Abstract
Aim
We evaluated the appropriateness of various chest compression (CC) depths among Thai population by comparing the calculated heart compression fraction (HCF) using mathematical methods based on chest computed tomography (CT) measurements.
Methods
This multicenter retrospective cross-sectional study was conducted from September 2014 to December 2020. Chest parameters included external anteroposterior diameter (EAPD), internal anteroposterior diameter (IAPD), heart anteroposterior diameter (HAPD), and non-cardiac soft tissue measured at the level of maximum left ventricular diameter (LVmax). We compared the HCFs as calculated from CT parameters using different CC depths at 5 cm, 6 cm, 1/4 of EAPD, and 1/3 of EAPD, with further subgroup analysis stratified by sex and BMI.
Results
A total of 2927 eligible adult patients with contrast-enhanced chest CT were included. The study group had mean age of 60.1 ± 14.7 years, mean BMI of 22 ± 4.4 kg/m2, and were 57% males. The mean HCFs were 41.5%, 53.5%, 42.4%, and 62.6%, for CC depths of 50 mm, 60 mm, 1/4 of EAPD, and 1/3 of EAPD respectively. HCF was significantly lower in male patients for all CC depths. Advanced age and higher BMI showed significant correlation with lower HCF for CC depths of 50 mm and 60 mm.
Conclusion
The CC depth measure of 50–60 mm demonstrated efficacy in maintaining HCF and coronary perfusion in the general population except for geriatric and obese individuals. Adjusting CC depth to 1/4–1/3 of the EAPD yielded better outcomes. Future research should prioritize determining individualized CC depths based on EAPD proportion.
Keywords: Cardiopulmonary resuscitation, Depth of cardiac compression, Heart compression fraction, Thai people
Introduction
Cardiopulmonary resuscitation (CPR) involves chest compressions (CCs) and artificial ventilation.1 High-quality CCs, as recommended by the 2020 guidelines from the European Resuscitation Council and the American Heart Association, include a CC depth of at least 5 cm while avoiding greater than 6-cm depth and a rate of 100–120 compressions per minute, with emphasis on complete chest recoil, minimal interruptions, and changing compressors every 2 minutes.2 These recommendations come from studies conducted in American and European populations. Stiell et al.3 found that a depth interval of 40.3 to 55.3 mm (peak, 45.6 mm) was associated with the maximum survival rate. Animal studies have shown that CC depth may vary with hemodynamic-directed CPR4 and that a depth of 50 or 60 mm may not be optimal for all body mass indexes (BMIs) and thoracic cage sizes.5, 6 Lee et al.7 proposed that the currently recommended CC depth (≥50 mm) may not be appropriate for CPR and instead recommended a depth between one-fourth (1/4) and one-third (1/3) of the external anteroposterior diameter (EAPD). Further research and consideration of various factors such as the BMI and thoracic cage size may be needed to determine the most appropriate CC depth for maximizing survival outcomes during CPR.
The use of computed tomography (CT) to calculate the heart compression fraction (HCF), which represents the blood flow generated during CPR, has been suggested for comparison of the appropriate CC depth7, 8, 9. Previous recommendations of a 50- and 60-mm CC depth were generalized and not categorized by factors such as sex, BMI, or specific diseases of the thoracic cage.
Studies have shown that patients with a higher BMI tend to have a higher EAPD, internal anteroposterior diameter (IAPD),7, 8 and heart anteroposterior diameter (HAPD)8 resulting in a lower HCF during CPR. Geriatric patients also tend to have a higher EAPD, IAPD, and HAPD and lower HCF than non-geriatric patients. This suggests that the recommended 50- to 60-mm CC depth may not be sufficient for patients with a higher BMI or geriatric populations.9
Furthermore, there is a lack of studies that have evaluated the appropriate CC depth in different populations, such as Southeast Asian or Thai people. We hypothesized that the Southeast Asian population, including Thai people, may have a smaller body size and smaller anteroposterior chest diameter than American and European populations, which could result in CCs of 5- to 6-cm depth being overly deep. Therefore, this study was performed to evaluate the appropriateness of the recommended CC depths by comparing the HCFs using mathematical methods based on contrast-enhanced chest CT parameters in Thai population.
Materials and methods
Study design and setting
We performed a multicenter retrospective cross-sectional study to evaluate the appropriateness of the recommended CC depths by comparing the HCFs as calculated from measurements on chest CT. The study was conducted at eight referral hospitals across all regions of Thailand (Ramathibodi Hospital, Saraburi Hospital, Chaophraya Yommarat Hospital, Nan Hospital, Yasothon Hospital, Chumphae Hospital, Maharaj Nakhon Si Thammarat Hospital, and Bangkok Hospital Chanthaburi). The study was approved by the Committee on Human Rights Related to Research, Faculty of Medicine, Ramathibodi Hospital, Mahidol University (IRB COA. MURA2019/866).
Selection and description of participants
We reviewed the electronic medical records and imaging data of patients who underwent contrast-enhanced chest CT from September 2014 to December 2020. The study included Thai adults aged ≥18 years and excluded patients with anatomical abnormalities such as pectus carinatum and excavatum, trauma-related deformities, dextrocardia, situs inversus, other cardiopulmonary structural deformities, and mediastinal shift.
Technical information
The types of CT equipment, settings, and image-viewing software used at each center are described in Supplement 1.
Sample size calculation and statistical analysis
The sample size was determined by estimating the infinite population mean with adjustment for cluster surveys. Based on a 59.3-mm (standard deviation, 13.1) residual EAPD in chest CT when compressed to a 5-cm depth among Thai people with a BMI of 25 to 29.99 kg/m2 as reported by Khunkhlai et al.10 the estimated sample size required for this study was 2,904 subjects with an error (d) of 1.00, alpha (α) of 0.05, beta (β) of 0.20, design effect of 4, and missing data rate of 10%.
