Abstract
Interstitial lung abnormalities (ILA), incidental findings on computed tomography scans, have raised concerns due to their association with worse clinical outcomes. Our meta-analysis, which included studies up to April 2023 from PubMed/MEDLINE, Embase, and Cochrane Library, aimed to clarify the impact of ILA on mortality, lung cancer development, and complications from lung cancer treatments. Risk ratios (RR) with 95% confidence intervals (CI) were calculated for outcomes. Analyzing 10 studies on ILA prognosis and 9 on cancer treatment complications, we found that ILA significantly increases the risk of overall mortality (RR 2.62, 95% CI 1.94–3.54; I2 = 90%) and lung cancer development (RR 3.85, 95% CI 2.64–5.62; I2 = 22%). Additionally, cancer patients with ILA had higher risks of grade 2 radiation pneumonitis (RR 2.28, 95% CI 1.71–3.03; I2 = 0%) and immune checkpoint inhibitor-related interstitial lung disease (RR 3.05, 95% CI 1.37–6.77; I2 = 83%) compared with those without ILA. In conclusion, ILA significantly associates with increased mortality, lung cancer risk, and cancer treatment-related complications, highlighting the necessity for vigilant patient management and monitoring.
Keywords: Immune checkpoint inhibitors, Interstitial lung diseases, Lung neoplasms, Mortality, Radiation pneumonitis
Subject terms: Epidemiology, Risk factors, Respiratory signs and symptoms, Respiratory tract diseases
Introduction
Interstitial lung disease (ILD) is characterized by inflammation and fibrosis of the lung interstitium, resulting in progressive lung damage and compromised respiratory function1. Within the spectrum of ILD, interstitial lung abnormalities (ILA) have emerged as incidental radiographic findings on computed tomography (CT) scans of the lungs2. The prevalence of ILA is 2–7% in the general population3–7 and 4–9% in individuals with a history of smoking3,7–11. Notably, a recent meta-analysis reported an ILA prevalence of 26% in familial pulmonary fibrosis cohorts7. Several risk factors have been proposed for the development of ILA, including advanced age, male sex, lower forced vital capacity (FVC)% predicted, smoking history, genetic mutations (e.g., MUC5B), and exposure to occupational and environmental pollutants3,4,7,12–15.
Several studies have reported an association between ILA and various clinical outcomes, including mortality, development of lung cancer, changes in lung function, and disease progression3,16–19. Additionally, there is increasing awareness regarding the influence of ILA on cancer treatment-related complications, including radiation therapy, immune checkpoint inhibitors (ICI), and surgery20–22. In 2020, the Fleischner Society standardized the definition of ILA as incidental findings on CT imaging, characterized by nondependent interstitial abnormalities involving more than 5% of any lung zone2. The standardized definition of ILA demonstrates the growing recognition of their distinct nature and emphasizes the importance of evaluating the clinical impact and implications of ILA for patient outcomes. Therefore, we aimed to conduct a comprehensive meta-analysis to thoroughly examine the association between ILA and various clinical outcomes such as mortality, development of lung cancer, and cancer treatment-related complications. We hypothesized that patients with ILA would demonstrate a poorer prognosis than those without ILA.
Methods
Literature search and study inclusion
This meta-analysis followed the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement23. The study protocol was registered in PROSPERO (CRD42023437679). A thorough literature search was performed using electronic databases including PubMed/MEDLINE, Embase, and the Cochrane Library to identify relevant articles. The search encompassed articles published between the inception of these databases and April 2023. The search strategy was developed using appropriate keywords, and specific search strategies are provided in Supplementary Tables S1–S3. Furthermore, we included all relevant studies cited in a previous comprehensive review2,24. To ensure comprehensive coverage, we manually searched the reference lists of relevant original and review articles to identify additional eligible studies.
The inclusion criteria for the studies were as follows: (1) randomized controlled trials, cohort studies, or case–control studies evaluating ILA; (2) clinical outcomes including mortality, lung cancer development, changes in lung function, and lung cancer treatment-related complications; (3) studies written in English; and (4) participants aged ≥ 18 years. The exclusion criteria were as follows: (1) animal studies or in vitro studies; (2) case reports or case series with a small sample size (less than 20); (3) conference abstracts or posters without full-text availability; and (4) duplicate studies.
While we conducted the literature search together, it should be noted that the studies included to examine cancer treatment-related complications focused exclusively on patients with cancer. To avoid potential bias in evaluating the overall prognosis of ILA based solely on this subgroup, we separately analysed these studies to specifically assess the complications related to cancer treatment in patients with ILA. In our study, when multiple studies were included in a single paper and their results were reported separately, each study was treated as an individual entity for the analysis. Even if the studies or populations were the same, we considered them separate studies if there were changes in the study pool or if different outcomes were reported.
Definition of ILA
According to the definition established by the Fleischner Society, ILA are defined as incidental findings on CT imaging, characterized by non-dependent changes that involve more than 5% of any lung zone, including ground-glass or reticular abnormalities, traction bronchiectasis, architectural distortion, honeycombing, and non-emphysematous cysts2. However, it is important to note that previous studies conducted before 2020 had different criteria for defining ILA, including additional lesions. Here, we provide detailed descriptions of the specific ILA definitions used in each study. In our study, “indeterminate ILA” referred to cases where radiological findings are consistent with ILA, but the extent of the lesion measures less than 5%2,18, although there is currently no universally accepted definition for it.
Study design and quality assessment
Two independent reviewers screened the titles and abstracts of the identified articles based on the predetermined criteria. Full-text articles that met the eligibility criteria were assessed for inclusion. Disagreements between the reviewers were resolved through discussion, and a third reviewer was consulted if needed for consensus.
Data extraction from the included studies followed a standardized approach using a predefined form. The extracted information included the study characteristics, patient demographics, ILA characteristics, clinical outcomes (mortality, lung cancer development, hospitalization, and changes in lung function), and cancer treatment-related complications. To assess the cause of death, we categorized the outcomes into respiratory, cardiovascular (CV), and lung cancer-related mortalities. Cancer treatment-related outcomes, such as radiation pneumonitis (RP), immune checkpoint inhibitor-related interstitial lung disease (ICI-ILD), and postoperative pulmonary complications (PPC), were assessed using the Common Terminology Criteria for Adverse Events guidelines. RP severity was further classified into ≥ grade (Gr) 2 and ≥ Gr 3 for detailed analysis.
