Abstract
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disorder affecting females across the lifespan. Eating disorders (EDs) are psychiatric conditions that may impact the development of PCOS and comorbidities including obesity, metabolic syndrome, and type 2 diabetes. The aim of this scoping review was to determine the prevalence of EDs and disordered eating, and to review the etiology of EDs in PCOS. The review was conducted using search terms addressing PCOS, EDs, and disordered eating in databases, including PubMed, Scopus, PsycINFO, and CINAHL. Structured interviews, self-administered questionnaires, chart review, or self-reported diagnosis were used to identify EDs in 38 studies included in the review. The prevalence of any ED in those with PCOS ranged from 0% to 62%. Those with PCOS were 3–6-fold more likely to have an ED and higher odds ratios (ORs) of an elevated ED score compared with controls. In those with PCOS, 30% had a higher OR of bulimia nervosa and binge ED was 3-fold higher compared with controls. Studies were limited on anorexia nervosa and other specified feeding or ED (such as night eating syndrome) and these were not reported to be higher in PCOS. To our knowledge, no studies reported on avoidant/restrictive food intake disorder, rumination disorder, or pica in PCOS. Studies showed strong associations between overweight, body dissatisfaction, and disordered eating in PCOS. The etiologic development of EDs in PCOS remains unclear; however, psychological, metabolic, hypothalamic, and genetic factors are implicated. The prevalence of any ED in PCOS varied because of the use of different diagnostic and screening tools. Screening of all individuals with PCOS for EDs is recommended and high-quality studies on the prevalence, pathogenesis of specific EDs, relationship to comorbidities, and effective interventions to treat ED in those with PCOS are needed.
Keywords: polycystic ovary syndrome, hyperandrogenism, insulin resistance, eating disorder, binge eating disorder, anorexia nervosa, bulimia nervosa
Statement of Significance.
Individuals with polycystic ovary syndrome (PCOS) have a high risk of eating disorders (EDs), in particular binge ED and bulimia nervosa. Further studies are required to explore the range of EDs in PCOS, particularly atypical anorexia nervosa. Early identification and management of an ED may prevent significant negative impacts on quality of life and health in those with PCOS.
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disorder affecting 6%–20% of females and impacting health and quality of life across the lifespan [[1], [2], [3], [4], [5], [6]]. The diagnostic criteria for PCOS includes the presence of 2 of the following: biochemical or clinical hyperandrogenism, menstrual-ovulatory dysfunction, and/or polycystic ovary morphology, with the exclusion of other endocrine disorders [7,8]. PCOS is highly associated with obesity, metabolic syndrome, diabetes, cardiovascular disease, and an increased incidence of infertility and pregnancy complications [[8], [9], [10], [11], [12], [13], [14], [15], [16]]. PCOS is also strongly associated with psychiatric disorders, including depression and anxiety [8,[16], [17], [18], [19], [20]].
Eating disorders (EDs) are psychiatric illnesses that have been reported in PCOS [8,18,21,[16], [22]]. EDs and disordered eating (DE) have been associated with a higher risk of depression, anxiety, and body dissatisfaction in those with PCOS [[23], [24], [25]]. EDs in PCOS may have pathophysiologic causes related to dysregulation of the hypothalamic-neuroregulation of appetite and satiety, associated with metabolic and endocrine factors [21,26,27]. There remain limited studies on the etiologic causes and the different types of EDs in PCOS [[28], [29], [30]].
EDs are clinically diagnosed using structured interviews according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) by the American Psychiatric Association [31]. These disorders are often characterized by disruptions in behaviors, thoughts, and attitudes toward food, eating, and body weight or shape [31,32]. The DSM-5 defines diagnostic criteria for anorexia nervosa (AN), bulimia nervosa (BN), binge ED (BED), other specified feeding or ED (OSFED), avoidant/restrictive feeding or ED (ARFID), pica, and rumination disorder (RD) (Table 1). DE refers to eating related symptoms with behavioral (e.g. bingeing and food restriction), cognitive (e.g. excessive dietary restraint and poor body image), and emotional (e.g. shame and anxiety) features. Both EDs and DE can have detrimental effects on quality of life and can lead to significant medical and psychosocial consequences [31].
TABLE 1.
Criteria for feeding and eating disorders from the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)
| Diagnosis | DSM-5 diagnostic criteria |
|---|---|
| Anorexia Nervosa (AN) | Restriction of energy intake leading to a significantly low body weight, intense fear of gaining weight, disturbance in the way in which one's body weight is experienced, denial of the seriousness of the current low body weight. Two subtypes: restricting type: (no binge eating or purging in the last 3 mo) and binge eating/purging type (binge eating or purging in the last 3 mo) |
| Bulimia Nervosa (BN) | Recurrent episodes of binge eating (eating a large amount of food in a discrete period of time with sense of lack of control) with compensatory behavior to prevent weight gain (e.g., vomiting, laxatives, diuretics, fasting, and excessive exercise) at least once a week for 3 mo. Self-evaluation is unduly influenced by body weight |
| Binge Eating Disorder (BED) | Recurrent episodes of binge eating that are associated with 3 (or more) of: eating rapidly, eating until uncomfortably full, eating large amounts when not hungry, eating alone because of being embarrassed, and guilt/disgust after overeating. Occurs at least once a week for 3 mo with absence of compensatory behaviors |
| Avoidant/Restrictive Food Intake Disorder (ARFID) | A feeding disturbance (e.g., lack of interest in food; sensory avoidance of food; and concern about consequences of eating) as manifested by failure to meet nutritional needs associated with 1 (or more) of: significant weight loss or faltering growth, nutritional deficiency, dependence on nutritional supplements, and interference with psychosocial functioning. Not better explained by lack of available food or by cultural practice. No evidence of body weight disturbance. Not better explained by another medical condition or if so, is severe enough to warrant additional clinical attention |
| Rumination disorder | Repeated regurgitation of food for a period of ≥1 mo. Regurgitated food may be rechewed, reswallowed, or spit out. Not better explained by another medical condition or if so, is severe enough to warrant additional clinical attention |
| Pica | Persistent eating of non-nutritive, nonfood substances for ≥1 mo that is inappropriate to the developmental level of the individual and is not part of a cultural or social practice. If occurring in the context of another condition, it is sufficiently severe to warrant additional clinical attention |
| Other Specified Feeding or Eating Disorder (OSFED) | Examples of presentations that can be specified using OSFED:
|
Table adapted from the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) [31].
The global prevalence of EDs has increased from 3.5% in 2000–2006 to 4.9% in 2007–2012, and to 7.8% in 2013–2018 [33]. The most common is BED, a disorder characterized by rapid consumption of large amounts of food without purging. It affects 1%–4% of adults [[34], [35], [36], [37], [38]] and 1%–2% of children and adolescents [39]. BN is characterized by rapid consumption of large amounts of food with purging and affects 0.41%–1.5% of adults [[33], [34], [35],37]. AN is a disorder characterized by fear of gaining weight, behaviors that interfere with maintaining weight, and severe body dysmorphia, and affects 0.2%–3% of adults [31,33,34,37,40,41]. The other recognized EDs are less common, and their prevalence is largely uncharacterized among the general population [31,33,42,43] (Table 2).
TABLE 2.
Prevalence of EDs in PCOS and in females in the global general population
| Eating disorder | PCOS (%) | General population (%) |
|---|---|---|
| Any eating disorder | 0–62 | 3.61–7.8 [33,37] |
| Binge eating disorder | 1.4–42 | 1.03–3.8 [33,34,37,38] |
| Bulimia nervosa | 0–12 | 0.41–1.5 [33,34,37] |
| Anorexia nervosa | 0–3.5 | 0.23–2.8 [33,37,41,43] |
| Other specified feeding or eating disorder | ||
| Atypical anorexia nervosa | 0 | 0.35 [37] |
| Low-frequency bulimia nervosa | 9.4 | 0.44 [37] |
| Low-frequency binge eating disorder | 16 | 0.38 [37] |
| Purging disorder | 1.2 | 0.23–0.60 [34,37] |
| Night eating syndrome | 12.93 | 1.6 [42] |
| Avoidant/restrictive food intake disorder | N/A | Unclear [31] |
| Rumination disorder | N/A | Unclear [31] |
| Pica | N/A | Unclear [31] |
Abbreviations: AN, anorexia nervosa; ARFID, avoidant/restrictive food intake disorder; BED, binge eating disorder; BN, bulimia nervosa; ED, eating disorder; EDE, Eating Disorder Examination; N/A, not applicable; NES, night eating syndrome; OSFED, other specified feeding or eating disorder; PCOS, polycystic ovary syndrome; RD, rumination disorder.
DE is a descriptive term used to describe a range of irregular eating behaviors such as body dissatisfaction, preoccupation with food, and dieting attempts that are abnormal but not sufficient to warrant a diagnosis using the DSM-5 [31]. DE is a strong predictor for development of an ED, and its prevalence is elevated worldwide [44,45]. Up to 43.8% of Australian people and 74.5% of American adult women have excessive concerns about their body weight/shape that can interfere with quality of life [46,47]. Two in 5 American women have used diet pills to lose weight [47], and 1 in 5 female Canadian adolescents report DE behaviors [48,49].
The coexistence of an ED can complicate first-line recommendations for intervention in PCOS, including diet and lifestyle modifications to target reductions or maintenance of body weight [7,18]. Body weight loss in the overweight-obese individual with PCOS can improve insulin resistance (IR), cardiometabolic risk factors, and menstrual function [7,[50], [51], [52], [53]]. However, a focus on weight loss for an individual with an ED may inadvertently reinforce harmful behaviors and exacerbate the ED [22]. Diet recommendations such as energy restriction may trigger ED symptoms, including restrictive eating, purging, and excessive exercise [54,55]. To add to the challenge, those with PCOS and obesity have been reported to be metabolically resistant to diet-lifestyle interventions to induce weight loss [51,56,57]. Failure to achieve a desired weight goal may then further exacerbate feelings of guilt, shame, and anxiety surrounding food intake and body image, potentially triggering or worsening an ED in those with PCOS [54].
Screening for an ED is recommended in PCOS; however, our understanding of the pathophysiology and prevalence of different types of EDs in PCOS across the lifespan remains limited [20,30,[16], [58], [59], [60], [61], [62]]. The aim of this scoping review was to determine the current state of knowledge on the prevalence of different types of EDs and to review the etiology of EDs in PCOS.
Methods
Review protocol
The PRISMA-ScR was used to guide the protocol of this scoping review [63]. Ethics approval and registration was not required for this study.
Eligibility criteria
The eligibility criteria for studies included in this scoping review are shown in Table 3. Eligible studies had to use a primary research study design; accordingly, a variety of study designs were eligible for inclusion (including cross-sectional, cohort, case–control, case studies, case series, randomized control trials, mixed methods, and qualitative studies). Because meta-analyses report new results, they were eligible for inclusion, whereas systematic reviews were not. Studies in any language other than English were excluded for lack of reliable translation methods.
TABLE 3.
Eligibility criteria for study inclusion in the scoping review
| Inclusion criteria |
|---|
|
| Exclusion criteria |
|
Abbreviations: AN, anorexia nervosa; ARFID, avoidant/restrictive food intake disorder; BED, binge eating disorder; BN, bulimia nervosa; ED, eating disorder; EDNOS, eating disorder not otherwise specified; OSFED, other specified feeding or eating disorder; PCOS, polycystic ovary syndrome; RCT, randomized controlled trial.
Information sources and search strategy
The databases searched were PubMed, Scopus, PsycINFO, and CINAHL. A search algorithm was developed for PubMed (Table 4), which was adapted for the other databases. Searches were completed up to and including 5 June 2023. Database searches were undertaken by 5 reviewers (SL-B, MM, RM, KN, SS). Search results from each database were exported into Covidence, a systematic review management software. Within Covidence, duplicate studies were removed. To confirm eligibility, the titles and abstracts were screened independently by all 5 reviewers during which any disagreements on eligibility were resolved by discussion. This was followed by full-text review by all 5 reviewers. Where full-text publications were unavailable, corresponding authors were contacted, and a 4-wk response was permitted (no requests for full texts were satisfied). The search strategy was augmented by 1 reviewer hand-searching through reference lists of papers relevant to the topic (including editorials and reviews) for additional studies that met the eligibility criteria.
TABLE 4.
Search algorithm for PubMed database
| Search algorithm for PubMed |
|---|
| ((PCOS) OR (PCO) OR (polycystic ovar∗)) AND ((eating disorder∗) OR (appetite disorder∗) OR (feeding disorder∗) OR (nutrition disorder∗) OR (disordered eating) OR (binge∗) OR (bulimi∗) OR (anorexi∗) OR (feeding disorder) OR (purging) OR (body image) OR (body dissatisfaction) OR (dieting)) |
Study selection
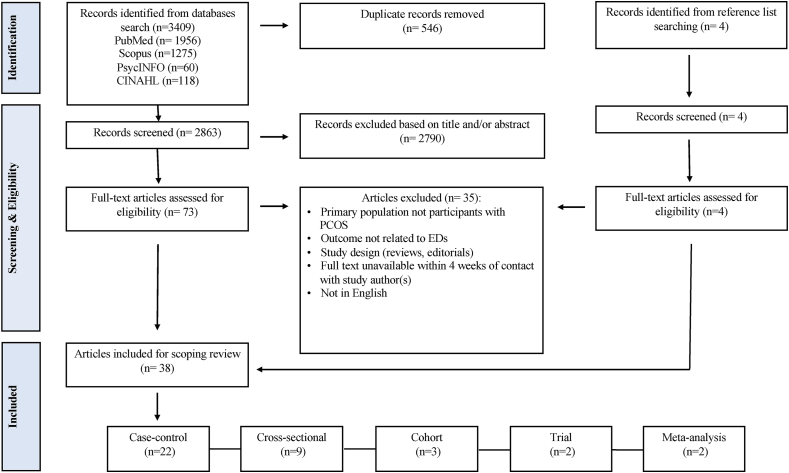
The database searches identified a total of 3409 records and a further 8 were identified from the reference lists of relevant publications. After removing duplicates (n = 546), 2863 publications underwent title and abstract screening based on which a further 2790 were excluded. A total of 73 studies were retrieved for full-text eligibility, and 35 were excluded with reasons including absence of PCOS in the study population, primary outcome not related to EDs, inappropriate study design, full text unavailable within 4 wk of contact with the author(s), and study not in English (Figure 1). This left 38 papers that met the eligibility criteria. Of the 38 included studies, 22 are case–control [1,28,[64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83]], 9 are cross-sectional [[84], [85], [86], [87], [88], [89], [90], [91], [92]], 3 are cohort [[93], [94], [95]], 2 are trials [96,97], and 2 are meta-analyses [29,62].
FIGURE 1.
Study eligibility, selection, and data extraction. ED, eating disorder; PCOS, polycystic ovary syndrome.
Data extraction and synthesis of results
Data from the 38 included studies were extracted by 1 reviewer and verified by 4 other reviewers for calibration. Data relating to author(s), year of publication, geographic location, study population characteristics (age and BMI [kg/m2]), PCOS diagnostic criteria, ED screening/diagnostic tools, ED scores, ED prevalence, and other key findings related to the outcome measures were extracted. Once extracted, data were described and qualitatively synthesized (Table 5).
TABLE 5.
