Abstract
The association between serum tumor necrosis factor receptor (TNFRs: TNFR1, TNFR2) levels and estimated glomerular filtration rate (eGFR) observed in patients with diabetes has not been comprehensively tested in healthy subjects with normal kidney function. It also remains unclear whether TNFR levels differ by age and sex, and between healthy subjects and diabetics. We measured serum TNFR levels in 413 healthy subjects and 292 patients with type 2 diabetes. In healthy subjects, TNFR levels did not differ between men and women. Additionally, TNFR2, but not TNFR1, levels increased with age. In multivariate analysis, TNFR1 was associated only with cystatin C-based eGFR (eGFR-CysC), whereas TNFR2 was associated with systolic blood pressure in addition to eGFR-CysC. Both TNFRs were associated with lower eGFR (eGFR-Cys < 90 mL/min/1.73 m2) even after adjustment for relevant clinical factors. Upon combining healthy subjects and patients with diabetes, the presence of diabetes and elevated glycated hemoglobin level were significant factors in determining TNFR levels. TNFR levels were associated with eGFR-CysC, but were not affected by age and sex in healthy subjects with normal kidney function. TNFR levels in patients with diabetes appeared to be higher than in healthy subjects.
Keywords: TNF receptor, Biomarker, Diabetes, eGFR, Kidney function, Healthy subject
Subject terms: Biomarkers, Endocrinology, Nephrology
Introduction
A growing body of evidence suggests that chronic inflammation plays an important role in the progression of chronic kidney disease (CKD), including diabetic kidney disease (DKD)1,2. Tumor necrosis factor (TNF)α is a central proinflammatory cytokine, which binds functionally distinct cell surface receptors, TNF receptors (TNFRs: TNFR1 and TNFR2)3,4. A number of studies have reported that elevated levels of TNFR predict decline in kidney function in patients with diabetes and CKD, and even in the general population5–11. TNFR levels correlate with both albuminuria/proteinuria and estimated glomerular filtration rate (eGFR)12,13. Moreover, several studies have reported that other factors, such as age, sex, body mass index (BMI), hypertension, glycated hemoglobin (HbA1c), high-density-lipoprotein cholesterol (HDL-C), and uric acid, also correlate with TNFR levels in patients with diabetes14,15.
However, it has not been comprehensively tested whether TNFR levels are associated with urinary albumin-to-creatinine ratio (UACR) and eGFR even in healthy subjects with normal kidney function. Additionally, it is unclear whether the TNFR levels in healthy subjects differ from those in patients with diabetes. Against this background, this study was established to shed light on these issues by measuring serum TNFRs and eGFR in 413 healthy subjects and 292 patients with type 2 diabetes.
Materials and methods
Healthy subjects and patients with type 2 diabetes
Japanese adults who worked at Juntendo University Hospital in Tokyo, Japan, participated in this study as healthy controls. An annual health check-up is held for the staff at this hospital. We included 3861 of these individuals who expressed a willingness to participate in the study as healthy controls. Of these, we randomly recruited 910 individuals for whom UACR had been measured for each age group and sex. Individuals with a past medical history including cancer, diabetes, hypertension, kidney disease, dyslipidemia, or other major pathological conditions, or HbA1c > 6.5%, systolic blood pressure (BP) > 140 mmHg, diastolic BP > 90 mmHg, and eGFR-creatinine (Cr) < 60 mL/min/1.73 m2, were excluded from this study. To apply these exclusion criteria, we used data from the annual health check-up in 2022. After the application of all exclusion criteria, the final group of healthy controls was made up of 413 Japanese adults aged between 25 and 69 years (Supplementary Fig. 1). This study was approved by the institutional review board (IRB) of Juntendo University Hospital, Tokyo, Japan (No. M20-0074). The study complied with the guidelines of the Declaration of Helsinki., with subjects being able to opt out via the hospital’s website.
