Abstract
Variants of human immunodeficiency virus type 1 (HIV-1) that are highly resistant to a number of nucleoside analog drugs have been shown to develop in some patients receiving 2′,3′-dideoxy-3′-azidothymidine therapy in combination with 2′,3′-dideoxycytidine or 2′,3′-dideoxyinosine. The appearance, in the reverse transcriptase (RT), of the Q151M mutation in such variants precedes the sequential appearance of three or four additional mutations, resulting in a highly resistant virus. Three of the affected residues are proposed to lie in the vicinity of the template-primer in the three-dimensional structure of the HIV-1 RT–double-stranded DNA complex. The amino acid residue Q151 is thought to be very near the templating base. The nucleoside analog resistance mutations in the β9-β10 (M184V) and the β5a (E89G) strands of HIV-1 RT were previously shown to increase the fidelity of deoxynucleoside triphosphate insertion. Therefore, we have examined wild-type HIV-1BH10 RT and two nucleoside analog-resistant variants, the Q151M and A62V/V75I/F77L/F116Y/Q151M (VILYM) RTs, for their overall forward mutation rates in an M13 gapped-duplex assay that utilizes lacZα as a reporter. The overall error rates for the wild-type, the Q151M, and the VILYM RTs were 4.5 × 10−5, 4.0 × 10−5, and 2.3 × 10−5 per nucleotide, respectively. Although the mutant RTs displayed minimal decreases in the overall error rates compared to wild-type RT, the error specificities of both mutant RTs were altered. The Q151M RT mutant generated new hot spots, which were not observed for wild-type HIV-1 RT previously. The VILYM RT showed a marked reduction in error rate at two of the predominant mutational hot spots that have been observed for wild-type HIV-1 RT.
Human immunodeficiency virus type 1 (HIV-1) infections are characterized by a high degree of genetic variation, which inevitably leads to facile generation of drug-resistant variants during treatment (16, 26). To date, five nucleoside analogs which target reverse transcriptase (RT) have been approved for treating infected individuals. Since monotherapies with each drug culminate in the emergence of resistant mutants, combinations of two or more drugs are preferred (8, 15, 22). One such combination is 2′,3′-dideoxy-3′-azidothymidine (AZT) with either 2′,3′-dideoxycytidine (ddC) or 2′,3′-dideoxyinosine (ddI) in simultaneous or sequential therapy (29, 30). However, the emergence of variant viruses that are resistant to one of the drugs in the regimen, as well as those that are resistant to multiple nucleoside analogs (29, 30), has been reported. Chronological studies of viral variants over an extended period (up to 48 months) revealed the appearance of a series of five mutations (13, 30). Of these, the Q151M mutation appears first and alone is sufficient to confer multidrug resistance to HIV-1, exhibiting a 10-fold increase in resistance to AZT, a 20-fold increase in resistance to ddC, and a 5-fold increase in resistance to ddI in cell culture virus replication assays (30). Subsequently, mutations appearing at four other sites (A62V, V75I, F77L, and F116Y) further increase the level of drug resistance. Viruses containing all five mutations exhibit up to a 320-fold increase in resistance to AZT, up to a 45-fold increase in resistance to ddC, and up to a 40-fold increase in resistance to ddI in cell culture (31). When recombinant purified RTs containing the Q151M alteration alone or the complete set of five mutations were studied via biochemical assays, they exhibited high-level resistance to ddATP, ddCTP, ddGTP, ddTTP, and AZT triphosphate (33, 34).
Mutations in RT that result in nucleoside analog resistance are thought to do so by decreasing the ability to bind and utilize a nucleoside analog while retaining the ability to utilize deoxynucleoside triphosphate (dNTP) substrates in the polymerization reaction (19, 33, 34). If these mutations affect the geometry of the dNTP-binding pocket to preferentially utilize the normal dNTP substrate, it is conceivable that the mutations may also impart an ability to favor the insertion of correctly base-paired dNTPs over incorrectly base-paired dNTPs. Indeed, it has recently been shown that the 2′,3′-dideoxy-3′-thiacytidine-resistant M184V RT mutant (11, 21, 35) and the multidrug-resistant E89G HIV-1 RT mutant (9, 27a) exhibit a significant increase in nucleotide insertion fidelity over that of the wild-type enzyme. Mutagenesis and biochemical studies have implicated residue Q151 of HIV-1 RT in dNTP-binding function (28). Furthermore, the Q151 residue lies within the LPQG motif that is conserved in RTs (23). The three-dimensional structure of HIV-1 RT complexed with double-stranded DNA reveals that the Q151 residue is in a position to interact with the first nucleotide of the single-stranded template (30). Additionally, the high-resolution X-ray structure of a fragment of the Moloney murine leukemia virus RT reveals that the residue Q190, which is analogous to Q151 of HIV-1 RT, interacts with the templating base and the incoming dNTP (12), suggesting that it may determine the fidelity of DNA synthesis. Three of the remaining four mutations that occur in AZT-ddI or AZT-ddC combination therapy also map to the DNA-protein interface, including residue 116, which may be involved in dNTP binding (30). Therefore, we have examined the effect of the Q151M mutation alone as well as in combination with the remaining four mutations (VILYM [see below]) on the overall fidelity of DNA synthesis by HIV-1 RT by using an M13-based forward mutation assay previously described by Kunkel and coworkers (3, 27). Our results show that the mutations cause a small change in the overall mutation frequency, but important differences in mutational specificities were evident when the three enzymes were compared.
