Abstract
Objectives
Antibodies to gp210 and sp100 are specific and unique anti-nuclear autoantibodies (ANAs) associated with primary biliary cholangitis (PBC). Importantly the presence of anti-gp210 and anti-sp100 responses is indicative of poor clinical outcomes. However, the utility of measuring titers of these antibodies remains unclear.
Materials and methods
Using the in-house purified gp210 (HSA108-C18) and sp100 (amino acid position 296–386), we quantitatively measured serum autoantibodies to gp210 and sp100 using chemiluminescence immunoassay (CLIA) in a very large cohort of 390 patients with PBC, including 259 cases with no prior ursodesoxycholic acid (UDCA) treatment and 131 cases with UDCA treatment. We also analyzed serial changes in anti-gp210 and anti-sp100 levels in 245 sequential samples from 88 patients.
Results
In our cross-sectional analysis, we detected anti-gp210 immunoglobulin G (IgG) and anti-sp100 IgG autoantibodies in 129 out of 390 (33.1%) and 80 out of 390 (20.5%) PBC patients, respectively. Multivariate analysis revealed that serum IgG (st.β = 0.35, P = 0.003) and gamma-glutamyltransferase (GGT) (st.β = 0.23, P = 0.042) levels at baseline were independently associated with anti-gp210 concentrations. In serial testing, we observed significant fluctuations in anti-gp210 antibody levels. These fluctuations reflected responsiveness to UDCA therapy, particularly in anti-gp210-positive patients with initially lower concentrations in the stages of disease.
Conclusions
Our study reflects that quantitative changes of anti-gp210 antibody are indicative of UDCA responses. There is a great need for newer metrics in PBC and we suggest that a more detailed and longer study of these unique ANAs is warranted.
Keywords: gp210, Primary biliary cholangitis, sp100, Quantitation, UDCA
Highlights
-
•
Anti-Nuclear autoantibodies (ANAs) to gp210 and sp100 specifically associated with primary biliary cholangitis (PBC).
-
•
Significant fluctuations in anti-gp210 antibody levels among PBC patients were observed in the serial testing.
-
•
Autoantibodies to gp210 fluctuations are indicative of UDCA responses.
1. Introduction
Primary biliary cholangitis (PBC) is a chronic autoimmune liver disease that damages intrahepatic bile ducts and leads to progressive cholestasis [1]. The most important serologic markers of PBC diagnosis are the anti-mitochondrial antibodies (AMAs), which occur in almost 95% of patients, and anti-nuclear autoantibodies (ANAs) to gp210 and sp100, especially in AMA-negative patients [2]. Evidences from different ethnic groups also indicate that anti-gp210 is associated with adverse outcomes and a worse prognosis in PBC [3]. The presence of autoantibodies against gp210 was found to be associated with disease severity and represented a hepatic failure type progression, indicating that the anti-gp210 antibody may potentially be useful as a prognostic indicator of PBC [[4], [5], [6], [7], [8], [9]]. Recent data from a UK retrospective study showed that the presence of anti-gp210 antibody was predictive of poor clinical phenotype, non-response to UDCA, and reduced transplant-free survival [10]. However, data on the clinical significance of anti-sp100 antibodies present in PBC are scarce and controversial [[11], [12], [13]].
Cross-sectional studies provide autoantibody data at one time point, which does not allow observation of the dynamic progression of disease. With PBC, the quantitative measurement of AMAs and their clinical significance were investigated by multiple studies [[14], [15], [16]]. AMAs levels varied greatly among PBC patients, but showed no correlation with disease progression and prognosis. However, There are conflicts regarding ANAs status during the clinical course of PBC. Nakamura et al. found that anti-gp210 titers changed from positive to negative under UDCA therapy during observation periods [7,9,17]. Wesierska-Gadek et al. also observed the change of reactivity to gp210 in sequential serum samples of 23 PBC patients [6]. A previous report by Itoh et al. seemed to argue variation of anti-gp210 titers throughout clinical course of PBC patients [18]. As regards anti-sp100 antibody, it seems that anti-sp100 levels remain stable over the course of the disease, according to some evidence supported by Zuchner et al. and Mytilinaiou et al. [11,12], but in Gatselis's study declining anti-sp100 titers correlated with Mayo risk score and response to UDCA therapy [13].
To further define the clinical significance of anti-gp210 and anti-sp100 antibodies, we quantitated anti-gp210 and anti-sp100 antibodies in serum samples of 259 PBC cases with no prior UDCA treatment (treatment-naïve) and 131 cases with UDCA treatment of at least one year. We then performed serial serum testing of 88 PBC patients during the course of UDCA treatment by microplate chemiluminescence immunoassay (CLIA). We evaluated the correlation of anti-gp210 and anti-sp100 quantitation with clinical parameters, liver cirrhosis severity, and UDCA therapy. The results of this study indicate that ANAs levels are likely modulated by UDCA therapy.
2. Materials and methods
2.1. Patients and sera
All PBC patients were recruited from the Jiangsu Province PBC Collaboration Group (JSPPCG) member hospitals with the approval of the research ethics boards of Southeast University in accordance with the guidelines of the Declaration of Helsinki. The diagnosis of PBC was based on criteria recommended by the American Association for the Study of Liver Diseases (AASLD) [2], excluding patients with history of schistosome infection, viral hepatitis including hepatitis B, C or D and alcoholic liver disease. Patients concurred with autoimmune liver hepatitis, as confirmed with liver pathology analysis, were also excluded from this study.