A separate cohort of patients with type 2 diabetes were recruited from Kure Medical Center and Chugoku Cancer Center, Hiroshima, Japan. In brief, 738 Japanese patients with diabetes agreed to participate in an observational follow-up study. Of these, we selected only 292 patients aged between 25 and 69 years, with type 2 diabetes, and eGFR-Cr > 60 mL/min/1.73 m2 (Supplementary Fig. 1). All patients with diabetes were undergoing treatment from a diabetes specialist, and 25.3% of them were undergoing insulin therapy. This study was approved by the ethics committee of Kure Medical Center and Chugoku Cancer Center (No. 26-06). Informed consent was obtained from all patients, and the study complied with the tenet of the Declaration of Helsinki.
Assessment of clinical variables
Serum Cr and cystatin C (CysC) levels were measured using standard enzymatic methods, and colloidal gold immunoassay [Nescauto GC Cystatin C (Nm); Alfresa Pharma Corp., Osaka, Japan], respectively. eGFR was calculated in accordance with Japanese Society of Nephrology guidelines using the following formula, which was designed for the Japanese population: eGFR-Cr (mL/min/1.73 m2) = 194 × [age (years)]−0.287 × [serum Cr (mg/ dL)]−1.094 × 0.739 (for women); eGFR-CysC (mL/min/1.73 m2) = [104 × (serum CysC)−1.019 × 0.996Age (× 0.929 for women)] − 816,17. Urinary albumin was analyzed by a nephelometry assay (N-assay TIA Micro Alb; Nittobo Medical Co., Ltd., Fukushima, Japan). UACR was used as an index of urinary albumin excretion, and expressed as milligrams of albumin per gram of Cr (mg/gCr). Non-HDL-C was defined as the difference between total cholesterol and HDL-C.
Measurement of tumor necrosis factor receptors 1 and 2 in serum
We measured serum TNFR1 and TNFR2 concentrations using Human TNF RI/TNFRSF1A and TNF RII/TNFRSF1B Quantikine ELISA kits (Cat. Nos. DRT100 and DRT200; R&D Systems, Minneapolis, MN, USA), respectively, as previously described18.
Statistical analysis
The characteristics of the participants are expressed as mean ± standard deviation (SD), median (25–75% interquartile range), or frequency (count and percentage). We used the Shapiro–Wilk test to assess the normality of distributions. TNFR and UACR are expressed as the median and 25–75% interquartile range, and other characteristics are expressed as the mean ± SD. Continuous variables were compared using the Mann–Whitney U test. Fisher’s exact test was used to compare categorical variables. Data with two independent variables were analyzed by two-way ANOVA. Spearman’s rank correlation analysis was used to analyze the correlations between two variables. Univariate and multivariate linear regression models were used to determine the factors that affect the serum TNFR levels. Univariate and multivariate logistic regression analyses were performed to examine the association of lower eGFR-CysC and TNFR levels. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA). A p value of < 0.05 was considered statistically significant.
Results
Clinical characteristics of healthy control and patients with diabetes
As shown in Table 1, the healthy subjects and the diabetes cohort consisted of 413 individuals (206 women, 49.9%) and 292 patients (143 women, 49.0%), respectively. We recruited healthy subjects and patients with diabetes whose eGFR-Cr was > 60 mL/min/1.73 m2. No significant difference was observed in the sex distribution or eGFR-Cr between the two cohorts. However, the patients with diabetes were older and had higher BMI, systolic BP, diastolic BP, HbA1c, uric acid, UACR, TNFR1, and TNFR2 than the healthy subjects. Meanwhile, healthy subjects had higher HDL-C and non-HDL-C than patients with diabetes.
Table 1.