Descriptive statistics were computed for all relevant variables and clinical characteristics. Continuous variables with normal distribution were presented as mean ± standard deviation or median [interquartile range] when non-parametric tests were used. An independent t-test or the Mann–Whitney U test was used to compare continuous variables between two groups, whereas analysis of variance was used for comparisons between more than two groups. Analysis of covariance was used to adjust for age and sex, which could influence the association between BMI and outcomes. Univariate and multivariate linear regressions were used to compare non-normally distributed continuous data among more than two groups.
Categorical data are presented as percentages using the chi-square test or Fisher’s exact test, as appropriate. We performed all data analyses using Stata version 16 (StataCorp LLC, College Station, TX, USA).
Data collection
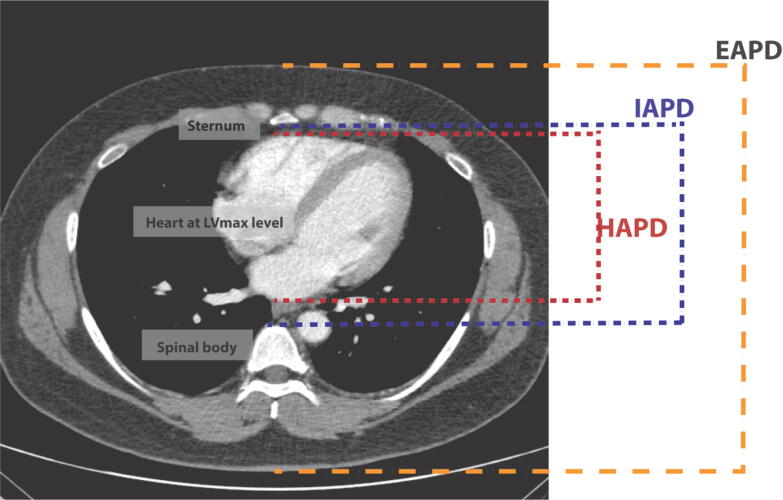
The data collected in this study comprised of general characteristics and chest CT measurements. Patient characteristics, including age, weight, height, BMI, sex, lung disease, heart disease, and geographic region, were extracted from medical records. BMI was categorized into four groups according to the World Health Organization guidelines: underweight (<18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). All chest parameters were measured at the midline of the axial level of LVmax (Fig. 1), including:
-
1.
EAPD (mm), the external anteroposterior distance from the skin overlying the sternum to the skin posterior to the spinous process on the back.
-
2.
IAPD (mm), the anteroposterior distance from the inner surface of the sternum to the anterior cortex of the vertebral body.
-
3.
HAPD (mm), the anteroposterior distance from the anterior to the posterior borders of the heart measured at midline, in line with the EAPD and IAPD axis.
Fig. 1.
Chest computed tomography measurements. Axial chest computed tomography image at the level of the LVmax. The external and internal chest anteroposterior and heart anteroposterior diameters were measured at the midline. The parameters used for heart compression fraction () were as follows: X is a CC depth of 50 mm, 60 mm, 1/4 of EAPD, or 1/3 of EAPD and d is the non-cardiac soft tissue (IAPD – HAPD). (LVmax, maximum left ventricular diameter; EAPD, external anteroposterior diameter; IAPD, internal anteroposterior diameter; HAPD, heart anteroposterior diameter.
Finally, the HCF was calculated using the following equation;
where X represents a CC depth of 50 mm, 60 mm, 1/4 of EAPD (mm), or 1/3 of EAPD (mm) and d represents the non-cardiac soft tissue diameter (mm) as calculated by subtracting HAPD from IAPD.
Results
Of the 3405 adult patients who had contrast-enhanced chest CT at 8 centers from 1 September 2014 to 31 December 2020, 478 patients were excluded by the exclusion criteria and missing medical record data (Fig. 2). The remaining 2927 patients met the eligibility criteria. The general characteristics of the study population, stratified by geographic regions of Thailand are presented in Table 1. The mean age of the total population was 60.1 ± 14.7 years, with no significant difference observed among regions. Most patients (57%) were male. The mean weight, height, and BMI of the population were 56.6 ± 12.6 kg, 160.6 ± 8.7 cm, and 22 ± 4.4 kg/m2, respectively.
Fig. 2.
Study flow chart. CT, computed tomography.
Table 1.
General characteristics of the study population stratified by geographic regions of the country.