Sera of the patients recruited in this study were collected between June 2013 and September 2021 and were stored at −80 °C until use. Patient information included demographic data (gender, age) and serum biochemical data (alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and gamma-glutamyltransferase (GGT), total bilirubin (TBIL), total protein (TP) and albumin (ALB) were collected from the participating hospitals. Serum immunoglobulin G (IgG), immunoglobulin M (IgM) and immunoglobulin A (IgA) concentrations were quantified by turbidimetric inhibition immunoassay (DENUO, Shanghai Zhicheng Biological Technology Co.,Ltd). The presence of liver cirrhosis was evaluated by computed tomography (CT), abdominal ultrasound examinations (UT) and magnetic resonance imaging (MRI). The albumin-bilirubin (ALBI) score, as a simple method to estimate hepatic function, has been extensively used [19,20]. PBC patients were grouped by hepatic cirrhosis or ALBI score.
In the cross-sectional analysis, 390 PBC patients were classified into two groups: (a) not treated with UDCA (259 subjects) and (b) treated with UDCA for at least one year (131 subjects) at a dose of 15 mg/kg/day. In the longitudinal study, 88 patients were included, among them 71 patients were followed up at least 12 months and treated with UDCA at a dose of 15 mg/kg/day. UDCA biochemical responses were defined based on Paris-II criteria [21].
All samples were analyzed for AMA-M2 using the in-house 3E based ELISA as previously described [22].
2.2. Quantitation of anti-gp210 and anti-sp100 by CLIA
In brief, the IgG against gp210 and sp100 in serum samples was assessed using the microplate CLIA. The 96-well microplates (Thermo Scientific) were coated with the in-house purified gp210 (HSA108-C18) antigen or sp100 antigen (AA position 296–386) for 50 ng/well at overnight 4 °C [23,24], and blocked with phosphate-buffered saline (PBS) buffer containing 1% bovine serum albumin (BSA) for 2 h at room temperature (RT). Serum samples were diluted (1:400) with PBS buffer containing 1% BSA and added to the plates, followed by incubation for 1 h at RT. The plates were then washed with PBS-Tween20 (0.05% v/v) and incubated with 1:40,000 diluted horseradish peroxidase (HRP)-conjugated anti-human IgG antibody (Millipore) for 1 h at RT. After washing, the plates were incubated with 100 μL of chromogenic substrate (luminol) for 15 min, and the chemiluminescent signal was detected using a microplate reader.
To determine the cutoff values for positive anti-gp210 and anti-sp100 levels, we used a mixed high positive serum as a standard reference and diluted it to seven different concentrations (1000, 500, 250, 125, 62.5, 31.25, 0 units/mL). Serum anti-gp210 concentrations that were greater than 100 unit/mL or anti-sp100 concentrations of more than 300 unit/mL (mean value + 3 SD of the concentrations of 150 healthy subjects) were arbitrarily determined as positive for the autoantibodies. The intra-assay and inter-assay coefficients of variation (CV) for anti-gp210 and anti-sp100 CLIA were both less than 20% (Table S1).
2.3. Immunoblotting for anti-gp210 or anti-sp100
In-house prepared and purified gp210 (HSA108-C18) and sp100 (AA position 296–386) proteins were separated by SDS-PAGE on 12% gels and then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrance was then blocked with 5% nonfat milk in PBS with 0.1% Tween-20 at RT for 1 h, and probed with patient sera diluted at 1:1000 overnight at 4 °C. After washing with PBS-Tween-20 for 4 times, and the membrane was incubated with HRP conjugated antibodies against human immunoglobulins at 1:40,000 dilution. Finally, the reactivities were detected by using ECL enhanced chemiluminescence western blotting detection reagents (Thermo fisher) and the ImageQuant LAS 4000 system (GE Healthcar).
2.4. Statistical analysis
Statistical analyses were performed using the statistical package of SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 8.0. Continuous variables were expressed as median (quartile) and tested using the Mann-Whitney U test to compare two groups. Categorical variables were presented with frequencies or percentages and tested using the chi-square test or Fisher's exact. Spearman's rho was used for correlation analysis. The association between antibody concentrations and biochemical parameters was analyzed using linear regression model. All statistical tests were 2-sided and P < 0.05 was considered significant.
3. Results
3.1. Clinical and immunological features of PBC patients
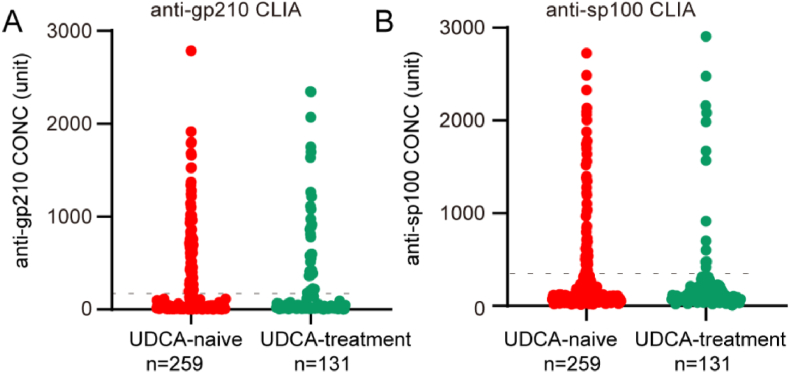
A total of 390 PBC patients, including 351 (90.0%) female and 39 (10.0%) male patients with mean age of 57.8 years, were quantitated for anti-gp210 and anti-sp100 antibodies (Fig. 1A and B). AMA-M2, anti-gp210 and anti-sp100 autoantibodies were present in 370 (94.9%), 129 (33.1%) and 80 (20.5%) patients, respectively. Among them, 259 patients were UDCA treatment-naïve and the other 131 patients were treated with UDCA for at least more than one year. The severity of hepatic cirrhosis examined by CT, UT or MRI was assessed in 360 available patients, including 163 cases with non-cirrhosis, and 197 cases with cirrhosis. Moreover, the application of the ALBI evaluation criterion resulted in a total of 382 PBC patients, including 136 cases with a score of 1, 173 cases with a score of 2, and 73 cases with a score of 3, respectively (Table 1).