Clinical characteristics of healthy subjects and patients with type 2 diabetes.
| Characteristics | Healthy subjects (n = 413) | Diabetics (n = 292) | U value | P value |
|---|---|---|---|---|
| Age (year) | 43 ± 12 | 57 ± 11 | 22,292 | < 0.0001 |
| Male (%) | 206 (49.9) | 143 (49.0) | NA | 0.81 |
| BMI | 22.1 ± 3.3 | 26.1 ± 5.1 | 29,797 | < 0.0001 |
| Systolic BP (mmHg) | 120 ± 15 | 138 ± 16 | 23,709 | < 0.0001 |
| Diastolic BP (mmHg) | 72 ± 11 | 81 ± 11 | 32,172 | < 0.0001 |
| HbA1c (%) | 5.5 ± 0.3 | 7.5 ± 1.2 | 2365 | < 0.0001 |
| HDL-C (mg/dL) | 67 ± 17 | 52 ± 12 | 92,155 | < 0.0001 |
| Non-HDL-C (mg/dL) | 141 ± 35 | 134 ± 33 | 67,391 | 0.008 |
| Uric acid (mg/dL) | 4.9 ± 1.3 | 5.1 ± 1.2 | 53,489 | 0.011 |
| UACR (mg/gCr) | 4.5 (3.4, 7.3) | 18.1 (7.1, 58.9) | 92,155 | < 0.0001 |
| eGFR-Cr (mL/min/1.73 m2) | 82 ± 13 | 85 ± 19 | 58,505 | 0.50 |
| eGFR-CysC (mL/min/1.73 m2) | 110 ± 19 | 97 ± 23 | 81,948 | < 0.0001 |
| TNFR1 (pg/mL) | 992 (873, 1126) | 1357 (1133, 1646) | 19,703 | < 0.0001 |
| TNFR2 (pg/mL) | 1951 (1717, 2218) | 2904 (2505, 3391) | 14,127 | < 0.0001 |
BMI body mass index, BP blood pressure, Cr creatinine, CysC cystatin C, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, HDL-C high-density-lipoprotein cholesterol, NA not applicable, TNF tumor necrosis factor, TNFR TNF receptor, UACR urinary albumin-to-creatinine ratio.
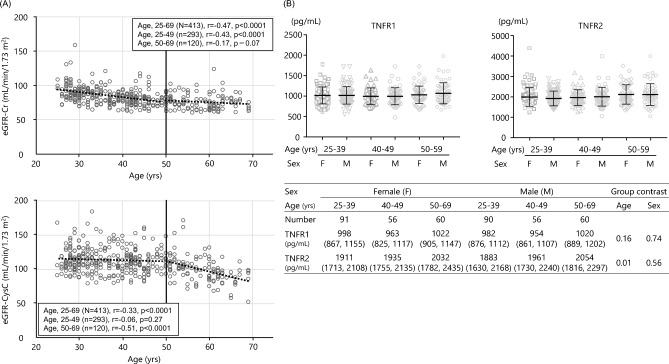
Circulating TNFR levels in each age group and sex in healthy subjects
As shown in Table 2, neither of the TNFR levels differed between the male and female groups. However, there was a significant difference in the TNFR2 levels between the age groups (p = 0.047).
Table 2.
Clinical characteristics of healthy subjects according to sex and age group.
| Variables | Women | Men | Group contrast | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25–29 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | 25–29 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | Age | Sex | |
| Number | 31 | 60 | 56 | 35 | 25 | 30 | 60 | 56 | 33 | 27 | ||
| TNFR1 | 981 (845, 1110) | 998 (888, 1157) | 963 (827, 1111) | 1036 (906, 1147) | 951 (905, 1103) | 978 (884, 1143) | 984 (869, 1097) | 954 (861, 1107) | 1023 (909, 1191) | 993 (854, 1205) | 0.40 | 0.43 |
| TNFR2 | 1944 (1773, 2122) | 1858 (1689, 2100) | 1896 (1724, 2169) | 1973 (1751, 2541) | 2091 (1867, 2421) | 1912 (1679, 2298) | 1840 (1605, 2113) | 1961 (1730, 2240) | 2089 (1905, 2321) | 2028 (1759, 2251) | 0.047 | 0.63 |
Abbreviations used in this table are the same as in Table 1.