| Characteristics | Total (N = 2927) |
Regions |
P | |||||
|---|---|---|---|---|---|---|---|---|
| North (n = 333) |
Northeast (n = 770) |
East (n = 246) |
Middle (n = 778) |
South (n = 490) |
Bangkok (n = 310) |
|||
| Age (years) | 60.1 ± 14.7 | 61.5 ± 12.6 | 60.2 ± 14.9 | 58.4 ± 14.3 | 60.2 ± 15.8 | 59.2 ± 15.4 | 60.7 ± 12.8 | 0.129 |
| Weight (kg) | 56.6 ± 12.6 | 51.3 ± 12.5 | 54.9 ± 12.4 | 61.3 ± 13.5 | 57.8 ± 11.9 | 55.7 ± 11.6 | 61.4 ± 12.6 | <0.001 |
| Height (cm) | 160.6 ± 8.7 | 157.9 ± 8.9 | 160.3 ± 8.6 | 162.4 ± 9.0 | 161 ± 8.7 | 161.2 ± 8.4 | 160.3 ± 8.2 | <0.001 |
| BMI (kg/m2) | 22 ± 4.4 | 20.5 ± 4.0 | 21.3 ± 4.3 | 23.2 ± 4.2 | 22.3 ± 4.2 | 21.5 ± 4.3 | 23.9 ± 4.3 | <0.001 |
| Sex, n (%) | ||||||||
| Male | 1668 (57.0) | 189 (56.8) | 460 (59.7) | 150 (61.0) | 435 (55.9) | 292 (59.6) | 142 (45.8) | 0.001 |
| Female | 1259 (43.0) | 144 (43.2) | 310 (40.3) | 96 (39.0) | 343 (44.1) | 198 (40.4) | 168 (54.2) | |
| Lung diseases, n (%) | ||||||||
| None | 1619 (55.3) | 170 (51.1) | 443 (57.5) | 191 (77.6) | 391 (50.3) | 263 (53.7) | 161 (51.9) | <0.001 |
| COPD/asthma | 140 (4.8) | 16 (4.8) | 54 (7.0) | 9 (3.7) | 33 (4.2) | 8 (1.6) | 20 (6.5) | |
| Lung cancer/metastasis | 885 (30.2) | 122 (36.6) | 156 (20.3) | 37 (15.0) | 289 (37.1) | 168 (34.3) | 113 (36.5) | |
| Tuberculosis | 283 (9.7) | 25 (7.5) | 117 (15.2) | 9 (3.7) | 65 (8.4) | 51 (10.4) | 16 (5.2) | |
| Heart diseases, n (%) | ||||||||
| None | 2826 (96.5) | 316 (94.9) | 739 (96.0) | 245 (99.6) | 757 (97.3) | 469 (95.7) | 300 (96.8) | 0.026 |
| IHD/arrhythmia | 101 (3.5) | 17 (5.1) | 31 (4.0) | 1 (0.4) | 21 (2.7) | 21 (4.3) | 10 (3.2) | |
| BMI groups, n (%) | ||||||||
| <18.5 | 635 (21.7) | 107 (32.1) | 191 (24.8) | 28 (11.4) | 147 (18.9) | 134 (27.3) | 28 (9.0) | <0.001 |
| 18.5–24.9 | 1667 (57.0) | 185 (55.6) | 450 (58.4) | 142 (57.7) | 455 (58.5) | 266 (54.3) | 169 (54.5) | |
| 25.0–29.9 | 478 (16.3) | 32 (9.6) | 96 (12.5) | 53 (21.5) | 142 (18.3) | 70 (14.3) | 85 (27.4) | |
| ≥30.0 | 147 (5.0) | 9 (2.7) | 33 (4.3) | 23 (9.3) | 34 (4.4) | 20 (4.1) | 28 (9.0) | |
| Size of chest compartments (mm) | ||||||||
| EAPD | 205.8 (25.9) | 197.9 (24.7) | 200.4 (25.2) | 214.3 (27) | 208.9 (25.8) | 203.9 (24) | 216.3 (25.3) | <0.001 |
| IAPD | 101.1 (16.8) | 98.9 (16.3) | 101.3 (17.7) | 104.5 (17.9) | 99.5 (15.5) | 100.3 (15.9) | 105.4 (17.5) | <0.001 |
| HAPD | 85.9 (13.6) | 82.9 (13.5) | 87.1 (15) | 87.9 (14) | 86.6 (11.9) | 82.7 (12.6) | 88.1 (14.4) | <0.001 |
| Non-cardiac soft tissue (mm)* | 13.4 [6.9, 21.6] | 14.4 [8.7, 21.4] | 12.5 [6, 20.4] | 15.5 [9.2, 22.2] | 11.2 [4.5, 18.8] | 16.7 [9.3, 24.4] | 16.5 [10.2, 23.9] | <0.001 |
Data are presented as mean ± standard deviation, n (%), or median [interquartile range].
BMI, body mass index; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; EAPD, external anteroposterior diameter; IAPD, internal anteroposterior diameter; HAPD, heart anteroposterior diameter.
Non-cardiac soft tissue: calculated by subtracting HAPD from IAPD.
The mean EAPD, IAPD, and HAPD and the median non-cardiac soft tissue diameter were 205.8 ± 25.9 mm, 101.1 ± 16.8 mm, 85.9 ± 13.6 mm, and 13.4 [6.9, 21.6] mm, respectively. Significant differences were observed among the geographic regions in sex, weight, height, BMI, lung disease, heart disease, and BMI groups, as well as in all chest parameters. When applying the mean chest compartment diameters of all patients to the formula [HCF = (X − d) / (HAPD) × 100], the mean HCFs were 41.5% (±14.0), 53.5% (±15.0), 42.4% (±10.6), and 62.6% (±10.7) for chest compression (CC) depths of 50 mm, 60 mm, 1/4 of EAPD, and 1/3 of EAPD respectively (Table 3).
Table 3.
Heart compression fractions (%) by depths of compression and characteristics of subjects.
