Abstract
Background
In early 2021, the Ministry of Public Health of Thailand announced heterologous regimens for COVID-19 vaccines using CoronaVac as the first dose followed by ChAdOx1 nCoV-19 at 3 weeks apart. Priority was given to individuals above 60 years old and those who had seven underlying conditions, including obesity. The vaccine regimen was evaluated for safety and immunogenicity in overweight populations in Chiang Mai, Thailand.
Methods
Participants who had a COVID-19 vaccination appointment for the heterologous prime-boost regimen were enrolled. Before each immunization and on day 28 following the second dosage, blood samples were taken, and were examined for anti-spike and neutralizing antibodies by using an indirect ELISA and virus neutralization assays. Safety profile of the vaccine regimen was assessed via a self-recorded diary of adverse events after each vaccination.
Results
No serious adverse events related to vaccination were reported during study period and the majority of adverse reactions were fatigue and pain at the injection site. The levels of anti-spike IgG were 26.3, 56.4 and 1752.1 BAU/mL at baseline, 21 days after first dose and 28 days after second dose, respectively. At 4 weeks after complete vaccination, the median inhibition rates of neutralizing antibody determined by surrogate neutralization assay against wild type, Delta and Omicron variants were 95.2, 85.0 and 3.8, respectively. Moreover, the NT50 level against wild type and Delta variants determined by pseudotyped virus neutralization assay were 133.3 and 41.7, respectively. The neutralizing activity against Omicron variant was almost lower than cutoff level for detection.
Conclusions
The heterologous CoronaVac-ChAdOx1vaccination was safe, well-tolerated and able to induce humoral immunity against wild-type and Delta variants but not against the Omicron variant in overweight population.
Keywords: Coronavirus disease (COVID-19), CoronaVac, ChAdOx1, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), Neutralizing antibodies
Introduction
Rapid development of effective vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) greatly reduce the morbidity and mortality rates from the global pandemic of coronavirus disease 2019 (COVID-19). As of March 30, 2023, the World Health Organization reported 183 and 199 vaccines in clinical and pre-clinical development, respectively [1]. The major platforms of COVID-19 vaccines include protein subunit, inactivated virus, viral vector-based and mRNA-based vaccines. Most of these vaccines are aimed at inducing neutralizing antibodies (NAbs) to the spike (S) protein of the virus [2].
During the COVID-19 pandemic, variants of SARS-CoV-2 owing to mutations have emerged at a significant rate worldwide [3]. This has resulted in changes in transmissibility, clinical presentation, and severity and has an impact on therapeutics and vaccines [4]. Thailand was affected by the B.1.617.2 (Delta) variant beginning in July 2021 followed by the B.1.1.529 (Omicron) in November 2021. Several studies have shown that these variants can infect individuals that have received vaccines. The neutralizing activity in serum samples obtained from vaccinated or naturally infected individuals reduced remarkably [5], [6], [7], [8]. This raises concerns regarding the efficacies of natural infection-derived immunity and vaccine-induced immunity.
In early 2021, an inactivated CoronaVac vaccine (Sinovac Biotech Ltd, Beijing, P.R. China) and a viral vector ChAdOx1 nCoV-19 vaccine (AstraZeneca; Vaxzevria™) were the first two available vaccines in Thailand. CoronaVac is made from SARS-CoV-2 virus particles, chemically inactivated with b-propiolactone, and combined with aluminum hydroxide to enhance the immune response. It induces antibody responses and to a lesser extent T cell responses. Clinical trials of CoronaVac have shown varying efficacy rates across different populations and regions. For example, trials conducted in Brazil initially reported an efficacy rate of around 50.4 %, while trials in Turkey reported a higher efficacy rate of around 83.5 %. ChAdOx1 nCoV-19 is a non-replicating adenoviral vector vaccine containing the surface gene that encodes the spike glycoproteins of SARS-CoV-2. The vaccine induces both antibodies and memory cells that provide protection against COVID-19. Overall, clinical trials of the ChAdOx1 nCoV-19 showed efficacy above 70 % [9]. Efficacy rates may be influenced by factors such as trial design, participant demographics, and the prevalence of virus variants.
