Abstract
Purpose
In view of conflicting reports on the ability of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to infect placental tissue, this study aimed to further evaluate the impact of inflammation and placental damage from symptomatic third-trimester maternal COVID-19 infection.
Materials and Methods
This case-control study included 32 placenta samples each from symptomatic COVID-19 pregnancy and normal non-COVID-19 pregnancy. The villous placental area’s inflammatory expression [angiotensin converting enzyme-2 (ACE-2), transmembrane protease serine-2 (TMPRSS2), interferon-γ (IFN-γ), interleukin-6 (IL-6), and SARS-CoV-2 spike protein] and apoptotic rate were examined using immunohistochemistry and Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) assay. Comparison and correlation analysis were used based on COVID-19 infection, placental SARS-CoV-2 spike protein evidence, and maternal severity status.
Results
Higher expressions of TMPRSS2, IFN-γ, and trophoblast apoptotic rate were observed in the COVID-19 group (p<0.001), whereas ACE-2 and IL-6 expressions were not significantly different from the control group (p>0.05). Additionally, SARS-CoV-2 spike protein was detected in 8 (25%) placental samples of COVID-19 pregnancy. COVID-19 subgroup analysis revealed increased IFN-γ, trophoblast, and stromal apoptosis (p<0.01). Moreover, the results of the current study revealed no correlation between maternal COVID-19 severity and placental inflammation as well as the apoptotic process.
Conclusion
The presence of SARS-CoV-2 spike protein as well as altered inflammatory and apoptotic processes may indicate the presence of placental disturbance in third-trimester maternal COVID-19 infection. The lack of correlation between placental disruption and maternal severity status suggests the need for more research to understand the infection process and any potential long-term impacts on all offsprings born to COVID-19-infected pregnant women.
Keywords: COVID-19, pregnancy, placenta, inflammation, apoptosis
Graphical Abstract
INTRODUCTION
More than 6 million people have died worldwide as a result of the highly contagious viral disease known as COVID-19.1 The transition of pandemic conditions to endemic is very likely to occur, even though current COVID-19 cases are still showing an increase in the surge in infection cases.2 Therefore, further research into the full impact on special populations, such as pregnancy, is still required.
Despite the fact that the susceptible pregnant population’s vulnerability to COVID-19 has been amply established by the rise in pregnancy morbidity, demand for intensive care, and use of mechanical ventilators, it turns out that the COVID-19’s effect on the placental unit and perinatal transmission is still a contentious issue due to inconsistent research findings.3
The placenta serves as a crucial barrier to infection, particularly severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Numerous studies have linked COVID-19 to morphological abnormalities in the placenta.4,5,6 Maternal vascular malformations, fibrin villi deposition, vilitis, intervillositis, and acute inflammation were the most prevalent aberrant histological findings.7 Despite inconsistencies, evidence suggests that the SARS-CoV-2 virus is present in the placenta,7,8,9 combined with the presence of the receptor, allowing the virus to infect the placenta.10
COVID-19 can cause excessive inflammation and cell damage in the lungs;11 therefore, the same thing could happen in the placenta if SARS-CoV-2 can reach this tissue. Due to the scarcity of original, matched control studies, the present study aimed to assess the further impact of specific inflammation and placental damage from symptomatic third-trimester maternal COVID-19 infection. This evidence is critical since recent data showed that placental disturbance and inflammation may contribute to the direct and long-term impacts on neonatal health outcomes, ranging from neurodevelopmental issues to autism disorders.12
MATERIALS AND METHODS
Sample collection, processing, and study design
This study used a case-control design, collecting placenta from symptomatic pregnant women who tested positive for SARS-CoV-2 nasopharyngeal polymerase chain reaction compared to controls who had negative test results during hospitalization. Placental samples were collected from pregnant women who were admitted during the COVID-19 pandemic between November 2020 and August 2021, during which pregnant women in Indonesia were still not vaccinated. We enrolled all placental samples from the third-trimester (gestational age ≥28 weeks) pregnancy at the delivery room of Dr. Soetomo General Academic Hospital (Surabaya, Indonesia) and excluded placental samples from pregnant women with medical conditions that might cause a cytokine response bias, such as COVID-19 vaccination history, obesity, hypertension in pregnancy, diabetes mellitus, autoimmune disease, renal disease, and other infectious diseases from patient’s medical history, as well as physical and laboratory examination.