Summary of studies included in the scoping review
| Study | Study characteristics | Participant characteristics Age (y): mean ± SD BMI (kg/m2): mean ± SD |
ED screening or diagnostic tool | Selected scores: mean ± SD |
Prevalence (%) |
||
|---|---|---|---|---|---|---|---|
| PCOS | Control | PCOS | Control | ||||
| Annagür, 2015 [84] | Design: cross-sectional Location: Turkey Sample size: 88 (PCOS: 88) PCOS diagnosis: Rotterdam |
PCOS Age: 22.26 ± 3.55 BMI: Sample mean <25 |
SCID-I for the DSM-IV | — | — | Any ED: 9% BED: 6.8% BN: 2.3% AN: 0% |
— |
| Asdaq, 2020 [85] | Design: cross-sectional Location: Saudi Arabia Sample size: 494 (PCOS: 116; control: 378) PCOS diagnosis: Rotterdam |
PCOS Age: 65% of sample ≥30 y, 35% of sample <30 y BMI: 61% of sample BMI “normal”, 39.5% of sample BMI “obese” |
Unvalidated binge eating questionnaire | — | — | Risk of BED 2.8 times greater in PCOS compared with controls (OR = 2.856, 95% CI: 1.328, 6.141) | — |
| Berni, 2018 [64] | Design: case–control Location: United Kingdom Sample size: 33876 (PCOS: 16938; control: 16938) PCOS diagnosis: Documented in medical chart |
PCOS Age: 26.9 ± 7.2 BMI: 29.86 ± 7.86 Control Age: 27.0 ± 7.36 BMI: 28.99 ± 7.01 |
Documented in medical chart | — | — | Any ED: 1.55%∗∗∗ | Any ED: 1.03%∗∗∗ |
| Cesta, 2016 [65] | Design: case–control Location: Sweden Sample size: 268235 (PCOS: 24385; control: 243850) PCOS diagnosis: NIH and Rotterdam |
PCOS Age: 28.0 ± 6.8 BMI: N/A Control Age: Matched to PCOS group, otherwise N/A BMI: N/A |
Documented in medical chart | — | — | Any ED: 2.45% AN: 0.57% BN: 0.73% Those with PCOS are more likely to have any ED (OR = 1.43, 95% CI: 1.31, 1.56) or BN (OR = 1.35, 95% CI: 1.15, 1.58), but not more likely to have AN (OR = 0.92, 95% CI: 0.78, 1.10) |
Any ED: 1.73% AN: 0.62% BN: 0.55% |
| Coban, 2019 [66] | Design: case–control Location: Turkey Sample size: 59 (PCOS: 28; control: 31) PCOS diagnosis: previously diagnosed |
PCOS Age: range from 13–19 y BMI: N/A but higher in PCOS compared with control group Control group Age: range from 13–19 y BMI: N/A but lower in control compared with PCOS group |
K-SADS-PL for the DSM-IV | Self-esteem score: 1.19 ± 0.93 | Self-esteem score: 1.52 ± 0.93 | AN: 0% BN: 0% |
AN: 0% BN: 0% |
| Dumoulin, 1996 [67] | Design: case–control Location: France Sample size: 92 (oligomenorrhea + EDNOS:13; oligomenorrhea + no ED: 61; control: 18) PCOS diagnosis: Polycystic ovaries on US plus elevated LH and testosterone |
Oligomenorrhea with EDNOS Age: 23.6 ± 1.6 BMI: 21.4 ± 1.6 Oligomenorrhea with no ED Age: 26.8 ± 0.8 BMI: 21.2 ± 0.3 Control Age: 29.2 ± 1.6 BMI: 21.5 ± 0.6 |
EAT-26 and psychiatric interview for the DSM-IV | — | — | — | — |
| Eyupoglu, 2022 [68] | Design: case–control Location: Turkey Sample size: 389 (PCOS: 232; control: 157) PCOS diagnosis: self-reported |
PCOS Age: 23 y BMI: 21.9 ± 2.6 Control Age: 23 BMI: 21.5 ± 2.9 |
TFEQ-18 | TFEQ-18 (baseline): 28.5 ± 5.1∗ |
TFEQ-18 (baseline): 27.3 ± 4.9∗ | — | — |
| Gökcen, 2020 [69] | Design: case–control Location: Turkey Sample size: 80 (PCOS: 40; control: 40) PCOS diagnosis: Rotterdam |
PCOS Age: 25.25 ± 4.79 BMI: 27.23 ± 6.60 Control Age: 24.83 ± 3.17 BMI: 25.37 ± 4.07 |
EDE-Q, TFEQ-R21 | EDE-Q: 2.32 ± 1.45∗∗∗ TFEQ-R21 EE: 56.26 ± 33.50∗ CR: 55.13 ± 28.97∗ UE:49.91 ± 20.99∗∗∗ |
EDE-Q: 1.29 ± 1.07∗∗∗ TFEQ-R21 EE: 44.58 ± 29.17∗ CR: 40.56 ± 19.86∗ UE: 32.21 ± 19.74∗∗∗ |
— | — |
| Greenwood, 2020 [93] | Design: prospective cohort Location: United States Sample size: 5389 (PCOS: 164; control: 5225) PCOS diagnosis: Rotterdam |
PCOS Age: 28.9 BMI: 28.1 Control Age: 30.26 BMI: 24.52 |
EDE-Q | EDE-Q: 2.33 ± 1.47∗∗∗ | EDE-Q: 1.52 ± 1.25∗∗∗ | — | — |
| Herriot, 2008 [94] | Design: retrospective cohort Location: United Kingdom Sample size: 88 (PCOS:88) PCOS diagnosis: by gynecologist or endocrinologist |
PCOS Age: 32.4 ± 9.1 BMI: Normal-weight group mean 21.4; Overweight group mean 32.8 |
Self-report of previous or current ED | — | — | Any ED: 17% | — |
| Hollinrake, 2007 [70] | Design: case–control Location: United States Sample size: 206 (PCOS: 103; control: 103) PCOS diagnosis: Rotterdam |
PCOS Age: 29.8 ± 6.2 BMI: 34.9 ± 8.5 Control Age: 30.7 ± 8.5 BMI: 25.4 ± 4.7 |
PRIME-MD PHQ for the DSM-IV | BED: 12.6%∗∗ BED: 30.5% (with depression) and 3% (no depression) |
BED: 1.9%∗∗ | ||
| Jahanfar, 1995 [71] | Design: case–control Location: Australia Sample size: 94 (PCOS: 42; control: 52) PCOS diagnosis: Presence of polycystic ovaries on US |
PCOS Age: 27.55 ± 9.31 BMI: 23.23 ± 5.06 Control Age: 30.42 ± 8.59 BMI: 23.22 ± 3.67 |
BITE | BITE: 5.35 ± 0.98∗∗∗ | BITE: 3.19 ± 0.83∗∗∗ | — | — |
| Jahanfar, 2005 [72] | Design: case–control Location: Iran Sample size: 154 (PCOS: 25; control: 129) PCOS diagnosis: elevated testosterone or LH/FSH ratio plus symptoms of hyperandrogenism and chronic anovulation plus polycystic ovaries on US |
PCOS Age: 21.77 ± 6.71 BMI: N/A |
BITE | BITE: 3.27 ± 5.51 | BITE: 2.06 ± 4.48 | — | — |
| Jeanes, 2017 [73] | Design: case–control Location: United Kingdom Sample size: 678 (PCOS: 583; control: 95) PCOS diagnosis: previous diagnosis by healthcare professional |
Lean PCOS Age: 31.3 ± 5.6 BMI: 22.5 ± 1.8 Overweight PCOS Age: 31.4 ± 7.6 BMI: 27.3 ± 1.4 Obese PCOS Age: 32.63 ± 7.3 BMI: 39.7 ± 7.3 Control Age: matched to lean PCOS BMI: matched to lean PCOS |
BITE, FCQ-T, TFEQ-R18, self-reported | FCQ-T: 114.0 ± 34.9 Binge eating symptom: 10.9 ± 7.8∗∗ Binge eating severity: 2.8 ± 2.1 |
FCQ-T: 105.6 ± 26.6 Binge eating symptom: 7.4 ± 6.0∗∗ Binge eating severity: 2.0 ± 2.2 |
Intake questionnaire: Any ED: 2% (lean), 1% (overweight), 2% (obese) Study results: BED: 13.6% (lean), 23.2% (overweight), 39.2% (obese) |
— |
| Jensterle, 2015 [97] | Design: nonrandomized trial Location: Slovenia Sample size: 36 (PCOS: 36) PCOS diagnosis: NICHD |
PCOS Age: 31.2 ± 7.8 BMI: 38.7 ± 0.1 |
TFEQ-R18 | TFEQ-R18 (baseline) UE: 36.8 ± 24.5∗∗∗ EE: 49.9 ± 33.33∗∗∗ CR: 52.8 ± 18.3 TFEQ-R18 (post) UE: 19.6.8 ± 18 ± 4∗∗∗ EE: 28.5 ± 26.9∗∗∗ CR: 52.5 ± 22.0 |
— | — | — |
| Jiskoot, 2022 [96] | Design: randomized controlled trial Location: The Netherlands Sample size: 179 (Care as usual PCOS: 60; lifestyle intervention SMS- PCOS: 61; lifestyle intervention SMS+ PCOS: 58) PCOS diagnosis: Rotterdam |
Care as usual (control) Age: 28.0 BMI: 30.6 Lifestyle intervention SMS-Age: 30.0 BMI: 33.5 Lifestyle intervention SMS+ Age: 28.0 BMI: 33.6 |
DEBQ, EDE-Q | EDE-Q (baseline): 1.7–2.1 [0.0–3.1] DEBQ: EE: 2.4–2.9 [1.9–3.5] Restrained eating:3.0–3.2 [2.7–3.6] External eating: 2.7–2.9 [ 2.2–3.3] Binge eating episodes (baseline): 41.7–57.1% |
— | — | — |
| Karacan, 2014 [74] | Design: case–control Location: Turkey Sample size: 94 (PCOS: 42; control: 52) PCOS diagnosis: Rotterdam |
PCOS Age:19.1 ± 2.3 BMI: 22.4 ± 3.8 Control Age: 19.7 ± 2.1 BMI: 21.4 ± 3.8 |
EAT-26 | EAT-26: 46.66 ± 17.03 | EAT-26: 48.21 ± 17.55 | — | — |
| Kerchner, 2009 [95] | Design: cohort Location: United States Sample size: 60 (PCOS with depression: 24; PCOS without depression: 36) PCOS diagnosis: Rotterdam |
PCOS with depression Age: 32.2 ± 6.3 BMI: 33.9 ± 8.3 PCOS without depression Age: 32.6 ± 6.3 BMI: 33.9 ± 8.3 |
PRIME-MD PHQ for the DSM-IV | — | — | BED: 25% (with depression) and 22% (without depression) | — |
| Larsson, 2016 [75] | Design: case–control Location: Sweden Sample size: 102 (PCOS: 72; control: 30) PCOS diagnosis: Rotterdam |
PCOS Age: 30.2 ± 4.4 BMI: 28.5 ± 7.2 Control Age: 27.8 ± 3.6 BMI: 24.6 ± 5.0 |
TFEQ-R21, EAT-40, psychiatric questionnaire for the DSM-IV | TFEQ-R21 CR: 41 ± 23 UE: 42 ± 20 EE: 44 ± 28 EAT-40: 7.8 ± 6.7∗∗∗ |
TFEQ-R21: CR: 37 ± 23 UE: 39 ± 15 EE: 37 ± 19 EAT-40: 16.4 ± 10.1∗∗∗ |
BN: 0% AN: 0.08% |
BN: 0% AN: 0.03% |
| Lee, 2017 [22] | Design: case–control Location: United States Sample size: 254 (PCOS: 148; control: 106) PCOS diagnosis: Rotterdam |
PCOS Age: 28.12 ± 5.13 BMI: 33.85 ± 8.9 Control Age: 31.85 ± 8.09 BMI: 26.82 ± 7.54 |
EDE-Q, NEQ, DSM-5, DSM-IV | EDE-Q: ∼2.4∗∗∗ NEQ: 16.67 ± 6.18∗ |
EDE-Q: ∼1.3∗∗∗ NEQ: 14.88 ± 5.43∗ |
Any ED: 28.38% AN: 0% BN: 6.08% BED: 17.57% NES: 12.93% |
Any ED: 18.87% AN: 0% BN: 5.66% BED: 10.38% NES: 12.38% |
| Lee, 2019 [29] | Design: meta-analysis Location: global Sample size: 860 (PCOS: 470; control: 390) PCOS diagnosis: Rotterdam or NIH |
N/A | EAT-26, EAT-40, MINI, EDE-Q, PRIME-MD PHQ, BITE, NEQ, TFEQ-R21 | Odds of an abnormally high ED score (OR = 3.05; 95% CI: 1.33, 6.99; 4 studies) higher in those with PCOS compared with controls | — | Odds of any ED diagnosis (OR = 3.87; 95% CI: 1.43, 10.49; 4 studies) higher in those with PCOS compared with controls | — |
| Lidaka, 2022 [76] | Design: case–control Location: Latvia Sample size: 129 (PCOS: 63; control: 66) PCOS diagnosis: 2018 ESHRE guidelines |
PCOS Age: 16 BMI: 89.9th percentile Control Age: 17 BMI: 46.9th percentile |
BES | BES: 12 Mild to severe binge eating: 37.7% |
BES: 12 Mild to severe binge eating: 36% |
— | — |
| Livadas, 2011 [86] | Design: cross-sectional Location: Greece Sample size: 130 (PCOS: 130) PCOS diagnosis: Rotterdam |
PCOS Age: 25.4 ± 6.2 BMI: 26.60 ± 7.01 |
Psychiatric interview for DSM (version unspecified), EAT-26 | EAT-26: 11.75 ± 9.6 (normal anxiety); 12.93 ± 4.2 (moderate anxiety); 17.69 ± 8.5 (severe anxiety) | — | Any ED: 0% | — |
| Månsson, 2008 [77] | Design: case–control Location: Sweden Sample size: 98 (PCOS: 49; control: 49) PCOS diagnosis: Rotterdam |
PCOS Age: N/A BMI: 29.1 ± 7.4 Control Age: N/A BMI: 23.5 ± 3.0 |
MINI for the DSM-IV | — | — | Any ED: 21%∗∗ BN: 12% Women with PCOS have significantly more EDs than age-matched controls (OR = 6.4, 95% CI: 1.3, 31) |
Any ED: 4%∗∗ BN: 4% |
| McCluskey, 1991 [78] | Design: case–control Location: United Kingdom Sample size: 261 (PCOS: 152; other endocrinopathy: 109) PCOS diagnosis: Conway et al. 1989 [82] |
PCOS Age: 27.5 ± 6.4 BMI: 24.4 ± 5.1 Other endocrinopathies Age: 32.3 ± 8.4 BMI: 25.4 ± 5.4 |
BITE for the DSM-III | BITE: 7.8 ± 6.1∗∗∗ | BITE: 5.2 ± 4.5∗∗∗ | BN: 6%∗∗ | BN: 1%∗∗ |
| Michelmore, 2001 [87] | Design: cross-sectional Location: United Kingdom Sample size: 230 PCOS diagnosis: Polycystic ovaries on US, plus one of: menstrual irregularity, acne, hirsutism, BMI >25 kg/m2, high testosterone, high LH |
PCOS Age: 21.5 BMI: 22.9 |
EDE for the DSM-IV | EDE: 0.9 | EDE: 0.8 | AN: 0% BN: 0% BED: 4% |
AN: 0% BN: 0% BED: 1% |
| Mizgier, 2020 [88] | Design: cross-sectional Location: Poland Sample size: 78 (PCOS: 78) PCOS diagnosis: Rotterdam |
Normal-weight PCOS Age: 16.29 ± 1.08 BMI: N/A Overweight/obese PCOS Age: 15.73 ± 1.66 BMI: N/A |
EAT-26 | EAT-26: 9.27 ± 5.67 (normal weight); 13.49 ± 9.54 (overweight/obese) | — | — | — |
| Morgan, 2008 [89] | Design: cross-sectional Location: United Kingdom Sample size: 80 (PCOS: 68; CAH: 9; acromegaly: 1; mixed endocrinopathies: 1; idiopathic hirsutism: 1) PCOS Diagnosis: N/A |
Hirsutism Age: 29 ± 5 BMI: N/A |
EDE, SCID-I for the DSM-IV | — | — | Any ED: 36.3% EDNOS: 22.5% BN: 12.6% AN: 1.3% Note that 85% of the sample had PCOS (whereas 100% had hirsutism) All of those diagnosed with an ED had PCOS. PCOS was the only endocrinopathy associated with ED |
— |
| Pirotta, 2019 [28] | Design: case–control Location: Australia Sample size: 899 (PCOS: 501; control: 398) PCOS diagnosis: Self-reported |
PCOS Age: 30.5 ± 5.9 BMI: 33.6 ± 9.3 Control Age: 22.8 ± 5.5 BMI: 24.3 ± 6.0 |
EDE-Q for the DSM-5 | EDE-Q: 2.30∗ | EDE-Q: 2.08∗ | Any ED: 62% BED: 29% Low-frequency BED: 16% Low-frequency BN: 9.4% BN: 5.2% Unspecified feeding or ED: 1.2% Purging disorder: 1.2% AN: 0%∗ Atypical AN: 0%∗∗∗ DE: 21%∗ |
Any ED: 56% BED: 23% Low-frequency BED: 12% Low-frequency BN: 8.0% BN: 4.5% Unspecified feeding or ED: 3% Purging disorder: 0.8% AN: 1.3%∗ Atypical AN: 5.8%∗∗∗ DE: 15%∗ |
| Rassi, 2010 [90] | Design: cross-sectional Location: Brazil Sample size: 72 (PCOS: 68; potential PCOS: 4) PCOS diagnosis: polycystic ovaries on US plus one or more of: menstrual irregularities, acne, hirsutism, and hyperandrogenism |
PCOS Age: 26.2 ± 5.05 BMI: N/A |
MINI for the DSM-IV | — | — | BED: 1.4% | — |
| Rodino, 2016 [79] | Design: case–control Location: Australia Sample size: Seeking IVF (total): 403 (PCOS: 55; non-PCOS: 348) PCOS diagnosis: Self-reported |
PCOS Age: 31.8 ± 4.4 BMI: 27.2 ± 6.6 Control Age: 34.9 ± 4.7 BMI: 24.7 ± 4.7 |
EDE-Q | EDE-Q Shape concern: 1.7 ± 1.3∗∗ |
EDE-Q Shape concern: 1.4 ± 1.3∗∗ |
PCOS status not associated with risk of binge eating | — |
| Sbaragli, 2007 [80] | Design: case–control Location: Italy Sample size: 302 (infertile couples: 81; pregnant couples: 70) PCOS diagnosis: N/A |
Infertile Age: 35 ± 5 BMI: N/A Pregnant Age: 31 ± 4 BMI: N/A |
SCID-1 for the DSM-IV | — | — | Females with endocrine infertility (i.e., PCOS) BED: 15%∗ |
Females with anatomical infertility: BED: 3%∗ Females with infertile male partner: BED: 0%∗ Females with functional infertility: BED: 25%∗ |
| Sirmans, 2014 [81] | Design: case–control Location: United States Sample size: 6756 (PCOS: 1689; control: 5067) PCOS diagnosis: “Probable PCOS” in medical chart |
PCOS Age: 25.24 BMI: N/A Control Age: 25.23 BMI: N/A |
Documented in medical chart | — | — | Any ED: 0.4% | Any ED: 0.3% |
| Suchta, 2023 [91] | Design: cross-sectional Location: Poland Sample size: 122 (PCOS: 122) PCOS diagnosis: Rotterdam |
PCOS Age: 26 ± 5.22 BMI: N/A |
Psychiatric interview, QBES for the DSM-5 | — | — | BED: 42% | — |
| Tay, 2019 [92] | Design: cross-sectional Location: Australia Sample size: 8467 (PCOS: 875; control: 7592) PCOS diagnosis: self-reported |
PCOS Age: 24.8 ± 1.7 BMI: 29.2 ± 7.9 Control Age: 24.6 ± 1.8 BMI: 25.3 ± 5.8 |
Self-reported | — | — | Any ED: 11%∗∗∗ AN: 3.5% BN: 3.4% Other ED: 6.4%∗∗∗ |
Any ED: 7.6%∗∗∗ AN: 3.4% BN: 2.6% Other ED: 3.4%∗∗∗ |
| Thannickal, 2020 [62] | Design: meta-analysis Sample size: 349,529 PCOS diagnosis: Rotterdam |