(The data in this paper are from a thesis to be submitted by L. F. Rezende in partial fulfillment of the requirements for a Ph.D. in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University.)
MATERIALS AND METHODS
Phage DNA and bacterial strains.
The gapped-duplex DNA substrate was derived from the bacteriophage M13mp2. The M13 phage was grown in Escherichia coli NR9099 [Δ(pro-lac) thi ara recA56/F′ (proAB lacIqZΔM15)] for the preparation of both single-stranded and replicative-form DNAs. E. coli MC1061 [hsdR hsdM+ araD Δ (ara leu) Δ(lacIPOZY) galU galK strA] was used for electroporation of the products of the fill-in reaction to produce phage. E. coli CSH50 [Δ(pro-lac) thi ara strA/F′ (proAB lacIqZΔM15 traD36)] was the α-complementation strain used to score for the mutant phage.
Enzymes.
Bacterial expression and purification of homodimers of wild-type HIV-1BH10 RT, the Q151M mutant RT, and the VILYM (containing the five mutations A62V, V75I, F77L, F116Y, and Q151M) mutant RT have been described by Ueno et al. (34). The specific activities of these RTs were found to be 500 U/mg for the wild-type enzyme, 833 U/mg for the Q151M RT, and 625 U/mg for the VILYM RT. One unit is defined as the amount of enzyme required to incorporate 1 nmol of dTMP into DNA on a poly(rA) · oligo(dT) template-primer at 37°C in 10 min.
Determination of forward mutation frequency.
The M13mp2 duplex DNA containing a single-stranded gap of 361 nucleotides (including the lacZ upstream regulatory sequences and the first 107 nucleotides of the open reading frame) was prepared as previously described (6) and used as a template-primer to perform the DNA synthesis reactions in vitro with purified wild-type and mutant RTs. DNA synthesis reactions were performed in a total volume of 25 μl containing 75 mM Tris-Cl (pH 8.0), 80 mM KCl, 6 mM MgCl2, 10 mM dithiothreitol, 500 μM (each) dATP, dCTP, dGTP, and dTTP (Boehringer Mannheim, Indianapolis, Ind.), 59 ng of gapped-duplex DNA, and 0.7 to 1.3 U of purified RT for 1 h at 37°C. Complete synthesis of the gapped region was confirmed by gel electrophoresis. Two independent fill-in reactions were performed with each enzyme.
Products of the fill-in reaction were electroporated (in two to five batches per fill-in reaction) into E. coli MC1061. After electroporation, the cells were allowed to recover for 10 min and then mixed with a log-phase culture of E. coli CSH50, and the mixed cells were overlaid, in top agar, on M9 plates containing 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) (Sigma) and 0.195 mM X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) (Labscientific, Inc). The plates were incubated at 37°C for approximately 15 h before scoring for mutant plaques that did not display the bright blue color of the wild-type M13mp2. These included both clear plaques and plaques with three grades of reduced color intensity (6). Mutant plaques were picked from the plates and stored in 1 ml of 0.9% saline at 4°C. Mutational frequencies were determined by dividing the number of confirmed mutant plaques by the total number of plaques screened. The background mutation frequency was determined by electroporating unfilled gapped-duplex DNA and scoring for mutants as described above. All mutants identified in the initial screen were confirmed by picking, resuspending, and replating with an equivalent amount of wild-type phage as described above.
Sequencing of phage DNA.
Single-stranded DNA was prepared from mutant plaques as described by Bebenek and Kunkel (6). Briefly, 50 μl of plaque supernatant and 200 μl of an overnight culture of E. coli CSH50 were added to 1.8 ml of 2xYT broth and incubated at 37°C for approximately 15 h. Culture supernatants were collected, and the phage were precipitated with one-fourth the volume of 15% polyethylene glycol–2.5 M NaCl for 6 min at 4°C. Phage were decoated by incubation with 50 μg of proteinase K for 30 min, and single-stranded DNA was precipitated by treatment with 1/10 volume of 5% hexadecyl trimethylammonium bromide in 0.5 mM NaCl for 10 min followed by centrifugation at 12,000 × g. The DNA pellets were resuspended in 1.2 M NaCl, and finally the single-stranded phage DNA was precipitated with ethanol.
The phage DNA was sequenced by using a Sequenase 2.0 DNA sequencing kit (Amersham Life Sciences, Arlington Heights, Ill.) with a primer that allowed the determination of the nucleotide sequence of the entire gap region (5′GCGCAGCTGTTGGGAAGGGCG3′). Sequenced templates were resolved on 6% sequencing gels with a GENOMYX DNA sequencer.
Calculation of error rates.
Error rates were calculated as described by Bebenek and Kunkel (6). Since two fill-in reactions were performed for each enzyme and the mixtures were separately electroporated, we calculated the mutation frequencies separately and derived the mean values and standard errors. The mean mutational frequency was corrected by subtracting the background mutational frequency. The corrected mean mutational frequency is multiplied by the percentage of all mutations represented by the particular class of mutations (e.g., base substitutions). This number was divided by 0.6 (the likelihood of expression of the newly synthesized strand in E. coli) and then divided by the total number of sites where this class of mutations can be detected within our 361-base target (6).