Correlations among TNFRs, eGFR, and clinical parameters
As shown in Table 3, in healthy subjects, a moderate correlation was observed between TNFR1 and TNFR2 (r = 0.58, p < 0.0001). Meanwhile, negative correlations were observed between the TNFRs and eGFR-CysC (TNFR1, r = − 0.38, p < 0.0001; TNFR2, r = − 0.35, p < 0.0001), while there were very weak correlations between the TNFRs and eGFR-Cr (TNFR1, r = − 0.10, p < 0.05; TNFR2, r = − 0.10, p < 0.05). TNFR2 was also associated with age (r = 0.11, p < 0.05) and systolic BP (r = 0.11, p < 0.05), but the correlations were very weak. The association between TNFR2 and age may have been due in part to the fact that eGFR-CysC was more strongly correlated with age for subjects over 50 years of age than for those under 50 years of age (Fig. 1). Indeed, as shown in Supplementary Table 1, multivariate regression analysis demonstrated that eGFR-CysC, but not eGFR-Cr, was associated with both TNFR1 and TNFR2. Systolic BP was still weakly associated with TNFR2, but age was not after adjustment. In patients with type 2 diabetes, TNFRs were associated with more clinical factors than in healthy subjects, in both univariate and multivariate regression analyses (Table 3 and Supplementary Table 2).
Table 3.
Spearman's correlation between TNFRs, eGFR, and clinical parameters in healthy subjects and patients with type 2 diabetes.
| Variables | Healthy subjects (n = 413) | Diabetics (n = 292) | ||||||
|---|---|---|---|---|---|---|---|---|
| TNFR1 | TNFR2 | GFR-Cr | GFR-CysC | TNFR1 | TNFR2 | GFR-Cr | GFR-CysC | |
| Age | 0.04 | 0.11* | − 0.47*** | − 0.33*** | 0.14* | 0.16** | − 0.48*** | − 0.31*** |
| BMI | 0.08 | 0.05 | − 0.21*** | − 0.04 | 0.17** | 0.11 | 0.20*** | − 0.05 |
| Systolic BP | 0.07 | 0.11* | − 0.14** | − 0.09 | 0.11 | 0.07 | − 0.07 | − 0.15* |
| Diastolic BP | 0.03 | 0.08 | − 0.16** | − 0.07 | 0.05 | 0.04 | 0.09 | 0.09 |
| HbA1c | 0.06 | 0.03 | − 0.18*** | − 0.12* | − 0.20*** | − 0.25*** | 0.17** | 0.08 |
| HDL-C | − 0.04 | 0.03 | − 0.01 | − 0.02 | − 0.08 | − 0.13* | − 0.10 | − 0.19** |
| Non-HDL-C | 0.04 | 0.05 | − 0.23*** | − 0.17*** | 0.19** | 0.13* | 0.10 | 0.05 |
| Uric acid | 0.01 | − 0.01 | − 0.12* | 0.13** | 0.17** | 0.14* | − 0.17** | 0.05 |
| UACR | − 0.004 | 0.02 | 0.11* | − 0.20*** | 0.38*** | 0.25*** | 0.13* | − 0.10 |
| eGFR-Cr | − 0.10* | − 0.10* | – | – | − 0.18** | − 0.19** | – | – |
| eGFR-CysC | − 0.38*** | − 0.35*** | 0.19*** | – | − 0.30*** | − 0.28*** | 0.31*** | – |
| TNFR2 | 0.58*** | – | – | – | 0.82*** | – | – | – |
Abbreviations used in this table are the same as in Table 1.
*p < 0.05, **p < 0.01, ***p < 0.001
Figure 1.
Associations of age and sex with eGFR and serum TNFR levels in healthy subjects. (A) Scatter plot of association between age and eGFR-Cr or eGFR-cysC. (B) Serum TNFR levels by each age and sex. The bars represent median and 25–75% interquartile range.