Confronted with limited COVID-19 vaccine supplies and to shorten the interval between prime and boost injection with the most effective vaccinations, the Ministry of Public Health of Thailand announced heterologous regimens for COVID-19 vaccines using CoronaVac as the first dose followed by ChAdOx1 nCoV-19 as the second dose at 3 weeks apart. People 60 years old or older and people with 7 underlying diseases, including obesity, were prioritized. As the eligibility criteria of people who could get vaccination were determined by the Thai government, most of vaccinees were overweight populations during the study period. There was very limited data on the immunogenicity, reactogenicity, or safety of such a vaccination regimen. We hypothesized that the regimen of prime-boost vaccination with CoronaVac followed by ChAdOx1 nCoV-19 would have high efficacy with good safety and tolerability profile and would achieve seropositivity of 98 % as observed for two AstraZeneca shots. This study was therefore conducted to evaluate safety and immunogenicity of a 2-dose heterologous prime-boost COVID-19 immunization regimen with CoronaVac vaccine followed by ChAdOx1 nCoV-19 vaccine in overweight populations in Chiang Mai, Thailand. In addition, the aim of all COVID-19 vaccination regimen was to induce immunity against the wild-type virus's spike protein, neutralizing abilities against SARS-CoV-2 variants may be compromised. The efficacy of vaccine-induced immunity against circulating variants of concern was therefore investigated.
Materials and methods
Ethics statement
All procedures involving human participants were performed in accordance with international ethical standards. Ethical approval was given by the ethics committees of the Research Institute for Health Sciences (20/64) and the Faculty of Medicine (MED-2564-08378), Chiang Mai University. Written informed consent was obtained from all participants before enrollment into the study.
Study design
This prospective observational study was conducted at the Research Institute for Health Sciences and the Faculty of Medicine, Chiang Mai University, Thailand between August and October 2021. People who had scheduled to receive the COVID-19 vaccines were invited to participate in this study. Participants received heterologous prime-boost vaccination by intramuscular injection into the deltoid muscle with CoronaVac vaccine (Sinovac, Beijing, P.R. China) as the first dose, followed by ChAdOx1 nCoV-19 (AZD1222, VaxzevriaTM) as the second dose at 21 days apart. Comorbidities were assessed by healthcare professionals through interviews. Medical examinations were also conducted by physicians every visit to assess the overall health of study participants. A diary self-record of adverse events following each immunization was used to evaluate the safety profile of the vaccine regimen. The diary contained checklists of occurrence and severity of local reactions at the injection site (redness, swelling, and pain), systemic reactions (fever, headache, and fatigue), other adverse events (diarrhea, nausea, numbness, faintness, and dyspnea) during the 10 days after vaccination (D0 is the vaccination date), and an open field for any adverse event during the rest of the period before the next visit. Serious adverse events were defined according to the criteria of International Conference on Harmonization - Good Clinical Practice (ICH-GCP). Blood samples were collected before each vaccination and at day 28 after the second vaccination. Sera were tested for anti-spike antibodies by indirect ELISA, and for neutralizing antibodies by surrogate and pseudotyped virus neutralization tests.
Study population
Adults 18 to 60 years of age who scheduled to receive vaccinations against COVID-19 at Maharaj Nakorn Chiang Mai Hospital were eligible for participation. The exclusion criteria included: a history of diagnosed COVID-19 or at high-risk of COVID-19 within 14 days before enrolment; having received vaccines against SARS-CoV-2 before enrolment or any inactivated or subunit vaccines within the past 14 days or any attenuated live vaccine within the past 28 days; having received blood products, blood components, or immunoglobulin within the past 90 days; a history of anaphylaxis in relation to vaccine component; having received immunosuppressive/immunomodulatory agents; and women in lactation, pregnancy, or planned pregnancy during the study period.
Determination of anti-spike antibodies by indirect enzyme-linked immunosorbent assay (ELISA)
Anti-SARS-CoV-2 spike protein immunoglobulin G (IgG) were determined by indirect enzyme-linked immunosorbent assay (ELISA) as described previously [10]. In brief, MaxiSorp™ ELISA plates (Thermo Fisher Scientific, Roskilde, Denmark) were coated with recombinant spike protein (GenScript, Piscataway, USA) in bicarbonate buffer (pH 9.6) and stored overnight at 4 °C. After washing with 0.05 % Tween-20 (Calbiochem, Gibbstown, USA) in phosphate buffer saline (PBS) and blocking with 2 % skimmed milk in PBS, 1:100 diluted serum samples and twofold serially diluted positive controls were added and incubated at 37 °C for 1 h. After washing, 1: 2000 horseradish peroxidase (HRP) conjugated goat anti-human IgG (Invitrogen, Carlsbad, USA) were added and incubated at 37 °C for 1 h. Then plates were washed before 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Life Technologies, Frederick, USA) was added to each well. After 20 min incubation, 0.2 M sulfuric acid was added to each well to stop the reaction. Plates were then read at 450 nm with a CLARIOstar® microtiter plate reader. Antibody levels were calculated from a standard curve of a twofold serially diluted positive control on each plate. The positive control was the WHO international standard anti-SARS-CoV-2 immunoglobulin (NIBSC, UK) which was assigned an arbitrary unitage of 1000 BAU/mL. Negative controls were participants' pooled sera obtained prior to COVID-19 pandemic. Serum samples were considered seropositive if the values were higher than the average of the negative controls plus three times their SD as previously described [10].