Fig. 1.
Concentrations of anti-gp210 and anti-sp100 in 390 PBC patients (259 PBC under UDCA-naïve and 131 PBC under UDCA treatment for at least one year), and correlation of anti-gp210 concentrations with clinical parameters. A, Concentrations of anti-gp210 among 390 PBC patients; B, Concentrations of anti-sp100 among 390 PBC patients.
Table 1.
Patients characteristics.
| PBC patients (n = 390) | UDCA-naïve treatment (n = 259) | UDCA treatment (n = 131) | |
|---|---|---|---|
| Gender (female/male) | 351/39 | 229/30 | 122/9 |
| Age (median, quartile) | 58.0 (16.0) | 58.0 (16.0) | 57.0 (14.0) |
| AMA-M2 +/− (%) | 370/20 (94.9) | 251/8 (96.9) | 119/12 (90.8) |
| Anti-gp210 +/− (%) | 129/261 (33.1) | 88/171 (34.2) | 41/90 (31.3) |
| Anti-sp100 +/− (%) | 80/310 (20.5) | 64/195 (24.7) | 16/115 (12.2) |
| ALBI scorea | |||
| 1/2/3 (%) | 136/173/73 (35.6/45.3/19.1) | 88/120/46 (34.6/47.2/18.1) | 48/53/27 (37.5/41.4/21.1) |
| Cirrhosisb | |||
| +/− (%) | 197/163 (54.7/45.3) | 114/131 (46.5/53.5) | 83/32 (72.2/27.8) |
Abbreviations are same as in the text.
available in 382 patients.
available in 360 patients.
3.2. Analysis of gp210 and sp100 auto-antibodies in UDCA naïve patients
We analyzed serum samples from 259 patients who had not received UDCA treatment. The presence of anti-sp100 antibody did not show any association with any biochemical or immunological markers. However, presence of anti-gp210 antibody was associated with increased serum IgM levels when compared to those without anti-gp210 antibody (Table 2). A multivariate linear regression model was used to identify the independent factors associated with anti-gp210 levels in patients who tested positive for anti-gp210. The results showed that serum IgG concentration, but not IgM concentration, was significantly correlated to anti-gp210 levels, although serum GGT showed weak significance (Table S2).
Table 2.
The clinical characteristics of PBC patients treated with UDCA or UDCA-naïve grouped by the presence of anti-gp210 autoantibodies.
| Characteristics | UDCA-naïve treatment |
P | UDCA- treatment |
P | ||
|---|---|---|---|---|---|---|
| gp210 + |
gp210 - |
gp210 + |
gp210 - |
|||
| n = 88 | n = 171 | n = 41 | n = 90 | |||
| Age | 58.0 (16.0) | 57.0 (17.0) | 0.96 | 53.0 (16.5) | 58.5 (14.0) | 0.54 |
| Female/Male | 74/14 | 155/16 | 0.13 | 34/7 | 88/2 | 0.01 |
| Tbil (μmol/L) | 20.6 (27.1) | 20.3 (20.7) | 0.73 | 20.1 (49.5) | 17.1 (15.2) | 0.18 |
| TP (g/L) | 74.0 (13.6) | 71.6 (13.8) | 0.02 | 68.7 (16.7) | 69.6 (13.2) | 0.12 |
| ALB (g/L) | 37.6 (12.3) | 37.6 (10.1) | 0.73 | 32.0 (14.3) | 38.7 (10.2) | 0.10 |
| ALT (U/L) | 68.8 (76.3) | 75.6 (95.0) | 0.19 | 40.5 (45.8) | 40.7 (72.1) | 0.54 |
| AST (U/L) | 78.0 (67.0) | 77.0 (81.8) | 0.85 | 55.0 (62.6) | 44.4 (67.0) | 0.67 |
| ALP (U/L) | 269.1 (300.1) | 240.6 (262.5) | 0.07 | 189.3 (157.1) | 141.8 (96.5) | 0.36 |
| GGT (U/L) | 351.1 (335.0) | 258.0 (310.1) | 0.04 | 124.0 (158.6) | 62.60 (108.3) | 0.24 |
| IgG (g/L) | 14.5 (9.8) | 12.2 (9.3) | 0.11 | 15.7 (13.3) | 9.9 (6.5) | 0.02 |
| IgM (g/L) | 4.1 (5.2) | 2.4 (3.2) | 2.56 × 10−4 | 2.6 (2.5) | 1.8 (1.4) | 0.06 |
| IgA (g/L) | 2.1 (1.9) | 2.0 (1.6) | 0.89 | 2.4 (1.8) | 1.9 (1.2) | 0.13 |
Abbreviations are same as in the text.
3.3. Analysis of gp210 and sp100 auto-antibodies qualitatively and quantitatively in PBC patients after UDCA treatment
In PBC patients who received UDCA treatment, we found that the anti-gp210 + group had significantly higher levels of IgG compared to the anti-gp210- group (Table 2). We also observed a significant increase in the prevalence of anti-gp210 antibodies with increasing ALBI score or the presence of hepatic cirrhosis in these patients (Table S3). However, by Pearson's correlation analysis, we did not observe any significant correlation between anti-gp210 levels and other biochemical markers, except for total bilirubin (TBIL) (Figure S1). Nevertheless, in our linear regression analysis, no parameter was found to be significant. On the other hand, in our analysis of sp100, we did not find any correlation between the prevalence or concentrations of anti-sp100 antibodies and any clinical parameters (Table S4).