The quality of the included studies was assessed using the Newcastle–Ottawa Scale25, which evaluates selection (representativeness, selection of the non-exposed cohort, ascertainment of exposure, outcome of interest not present at start of study), comparability, and outcome (assessment of outcome, length of follow-up, and adequacy of follow-up). Scores higher than 7 indicate low risk of bias, scores ranging from 5 to 7 indicate moderate risk, and scores below 5 indicate high risk. Two independent reviewers conducted the assessment with a third-party arbitrator involved in resolving disagreements and ensuring consensus.
Statistical analysis
To compare the prognosis between the two groups, the risk ratio (RR) with the corresponding 95% confidence interval (CI) was calculated for dichotomous outcomes. The heterogeneity of the included studies was assessed using the I2 statistic. I2 values ≤ 40% were considered insignificant, values from 30 to 60% indicated moderate heterogeneity, values from 50 to 90% denoted substantial heterogeneity, and values ≥ 75% indicated considerable heterogeneity. Due to the heterogeneity of the studies, a random-effects model was employed to estimate effect sizes. Subgroup analyses were performed based on the study population, specifically comparing the general population and high-risk group for lung cancer in terms of overall mortality. Subgroup analyses of the other outcomes could not be conducted because of the limited number of included studies. Sensitivity analyses for overall mortality were performed based on the study design (cohort vs. case–control) and ILA definition of the Fleischner Society. Publication bias was not evaluated using funnel plots, given the limited number of included studies (n < 10). Statistical significance was defined as a P value < 0.05. All data analyses were conducted using Review Manager version 5.4.1.
Results
Description of included studies
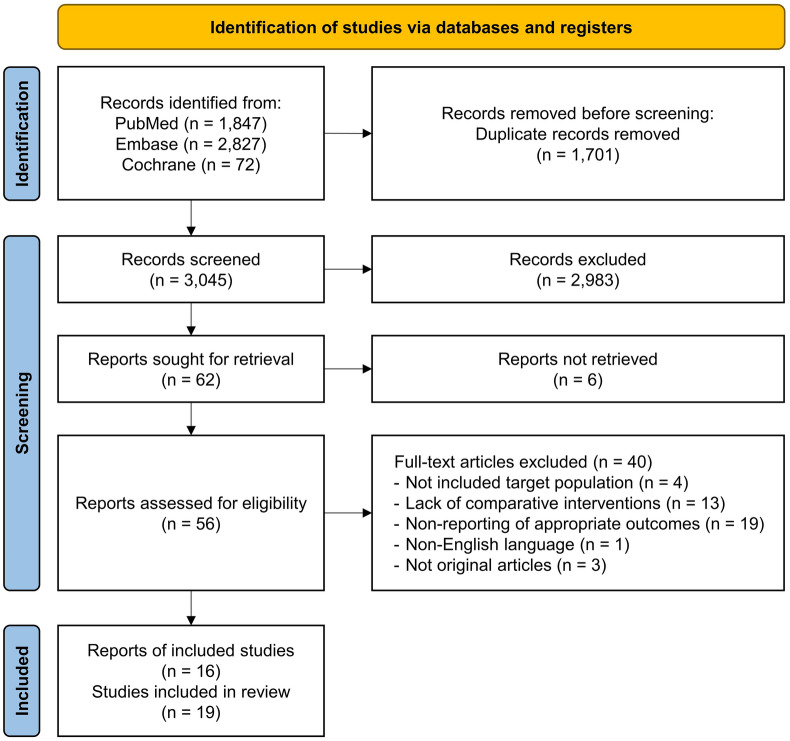
The search identified 4746 records. After removing duplicates and screening, a total of 19 studies from 16 articles were included (Fig. 1). Of the 19 studies, 10 were studies on the prognosis of ILA, and the remaining 9 studies were on cancer treatment-related complications (Table 1).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram illustrating the study selection process.
Table 1.
Characteristics of the included studies for prognosis and cancer treatment-related complications.