The disease severity of COVID-19 was categorized as mild, moderate, or severe-critical according to the WHO guidelines.13 Additionally, the control placentas from normal third-trimester pregnant women in the same period were also collected. At the time of delivery, a 2×2 cm entire thickness of placental tissue was placed in 10% neutral buffered formalin and the immunopathological result was evaluated by a board-certified pathologist from Dr. Soetomo General Academic Hospital’s Department of Anatomical Pathology. For descriptive purposes, additional maternal demographic, clinical, and delivery outcomes were obtained.
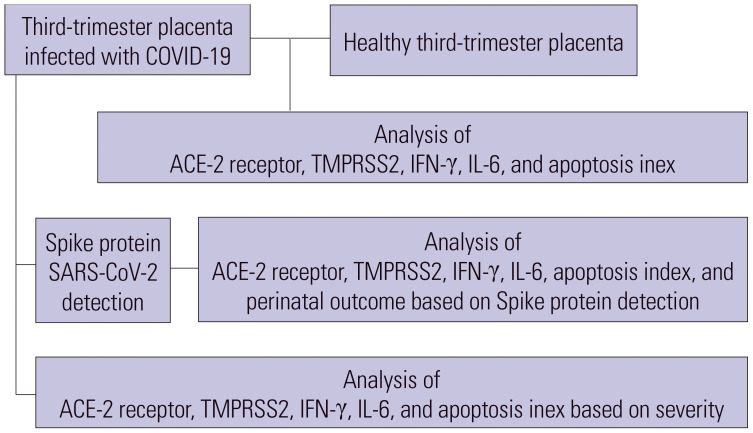
The study started by comparing the entry receptor (angiotensin converting enzyme-2, ACE-2), protease (transmembrane protease serine-2, TMPRSS2), inflammatory cytokine (interferon-γ, IFN-γ and interleukin-6, IL-6), and apoptosis index between third-trimester SARS-COV-2-infected placenta and healthy control placenta. This study then assessed the presence of SARS-COV-2 spike protein in the COVID-19-infected placenta subgroup and examined the variations in the apoptosis index, protein expression, and perinatal output from the SARS-CoV-2 spike protein-detected placenta in that subgroup. Finally, the study evaluated all of those expressions in the subgroup of COVID-19-infected placentas based on the severity of the infection (Fig. 1).
Fig. 1. Study design. SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ACE-2, angiotensin converting enzyme-2; TMPRSS2, transmembrane protease serine-2; IFN-γ, interferon-γ; IL-6, interleukin-6.
Immunohistopathological analysis
The samples were paraffin-embedded. The primary antibody was stained on 10-+m paraffin sections after routine rehydration and antigen retrieval. For antigen retrieval, samples were incubated for 15 min in sodium citrate buffer and blocked for 20 min with 3% hydrogen peroxide at room temperature. The blocking buffer was added to the samples at 37℃ for 30 minutes, followed by incubating with antibodies. All cases were stained by immunohistochemistry with ACE-2 antibody (bs-1004R; Bioss Antibody, Woburn, MA, USA), TMPRSS2 antibody (bs-6285R; Bioss Antibody), IFN-γ antibody (bs-0480R; Bioss Antibody), IL-6 antibody (bs-4539R; Bioss Antibody), and SARS-CoV-2 spike antibody (ABIN1030641; GmbH, Aachen, Germany). The expression of each antibody was examined in five fields of view with 400× magnification (Nikon H600L light microscope, Nikon Instruments, Tokyo, Japan) with positive expression shown by the red-brown chromogen and focused on placental villous to examine the fetal side’s pathological conditions. A modified semi-quantitative immunoreactive score (IRS) by Remmele and Stegner was used for all placental expressions except for the SARS-CoV-2 spike protein. This method relied on both the percentage of positive cells and the intensity of the color reaction.14,15 The IRS was calculated by multiplying the number of stained cells (0=no positive cells; 1≤10%; 2=11%–50%; 3=51%–80%; 4≥80%) and staining intensity (0=no color reaction; 1=low; 2=moderate; 3=intense). Each sample’s value was determined using an average between 0–12 for each field of view, whereas we only employed a qualitative (positive and negative) evaluation for the SARS-CoV-2 spike antibody expression.