3.4. Comparison of anti-gp210 and sp100 status between PBC patients with and without UDCA treatment
Among the 131 PBC patients treated with UDCA for at least one year, the frequency of anti-sp100 antibodies was significantly lower than that before UDCA treatment (24.7% vs 12.2%, P = 0.004) (Table 3). However, there was no significant difference in the frequency of anti-gp210 antibodies between UDCA-treated and UDCA-naïve PBC patients (34.0% vs 33.1%, P = 0.60) (Table 3). Subgroup analysis according to ALBI score showed that the difference in anti-sp100 antibody frequency between UDCA-treated and UDCA-naïve patients was significant in patients with ALBI score 1 (Table S5).
Table 3.
Seropositivity of ANAs stratified by UDCA therapy.
| UDCA-naïve treatment n = 259 | UDCA treatment n = 131 | P | |
|---|---|---|---|
| anti-gp210 | 88/171 (34.0%) | 41/90 (31.3%) | 0.60 |
| anti-sp100 | 64/195 (24.7%) | 16/115 (12.2%) | 4.00 × 10−3 |
3.5. Serological conversion of autoantibodies against gp210 and sp100 in PBC patients during the UDCA treatment
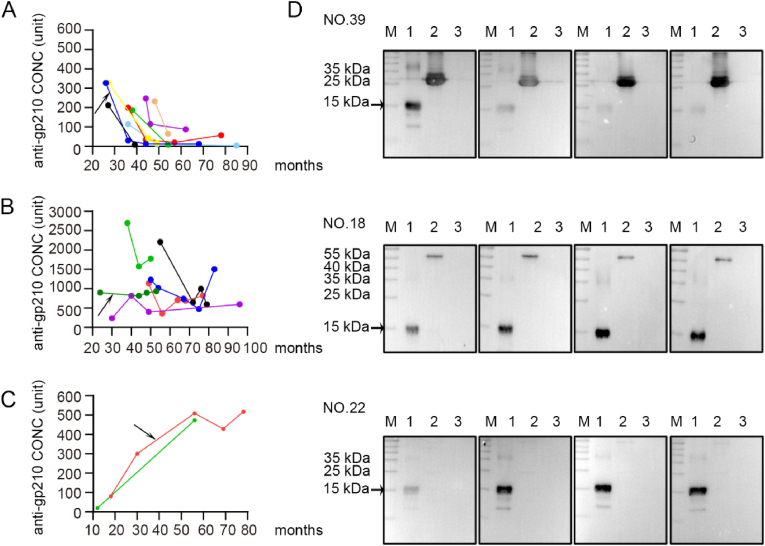
In this study, a total of 88 PBC patients were followed for a varying period of 6–75 months, from June 2013 to June 2021. Of the 88 patients, 13 were initially positive for anti-sp100 antibodies and 29 were initially positive for anti-gp210 antibodies. During the entire observation period, it was found that 8 out of 29 anti-gp210 seropositive patients, who had initially lower anti-gp210 levels, became seronegative or slightly positive, whereas 21 patients with high anti-gp210 levels remained positive with small fluctuations (Fig. 2A and B). On the other hand, only 2 out of 59 initially anti-gp210 negative patients (3.4%) developed the gp210 autoantibody during the follow-up (Fig. 2C). The western blotting analysis of sequential serum samples from some representative patients is shown in Fig. 2D and Figure S2.
Fig. 2.
The fluctuations of serum autoantibodies against gp210 in patients with PBC. A, anti-gp210 autoantibodies levels were initially lower and changed to negative or weakly positive after the initiation of UDCA treatment in eight patients; B, anti-gp210 autoantibodies levels were sustained at a relatively high concentrations and consistently positive in 21 patients (6 patients were representative shown in Fig. 2B); C, two patients developed anti-gp210 autoantibodies during the observation time; D, Reactivity to gp210 protein of sequential serum samples in 3 representative PBC patients by western blotting. Reactivity of patient NO.18 with stable anti-gp210 positivity; Reactivity of patient NO.22 with increasing anti-gp210 levels; Reactivity of patient NO.39 who lost anti-gp210. Proteins in lane 1 and lane 3 were purified mHSA108-gp210C18 (200 ng) and mHSA108 (200 ng). Proteins in lane 2 were purified BPO (approximately 55 kDa) (patients NO.18 and NO.22) and purified PDC-E2 antigen containing outer and inner lipoyl domain (approximately 30 kDa) (patients NO.39). Reactivity to gp210 protein of more sequential serum samples were in supplementary materials.
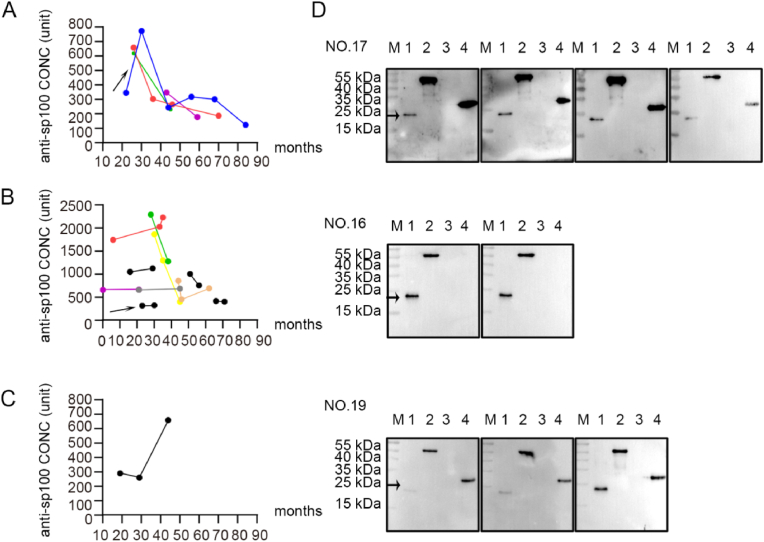
Among the 13 PBC patients who were initially positive for anti-sp100 antibodies, 4 patients (30.8%) changed to negative or weakly positive, and 9 patients (69.2%) remained serologically positive against sp100 during the entire observation period. In the 75 initially anti-sp100 seronegative patients, only one patient became positive with concentration escalation during the treatment (Fig. 3, Figure S3).