N/A | Not specified | — | — | Compared with control: AN: OR = 0.92, 95% CI: 0.78, 1.10 (3 studies) BN: OR = 1.37, 95% CI: 1.17, 1.60 (5 studies) BED: OR = 2.95, 95% CI: 1.61, 5.42 (4 studies) Any ED: OR = 3.68, 95% CI: 1.38, 9.81 (4 studies) |
— |
| Wang, 2021 [82] | Design: case–control Location: The Netherlands Sample size: 491 (PCOS: 170; control: 321) PCOS diagnosis: Rotterdam |
PCOS Age: 28.0 ± 4.2 BMI: 36.0 ± 3.5 Control Age: 30.8 ± 4.4 BMI: 36 ± 3.3 |
DEBQ | DEBQ: EE: 34.6 ± 11.2 External eating: 27.7 ± 6.3 Restricted eating: 32.3 ± 5.9 |
DEBQ: EE: 34.1 ± 11.3 External eating: 27.5 ± 5.8 Restricted eating: 31.7 ± 6.1 |
— | — |
| Weiner, 2004 [83] | Design: case–control Location: United States Sample size: 54 (PCOS: 27; control: 27) PCOS diagnosis: oligo/amenorrhea + hyperandrogenemia + infertility, hirsutism, acne, or androgenetic alopecia + exclusion of other endocrinopathy |
PCOS Age: 28.19 ± 4.84 BMI: 37.7 ± 8.46 Control Age: 30.07 ± 6.48 BMI: 36.89 ± 7.24 |
EDI-2 (selected subscales) | No difference in binge eating between groups | No difference in binge eating between groups | — | — |
Abbreviations: AN, anorexia nervosa; BED, binge eating disorder; BES, binge eating scale; BITE, Bulimic Investigatory Test, Edinburgh; CR, cognitive restraint; DE, disordered eating; DEBQ, Dutch Eating Behavior Questionnaire; DSM, Diagnostic and Statistical Manual of Mental Disorders; EAT, eating attitudes test; ED, eating disorder; EDI, Eating Disorder Inventory; EDE, Eating Disorder Examination; EDE-Q. Eating Disorder Examination Questionnaire; EE, emotional eating; EDNOS, Eating disorder not otherwise specified; ESHRE, European Society of Human Reproduction and Embryology; FCQ-T, Food Cravings Questionnaire—Trait; FSH, follicle-stimulating hormone; IVF, in vitro fertilization; K-SADS-PL, Schedule for Affective Disorders and Schizophrenia in School Age Children; LH, luteinizing hormone; MINI, Mini-International Neuropsychiatric Interview; NEQ, night eating questionnaire; NICHD, National Institute of Child Health and Human Development; PCOS, polycystic ovary syndrome; PRIME-MD PHQ, PRIME-MD Patient Health Questionnaire; QBES, Questionnaire for Binge Eating Screening; RCT, randomized controlled trial; SCID-I, Structured Clinical Interview 1; SMS, short message service; TFEQ, Three-Factor Eating Questionnaire; UE, uncontrolled eating; US, ultrasound.
∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
Results
Study and participant characteristics
Study and participant characteristics are summarized in Table 5. Six of the included studies were conducted in the United States [22,70,81,83,93,95], 6 in the United Kingdom [64,73,78,87,89,94], 5 in Turkey [66,68,69,74,84], 4 in Australia [28,71,79,92], 3 in Sweden [65,75,77], 2 in the Netherlands [82,96], and 2 in Poland [88,91]. One each of the remaining studies was conducted in Saudi Arabia [85], France [67], Iran [72], Slovenia [97], Latvia [76], Greece [86], Brazil [90], and Italy [80]. Both the included meta-analyses employed literature from combinations of countries [29,62].
Different criteria were used to diagnose PCOS in studies. A PCOS diagnosis is defined in the international evidence-based guideline for the assessment and management of PCOS (Supplemental Table 1) and the Rotterdam criteria was the most commonly used criteria in 17 studies [22,29,62,69,70,[74], [75], [76], [77],82,[84], [85], [86],88,91,93,[95], [96], [97]]. Eight studies relied upon a self-reported diagnosis or diagnosis found in the participant’s medical chart [28,64,66,68,73,79,81,92]. Several studies relied on other methods, such as solely polycystic ovary morphology on ultrasound [71], polycystic ovaries on ultrasound plus 1 or more other biochemical and clinical features of PCOS [67,78,83,87,90], other clinical and biochemical measures [72], and otherwise unspecified diagnostic criteria [80,89,94] for defining PCOS.
The age of all included participants ranged from 13 y [66,76] to 35.5 ± 5 y [80], whereas BMI ranged from 21.2 ± 0.3 kg/m2 [67] to 36.89 ± 7.24 kg/m2 [83]. However, 10 studies did not report BMI data for ≥1 study subgroup [29,62,65,66,72,80,[88], [89], [90], [91]] and 4 studies did not report age data for ≥1 study subgroup [29,62,65,77].
Of 25 studies that reported on prevalence of EDs in PCOS, 13 reported on BED, 12 reported on BN, 10 reported on AN, 2 reported on OSFED [including night eating syndrome (NES), low-frequency BN, low-frequency BED, purging disorder, and atypical anorexia], 1 reported on ED not otherwise specified (EDNOS), and 0 reported on ARFID, pica, and RD. Thirteen studies reported on the prevalence of any ED in PCOS (Table 5).
Tools used for screening and diagnosis of EDs and DE
A total of 15 tools were used in 38 studies to explore ED and DE behaviors in those with PCOS (Table 6) [[98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114], [115]]. To determine the prevalence of individuals with EDs, 12 studies used structured interviews with participants. These included the Structured Clinical Interview 4 [80,84,89], the Schedule for Affective Disorders and Schizophrenia in School Age Children interviews [66], the semistructured Eating Disorder Examination (EDE) [87,89], the Mini-International Neuropsychiatric Interview [77,90], and other psychiatric interviews [67,75,86,91]. Of these, all used the DSM-IV diagnostic criteria for diagnosing an ED, whereas 1 study [91] used the most current DSM-5 criteria, and another [86] did not specify the edition used.
TABLE 6.
Tools used in the assessment of eating disorders
| Instrument name | Details | Tests for | Psychometric data |
|---|---|---|---|
| Structured interviews | |||
| Structured Clinical Interview (SCID) [98] | SCID for DSM-5 was developed for making the major diagnoses by DSM-5 criteria. It has now been superseded by the SCID for DSM-5, which has a specific version and module for assessing for the feeding and eating disorders [98] | EDs—all DSM-defined EDs | Currently no psychometric data available for the SCID-5 eating and feeding disorder diagnoses (has been shown to be suitable for the schizophrenia spectrum but less so for the anxiety spectrum) [99] |
| Eating Disorder Examination (EDE) [100] | Used to identify frequency of DE behaviors over the past 29 d and to provide 4 subscale scores: 1) Restraint, 2) Eating Concern, 3) Shape Concern, and 4) Weight Concern. Community norms are reported and include mean score of 0.932 ± 0.805 for the global EDE [100] | EDs—AN, BN, BED | A systematic review of the EDE supports the reliability and validity of its scores [101] |
| Mini-International Neuropsychiatric Interview (MINI) [102] | Brief structured diagnostic interview for the major psychiatric disorders by DSM-III, DSM-IV, and DSM-5 | EDs—AN, BN | Found to have 90% sensitivity for AN, 99% sensitivity for BN, 100% specificity for AN, and 99% specificity for BN [101] |
| Schedule for Affective Disorders and Schizophrenia in School Age Children (K-SADS-PL) [103] | Semistructured diagnostic interview designed to assess current and past episodes of psychopathology in those aged 6–18 according to DSM-III and DSM-IV criteria | EDs—all DSM-defined EDs | Suggested to generate reliable and valid child psychiatric diagnoses [103] |
| Self-administered questionnaires | |||
| Eating Attitudes Test (EAT-40 and EAT-26) [104,105] | Used to identify ED risk based on behaviors, feelings, and attitudes associated with eating. The EAT-40 (40-item questionnaire) was developed assess for AN. A cutoff score of ≥30 is considered a high risk, and 21–30 is a moderate risk. The EAT-26 (26-item questionnaire) was later developed to screen for AN, BN, and BED. A score of >20 is considered a high risk | EDs—AN, BN, BED | For both EAT-40 and EAT-26 internal consistency has been shown to be good (Cronbach’s alpha for the total scores found to be 0.83 or higher) [108]. The EAT-26 has 88% sensitivity and 96% specificity for DE [106] |
| Eating Disorder Examination Questionnaire (EDE-Q) | 28-item questionnaire adapted from the EDE interview. Assesses ED behaviors over the past 28 d and uses the same subscales as the EDE Community norms are reported and include a mean score of 1.554 ± 1.213 (Global EDE-Q) [100] |
EDs—AN, BN, BED | The EDE-Q has 83% sensitivity and 96% specificity for disordered eating [110]. Some work suggests that the EDE-Q may generate higher scores than the EDE interview for items including binge eating and shape concerns [101] |
| Bulimic Investigatory Test, Edinburgh (BITE) | 33-item questionnaire that assesses symptoms of DSM-III BN. Two subscales: 1) Symptoms and 2) Severity [107] | EDs—BN | Shown to have satisfactory reliability and validity for DSM-III criteria of BN [108] |
| PRIME-MD Patient Health Questionnaire (PRIME-MD PHQ) | 26-item questionnaire that screens for 5 of the most common groups of disorders in primary care (depressive, anxiety, alcohol, somatoform, and EDs) followed by a clinician evaluation [109] | EDs—AN, BN, BED | The PRIME-MD PHQ has diagnostic validity comparable with the original clinician-administered PRIME-MD [109] |
| Eating Disorder Inventory (EDI) | Measures behaviors associated with AN and BN. The latest version, the EDI-3, has 91 items and 12 subscales [107] | EDs—AN, BN | Validation studies have shown good internal consistency in EDI-3 subscales and ability to discriminate between cases and controls [110] |
| Night Eating Questionnaire (NEQ) | 14-item questionnaire assessing for NES. Four subscales: 1) Nocturnal Ingestions, 2) Evening Hyperphagia, 3) Morning Anorexia, and 4) Mood/Sleep [107] | ED—NES | Shown to be a valid measure of severity of NES [111] |
| Three-Factor Eating Questionnaire (TFEQ-R21 and TFEQ-R18) | The full TFEQ is a 51-item self-report measure of eating behavior. It has 3 subscales: 1) Cognitive Restraint of Eating, 2) Hunger, and 3) Disinhibition. The TFEQ-R18 was the first shortened version developed and was later refined to become the TFEQ-R21 | DE—Eating behaviors | Cronbach’s alpha for the 3 subscales found to be good at 0.85 or higher [116] for the full TFEQ Psychometric properties of the TFEQ-R21 and TFEQ-R18 are both acceptable [112] |
| Food Cravings Questionnaire—Trait (FCQ-T) | 39-item questionnaire designed to measure the frequency and intensity of food cravings. Has 9 subscales that relate to lack of control over eating, preoccupations about food, and food cravings [73] | DE—Eating behaviors | Studies support reliability and validity of the FCQ but findings about the 9 subscales are inconsistent [113] |
| Dutch Eating Behavior Questionnaire (DEBQ) | 33-item questionnaire to assess eating behaviors related to obesity. 3 subscales: 1) Emotional Eating, 2) External Eating, and 3) Restrained Eating [107] | DE – Eating behaviors (in obesity) | Cronbach’s alpha for the DEBQ subscales found to range from 0.80 to 0.95 [114] |
| Binge Eating Scale (BES) | 16-item questionnaire assessing binge eating severity by behaviors and cognitive symptoms | DE—binge eating behaviors | BES scores have been shown to differentiate between those with no, moderate, and severe levels of binge eating [115] |
| Questionnaire for Binge Eating Screening (QBES) | Consists of 4 yes or no questions to screen for binge eating. One or more “yes” responses indicate the presence of binge eating [91] | DE—binge eating behaviors | Psychometrics of this screening tool have not yet been evaluated [91] |
Abbreviations: BES, Binge Eating Scale; BITE, Bulimic Investigatory Test, Edinburgh; DE, disordered eating; DEBQ, Dutch Eating Behavior Questionnaire; DSM, Diagnostic and Statistical Manual of Mental Disorders; EAT, Eating Attitudes Test; EDI, Eating Disorder Inventory; EDE, Eating Disorder Examination; EDE-Q, Eating Disorder Examination Questionnaire; FCQ-T, Food Cravings Questionnaire—Trait; K-SADS-PL, Schedule for Affective Disorders and Schizophrenia in School Age Children; MINI, Mini-International Neuropsychiatric Interview; NEQ, Night Eating Questionnaire; NES, night eating syndrome; PRIME-MD PHQ, PRIME-MD Patient Health Questionnaire; QBES, Questionnaire for Binge Eating Screening; SCID, Structured Clinical Interview; TFEQ, Three-Factor Eating Questionnaire.
Thirty studies used ≥1 self-administered questionnaire to assess eating behaviors in participants. One study used the original 40-question Eating Attitude Test (EAT-40) [75] and 4 studies used the shorter 26-question test (EAT-26) [67,74,86,88]. Six studies used the Eating Disorder Examination Questionnaire (EDE-Q) [22,28,69,79,93,96], that is a 28-item self-report questionnaire adapted from the EDE interview. None of the studies used the original 51-item Three-Factor Eating Questionnaire (TFEQ), and 4 studies used the condensed TFEQ-R21 [69,75] and TFEQ-R18 [73,97] versions. Four studies explored BN behaviors using the Bulimic Investigatory Test, Edinburgh (BITE) [[71], [72], [73],78], based on the DSM-III criteria for BN. Two studies screened for EDs through a nonspecific psychiatric disorder questionnaire, the PRIME-MD Patient Health Questionnaire [70,95]. Two studies used the Dutch Eating Behavior Questionnaire [[82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96]] and another used the second version of the Eating Disorders Inventory [83]. The other tools used were the Food Cravings Questionnaire Trait to assess for to lack of control over eating and preoccupations about food [73], the Night Eating Questionnaire to assess for presence of NES [22], the Binge Eating Scale to assess binge-eating severity [76], the unvalidated Questionnaire for Binge Eating Screening to screen for binge eating [91], and other unvalidated binge eating questionnaires [85]. Six studies relied upon ED diagnosis being reported in the patient’s chart or on self-reported diagnosis [64,65,73,81,92,94].