Surrogate virus neutralization assay
NAbs against wild-type (Wuhan), Delta (B.1.617.2) and Omicron (B.1.1.529) variants were determined using a surrogate virus neutralization test as previously described [11] with modifications. Briefly, horseradish peroxidase (HRP)–RBD of wild-type (GenScript catalog no. Z03594-100), Delta (Delta RBD-HRP, GenScript catalog no. Z03614-20) or Omicron (Omicron RBD-HRP, GenScript catalog no. Z03730-100) variants was pre-incubated with diluted serum or control at 37 °C for 30 min. Then, the mixture was added to the hACE2 (GenScript, catalog no. Z03516-100) protein-coated ELISA plate and incubated at 37 °C for 30 min. After washing, TMB substrate solution was added followed by the stop solution after 30 min incubation period at room temperature in darkness. The absorbance of the final solution was read at 450 nm on a CLARIOstar® microtiter plate reader (Ortenberg, Germany). The cut-off level for seroconversion was established by taking the mean percentage of inhibition of each negative sera plus three standard deviations, which was 30 % as described previously [10].
Pseudotyped virus neutralization assay
Pseudotyped virus neutralization assays were also used to evaluate neutralizing activity of antibodies against wild-type (Wuhan), Delta and Omicron variants. SARS-CoV-2 spike – pseudotyped lentiviruses were produced as previously described [12] with modification. Briefly, HEK293T cells were co-transfected with plasmid containing lentiviral backbone encoding firefly luciferase and ZsGreen (addgene plasmid #164432), plasmid encoding optimized HIV gag-pol (addgene plasmid #164441), plasmid encoding HIV-1 Tat accessory protein (addgene plasmid #164442), plasmid encoding HIV-1 Rev accessory protein (addgene plasmid #164443) and plasmid encoding SARS-CoV-2 wild-type Spike Protein (addgene plasmid #164436) or plasmid encoding SARS-CoV-2 Delta variant Spike Protein (addgene plasmid #172320) or plasmid encoding SARS-CoV-2 Omicron variant Spike Protein (addgene plasmid #179907) using Lipofectamine 3000 transfection reagent (Invitrogen, MA, USA). Peudotyped virus supernatants were harvested at 48 h after transfection and stored at −70 °C until use. Almost all of the plasmids were gifts from Alejandro Balazs except the plasmid encoding SARS-CoV-2 Delta variant Spike Protein, which was a gift from David Nemazee. For the pseudotyped virus neutralization assays, heat-inactivated sera were diluted 1:10 and then serially diluted 4-fold for 6 dilutions. Diluted sera were incubated with peudotyped virus at 37 °C for 1 h. ACE2 – TMPRSS2 expressing HEK293T cells (NIBSC # 101008, London, UK) were then added to the mixture and incubated at 37 °C 5 % CO2 for 48 h. Viral infection was determined by measuring the luciferase activity after addition of Bright-Glo luciferase reagent (Promega, Madison, WI). Luciferase activity was measured using a CLARIOstar® microtiter plate reader and expressed as relative light units (RLU). Cell alone or infected cells with Pseudotyped virus were used as 0 % or 100 % inhibition rates, respectively. Data was presented as 50 % pseudotyped virus neutralizing titers (PVNT50) which was defined as the reciprocal serum dilution that caused a 50 % reduction of pseudotyped viral infection. Pooled sera obtained before COVID-19 pandemic was used as negative control with PVNT50 value less than 10.
Sample size calculation
We hypothesized that the regimen of prime-boost vaccination with CoronaVac followed by ChAdOx1 nCoV-19 would achieve 98 % seropositivity of neutralizing anti-spike antibodies at 28 days after booster dose determined by surrogate neutralization assay as observed for two ChAdOx1 nCoV-19 shots. We also expected a similar seroconversion rate of neutralizing antibodies in this regimen. A minimum sample of 31 samples was required to achieve a 80 % power of estimation and testing based on a target significance level of 0.05.
Statistical analysis
GraphPad Prism version 9.5.1 was used for data and statistical analysis. Baseline descriptive characteristics of the study population were summarized as frequency with percentage for categorical variables, and as medians with interquartile range (IQR) or mean with standard deviation (SD) depending on data distribution. Statistical comparisons between multiple time-points or SARS-CoV-2 variants were performed using the Friedman test with Dunn's multiple comparisons test. The difference in humoral immune responses between participants with and without comorbidities was analyzed using the Mann-Whitney test. Statistical significance was considered as two-tailed probability with a p-value less than 0.05.