Statistical analysis.
Differences between mutation frequencies were calculated by using the unpaired t test. Differences in the proportions of errors at specific sites were calculated by using the two-tailed Fisher’s exact test.
RESULTS
Mutational frequencies of wild-type and multidrug-resistant RTs.
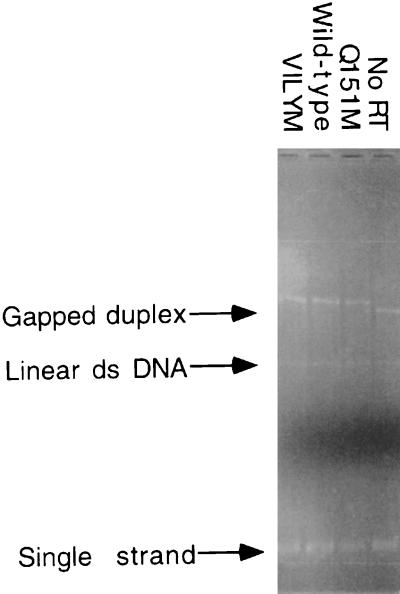
We used the M13-based forward assay to assess the overall fidelity of the wild-type, Q151M, and VILYM (A62V/V75I/F77L/Q151M/F116Y) RTs. This assay allows the measurement of both the overall fidelity and the rates of specific mutations by each mutant RT. The M13 gapped-duplex substrate contained a single-stranded gap over the regulatory sequences and some of the coding region of the lacZα gene. All three enzymes were capable of synthesizing DNA, resulting in gap closure as determined by agarose gel electrophoresis (Fig. 1).
FIG. 1.
Ethidium bromide-stained agarose gel showing synthesis of DNA across the gapped-duplex DNA substrate with recombinant, purified HIV-1 RT. Complete synthesis of DNA across the gapped-duplex DNA substrate was confirmed on a 0.8% agarose gel run at 25 V for 20 h. The synthesis is indicated by the gel mobility shift observed between the initial gapped-duplex DNA (no RT) and the products obtained after filling in by wild-type, Q151M, and the VILYM RTs. ds, double stranded.
After screening of approximately 19,000 to 24,000 plaques per enzyme, generated from gap fill-in reactions by the wild-type, Q151M, and VILYM RTs, the background-adjusted overall mutation frequencies were determined to be 64 × 10−4 ± 9 × 10−4, 55 × 10−4 ± 9 × 10−4, and 31 × 10−4 ± 4 × 10−4, respectively (Table 1). These mutation frequencies corresponded to overall error rates of 4.5 × 10−5, 4.0 × 10−5, and 2.3 × 10−5 per nucleotide, respectively. Statistical analysis revealed that the mutation frequencies of the two variant RTs were not substantially altered from that of wild-type RT (P > 0.05 for both comparisons by the unpaired t test; P = 0.6 for wild-type RT compared to Q151M RT, and P = 0.075 for wild-type RT compared to VILYM RT).
TABLE 1.
Overall mutation frequencies of the three RTs
| RT | No. of plaques screeneda | No. of mutantsa | Mutation frequency (10−4) |
|---|---|---|---|
| Wild type | 18,798 | 161 | 64 ± 9 |
| Q151M | 19,145 | 121 | 55 ± 9 |
| VILYM | 23,828 | 111 | 31 ± 4 |
Numbers represent pooled totals from two independent fill-in reactions.
Spectra of mutations by wild-type and multidrug-resistant HIV-1 RTs.
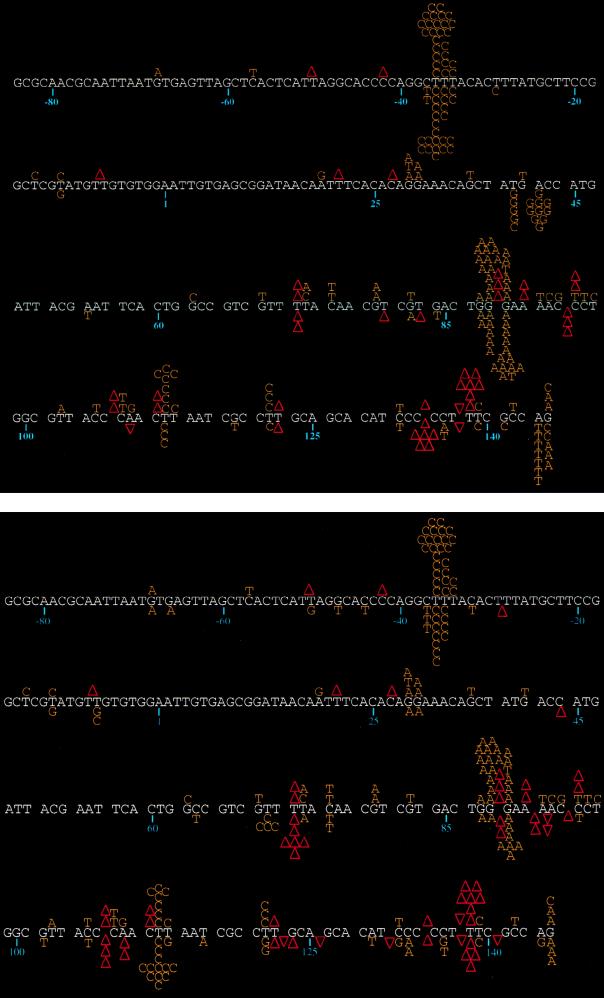
In order to determine the specificity of mutations generated during the gap fill-in DNA synthesis reaction, M13 templates from 117 plaques representing the mutations induced by the wild-type enzyme, 103 plaques representing the errors by the Q151M RT, and 101 plaques representing those by the VILYM RT were randomly selected from each independent electroporation for sequence determination. A comparison of the mutational spectra of the wild-type and Q151M RTs is shown in the top panel of Fig. 2, while that for wild-type and VILYM RTs is shown in the bottom panel of Fig. 2. It is readily apparent from these mutational spectra that there are differences in the error specificities of the wild-type and the mutant RTs. An examination of the mutation spectrum generated by the wild-type RT reveals four prominent mutational hot spots (consisting of at least six errors at a given site), each occurring at runs of nucleotides. Of these, three are substitution hot spots located at the run of Ts at positions −36 to −34, the run of Gs at positions 88 to 90, and the first of two Ts at positions 112 to 113. The fourth is a frameshift hot spot located in the run of Ts at positions 137 to 139. Three milder hot spots (consisting of three errors at a given site) are also observed for wild-type HIV-1 RT: one at the first of two Gs at position 29, a second at the first T of the pair at position 121, and a third at the last G in the stretch of sequence shown (Fig. 2).