Fig. 3.
The fluctuations of serum autoantibodies against sp100 in patients with PBC. A, anti-sp100 autoantibodies levels were initially lower and changed to negative or weakly positive after the initiation of UDCA treatment in four patients; B, anti-sp100 autoantibodies levels were sustained at a relatively high concentrations and consistently positive in 11 patients; C, one patient developed anti-sp100 autoantibodies during follow-up; D, Reactivity to sp100 protein of sequential serum samples in 3 representative PBC patients. Reactivity of patient NO.16 with stable anti-sp100 positivity; Reactivity of patient NO.17 who lost anti-sp100; Reactivity of patient NO.19 with increasing anti-sp100 concentrations; lane 1, sp100 protein (23 kDa), lane 2, BPO (approximately 55 kDa), lane 3, mHSA108, lane 4, PDC-E2 antigen containing outer and inner lipoyl domain (approximately 30 kDa). Reactivity to sp100 protein of more sequential serum samples were in supplementary materials.
3.6. Changes in anti-gp210 levels occur in early-stage patients and reflect response to UDCA
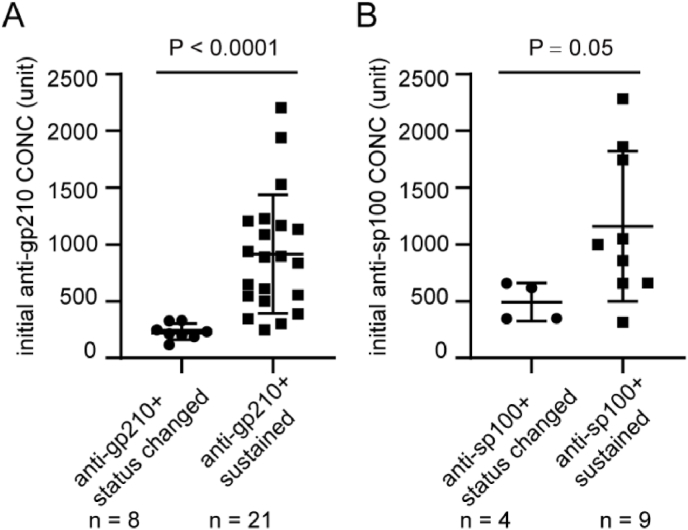
Among the eight patients who showed seroconversion of anti-gp210 antibody, six (75.0%) were in the early stage of the disease with non-cirrhosis, while two (25.0%) had mild fibrosis. Notably, six of these patients (75.0%) responded positively to UDCA treatment. However, among the 21 patients who had high anti-gp210 levels during observation (Fig. 4A), 10 patients (50.0%) had mild or severe cirrhosis, while the other 10 patients (50.0%) had no cirrhosis, except for one patient whose data was missing.
Fig. 4.
The comparison of ANAs concentrations between patients with antibodies status change and those with consistently high levels. A, initial levels of anti-gp210 in patients in Fig. 2A and B; B, initial levels of anti-sp100 in patients in Fig. 3A and B.
Out of the 21 patients, 18 were followed up for more than one year, and seven of them (38.9%) showed a positive response to UDCA therapy (Table S6). Among these seven patients, five had no cirrhosis, and two had cirrhosis. The remaining 11 patients (five with no cirrhosis and six with cirrhosis) did not respond to UDCA.
In the case of the four patients who showed a change from positive to negative for anti-sp100 antibody, their corresponding concentrations at baseline were significantly lower than those who remained anti-sp100 positive during observation (Fig. 4B). All four patients had a score of 1/2. Unfortunately, no statistical data could be shown due to a lack of detailed clinical data or a short follow-up (Table S6).
4. Discussion
Primary biliary cholangitis (PBC) is a multifaceted immune-mediated disease that can be diagnosed through serum markers, including AMA-M2 or specific antinuclear antibodies (ANAs) such as anti-gp210 or anti-sp100. In this study, we conducted a quantitative analysis of anti-gp210 and anti-sp100, and evaluated their clinical significance in cross-sectional and longitudinal studies, respectively.
Previous studies have suggested that increased levels of IgM in PBC patients may indicate an enhanced innate immune response [25]. In our study, we found that anti-gp210 antibodies in UDCA-naive patients were associated with qualitative changes in serum IgM and quantitative changes in IgG, suggesting that anti-gp210 antibodies may arise from infection or an altered liver-gut microbial balance. The gp210 epitope has been found to be homologous to the protein products of the Escherichia coli mutY gene and Salmonella typhimurium mutB gene, with an exact identity of six consecutive amino acids [26]. This similarity suggests that anti-gp210 antibodies may arise due to molecular mimicry of bacterial antigenic determinants Our search of human gut microbial DNA sequences revealed similar coding sequences in Sphingobacteriales bacterium, Bacteroides sp. CAG:1060_57_27, and Faecalibacterium prausnitzii (unpublished data). Further evidence came from Tang et al., who found that gut faecalibacterium was decreased in gp210-positive patients compared to gp210-negative patients, indicating that anti-gp210 antibody is associated with a change in gut microbiome [27]. Therefore, the quantity of anti-gp210 antibodies is perceivably associated with increased IgG concentration, indicating a response to increased bacterial infection.
