral cancer: an update on current knowledge. Oral Oncol 50:818–824 [DOI] [PubMed] [Google Scholar]
- 48.Xin Z, Tong Z, Tan J, Liu C (2021) MicroRNA-145-5p aggravates cell apoptosis and oxidative stress in tongue squamous cell carcinoma. Exp Ther Med 21:373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uma Maheswari TN, Nivedhitha MS, Ramani P (2020) Expression profile of salivary micro RNA-21 and 31 in oral potentially malignant disorders. Braz Oral Res 34:e002 [DOI] [PubMed] [Google Scholar]
- 50.Prasad SR, Pai A, Shyamala K, Yaji A (2020) Expression of salivary miRNA 21 in oral submucous fibrosis (OSMF): an observational study. Microrna 9:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaunein N et al (2021) A systematic review of MicroRNA signatures associated with the progression of leukoplakia with and without epithelial dysplasia. Biomolecules 11:1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cervigne NK et al (2009) Identification of a microRNA signature associated with progression of leukoplakia to oral carcinoma. Hum Mol Genet 18:4818–4829 [DOI] [PubMed] [Google Scholar]
- 53.Chen C-M et al (2021) Exosome-derived microRNAs in oral squamous cell carcinomas impact disease prognosis. Oral Oncol 120:105402 [DOI] [PubMed] [Google Scholar]
- 54.Yoon AJ et al (2020) MicroRNA-based risk scoring system to identify early-stage oral squamous cell carcinoma patients at high-risk for cancer-specific mortality. Head Neck 42:1699–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahmood N et al (2019) Circulating miR-21 as a prognostic and predictive biomarker in oral squamous cell carcinoma. Pak J Med Sci Q 35:1408–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings are not available openly and are available with corresponding author upon reasonable request.