FIG. 2.
Spectra of mutations generated by wild-type and nucleoside analog-resistant HIV-1 RTs. The wild-type lacZα sequence is represented, with the corresponding nucleotide positions indicated below the sequence. Mutations created by the wild-type HIV-1 RT are shown above the lacZα sequence, and those generated by one of the mutant RTs are shown below the sequence. Base substitutions are noted directly above or below the lacZα sequence and represent the nucleotide in the mutated template. Frameshift mutations are indicated with an upright triangle for single-base deletions and with an inverted triangle for single-base insertions. Since it cannot be determined exactly which base in a run of nucleotides has been deleted or inserted, the frameshift events are indicated in the middle of the run. (Top) Comparison of the spectra of mutations generated by the wild-type and Q151M variant HIV-1 RTs. (Bottom) Comparison of wild-type and VILYM RTs. In addition to the mutations shown here, two templates generated by the VILYM RT contained large deletions (see text).
The mutational spectrum generated by the Q151M RT retains both the hot spot at the run of Ts at positions −36 to −34 and that at the run of Gs at positions 88 to 90 (Fig. 2, top; Table 2). However, the frameshift hot spot observed at positions 137 to 139 with the wild-type HIV-1 RT is significantly reduced (10-fold; P < 0.05 by the two-tailed Fisher’s exact test). Three new prominent hot spots were created by the Q151M RT: an A-to-G mutation at nucleotide 42 (P < 0.0005 by the two-tailed Fisher’s exact test), a frameshift hot spot at the run of Cs located at positions 132 to 136 (P < 0.06 by the two-tailed Fisher’s exact test), and an A-to-T base substitution hot spot at position 144 (P < 0.01 by the two-tailed Fisher’s exact test). A milder T-to-G hot spot also appears at position 40 (P < 0.03 by the two-tailed Fisher’s exact test). All but one (the frameshift hot spot at positions 132 to 136; P < 0.06 by the two-tailed Fisher’s exact test) of the new hot spots detected with the Q151M RT were previously not observed with the wild-type HIV-1 RT at these new positions (3, 4, 10, 17, 18).
TABLE 2.
Comparison of error rates at specific hot spots
| Mutation type and position | Wild-type RT
|
Q151M RT
|
VILYM RT
|
|||
|---|---|---|---|---|---|---|
| No. of errors | Error rate | No. of errors | Error rate | No. of errors | Error rate | |
| Base substitutions | ||||||
| −36 | 20 | 1/580 | 17 | 1/670 | 7a | 1/2,900 |
| 40 | 0 | CDd | 5a | 1/2,300 | 0 | CD |
| 42 | 0 | CD | 10b | 1/1,100 | 0 | CD |
| 89 | 13 | 1/880 | 5 | 1/2,300 | 1b | 1/21,000 |
| 90 | 6 | 1/1,900 | 13c | 1/870 | 7 | 1/2,900 |
| 144 | 0 | CD | 6a | 1/1,800 | 1 | 1/21,000 |
| Frameshifts | ||||||
| 132–136 | 1 | 1/47,000 | 6c | 1/7,600 | 1 | 1/83,000 |
| 137–139 | 8 | 1/4,700 | 1a | 1/34,000 | 5 | 1/12,000 |
| All | 123 | 1/22,000 | 104 | 1/25,000 | 106 | 1/44,000 |
P < 0.05 by Fisher’s exact test when compared to errors made by the wild-type enzyme.
P ≤ 0.002 by Fisher’s exact test when compared to errors made by the wild-type enzyme.
P < 0.06 by Fisher’s exact test when compared to errors made by the wild type enzyme.