| Characteristics | 50 mms depth |
60 mms depth |
1/4 of EAPD depth |
1/3 of EAPD depth |
||||
|---|---|---|---|---|---|---|---|---|
| mean (SD) | P | mean (SD) | P | mean (SD) | P | mean (SD) | P | |
| All patient | 41.5. ± 14.0 | 53.5. ± 15.0 | 42.4. ± 10.6 | 62.6. ± 10.7 | ||||
| Sex | ||||||||
| Male | 38.3 ± 13.5 | <0.001 | 49.8 ± 14.2 | <0.001 | 40 ± 10.2 | <0.001 | 59.8 ± 9.7 | <0.001 |
| Female | 46 ± 13.7 | 58.5 ± 14.8 | 45.6 ± 10.3 | 66.2 ± 10.8 | ||||
| BMI groups | ||||||||
| <18.5 | 47.8 ± 14.9 | <0.001 | 61.2 ± 15.9 | <0.001 | 40.9 ± 10.7 | <0.001 | 60.9 ± 10.2 | <0.001 |
| 18.5–24.9 | 41.4 ± 13.4 | 53.3 ± 14.1 | 42.3 ± 10.7 | 62.5 ± 10.8 | ||||
| 25–29.9 | 36.6 ± 12.5 | 47.4 ± 13.1 | 43.7 ± 10.1 | 64 ± 10.5 | ||||
| >=30 | 33.5 ± 11.8 | 43.6 ± 12.5 | 44.7 ± 10.6 | 65.3 ± 11.2 | ||||
| Regions | ||||||||
| North | 42.1 ± 13.7 | <0.001 | 54.5 ± 14.8 | <0.001 | 40.4 ± 9.6 | <0.001 | 60.5 ± 9.3 | <0.001 |
| North East | 42.4 ± 14.3 | 54.2 ± 15.5 | 41.4 ± 10.1 | 60.9 ± 10 | ||||
| East | 39.3 ± 13.5 | 50.9 ± 14.6 | 42.4 ± 9.6 | 62.9 ± 9.8 | ||||
| Middle | 43.6 ± 13.7 | 55.4 ± 14.4 | 45.6 ± 11.2 | 65.9 ± 11.7 | ||||
| South | 40.1 ± 14.8 | 52.5 ± 15.7 | 40.4 ± 11 | 61.2 ± 10.8 | ||||
| Bangkok | 38.2 ± 13.5 | 49.8 ± 14.5 | 41.9 ± 9.7 | 62.6 ± 9.5 | ||||
| Heart diseases | ||||||||
| None | 41.5 ± 14.1 | 0.141 | 53.5 ± 15.1 | 0.353 | 42.3 ± 10.6 | 0.078 | 62.5 ± 10.7 | 0.519 |
| IHD/arrhythmia | 43.6 ± 13 | 54.9 ± 13.8 | 44.2 ± 9.7 | 63.2 ± 9.6 | ||||
| Lung diseases | ||||||||
| None | 41.5 ± 13.3 | <0.001 | 53.2 ± 14.3 | <0.001 | 42.9 ± 10.1 | 0.026 | 63 ± 10.4 | 0.102 |
| COPD/Asthma | 43.3 ± 15.5 | 55.6 ± 16.6 | 41.6 ± 11 | 61.6 ± 11 | ||||
| Lung cancer/metastasis | 40.6 ± 14.8 | 52.5 ± 15.6 | 41.8 ± 11.3 | 62.1 ± 11.2 | ||||
| Tuberculosis | 44.6 ± 15.3 | 57.3 ± 16.5 | 41.6 ± 10.9 | 61.9 ± 10.3 | ||||
BMI, body mass index; COPD, chronic obstructive pulmonary disorder; EAPD, external anteroposterior diameter; mm, millimeter; kg, kilogram; IHD, ischemic heart disease; NE, north east; SD, standard deviation.
Table 2 presents the chest compartment diameters and the mean depth of 1/4 and 1/3 of EAPD stratified by sex, BMI, regions, heart diseases, and lung diseases. The mean diameters of all chest compartments were longer in men than in women, with significant differences observed in EAPD (209 ± 25.3 vs. 201.6 ± 26.2 mm, P < 0.01), IAPD (105.5 ± 16.5 vs. 95.3 ± 15.2 mm, P < 0.01), HAPD (88.9 ± 13.7 vs. 82.1 ± 12.6 mm, P < 0.01), and non-cardiac soft tissue (14.9 [8.2, 23.6] vs. 11.8 [5.9, 19.1] mm; P < 0.01). Table 3 presents the HCF categorized by depths of compression and characteristics of the subjects. A lower HCF was observed in males compared to females, with significant differences at various compression depths: 50 mm (38.3% ± 13.5% vs. 46.0% ± 13.7%, P < 0.01), 60 mm (49.8% ± 14.2% vs. 58.5% ± 14.8%, P < 0.01), 1/3 of EAPD (59.8% ± 9.7% vs. 66.2% ± 10.8%, P < 0.01) and 1/4 of EAPD (40.0% ± 10.2% vs. 45.6% ± 10.3%, P < 0.01).
Table 2.
Chest compartment diameters (millimeters) stratified by patient characteristics.
| Characteristics | External anteroposterior diameter |
Internal anteroposterior diameter |
1/4 of EAPD |
1/3 of EAPD |
Heart anteroposterior diameter |
Non-cardiac soft tissue |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | P | mean ± SD | P | mean ± SD | P | mean ± SD | P | mean ± SD | P | Median (IQR) | P | |
| Sex | ||||||||||||
| Male | 209 ± 25.3 | <0.001 | 105.5 ± 16.5 | <0.001 | 52.2 ± 6.3 | <0.001 | 69.7 + 8.4 | <0.001 | 88.9 ± 13.7 | <0.001 | 14.9 ± 8.2, 23.6 | <0.001 |
| Female | 201.6 ± 26.2 | 95.3 ± 15.2 | 50.4 ± 6.5 | 67.2 ± 8.7 | 82.1 ± 12.6 | 11.8 ± 5.9, 19.1 | ||||||
| BMI groups | ||||||||||||
| <18.5 | 181.7 ± 18.3 | <0.001 | 90.7 ± 14.4 | <0.001 | 45.4 ± 4.6 | <0.001 | 60.5 ± 6.1 | <0.001 | 76.6 ± 11.5 | <0.001 | 12.4 ± 6.3, 20.4 | 0.006 |
| 18.5–24.9 | 205 ± 20.1 | 100.8 ± 14.7 | 51.2 ± 5 | 68.3 ± 6.7 | 85.7 ± 11.6 | 13.2 ± 6.9, 21.8 | ||||||
| 25–29.9 | 228.1 ± 18.6 | 110.7 ± 16.6 | 57 ± 4.6 | 76 ± 6.2 | 94.7 ± 13 | 14.6 ± 7.6, 21.7 | ||||||