Results
Characteristics of study populations
A total of 34 adults participated throughout the study period. Demographic characteristics are presented in Table 1. Of 34 participants, 20 (58.8 %) were female with a median age (interquartile range; IQR) of 38.5 years (IQR 27.3–47.0) and 14 (41.2 %) had comorbidities. All participants were considered overweight or obese with mean (SD) body mass index (BMI) of 32.4 (4.8). The majority of participants were obese (91.2 %). Six participants (17.6 %) had smoking habits and 12 participants (35.3 %) had alcohol consumption.
Table 1.
Demographic characteristics of participants.
| Variables | n = 34 |
|---|---|
| Age (years), median (IQR) | 38.5 (27.3–47.0) |
| Gender, n (%) | |
| Female | 20 (58.8) |
| Male | 14 (41.2) |
| BMI (kg/m2), mean (SD) | 32.4 (4.8) |
| Overweight, n (%) | 3 (8.8) |
| Obese, n (%) | 31 (91.2) |
| Comorbidities, n (%) | 14 (41.2) |
| Diabetes mellitus | 1 (2.9) |
| Hypertension | 5 (14.7) |
| Dyslipidemia | 4 (11.8) |
| Neurological disorders | 3 (8.8) |
| Gout | 2 (5.9) |
| Dyspepsia | 3 (8.8) |
| Asthma | 1 (2.9) |
| Allergic rhinitis | 2 (5.9) |
| Smokinga, n (%) | 6 (17.6) |
| Alcohol consumptionb, n (%) | 12 (35.3) |
Abbreviation: BMI: body mass index, IQR: interquartile range, SD: standard deviation. aSmoking status is defined as current cigarette smoking every day or some days. bAlcohol consumption status is defined as current regular alcohol consumption.
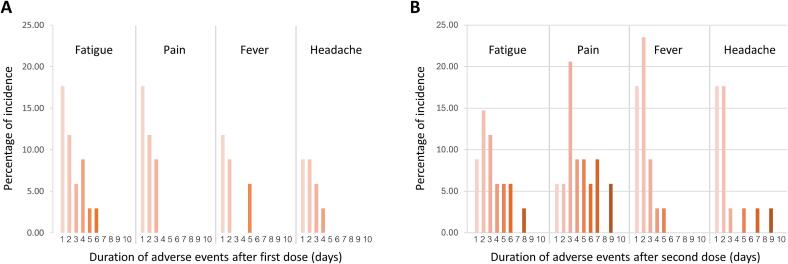
The participants were observed for at least 30 min after vaccination to detect any immediate adverse events. Participants were asked to record any adverse events for 10 days after each vaccination. Twenty-four (70.6 %) and 30 (88.2 %) participants had at least one adverse event after the first and second dose vaccination, respectively. The occurrence of post-vaccination adverse events is summarized in Table 2. The most common adverse reactions after the first and second dose were fatigue (50.0 %) and pain at the injection site (70.6 %), respectively. Among the reported adverse reactions, fever (26.5 % to 55.9 %), pain at injection site (38.2 % to 70.6 %), headache (26.5 % to 47.1 %), swelling (5.9 % to 14.7 %) and dyspnea (2.9 % to 11.8 %) were markedly increased after the second dose vaccination. Moreover, resolution time of fatigue, pain, fever and headache were also increased after the second dose vaccination (Fig. 1).
Table 2.
Adverse reactions within 10 days after each vaccination.
| Adverse reactions | 1st dose, n (%) | 2nd dose, n (%) |
|---|---|---|
| Fatigue | 17 (50.0) | 19 (55.9) |
| Pain (injection site) | 13 (38.2) | 24 (70.6) |
| Fever | 9 (26.5) | 19 (55.9) |
| Headache | 9 (26.5) | 16 (47.1) |
| Diarrhea | 5 (14.7) | 2 (5.9) |
| Redness | 4 (11.8) | 3 (8.8) |
| Nausea | 3 (8.8) | 1 (2.9) |
| Swelling | 2 (5.9) | 5 (14.7) |
| Numbness | 2 (5.9) | 2 (5.9) |
| Faintness | 1 (2.9) | 1 (2.9) |
| Dyspnea | 1 (2.9) | 4 (11.8) |
Fig. 1.
Duration of adverse events. The percentage of incidence of adverse event duration after (A) first dose and (B) second dose.
Anti-spike Ab levels
Anti-spike Ab responses before and after each vaccination were determined by indirect ELISA and the arbitrary units were calculated from the WHO international standard anti-SARS-CoV-2 immunoglobulin as described in materials and methods. Sera taken before vaccination did not contain Abs specific to spike proteins (Fig. 2). The levels of Abs against spike proteins increased significantly after each vaccination. The median of anti-spike IgG level in serum at 21 days after the first vaccination was 56.4 BAU/mL (IQR 28.4–90.1), which significantly increased from baseline levels (26.3 BAU/mL; IQR 18.4–35.7, p = 0.002). The median anti-spike IgG level increased to 1752.1 BAU/mL (1105.5–2193.1) in serum at 28 days after the second vaccination. This was statistically significantly higher compared to baseline (p < 0.0001) and compared to 21 days after the first vaccination (p < 0.0001).