Prevalence of EDs in PCOS
The prevalence of each ED in PCOS is summarized and compared with the prevalence of EDs in females among the general population in different populations (Table 2).
Prevalence of any ED
The prevalence of any ED in those with PCOS ranged from 0% to 62% as reported in 13 studies [22,28,62,64,65,73,77,81,84,86,89,92,94]. Two large epidemiologic studies found that the prevalence of any ED in those with PCOS was 1.55% [64] and 2.45% [65], and this was significantly higher in age-matched controls that was 1.03% [64] and 1.73% [65], respectively. A case–control study found the prevalence of any ED was 21% in PCOS compared with 4% in controls [77]. Similarly, a cohort study found the prevalence of any ED to be 11% in PCOS compared with 7.6% in controls [92]. A meta-analysis concluded that those with PCOS were 3–4 times more likely to have any ED [odds ratio (OR): 3.68; 95% confidence interval (CI): 1.38, 9.81] compared with females without PCOS [62]. The highest OR reported in the literature indicated that those with PCOS had 6-fold higher prevalence of EDs compared with age-matched controls (OR: 6.4; 95% CI: 1.3, 31) [77]. In another cohort study composed of females with endocrinopathies, all those diagnosed with an ED had PCOS, and PCOS was the only endocrine diagnosis associated with the presence of an ED [89].
On the other hand, 3 case–control studies found no significant difference in prevalence of any ED in PCOS compared with controls [22,28,81]. One case–control study found low prevalence of EDs in both PCOS (0.4%) and non-PCOS participants (0.3%) [81]. Two case–control studies found elevated prevalence of EDs in both PCOS (28.38% and 62%, respectively) and non-PCOS participants (18.87% and 56%, respectively) [22,28]. The latter 2 studies concluded that DEs, but not EDs, were more prevalent in PCOS. Four studies reported on ED prevalence in PCOS without comparison with a non-PCOS control group. These studies reported a prevalence of any ED to be 0% in a Greek sample [86], 1.7% in a UK sample [94] and 9% in a group of Turkish university students [84]. One study compared ED prevalence in PCOS based on body weight and reported ED prevalence in lean, overweight, and obese groups as nonsignificant at 2%, 2%, and 1%, respectively [73]. Collectively, studies demonstrate an increased prevalence of EDs in those with PCOS with no apparent impact of body weight.
ED scores
Many studies have reported ED scores without measuring prevalence of an ED in PCOS. Three studies found no difference in ED scores between those with and without PCOS [74,82,97]. Seven studies found higher scores on the TFEQ-18 and TFEQ-21 [68,69], EDE-Q [22,69,93,96], EAT [75], and BITE [73] scales in those with PCOS compared with controls. In one study, 12.16% of those with PCOS had elevated EDE-Q scores compared with 2.83% of controls [22]. Results from a meta-analysis showed that those with PCOS had higher odds of having elevated ED scores when compared with controls (OR: 3.05; 95% CI: 1.33, 6.99) [29].
One study showed higher EDE-Q and TFEQ-21 scores in overweight-obese women with PCOS compared with overweight-obese controls but no difference in scores between the groups in those with BMI <25 kg/m2 [69]. Similarly, another study found that those with PCOS and BMI >25 kg/m2 had median EDE-Q scores higher than the mean reported in the general population (1.7–2.1 compared with 1.29) [96]. The association between ED score and overweight in PCOS was also supported in another study that found that EAT-26 score ≥20 was correlated with a 7-fold (OR: 6.88; 95% CI: 1.35, 34.97) increased OR with overweight or obesity [88]. Another study reported a BMI increase of 1 kg/m2 corresponded to 15% increased odds of scoring in highest EDE-Q score tertile in those with PCOS [93]. However, another study in adolescents with PCOS found no significant difference in EAT-26 scores between those with overweight/obesity and PCOS and those without overweight/obesity and PCOS [88]. Together, these findings suggest that there may be an association between overweight and ED in those with PCOS; however, this may depend on the type of ED.
Prevalence of AN
Ten studies reported the prevalence of AN in PCOS and 5 of these found the prevalence of AN to be 0% in PCOS [22,28,66,84,87]. The highest prevalence of AN reported in a PCOS sample was 3.5%, but this result was insignificantly different to the 3.4% prevalence found in controls [92]. In this case, the authors indicated that AN prevalence may have been unusually high in their large Australian population sample because of reliance on self-reported AN and PCOS diagnoses [92]. Other papers found that AN prevalence in PCOS was 0.08% [75], 0.57% [65], and 1.3% [89]. Both a large epidemiologic study [65] and a meta-analysis [62] concluded that females with PCOS are not any more likely to have AN compared with controls (OR: 0.92, 95% CI: 0.78, 1.10). Overall, the literature suggests that although AN occurs in those with PCOS, it is of low prevalence compared with other EDs.
Prevalence of BN
The prevalence of BN in those with PCOS ranged from 0% to 12% as reported in 13 studies [22,28,62,65,66,71,72,75,77,78,84,87,92]. Early work published in 1991 and 1995 found that people with PCOS scored higher on the BITE questionnaire and were 6 times more likely to have subclinical BN when compared with controls [71,78]. In these studies, subclinical BN was defined as a score of 10–19 on the BITE, where a score above 20 suggests a high risk of having BN. In the context of recent studies and use of other ED assessment tools, subclinical BN would be categorized as OSFED or DE. Interestingly, a decade later, one research team reported no difference in BITE scores in PCOS compared with controls in a cohort study that used PCOS diagnostic criteria and a large sample size [72]. Certain studies have found elevated rates of BN in PCOS at 12% [77], 6.08% [22], 5.2% [28], and 3.4% [92]; however, these were not significantly different from non-PCOS control groups. Three studies found 0% BN prevalence in PCOS [66,75,87]. Both a large epidemiologic study [65] and a meta-analysis [62] have concluded that individuals with PCOS have 30% higher odds of having BN compared with controls (OR: 1.35, 95% CI: 1.15, 1.58 and OR: 1.37, 95% CI: 1.17, 1.60, respectively).
Prevalence of BED
The prevalence of BED in those with PCOS ranges from 1.4% to 42% as reported in 15 studies [22,28,62,70,73,76,79,80,[83], [84], [85],87,91,95]. One study found elevated rates of BED in PCOS (12.6%) compared with controls (1.9%) [70] and another found a 2.8-fold greater risk of BED in PCOS; however, the latter study did not employ a validated BED questionnaire [85]. A meta-analysis of 4 studies concluded that those with PCOS were more likely to have BED compared with controls (OR: 2.95, 95% CI: 1.61, 5.42) [62]. On the other hand, 6 studies found that BED was not associated with PCOS [22,28,76,79,83,87]. Another 5 studies reported on BED prevalence in PCOS but without comparison with a non-PCOS control group. These studies reported prevalence of BED in PCOS to be 1.4% [90], 6.8% [84], 15% [80], and 42% [91]. One study assessed the relationship between BED and BMI and observed prevalence to be 13.6% in lean, 23.2% in overweight, and 39.2% in obese individuals with PCOS [73], suggesting a significant positive association between body weight and BED risk. BED is also hypothesized to be associated with depression. One study found that BED prevalence was 30.5% in those with both PCOS and depression, whereas it was only 3% in those with PCOS and no depression [70]. However, another study found that risk of BED in those with PCOS and depression was similar to that of those with PCOS and no depression (25% and 22%, respectively) [95].
Prevalence of OSFEDs
Two studies reported on OSFED prevalence in PCOS (which includes NES, low-frequency and/or limited duration BN, low-frequency and/or limited duration BED, purging disorder, and atypical anorexia) [22,28]. NES prevalence was found to be 12.93% in PCOS compared with 12.38% in controls [22]. Although the difference between groups was not significant in this large case–control study (n = 254), the prevalence of NES was high compared with a population study that reported the prevalence of NES to be 1.6% [42] (Table 5). In another study, the prevalence of both BN and BED of low-frequency and/or limited duration was found to be similar and not significantly different between those with and without PCOS (9.4% compared with 8%; 16% compared with 12%, respectively) [28]. Purging disorder was found to not be significantly different in those with (1.2%) and without PCOS (1.2%) [28]. Atypical anorexia was reported at 0% in individuals with PCOS, whereas controls had a prevalence of 5.8% [28]. Of note, no studies reported on the prevalence of ARFID, RD, or pica in a PCOS population.
Discussion
EDs are increased in PCOS
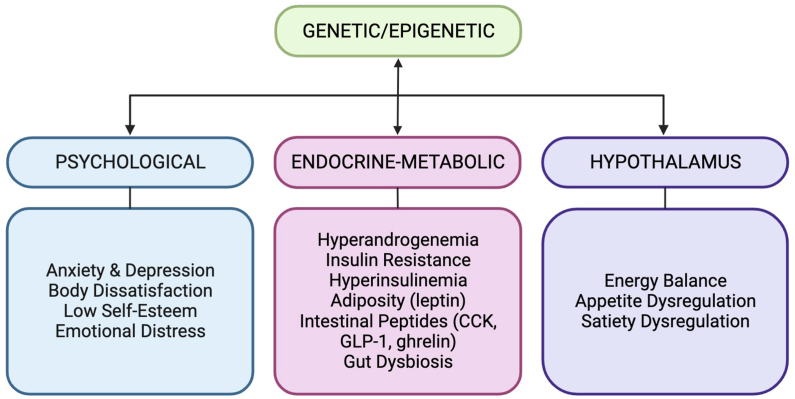
EDs have been proposed to be related to the disruption of the hypothalamic–pituitary–ovarian axis through metabolic, genetic, or psychological factors and these can be associated with altered eating and food-seeking behaviors in PCOS [21,65,67]. EDs can profoundly impact quality of life and have been proposed to contribute to the pathology of PCOS related to adiposity, IR, and androgen excess [21,26,116,117]. In the international guidelines for assessment and management of PCOS, it is recommended that ED and DE should be taken into account and subsequent referral of patients for diagnoses and psychological management [8]. In this scoping review we have investigated the prevalence of the full range of medically diagnosed EDs in individuals with PCOS using the DSM and other screening methodologies. We report that individuals with PCOS are 3–6-fold more likely to have an ED and have higher ORs of an elevated ED score compared with controls. PCOS is associated with an increased prevalence of an ED, in particular BED and BN but not AN, NES, or atypical AN. EDs have a low prevalence in the general population; therefore, data on the prevalence of a range of specific ED diagnoses are limited, particularly in those with PCOS; therefore, the prevalence of EDs in PCOS is likely to be underestimated. There are very limited studies on NES, atypical AN, pica, RD, and ARFID in PCOS. Diagnosis also depends on the tool used to assess for an ED and these vary across studies. The relationship between body weight and an ED may depend on the type of ED, and requires further investigation. Body weight does not appear to be associated with the prevalence of an ED; however, ED scores are higher in PCOS individuals with overweight-obesity compared with BMI-matched controls and BED is increased with BMI [73,88,93,96]. The proposed etiology of EDs in PCOS is multifactorial involving genetic, metabolic, endocrine, and psychological factors and remains understudied [20,21,26,29]. Further investigations are required to understand the pathophysiology and risk of specific types of EDs in PCOS. The pathophysiologic mechanisms that underlie EDs in PCOS are discussed below and summarized in Figure 2.
FIGURE 2.
Multifactorial etiology of eating disorders in polycystic ovary syndrome. CCK, cholecystokinin: GLP-1, glucagon-like-peptide-1.
Psychological risk factors for EDs in PCOS
Emerging evidence suggests that body weight dissatisfaction is associated with the risk of EDs in individuals with PCOS [30,66]. Studies have consistently revealed higher prevalence of body dissatisfaction, perception of being overweight, and multiple weight-loss attempts in those with PCOS compared with controls, independent of BMI [30,83,118,119]. The connection between the pursuit of body weight loss and BED risk is well established, with a 10-fold higher prevalence of BED in adults seeking weight loss compared with the general population [120]. The first-line intervention in those with overweight-obesity in PCOS is targeting diet and lifestyle habits to promote healthy eating behaviors and reduce body weight by 5%–10% [7]. Therefore, it is not surprising that those with PCOS who experience body dissatisfaction and/or overweight-obesity may feel psychological pressure to lose weight, and this may promote the development of an ED [30,66]. Energy-restricted diets and restrictive eating patterns are contributors to BED development [96] and several studies have shown that those with PCOS self-report frequently dieting to reduce body weight [30]. However, other studies show that obese individuals with PCOS who report dieting to lose weight have similar binge eating symptom scores compared with obese PCOS women who are not dieting [73]. Another case–control study found that scores for body dissatisfaction, eating attitudes, and self-esteem were not higher in young adults with PCOS compared with non-PCOS controls [74]. However, both groups had very high scores on the EAT-26 (46.66 ± 17.03 and 48.21 ± 17.55, respectively), where a score of >20 is considered a high risk of an ED [74] (Table 6).
It has been suggested that treating childhood and adolescent obesity as a root cause of PCOS through weight reduction strategies may prevent development of BED and PCOS later in life [[121], [122], [123]]. However, it has been proposed that PCOS may be a result of psychological distress related to dieting and altered food-seeking behaviors during childhood and puberty, contributing to the development of an ED and obesity [27]. Treatment of adolescents with obesity and PCOS using energy-restricted diets rather than promoting healthy eating patterns and health-promoting behaviors may contribute to development of an ED because of increased pressure to lose weight, emotional distress associated with body weight, and body dissatisfaction [30,61,74,124,125].
It has been proposed that menstrual irregularities, hirsutism, overweight, and anxiety about fertility in PCOS are in conflict with accepted societal views of femininity, and these factors contribute to emotional distress and low self-esteem that may lead to altered food-seeking and consumption behaviors to cope [78,[126], [127], [128]]. Self-esteem building with guidance from a psychotherapist or counselor to reframe negative self-talk, cultivate self-compassion, and improve confidence may reduce the risk of ED development, without explicitly focusing on body weight change [92].
Individuals with PCOS are at higher risk for psychological comorbidities, such as anxiety and depressive disorders [[16], [20]]. In several studies, it has been shown that anxiety and depressive disorders are associated with an increased risk of an ED in those with PCOS [22,64,86,93]. One study found that BED prevalence was 30.5% in those with both PCOS and depression, whereas it was only 3% in those with PCOS and no depression [70]. It has been proposed that those with PCOS may be highly sensitive and hyper-responsive to adrenocorticotropic hormone leading to higher production of cortisol that stimulates adrenal androgen production and related hyperandrogenic symptoms (acne, hirsutism, and hunger) [129,130]. Thus, a vicious cycle may ensue of hyperandrogenism symptoms associated with psychological distress, and further release of adrenal cortisol contributing to an exacerbation of PCOS symptoms [129]. It has been reported that individuals with PCOS can lack intrinsic motivation to undertake lifestyle changes or perform regular physical activity [58]. This lack of motivation may be associated with a vicious cycle of body weight gain, body dissatisfaction, and psychological distress, and these may contribute to barriers to implement healthy diet-lifestyle behaviors [58]. Therefore, psychological comorbidities and EDs may coexist in those with PCOS and screening for these conditions can be part of the global health assessment with appropriate referrals to psychotherapy [8,18].
Metabolic and hormonal factors increase ED risk in PCOS
Hyperandrogenemia
Androgens are considered orexigenic, stimulating appetite, disrupting impulse control, and have been associated with binge eating behaviors in those with PCOS [66,67, 97,125,131,132]. Indeed, the androgen receptor is expressed in the arcuate and ventromedial nuclei involved in energy balance and food intake [133,134]. It has been reported that BN is associated with higher serum levels of androgens independent of PCOS status [65,67,132,[135], [136], [137]]; however, other studies have not found the same association [138,139]. A placebo-controlled trial found that flutamide, an androgen receptor antagonist, improved symptoms of BN, binge eating behaviors, and food cravings [140]. In another trial, an antiandrogenic oral contraceptive was shown to improve hunger and lower self-induced vomiting in those with BN [135]. The results of these trials suggest that BN may be associated with hyperandrogenism and androgen receptor mediated effects on the feeding center related to BN and BED.
Elevated testosterone in-utero may predispose individuals to PCOS and simultaneous ED development [141,142], whereas others suggest decreased ED risk [[143], [144], [145]]. Serum testosterone levels are reported to be higher in females with BN and lower in females with AN [132,146]. This may help to explain why AN is not commonly reported in PCOS [28].