TNFRs as early markers of decline in kidney function
In healthy subjects, both eGFR-Cr and eGFR-CysC were associated with many demographic/clinical parameters, including age, HbA1c, non-HDL-C, uric acid, UACR, and TNFRs, although eGFR-Cr and eGFR-CysC were associated weakly with each other (r = 0.19, p < 0.001) (Table 3). Age and UACR remained significant clinical predictors of lower eGFR-CysC (< 90 mL/min/1.73 m2) in the multivariate analysis (model 1) (Table 4). Next, we assessed the independent effect of each TNFR on lower eGFR-CysC by adding them to model 1 (models 2–3) (Table 4). In these models, both TNFRs remained significant, in addition to age and UACR.
Table 4.
Multivariate analysis of variables associated with eGFR decline (eGFR-CysC < 90 mL/min/1.73 m2) in healthy subjects.
| Variables | Per 1 increase | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| Age | 1 year | 1.07 (1.03–1.10) | < 0.0001 | 1.08 (1.05–1.11) | < 0.0001 | 1.08 (1.05–1.11) | < 0.0001 |
| Uric acid | 1 mg/dL | 0.79 (0.61–1.03) | 0.08 | – | – | – | – |
| HbA1c | 1% | 2.93 (0.85–10.09) | 0.09 | – | – | – | – |
| Non-HDL-C | 1 mg/dL | 1.00 (0.93–1.01) | 0.57 | – | – | – | – |
| UACR | log 1SD = 0.28 | 1.43 (1.09–1.89) | 0.011 | 1.57 (1.17–2.11) | 0.003 | 1.49 (1.12–1.99) | 0.006 |
| TNFR1 | log 1SD = 0.09 | – | – | 2.51 (1.76–3.59) | < 0.0001 | – | – |
| TNFR2 | log1 SD = 0.09 | – | – | – | – | 1.88 (1.38–2.57) | < 0.0001 |
Model 1: age, uric acid, HbA1c, non-HDL-C, and UACR.
Model 2: age, UACR, and TNFR1.
Model 3: age, UACR, and TNFR2.
Abbreviations used in this table are the same as in Table 1.
CI confidence interval, OR odds ratio.
TNFR levels are associated with presence of diabetes and higher glycated hemoglobin
To determine whether TNFR values are higher in patients with type 2 diabetes mellitus than in healthy controls, the two groups were combined into a single group and subjected to univariate and multivariate stepwise regression analyses to identify factors that define TNFR values. TNFRs were found to be associated with many clinical parameters (Table 5, Supplementary Tables 3 and 4). The presence of diabetes and elevated HbA1c level were revealed to be significant factors in determining TNFR levels in multivariate regression analysis, indicating that TNFR levels in patients with diabetes appeared to be higher than in healthy subjects.
Table 5.
Multivariate stepwise regression analysis of the factors associated with tumor necrosis factor receptor 1 or tumor necrosis factor receptor 2 in the combined group of healthy subjects and patients with type 2 diabetes.
| Variables | Estimate | SE | t value | P |
|---|---|---|---|---|
| TNFR1 | ||||
| Age | − 0.0006 | 0.0340 | − 1.943 | 0.052 |
| Sex | 0.0067 | 0.0070 | 9.556 | < 0.0001 |
| BMI | 0.0016 | 0.0008 | 1.967 | 0.0049 |
| Diabetes | 0.0767 | 0.0094 | 8.180 | < 0.0001 |
| Non-HDL | − 0.0003 | 0.0001 | − 2.773 | 0.006 |
| eGFR-CysC | − 0.0024 | 0.0002 | − 13.673 | < 0.0001 |
| ACR | 0.0452 | 0.0066 | 6.855 | < 0.0001 |
| TNFR2 | ||||
| Sex | 0.0628 | 0.0074 | 8.526 | < 0.0001 |
| Diabetes | 0.1167 | 0.0086 | 13.521 | < 0.0001 |
| Non-HDL | − 0.0003 | 0.0001 | − 3.061 | 0.003 |
| eGFR-CysC | − 0.0023 | 0.0002 | − 12.725 | < 0.0001 |
| UACR | 0.0375 | 0.0070 | 5.352 | < 0.0001 |
Abbreviations used in this table are the same as in Table 1.