Fig. 2.
Levels of anti-spike IgG antibodies against SARS CoV-2 wild type. Each symbol represents the antibody level in serum from individual participants before vaccination (Before 1st dose), at 21 days after the first vaccination (Before 2nd dose) and at 28 days after the second vaccination (4 weeks post 2nd dose). The median and interquartile range are shown as solid lines on dot plots. The horizontal dashed line indicates the cutoff value which was calculated from the mean level of anti-spike Abs before vaccination plus 3 times the standard deviation (cutoff = 98.98). ** indicates p-value = 0.002 and **** indicates p-value < 0.0001.
Neutralizing antibody levels against variants of concern by sVNT
Neutralizing antibody activities against variants of concern were evaluated by using the SARS-CoV-2 surrogate virus neutralization test. At 28 days after the second vaccination, sera from all participants inhibited SARS-CoV-2 wild type variant with the median inhibition rate of 95.2 (IQR 89.1–97.4) (Fig. 3). There were differential reductions in neutralization against Delta and Omicron variants. Sera from 33 (97.1 %) of participants inhibited Delta whereas sera from only 3 (9.1 %) participants inhibited Omicron variants. The median inhibition rates against Delta (85.0; IQR 72.8–94.2) and Omicron (3.8; IQR –1.0–11.2) were statistically significantly lower than that of the wild type (p = 0.0001 and p < 0.0001, respectively). The inhibition rate against Omicron was also significantly lower than that of the Delta variant (p = 0.0001).
Fig. 3.
Neutralizing activities against wild type, Delta and Omicron variants of SARS CoV-2 determined by surrogate virus neutralization assay. The data points are the inhibition rate in percent from each participant against wild type, Delta and Omicron strains at 28 days after completion of two immunizations. The black solid lines on the dot plots indicate the median and interquartile range. The horizontal dashed line indicates the cutoff value of 30 %. *** indicates p-value = 0.0001 and **** indicates p-value < 0.0001.
Neutralizing antibody levels against variants of concern by pseudotyped virus neutralization assay
A pseudotyped virus neutralization assay was performed to evaluate the NT50 against the wild-type, Delta, and Omicron variants in serum at 28 days after the second vaccination (Fig. 4). The median of NT50 was highest against the wild type variant (133.3; IQR 54.6–288.3) and lower for Delta (41.7; IQR 21.9–133.9). Compared with the wild type, the neutralizing activities were statistically significantly reduced against the Delta variant (p = 0.013). For the Omicron variant, serum from only 6 participants had Nab levels above the cutoff value (cutoff = 10.0). The neutralizing activity against the Omicron variant was significantly lower than the wild type (p < 0.0001) and Delta variants (p < 0.0001).
Fig. 4.
Neutralizing antibody titers against wild type, Delta and Omicron variants of SARS-CoV-2 determined by pseudotyped virus neutralization assay. The data points represent 50 % neutralizing titer (NT50) which was the reciprocal serum dilution to achieve 50 % viral neutralization. The solid lines on the dot plots present the median and interquartile range. The horizontal dashed line indicates the cutoff value of 10. * indicates p-value = 0.013 and **** indicates p-value < 0.0001.
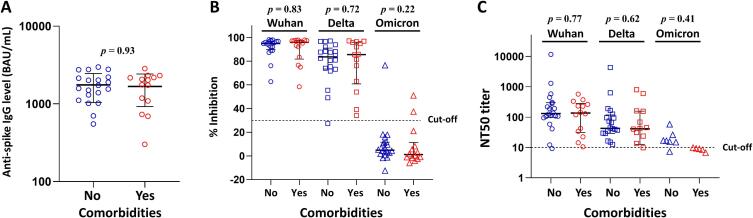
Comorbidities and Ab responses
We next examined whether pre-existing comorbidities might have an effect on Ab responses to the vaccines. As shown in Fig. 5A, the median levels of anti-spike Abs at 28 days following second vaccination were not different between participants who had and did not have comorbidities (p = 0.93). Neutralizing activities against SARS-CoV-2 wild type, Delta and Omicron variants determined by sVNT (Fig. 5B; p = 0.83, p = 0.72, p = 0.22, respectively) or pseudotyped virus neutralization assay (Fig. 5C; p = 0.77, p = 0.62, p = 0.41, respectively) were also not statistically different between the two groups.
Fig. 5.