Other studies have hypothesized that decreased serum estrogen levels, rather than increased androgen levels, increase the risk of an ED in PCOS [69,147]. Estrogen and the estrogen receptor are considered anorexigenic, promoting satiety that may be protective against emotional eating [69,131,148,149]. Although estrogen levels may not be reduced or may be normal in PCOS, excess luteinizing hormone (LH) may reduce aromatization of androgens to estrogen in ovarian granulosa cells, and this may be associated with emotional eating [131,150,151]. Overall, the literature supports the association of hyperandrogenemia and dysregulation of hypothalamic-neuroregulation of energy balance and food intake and elevated ED scores, and these may contribute to ED risk in those with PCOS [93].
BN has also been associated with PCOS and hyperandrogenemia [131,139]. A case–control study reported that a diagnosis of PCOS occurred in 16.6% of BN cases compared with 1.7% in controls without BN, and those with BN had higher prevalence of menstrual disturbances, hirsutism, high testosterone/sex hormone-binding globulin (SHBG) ratio, and low estrogen levels [131]. Another study reported that 10 of 12 females with BN had polycystic ovary morphology; however, no difference in serum LH, follicle-stimulating hormone, testosterone, or SHBG levels was reported between those with BN and controls [139]. In a 9-year longitudinal follow-up study of 8 females originally treated for BN, all 5 females who had persistent BN also had polycystic ovaries [137]. In contrast, 0% of individuals with BN and 23% of individuals with EDNOS were found to have polycystic ovarian morphology [152]. These findings corroborate with our results that show 30% higher odds of those with PCOS having BN compared with controls [62,65]. There appears to be an association between ED, particularly BN and ovarian follicular morphology and further research to examine this relationship is warranted.
Insulin resistance
IR is common in those affected by PCOS, including lean individuals with PCOS [153]. Hyperinsulinemia can lead to postprandial hypoglycemia, which occurs when blood glucose levels fall <4 mmol/L 2–5 h after a meal. Symptoms of postprandial hypoglycemia can include mood changes, tremor, dizziness, and hunger. Hypoglycemia can therefore cause individuals with PCOS to crave and seek out carbohydrate-rich foods and beverages [154,155]. Studies have found that ≤50% of lean and 66% of obese individuals with PCOS have postprandial hypoglycemia, and this may contribute to mood changes and hunger [[156], [157], [158]].
Binge eating episodes may contribute to excess energy intake, visceral adiposity, and IR [26,159]. BED may contribute to postprandial hyperinsulinemia and contribute to IR via pancreas β-cell dysfunction and peripheral IR [160,161]. IR can result in increased free serum testosterone via decreased hepatic SHBG, and androgens may contribute to changes in eating behavior [66,67,97,125,[131], [132], [133],161]. In a study of individuals with PCOS (n = 164), fasting serum insulin, 2-h postprandial blood glucose, and homeostatic model assessment of IR were positively correlated with EDE-Q scores [93], whereas other studies found no association between insulin levels in patients with EDs, psychiatric disorders (mood disorders, anxiety disorders, and EDs), and PCOS [84,86]. The high prevalence of IR and reactive hypoglycemia may contribute to increased carbohydrate and food craving scores in PCOS [73,155]. Studies have suggested that primary management of PCOS should target treatment of IR rather than weight loss [59]. One study found that a low glycemic index diet and a hypocaloric diet in overweight individuals with PCOS reduced symptoms of carbohydrate cravings, hunger, and tiredness [94]. Further research is needed to test whether low glycemic index diets and other diet regimes may improve reactive hypoglycemia, carbohydrate cravings, and ED behaviors in PCOS [129,156,[162], [163], [164], [165], [166]].
IR has been linked to increased cortisol and lower central nervous system serotonin production and may contribute to risk of mood and depressive symptoms [70]. In a case–control study in those with PCOS (n = 12) and controls (n = 12) matched for age, BMI, and eating behavior, it was found that those with PCOS had more adverse mood symptoms [156].
Adiposity
An association between ED and overweight-obesity in PCOS is reported and appears to be associated with the type of ED [69,72,88,93,96]. The prevalence of EDs in people with obesity and PCOS was 39.2%, compared with 23.2% and 13.6% in those with overweight and normal body weight with PCOS, respectively [73]. Our review results indicate that those with PCOS who are overweight are at higher risk for BED and BN. However, some studies show that EDs in PCOS are independent of BMI or visceral adiposity in normal-weight and overweight individuals. In a case–control study, binge eating symptom scores were higher in lean participants with and without PCOS [73]. Obesity independent of PCOS was shown to be associated with higher risk of binge eating (OR: 7.915, 95% CI: 3.421, 18.312) [79].
Increased adiposity may contribute to the development of EDs via impairment of the hypothalamic-neuroregulation of energy balance and food intake, and this applies to those with PCOS and overweight-obesity as we observed in several studies [67,68,88,93,96]. The arcuate nuclei contain pro-opiomelanocortin (POMC)/cocaine amphetamine-regulated transcript (CART), neuropeptide Y (NPY)/agouti-related peptide (AGRP), and kisspeptin neurons that coregulate energy balance and the hypothalamic–pituitary–ovarian axis to impact estrogen and testosterone production and reproduction [72,167]. Adipose tissue produces adipokines, including leptin that inhibits appetite and promotes satiety [72]. In those with higher levels of adiposity, there is often increased leptin and ensuing leptin resistance that can impair activation of anorexigenic POMC/CART signaling to inhibit food-energy intake and lack of inhibition of orexigenic pathways NPY/AGRP that promote food-energy intake, resulting in impaired satiety and this may lead to the development of BED [28,72]. In addition, hyperinsulinemia and IR commonly coexist with visceral adiposity and may further inhibit satiety signals and simultaneously promote ovarian androgen production [28].
In examining the relationship between AN and PCOS, one study found that BMI was higher in those with AN and PCOS (19.5 ± 2.5) compared with AN alone (16.9 ± 0.7) [124]. We only identified one study on atypical AN and the prevalence in PCOS was 0% compared with non-PCOS controls [28]. Diagnostic criteria of atypical AN is a more recent addition to OFSED in the DSM-5; therefore, the prevalence of atypical AN in PCOS may be underestimated [31]. AN is commonly associated with low BMI and weight loss; therefore, awareness and screening for atypical AN may not regularly occur in PCOS. It may be useful to screen for atypical AN in those with PCOS, because in atypical AN, BMI is often within or above the normal range, and this may be the case in those with PCOS [28,124].
PCOS diet, lifestyle, and weight management recommendations need to be sensitive to EDs so as not to perpetuate DE behaviors. One way to potentially achieve this is through a weight-neutral approach and a focus on health eating patterns and lifestyle behaviors [8,28,58]. Weight-neutral approaches that focus on intuitive eating, stress management, physical activity, and healthy eating, independent of body weight have been successful in improving BN in non-PCOS populations [168,169]. Research is needed to evaluate the impact of weight-neutral programs and the impact on modulation of an ED in those affected by PCOS.
Appetite dysregulation
Appetite dysregulation appears to be a contributor to ED risk in PCOS and can be associated with altered leptin and insulin metabolism, as described above. Individuals with obesity and PCOS were found to have higher energy intake and an earlier return of hunger after an ad libitum meal compared with controls with obesity [170]. A randomized controlled trial found that those with PCOS were less satiated and felt hungrier after meals compared to those without PCOS [171].
Endogenous opioids have been linked to hedonic aspects of eating and sensory reward processes, stimulating a desire for high fat and glucose foods through μ-opioid receptors within the amygdala and nucleus accumbens [97]. IR has been found to stimulate central opioid activity in PCOS, potentially contributing to higher risk for binge eating [172]. Ghrelin, a hormone that signals that the stomach is empty to promote appetite and food-seeking behavior, has been found to be associated with IR in those with PCOS [74]. Because of imbalances in ghrelin levels, those with PCOS may experience feelings of hunger leading to food-seeking behaviors and overeating [171]. On the other hand, glucagon-like peptide-1 (GLP-1), a hormone released from the ileum in response to feeding to enhance insulin secretion, also promotes satiety and reduces energy intake [97]. Impaired secretion of GLP-1 has been demonstrated in in PCOS [97]. Studies support the role of GLP-1 receptor agonist medications (such as semaglutide and liraglutide) that improve insulin sensitivity, reduce appetite, and reduce food-seeking behavior in those with PCOS [97,173,174]. Cholecystokinin (CCK) is a satiety-promoting hormone secreted in response to dietary lipid in the duodenum and has been reported to have reduced secretion in PCOS [155]. CCK stimulates gallbladder contraction, inhibits gastric emptying, and signals the hypothalamic arcuate nuclei to reduce food intake. Impaired CCK production has been observed in PCOS and may lead to decreased satiety and potentially contribute to overeating or EDs [155].
Gut microbiota dysbiosis
A growing body of evidence suggests that gut microbiota dysbiosis plays a role in the development of psychiatric disorders, obesity, IR, and PCOS [[175], [176], [177]]. The microbiome phenotype can affect how nutrients are absorbed and can influence the effects of peptides, CCK, GLP-1, and ghrelin [27]. It has been shown that food restriction, a characteristic of EDs, is associated with altered gut microbiota composition [175]. A Japanese study found that females with AN had significantly lower amounts of total gut bacteria and obligate anaerobes including Clostridium coccoides, Clostridium leptum subgroup, and Bacteroides fragilis [178]. Gut microbes can be influenced by hormonal signals that are affected by psychological stress caused by EDs [175]. At this time the mechanisms of how the microbiome is directly linked to ED development in PCOS remain unclear.
Taste perception
Altered taste perception has been proposed to contribute to ED development in PCOS; however, more studies are needed in this area. Those with PCOS were found to have lower sour, salty, and total taste scores compared with controls, and higher free androgen index was associated with lower total taste score [179]. Oral contraceptive treatment was shown to correct hyperandrogenism but did not affect total taste score. No conclusion could be drawn about taste perception and food cravings in those with PCOS and EDs, because participants with EDs were excluded from the trial [179].
Genetics and increased ED risk in PCOS
Most PCOS phenotypes do not show a clear Mendelian pattern of inheritance and the genetic loci linked to PCOS to date account for only 10% of its heritability, which is estimated at 70% [[180], [181], [182]]. Genes that may contribute to the risk of developing PCOS include gene variants of obesity, insulin, steroid hormone regulation, insulin signaling, and ovarian function [183]. Overall, PCOS is a polygenic and multifactorial disorder, and epigenetic modifications resulting from hormonal dysregulation of the maternal uterine environment may also contribute the development of PCOS [180,183].
Genetics may contribute to the risk of developing EDs [184]. Twin and family studies have demonstrated that heritable genetic traits related to hypothalamic-neuroregulation appetite and energy balance may play a role in BN development [71,184]. However, genetics alone may not appear to be sufficient to cause an ED, because environmental and psychological factors play a significant role in the development of EDs [72]. A large epidemiologic study found that the odds of siblings of those with PCOS with unclear ED status (both genetically male and female) having AN, BN, and any ED were not different (OR: 0.88, 95% CI: 0.68, 1.14; OR: 1.20, 95% CI: 0.91, 1.59; OR: 0.94, 95% CI: 0.81, 1.10, respectively) [65]. However, elevated risk in female siblings was found for other psychiatric disorders, such as depressive, anxiety, and schizophrenia disorders [65]. No shared genetic link between PCOS and psychiatric disorders (depression, anxiety, schizophrenia, and bipolar disorder) has been observed; however, this study did not examine EDs [185]. Therefore, genetic predisposition to psychiatric disorders may contribute but does not appear to be the strongest predictor of possible shared etiology between PCOS and EDs.
Limitations of the review
This scoping review represents a summary of medically diagnosed EDs and those identified using different ED assessment screening tools. The findings on the prevalence of EDs in PCOS compared with controls varied and had large ranges, which may be because of the array of diagnostic and screening tools used. There was no consistent method for the assessment of EDs used in PCOS studies and we did not restrict the review to a specific type of tool to assess EDs and DE (TABLE 5, TABLE 6). The methods used to diagnose EDs have evolved as new versions of the DSM have been published by the American Psychiatric Association; therefore, our review included studies that used the DSM-III, DSM-IV, and DSM-5. Different PCOS diagnostic criteria were used in studies and this may have resulted in different prevalence rates of EDs. However, the Rotterdam diagnostic criteria was mostly used by studies. A strength of the study was inclusion of a range of tools and diagnostic criteria to assess the prevalence of an ED and this allowed us to report on the scope of EDs and DE in PCOS.
Summary
Individuals with PCOS are at high risk of EDs, particularly BED and BN. However, the degree of risk for specific subtypes of EDs remains largely unclear. The pathophysiology of EDs in PCOS involves multiple pathways including genetic, metabolic, endocrine and psychological factors [20,21,26,29]. Body weight dissatisfaction, anxiety, and depression have been identified as risk factors for ED development in those with PCOS. Furthermore, those with PCOS may have altered eating and food-seeking behaviors related to inherent hyperandrogenism. Several studies have proposed a bidirectional relationship between hyperandrogenism and hyperinsulinemia in contributing to the development of ED in PCOS, particularly BED and BN, wherein hyperinsulinemia can predispose individuals to binge eating, whereas binge eating behaviors may contribute to hyperinsulinemia, adiposity, and androgen excess [26]. Taste perception, gut microbiome dysbiosis, and genetic dysregulation of the hypothalamic-neuroregulation of energy metabolism have been hypothesized to play a role in EDs in PCOS.
Research implications
High-quality studies on the prevalence of EDs in individuals with PCOS are needed that employ large cohorts and assessment of rare ED subtypes, such as NES, atypical AN, pica, RD, and ARFID. Ideally, future studies should use clinical interviews with a registered psychologist or psychiatrist to clinically diagnose EDs [58]. Alternatively, studies could use multiple validated ED instruments and avoid reliance on self-reported ED or DE symptoms. There are no studies assessing the development or change in ED diagnosis or prevalence over time. Furthermore, differences in prevalence based on ethnicity, gender, and impact of management strategies including counseling, diet and lifestyle, and impact of body weight discrimination on EDs in PCOS would be of interest.
Clinical implications
EDs impact quality of life and health in individuals with PCOS across the lifespan [186]. It is recommended that clinicians and healthcare providers be alert to EDs and DE, regardless of a patient’s body weight, especially in the context of weight management and lifestyle interventions [8]. If an ED is detected, patients should be offered appropriate therapy through a clinical psychiatrist and/or psychologist and/or registered dietitian [8,18]. The care of a patient with both PCOS and an ED requires specialized and comprehensive care involving a multidisciplinary team of health care providers. Although prioritizing weight loss and/or weight management in those with obesity and PCOS can improve preconception health, menstrual function, insulin sensitivity, and hyperandrogenism, it is essential to acknowledge the serious potential harm of placing emphasis on weight loss in someone with an ED or DE behaviors [8,22,[50], [51], [52], [53], [54]]. Weight loss regimes encouraged by a clinician without follow-up care or referral to a registered dietitian may be interpreted as promotion of an ideal body weight, reinforcing feelings of despair, worsening body dissatisfaction that may increase the risk of restrictive dietary behaviors and exacerbate an ED [29]. A cautious, compassionate, weight-neutral, and self-esteem building approach may be necessary during ED treatment to prevent prioritizing achieving an arbitrary weight goal at the expense of mental health and overall wellbeing.
Conclusions
This scoping review has shown that individuals with PCOS have a high risk of developing EDs, especially BN and BED. The etiology of ED development in PCOS is not yet fully understood, but it is proposed to include psychological (body dissatisfaction, anxiety, depression), metabolic (hyperandrogenism, IR, adiposity, appetite dysregulation, gut dysbiosis), and genetic predisposition. High-quality studies are needed to rigorously assess the prevalence of EDs in individuals with PCOS and to better understand the relationship between risk factors and ED development. Early identification and management of an ED may prevent significant negative impact on quality of life in those with PCOS.
Author contributions
The authors’ responsibilities were as follows – SL-B, DV: designed the research; SL-B, MM, RM, KN, SS: conducted the research; SL-B: analyzed the data; SL-B, DV: wrote the paper; MG: reviewed the manuscript; DV: responsible for final content; and all authors: read and approved the final manuscript.
Conflict of interest
The authors report no conflicts of interest.
Funding
SL-B was funded by a Women and Children's Health Research Institute Studentship to complete this research. This research was supported by the Women and Children’s Health Research Institute and the Alberta Women's Health Foundation.