| >=30 | 246.6 ± 20.8 | 117.9 ± 17.7 | 61.6 ± 5.2 | 82.2 ± 6.9 | 101 ± 14.7 | 15.8 ± 9.2, 23.8 | ||||||
| Regions | ||||||||||||
| North | 197.9 ± 24.7 | <0.001 | 98.9 ± 16.3 | <0.001 | 49.5 ± 6.2 | <0.001 | 65.9 ± 8.2 | <0.001 | 82.9 ± 13.5 | <0.001 | 14.4 ± 8.7, 21.4 | <0.001 |
| North East | 200.4 ± 25.2 | 101.3 ± 17.7 | 50.1 ± 6.3 | 66.8 ± 8.4 | 87.1 ± 15 | 12.5 ± 6, 20.4 | ||||||
| East | 214.3 ± 27 | 104.5 ± 17.9 | 53.6 ± 6.7 | 71.4 ± 9 | 87.9 ± 14 | 15.5 ± 9.2, 22.2 | ||||||
| Middle | 208.9 ± 25.8 | 99.5 ± 15.5 | 52.2 ± 6.4 | 69.6 ± 8.6 | 86.6 ± 11.9 | 11.2 ± 4.5, 18.8 | ||||||
| South | 203.9 ± 24 | 100.3 ± 15.9 | 51 ± 6.0 | 67.9 ± 8.0 | 82.7 ± 12.6 | 16.7 ± 9.3, 24.4 | ||||||
| Bangkok | 216.3 ± 25.3 | 105.4 ± 17.5 | 54.1 ± 6.3 | 72.1 ± 8.4 | 88.1 ± 14.4 | 16.5 ± 10.2, 23.9 | ||||||
| Heart diseases | ||||||||||||
| None | 205.8 ± 26 | 0.821 | 101 ± 16.8 | 0.426 | 51.4 ± 6.5 | 0.821 | 68.6 ± 8.7 | 0.821 | 85.7 ± 13.5 | <0.001 | 13.6 ± 7.1, 21.7 | <0.001 |
| IHD/arrhythmia | 205.3 ± 23.4 | 102.4 ± 17.2 | 51.3 ± 5.8 | 68.4 ± 7.8 | 91.4 ± 16.1 | 7.6 ± 3.1, 16.1 | ||||||
| Lung diseases | ||||||||||||
| None | 207.9 ± 25.8 | <0.001 | 102 ± 16.5 | <0.001 | 52 ± 6.4 | <0.001 | 69.3 ± 8.6 | <0.001 | 87.3 ± 13.7 | <0.001 | 13 ± 7.1, 20.5 | 0.032 |
| COPD/Asthma | 198.1 ± 28.9 | 98.6 ± 18.1 | 49.5 ± 7.2 | 66 ± 9.6 | 83.6 ± 14.2 | 13.4 ± 6.1, 21.8 | ||||||
| Lung cancer/metastasis | 206.8 ± 24.8 | 101.6 ± 16.8 | 51.7 ± 6.2 | 68.9 ± 8.2 | 85.4 ± 12.9 | 14.8 ± 7, 23.4 | ||||||
| Tuberculosis | 194.4 ± 25 | 95.9 ± 16.6 | 48.6 ± 6.2 | 64.8 ± 8.3 | 81 ± 14.1 | 12.7 ± 6.6, 21.9 | ||||||
EAPD, external anteroposterior diameter; IAPD, internal anteroposterior diameter; HAPD, heart anteroposterior diameter; HCF, heart compression fraction; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; SD, standard deviation; IQR, interquartile range.
When stratified by BMI, all chest compartment diameters were significantly shorter in individuals with a BMI of <18.5 kg/m2. In Table 2, the mean CC depth of 1/4 of EAPD varied from 45.4 ± 4.6 mm in the BMI < 18.5 group up to 61.6 ± 5.2 mm in the BMI ≥ 30 group. Similarly, the mean CC depth at 1/3 of EAPD ranged from 60.5 ± 6.1 mm in the BMI < 18.5 group to 82.2 ± 6.9 mm in the BMI ≥ 30 group. In Table 3, The HCFs at CC depths of 50 mm and 60 mm were observed to be lower in individuals with higher BMI. In contrast, the HCFs at CC depths of 1/4 and 1/3 of EAPD were greater in individuals with higher BMI. Significant differences in the chest compartment diameters and HCFs were observed across the different regions of Thailand.
The HAPD was significantly greater in patients with than without ischemic heart disease/arrhythmia, with values of 91.4 ± 16.1 vs. 85.7 ± 13.5 mm, respectively (P < 0.01). However, the HCF did not differ significantly between the two groups. Among patients with lung disease (chronic obstructive pulmonary disease/asthma, lung cancer/metastasis, and tuberculosis), the EAPD, IAPD, and HAPD were significantly smaller than in patients without lung disease. The HCF at CC depths of both 50 and 60 mm was higher in patients with tuberculosis than in patients with chronic obstructive pulmonary disease/asthma, patients without lung disease, and patients with lung cancer/metastasis. However, the HCFs at CC depths of 1/4 and 1/3 of EAPD were higher in patients without lung diseases.
Factors associated with the HCF at CC depths of 50 and 60 mm by univariate and multivariate linear regression models are shown in Table 4 and Table 5, respectively. Advanced age and a higher BMI group were independent prognostic factors significantly associated with a lower HCF at CC depths of both 50 mm and 60 mm. Female sex and residence in the Northeast or Middle regions were significantly associated with a higher HCF at both CC depths. Fig. 3 displays a box plot of residual EAPD after subtracting 50 mm, 60 mm, 1/4 and 1/3 of EAPD compression depths. The CC depth at 1/3 of EAPD yielded the most significantly reduced residual EAPD compared to depths of 60 mm, 1/4 of EAPD, and 50 mm, respectively.