| Study, year | Design | Site | Study population | ILA definition | Sample sizea | Age, years | Male | Follow-up, year | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| Prognosis | ||||||||||
| Putman, 20163 | FHS | Cohort study | USA | Health screening | Compatible with Fleischner Society definitionb, but including the presence of centrilobular nodularity lesion | 2633 (177/1086) |
ILA: 70 ± 12 Non-ILA: 56 ± 11 |
ILA: 88 (50) Non-ILA: 695 (51) |
4 (3–5) | Mortality |
| AGES-Reykjavik | Cohort study | Iceland | Birth cohort | 5320 (378/1726) |
ILA: 78 ± 6 Non-ILA: 76 ± 5 |
ILA: 206 (54) Non-ILA: 1306 (41) |
9 (7–10) | Mortality | ||
| COPDGene | Case–control | USA | Smoking | 2068 (156/739) |
ILA: 64 ± 9 Non-ILA: 60 ± 9 |
ILA: 76 (49) Non-ILA: 609 (52) |
7 (7–7) | Mortality | ||
| ECLIPSE | Cohort study | 12 countries | Smoking | 1670 (157/985) |
ILA: 64 ± 8 Non-ILA: 62 ± 7 |
ILA: 116 (74) Non-ILA: 346 (66) |
3 (3–3) | Mortality | ||
| Ash, 20179 | COPDGene | Case–control | USA | Smoking | Non-dependent changes affecting more than 10% of any lung zone, including reticular or GGA, diffuse centrilobular nodularity, non-emphysematous cysts, HC, or traction BE | 8266 (1069/NR) | 60 ± 9 | 4256 (52) | 6 ± 2 | Mortality |
| Hoyer, 201811 | DLCST | Cohort study | Denmark | Lung cancer high risk | GGA, HC, reticulation, pleural nodules, centrilobular nodules, paraseptal/subpleural nodules, mosaic attenuation, and mass | 1990 (332/NR) | 63 ± 6 | 956 (56) | 12 (11–12) | Mortality, cause specific mortality, lung cancer incidence, hospitalization |
| Axelsson, 202026 | AGES-Reykjavik | Cohort study | Iceland | Birth cohort | Compatible with Fleischner Society definitionb, but including the presence of centrilobular nodularity lesion | 5270 (375/1712) |
ILA: 78 ± 6 Non-ILA: 76 ± 5 |
ILA: 205 (55) Non-ILA: 1296 (41) |
9 (7–10) | Lung cancer incidence |
| Lee 1, 202217 | Cohort study | South Korea | Heath screening | Compatible with Fleischner Society definitionb | 840 (55/NR) | 59 ± 7 | 564 (67) | 11 ± 1 | Mortality, cause specific mortality, lung cancer incidence | |
| Lee 2, 202218 | Cohort study | South Korea | Heath screening | Compatible with Fleischner Society definitionb | 2765 (94/119) | 59 ± 7 | 2068 (75) | 12 (11–13) | Mortality, cause specific mortality, lung cancer incidence | |
| Patel, 202316 | CTLS | Case–control | USA | Smoking | Compatible with Fleischner Society definitionb | 1669 (41/101) | 63 ± 6 | 956 (56) | 6 ± 2 | Mortality, cause specific mortality, lung cancer incidence, hospitalization |
| Cancer treatment-related complications | ||||||||||
| Yamaguchi, 201427 | Retrospective cohort | Japan | Thoracic cancer with RTx | Compatible with Fleischner Society definitionb, but including the presence of centrilobular nodularity lesion | 62 (11) | 69 (43–86) | 57 (92) | 12 | RP ≥ Gr 2 | |
| Li, 201828 | Retrospective cohort | China | SCLC with RTx | Reticular abnormalities, traction BE, bilateral independent GGA, HC, and non-emphysematous cysts | 95 (15) | 61 (42–80) | 85 (89) | 13 (3–29) | RP ≥ Gr 2 | |
| Nakanishi, 201929 | Retrospective cohort | Japan | Advanced NSCLC with ICI | Compatible with Fleischner Society definitionb, but including the presence of centrilobular nodularity lesion | 83 (3) | 68 (34–85) | 133 (67) | 0.3 (0–1) | ICI-ILD | |
| Shimoji, 202030 | Retrospective cohort | Japan | Nonlung cancer with ICI | Compatible with Fleischner Society definitionb, but including the presence of centrilobular nodularity lesion and without any limitations on their extent | 199 (37) | 66 (20–93) | 58 (70) | NR | ICI-ILD | |
| Daido, 202231 | Retrospective cohort | Japan | Locally advanced NSCLC with ICI after CRT | Compatible with Fleischner Society definitionb, but including the presence of centrilobular nodularity lesion | 148 (56) | 74 (43–86) | 106 (72) | NR | ICI-ILD | |
| Im, 202222c | Case–control | South Korea | Underwent curative lung resection | Compatible with Fleischner Society definitionb | 300 (50) | 69 ± 7 | 266 (89) | 4 (2–5) | PPC | |
| Murata, 202220 | Retrospective cohort | Japan | Advanced or recurrent NSCLC with ICIs | Compatible with Fleischner Society definitionb | 264 (57) | 70 (63–75) | 109 (74) | 1 | ICI-ILD | |
| Jeong, 202332d | Retrospective cohort | South Korea | Unresectable NSCLC with RTx | Compatible with Fleischner Society definitionb | 201 (44) | 65 ± 7 | 188 (94) | 2 ± 1 | RP ≥ Gr 2 | |
| Ito, 202333e | Retrospective cohort | Japan | NSCLC with RTx | Compatible with Fleischner Society definitionb | 175 (64) |
ILA: 72 (60–86) Non-ILA: 71 (41–60) |
ILA: 52 (81) Non-ILA: 82 (78) |
2 (1–10) | RP ≥ Gr 2 | |
Data are expressed as mean ± standard deviation, median (interquartile range), or number (%).
aNumber (ILA/indeterminate ILA) in studies on prognosis and number (ILA) in studies on cancer treatment-related complications. bIncidental CT findings of non-dependent abnormalities, such as ground-glass or reticular abnormalities, lung distortion, traction bronchiectasis, honeycombing, and non-emphysematous cysts observed in more than 5% of any lung zone (i.e., upper, middle, and lower lung zones demarcated by the levels of the inferior aortic arch and right inferior pulmonary vein) during complete or partial chest CT examinations (e.g., abdominal or cardiac CT) where interstitial disease was not suspected. cIncluded idiopathic pulmonary fibrosis (n = 50). dIncluded indeterminate ILA (n = 24). eIncluded ILD (n = 6).
BE bronchiectasis, CRT chemoradiotherapy, CT computed tomography, GGA ground-glass abnormalities, Gr grade, HC honeycombing, ICI immune checkpoint inhibitor, ICI-ILD immune checkpoint inhibitor-related interstitial lung disease, ILA interstitial lung abnormalities, NR not recorded, NSCLC non-small cell lung cancer, PPC postoperative pulmonary complications, RP radiation pneumonitis, RTx radiation therapy, SCLC small cell lung cancer.
The methodological quality of studies assessing the prognosis of ILA was high, with a mean score of 7.1 ± 0.7 (range 6–8), and 7 out of 10 studies scoring ≥ 7. However, studies on cancer treatment-related complications had a moderate overall methodological quality, with a mean score of 5.3 ± 1.4 (Supplementary Table S4).