Our results supported the notion that persistence of anti-gp210 antibodies after UDCA treatment is a risk factor for the progression to end-stage hepatic failure, while their disappearance after therapy indicates a more favorable clinical course in PBC, as reported by Nakamura et al. [9]. We observed that serum conversion of anti-gp210 antibodies after UDCA treatment mainly occurred in early-stage patients with lower initial antibody concentrations. In our study, the conversion rate was relatively high, occurring in 24.1% of patients with initial gp210 antibody positivity. Higher concentrations of anti-gp210 antibodies, rather than mere seropositivity, before UDCA treatment were associated with increased serum IgG, which is an indicator of a more severe autoimmune response.
We found that anti-gp210 concentrations were positively correlated with serum GGT only in the anti-gp210+ patients with ALBI score 1, and with serum IgG only in the anti-gp210+ patients with ALBI score 2/3, as patients were grouped by ALBI scores Figure S4, Table S7). This suggests that in PBC patients undergoing UDCA-naïve treatment, anti-gp210 levels correlate with serum GGT in mild cases and with serum IgG in severe cases. As previously reported, in rat models with cholestasis or treatment with cell type-specific hepatotoxicants, injured bile duct cells, but not hepatocytes, were the source of the increased serum GGT, indicating that the increase in serum GGT activity reflects biliary injury and cholangiolar proliferation [28,29]. Thus, in the early stage of PBC, it is speculated that anti-gp210 expression levels are potentially associated with bile duct injury severity rather than inflammation of hepatocytes. In AIH, IgG serum levels are associated with histologic activity, and their elevation is a risk factor for reduced transplantation-free survival in PSC [30,31]. Therefore, it is hypothesized that anti-gp210 production may be involved in the progression of hepatic cirrhosis in the late stage of PBC, as the balance of the immune system is further impaired.
UDCA is the current first-line therapy for PBC and its mechanisms of action are diverse [32]. One notable effect of UDCA treatment on PBC patients is the reduction in anti-sp100 seropositivity, as reported in this study. However, in our study, we did not observe a similar effect on the seropositivity of anti-gp210 antibodies after UDCA treatment. We attribute this failure to the inclusion of patients with different disease severities in our study. Specifically, nearly half of the anti-gp210 positive PBC patients who were treated with UDCA for more than one year had already developed decompensated liver cirrhosis. This proportion is much higher than that observed in patients with UDCA-naïve treatment (25%), which suggests that the UDCA effect on changing the status of anti-gp210 antibodies may have been eliminated by the advanced disease state of these patients.
According to evidence supported by several research groups, ANAs status in the course of PBC was controversial [6,7,9,[11], [12], [13],17,18]. In this study, we observed that ANAs levels varied throughout the clinical course of PBC and that they were potentially responsive to UDCA therapy, particularly in patients with relatively low ANAs levels in the early stage of the disease. Our findings are consistent with previous reports by Nakamura et al., who found that 20% of anti-gp210-positive patients lost their serum reactivity under UDCA treatment [8,33]. Therefore, it is suggested that quantifying anti-gp210 levels may be useful in monitoring the effect of UDCA in early-stage PBC patients, as it reflects the beneficial immunomodulatory effects of UDCA in regulating the immune status of ANAs-secreting plasmablasts.
The contribution of PBC-related autoantibodies to disease development and pathogenesis is currently unclear. However, data from PBC murine models suggests that cytokines may play an important role in the production of ANAs and AMAs. Specifically, in dnTGF-βRII mice, IL-23, IL-17, and IL-6 were found to modulate anti-gp210 generation [34]. Additionally, a recent study in the p40−/−IL-2Rα−/− PBC murine model suggested that cytokines such as IL-21 or IFN-γ were responsible for AMA levels [35]. Therefore, we hypothesize that fluctuations in autoantibody concentration may be a concomitant state of the disease and may reflect interference with the immune system under UDCA treatment to some extent.
There are several limitations to the current study. Firstly, analyses by crudely defining patients groups according to hepatic cirrhosis (CT, UT or MRI) or AIBL scores (TBIL and ALB), may result in deviation from the actual disease pathology of the patients. Secondly, due to the retrospective nature of the study, there were limitations in terms of sample size, follow-up time, and availability of complete clinical data. Therefore, further studies involving larger PBC cohorts with longer follow-up and comprehensive clinicopathological and immunological data are needed to validate the significance of PBC-specific autoantibody fluctuations.
Finally, a larger and more important question is why such unique autoantibodies are produced. Typical blast searches have failed to identify viable mimics. However molecular mimicry exists, not only at a primary amino acid level, but also at secondary and tertiary levels. The identification of comformational epitopes and therefore potential mimics is a challenge not only here but for many other autoimmune diseases and remains a major gap in our understanding of disease etiology.
5. Conclusions
In conclusion, our study reveals that the presence of anti-gp210 antibody, rather than their levels, was associated with disease severity in PBC patients. We also found that quantitative CLIA was useful in monitoring responsiveness of UDCA treatment. However, it is important to note that, given the limitations of our data, ANA levels cannot serve as a reliable predictor of disease progression. Nonetheless we encourage long term follow up of patients, study of patients following transplant and potential usage of these unique autoantibodies to identify patients in their preclinical phase of disease.
Financial support statement
This work was supported in part by grants from the National Natural Science Foundation of China (No. 81870397 to X.L.; No. 82000534 to C.W; No. 82073156 to W.C.; 82170370 to X.S.), from Shenzhen Kangzhe Pharmaceutical Co.,Ltd (URC-126/PBC to W.C, W.Z. and X.L.), the Fifth Suzhou Health Talent Project (GSWS201903), and the Wuxi Municipal Health Commission (M202117).