Terminal deoxynucleotidyl transferase dUTP Nick-End Labeling (TUNEL) assay kit (Neogen Corporation, Lansing, MI, USA) was used to detect apoptotic cells from the samples. Deparaffinized 5-+m paraffin sections were hydrated and rinsed with 0.1-M phosphate buffer (pH 7.4) before blocking with 3% bovine serum albumin and 20% fetal bovine serum. The sections were incubated for 60 minutes at 37℃ with a TUNEL reaction mixture. With a 400× magnification of the microscope, five fields of view were used for the quantitative analysis, and the apoptosis index (calculated by dividing the number of apoptotic cells by the total cells multiplied by 100) was counted.16 The cell nuclei of apoptotic cells from the samples were stained brownish-black with methyl green as a counterstain.
Statistical analysis
The data were analyzed using SPSS ver. 25 (IBM; Armonk, NY, USA). Descriptive statistics were used to assess categorical variables and chi-square test was used for analysis with Fischer’s exact test as an alternative. The numerical variables with normal distributions were analyzed using independent t-test values, and the result are presented as mean±standard deviation (SD). Meanwhile, the non-normally distributed numerical variables were analyzed using the Mann-Whitney U test, and the result are presented as median values (interquartile range). The correlation between COVID-19 severity and numerical variables was analyzed using Spearman’s rho correlation coefficient. Statistical significance was set at p<0.05.
Study approval
The ethical committee of Dr. Soetomo General Academic Hospital approved this study (No. 0099/KEPK/XI/2020). Informed consent was obtained from all patients before the study began.
RESULTS
Among 64 pregnant women included in this study, 32 (50%) had confirmed symptomatic COVID-19 infection. Both groups were comparable in terms of basic maternal characteristics, laboratory results, and delivery outcomes (Table 1).
Table 1. Basic Characteristics of Subjects Included in the Study (n=64).
| Characteristics | Normal pregnancy (n=32) | Pregnancy with COVID-19 (n=32) | p value | |
|---|---|---|---|---|
| Basic maternal characteristics | ||||
| Maternal age (yr) | 31.41±5.35 | 30.37±5.36 | 0.444 | |
| Gestational age (weeks) | 36.5 (3) | 37 (4.75) | 0.189 | |
| Primigravida | 8 (25) | 9 (28.1) | 0.777 | |
| Body mass index (kg/m2) | 26.04±4.09 | 25.27±3.62 | 0.431 | |
| Delivery outcomes | ||||
| Cesarean section | 28 (87.5) | 24 (75) | 0.200 | |
| Birthweight (grams) | 2712.03±661.85 | 2745±712.25 | 0.844 | |
| Low Apgar score 1 min | 10 (31.35) | 13 (40.46) | 0.603 | |
| Low Apgar score 5 min | 4 (12.5) | 5 (15.6) | >0.999 | |
Numeric values are presented as mean±standard deviation and median (interquartile range) for non-parametric data. Categorical data are presented as n (%).
Low Apgar score: Apgar score <7.
COVID-19-infected villous trophoblasts expressed higher TMPRSS2, IFN-γ, and apoptosis index
In order to explore placental inflammation and apoptosis, we initially checked the expression of ACE-2 and TMPRSS2 to gauge the possibility of SARS-CoV-2 entry in the placenta. We found lower non-significant expression of ACE-2 with significantly higher TMPRSS2 expression in the COVID-19 group (4.76±1.37 vs. 2.61±1.04; p<0.001). Next, we looked for signs of placental inflammation and apoptosis brought on by SARS-CoV-2 infection. Our findings demonstrated that the COVID-19 group had significantly greater levels of IFN-γ (2.36±0.92 vs. 1.67±0.47; p<0.001) but non-significantly higher levels of IL-6. All of the placental expressions could be seen from the trophoblast cells in the placental villous. A greater apoptotic index was observed in the villous trophoblast side of the COVID-19 group (43.35±15.7 vs. 28.32±8.9; p<0.001), but none in the stromal area. Detailed expressions in placental inflammation and apoptosis are shown in Fig. 2.
Fig. 2. Immunohistochemical characteristics (with 400× magnification) and their expressions on (A) ACE-2, (B) TMPRSS2, (C) IFN-γ, (D) IL-6, and (E) trophoblast and stromal apoptosis between COVID-19 and normal placenta (thin arrow: trophoblast expression, thick arrow: stromal expression). ACE-2, angiotensin converting enzyme-2; TMPRSS2, transmembrane protease serine-2; IFN-γ, interferon-γ; IL-6, interleukin-6.