Among ten studies on the prognosis of ILA3,9,11,16–18,26, five focused on the general population3,17,18,26, while the remaining five specifically studied high-risk groups for lung cancer, such as individuals who smoke heavily3,9,11,16. The sample sizes ranged from 840 to 5320, with follow-up durations ranging from 3 to 12 years. The prevalence of ILA varied across the populations studied. In the general population, the prevalence of ILA was reported to be 3–7%, while that of populations at a higher risk of developing lung cancer was 2% and 17%, respectively. Six studies included indeterminate ILA, with a prevalence ranging from 4 to 59%. The definitions of ILA used in these studies varied depending on whether they were published before or after the 2020 Fleischner Society definition. Some earlier studies included centrilobular nodules or used a disease extent of 10% in their definitions. We could not find comparative studies on pulmonary function in participants with and without ILA, and combining hospitalization data was not feasible because of methodological variations.
In nine studies on cancer treatment-related complications20,22,27–33, the prevalence of ILA ranged from 4 to 38%, and the follow-up periods varied from 0 to 12 years. Four studies adhered to the Fleischner Society definition, whereas the others had minor differences in ILA criteria. Four studies examined RP27,28,32,33, and another four studied ICI-ILD20,29–31. Notably, a single study focused on PPC22, which precluded the possibility of a comprehensive meta-analysis on PPC. A meta-analysis of the relationship between indeterminate ILA and cancer treatment-related complications was limited owing to the small number of studies.
Impact of ILA on mortality
The ILA group showed a higher risk of overall mortality than the non-ILA group (RR 2.62, 95% CI 1.94–3.54), with significant heterogeneity (I2 = 90%). Subgroup analysis based on lung cancer risk consistently demonstrated increased overall mortality in the ILA group. Specifically, in the general population subgroup, the RR was even higher (RR 3.83, 95% CI 1.88–7.79), with greater heterogeneity (I2 = 95%) than that in the ILA group in the lung cancer risk population (RR 2.04, 95% CI 1.44–2.89; I2 = 80%) (Fig. 2a).
Figure 2.
Meta-analyses of overall and cause-specific mortality between ILA and non-ILA groups. (a) Overall mortality; (b) Lung cancer-related mortality; (c) Respiratory disease-related mortality; (d) Cardiovascular disease-related mortality. CI confidence interval, ILA interstitial lung abnormalities, M–H Mantel–Haenszel method.
Regarding cause-specific mortality, significantly higher rates were observed in the ILA group than those in the non-ILA group for lung cancer-related mortality (RR 4.18, 95% CI 2.86–6.10; I2 = 23%) and respiratory-related mortality (RR 11.01, 95% CI 3.84–31.59; I2 = 77%) (Fig. 2b,c). However, the difference in CV-related mortality between the ILA and non-ILA groups did not reach statistical significance (RR 3.76, 95% CI 0.92–15.31; I2 = 57%) (Fig. 2d).
Impact of indeterminate ILA on mortality
The indeterminate ILA group also exhibited higher mortality rates than the non-ILA group (RR 1.74, 95% CI 1.33–2.27; I2 = 79%) (Fig. 3a). Specifically, lung cancer-related mortality was significantly higher in the indeterminate ILA group than in the non-ILA group (RR 1.70, 95% CI 1.23–2.34; I2 = 0%) (Fig. 3b). However, there was no statistically significant difference in the risk of respiratory-related mortality between the two groups, although the RR was above 1 (RR 1.50, 95% CI 0.22–10.36; I2 = 69%) (Fig. 3c). CV-related mortality could not be included in the meta-analysis because of the limited data from only one study.
Figure 3.
Meta-analyses of overall and cause-specific mortality between the indeterminate ILA and non-ILA groups. (a) Overall mortality; (b) Lung cancer-related mortality; (c) Respiratory disease-related mortality. CI confidence interval, ILA interstitial lung abnormalities, M–H Mantel–Haenszel method.
Impact of ILA on lung cancer development
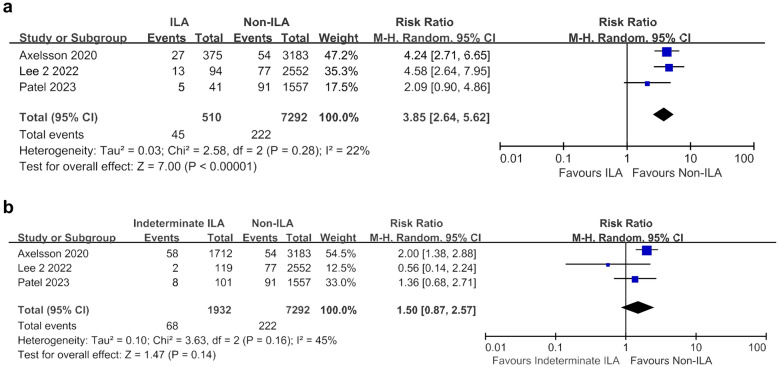
ILA were associated with a significantly higher incidence of lung cancer compared to the non-ILA group (RR 3.85, 95% CI 2.64–5.62; I2 = 22%) based on the analysis of three studies (Fig. 4a). However, the indeterminate ILA group did not show a statistically significant increase in lung cancer risk compared to the non-ILA group (RR 1.50, 95% CI 0.87–2.57; I2 = 45%) (Fig. 4b).
Figure 4.
Meta-analyses of lung cancer development. (a) Between ILA and non-ILA groups; (b) Between indeterminate ILA and non-ILA groups. CI confidence interval, ILA interstitial lung abnormalities, M–H Mantel–Haenszel method.
Impact of ILA on cancer treatment-related complications
In patients with lung cancer, the ILA group was found to be associated with a higher risk of ≥ Gr 2 RP (RR 2.28, 95% CI 1.71–3.03; I2 = 0%) and ≥ Gr 3 RP (RR 7.21, 95% CI 4.47–11.64; I2 = 0%) than the non-ILA group (Fig. 5a,b). ICI-ILD was significantly more common in the ILA group than in the non-ILA group (RR 3.05, 95% CI 1.37–6.77; I2 = 83%) (Fig. 5c).
Figure 5.
Meta-analyses of cancer treatment -related complications between ILA and non-ILA groups. (a) Radiation pneumonitis ≥ grade 2; (b) Radiation pneumonitis ≥ grade 3; (c) Immune checkpoint inhibitor-related interstitial lung disease. CI confidence interval, ILA interstitial lung abnormalities, M–H Mantel–Haenszel method.