CD, Cannot be determined.
The mutational spectrum generated by the VILYM RT also shows variation from that of the wild-type RT (Fig. 2, bottom). The two major base substitution hot spots at positions −36 and 89 show marked reductions in mutations, i.e., 5.5-fold (P < 0.03 by the two-tailed Fisher’s exact test) and 25-fold (P < 0.002 by the two-tailed Fisher’s exact test), respectively (Table 2). The strongest mutational hot spot (12 mutations) for base substitutions by VILYM RT is position 112, which was also a hot spot (6 mutations) in the spectrum generated by the wild-type RT.
Some of the templates sequenced showed multiple mutations, ranging from two to six per template. Approximately 6% of the sequenced templates generated by the wild-type and the VILYM RTs contained mutations at multiple sites. However, only 1% of templates generated by the Q151M RT contained multiple mutations. While this rate of multiple mutations may be higher than one would expect considering the mutational frequency, many of the second mutations would have been silent if found alone and thus not counted in the mutation frequency.
In addition to templates containing mutations involving only a few (one to three) nucleotides per site, the VILYM RT generated two mutants with large deletions of 86 and 91 bp. One of these deletions began at nucleotide 145, which should be the first nucleotide synthesized in our gap-filling DNA synthesis reactions, and ended at nucleotide 59. The other began six nucleotides upstream of the priming site and ended at position 60.
The distributions of mutations generated by the RTs in this study varied slightly. Unlike in a previous study (3), which revealed an approximately 1:1 ratio of frameshifts to base substitutions for HIV-1 RT, in the present study wild-type RT and its Q151M and VILYM variants generated approximately four, seven, and three times more substitutions than frameshifts, respectively (frameshift mutations constituting 22, 16, and 30%, respectively, of total mutations). We believe that this discrepancy can be attributed to the different molecular clones from which the RTs used in the two studies were derived. The wild-type and mutant RTs studied here differed not only in the amounts of frameshift errors made but also in the types of frameshift errors made. While all enzymes showed a preference for 1-base deletions over 1-base insertions, the wild-type enzyme created the fewest insertions (0.8%), the Q151M RT generated slightly more insertions (1.9%), and the VILYM RT generated the most insertions (6.6%).
The rate of formation of base substitution errors paralleled the pattern seen in the overall error rate; i.e., it was lower in the nucleoside analog-resistant RTs than in the wild-type RT (Table 3). Base substitutions can be generated by either the direct misinsertion of a nucleotide or by properly base-paired insertion during transient misalignment of a template or primer (4). Misalignment-generated base substitutions generally occur at the end of a run of nucleotides (4, 7). We have classified the base substitutions in our spectrum as those that most likely arise by misalignment (mutations at the ends of runs of nucleotides) and those that most likely arise by direct misinsertion (mutations not in or adjacent to runs of nucleotides). Base substitutions in the middle of runs, which form 0.8% of the detectable mutations, or at the end of a 2-nucleotide run, which form 5.1% of the detectable mutations, are put into both categories, since the mechanism of their generation cannot be accurately assessed. We calculated the rate of generation of base substitution by each mechanism. Using this method of analysis, we found that the proportion of misalignment-mediated errors made by the VILYM RT was significantly less than that for the wild-type RT (P = 0.04 by the two-tailed Fisher’s exact test) (Table 3).
TABLE 3.
Summary of error rates for various classes of mutations
| Mutation type | Wild-type RT
|
Q151M RT
|
VILYM RT
|
|||
|---|---|---|---|---|---|---|
| No. of errors | Error rate | No. of errors | Error rate | No. of errors | Error rate | |
| All classes | 123 | 1/22,000 | 104 | 1/25,000 | 106 | 1/44,000 |
| Frameshifts | 28 | 1/61,000 | 17 | 1/99,000 | 32 | 1/94,000 |
| At runs | 25 | 1/34,000 | 15 | 1/55,000 | 27 | 1/56,000 |
| At nonruns | 3 | 1/290,000 | 2 | 1/430,000 | 5 | 1/310,000 |
| Base substitutionsa | 95 | 1/13,000 | 87 | 1/14,000 | 72 | 1/30,000 |
| Misalignment | 60 | 1/5,800 | 48 | 1/7,100 | 37b | 1/17,000 |
| Miscoding | 65 | 1/19,000 | 53 | 1/23,000 | 53 | 1/41,000 |
The total number of base substitutions does not equal the sum of the numbers possibly arising from misalignment or miscoding, because some mutations could not be classified as one or the other and were put in both classes. See text for details.
P < 0.05 by Fisher’s exact test when compared to errors made by the wild-type enzyme.
DISCUSSION
Fidelity studies of HIV-1 RT, using either gel-based primer extension assays or the gap-filling assay with the lacZα gene, have shown that this polymerase has a high error rate (24, 27). More recent studies on the nucleoside analog-resistant RTs, such as those with M184V or E89G alterations, have shown an increase of fidelity (9, 11, 21, 27a, 35). However, those studies employed a gel-based primer extension assay, which allows one to specifically measure the efficiency of misinsertion and does not allow detection of changes in the efficiency of mispair extension or the generation of frameshift mutations. Furthermore, the assay allows for the measurement of misinsertion fidelity under conditions where the enzyme does not have the option to make the correct insertion. In this study, we have used the M13-based forward mutation assay to assess the overall fidelities and mutational specificities of mutant RTs which are parts of variant viruses seen in a subset of patients receiving long-term combination therapy with AZT and ddI or with AZT and ddC. We were able to show that while no significant difference in overall fidelity was observed, important differences in the mutational specificity were seen when wild-type and mutant enzymes were compared.