Evidence of SARS-CoV-2 placental villous infection and related disturbance
The villous trophoblast expression of the SARS-CoV-2 spike protein in eight samples (25%) of the maternal COVID-19 infection group provided proof that SARS-CoV-2 can be present in the placenta (Fig. 3). In the non-COVID-19 group, no spike protein expression was observed. Additional investigation revealed that the expressions of IFN-γ (3.1±1.01 vs. 2.11±0.75; p=0.006), apoptosis in villous trophoblast (56.66±14.28 vs. 38.92±13.71; p=0.004), and stromal area (57.79±11.08 vs. 41.02±14.49; p=0.006) were much higher in the SARS-CoV-2 spike protein-positive subgroup. There were no significant differences in the expressions of placental ACE-2, TMPRSS2, IL-6, and perinatal outcomes (birthweight and Apgar Score) (Fig. 2). Moreover, no vertical transmission was detected across the whole COVID-19 group from the first 24-hour oropharyngeal swab.
Fig. 3. Immunohistochemical image of the SARS-CoV-2 spike protein on COVID-19-infected placenta (thin arrow) with box-plot comparison of the spike protein-positive and spike protein-negative subgroups in the COVID-19 placenta for ACE-2, TMPRSS2, IFN-γ, IL-6, trophoblast-stromal apoptosis expression, and perinatal outcome (birthweight, Apgar score 1 minute and 5 minutes). SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ACE-2, angiotensin converting enzyme-2; TMPRSS2, transmembrane protease serine-2; IFN-γ, interferon-γ; IL-6, interleukin-6.
Maternal severity of COVID-19 infection is independent of villous placental inflammation or apoptosis
The link between maternal severity and placental disruption was further examined using a COVID-19 subgroup analysis. Eight (25%) women in the COVID-19 group experienced severe symptoms, compared to 13 (40.6%) and 11 (34.4%) cases with mild and moderate symptoms, respectively. There was no significant difference (p=0.414) in the number of days between the onset of symptoms and childbirth for the mild [5 (2–7) days], moderate [4 (3–7) days], and severe subgroups [5 (3–5) days]. Furthermore, none of the placental expressions examined in this study had a correlation with the severity of sickness in COVID-19 pregnant women, according to correlation analysis based on maternal COVID-19 severity levels (Table 2) including the SARS-CoV-2 spike protein expression (p=0.803).
Table 2. Correlation Analysis According to Severity Levels.
| Placental expression | Mild (n=13) | Moderate (n=11) | Severe (n=8) | p value |
|---|---|---|---|---|
| Angiotensin-converting enzyme 2 | 1.74±0.71 | 1.85±0.72 | 1.67±0.45 | 0.604 |
| Transmembrane serine protease 2 | 4.71±1.58 | 4.51±1.29 | 5.2±1.18 | 0.492 |
| Spike protein SARS-CoV-2 | 2 (15.4) | 5 (45.5) | 1 (12.5) | 0.803 |
| Interferon-γ | 2.34±1.13 | 2.47±0.86 | 2.22±0.64 | 0.956 |
| Interleukin-6 | 1.6 (0.4) | 1.8 (0.6) | 1.6 (0.4) | 0.124 |
| Trophoblast apoptosis | 37.71±15.91 | 52.36±10.88 | 40.12±17.03 | 0.136 |
| Stromal apoptosis | 45.71±17.41 | 51.54±10.73 | 35.71±14.26 | 0.302 |
SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Numeric values are presented as mean±standard deviation and median (interquartile range) for non-parametric data. Categorical data are presented as n (%).
DISCUSSION
SARS-CoV-2 is a novel coronavirus causing global health concerns, including fetoplacental problems among infected pregnant women. Preterm delivery, poor fetal vascular perfusion, and neonatal morbidity are all more likely in this population.17 Since the placenta is a vital organ that serves as the maternal-fetal gestational interface, defects in the placenta may contribute to feto-maternal morbidity. Inflammatory and infectious disorders are recognized to demand a histological assessment of the placenta.18 Albeit more than 2 years of the pandemic, the placental pathology in SARS-CoV-2 infection as well as the maternal-neonatal transmission mechanisms of COVID-19 are still poorly understood. By minimizing other inflammation’s impact and comparing with healthy controls, specific research studies on symptomatic, third-trimester maternal SARS-CoV-2 infections in this study are expected to further advance knowledge in this area.