Comparison of humoral immune responses at 28 days after second vaccination between overweight individuals with and without comorbidities. (A) Level of anti-spike IgG antibody. (B) Neutralizing activities against wild type, Delta and Omicron variants measured by sVNT. (C) NT50 against wild type, Delta and Omicron variants. The solid lines on the dot plots indicate the median and interquartile range. Mann-Whitney test was used to test for significant differences between groups.
Discussion
Our findings in this study show that the heterologous prime-boost immunization with CoronaVac vaccine and ChAdOx1 nCoV-19 vaccine was safe and well-tolerated with no vaccine-related serious adverse events (AE) in an overweight population. Moreover, the level of anti-spike IgG against wild-type SARS-CoV-2 increased significantly after each vaccination. Upon completion of this vaccination regimen, the high level of neutralizing antibodies was demonstrated against wild-type SARS-CoV-2. However, this neutralizing activity declined and was lost against Delta and Omicron variants, respectively. As far as we know, this is the first study concerning the heterologous prime-boost immunization with CoronaVac vaccine and ChAdOx1 nCoV-19 vaccine in an overweight population.
In our study, the recorded AE rate of the heterologous prime-boost immunization in an overweight population was 67.7 % and 85.3 % after receiving the first and second dose, respectively. These findings were consistent with previous studies of similar vaccine regimens in general populations [13], [14]. All AEs in the overweight population in this study were of mild or moderate severity without any serious AEs which was similar to earlier study in general populations [8], [13], [14].
The efficacy of the heterologous prime-boost immunization in an overweight population in this study was comparable to the general populations reported by other study groups in terms of the level and seropositivity rate for anti-spike antibodies [8], [13], [14], [15], [16], [17], [18], [19]. However, the results of this study could not absolutely be compared with previous studies because of differences in types of antigens for anti-spike antibody detection. S1 subunit of SARS-CoV-2 spike protein was used as antigen in our study whereas SARS-CoV-2 spike RBD was used in previous studies. Nevertheless, the anti-spike antibody level was strongly positively correlated with anti-RBD antibody levels [10], suggesting that the level of anti-spike antibodies was comparable to the level of anti-RBD antibodies. Previous studies demonstrated that obesity is associated with reduced antibody responses to COVID-19 vaccines [20], [21] but studies on effectiveness of CoronaVac and ChAdOx1 heterologous prime-boost vaccines against variants of concern, including Omicron specific to overweight populations are limited. Impact of vaccination regimen on variants of concern in individuals with obesity may require further research.
Our study showed that the heterologous CoronaVac-ChAdOx1 regimen induced the highest NAb level (neutralizing activity) against the wild-type, followed by the Delta variant. It was lowest against the Omicron variant in this overweight population. This is consistent with previous studies in general populations who received a similar SARS-CoV-2 vaccine regimen [13], [22]. These findings support the notion that the heterologous CoronaVac-ChAdOx1vaccine regimen has the potential to induce sufficient humoral immune responses against the wild-type and Delta variants, but not the Omicron variant. This is in line with previous studies which reported that neutralizing activity generated by the primary series of some COVID-19 vaccines may not be sufficient to provide strong protection against the Omicron variant [23], [24]. A decline in neutralizing activity against Delta and Omicron variants observed in our study also suggests reduced effectiveness of the vaccine in preventing infection with these strains which may compromise the protection. This could lead to the need for booster doses or updated vaccines tailored to address emerging variants, which would be especially relevant for overweight individuals who may have a higher risk of complications from COVID-19 [25]. It should be noted that the heterologous prime-boost regimen CoronaVac and ChAdOx1 vaccines has been discontinued for use in Thailand. The current dominant SARS-CoV-2 in Thailand is the Omicron JN.1 strain [26]. As of March 11, 2024, Thailand continued to face challenges related to COVID-19 infections (reported less than 100 cases/day), hospitalizations, and severe cases [27]. Current COVID-19 vaccine provided by Thai government is the bivalent Pfizer-BioNTech COVID-19 Vaccine.
Limitations of this study include the small sample size and the absence of a general or normal weight population for comparison. At the time of enrollment, overweight populations were given priority according to national policy which could introduce a selection bias. Overweight individuals may have different baseline characteristics that could influence vaccine responses and outcomes; therefore, the findings may not be generalizable to the wider population. The efficacy and safety of the CoronaVac followed by AstraZeneca regimen observed in such small sample size may not reflect outcomes in overweight individuals more broadly.
In conclusion, this study demonstrated that heterologous CoronaVac-ChAdOx1vaccine regimen was safe and well-tolerated in an overweight population, and can induce satisfactory humoral immune responses against the wild-type and Delta variants, but induces inadequate responses against the Omicron variant. The potential need for booster doses or updated vaccines against emerging variants would be particularly important for overweight people who might be more susceptible to COVID-19-related complication.