Acknowledgments
We would like to thank psychiatrist Dr Michele Foster for explanations of the changes to the DSM-5 diagnostic criteria that were helpful in critically appraising the literature.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.advnut.2024.100193.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Azziz R., Woods K.S., Reyna R., Key T.J., Knochenhauer E.S., Yildiz B.O. The prevalence and features of the polycystic ovary syndrome in an unselected population. J. Clin. Endocrinol. Metab. 2004;89(6):2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 2.Yildiz B.O., Bozdag G., Yapici Z., Esinler I., Yarali H. Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum. Reprod. 2012;27(10):3067–3073. doi: 10.1093/humrep/des232. [DOI] [PubMed] [Google Scholar]
- 3.Azziz R., Carmina E., Chen Z., Dunaif A., Laven J.S., Legro R.S., et al. Polycystic ovary syndrome. Nat. Rev. Dis. Primers. 2016;2 doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- 4.Cooney L.G., Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil. Steril. 2018;110(5):794–809. doi: 10.1016/j.fertnstert.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Bozdag G., Mumusoglu S., Zengin D., Karabulut E., Yildiz B.O. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 2016;31(12):2841–2855. doi: 10.1093/humrep/dew218. [DOI] [PubMed] [Google Scholar]
- 6.Hart R., Doherty D.A. The potential implications of a PCOS diagnosis on a woman's long-term health using data linkage. J. Clin. Endocrinol. Metab. 2015;100(3):911–919. doi: 10.1210/jc.2014-3886. [DOI] [PubMed] [Google Scholar]
- 7.Teede H.J., Misso M.L., Costello M.F., Dokras A., Laven J., Moran L., et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018;110(3):364–379. doi: 10.1016/j.fertnstert.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teede H.J., Tay C.T., Laven J.J.E., Dokras A., Moran L.J., Piltonen T.T., et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2023;108(10):2447–2469. doi: 10.1210/clinem/dgad463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirotta S., Joham A., Grieger J.A., Tay C.T., Bahri-Khomami M., Lujan M., et al. Obesity and the risk of infertility, gestational diabetes, and type 2 diabetes in polycystic ovary syndrome. Semin. Reprod. Med. 2020;38(6):342–351. doi: 10.1055/s-0041-1726866. [DOI] [PubMed] [Google Scholar]
- 10.Lim S.S., Kakoly N.S., Tan J.W.J., Fitzgerald G., Bahri-Khomami M., Joham A.E., et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes. Rev. 2019;20(2):339–352. doi: 10.1111/obr.12762. [DOI] [PubMed] [Google Scholar]
- 11.Glintborg D., Rubin K.H., Nybo M., Abrahamsen B., Andersen M. Cardiovascular disease in a nationwide population of Danish women with polycystic ovary syndrome. Cardiovasc. Diabetol. 2018;17(1):37. doi: 10.1186/s12933-018-0680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin K.H., Glintborg D., Nybo M., Abrahamsen B., Andersen M. Development and risk factors of type 2 diabetes in a nationwide population of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017;102(10):3848–3857. doi: 10.1210/jc.2017-01354. [DOI] [PubMed] [Google Scholar]
- 13.Palomba S., de Wilde M.A., Falbo A., Koster M.P., La Sala G.B., Fauser B.C. Pregnancy complications in women with polycystic ovary syndrome. Hum. Reprod. Update. 2015;21(5):575–592. doi: 10.1093/humupd/dmv029. [DOI] [PubMed] [Google Scholar]
- 14.Bahri-Khomami M., Joham A.E., Boyle J.A., Piltonen T., Silagy M., Arora C., et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity—a systematic review, meta-analysis, and meta-regression. Obes. Rev. 2019;20(5):659–674. doi: 10.1111/obr.12829. [DOI] [PubMed] [Google Scholar]
- 15.Riestenberg C., Jagasia A., Markovic D., Buyalos R.P., Azziz R. Health care-related economic burden of polycystic ovary syndrome in the United States: pregnancy-related and long-term health consequences. J. Clin. Endocrinol. Metab. 2022;107(2):575–585. doi: 10.1210/clinem/dgab613. [DOI] [PubMed] [Google Scholar]
- 16.Vine D.F., Ghosh M., Wang T. Increased prevalence of health outcomes across the lifespan in those affected by polycystic ovary syndrome: a Canadian population cohort study. Can J Cardiol. 2023;6:314–326. doi: 10.1016/j.cjc0.2023.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry J.A., Kuczmierczyk A.R., Hardiman P.J. Anxiety and depression in polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 2011;26(9):2442–2451. doi: 10.1093/humrep/der197. [DOI] [PubMed] [Google Scholar]
- 18.Dokras A., Stener-Victorin E., Yildiz B.O., Li R., Ottey S., Shah D., et al. Androgen Excess-Polycystic Ovary Syndrome Society: position statement on depression, anxiety, quality of life, and eating disorders in polycystic ovary syndrome. Fertil. Steril. 2018;109(5):888–899. doi: 10.1016/j.fertnstert.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Veltman-Verhulst S.M., Boivin J., Eijkemans M.J., Fauser B.J. Emotional distress is a common risk in women with polycystic ovary syndrome: a systematic review and meta-analysis of 28 studies. Hum. Reprod. Update. 2012;18(6):638–651. doi: 10.1093/humupd/dms029. [DOI] [PubMed] [Google Scholar]
- 20.Blay S.L., Aguiar J.V., Passos I.C. Polycystic ovary syndrome and mental disorders: a systematic review and exploratory meta-analysis. Neuropsychiatr. Dis. Treat. 2016;12:2895–2903. doi: 10.2147/NDT.S91700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burnatowska E., Wikarek A., Oboza P., Ogarek N., Glinianowicz M., Kocelak P., et al. Emotional eating and binge eating disorders and night eating syndrome in polycystic ovary syndrome-a vicious circle of disease: a systematic review. Nutrients. 2023;15(2):295. doi: 10.3390/nu15020295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee I., Cooney L.G., Saini S., Smith M.E., Sammel M.D., Allison K.C., et al. Increased risk of disordered eating in polycystic ovary syndrome. Fertil. Steril. 2017;107(3):796–802. doi: 10.1016/j.fertnstert.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Cooney L.G., Lee I., Sammel M.D., Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum. Reprod. 2017;32(5):1075–1091. doi: 10.1093/humrep/dex044. [DOI] [PubMed] [Google Scholar]
- 24.Cinar N., Kizilarslanoglu M.C., Harmanci A., Aksoy D.Y., Bozdag G., Demir B., et al. Depression, anxiety and cardiometabolic risk in polycystic ovary syndrome. Hum. Reprod. 2011;26(12):3339–3345. doi: 10.1093/humrep/der338. [DOI] [PubMed] [Google Scholar]
- 25.Sander J., Moessner M., Bauer S. Depression, anxiety and eating disorder-related impairment: moderators in female adolescents and young adults. Int. J. Environ. Res. Public Health. 2021;18(5):2779. doi: 10.3390/ijerph18052779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paganini C., Peterson G., Stavropoulos V., Krug I. The overlap between binge eating behaviors and polycystic ovarian syndrome: an etiological integrative model. Curr. Pharm. Des. 2018;24(9):999–1006. doi: 10.2174/1381612824666171204151209. [DOI] [PubMed] [Google Scholar]
- 27.Steegers-Theunissen R.P.M., Wiegel R.E., Jansen P.W., Laven J.S.E., Sinclair K.D. Polycystic ovary syndrome: a brain disorder characterized by eating problems originating during puberty and adolescence. Int. J. Mol. Sci. 2020;21(21):8211. doi: 10.3390/ijms21218211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pirotta S., Barillaro M., Brennan L., Grassi A., Jeanes Y.M., Joham A.E., et al. Disordered eating behaviours and eating disorders in women in Australia with and without polycystic ovary syndrome: a cross-sectional study. J. Clin. Med. 2019;8(10):1682. doi: 10.3390/jcm8101682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee I., Cooney L.G., Saini S., Sammel M.D., Allison K.C., Dokras A. Increased odds of disordered eating in polycystic ovary syndrome: a systematic review and meta-analysis, Eat. Weight Disord. 2019;24(5):787–797. doi: 10.1007/s40519-018-0533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee I., Dokras A. Mental health and body image in polycystic ovary syndrome. Curr. Opin. Endocr. Metab. Res. 2020;12:85–90. doi: 10.1016/j.coemr.2020.04.004. [DOI] [Google Scholar]
- 31.American Psychiatric Association . 5th ed. CBS Publishers and Distributors PTY LTD 2017, US; 2013. Diagnostic and Statistical Manual of Mental Disorders. [DOI] [Google Scholar]
- 32.Birmingham C.L., Su J., Hlynsky J.A., Goldner E.M., Gao M. The mortality rate from anorexia nervosa. Int. J. Eat. Disord. 2005;38(2):143–146. doi: 10.1002/eat.20164. [DOI] [PubMed] [Google Scholar]
- 33.Galmiche M., Déchelotte P., Lambert G., Tavolacci M.P. Prevalence of eating disorders over the 2000-2018 period: a systematic literature review. Am. J. Clin. Nutr. 2019;109(5):1402–1413. doi: 10.1093/ajcn/nqy342. [DOI] [PubMed] [Google Scholar]
- 34.Gauvin L., Steiger H., Brodeur J.M. Eating-disorder symptoms and syndromes in a sample of urban-dwelling Canadian women: contributions toward a population health perspective. Int. J. Eat. Disord. 2009;42(2):158–165. doi: 10.1002/eat.20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler R.C., Berglund P.A., Chiu W.T., Deitz A.C., Hudson J.I., Shahly V., et al. The prevalence and correlates of binge eating disorder in the World Health Organization World Mental Health Surveys. Biol. Psychiatry. 2013;73(9):904–914. doi: 10.1016/j.biopsych.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erskine H.E., Whiteford H.A. Epidemiology of binge eating disorder. Curr. Opin. Psychiatry. 2018;31(6):462–470. doi: 10.1097/YCO.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 37.Micali N., Martini M.G., Thomas J.J., Eddy K.T., Kothari R., Russell E., et al. Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: a population-based study of diagnoses and risk factors. BMC Med. 2017;15(1):12. doi: 10.1186/s12916-016-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinzl J.F., Traweger C., Trefalt E., Mangweth B., Biebl W. Binge eating disorder in females: a population-based investigation. Int. J. Eat. Disord. 1999;25(3):287–292. doi: 10.1002/(sici)1098-108x(199904)25:33.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 39.Kjeldbjerg M.L., Clausen L. Prevalence of binge-eating disorder among children and adolescents: a systematic review and meta-analysis. Eur. Child Adolesc. Psychiatry. 2023;32(4):549–574. doi: 10.1007/s00787-021-01850-2. [DOI] [PubMed] [Google Scholar]
- 40.van Eeden A.E., van Hoeken D., Hoek H.W. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr. Opin. Psychiatry. 2021;34(6):515–524. doi: 10.1097/yco.0000000000000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Favaro A., Ferrara S., Santonastaso P. The spectrum of eating disorders in young women: a prevalence study in a general population sample. Psychosom. Med. 2003;65(4):701–708. doi: 10.1097/01.psy.0000073871.67679.d8. [DOI] [PubMed] [Google Scholar]
- 42.Striegel-Moore R.H., Dohm F.A., Hook J.M., Schreiber G.B., Crawford P.B., Daniels S.R. Night eating syndrome in young adult women: prevalence and correlates. Int. J. Eat. Disord. 2005;37(3):200–206. doi: 10.1002/eat.20128. [DOI] [PubMed] [Google Scholar]
- 43.Hoek H.W., van Hoeken D. Review of the prevalence and incidence of eating disorders. Int. J. Eat. Disord. 2003;34(4):383–396. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 44.Urquhart C.S., Mihalynuk T.V. Disordered eating in women: implications for the obesity pandemic. Can. J. Diet. Pract. Res. 2011;72(1):e115–e125. doi: 10.3148/72.1.2011.50. [DOI] [PubMed] [Google Scholar]
- 45.Gusella J., Goodwin J., van Roosmalen E. 'I want to lose weight': early risk for disordered eating? Paediatr. Child Health. 2008;13(2):105–110. doi: 10.1093/pch/13.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dias Santana D., Mitchison D., Gonzalez-Chica D., Touyz S., Stocks N., Appolinario J.C., et al. Associations between self-reported diabetes mellitus, disordered eating behaviours, weight/shape overvaluation, and health-related quality of life. J. Eat. Disord. 2019;7(1):35. doi: 10.1186/s40337-019-0266-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reba-Harrelson L., Von Holle A., Hamer R.M., Swann R., Reyes M.L., Bulik C.M. Patterns and prevalence of disordered eating and weight control behaviors in women ages 25-45, Eat. Weight Disord. 2009;14(4):e190–e198. doi: 10.1007/BF03325116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jonat L.M., Birmingham C.L. Disordered eating attitudes and behaviours in the high-school students of a rural Canadian community, Eat. Weight Disord. 2004;9(4):285–289. doi: 10.1007/BF03325083. [DOI] [PubMed] [Google Scholar]
- 49.Leichner P., Arnett J., Rallo J.S., Srikameswaran S., Vulcano B. An epidemiologic study of maladaptive eating attitudes in a Canadian school age population. Int. J. Eat. Disord. 1986;5(6):969–982. doi: 10.1002/1098-108X(198609)5:63.0.CO;2-P. [DOI] [Google Scholar]
- 50.Norman R.J., Davies M.J., Lord J., Moran L.J. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol. Metab. 2002;13(6):251–257. doi: 10.1016/s1043-2760(02)00612-4. [DOI] [PubMed] [Google Scholar]
- 51.Lie Fong S., Douma A., Verhaeghe J. Implementing the international evidence-based guideline of assessment and management of polycystic ovary syndrome (PCOS): how to achieve weight loss in overweight and obese women with PCOS? J. Gynecol. Obstet. Hum. Reprod. 2021;50(6) doi: 10.1016/j.jogoh.2020.101894. [DOI] [PubMed] [Google Scholar]
- 52.Moran L.J., Hutchison S.K., Norman R.J., Teede H.J. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst. Rev. 2011;(2):CD007506. doi: 10.1002/14651858.CD007506.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Awoke M.A., Earnest A., Joham A.E., Hodge A.M., Teede H.J., Brown W.J., et al. Weight gain and lifestyle factors in women with and without polycystic ovary syndrome. Hum. Reprod. 2021;37(1):129–141. doi: 10.1093/humrep/deab239. [DOI] [PubMed] [Google Scholar]
- 54.Memon A.N., Gowda A.S., Rallabhandi B., Bidika E., Fayyaz H., Salib M., et al. Have our attempts to curb obesity done more harm than good? Cureus. 2020;12(9) doi: 10.7759/cureus.10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patton G.C., Selzer R., Coffey C., Carlin J.B., Wolfe R. Onset of adolescent eating disorders: population based cohort study over 3 years. BMJ. 1999;318(7186):765–768. doi: 10.1136/bmj.318.7186.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoeger K.M., Dokras A., Piltonen T. Update on PCOS: consequences, challenges, and guiding treatment. J. Clin. Endocrinol. Metab. 2021;106(3):e1071–e1083. doi: 10.1210/clinem/dgaa839. [DOI] [PubMed] [Google Scholar]
- 57.Pasquali R., Gambineri A., Cavazza C., Ibarra Gasparini D., Ciampaglia W., Cognigni G.E., et al. Heterogeneity in the responsiveness to long-term lifestyle intervention and predictability in obese women with polycystic ovary syndrome. Eur. J. Endocrinol. 2011;164(1):53–60. doi: 10.1530/EJE-10-0692. [DOI] [PubMed] [Google Scholar]
- 58.Ee C., Pirotta S., Mousa A., Moran L., Lim S. Providing lifestyle advice to women with PCOS: an overview of practical issues affecting success. BMC Endocr. Disord. 2021;21(1):234. doi: 10.1186/s12902-021-00890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Farkas J., Rigó A., Demetrovics Z. Psychological aspects of the polycystic ovary syndrome. Gynecol. Endocrinol. 2014;30(2):95–99. doi: 10.3109/09513590.2013.852530. [DOI] [PubMed] [Google Scholar]
- 60.Krępuła K., Bidzińska-Speichert B., Lenarcik A., Tworowska-Bardzińska U. Psychiatric disorders related to polycystic ovary syndrome. Endokrynol. Pol. 2012;63(6):488–491. [PubMed] [Google Scholar]
- 61.Kriti V., Kumari S., Joshi S. Body image and self- esteem in girls with polycystic ovary syndrome (PCOS): the Indian scenario. Mind Soc. 2022;11(01):82–88. doi: 10.56011/mind-mri-111-202211. [DOI] [Google Scholar]
- 62.Thannickal A., Brutocao C., Alsawas M., Morrow A., Zaiem F., Murad M.H., et al. Eating, sleeping and sexual function disorders in women with polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Clin. Endocrinol. (Oxf). 2020;92(4):338–349. doi: 10.1111/cen.14153. [DOI] [PubMed] [Google Scholar]