Sensitivity analyses for overall mortality
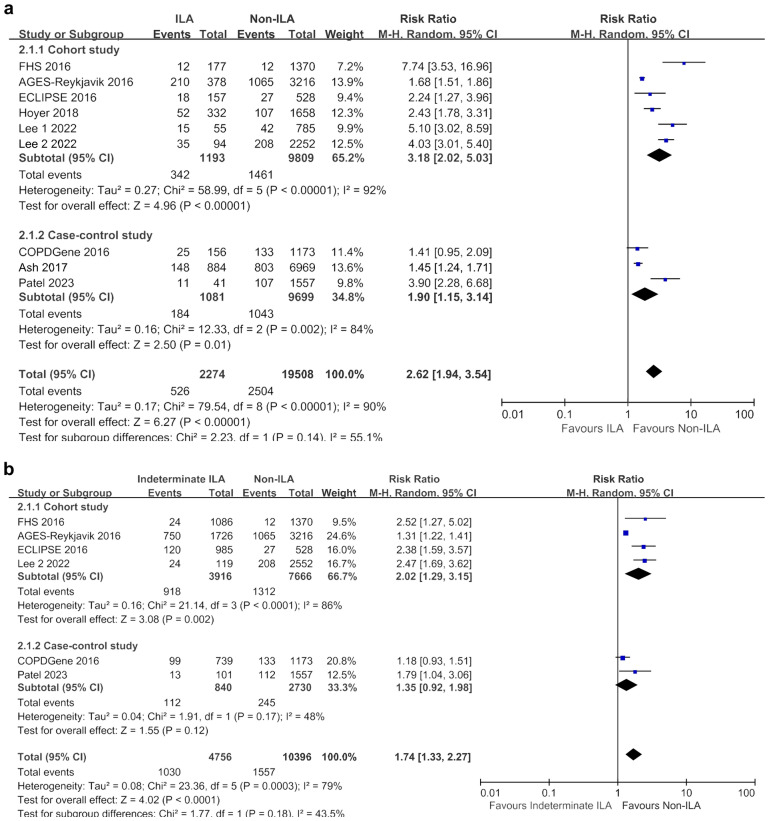
In the sensitivity analyses conducted by dividing according to study design, the observational studies (n = 6) indicated higher mortality in both ILA (RR 3.18, 95% CI 2.02–5.03; I2 = 92%) and indeterminate ILA (RR 2.02, 95% CI 1.29–3.15; I2 = 86%) groups compared with the non-ILA group (Fig. 6). However, in groups where only case–control studies were conducted (n = 4), ILA showed higher overall mortality compared with the non-ILA group (RR 1.90, 95% CI 1.15–3.14; I2 = 84%), while indeterminate ILA demonstrated only a tendency towards higher mortality (RR 1.35, 95% CI 0.92–1.98; I2 = 48%).
Figure 6.
Sensitivity analyses based on study design for overall mortality. (a) Between ILA and non-ILA groups; (b) Between indeterminate ILA and non-ILA groups. CI confidence interval, ILA interstitial lung abnormalities, M–H Mantel–Haenszel method.
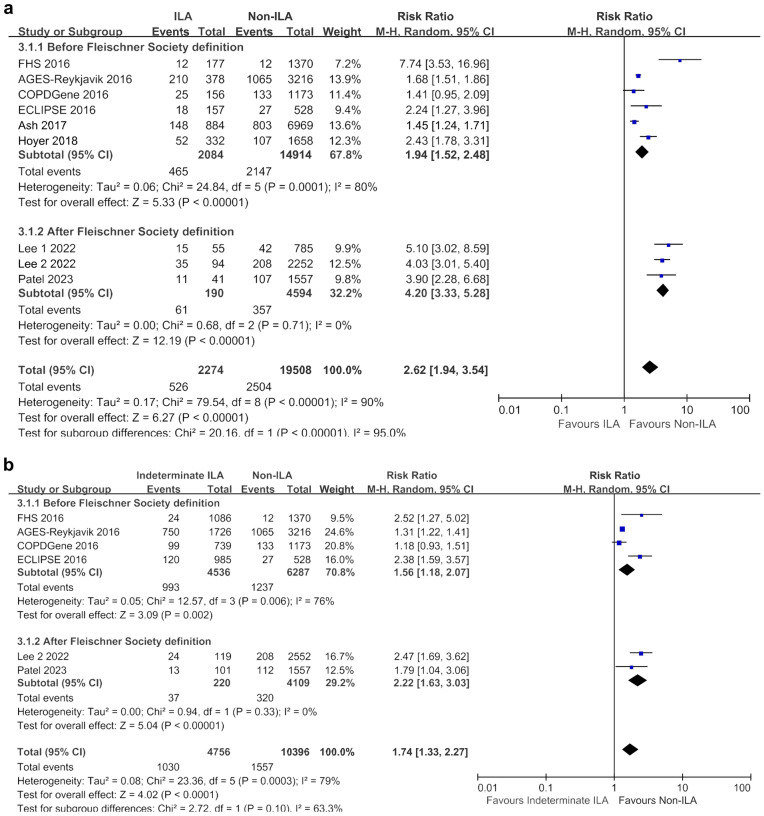
Regardless of adherence to the Fleischner Society's 2020 ILA definition, the ILA group exhibited higher mortality rates compared with the non-ILA group (2020 ILA definition: RR 4.20, 95% CI 3.33–5.28; I2 = 32.2%; non-2020 ILA definition: RR 1.94, 95% CI 1.52–2.48; I2 = 80.0%) (Fig. 7). Similarly, the indeterminate ILA group also showed higher mortality rates than the non-ILA group (2020 ILA definition: RR 2.22, 95% CI 1.63–3.03; I2 = 0%; non-2020 ILA definition: RR 1.56, 95% CI 1.18–2.07; I2 = 32.2%).
Figure 7.
Sensitivity analyses of studies based on Fleischner Society definition of ILA for overall mortality. (a) Between ILA and non-ILA groups; (b) Between indeterminate ILA and non-ILA groups. CI confidence interval, ILA interstitial lung abnormalities, M–H Mantel–Haenszel method.