Table 4.
Association between HCF and covariates at CC depth of 50 mm.
| Variables | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| Coefficients | P | Coefficients | P | ||
| Age | −0.1 (−0.2, −0.1) | <0.001 | −0.1 (−0.2, −0.1) | <0.001 | |
| Sex | Male | Reference | Reference | ||
| Female | 7.8 (6.8, 8.8) | <0.001 | 8.4 (7.4, 9.3) | <0.001 | |
| BMI groups | <18.5 kg/m2 | Reference | Reference | ||
| 18.5–24.9 kg/m2 | −6.5 (−7.7, −5.2) | <0.001 | −6.8 (−8.0, −5.7) | <0.001 | |
| 25.0–29.9 kg/m2 | −11.2 (−12.8, −9.6) | <0.001 | −12.1 (−13.6, −10.5) | <0.001 | |
| ≥30.0 kg/m2 | −14.3 (−16.7, −11.9) | <0.001 | −15.6 (−17.9, −13.2) | <0.001 | |
| Regions | Bangkok | Reference | Reference | ||
| North | 4.0 (1.8, 6.1) | <0.001 | 1.9 (−0.2, 3.9) | 0.068 | |
| Northeast | 4.3 (2.4, 6.1) | <0.001 | 2.9 (1.2, 4.6) | 0.001 | |
| East | 1.1 (−1.3, 3.5) | 0.357 | 1.6 (−0.6, 3.8) | 0.148 | |
| Middle | 5.5 (3.6, 7.3) | <0.001 | 4.7 (3.0, 6.4) | <0.001 | |
| South | 2.0 (−0.1, 4.0) | 0.051 | 0.7 (−1.3, 2.5) | 0.513 | |
| Heart diseases | None | Reference | N/A | ||
| IHD/arrhythmia | 2.1 (−0.7, 4.9) | 0.141 | N/A | ||
| Lung diseases | None | Reference | Reference | ||
| COPD/asthma | 1.9 (−0.6, 4.3) | 0.139 | 1.1 (−1.2, 3.4) | 0.336 | |
| Lung cancer/metastasis | −1.0 (−2.1, 0.3) | 0.118 | −1.0 (−2.1, 0.2) | 0.078 | |
| Tuberculosis | 3.2 (1.4, 4.9) | 0.001 | 1.2 (−0.5, 2.9) | 0.156 | |
HCF, heart compression fraction; CC, chest compression; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; mm, millimeter; N/A, not applicable.
Table 5.
Association between HCF and covariates at CC depth of 60 mm.
| Variables | Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|
| Coefficients | P | Coefficients | P | ||
| Age | −0.1 (−0.2, −0.1) | <0.001 | −0.1 (−0.2, −0.1) | <0.001 | |
| Sex | Male | Reference | Reference | ||
| Female | 8.7 (7.7, 9.8) | <0.001 | 9.5 (8.5, 10.5) | <0.001 | |
| BMI groups | <18.5 kg/m2 | Reference | Reference | ||
| 18.5–24.9 kg/m2 | −7.9 (−9.2, −6.6) | <0.001 | −8.3 (−9.5, −7.1) | <0.001 | |
| 25.0–29.9 kg/m2 | −13.9 (−15.5, −12.2) | <0.001 | −14.7 (−16.3, −13.1) | <0.001 | |
| ≥30.0 kg/m2 | −17.6 (−20.1, −15.0) | <0.001 | −18.9 (−21.3, −16.5) | <0.001 | |
| Regions | Bangkok | Reference | Reference | ||
| North | 4.7 (2.4, 7.0) | <0.001 | 2.1 (−0.1, 4.2) | 0.053 | |
| Northeast | 4.5 (2.5, 6.4) | <0.001 | 2.7 (0.9, 4.5) | 0.004 | |
| East | 1.1 (−1.5, 3.6) | 0.389 | 1.7 (−0.6, 4.0) | 0.141 | |
| Middle | 5.6 (3.6, 7.5) | <0.001 | 4.6 (2.8, 6.3) | <0.001 | |
| South | 2.7 (0.6, 4.9) | 0.013 | 1.0 (–1.0, 2.9) | 0.334 | |
| Heart diseases | None | Reference | N/A | ||
| IHD/arrhythmia | 1.5 (−1.6, 4.4) | 0.352 | N/A | ||
| Lung diseases | None | Reference | Reference | ||
| COPD/asthma | 2.4 (−0.2, 5.0) | 0.07 | 1.5 (−0.9, 3.9) | 0.211 | |
| Lung cancer/metastasis | −0.7 (−2.0, 0.6) | 0.275 | −0.8 (−1.9, 0.4) | 0.171 | |
| Tuberculosis | 4.2 (2.3, 6.0) | <0.001 | 1.8 (0.1, 3.5) | 0.043 | |
HCF, heart compression fraction; CC, chest compression; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IHD, ischemic heart disease; mm, millimeter; NA, not applicable.
Fig. 3.
Box plot of residual EAPD after subtracting 50 mm, 60 mm, 1/4 EAPD and 1/3 of EAPD compression depths. EAPD, external anteroposterior diameter; mm, millimeter.
Discussion
Our study is the first multicenter nationwide study conducted across Thailand to evaluate the appropriateness of recommended chest compression depths by comparing the HCFs calculated by mathematical methods using parameters measured on contrast-enhanced chest CT images. The mean HCFs of the total population were 41.5%, 53.5%, 42.4%, and 62.6% for chest compression (CC) depths of 50 mm, 60 mm, 1/4 of EAPD, and 1/3 of EAPD, respectively. This HCF range appeared to be more effective in generating blood flow during CPR than that observed in a previous Asian population study conducted in Korea8 in which the mean HCF in individuals of normal weight ranged from 35.0% to 47.3% for CC depth of 5 to 6 cm. However, our study had limited data for comparison with Caucasian populations, and it is important to consider potential differences in body size, chest anatomy, and other factors between different populations when interpreting study results and making recommendations for CPR practice.