It is well-established that SARS-CoV-2 infections of host cells require binding to the ACE-2 cell entry receptors with TMPRSS2 activation.19 Several studies have analyzed these SARS-CoV-2 entry receptors in the placenta.20,21,22 Our observation revealed that the trophoblast villous cells expressed both ACE-2 and a significantly higher amount of TMPRSS2 in the COVID-19 group, which has also been reported in previous studies.10,23,24 This protease increment showed facilitation and active priming in host cells, which will be an important factor in the entry of viral infections.19,23 Lower levels of ACE2 protein in placentas from COVID-19-positive pregnancies have also been reported in a previous study, hinting that SARS-CoV-2 infection and inflammation may directly or indirectly downregulate ACE2 expression as a result of active shedding.20
This study, to our knowledge, is the first to assess IFN-γ expression directly on the COVID-19-infected placenta. This viral-specific inflammatory marker is found to increase significantly without being accompanied by a significant increase in the typical COVID-19 severe inflammatory marker, IL-6. These findings are consistent with a number of other placental diseases, including toxoplasmosis, where elevated IFN-γ are necessary to prevent infected cells from migrating and replicating.25 One of the causes of this high cytokine production, which is likely to have limited the movement of the virus, can be the migration of immune cells including macrophages, T cells, and NK cells obtained in the COVID-19-infected placenta.24,26 The analysis of placental RNA24 and the COVID-19-infected cell line23 also revealed significant levels of inflammatory and anti-inflammatory cytokines, including IL-6, which was also shown to be elevated in trophoblast.27
In the structural analysis of the placenta, it was possible to observe alterations that suggest an intense apoptotic process of placental tissues in the COVID-19-positive group as indicated by a higher apoptotic index, especially in the villous trophoblast cells. Other studies also showed an increase in COVID-19-infected placental apoptosis, although indirectly, including an increase of proapoptotic Bax protein27 and Caspase 3/7 induction in the infected placental cell line.23 In particular, interferon-γ, which was elevated in this study, is known to induce trophoblast apoptosis,28 which also occurs in other clinical circumstances, including preeclampsia and toxoplasma infection.25,29
It has been widely reported that SARS-CoV-2 infection is accompanied by an aggressive systemic inflammatory response known as a cytokine storm, and this inflammatory condition may lead to the occurrence of histopathologic anomalies related to inflammation, which may further harm the placenta.30,31 Intriguingly, the current study’s findings revealed no correlation between maternal COVID-19 severity and placental inflammation or apoptosis. The increased apoptotic process in trophoblasts and IFN-γ levels expression due to direct placental infection of SARS-CoV-2 might explain the potential mechanisms, supported by the detection of the SARS-CoV-2 spike protein with significantly higher inflammation and apoptosis when this viral protein evidence was detected. Argueta’s study, which infected placental cells with the SARS-CoV-2 virus and caused an increase in inflammatory and apoptotic responses, also supports this mechanism.24
Despite the fact that the previous study revealed approximately 3.2% vertical transmission of COVID-19 in the third trimester,32 no newborns in the present study tested positive for SARS-CoV-2. Notably, the sampling method (oral, umbilical, neonatal blood) may have contributed to the vertical transmission incidence. Furthermore, the low amount of maternal SARS-CoV-2 viremia evidence may also make it difficult for vertical transmission to occur.33 Although some studies have produced mixed results,20,23 spike protein expression which is not always detected in the COVID-19 group supports this condition.