CRediT authorship contribution statement
Chan Wang: Writing – original draft, Funding acquisition, Data curation. Zhuye Qin: Writing – original draft. Mingming Zhang: Data curation. Yaping Dai: Resources, Funding acquisition. Luyao Zhang: Validation, Data curation. Wenyan Tian: Resources. Yuhua Gong: Resources. Sufang Chen: Resources. Can Yang: Validation, Data curation. Ping Xu: Resources. Xingjuan Shi: Formal analysis. Weifeng Zhao: Funding acquisition. Suraj Timilsina: Writing – review & editing. M. Eric Gershwin: Writing – review & editing. Weichang Chen: Writing – review & editing, Funding acquisition. Fang Qiu: Writing – review & editing, Supervision, Data curation. Xiangdong Liu: Writing – review & editing, Writing – original draft, Supervision, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Acknowledgment
We thank all participating members of The Jiangsu Provincial PBC Collaboration Group for providing patient samples and clinical information. We would like to thank the patients for their participation in this study.
Handling editor: Y Renaudineau
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2024.100239.
Contributor Information
Weichang Chen, Email: weichangchen@126.com.
Fang Qiu, Email: 13675107990@163.com.
Xiangdong Liu, Email: xiangdongliu@seu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Kaplan M.M., Gershwin M.E. Primary biliary cirrhosis. N. Engl. J. Med. 2005;353(12):1261–1273. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 2.Lindor K.D., Bowlus C.L., Boyer J., Levy C., Mayo M. Primary biliary cholangitis: 2018 Practice guidance from the American association for the study of liver diseases. Hepatology. 2019;69(1):394–419. doi: 10.1002/hep.30145. [DOI] [PubMed] [Google Scholar]
- 3.Cristoferi L., Gerussi A., Invernizzi P. Anti-gp210 and other anti-nuclear pore complex autoantibodies in primary biliary cholangitis: what we know and what we should know. Liver Int. : official journal of the International Association for the Study of the Liver. 2021;41(3):432–435. doi: 10.1111/liv.14791. [DOI] [PubMed] [Google Scholar]
- 4.Itoh S., Ichida T., Yoshida T., Hayakawa A., Uchida M., Tashiro-Itoh T., et al. Autoantibodies against a 210 kDa glycoprotein of the nuclear pore complex as a prognostic marker in patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 1998;13(3):257–265. doi: 10.1111/j.1440-1746.1998.01553.x. [DOI] [PubMed] [Google Scholar]
- 5.Invernizzi P., Podda M., Battezzati P.M., Crosignani A., Zuin M., Hitchman E., et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J. Hepatol. 2001;34(3):366–372. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 6.Wesierska-Gadek J., Penner E., Battezzati P.M., Selmi C., Zuin M., Hitchman E., et al. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology. 2006;43(5):1135–1144. doi: 10.1002/hep.21172. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura M., Kondo H., Mori T., Komori A., Matsuyama M., Ito M., et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45(1):118–127. doi: 10.1002/hep.21472. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura M., Kondo H., Tanaka A., Komori A., Ito M., Yamamoto K., et al. Autoantibody status and histological variables influence biochemical response to treatment and long-term outcomes in Japanese patients with primary biliary cirrhosis. Hepatol. Res. 2015;45(8):846–855. doi: 10.1111/hepr.12423. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura M., Shimizu-Yoshida Y., Takii Y., Komori A., Yokoyama T., Ueki T., et al. Antibody titer to gp210-C terminal peptide as a clinical parameter for monitoring primary biliary cirrhosis. J. Hepatol. 2005;42(3):386–392. doi: 10.1016/j.jhep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Haldar D., Janmohamed A., Plant T., Davidson M., Norman H., Russell E., et al. Antibodies to gp210 and understanding risk in patients with primary biliary cholangitis. Liver Int. : official journal of the International Association for the Study of the Liver. 2021;41(3):535–544. doi: 10.1111/liv.14688. [DOI] [PubMed] [Google Scholar]
- 11.Zuchner D., Sternsdorf T., Szostecki C., Heathcote E.J., Cauch-Dudek K., Will H. Prevalence, kinetics, and therapeutic modulation of autoantibodies against Sp100 and promyelocytic leukemia protein in a large cohort of patients with primary biliary cirrhosis. Hepatology. 1997;26(5):1123–1130. doi: 10.1002/hep.510260506. [DOI] [PubMed] [Google Scholar]
- 12.Mytilinaiou M.G., Meyer W., Scheper T., Rigopoulou E.I., Probst C., Koutsoumpas A.L., et al. Diagnostic and clinical utility of antibodies against the nuclear body promyelocytic leukaemia and Sp100 antigens in patients with primary biliary cirrhosis. Clinica chimica acta; international journal of clinical chemistry. 2012;413(15–16):1211–1216. doi: 10.1016/j.cca.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Gatselis N.K., Zachou K., Norman G.L., Gabeta S., Papamichalis P., Koukoulis G.K., et al. Clinical significance of the fluctuation of primary biliary cirrhosis-related autoantibodies during the course of the disease. Autoimmunity. 2013;46(7):471–479. doi: 10.3109/08916934.2013.801461. [DOI] [PubMed] [Google Scholar]
- 14.Van Norstrand M.D., Malinchoc M., Lindor K.D., Therneau T.M., Gershwin M.E., Leung P.S., et al. Quantitative measurement of autoantibodies to recombinant mitochondrial antigens in patients with primary biliary cirrhosis: relationship of levels of autoantibodies to disease progression. Hepatology. 1997;25(1):6–11. doi: 10.1002/hep.510250103. [DOI] [PubMed] [Google Scholar]