SE standard error.
Discussion
In this study, we demonstrated that serum TNFR levels are associated with eGFR-CysC, but not eGFR-Cr, even in healthy subjects with normal kidney function, as well as in patients with type 2 diabetes and normal kidney function. The findings also showed that neither of the TNFRs was affected by age and sex in healthy subjects. Moreover, patients with type 2 diabetes had higher TNFR levels than healthy subjects.
Because the decline in eGFR with age is expected to affect TNFR excretion, resulting in lower fractional TNFR excretion with age, the population with eGFR-Cr < 60 mL/min/1.73 m2 was excluded from this study. We previously reported that TNFR levels increased with declining eGFR-CysC in patients with type 2 diabetes and normal kidney function, and that the increase in serum TNFRs might result from their increased systemic production, including in the kidney, rather than being a simple reflection of GFR decline19. Building on this previous work, the present study demonstrated that the correlation between TNFR levels and eGFR-CysC was observed even in healthy subjects with normal kidney function.
Franceschi et al.20 proposed the concept of inflammaging, referring to chronic low-grade inflammation that is exacerbated with age and affects the acquired and innate immune systems. The elderly exhibit an inflammaging-like phenotype featuring increases in inflammatory markers such as inflammatory cytokines in the blood and activation of inflammatory signals in the tissues, suggesting the induction of chronic low-level inflammation21. In the present study, eGFR-CysC did not change from age 20 to 49, but decreased after age 50. Therefore, age-related elevation in TNFR levels after the age of 50 may simply reflect declining eGFR-CysC, given the finding of multivariate analysis that the relationship between TNFR2 and age disappeared after adjustment for eGFR-CysC. In other words, the increase in inflammatory markers with aging may instead reflect the effects of declining kidney function. Further research is needed to elaborate on the exact mechanisms involved in the increase of TNFRs.
A post hoc analysis of cardiovascular outcome trials involving relatively preserved kidney function in patients with type 2 diabetes treated with a sodium-glucose co-transporter protein-2 (SGLT2) inhibitor, canagliflozin, reported two major findings. Not only were baseline TNFR levels associated with the progression of kidney disease, but also a smaller difference in TNFR levels between baseline and 1 year after SGLT2 inhibitor treatment was associated with better subsequent kidney outcomes22,23. Moreover, it has been reported that TNFR levels are associated with histological findings in renal tissues, such as mesangial fractional volume and percentage of endothelial cell fenestration, in early diabetic nephropathy24. Therefore, the measurement of TNFR levels may be valuable not only for predicting kidney biopsy findings and future kidney outcomes, but also as a marker of treatment responsiveness, suggesting potential therapeutic applications. Additionally, the association between longitudinal changes in TNFR and the subsequent risk of end stage kidney disease or kidney function decline has been reported in studies such as the VA NEPHRON-D trial involving advanced DKD patients and the AASK trial targeting CKD patients with hypertension25. A report has also been published indicating that the Janus kinase inhibitor baricitinib lowers TNFR levels in patients with type 2 diabetes, while another report suggested that the renin-angiotensin-system inhibitor losartan does not have the same effect26. There is thus a need for further investigation to determine how TNFR concentrations change with different treatments.
As shown in Supplementary Table 5, the levels of TNFR in the Native American Pima tribe with type 2 diabetes appeared to be higher than those in Japanese patients with type 2 diabetes. This could be explained by the Japanese having lower kidney function but similar levels of albuminuria compared with Pima Native Americans10,24. Considering how high circulating TNFR2 level and TNFR2 mRNA level are in fat tissue in obese individuals compared with those in others, it cannot be ruled out that BMI is somewhat involved in the elevated TNFR levels observed in Pima Native Americans with type 2 diabetes27. However, in the present study, BMI was not associated with TNFR levels in either healthy subjects or patients with type 2 diabetes, suggesting that this tribe’s TNFR levels are elevated by genetic or other factors.