The detection of an apoptosis predominance in the villous trophoblast area rather than the stroma, as well as elevated levels of IFN-cytokines rather than IL-6 levels, may point to a mechanism by which the placenta delivers an efficient antiviral response at the fetal villous placenta. The placental barrier function of syncytiotrophoblast and passive immunisation caused by IgG transfer from the mother’s antibodies may prevent intrauterine transmission to the fetus. Despite reports of transplacental transfer, vertical transmission has up been a contentious issue to date.31,34
In the published literature, there were several proven cases of direct placenta infection from SARS-CoV-2 positive pregnant women, which varied from asymptomatic6,9,35 to requiring NICU admission.36 The possibility of pathogen penetration from the mother’s bloodstream into the intervillous space, which is plausible in the study given the evidence of SARS-CoV-2 entrance receptor expression, spikes protein to trophoblast inflammation, and damage shown by increased apoptosis, makes exposure to trophoblast villi one of the mechanisms of placental infection.10,34
The novelty of this study is that it provides a comprehensive picture of placental abnormalities brought on by SARS-CoV-2 infection, based on the evidence of viral entry via receptor binding to an inflammatory response and apoptotic damage. However, this study does have certain shortcomings. First, we did not examine other fetal samples (amniotic, umbilical, etc.) as it is difficult to do so in the midst of COVID-19 attacks, so as to more thoroughly explain the likelihood of fetal transfer. Additionally, due to the likelihood of different immune responses during previous trimesters, this study solely examined the maternal COVID-19 infection, which only occurs in the third trimester, making it inapplicable to explain placental infection and disruption at other periods of pregnancy. Due to the pregnancy’s increasing level of progesterone and its specific anti-inflammatory dominance, this study attempted to limit the gestational age period with homogenous gestational age characteristics between groups in the third trimester. However, given the high incidence of COVID-19 discovered during third trimester, it is crucial to learn the state of the placental infection during this period. Moreover, the reduced ability of the placental barrier due to weakened trophoblast integrity increases in the third trimester; therefore, it is vital to be aware of this.34,37
Although the present study demonstrated no direct effect of SARS-CoV-2 infection on early neonatal morbidity, the observation that SARS-CoV-2 can infect the placenta to impact fetal development is of great relevance and indicates that further and more strict monitoring of infants and long-term follow-up should be undertaken in SARS-CoV-2 infected mothers, to examine varying evidence of infection and pandemic impact on long-term neurodevelopmental issues in children.38,39,40
In conclusion, we observed that SARS-CoV-2 infection was able to reach the placenta based on the positive results in the viral and entry receptor immunohistochemistry analyses of the samples. Moreover, the alteration of the inflammatory and apoptosis processes from the histopathological analysis was also found in the infected placenta. The identification of these alterations contributes to a better understanding of the pathogenesis of infection in the maternal-fetal context. The inflammatory conditions and placental damage demonstrated in this study showed that pregnancy had a unique and vulnerable impact owing to SARS-CoV-2 infection. Although the short-term impact on perinatal health was not found in this study, the possibility of the long-term impact of the feto-maternal outcome will require additional attention.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Soetomo Academic General Hospital for the great support regarding this work.
This study was conducted in Dr. Soetomo General Hospital, Surabaya, Indonesia during the COVID-19 pandemic in Indonesia (between November 2020 and August 2021).
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Manggala Pasca Wardhana, Erry Gumilar Dachlan, and Kuntaman Kuntaman.
- Data curation: Manggala Pasca Wardhana, Almira Aulia Shahnaz, Dahlia Ningrum, and Nareswari Imanadha Cininta Marcianora.
- Formal analysis: Manggala Pasca Wardhana, Rozi Aditya Aryananda, and Nareswari Imanadha Cininta Marcianora.
- Funding acquisition: Manggala Pasca Wardhana.
- Investigation: Manggala Pasca Wardhana, Almira Aulia Shahnaz, Dahlia Ningrum, and Nareswari Imanadha Cininta Marcianora.
- Methodology: Manggala Pasca Wardhana, Rozi Aditya Aryananda, Salsabila Nabilah Rifdah, and Ifan Ali Wafa.
- Project administration: Manggala Pasca Wardhana, Almira Aulia Shahnaz, Dahlia Ningrum, and Nareswari Imanadha Cininta Marcianora.
- Resources: Manggala Pasca Wardhana, Almira Aulia Shahnaz, Dahlia Ningrum, Nareswari Imanadha Cininta Marcianora, and Grace Ariani.
- Software: Manggala Pasca Wardhana, Rozi Aditya Aryananda, Salsabila Nabilah Rifdah, and Ifan Ali Wafa.
- Supervision: Manggala Pasca Wardhana, Erry Gumilar Dachlan, Kuntaman Kuntaman, Budi Utomo, and Jan MM Van Lith.
- Validation: Manggala Pasca Wardhana, Erry Gumilar Dachlan, Kuntaman Kuntaman, and Budi Utomo.
- Visualization: Manggala Pasca Wardhana, Almira Aulia Shahnaz, and Dahlia Ningrum.