Discussion
Our comprehensive meta-analysis, the first of its kind, demonstrates that ILA are associated with an elevated risk of overall mortality, with a notable increase in lung cancer and respiratory-related deaths, and an elevated incidence of lung cancer. Consistent results for overall mortality were observed in analyses based on study design and the 2020 ILA definition Fleischner Society. Our study also demonstrates that ILA are associated with a higher risk of RP and ICI-ILD in patients with lung cancer, and that indeterminate ILA are associated with higher overall and lung cancer mortality rates. This meta-analysis provides a systematic examination of ILA prognosis across various outcomes, making a valuable contribution to the existing literature.
Our findings are consistent with previous research demonstrating that ILA and indeterminate ILA are associated with increased mortality compared to non-ILA individuals3,7,9,11,16–18. Recent meta-analyses reported a higher pooled mortality risk in individuals with ILAs compared to those without (odds ratio (OR), 3.56; 95% CI 2.19–5.81)7, which aligns with our findings. However, our study extends its analysis by examining cause-specific mortality in the ILA, specifically identifying associations with lung cancer and respiratory-related causes of death. Although ILA are typically defined in the absence of respiratory symptoms or abnormal lung function, studies have shown associations between ILA and impaired exercise capacity and decline in pulmonary function34–37. Lee et al. found that among patients with chronic obstructive lung disease (n = 363), those with ILA were older, had lower forced expiratory volume in 1 s (FEV1) and FVC, and a significantly higher rate of annual decline in FEV1 and FVC in patients with progressive ILA than in those with stable or improved ILA34. In the AGES-Reykjavik cohort study (n = 375), Axelsson et al. reported that the presence of ILA was associated with decreased physical function, including decreased grip strength (OR 1.21, 95% CI 1.02–1.42), knee extension strength (OR 1.23, 95% CI 1.07–1.41), gait speed (OR 1.06, 95% CI 1.01–1.12), and thigh muscle mass (OR 1.14, 95% CI 1.05–1.23) in multivariable models35. These findings suggest that although ILA may not be identical to ILD, they can serve as early indicators of lung disease, thereby increasing mortality risk. Furthermore, the progression rate of ILA, which ranges from 6 to 80.5%, supports these results5,6,8,38. Park et al. reported that among Korean individuals with ILA who underwent consecutive chest CT scans for health screening (n = 200), 80.5% showed ILA progression with the median time to ILA progression being 3.2 years38. Our findings also align with the concept of ILA as early surrogate markers for respiratory diseases associated with higher mortality, especially in respiratory- and lung cancer-related deaths. Furthermore, differences in baseline characteristics such as age and comorbidities between the ILA and non-ILA groups may have influenced the higher mortality rates observed in the ILA group3,39. However, even after adjusting for these differences in several previous studies3,9,11,16,39, the ILA group consistently demonstrated a higher mortality rate, indicating that ILA themselves contribute to increased mortality. Furthermore, in our research, the heightened mortality observed in ILA cases may be linked to the progression of ILA to ILD. In an observational, retrospective multicenter study, 17% of fibrotic ILA cases (n = 59) and 6% of non-fibrotic ILA cases (n = 35) progressed to ILD over a median follow-up period of 12 years, with no instances of ILD progression during the same period observed in the non-ILA group (n = 2552) or the equivocal ILA group (n = 119)18. In another retrospective study of lung cancer patients who underwent surgical resection, six individuals developed ILD during follow-up: one with equivocal ILAs (4.5%) and five with fibrotic ILAs (19.2%), with no cases in the non-ILA group (n = 291) over a median follow-up period of 1313 days40. These findings collectively suggest in our study that the higher mortality rate observed in ILA cases, particularly the increased respiratory-related mortality, could be indicative of the potential for progression to ILD. Additionally, these findings suggest that ILA could serve as an early marker for ILD, emphasizing the clinical significance and stressing the importance of early detection and management in patients with these abnormalities.
In our study, ILA were found to affect the development of lung cancer. Smoking and age are well-established risk factors for lung cancer. The higher prevalence of individuals who smoke and older adults in the ILA group, which is consistent with previous studies3,9,18,39, could contribute to an increased risk of lung cancer. ILA and lung cancer may share other common risk factors, such as environmental exposure or air pollution41,42. Sack et al. reported an increased risk of ILA associated with self-reported vapour/gas exposure in currently employed individuals (OR 1.97, 95% CI 1.16–3.35) and those under 65 years old (OR 1.76, 95% CI 1.09–2.84) in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort (n = 2312) during 10-year follow-up14. In addition, higher levels of ambient nitrogen oxide tended to increase the risk of ILA (OR 1.62, 95% CI 0.97–2.71; P = 0.06), particularly in individuals who do not smoke (OR 2.60, 95% CI 1.20–5.61; P = 0.02) in the MESA cohort (n = 6813)43. Additionally, the Framingham Heart Study showed an association between ILA and air pollution, specifically elemental carbon15. These shared risk factors may contribute to a higher incidence of lung cancer in patients with ILA.
We also found that ILA were significantly associated with the occurrence of RP and ICI-ILD in patients with cancer. Pre-existing ILD has been identified as a risk factor for RP or ICI-ILD development in previous studies44–48. Ueki et al. reported that the presence of ILD was significantly associated with grade ≥ 2 RP (hazard ratio 5.52, 95% CI 2.43–12.5) on multivariable analysis in patients with stage I non-small cell lung cancer (NSCLC) who underwent stereotactic body radiation therapy (n = 157)47. Yamaguchi et al. similarly demonstrated that pre-existing ILD was a significant risk factor for ICI-ILD on the multivariable model (OR 5.92, 95% CI 2.07–18.54) in 313 patients with cancer, including NSCLC (n = 96)48. Therefore, ILA, as surrogate markers of early ILD, may indicate an increased risk of treatment-related complications such as RP and ICI-ILD.