- 63.Tricco A.C., Lillie E., Zarin W., O'Brien K.K., Colquhoun H., Levac D., et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann. Intern. Med. 2018;169(7):467–473. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 64.Berni T.R., Morgan C.L., Berni E.R., Rees D.A. Polycystic ovary syndrome is associated with adverse mental health and neurodevelopmental outcomes. J. Clin. Endocrinol. Metab. 2018;103(6):2116–2125. doi: 10.1210/jc.2017-02667. [DOI] [PubMed] [Google Scholar]
- 65.Cesta C.E., Månsson M., Palm C., Lichtenstein P., Iliadou A.N., Landén M. Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016;73:196–203. doi: 10.1016/j.psyneuen.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 66.Çoban Ö.G., Tulacı Ö.D., Adanır A.S., Önder A. Psychiatric disorders, self-esteem, and quality of life in adolescents with polycystic ovary syndrome. J. Pediatr. Adolesc. Gynecol. 2019;32(6):600–604. doi: 10.1016/j.jpag.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 67.Dumoulin S.C., de Glisezinski I., Saint-Martin F., Jamrozik S.I., Barbe P., Thouvenot J.P., et al. Hormonal changes related to eating behavior in oligomenorrheic women. Eur. J. Endocrinol. 1996;135(3):328–334. doi: 10.1530/eje.0.1350328. [DOI] [PubMed] [Google Scholar]
- 68.Eyupoglu N.D., Aksun S., Ozturk M., Yildiz B.O. Impact of social isolation during COVID-19 pandemic on health behaviors and weight management in women with polycystic ovary syndrome, Eat. Weight Disord. 2022;27(7):2407–2413. doi: 10.1007/s40519-022-01369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Başar Gökcen B., Akdevelioğlu Y., Canan S., Bozkurt N. Increased risk of eating disorders in women with polycystic ovary syndrome: a case-control study. Gynecol. Endocrinol. 2020;36(9):764–767. doi: 10.1080/09513590.2020.1744554. [DOI] [PubMed] [Google Scholar]
- 70.Hollinrake E., Abreu A., Maifeld M., Van Voorhis B.J., Dokras A. Increased risk of depressive disorders in women with polycystic ovary syndrome. Fertil. Steril. 2007;87(6):1369–1376. doi: 10.1016/j.fertnstert.2006.11.039. [DOI] [PubMed] [Google Scholar]
- 71.Jahanfar S., Eden J.A., Nguyent T.V. Bulimia nervosa and polycystic ovary syndrome. Gynecol. Endocrinol. 1995;9(2):113–117. doi: 10.3109/09513599509160199. [DOI] [PubMed] [Google Scholar]
- 72.Jahanfar S.H., Maleki H., Mosavi A.R. Subclinical eating disorder, polycystic ovary syndrome—is there any connection between these two conditions through leptin—a twin study. Med. J. Malaysia. 2005;60(4):441–446. [PubMed] [Google Scholar]
- 73.Jeanes Y.M., Reeves S., Gibson E.L., Piggott C., May V.A., Hart K.H. Binge eating behaviours and food cravings in women with polycystic ovary syndrome. Appetite. 2017;109:24–32. doi: 10.1016/j.appet.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 74.Karacan E., Caglar G.S., Gürsoy A.Y., Yilmaz M.B. Body satisfaction and eating attitudes among girls and young women with and without polycystic ovary syndrome. J. Pediatr. Adolesc. Gynecol. 2014;27(2):72–77. doi: 10.1016/j.jpag.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Larsson I., Hulthén L., Landén M., Pålsson E., Janson P., Stener-Victorin E. Dietary intake, resting energy expenditure, and eating behavior in women with and without polycystic ovary syndrome. Clin. Nutr. 2016;35(1):213–218. doi: 10.1016/j.clnu.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Lidaka L., Lazdane G., Kivite-Urtane A., Gailite L., Dzivite-Krisane I., Stokenberga I. Health-related quality of life and binge eating among adolescent girls with PCOS. Clin. Exp. Obstet. Gynecol. 2022;49(3):57. doi: 10.31083/j.ceog4903057. [DOI] [Google Scholar]
- 77.Månsson M., Holte J., Landin-Wilhelmsen K., Dahlgren E., Johansson A., Landén M. Women with polycystic ovary syndrome are often depressed or anxious—a case control study. Psychoneuroendocrinology. 2008;33(8):1132–1138. doi: 10.1016/j.psyneuen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 78.McCluskey S., Evans C., Lacey J.H., Pearce J.M., Jacobs H. Polycystic ovary syndrome and bulimia. Fertil. Steril. 1991;55(2):287–291. [PubMed] [Google Scholar]
- 79.Rodino I.S., Byrne S., Sanders K.A. Obesity and psychological wellbeing in patients undergoing fertility treatment. Reprod. Biomed. Online. 2016;32(1):104–112. doi: 10.1016/j.rbmo.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 80.Sbaragli C., Morgante G., Goracci A., Hofkens T., De Leo V., Castrogiovanni P. Infertility and psychiatric morbidity. Fertil. Steril. 2008;90(6):2107–2111. doi: 10.1016/j.fertnstert.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 81.Sirmans S.M., Parish R.C., Blake S., Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J. Investig. Med. 2014;62(6):868–874. doi: 10.1097/01.JIM.0000446834.90599.5d. [DOI] [PubMed] [Google Scholar]
- 82.Wang Z., Groen H., Cantineau A.E.P., van Elten T.M., Karsten M.D.A., van Oers A.M., et al. Dietary intake, eating behavior, physical activity, and quality of life in infertile women with PCOS and obesity compared with non-PCOS obese controls. Nutrients. 2021;13(10):3526. doi: 10.3390/nu13103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weiner C.L., Primeau M., Ehrmann D.A. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom. Med. 2004;66(3):356–362. doi: 10.1097/01.psy.0000127871.46309.fe. [DOI] [PubMed] [Google Scholar]
- 84.Annagür B.B., Kerimoglu Ö.S., Tazegül A., Gündüz Ş., Gençoglu B.B. Psychiatric comorbidity in women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2015;41(8):1229–1233. doi: 10.1111/jog.12696. [DOI] [PubMed] [Google Scholar]
- 85.Asdaq S.M.B., Jomah S., Hasan R., Al-Baroudi D., Alharbi M., Alsubaie S., et al. Impact of polycystic ovary syndrome on eating behavior, depression and health related quality of life: a cross-sectional study in Riyadh. Saudi J. Biol. Sci. 2020;27(12):3342–3347. doi: 10.1016/j.sjbs.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Livadas S., Chaskou S., Kandaraki A.A., Skourletos G., Economou F., Christou M., et al. Anxiety is associated with hormonal and metabolic profile in women with polycystic ovarian syndrome. Clin. Endocrinol. (Oxf). 2011;75(5):698–703. doi: 10.1111/j.1365-2265.2011.04122.x. [DOI] [PubMed] [Google Scholar]
- 87.Michelmore K.F., Balen A.H., Dunger D.B. Polycystic ovaries and eating disorders: are they related? Hum. Reprod. 2001;16(4):765–769. doi: 10.1093/humrep/16.4.765. [DOI] [PubMed] [Google Scholar]
- 88.Mizgier M., Jarząbek-Bielecka G., Opydo-Szymaczek J., Wendland N., Więckowska B., Kędzia W. Risk factors of overweight and obesity related to diet and disordered eating attitudes in adolescent girls with clinical features of polycystic ovary syndrome. J. Clin. Med. 2020;9(9):3041. doi: 10.3390/jcm9093041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morgan J., Scholtz S., Lacey H., Conway G. The prevalence of eating disorders in women with facial hirsutism: an epidemiological cohort study. Int. J. Eat. Disord. 2008;41(5):427–431. doi: 10.1002/eat.20527. [DOI] [PubMed] [Google Scholar]
- 90.Rassi A., Veras A.B., dos Reis M., Pastore D.L., Bruno L.M., Bruno R.V., et al. Prevalence of psychiatric disorders in patients with polycystic ovary syndrome. Compr. Psychiatry. 2010;51(6):599–602. doi: 10.1016/j.comppsych.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 91.Suchta K., Smolarczyk R., Czajkowski K., Rudnicka E., Kokoszka A. Binge eating disorder-the point prevalence among polish women with polycystic ovary syndrome and validity of screening tool for this population. Int. J. Environ. Res. Public Health. 2022;20(1):546. doi: 10.3390/ijerph20010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tay C.T., Teede H.J., Hill B., Loxton D., Joham A.E. Increased prevalence of eating disorders, low self-esteem, and psychological distress in women with polycystic ovary syndrome: a community-based cohort study. Fertil. Steril. 2019;112(2):353–361. doi: 10.1016/j.fertnstert.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 93.Greenwood E.A., Pasch L.A., Cedars M.I., Huddleston H.G. Obesity and depression are risk factors for future eating disorder-related attitudes and behaviors in women with polycystic ovary syndrome. Fertil. Steril. 2020;113(5):1039–1049. doi: 10.1016/j.fertnstert.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 94.Herriot A.M., Whitcroft S., Jeanes Y. An retrospective audit of patients with polycystic ovary syndrome: the effects of a reduced glycaemic load diet. J. Hum. Nutr. Diet. 2008;21(4):337–345. doi: 10.1111/j.1365-277X.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- 95.Kerchner A., Lester W., Stuart S.P., Dokras A. Risk of depression and other mental health disorders in women with polycystic ovary syndrome: a longitudinal study. Fertil. Steril. 2009;91(1):207–212. doi: 10.1016/j.fertnstert.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 96.Jiskoot G., de Loos A.D., Timman R., Beerthuizen A., Laven J., Busschbach J. Changes in eating behavior through lifestyle treatment in women with polycystic ovary syndrome (PCOS): a randomized controlled trial. J. Eat. Disord. 2022;10(1):69. doi: 10.1186/s40337-022-00593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jensterle M., Janez A., Vrtovec B., Meden-Vrtovec H., Pfeifer M., Prezelj J., et al. Decreased androgen levels and improved menstrual pattern after angiotensin II receptor antagonist telmisartan treatment in four hypertensive patients with polycystic ovary syndrome: case series. Croat. Med. J. 2007;48(6):864–870. doi: 10.3325/cmj.2007.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gorgens K.A. Springer eBooks, American Psychiatry Association, Washington; 2011. Structured Clinical Interview for DSM-IV (SCID-I/SCID-II) pp. 2410–2417. [DOI] [Google Scholar]
- 99.Shabani A., Masoumian S., Zamirinejad S., Hejri M., Pirmorad T., Yaghmaeezadeh H. Psychometric properties of Structured Clinical Interview for DSM-5 Disorders-Clinician Version (SCID-5-CV) Brain Behav. 2021;11(5) doi: 10.1002/brb3.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fairburn C.G., Beglin S.J. Assessment of eating disorders: interview or self-report questionnaire? Int. J. Eat. Disord. 1994;16(4):363–370. [PubMed] [Google Scholar]
- 101.Berg K.C., Peterson C.B., Frazier P., Crow S.J. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: a systematic review of the literature. Int. J. Eat. Disord. 2012;45(3):428–438. doi: 10.1002/eat.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):22–57. [PubMed] [Google Scholar]
- 103.Kaufman J., Birmaher B., Brent D., Rao U., Flynn C., Moreci P., et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 104.Garner D.M., Garfinkel P.E. The Eating Attitudes Test: an index of the symptoms of anorexia nervosa. Psychol. Med. 1979;9(2):273–279. doi: 10.1017/s0033291700030762. [DOI] [PubMed] [Google Scholar]
- 105.Garner D.M., Olmsted M.P., Bohr Y., Garfinkel P.E. The Eating Attitudes Test: psychometric features and clinical correlates. Psychol. Med. 1982;12(4):871–878. doi: 10.1017/s0033291700049163. [DOI] [PubMed] [Google Scholar]
- 106.Mann A.H., Wakeling A., Wood K., Monck E., Dobbs R., Szmukler G. Screening for abnormal eating attitudes and psychiatric morbidity in an unselected population of 15-year-old schoolgirls. Psychol. Med. 1983;13(3):573–580. doi: 10.1017/s0033291700047991. [DOI] [PubMed] [Google Scholar]
- 107.Schaefer L.M., Crosby R.D., Machado P.P.P. A systematic review of instruments for the assessment of eating disorders among adults. Curr. Opin. Psychiatry. 2021;34(6):543–562. doi: 10.1097/YCO.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henderson M., Freeman C.P. A self-rating scale for bulimia. The 'BITE'. Br. J, Psychiatry. 1987;150:18–24. doi: 10.1192/bjp.150.1.18. [DOI] [PubMed] [Google Scholar]
- 109.Spitzer R.L., Kroenke K., Williams J.B. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study, Primary Care Evaluation of Mental Disorders, Patient Health Questionnaire. JAMA. 1999;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 110.Clausen L., Rosenvinge J.H., Friborg O., Rokkedal K. Validating the Eating Disorder Inventory-3 (EDI-3): a comparison between 561 female eating disorders patients and 878 females from the general population. J. Psychopathol. Behav. Assess. 2011;33(1):101–110. doi: 10.1007/s10862-010-9207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Allison K.C., Lundgren J.D., O'Reardon J.P., Martino N.S., Sarwer D.B., Wadden T.A., et al. The Night Eating Questionnaire (NEQ): psychometric properties of a measure of severity of the night eating syndrome. Eat. Behav. 2008;9(1):62–72. doi: 10.1016/j.eatbeh.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 112.Cappelleri J.C., Bushmakin A.G., Gerber R.A., Leidy N.K., Sexton C.C., Lowe M.R., et al. Psychometric analysis of the Three-Factor Eating Questionnaire-R21: results from a large diverse sample of obese and non-obese participants. Int. J. Obes. 2009;33(6):611–620. doi: 10.1038/ijo.2009.74. [DOI] [PubMed] [Google Scholar]
- 113.Meule A. Twenty years of the Food Cravings Questionnaires: a comprehensive review. Curr. Addict. Rep. 2020;7(1):30–43. doi: 10.1007/s40429-020-00294-z. [DOI] [Google Scholar]
- 114.Van Strien T., Frijters J.E., Bergers G.P., Defares P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int. J. Eat. Disord. 1986;5(2):295–315. doi: 10.1002/1098-108X(198602)5:23.0.CO;2-T. [DOI] [Google Scholar]
- 115.Gormally J., Black S., Daston S., Rardin D. The assessment of binge eating severity among obese persons, Addict. Behav. 1982;7(1):47–55. doi: 10.1016/0306-4603(82)90024-7. [DOI] [PubMed] [Google Scholar]
- 116.de la Rie S.M., Noordenbos G., van Furth E.F. Quality of life and eating disorders. Qual. Life Res. 2005;14(6):1511–1522. doi: 10.1007/s11136-005-0585-0. [DOI] [PubMed] [Google Scholar]
- 117.Krug I., Giles S., Paganini C. Binge eating in patients with polycystic ovary syndrome: prevalence, causes, and management strategies. Neuropsychiatr. Dis. Treat. 2019;15:1273–1285. doi: 10.2147/NDT.S168944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thomas S.A., Fan A., Pastore L.M. A review of the impact of body image on quality of life in women with polycystic ovary syndrome. J. Psychol. Res. 2014;9(17):17–32. [Google Scholar]
- 119.Pesonen E., Nurkkala M., Niemelä M., Morin-Papunen L., Tapanainen J.S., Jämsä T., et al. Polycystic ovary syndrome is associated with weight-loss attempts and perception of overweight independent of BMI: a population-based cohort study. Obesity (Silver Spring) 2023;31(4):1108–1120. doi: 10.1002/oby.23681. [DOI] [PubMed] [Google Scholar]
- 120.Cossrow N., Pawaskar M., Witt E.A., Ming E.E., Victor T.W., Herman B.K., et al. Estimating the prevalence of binge eating disorder in a community sample from the United States: comparing DSM-IV-TR and DSM-5 criteria. J. Clin. Psychiatry. 2016;77(8):e968–e974. doi: 10.4088/JCP.15m10059. [DOI] [PubMed] [Google Scholar]
- 121.Kolnikaj T.S., Herman R., Janež A., Jensterle M. Assessment of eating disorders and eating behavior to improve treatment outcomes in women with polycystic ovary syndrome. Life (Basel) 2022;12(11):1906. doi: 10.3390/life12111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anderson A.D., Solorzano C.M., McCartney C.R. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32(3):202–213. doi: 10.1055/s-0034-1371092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stabouli S., Erdine S., Suurorg L., Jankauskienė A., Lurbe E. Obesity and eating disorders in children and adolescents: the bidirectional link. Nutrients. 2021;13(12):4321. doi: 10.3390/nu13124321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pinhas-Hamiel O., Pilpel N., Carel C., Singer S. Clinical and laboratory characteristics of adolescents with both polycystic ovary disease and anorexia nervosa. Fertil. Steril. 2006;85(6):1849–1851. doi: 10.1016/j.fertnstert.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 125.Bellver J., Rodríguez-Tabernero L., Robles A., Muñoz E., Martínez F., Landeras J., et al. Polycystic ovary syndrome throughout a woman's life. J. Assist. Reprod. Genet. 2018;35(1):25–39. doi: 10.1007/s10815-017-1047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bazarganipour F., Ziaei S., Montazeri A., Foroozanfard F., Kazemnejad A., Faghihzadeh S. Body image satisfaction and self-esteem status among the patients with polycystic ovary syndrome, Iran. J. Reprod. Med. 2013;11(10):829–836. [PMC free article] [PubMed] [Google Scholar]