- Writing—original draft: Manggala Pasca Wardhana, Salsabila Nabilah Rifdah, and Ifan Ali Wafa.
- Writing—review & editing: Manggala Pasca Wardhana, Erry Gumilar Dachlan, Jan MM Van Lith, Kuntaman Kuntaman, and Budi Utomo.
- Approval of final manuscript: all authors.
References
- 1.Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19) Treasure Island, FL: StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 2.Ioannidis JPA. The end of the COVID-19 pandemic. Eur J Clin Invest. 2022;52:e13782. doi: 10.1111/eci.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellington S, Strid P, Tong VT, Woodworth K, Galang RR, Zambrano LD, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–775. doi: 10.15585/mmwr.mm6925a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi B, Chandi A, Srinivasan R, Saini SS, Prasad GRV, Puri GD, et al. The placental pathology in coronavirus disease 2019 infected mothers and its impact on pregnancy outcome. Placenta. 2022;127:1–7. doi: 10.1016/j.placenta.2022.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebutini PZ, Zanchettin AC, Stonoga ETS, Prá DMM, de Oliveira ALP, Dezidério FDS, et al. Association between COVID-19 pregnant women symptoms severity and placental morphologic features. Front Immunol. 2021;12:685919. doi: 10.3389/fimmu.2021.685919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hecht JL, Quade B, Deshpande V, Mino-Kenudson M, Ting DT, Desai N, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. doi: 10.1038/s41379-020-0639-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hosier H, Farhadian SF, Morotti RA, Deshmukh U, Lu-Culligan A, Campbell KH, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. doi: 10.1172/JCI139569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Algarroba GN, Rekawek P, Vahanian SA, Khullar P, Palaia T, Peltier MR, et al. Visualization of severe acute respiratory syndrome coronavirus 2 invading the human placenta using electron microscopy. Am J Obstet Gynecol. 2020;223:275–278. doi: 10.1016/j.ajog.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patanè L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2:100145. doi: 10.1016/j.ajogmf.2020.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouyang Y, Bagalkot T, Fitzgerald W, Sadovsky E, Chu T, Martínez-Marchal A, et al. Term human placental trophoblasts express SARS-CoV-2 entry factors ACE2, TMPRSS2, and furin. mSphere. 2021;6:e00250–e00221. doi: 10.1128/mSphere.00250-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daş T, Buğra A, Arslan MN, Ziyade N, Buyuk Y. Evaluation of postmortem pathological changes in the lung in SARS-CoV-2 RT-PCR positive cases. J Surg Med. 2021;5:1113–1120. doi: 10.1007/s11845-021-02638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soullane S, Spence AR, Abenhaim HA. Association of placental pathology and gross morphology with autism spectrum disorders. Autism Res. 2022;15:531–538. doi: 10.1002/aur.2658. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. COVID-19 clinical management: living guidance, 25 January 2021. Genava: World Health Organization; 2021. [Google Scholar]
- 14.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] Pathologe. 1987;8:138–140. German. [PubMed] [Google Scholar]
- 15.Wardhana MP, Wicaksono B, Aditiawarman, Widjiati, Ardian M, Cahyani MD, et al. L-arginine protective effect on systemic blood pressure and placental expression of endoglin, transforming growth factor-β1 in the preeclampsia mice model. Int J Women’s Health Reprod Sci. 2021;9:29–34. [Google Scholar]
- 16.Kokawa K, Shikone T, Otani T, Nishiyama R, Ishii Y, Yagi S, et al. Apoptosis and the expression of Bax and Bcl-2 in hyperplasia and adenocarcinoma of the uterine endometrium. Hum Reprod. 2001;16:2211–2218. doi: 10.1093/humrep/16.10.2211. [DOI] [PubMed] [Google Scholar]