Our observations suggest that women tend to have higher calculated HCF values than men because of their smaller EAPD, IAPD, and HAPD.11 The differences observed for the different regions of Thailand may be related to variations of EAPD and IAPD within these groups. We also considered variations in chest AP diameter, BMI, age, and abnormal heart–lung structures as factors that might suggest requirement for specific CC depths. Our findings align with prior studies conducted in Asia7, 8, 9 indicating that the CC depths of 50–60 mm may not yield adequate cardiac output during CPR, particularly in older individuals and patients with higher BMI. In addition, based on mathematical calculation of HCF, a fixed CC depth (50 mm and 60 mm) and an adjusted CC depth by the proportions of EAPD showed different results. Decreasing HCF among higher BMI groups was observed when 50 mm and 60 mm CC depths were applied whereas the adjusted CC depth by chest proportions yielded slightly increasing HCF with higher BMI. Therefore, the optimal target depth for chest compressions in obese and geriatric individuals may need to be individualized. The multivariable regression further supported the association of advanced age and higher BMI with a lower HCF at CC depths of both 50 and 60 mm. However, female sex was associated with a higher HCF at CC depths of both 50 and 60 mm after adjustment for age and BMI. These results might point out that flexible compression depth based on human morphology is better than fixed level of depth. Lee et al.7 proposed that a residual chest depth of less than 20 mm may injure intrathoracic structures. Fig. 3 demonstrates that although the CC depth at 1/3 of EAPD yielded a more effective HCF values, the residual EAPD after subtracting 1/3 of EAPD was the lowest compared to the CC depths of 60 mm, 1/4 of EAPD, and 50 mm. This reduction may occasionally increase the risk of injury to intrathoracic organs.
A report in Thai population stated that a “super-aged society” would be reached in 2031.12 Further studies are needed to investigate the optimal CC depth in geriatric patients who experience cardiac arrest compared with other patient populations to ensure that sufficient high-quality CCs are delivered. The HAPD was higher in the higher BMI group of our study, which is consistent with the association between obesity and cardiomyopathy, clinically characterized by left ventricular dilation and hypertrophy.8, 13 Further studies are needed to determine the optimal CC depth in obese patients experiencing cardiac arrest, considering the tradeoff between achieving proper cardiac output and the potential complications such as liver injury, rib and sternal fractures, and others.
This study has several limitations. First, estimation of the HCF using a mathematical formula may not directly reflect the actual ejection fraction or cardiac output. Two theories have been proposed to explain the mechanism of blood flow during CPR: the cardiac pump theory and the thoracic pump theory. However, the HCF formula that we used only considers the cardiac pump theory and does not incorporate the potential effects of the thoracic pump theory, which suggests that changes in the chest wall and pulmonary dynamics also influence blood flow during CCs.9
Second, there may have been selection or misclassification bias in our study because we included patients who underwent chest CT scans for various reasons, including emergency conditions, urgent conditions, and normally scheduled procedures, which could have resulted in the inclusion of patients with abnormal anatomical structures of the thorax. As a result, these patients may have had different physiological conditions than the general population. This might impact the generalizability of our findings.
Third, the chest CT scans were performed with both arms in a raised position and maintained in an inspiratory state during the scan. Notably, however, this may not represent the actual physiological position during CPR, during which the arms are typically placed down and positive-pressure ventilation may be administered.
Fourth, errors in image-based measurement of thoracic and heart parameters can occur for various reasons, including incorrect positioning of the patient during imaging, breathing artifacts, image resolution and image quality, observer variability, and equipment and software errors. To minimize errors during image analysis, we followed standardized measurement techniques, ensured proper patient positioning, used high-quality images with optimal resolution, and accounted for potential observer variability.
Finally, the appropriateness of the mean depth of CCs during CPR for maximizing the HCF may be associated with multiple factors such as age, sex, BMI, and the underlying disease, all of which can affect the intrathoracic anatomy. These factors may need to be individualized. In the future, advancements in CPR feedback devices and mechanical CPR may incorporate novel algorithms aimed at adjusting optimal compression depth recommendations tailored for obese or geriatric patients, or variations based on EAPD or IAPD measurements. It is important to consider monitoring parameters such as end-tidal carbon dioxide or diastolic blood pressure during CPR to assess the adequacy of coronary perfusion and improve survival and neurological outcomes. Further prospective studies are needed to evaluate the factors associated with the appropriate CC depth to enhance favorable survival outcomes.
Conclusion
Our study demonstrated that the absolute CC depth measure of 50 mm to 60 mm may unequivocally exhibit efficacy in maintaining HCF and ensure coronary perfusion in the general population. However, this absolute measure may not optimize coronary blood flow in older and obese individuals. Conversely, adjusting the CC depth by proportion to 1/4 to 1/3 of the EAPD yields more favorable HCF outcomes. Nevertheless, it is essential to consider the potential risk of intrathoracic injury resulting from decreased residual EAPD. Future research should prioritize determining individualized CC depths based on EAPD proportion and advancing CPR device technology accordingly to enhance patient outcomes.
Mathematical formula
Heart compression fraction = [(X − d)/heart anteroposterior diameter] × 100%.
Where X is a CC depth of 50 mm, 60 mm, 1/4 of EAPD (mm), or 1/3 of EAPD (mm) and d is non-cardiac soft tissue diameter (mm).
Trial registry
This trial was retrospectively registered on 2 February 2023 in the Thai Clinical Trial Registry (identification number TCTR 20230202009).
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Committee on Human Rights Related to Research, Faculty of Medicine, Ramathibodi Hospital, Mahidol University (IRB COA. MURA2019/866, Date: September 5, 2019).