By using the M13-based gap-filling in vitro mutation assay, the mutation frequency of HIV-1NY5 RT has been estimated to be 400 × 10−4 nucleotides (27). In this study, however, the wild-type HIV-1BH10 RT had a mutation frequency of 64 × 10−4. Thus, the wild-type RT used here displays an approximately 6.5-fold-lower mutation frequency in vitro than that reported for RT derived from HIV-1NY5 (27). A second difference between the previous in vitro study and this study is the proportion of frameshift mutations. The ratios of substitutions to frameshifts observed here were 4.5:1, 6.25:1, and 3.3:1 for wild-type, Q151M, and VILYM RTs, respectively. These ratios contrast with a 1:1 ratio observed previously by Bebenek et al. (3). These differences cannot be explained merely by the fact that the strains of HIV used in these two studies are different. In recent studies, the HIV-1NY5 RT has also been shown to display decreased affinity for template (compared with HxB2-derived RT), as revealed by a 279-fold decrease in the Km for the template-primer (2) and a greater-than-200-fold increase in the Koff on poly(rA) · oligo(dT) template-primers (14). Poor affinity of RT for the template-primer is thought to promote poorer processivity (1), which in turn can decrease the fidelity of the polymerase (32). Subsequent studies by Bebenek et al. using HIV-1HxB2 RT showed lower mutation frequencies (200 × 10−4 to 210 × 10−4) (5). We have also performed the forward assay with HIV-1HxB2 RT and have found a mutation frequency of 97 × 10−4, similar to that we have found for RT from HIV-1BH10 reported here (24a). When comparing the wild-type and nucleoside analog-resistant RTs from this study, the increases in fidelity are found to be small (1.1-fold for the single mutant and 2.0-fold for the quintuple mutant) and do not reach the difference seen between RTs of different strains, making the difference seem negligible. The fidelities of Moloney murine leukemia virus RT and avian myeloblastosis virus RT have been studied previously by using this assay (27), and the mutation frequencies were found to be 22 × 10−4 and 42 × 10−4, respectively, which are similar to the mutation frequencies determined for HIV-1 RT in this study.
Interestingly, differences between the mutational specificities of the wild-type and mutant enzymes were revealed by our studies. It is well documented that the context of a nucleotide site affects the mutation frequency (20, 25). Our data suggest that the overall structure of the enzyme template-primer-binding pocket may also affect which portions of the template are susceptible to mutation. The Q151M RT showed the generation of new hot spots neither previously reported for HIV-1 RT (3, 10, 17, 18) nor observed in our laboratory (24a). Three of these sites (positions 40, 42, and 144) showed statistically significant increases in proportions of mutations (P < 0.05 by the two-tailed Fisher’s exact test), with one site (position 42) showing an increase at a more rigorous degree of significance (P < 0.002 by the two-tailed Fisher’s exact test) that is necessary to avoid false positives due to type I error. The Q151 residue in HIV-1 RT maps to the loop between the αE and β8 region of the RT, near the single-stranded DNA of the template base (30), and is proposed to make contact with the deoxyribose backbone of the templated base (28). The amino acid change from a glutamine to a methionine reduces the size of the side chain. This may add flexibility to the template base, changing the context specificity in which mutations are made. The Q151 residue has been shown to be involved in dNTP binding (28), which may also allow for changes in the nucleotide base pairing allowed by the enzyme. The most striking change in the multiply mutated nucleoside analog-resistant RT is the decrease in misalignment-mediated substitutions and the decrease in mutations at two common mutation sites, −36 and 89. The presence of multiple mutations in this RT, however, precludes any speculations on mechanistic explanations for this phenotype.
We and others (9, 11, 21, 27a, 35) have previously reported coexistence of nucleoside analog resistance with an increase in dNTP insertion fidelity. In this study, however, we find only modest increases in fidelity with another class of nucleoside analog-resistant mutants. The in vivo effects of the alteration of mutational specificity by these RT mutations in vitro need to be assessed. It is conceivable that dramatic changes in the mutational specificity of RT could alter the course of evolution of the HIV-1 quasispecies in infected individuals. It is, however, unclear whether the small changes in error specificity observed here will have a major impact on viral variation. This remains to be assessed via a single-cycle infection assay.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI-30861 and AI40375 (to V.R.P.). L.F.R. acknowledges support from institutional NIGMS predoctoral training grant T32-GM07491.
We thank T. A. Kunkel (National Institute for Environmental Health Sciences) for providing the reagents for the M13 forward mutation assay, B. D. Preston (University of Utah) for M13mp2 DNA, W. C. Drosopoulos for several helpful discussions, Deborah Stemp and Clark Choi for their relentless enthusiasm while preparing hundreds of templates for sequencing, Gloria Ho (Albert Einstein College of Medicine) for assistance with the statistical analysis, and the Oligonucleotide Synthesis Facility of the Albert Einstein College of Medicine’s Cancer Center for DNA oligonucleotides.