- 15.Tana M.M., Shums Z., Milo J., Norman G.L., Leung P.S., Gershwin M.E., et al. The significance of autoantibody changes over time in primary biliary cirrhosis. Am. J. Clin. Pathol. 2015;144(4):601–606. doi: 10.1309/AJCPQV4A7QAEEFEV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colapietro F., Lleo A., Generali E. Antimitochondrial antibodies: from Bench to Bedside. Clin. Rev. Allergy Immunol. 2022;63(2):166–177. doi: 10.1007/s12016-021-08904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M., Kondo H., Tanaka A., Komori A., Ito M., Yamamoto K., et al. Autoantibody status and histological variables influence biochemical response to treatment and long-term outcomes in Japanese patients with primary biliary cirrhosis. Hepatol. Res. : the official journal of the Japan Society of Hepatology. 2015;45(8):846–855. doi: 10.1111/hepr.12423. [DOI] [PubMed] [Google Scholar]
- 18.Itoh S., Ichida T., Yoshida T., Hayakawa A., Uchida M., Tashiro-Itoh T., et al. Autoantibodies against a 210 kDa glycoprotein of the nuclear pore complex as a prognostic marker in patients with primary biliary cirrhosis. J. Gastroenterol. Hepatol. 1998;13(3):257–265. doi: 10.1111/j.1440-1746.1998.01553.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnson P.J., Berhane S., Kagebayashi C., Satomura S., Teng M., Reeves H.L., et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J. Clin. Oncol. : official journal of the American Society of Clinical Oncology. 2015;33(6):550–558. doi: 10.1200/JCO.2014.57.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demirtas C.O., D'Alessio A., Rimassa L., Sharma R., Pinato D.J. ALBI grade: evidence for an improved model for liver functional estimation in patients with hepatocellular carcinoma. JHEP reports : innovation in hepatology. 2021;3(5) doi: 10.1016/j.jhepr.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corpechot C., Chazouilleres O., Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J. Hepatol. 2011;55(6):1361–1367. doi: 10.1016/j.jhep.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 22.Qiu F., Tang R., Zuo X., Shi X., Wei Y., Zheng X., et al. A genome-wide association study identifies six novel risk loci for primary biliary cholangitis. Nat. Commun. 2017;8 doi: 10.1038/ncomms14828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J., Zhang H., Wang C., Jiang P., Han C., Dai Y., et al. Increased sensitivity of gp210 autoantibody detection using a newly designed gp210 antigen. J. Immunol. Methods. 2022;501 doi: 10.1016/j.jim.2021.113211. [DOI] [PubMed] [Google Scholar]
- 24.Wang C., Zheng X., Jiang P., Tang R., Gong Y., Dai Y., et al. Genome-wide association studies of specific antinuclear autoantibody Subphenotypes in primary biliary cholangitis. Hepatology. 2019;70(1):294–307. doi: 10.1002/hep.30604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas H.C., Holden R., Jones J.V., Peacock D.B. Immune response to phi X 174 in man. 5. Primary and secondary antibody production in primary biliary cirrhosis. Gut. 1976;17(11):844–848. doi: 10.1136/gut.17.11.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickowitz R.E., Worman H.J. Autoantibodies from patients with primary biliary cirrhosis recognize a restricted region within the cytoplasmic tail of nuclear pore membrane glycoprotein Gp210. J. Exp. Med. 1993;178(6):2237–2242. doi: 10.1084/jem.178.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang R., Wei Y., Li Y., Chen W., Chen H., Wang Q., et al. Gut microbial profile is altered in primary biliary cholangitis and partially restored after UDCA therapy. Gut. 2018;67(3):534–541. doi: 10.1136/gutjnl-2016-313332. [DOI] [PubMed] [Google Scholar]
- 28.Bulle F., Mavier P., Zafrani E.S., Preaux A.M., Lescs M.C., Siegrist S., et al. Mechanism of gamma-glutamyl transpeptidase release in serum during intrahepatic and extrahepatic cholestasis in the rat: a histochemical, biochemical and molecular approach. Hepatology. 1990;11(4):545–550. doi: 10.1002/hep.1840110404. [DOI] [PubMed] [Google Scholar]
- 29.Leonard T.B., Neptun D.A., Popp J.A. Serum gamma glutamyl transferase as a specific indicator of bile duct lesions in the rat liver. Am. J. Pathol. 1984;116(2):262–269. http://www.ncbi.nlm.nih.gov/pubmed/6147091 [PMC free article] [PubMed] [Google Scholar]
- 30.Hippchen T., Sauer P., Goppert B., Schirmacher P., Gotthardt D.N., Weiss K.H., et al. Association between serum IgG level and clinical course in primary sclerosing cholangitis. BMC Gastroenterol. 2019;19(1):153. doi: 10.1186/s12876-019-1075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luth S., Herkel J., Kanzler S., Frenzel C., Galle P.R., Dienes H.P., et al. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J. Clin. Gastroenterol. 2008;42(8):926–930. doi: 10.1097/MCG.0b013e318154af74. [DOI] [PubMed] [Google Scholar]
- 32.Paumgartner G., Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36(3):525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura M., Komori A., Ito M., Kondo H., Aiba Y., Migita K., et al. Predictive role of anti-gp210 and anticentromere antibodies in long-term outcome of primary biliary cirrhosis. Hepatol. Res. 2007;37(Suppl 3):S412–S419. doi: 10.1111/j.1872-034X.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang C.Y., Leung P.S., Yang G.X., Kenny T.P., Zhang W., Coppel R., et al. Epitope-specific anti-nuclear antibodies are expressed in a mouse model of primary biliary cirrhosis and are cytokine-dependent. Clin. Exp. Immunol. 2012;168(3):261–267. doi: 10.1111/j.1365-2249.2012.04577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Y.F., Yao Y., Ma M., Yang S.H., Jiang P., Wang J., et al. The proinflammatory cytokines IL-18, IL-21, and IFN-gamma differentially regulate liver inflammation and anti-mitochondrial antibody level in a murine model of primary biliary cholangitis. Journal of immunology research. 2022;2022 doi: 10.1155/2022/7111445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.