- 127.Kitzinger C., Willmott J. 'The thief of womanhood': women's experience of polycystic ovarian syndrome. Soc. Sci. Med. 2002;54(3):349–361. doi: 10.1016/s0277-9536(01)00034-x. [DOI] [PubMed] [Google Scholar]
- 128.Kogure G.S., Ribeiro V.B., Lopes I.P., Furtado C.L.M., Kodato S., Silva de Sá M.F., et al. Body image and its relationships with sexual functioning, anxiety, and depression in women with polycystic ovary syndrome. J. Affect. Disord. 2019;253:385–393. doi: 10.1016/j.jad.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 129.Barry J.A., Qu F., Hardiman P.J. An exploration of the hypothesis that testosterone is implicated in the psychological functioning of women with polycystic ovary syndrome (PCOS) Med. Hypotheses. 2018;110:42–45. doi: 10.1016/j.mehy.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 130.McKenna T.J., Cunningham S.K. Adrenal androgen production in polycystic ovary syndrome. Eur. J. Endocrinol. 1995;133(4):383–389. doi: 10.1530/eje.0.1330383. [DOI] [PubMed] [Google Scholar]
- 131.Naessén S., Carlström K., Garoff L., Glant R., Hirschberg A.L. Polycystic ovary syndrome in bulimic women—an evaluation based on the new diagnostic criteria. Gynecol, Endocrinol. 2006;22(7):388–394. doi: 10.1080/09513590600847421. [DOI] [PubMed] [Google Scholar]
- 132.Sundblad C., Bergman L., Eriksson E. High levels of free testosterone in women with bulimia nervosa. Acta Psychiatr. Scand. 1994;90(5):397–398. doi: 10.1111/j.1600-0447.1994.tb01613.x. [DOI] [PubMed] [Google Scholar]
- 133.Fan W., Yanase T., Nishi Y., Chiba S., Okabe T., Nomura M., et al. Functional potentiation of leptin-signal transducer and activator of transcription 3 signaling by the androgen receptor. Endocrinology. 2008;149(12):6028–6036. doi: 10.1210/en.2008-0431. [DOI] [PubMed] [Google Scholar]
- 134.Morford J.J., Wu S., Mauvais-Jarvis F. The impact of androgen actions in neurons on metabolic health and disease. Mol. Cell. Endocrinol. 2018;465:92–102. doi: 10.1016/j.mce.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Naessén S., Carlström K., Byström B., Pierre Y., Hirschberg A.L. Effects of an antiandrogenic oral contraceptive on appetite and eating behavior in bulimic women. Psychoneuroendocrinology. 2007;32(5):548–554. doi: 10.1016/j.psyneuen.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 136.Algars M., Huang L., Von Holle A.F., Peat C.M., Thornton L.M., Lichtenstein P., et al. Binge eating and menstrual dysfunction. J. Psychosom. Res. 2014;76(1):19–22. doi: 10.1016/j.jpsychores.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Morgan J.F., McCluskey S.E., Brunton J.N., Hubert Lacey J. Polycystic ovarian morphology and bulimia nervosa: a 9-year follow-up study. Fertil. Steril. 2002;77(5):928–931. doi: 10.1016/s0015-0282(02)03063-7. [DOI] [PubMed] [Google Scholar]
- 138.Naessén S., Söderqvist G., Carlström K. So similar and so different: circulating androgens and androgen origin in bulimic women. J. Steroid Biochem. Mol. Biol. 2019;185:184–188. doi: 10.1016/j.jsbmb.2018.08.013. [DOI] [PubMed] [Google Scholar]
- 139.Raphael F.J., Rodin D.A., Peattie A., Bano G., Kent A., Nussey S.S., et al. Ovarian morphology and insulin sensitivity in women with bulimia nervosa. Clin. Endocrinol. (Oxf). 1995;43(4):451–455. doi: 10.1111/j.1365-2265.1995.tb02617.x. [DOI] [PubMed] [Google Scholar]
- 140.Sundblad C., Landén M., Eriksson T., Bergman L., Eriksson E. Effects of the androgen antagonist flutamide and the serotonin reuptake inhibitor citalopram in bulimia nervosa: a placebo-controlled pilot study. J. Clin. Psychopharmacol. 2005;25(1):85–88. doi: 10.1097/01.jcp.0000150222.31007.a9. [DOI] [PubMed] [Google Scholar]
- 141.Filippou P., Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum. Reprod. Update. 2017;23(4):421–432. doi: 10.1093/humupd/dmx013. [DOI] [PubMed] [Google Scholar]
- 142.Kothari R., Gafton J., Treasure J., Micali N. 2D:4D ratio in children at familial high-risk for eating disorders: the role of prenatal testosterone exposure. Am. J. Hum. Biol. 2014;26(2):176–182. doi: 10.1002/ajhb.22495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Culbert K.M., Breedlove S.M., Burt S.A., Klump K.L. Prenatal hormone exposure and risk for eating disorders: a comparison of opposite-sex and same-sex twins. Arch. Gen. Psychiatry. 2008;65(3):329–336. doi: 10.1001/archgenpsychiatry.2007.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Culbert K.M., Breedlove S.M., Sisk C.L., Burt S.A., Klump K.L. The emergence of sex differences in risk for disordered eating attitudes during puberty: a role for prenatal testosterone exposure. J. Abnorm. Psychol. 2013;122(2):420–432. doi: 10.1037/a0031791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Smith A.R., Hawkeswood S.E., Joiner T.E. The measure of a man: associations between digit ratio and disordered eating in males. Int. J. Eat. Disord. 2010;43(6):543–548. doi: 10.1002/eat.20736. [DOI] [PubMed] [Google Scholar]
- 146.Monteleone P., Luisi M., Colurcio B., Casarosa E., Monteleone P., Ioime R., et al. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom. Med. 2001;63(1):62–68. doi: 10.1097/00006842-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 147.Klump K.L., O'Connor S.M., Hildebrandt B.A., Keel P.K., Neale M., Sisk C.L., et al. Differential effects of estrogen and progesterone on genetic and environmental risk for emotional eating in women. Clin. Psychol. Sci. 2016;4(5):895–908. doi: 10.1177/2167702616641637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Asarian L., Geary N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(11):R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xu Y., Nedungadi T.P., Zhu L., Sobhani N., Irani B.G., Davis K.E., et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14(4):453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Badawy A., Elnashar A. Treatment options for polycystic ovary syndrome. Int. J. Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schiffer L., Kempegowda P., Arlt W., O'Reilly M.W. Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur. J. Endocrinol. 2017;177(3):R125–R143. doi: 10.1530/EJE-17-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Resch M., Szendei G., Haász P. Bulimia from a gynecological view: hormonal changes. J. Obstet. Gynaecol. 2004;24(8):907–910. doi: 10.1080/01443610400018924. [DOI] [PubMed] [Google Scholar]
- 153.Amisi C.A. Markers of insulin resistance in polycystic ovary syndrome women: an update. World J. Diabetes. 2022;13(3):129–149. doi: 10.4239/wjd.v13.i3.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heller R.F., Heller R.F. Hyperinsulinemic obesity and carbohydrate addiction: the missing link is the carbohydrate frequency factor. Med. Hypotheses. 1994;42(5):307–312. doi: 10.1016/0306-9877(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 155.Hirschberg A.L., Naessén S., Stridsberg M., Byström B., Holtet J. Impaired cholecystokinin secretion and disturbed appetite regulation in women with polycystic ovary syndrome. Gynecol. Endocrinol. 2004;19(2):79–87. doi: 10.1080/09513590400002300. [DOI] [PubMed] [Google Scholar]
- 156.Barry J.A., Bouloux P., Hardiman P.J. The impact of eating behavior on psychological symptoms typical of reactive hypoglycemia. A pilot study comparing women with polycystic ovary syndrome to controls. Appetite. 2011;57(1):73–76. doi: 10.1016/j.appet.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 157.Altuntas Y., Bilir M., Ucak S., Gundogdu S. Reactive hypoglycemia in lean young women with PCOS and correlations with insulin sensitivity and with beta cell function. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005;119(2):198–205. doi: 10.1016/j.ejogrb.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 158.Kasim-Karakas S.E., Cunningham W.M., Tsodikov A. Relation of nutrients and hormones in polycystic ovary syndrome. Am. J. Clin. Nutr. 2007;85(3):688–694. doi: 10.1093/ajcn/85.3.688. [DOI] [PubMed] [Google Scholar]
- 159.Dumesic D.A., Abbott D.H., Sanchita S., Chazenbalk G.D. Endocrine-metabolic dysfunction in polycystic ovary syndrome: an evolutionary perspective. Curr. Opin. Endocr. Metab. Res. 2020;12:41–48. doi: 10.1016/j.coemr.2020.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen Z., Watanabe R.M., Stram D.O., Buchanan T.A., Xiang A.H. High calorie intake is associated with worsening insulin resistance and β-cell function in Hispanic women after gestational diabetes mellitus. Diabetes Care. 2014;37(12):3294–3300. doi: 10.2337/dc14-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McCluskey S.E., Lacey J.H., Pearce J.M. Binge-eating and polycystic ovaries. Lancet. 1992;340(8821):723. doi: 10.1016/0140-6736(92)92257-g. [DOI] [PubMed] [Google Scholar]
- 162.Douglas C.C., Gower B.A., Darnell B.E., Ovalle F., Oster R.A., Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil. Steril. 2006;85(3):679–688. doi: 10.1016/j.fertnstert.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Marsh K.A., Steinbeck K.S., Atkinson F.S., Petocz P., Brand-Miller J.C. Effect of a low glycemic index compared with a conventional healthy diet on polycystic ovary syndrome. Am. J. Clin. Nutr. 2010;92(1):83–92. doi: 10.3945/ajcn.2010.29261. [DOI] [PubMed] [Google Scholar]
- 164.Moran L.J., Ko H., Misso M., Marsh K., Noakes M., Talbot M., et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. J. Acad. Nutr. Diet. 2013;113(4):520–545. doi: 10.1016/j.jand.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 165.Saei Ghare Naz M., Jahanfar S., Ramezani Tehrani F. An overview on effects of micronutrients and macronutrients interventions in management of polycystic ovary syndrome. Clin. Nutr. ESPEN. 2022;52:218–228. doi: 10.1016/j.clnesp.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 166.Moslehi N., Zeraattalab-Motlagh S., Rahimi Sakak F., Shab-Bidar S., Tehrani F.R., Mirmiran P. Effects of nutrition on metabolic and endocrine outcomes in women with polycystic ovary syndrome: an umbrella review of meta-analyses of randomized controlled trials. Nutr. Rev. 2023;81(5):555–577. doi: 10.1093/nutrit/nuac075. [DOI] [PubMed] [Google Scholar]
- 167.Yeo S.H., Colledge W.H. The role of Kiss1 neurons as integrators of endocrine, metabolic, and environmental factors in the hypothalamic-pituitary-gonadal axis. Front. Endocrinol. 2018;9:188. doi: 10.3389/fendo.2018.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mensinger J.L., Calogero R.M., Stranges S., Tylka T.L. A weight-neutral versus weight-loss approach for health promotion in women with high BMI: a randomized-controlled trial. Appetite. 2016;105:364–374. doi: 10.1016/j.appet.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 169.Dugmore J.A., Winten C.G., Niven H.E., Bauer J. Effects of weight-neutral approaches compared with traditional weight-loss approaches on behavioral, physical, and psychological health outcomes: a systematic review and meta-analysis. Nutr. Rev. 2020;78(1):39–55. doi: 10.1093/nutrit/nuz020. [DOI] [PubMed] [Google Scholar]
- 170.Japur C.C., Diez-Garcia R.W., de Oliveira Penaforte F.R., das Graças Pena G., de Araújo L.B., de Sá M.F.S. Insulin, ghrelin and early return of hunger in women with obesity and polycystic ovary syndrome. Physiol. Behav. 2019;206:252–258. doi: 10.1016/j.physbeh.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 171.Moran L.J., Noakes M., Clifton P.M., Wittert G.A., Tomlinson L., Galletly C., et al. Ghrelin and measures of satiety are altered in polycystic ovary syndrome but not differentially affected by diet composition. J. Clin. Endocrinol. Metab. 2004;89(7):3337–3344. doi: 10.1210/jc.2003-031583. [DOI] [PubMed] [Google Scholar]
- 172.Berent-Spillson A., Love T., Pop-Busui R., Sowers M., Persad C.C., Pennington K.P., et al. Insulin resistance influences central opioid activity in polycystic ovary syndrome, Fertil. Steril. 2011;95(8):2494–2498. doi: 10.1016/j.fertnstert.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Salamun V., Jensterle M., Janez A., Vrtacnik Bokal E. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur. J. Endocrinol. 2018;179(1):1–11. doi: 10.1530/EJE-18-0175. [DOI] [PubMed] [Google Scholar]
- 174.Bednarz K., Kowalczyk K., Cwynar M., Czapla D., Czarkowski W., Kmita D., et al. The role of GLP-1 receptor agonists in insulin resistance with concomitant obesity treatment in polycystic ovary syndrome. Int. J. Mol. Sci. 2022;23(8):4334. doi: 10.3390/ijms23084334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lam Y.Y., Maguire S., Palacios T., Caterson I.D. Are the gut bacteria telling us to eat or not to eat? Reviewing the role of gut microbiota in the etiology, disease progression and treatment of eating disorders. Nutrients. 2017;9(6):602. doi: 10.3390/nu9060602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rackers H.S., Thomas S., Williamson K., Posey R., Kimmel M.C. Emerging literature in the microbiota-brain axis and perinatal mood and anxiety disorders. Psychoneuroendocrinology. 2018;95:86–96. doi: 10.1016/j.psyneuen.2018.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.He F.F., Li Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: a review. J. Ovarian Res. 2020;13(1):73. doi: 10.1186/s13048-020-00670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Morita C., Tsuji H., Hata T., Gondo M., Takakura S., Kawai K., et al. Gut dysbiosis in patients with anorexia nervosa. PLOS ONE. 2015;10(12) doi: 10.1371/journal.pone.0145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cetik S., Acikgoz A., Yildiz B.O. Investigation of taste function and eating behavior in women with polycystic ovary syndrome. Appetite. 2022;168 doi: 10.1016/j.appet.2021.105776. [DOI] [PubMed] [Google Scholar]
- 180.Stener-Victorin E., Deng Q. Epigenetic inheritance of polycystic ovary syndrome—challenges and opportunities for treatment. Nat. Rev. Endocrinol. 2021;17(9):521–533. doi: 10.1038/s41574-021-00517-x. [DOI] [PubMed] [Google Scholar]
- 181.Dapas M., Dunaif A. Deconstructing a syndrome: genomic insights into PCOS causal mechanisms and classification. Endocr. Rev. 2022;43(6):927–965. doi: 10.1210/endrev/bnac001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Day F., Karaderi T., Jones M.R., Meun C., He C., Drong A., et al. Large-scale genome-wide meta-analysis of polycystic ovary syndrome suggests shared genetic architecture for different diagnosis criteria. PLOS Genet. 2018;14(12) doi: 10.1371/journal.pgen.1007813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khan M.J., Ullah A., Basit S. Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl. Clin. Genet. 2019;12:249–260. doi: 10.2147/TACG.S200341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Berrettini W. The genetics of eating disorders. Psychiatry (Edgmont). 2004;1(3):18–25. [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang X., Deng Q., Stener-Victorin E. Is there a shared genetic basis and causal relationship between polycystic ovary syndrome and psychiatric disorders: evidence from a comprehensive genetic analysis. Hum. Reprod. 2021;36(8):2382–2391. doi: 10.1093/humrep/deab119. [DOI] [PubMed] [Google Scholar]
- 186.Parry S.A., Woods R.M., Hodson L., Hulston C.J. A single day of excessive dietary fat intake reduces whole-body insulin sensitivity: the metabolic consequence of binge eating. Nutrients. 2017;9(8):818. doi: 10.3390/nu9080818. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.