REFERENCES
- 1.Abbotts J, Bebenek K, Kunkel T A, Wilson S H. Mechanism of HIV-1 reverse transcriptase. J Biol Chem. 1993;268:10312–10323. [PubMed] [Google Scholar]
- 2.Beard W A, Wilson S H. Kinetic analysis of template-primer interactions with recombinant forms of HIV-1 reverse transcriptase. Biochemistry. 1993;32:9745–9753. doi: 10.1021/bi00088a029. [DOI] [PubMed] [Google Scholar]
- 3.Bebenek K, Abbotts J, Roberts J D, Wilson S H, Kunkel T A. Specificity and mechanism of error-prone replication by human immunodeficiency virus-1 reverse transcriptase. J Biol Chem. 1989;264:16948–16956. [PubMed] [Google Scholar]
- 4.Bebenek K, Abbotts J, Wilson S H, Kunkel T A. Error-prone polymerization by HIV-1 reverse transcriptase. J Biol Chem. 1993;268:10324–10334. [PubMed] [Google Scholar]
- 5.Bebenek K, Beard W A, Casas-Finet J R, Kim H R, Darden T A, Wilson S H, Kunkel T A. Reduced frameshift fidelity and processivity of HIV-1 reverse transcriptase mutants containing alanine substitutions in helix H of the thumb subdomain. J Biol Chem. 1995;270:19516–19523. doi: 10.1074/jbc.270.33.19516. [DOI] [PubMed] [Google Scholar]
- 6.Bebenek K, Kunkel T A. Analyzing fidelity of DNA polymerases. Methods Enzymol. 1995;262:217–232. doi: 10.1016/0076-6879(95)62020-6. [DOI] [PubMed] [Google Scholar]
- 7.Bebenek K, Kunkel T A. The fidelity of retroviral reverse transcriptase. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 85–102. [Google Scholar]
- 8.Collier A C, Coombs R W, Schoenfeld D A, Bassett R, Baruch A, Corey L. Combination therapy with zidovudine, didanosine and saquinavir. Antiviral Res. 1996;29:99. doi: 10.1016/0166-3542(95)00928-0. [DOI] [PubMed] [Google Scholar]
- 9.Drosopoulos W C, Prasad V R. Increased polymerase fidelity of E89G, a nucleoside analog-resistant variant of human immunodeficiency virus type 1 reverse transcriptase. J Virol. 1996;70:4834–4838. doi: 10.1128/jvi.70.7.4834-4838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eckert K A, Kunkel T A. Fidelity of DNA synthesis catalyzed by human DNA polymerase α and HIV-1 reverse transcriptase: effect of reaction pH. Nucleic Acids Res. 1993;21:5212–5220. doi: 10.1093/nar/21.22.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Essink B B O, Back N K T, Berkhout B. Increased polymerase fidelity of the 3TC-resistant variants of HIV-1 reverse transcriptase. Nucleic Acids Res. 1997;25:3212–3217. doi: 10.1093/nar/25.16.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgiadis M M, Jessen S M, Ogata C M, Telesnitsky A, Goff S P, Hendrickson W A. Mechanistic implications from the structure of a catalytic fragment of Moloney murine leukemia virus reverse transcriptase. Structure. 1995;3:879–892. doi: 10.1016/S0969-2126(01)00223-4. [DOI] [PubMed] [Google Scholar]
- 13.Iversen A K N, Shafer R W, Wehrly K, Winters M A, Mullins J I, Chesebro B, Merigan T C. Multidrug-resistant human immunodeficiency virus type 1 strains resulting from combination antiretroviral therapy. J Virol. 1996;70:1086–1090. doi: 10.1128/jvi.70.2.1086-1090.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaju M, Beard W A, Wilson S H. Human immunodeficiency virus type 1 reverse transcriptase. J Biol Chem. 1995;270:9740–9747. doi: 10.1074/jbc.270.17.9740. [DOI] [PubMed] [Google Scholar]
- 15.Johnson V A. Combination therapy for HIV-1 infection-overview: preclinical and clinical analysis of antiretroviral combinations. Antiviral Res. 1996;29:35–39. doi: 10.1016/0166-3542(95)00912-4. [DOI] [PubMed] [Google Scholar]
- 16.Larder B A. Inhibitors of HIV reverse transcriptase as antiviral agents and drug resistance. In: Skalka A M, Goff S P, editors. Reverse transcriptase. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1993. pp. 205–222. [Google Scholar]
- 17.Mansky L M. The mutation rate of human immunodeficiency virus type 1 is influenced by the vpr gene. Virology. 1996;222:391–400. doi: 10.1006/viro.1996.0436. [DOI] [PubMed] [Google Scholar]
- 18.Mansky L M, Temin M. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol. 1995;69:5087–5094. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin J L, Wilson J E, Haynes R B, Furman P A. Mechanism of resistance of human immunodeficiency virus type 1 to 2′,3′-dideoxyinosine. Proc Natl Acad Sci USA. 1993;90:6135–6139. doi: 10.1073/pnas.90.13.6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendelman L V, Boosalis M S, Petruska J, Goodman M F. Nearest neighbor influences on DNA polymerase insertion fidelity. J Biol Chem. 1989;264:14415–14423. [PubMed] [Google Scholar]
- 21.Pandey V N, Kaushik N, Rege N, Sarafianos S G, Yadav P N S, Modak M J. Role of methionine 184 in human immunodeficiency virus type 1 reverse transcriptase in the polymerase function and fidelity of DNA synthesis. Biochemistry. 1996;35:2168–2179. doi: 10.1021/bi9516642. [DOI] [PubMed] [Google Scholar]
- 22.Perrin L, Hirschel B. Combination therapy in primary HIV infection. Antiviral Res. 1996;29:87–89. doi: 10.1016/0166-3542(95)00925-6. [DOI] [PubMed] [Google Scholar]
- 23.Poch O, Sauvaget I, Delarue M, Tordo N. Identification of four conserved motifs among the RNA-dependent encoding elements. EMBO J. 1989;8:3867–3874. doi: 10.1002/j.1460-2075.1989.tb08565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Preston B D, Poiesz B J, Loeb L A. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–1171. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 24a.Rezende, L. F., et al. Unpublished observations.