- 17.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55:586–592. doi: 10.1002/uog.22014. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taglauer E, Benarroch Y, Rop K, Barnett E, Sabharwal V, Yarrington C, et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. doi: 10.1016/j.placenta.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bloise E, Zhang J, Nakpu J, Hamada H, Dunk CE, Li S, et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol. 2021;224:298.e1–298.e8. doi: 10.1016/j.ajog.2020.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faure-Bardon V, Isnard P, Roux N, Leruez-Ville M, Molina T, Bessieres B, et al. Protein expression of angiotensin-converting enzyme 2, a SARS-CoV-2-specific receptor, in fetal and placental tissues throughout gestation: new insight for perinatal counseling. Ultrasound Obstet Gynecol. 2021;57:242–247. doi: 10.1002/uog.22178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agostinis C, Toffoli M, Spazzapan M, Balduit A, Zito G, Mangogna A, et al. SARS-CoV-2 modulates virus receptor expression in placenta and can induce trophoblast fusion, inflammation and endothelial permeability. Front Immunol. 2022;13:957224. doi: 10.3389/fimmu.2022.957224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argueta LB, Lacko LA, Bram Y, Tada T, Carrau L, Rendeiro AF, et al. Inflammatory responses in the placenta upon SARS-CoV-2 infection late in pregnancy. iScience. 2022;25:104223. doi: 10.1016/j.isci.2022.104223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert-Gangneux F, Murat JB, Fricker-Hidalgo H, Brenier-Pinchart MP, Gangneux JP, Pelloux H. The placenta: a main role in congenital toxoplasmosis? Trends Parasitol. 2011;27:530–536. doi: 10.1016/j.pt.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Juttukonda LJ, Wachman EM, Boateng J, Jain M, Benarroch Y, Taglauer ES. Decidual immune response following COVID-19 during pregnancy varies by timing of maternal SARS-CoV-2 infection. J Reprod Immunol. 2022;151:103501. doi: 10.1016/j.jri.2022.103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fatih TAŞ, Erdemci F, Fırat AŞIR, Maraşli M, Deveci E. Histopathological examination of the placenta after delivery in pregnant women with COVID-19. J Health Sci Med. 2022;5:868–874. [Google Scholar]
- 28.Sun QH, Peng JP, Xia HF, Yang Y. IFN-γ promotes apoptosis of the uterus and placenta in pregnant rat and human cytotrophoblast cells. J Interferon Cytokine Res. 2007;27:567–578. doi: 10.1089/jir.2006.0106. [DOI] [PubMed] [Google Scholar]
- 29.Sheibak N, Mahmoudzadeh-Sagheb H, Moudi B, Heidari Z. Elevated immunoexpression of interferon-gamma in placenta tissue samples from pregnancies complicated with preeclampsia compared to the placenta previa. Pregnancy Hypertens. 2020;22:175–180. doi: 10.1016/j.preghy.2020.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Di Girolamo R, Khalil A, Alameddine S, D’Angelo E, Galliani C, Matarrelli B, et al. Placental histopathology after SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2021;3:100468. doi: 10.1016/j.ajogmf.2021.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celik E, Vatansever C, Ozcan G, Kapucuoglu N, Alatas C, Besli Y, et al. Placental deficiency during maternal SARS-CoV-2 infection. Placenta. 2022;117:47–56. doi: 10.1016/j.placenta.2021.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224:35–53.e3. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bwire GM, Majigo MV, Njiro BJ, Mawazo A. Detection profile of SARS-CoV-2 using RT-PCR in different types of clinical specimens: a systematic review and meta-analysis. J Med Virol. 2021;93:719–725. doi: 10.1002/jmv.26349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heerema-McKenney A. Defense and infection of the human placenta. APMIS. 2018;126:570–588. doi: 10.1111/apm.12847. [DOI] [PubMed] [Google Scholar]
- 35.Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, Spinillo A, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11:5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wardhana MP, Aditiawarman, Ernawati, Maniora NC, Aditya R, Gumilar KE, et al. SARS-CoV-2 antibody test for the hospitalised emergency obstetric cases: useful or wasteful. New Armen Med J. 2021;15:56–63. [Google Scholar]
- 38.McDonald AJ, Mew EJ, Hawley NL, Lowe SR. Anticipating the long-term neurodevelopmental impact of the COVID-19 pandemic on newborns and infants: a call for research and preventive policy. J Affect Disord Rep. 2021;6:100213. doi: 10.1016/j.jadr.2021.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brum AC, Vain NE. Impact of perinatal COVID on fetal and neonatal brain and neurodevelopmental outcomes. Semin Fetal Neonatal Med. 2023;28:101427. doi: 10.1016/j.siny.2023.101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shook LL, Sullivan EL, Lo JO, Perlis RH, Edlow AG. COVID-19 in pregnancy: implications for fetal brain development. Trends Mol Med. 2022;28:319–330. doi: 10.1016/j.molmed.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]