Informed consent statement
The requirement for patient consent was waived because the data were retrospectively collected and anonymous.
Funding
This work was supported by a Faculty of Medicine Ramathibodi Hospital Mahidol University grant (“Faculty Income Scholarship 2021”).
Submission declaration and verification
This article has not been published previously, is not under consideration for publication elsewhere, is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder.
Declaration of generative AI in scientific writing
During the preparation of this work, the authors used ChatGPT/Open AI to check grammar and spelling. After using this service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CRediT authorship contribution statement
Pitsucha Sanguanwit: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Methodology, Funding acquisition, Formal analysis, Data curation, Conceptualization. Nitima Saksobhavivat: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Phatthranit Phattharapornjaroen: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Pongsakorn Atiksawedparit: Writing – review & editing, Visualization, Supervision, Software, Methodology, Formal analysis, Data curation, Conceptualization. Phanorn Chalermdamrichai: Writing – review & editing, Visualization, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis, Conceptualization. Ratchanee Saelee: Writing – review & editing, Visualization, Supervision, Methodology, Formal analysis, Conceptualization. Pongthorn Jantataeme: Writing – review & editing, Validation, Investigation. Krittaya Na Petvicharn: Writing – review & editing, Validation, Investigation. Napas Lawantuksin: Writing – review & editing, Validation, Investigation. Possawee Paosaree: Writing – review & editing, Validation, Investigation. Patcharaporn Klongklaew: Writing – review & editing, Validation, Investigation. Aphichai Prakongsin: Writing – review & editing, Validation, Investigation. Dhawankorn Walanchaphruk: Writing – review & editing, Validation, Investigation. Pornpun Wattanaruengchai: Writing – review & editing, Validation, Investigation. Suwitchaya Surapornpaiboon: Writing – review & editing, Validation, Investigation. Maesaya Chartkul: Writing – review & editing, Validation, Investigation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We wish to acknowledge the contributions of all the medical staff, nursing staff, and radiological technicians of the Emergency Department of Ramathibodi Hospital, Chakri Naruebodindra Medical Institute, Maharaj Nakhon Si Thammarat Hospital, Chumphae Hospital, Yasothon Hospital, Saraburi Hospital, Chaophraya Yommarat Hospital, Nan Hospital, and Bangkok Hospital Chanthaburi. We also thank Angela Morben, DVM, ELS, from Edanz (www.edanz.com/ac), for editing a draft of this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100605.
Contributor Information
Pitsucha Sanguanwit, Email: Pitsucha.san@mahidol.edu.
Nitima Saksobhavivat, Email: nitima.sak@mahidol.edu.
Phatthranit Phattharapornjaroen, Email: Phatthranit.pha@mahidol.edu.
Pongsakorn Atiksawedparit, Email: pongsakorn.ati@mahidol.edu.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Panchal A.R., Bartos J.A., Cabañas J.G., et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 2.Idris A.H., Guffey D., Pepe P.E., et al. Chest compression rates and survival following out-of-hospital cardiac arrest. Crit Care Med. 2015;43:840–848. doi: 10.1097/CCM.0000000000000824. [DOI] [PubMed] [Google Scholar]
- 3.Stiell I.G., Brown S.P., Christenson J., et al. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit Care Med. 2012;40:1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutton R.M., Friess S.H., Bhalala U., et al. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C.-H., Huang C.-H., Chang W.-T., et al. Associations between body size and outcomes of adult in-hospital cardiac arrest: a retrospective cohort study. Resuscitation. 2018;130:67–72. doi: 10.1016/j.resuscitation.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Pei-Chuan Huang E., Fu C.-M., Chang W.-T., et al. Associations of thoracic cage size and configuration with outcomes of adult in-hospital cardiac arrest: a retrospective cohort study. J Formosan Med Assoc. 2021;120:371–379. doi: 10.1016/j.jfma.2020.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Lee S.H., Kim D.H., Kang T.S., et al. The uniform chest compression depth of 50 mm or greater recommended by current guidelines is not appropriate for all adults. Am J Emerg Med. 2015;33:1037–1041. doi: 10.1016/j.ajem.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 8.Lee H., Oh J., Lee J., et al. Retrospective study using computed tomography to compare sufficient chest compression depth for cardiopulmonary resuscitation in obese patients. J Am Heart Assoc. 2019;8:e013948. doi: 10.1161/JAHA.119.013948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo K.H., Oh J., Lee H., et al. Comparison of heart proportions compressed by chest compressions between geriatric and nongeriatric patients using mathematical methods and chest computed tomography: a retrospective study. Ann Geriatr Med Res. 2018;22:130–136. doi: 10.4235/agmr.2018.22.3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khunkhlai N.A.P., Kaewlai R., Jenjitranant P., Dissaneevate K., Khruekarnchana P. Estimation of optimal chest compression depth based on chest computed tomography in Thai patients. Resuscitation. 2017;118:e56. [Google Scholar]
- 11.Jin S.Y., Oh S.B., Kim Y.O. Estimation of optimal pediatric chest compression depth by using computed tomography. Clin Exp Emerg Med. 2016;3:27–33. doi: 10.15441/ceem.16.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Working Committee of the Report on the Situation of the Thai Elderly 2014. Situation of the Thai Elderly 2014. The Foundation of Thai Gerontology Researchand Development Institute (TGRI) and Institute for Population and Social Researh, Mahidol University; 2016. https://www.dop.go.th/download/knowledge/knowledge_th_20161608145327_1.pdf.
- 13.Bhatheja S., Panchal H.B., Ventura H., Paul T.K. Obesity cardiomyopathy: pathophysiologic factors and nosologic reevaluation. Am J Med Sci. 2016;352:219–222. doi: 10.1016/j.amjms.2016.05.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.