The impact of various factors on TNFR levels in patients with diabetes makes it difficult to definitively state that these levels independently differ between Japanese and Caucasian populations. However, the TNFR levels in Japanese patients with type 2 diabetes tend to be lower than those in their Caucasian equivalents28–30. Further validation is needed to determine whether there are ethnic differences in TNFR levels and what clinical factors influence TNFR levels.
Our study is associated with several limitations that warrant mentioning. We used serum cystatin C-based estimates of eGFR, which are less accurate than direct measurements. Moreover, only Japanese subjects were enrolled in this work. Therefore, the results may not apply to other ethnic groups or DKD patients with kidney function decline. Furthermore, we assembled the healthy subjects from among those who worked at Juntendo University Hospital. Therefore, many of the recruited subjects are likely to have a better understanding of disease than the general healthy population. As such, this control group may have been healthier than the general healthy population.
In conclusion, we revealed that the serum TNFR levels in healthy Japanese subjects are not affected by age or sex, but are related to eGFR even in those with normal kidney function. Further studies are needed to collect a larger number of appropriate subjects from multiple centers and to clarify reference values not only in healthy subjects from the Japanese population but also from other ethnic groups.
Supplementary Information
Acknowledgements
We thank staff at the Department of Research Support Utilizing Bioresource Bank, Juntendo University Graduate School of Medicine, for allowing us to use their facilities. We also thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author contributions
T.G. analyzed and interpreted the patient data and was a major contributor to writing the manuscript. H.T., K.T., T.O., M.W. and Y.H. investigated the patient data. M.M. and T.S. performed the ELISA. Y.S., Y.T., and N.K. revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was partially funded by JSPS KAKENHI Grant Number JP20K08617 to TG.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-57265-x.
References
- 1.Tomino Y, Gohda T. The prevalence and management of diabetic nephropathy in Asia. Kidney Dis. 2015;1:52–60. doi: 10.1159/000381757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gohda T, et al. Concept of diabetic kidney disease: Paradigm shift from albuminuria-based to GFR-based kidney disease. Juntendo Med. J. 2019;65:510–516. doi: 10.14789/jmj.2019.65.JMJ19-R16. [DOI] [Google Scholar]
- 3.Gohda T, Tomino Y. Novel biomarkers for the progression of diabetic nephropathy: soluble TNF receptors. Curr. Diab. Rep. 2013;13:560–566. doi: 10.1007/s11892-013-0385-9. [DOI] [PubMed] [Google Scholar]
- 4.Murakoshi M, Gohda T, Suzuki Y. Circulating tumor necrosis factor receptors: A potential biomarker for the progression of diabetic kidney disease. Int. J. Mol. Sci. 2020;21:1957. doi: 10.3390/ijms21061957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niewczas MA, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J. Am. Soc. Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gohda T, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J. Am. Soc. Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatraju PK, Zelnick LR, Shlipak M, Katz R, Kestenbaum B. Association of soluble TNFR-1 concentrations with long-term decline in kidney function: The multi-ethnic study of atherosclerosis. J. Am. Soc. Nephrol. 2018;29:2713–2721. doi: 10.1681/ASN.2018070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gohda T, et al. Association between kidney function decline and baseline TNFR levels or change ratio in TNFR by febuxostat chiefly in non-diabetic CKD patients with asymptomatic hyperuricemia. Front. Med. 2021;8:634932. doi: 10.3389/fmed.2021.634932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarnak MJ, et al. Plasma biomarkers as risk factors for incident CKD. Kidney Int. Rep. 2022;7:1493–1501. doi: 10.1016/j.ekir.2022.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakoshi M, et al. Progranulin and its receptor predict kidney function decline in patients with type 2 diabetes. Front. Endocrinol. 2022;13:849457. doi: 10.3389/fendo.2022.849457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murakoshi M, et al. Circulating tumor necrosis factor-related biomarkers predict kidney function decline in Japanese patients with diabetes: An observational cohort study. Diabetes Res. Clin. Pract. 2023;206:111017. doi: 10.1016/j.diabres.2023.111017. [DOI] [PubMed] [Google Scholar]