Financial support
This study was partially supported by Research Institute for Health Sciences and Chiang Mai University. The funding resource had no role in the study design, data collection, analysis, interpretation or report of this study.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author used ChatGPT for editing English grammar. After using this tool, the author reviewed and edited the content as needed and take full responsibility for the content of the publication.
CRediT authorship contribution statement
Kriangkrai Chawansuntati: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. Supachai Sakkhachornphop: Methodology. Sayamon Hongjaisee: Conceptualization, Methodology, Writing – review & editing. Saranta Freeouf: Formal analysis, Methodology. Patumrat Sripan: Formal analysis. Nattaya Nusartsang: Methodology. Romanee Chaiwarith: Conceptualization, Investigation. Tavitiya Sudjaritruk: Conceptualization, Investigation. Khuanchai Supparatpinyo: Funding acquisition. Jiraprapa Wipasa: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to thank all participants for their participation, Gernot Huber and Marisa Guptarak for editorial assistance, and the staff of the department of internal medicine and the department of pediatrics, Faculty of Medicine, Chaing Mai University for recruiting participants. We are grateful to Kornkamon Kingkaew for her technical assistance.
Contributor Information
Kriangkrai Chawansuntati, Email: kriangkrai.ch@cmu.ac.th.
Supachai Sakkhachornphop, Email: supachai.sak@cmu.ac.th.
Sayamon Hongjaisee, Email: sayamon.ho@cmu.ac.th.
Patumrat Sripan, Email: patumrat.sripan@cmu.ac.th.
Nattaya Nusartsang, Email: nattaya.n@cmu.ac.th.
Romanee Chaiwarith, Email: romanee.c@cmu.ac.th.
Tavitiya Sudjaritruk, Email: tavitiya.s@cmu.ac.th.
Khuanchai Supparatpinyo, Email: khuanchai.s@cmu.ac.th.
Jiraprapa Wipasa, Email: jiraprapa.wipasa@cmu.ac.th.
Data availability
Data will be made available on request.
References
- 1.World Health Organization. COVID-19 vaccine tracker and landscape. 2023. (https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines).
- 2.Teijaro J.R., Farber D.L. COVID-19 vaccines: modes of immune activation and future challenges. Nat. Rev. Immunol. 2021;21:195–197. doi: 10.1038/s41577-021-00526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hillary V.E., Ceasar S.A. An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines. Heliyon. 2023;9:e13952. doi: 10.1016/j.heliyon.2023.e13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khateeb J., Li Y., Zhang H. Emerging SARS-CoV-2 variants of concern and potential intervention approaches. Crit. Care. 2021;25:244. doi: 10.1186/s13054-021-03662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen X., Chen Z., Azman A.S., Sun R., Lu W., Zheng N., et al. Neutralizing antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Variants induced by natural infection or vaccination: a systematic review and pooled analysis. Clin. Infect. Dis. 2022;74:734–742. doi: 10.1093/cid/ciab646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi J.Y., Lee Y.J., Ko J.H., Kim S.H., Kim H.J., Lee H.W., et al. Neutralizing activity against SARS-CoV-2 Delta and omicron Variants following a third BNT162b2 booster dose according to three homologous or heterologous COVID-19 vaccination schedules. Front Cell Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.948014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence - 25 U.S. Jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71:132-8. [DOI] [PMC free article] [PubMed]
- 8.Mahasirimongkol S., Khunphon A., Kwangsukstid O., Sapsutthipas S., Wichaidit M., Rojanawiwat A., et al. The pilot study of immunogenicity and adverse events of a COVID-19 vaccine regimen: priming with inactivated whole SARS-CoV-2 vaccine (CoronaVac) and boosting with the adenoviral vector (ChAdOx1 nCoV-19) vaccine. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10040536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman M.M., Masum M.H.U., Wajed S., Talukder A. A comprehensive review on COVID-19 vaccines: development, effectiveness, adverse effects, distribution and challenges. Virusdisease. 2022;33:1–22. doi: 10.1007/s13337-022-00755-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winichakoon P., Wipasa J., Chawansuntati K., Salee P., Sudjaritruk T., Yasri S., et al. Diagnostic performance between in-house and commercial SARS-CoV-2 serological immunoassays including binding-specific antibody and surrogate virus neutralization test (sVNT) Sci. Rep. 2023;13:34. doi: 10.1038/s41598-022-26202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan C.W., Chia W.N., Qin X., Liu P., Chen M.I., Tiu C., et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020;38:1073–1078. doi: 10.1038/s41587-020-0631-z. [DOI] [PubMed] [Google Scholar]