Our study has several limitations that should be acknowledged. First, the included studies exhibited heterogeneity in terms of study design, sample size, follow-up duration, and ILA definition, which may have introduced potential bias and affected the generalizability of the results. Specifically, our study, predominantly composed of cohort studies, was susceptible to biases due to this heterogeneity. To mitigate this issue, sensitivity analyses were conducted focusing on (observational vs. case–control studies), which showed consistent trends towards an increased risk of mortality in both ILA and indeterminate ILA patients. Although consistent trends were also found in sensitivity analyses focusing on the Fleischner Society definition, further meta-analyses that include recent studies following the standardized definition of ILA in 2020 would provide valuable insights. Second, the majority of the included studies focused on specific populations at a high risk of lung cancer, which may restrict the applicability of our findings to the general population. However, similar results were observed in the subgroup analyses focusing on the general population. Third, our analysis primarily focused on mortality outcomes, with limited data available on other important clinical parameters, such as pulmonary function decline, hospitalization, or development of ILD. Studies examining diverse outcomes will enhance our understanding of the implications of ILA. Lastly, our analysis predominantly used case–control and retrospective studies from East Asian countries, limiting generalization to other regions. Future research should include more diverse populations and prospective study designs to validate and expand our findings. Despite these limitations, our study provided valuable insights into the prognosis of patients with ILA.
Conclusion
In conclusion, our study emphasizes the strong association between ILA and important clinical outcomes, such as mortality, lung cancer development, and cancer treatment-related complications. These findings underscore the importance of close monitoring and managing patients with ILA, as they may require personalized interventions and comprehensive care. Further research is required to better understand the underlying mechanisms and develop effective strategies for the prevention and treatment of ILA and their associated complications.
Supplementary Information
Acknowledgements
The authors would like to thank Sangkeun Hyon, Dong Won Shin and Saeam Kim (Seoul Medical Library, Soonchunhyang University Seoul Hospital, Seoul, Republic of Korea) for their help in reviewing and editing the search strategy. The authors are grateful for the Soonchunhyang Systematic Literature Review and Meta-Analysis Research Exchange Association.
Author contributions
H.Y. takes full responsibility for the content of this manuscript, including data and analysis. H.Y. made substantial contributions to the conception and design of the study. J.S., S.P. and H.Y. made substantial contributions to the analysis and interpretation of the data. J.S., S.P. and E.C.Y. drafted the initial manuscript. All authors discussed the results and reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Soonchunhyang University Research Fund.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jinwoo Seok and Shinhee Park.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-57831-3.
References
- 1.Antoniou KM, et al. Interstitial lung disease. Eur. Respir. Rev. 2014;23:40–54. doi: 10.1183/09059180.00009113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hatabu H, et al. Interstitial lung abnormalities detected incidentally on CT: A Position Paper from the Fleischner Society. Lancet Respir. Med. 2020;8:726–737. doi: 10.1016/s2213-2600(20)30168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Putman RK, et al. Association between interstitial lung abnormalities and all-cause mortality. JAMA. 2016;315:672–681. doi: 10.1001/jama.2016.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGroder CF, et al. Incidence of interstitial lung abnormalities: The MESA lung study. Eur. Respir. J. 2023 doi: 10.1183/13993003.01950-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsushima K, et al. The radiological patterns of interstitial change at an early phase: Over a 4-year follow-up. Respir. Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Araki T, et al. Development and progression of interstitial lung abnormalities in the Framingham heart study. Am. J. Respir. Crit. Care Med. 2016;194:1514–1522. doi: 10.1164/rccm.201512-2523OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant-Orser A, Min B, Elmrayed S, Podolanczuk AJ, Johannson KA. Prevalence, risk factors, and outcomes of adult interstitial lung abnormalities: A systematic review and meta-analysis. Am. J. Respir. Crit. Care Med. 2023;208:695–708. doi: 10.1164/rccm.202302-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin GY, et al. Interstitial lung abnormalities in a CT lung cancer screening population: Prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ash SY, et al. Clinical and genetic associations of objectively identified interstitial changes in smokers. Chest. 2017;152:780–791. doi: 10.1016/j.chest.2017.04.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sverzellati N, et al. Interstitial lung diseases in a lung cancer screening trial. Eur. Respir. J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 11.Hoyer N, et al. Interstitial lung abnormalities are associated with increased mortality in smokers. Respir. Med. 2018;136:77–82. doi: 10.1016/j.rmed.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Pompe E, et al. Computed tomographic findings in subjects who died from respiratory disease in the National Lung Screening Trial. Eur. Respir. J. 2017 doi: 10.1183/13993003.01814-2016. [DOI] [PubMed] [Google Scholar]
- 13.Washko GR, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad. Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sack CS, et al. Occupational exposures and subclinical interstitial lung disease. The MESA (Multi-Ethnic Study of Atherosclerosis) Air and Lung Studies. Am. J. Respir. Crit. Care Med. 2017;196:1031–1039. doi: 10.1164/rccm.201612-2431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice MB, et al. Ambient air pollution exposure and risk and progression of interstitial lung abnormalities: The Framingham Heart Study. Thorax. 2019;74:1063–1069. doi: 10.1136/thoraxjnl-2018-212877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel AS, et al. Interstitial lung abnormalities in a large clinical lung cancer screening cohort: Association with mortality and ILD diagnosis. Respir. Res. 2023;24:49. doi: 10.1186/s12931-023-02359-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JE, et al. Relationship between incidental abnormalities on screening thoracic computed tomography and mortality: A long-term follow-up analysis. Korean J. Radiol. 2022;23:998–1008. doi: 10.3348/kjr.2022.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JE, et al. Prevalence and long-term outcomes of CT interstitial lung abnormalities in a health screening cohort. Radiology. 2023;306:e221172. doi: 10.1148/radiol.221172. [DOI] [PubMed] [Google Scholar]