- 25.Ricchetti M, Buc H. Reverse transcriptases and genomic variability: the accuracy of DNA replication is enzyme specific and sequence dependent. EMBO J. 1990;9:1583–1593. doi: 10.1002/j.1460-2075.1990.tb08278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richman D D. HIV drug resistance. AIDS Res Hum Retroviruses. 1992;8:1065–1071. doi: 10.1089/aid.1992.8.1065. [DOI] [PubMed] [Google Scholar]
- 27.Roberts J D, Bebenek K, Kunkel T A. The accuracy of reverse transcriptase from HIV-1. Science. 1988;242:1171–1173. doi: 10.1126/science.2460925. [DOI] [PubMed] [Google Scholar]
- 27a.Rubinek T, Bakhanashvili M, Hizi A. The fidelity of 3′ misinsertion and mispair extension during DNA synthesis exhibited by two drug-resistant mutants of the reverse transcriptase of human immunodeficiency virus type 1 with Leu74Val and Glu89Gly. Eur J Biochem. 1997;247:238–247. doi: 10.1111/j.1432-1033.1997.00238.x. [DOI] [PubMed] [Google Scholar]
- 28.Sarafianos S G, Pandey V N, Kaushik N, Modak M J. Glutamine 151 participates in the substrate dNTP binding function of HIV-1 reverse transcriptase. Biochemistry. 1995;34:7207–7216. doi: 10.1021/bi00021a036. [DOI] [PubMed] [Google Scholar]
- 29.Shafer R W, Kozal M J, Winters M A, Iversen A K N, Katzenstein D A, Ragnei M V, Meyer III W A, Gupta P, Rasheed S, Coombs R, Katzman M, Fiscus S, Merrigan T C. Combination therapy with zidovudine and didanosine selects for drug-resistant human immunodeficiency virus type 1 strains with unique patterns of pol gene mutations. J Infect Dis. 1994;169:722–729. doi: 10.1093/infdis/169.4.722. [DOI] [PubMed] [Google Scholar]
- 30.Shirasaka T, Kavlick M F, Ueno T, Gao W, Kojima E, Alcaide M L, Chokekijchai S, Roy B M, Arnold E, Yarchoan R, Mitsuya H. Emergence of human immunodeficiency virus type 1 variants with resistance to multiple dideoxynucleosides in patients receiving therapy with dideoxynucleosides. Proc Natl Acad Sci USA. 1995;92:2398–2402. doi: 10.1073/pnas.92.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirasaka T, Yarchoan R, O’Brien M C, Husson R N, Anderson B D, Kojima E, Shimada T, Border S, Mitsuya H. Changes in drug sensitivity of human immunodeficiency virus type 1 during therapy with azidothymidine, dideoxycytidine, and dideoxyinosine: an in vitro comparative study. Proc Natl Acad Sci USA. 1993;90:562–566. doi: 10.1073/pnas.90.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temin H M. Retrovirus variation and reverse transcription: abnormal transfers result in retrovirus genetic variation. Proc Natl Acad Sci USA. 1993;90:6900–6903. doi: 10.1073/pnas.90.15.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno T, Mitsuya H. Comparative enzymatic study of HIV-1 reverse transcriptase resistant to 2′,3′-dideoxynucleotide analogs using the single-nucleotide incorporation assay. Biochemistry. 1997;36:1092–1099. doi: 10.1021/bi962393d. [DOI] [PubMed] [Google Scholar]
- 34.Ueno T, Shirasaka T, Mitsuya H. Enzymatic characterization of human immunodeficiency virus reverse transcriptase resistant to multiple 2′,3′-dideoxynucleoside 5′-triphosphates. J Biol Chem. 1995;270:23605–23611. doi: 10.1074/jbc.270.40.23605. [DOI] [PubMed] [Google Scholar]
- 35.Wainberg M A, Drosopoulos W C, Salomon H, Hsu M, Borkow G, Parniak M A, Gu Z, Song Q, Manne J, Islam S, Castriota G, Prasad V R. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science. 1996;271:1282–1285. doi: 10.1126/science.271.5253.1282. [DOI] [PubMed] [Google Scholar]