- 12.Kamei N, et al. Association between circulating tumor necrosis factor-related biomarkers and estimated glomerular filtration rate in type 2 diabetes. Sci. Rep.-Uk. 2018;8:15302. doi: 10.1038/s41598-018-33590-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gohda T, et al. Clinical predictive biomarkers for normoalbuminuric diabetic kidney disease. Diabetes Res. Clin. Pract. 2018;141:62–68. doi: 10.1016/j.diabres.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Dzienis-Straczkowska S, et al. Soluble tumor necrosis factor-alpha receptors in young obese subjects with normal and impaired glucose tolerance. Diabetes Care. 2003;26:875–880. doi: 10.2337/diacare.26.3.875. [DOI] [PubMed] [Google Scholar]
- 15.Miyazawa I, et al. Association between serum soluble TNFalpha receptors and renal dysfunction in type 2 diabetic patients without proteinuria. Diabetes Res. Clin. Pract. 2011;92:174–180. doi: 10.1016/j.diabres.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo S, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. GFR estimation using standardized serum cystatin C in Japan. Am. J. Kidney Dis. 2013;61:197–203. doi: 10.1053/j.ajkd.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Gohda T, et al. Circulating TNF receptors 1 and 2 Predict mortality in patients with end-stage renal disease undergoing dialysis. Sci. Rep. 2017;7:43520. doi: 10.1038/srep43520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gohda T, et al. Fractional excretion of tumor necrosis factor receptor 1 and 2 in patients with type 2 diabetes and normal renal function. J. Diabetes Investig. 2021;12:382–389. doi: 10.1111/jdi.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi C, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 21.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sen T, et al. Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia. 2021;64:2147–2158. doi: 10.1007/s00125-021-05512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waijer SW, et al. Association between TNF receptors and KIM-1 with kidney outcomes in early-stage diabetic kidney disease. Clin. J. Am. Soc. Nephrol. 2022;17:251–259. doi: 10.2215/CJN.08780621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavkov ME, et al. Tumor necrosis factor receptors 1 and 2 are associated with early glomerular lesions in type 2 diabetes. Kidney Int. 2016;89:226–234. doi: 10.1038/ki.2015.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen TK, et al. Longitudinal TNFR1 and TNFR2 and kidney outcomes: Results from AASK and VA NEPHRON-D. J. Am. Soc. Nephrol. 2022;33:996–1010. doi: 10.1681/ASN.2021060735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niewczas MA, et al. A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat. Med. 2019;25:805–813. doi: 10.1038/s41591-019-0415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Arner P, Atkinson RL, Spiegelman BM. Differential regulation of the p80 tumor necrosis factor receptor in human obesity and insulin resistance. Diabetes. 1997;46:451–455. doi: 10.2337/diab.46.3.451. [DOI] [PubMed] [Google Scholar]
- 28.Saulnier PJ, et al. Association of circulating biomarkers (Adrenomedullin, TNFR1, and NT-proBNP) with renal function decline in patients with Type 2 diabetes: A French prospective cohort. Diabetes Care. 2017;40:367–374. doi: 10.2337/dc16-1571. [DOI] [PubMed] [Google Scholar]
- 29.Oshima M, et al. Comparison of circulating biomarkers in predicting diabetic kidney disease progression with autoantibodies to erythropoietin receptor. Kidney Int. Rep. 2021;6:284–295. doi: 10.1016/j.ekir.2020.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pavkov ME, et al. Elevation of circulating TNF receptors 1 and 2 increases the risk of end-stage renal disease in American Indians with type 2 diabetes. Kidney Int. 2015;87:812–819. doi: 10.1038/ki.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.