- 19.Sanders JL, et al. The relationship between interstitial lung abnormalities, mortality, and multimorbidity: A cohort study. Thorax. 2022 doi: 10.1136/thoraxjnl-2021-218315. [DOI] [PubMed] [Google Scholar]
- 20.Murata D, et al. Clinical significance of interstitial lung abnormalities and immune checkpoint inhibitor-induced interstitial lung disease in patients with non-small cell lung cancer. Thorac. Cancer. 2023;14:73–80. doi: 10.1111/1759-7714.14718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Daido W, et al. Pre-existing interstitial lung abnormalities are independent risk factors for interstitial lung disease during durvalumab treatment after chemoradiotherapy in patients with locally advanced non-small lung cancer. J. Clin. Oncol. 2022 doi: 10.1200/JCO.2022.40.16_suppl.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Im Y, et al. Impact of interstitial lung abnormalities on postoperative pulmonary complications and survival of lung cancer. Thorax. 2023;78:183–190. doi: 10.1136/thoraxjnl-2021-218055. [DOI] [PubMed] [Google Scholar]
- 23.Page MJ, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hata A, Schiebler ML, Lynch DA, Hatabu H. Interstitial lung abnormalities: State of the art. Radiology. 2021;301:19–34. doi: 10.1148/radiol.2021204367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 26.Axelsson GT, et al. The associations of interstitial lung abnormalities with cancer diagnoses and mortality. Eur. Respir. J. 2020 doi: 10.1183/13993003.02154-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi S, et al. Radiotherapy for thoracic tumors: Association between subclinical interstitial lung disease and fatal radiation pneumonitis. Int. J. Clin. Oncol. 2015;20:45–52. doi: 10.1007/s10147-014-0679-1. [DOI] [PubMed] [Google Scholar]
- 28.Li F, et al. Preexisting radiological interstitial lung abnormalities are a risk factor for severe radiation pneumonitis in patients with small-cell lung cancer after thoracic radiation therapy. Radiat. Oncol. 2018;13:82. doi: 10.1186/s13014-018-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakanishi Y, et al. Pre-existing interstitial lung abnormalities are risk factors for immune checkpoint inhibitor-induced interstitial lung disease in non-small cell lung cancer. Respir. Investig. 2019;57:451–459. doi: 10.1016/j.resinv.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Shimoji K, et al. Association of preexisting interstitial lung abnormalities with immune checkpoint inhibitor-induced interstitial lung disease among patients with nonlung cancers. JAMA Netw. Open. 2020;3:e2022906. doi: 10.1001/jamanetworkopen.2020.22906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daido W, et al. Pre-existing interstitial lung abnormalities are independent risk factors for interstitial lung disease during durvalumab treatment after chemoradiotherapy in patients with locally advanced non-small-cell lung cancer. Cancers (Basel) 2022 doi: 10.3390/cancers14246236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong WG, et al. Effect of interstitial lung abnormality on concurrent chemoradiotherapy-treated stage III non-small cell lung cancer patients. Anticancer Res. 2023;43:1797–1807. doi: 10.21873/anticanres.16333. [DOI] [PubMed] [Google Scholar]
- 33.Ito M, et al. Subpleural fibrotic interstitial lung abnormalities are implicated in non-small cell lung cancer radiotherapy outcomes. Radiol. Oncol. 2023;57:229–238. doi: 10.2478/raon-2023-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TS, et al. Interstitial lung abnormalities and the clinical course in patients with COPD. Chest. 2021;159:128–137. doi: 10.1016/j.chest.2020.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Axelsson GT, et al. Interstitial lung abnormalities and physical function. ERJ Open Res. 2018 doi: 10.1183/23120541.00057-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Washko GR, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N. Engl. J. Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doyle TJ, et al. Interstitial lung abnormalities and reduced exercise capacity. Am. J. Respir. Crit. Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park S, et al. Long-term follow-up of interstitial lung abnormality: Implication in follow-up strategy and risk thresholds. Am. J. Respir. Crit. Care Med. 2023;208:858–867. doi: 10.1164/rccm.202303-0410OC. [DOI] [PubMed] [Google Scholar]
- 39.Sanders JL, et al. The relationship between interstitial lung abnormalities, mortality, and multimorbidity: A cohort study. Thorax. 2023;78:559–565. doi: 10.1136/thoraxjnl-2021-218315. [DOI] [PubMed] [Google Scholar]
- 40.Shin YJ, Yi JG, Kim MY, Son D, Ahn SY. Radiologic progression of interstitial lung abnormalities following surgical resection in patients with lung cancer. J. Clin. Med. 2023 doi: 10.3390/jcm12216858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malhotra J, Malvezzi M, Negri E, La Vecchia C, Boffetta P. Risk factors for lung cancer worldwide. Eur. Respir. J. 2016;48:889–902. doi: 10.1183/13993003.00359-2016. [DOI] [PubMed] [Google Scholar]
- 42.Hamra GB, et al. Lung cancer and exposure to nitrogen dioxide and traffic: A systematic review and meta-analysis. Environ. Health Perspect. 2015;123:1107–1112. doi: 10.1289/ehp.1408882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sack C, et al. Air pollution and subclinical interstitial lung disease: The Multi-Ethnic Study of Atherosclerosis (MESA) air-lung study. Eur. Respir. J. 2017 doi: 10.1183/13993003.00559-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen H, et al. Treatment-related toxicity in patients with early-stage non-small cell lung cancer and coexisting interstitial lung disease: A systematic review. Int. J. Radiat. Oncol. Biol. Phys. 2017;98:622–631. doi: 10.1016/j.ijrobp.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Glick D, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival in patients treated with lung stereotactic body radiation therapy (SBRT) Clin. Lung Cancer. 2018;19:e219–e226. doi: 10.1016/j.cllc.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 46.Okubo M, et al. Predicting risk factors for radiation pneumonitis after stereotactic body radiation therapy for primary or metastatic lung tumours. Br. J. Radiol. 2017;90:20160508. doi: 10.1259/bjr.20160508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueki N, et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J. Thorac. Oncol. 2015;10:116–125. doi: 10.1097/jto.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 48.Yamaguchi T, et al. Pre-existing interstitial lung disease is associated with onset of nivolumab-induced pneumonitis in patients with solid tumors: A retrospective analysis. BMC Cancer. 2021;21:924. doi: 10.1186/s12885-021-08661-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.