- 12.Crawford K.H.D., Eguia R., Dingens A.S., Loes A.N., Malone K.D., Wolf C.R., et al. Protocol and reagents for pseudotyping lentiviral Particles with SARS-CoV-2 spike protein for neutralization assays. Viruses. 2020;12 doi: 10.3390/v12050513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudjaritruk T., Mueangmo O., Saheng J., Winichakoon P., Salee P., Wongjak W., et al. Comparison of immunogenicity and reactogenicity of five Primary series of COVID-19 vaccine regimens against circulating SARS-CoV-2 Variants of concern among healthy Thai populations. Vaccines (Basel) 2023;11 doi: 10.3390/vaccines11030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanlapakorn N., Suntronwong N., Phowatthanasathian H., Yorsaeng R., Vichaiwattana P., Thongmee T., et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: a prospective cohort study. Hum. Vaccin. Immunother. 2022;18 doi: 10.1080/21645515.2022.2029111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen G., Jungsomsri P., Sangwongwanich J., Tawinprai K., Siripongboonsitti T., Porntharukchareon T., et al. Immunogenicity and reactogenicity after heterologous prime-boost vaccination with CoronaVac and ChAdox1 nCov-19 (AZD1222) vaccines. Hum. Vaccin. Immunother. 2022;18 doi: 10.1080/21645515.2022.2052525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Hou L., Guo X., Jin P., Wu S., Zhu J., et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat. Med. 2022;28:401–409. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niyomnaitham S., Quan Toh Z., Wongprompitak P., Jansarikit L., Srisutthisamphan K., Sapsutthipas S., et al. Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: an open-label randomized study in healthy Thai adults. Hum. Vaccin. Immunother. 2022;18 doi: 10.1080/21645515.2022.2091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tawinprai K., Jungsomsri P., Pinijnai O., Tavonvunchai F., Lievjaroen A., Suwannaroj P., et al. Immunogenicity and reactogenicity of heterologous prime-boost vaccination with inactivated COVID-19 and ChAdOx1 nCoV-19 (AZD1222) vaccines, a quasi-experimental study. Hum. Vaccin. Immunother. 2023 doi: 10.1080/21645515.2023.2206360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wanlapakorn N., Suntronwong N., Phowatthanasathian H., Yorsaeng R., Thongmee T., Vichaiwattana P., et al. Immunogenicity of heterologous inactivated and adenoviral-vectored COVID-19 vaccine: real-world data. Vaccine. 2022;40:3203–3209. doi: 10.1016/j.vaccine.2022.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watanabe M., Balena A., Masi D., Tozzi R., Risi R., Caputi A., et al. Rapid weight loss, central obesity improvement and blood glucose reduction are associated with a stronger adaptive immune response following COVID-19 mRNA vaccine. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaborit B., Fernandes S., Loubet P., Ninove L., Dutour A., Cariou B., et al. Early humoral response to COVID-19 vaccination in patients living with obesity and diabetes in France. The COVPOP OBEDIAB study with results from the ANRS0001S COV-POPART cohort. Metabolism. 2023;142 doi: 10.1016/j.metabol.2023.155412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liwsrisakun C., Pata S., Laopajon W., Takheaw N., Chaiwong W., Duangjit P., et al. Humoral and cellular immune responses against SARS-CoV-2 variants of concern induced by heterologous CoronaVac/ChAdOx-1 versus homologous ChAdOx-1 vaccination in the elderly. Asian Pac. J. Allergy Immunol. 2023 doi: 10.12932/AP-120822-1434. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., Ng S.S., Chan K.C.K., Ko F.W., et al. Neutralizing antibodies against the SARS-CoV-2 omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat. Med. 2022;28:486–489. doi: 10.1038/s41591-022-01704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos G.R.F., Almeida N.B.F., Filgueiras P.S., Corsini C.A., Gomes S.V.C., de Miranda D.A.P., et al. Booster dose of BNT162b2 after two doses of CoronaVac improves neutralization of SARS-CoV-2 omicron variant. Commun. Med. (Lond.) 2022;2:76. doi: 10.1038/s43856-022-00141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Paula Silva-Lalucci M.P., Marques D.C.S., Valdes-Badilla P., Andreato L.V., Magnani Branco B.H. Obesity as a risk factor for complications and mortality in individuals with SARS-CoV-2: a systematic review. Nutrients. 2024;16 doi: 10.3390/nu16040543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.World Health Organization. Update on COVID-19 in Thailand: 7 February 2024. 2024. (https://www.who.int/thailand/news/detail/06-02-2024-update-on-covid-19-in-thailand--7-february-2024).
- 27.Department of Disease Control, Ministry of Public Health, Thailand. COVID-19 Dashboard. 2024. (https://ddc.moph.go.th/covid19-dashboard/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.