Abstract
Aim
We sought to determine if higher plasma levels of brain injury biomarkers neurofilament light (NfL), phosphorylated tau 181 (pT181), tau, and ubiquitin C-terminal hydrolase L1 (UCHL1) were associated with unfavorable outcomes in children supported on extracorporeal membrane oxygenation (ECMO) with and without preceding cardiac arrest.
Methods
We conducted a secondary analysis of a two-center prospective observational study of ECMO patients 0-<18 years. Plasma concentrations of NfL, pT181, tau, and UCHL1 were measured on ECMO days 1, 2 and 3. Unfavorable outcome was defined as in-hospital mortality or discharge Pediatric Cerebral Performance Category (PCPC) >2 with decline from baseline PCPC among survivors.
Results
Among 88 children on ECMO, mean tau levels were significantly higher on each of the first three ECMO days in children who underwent extracorporeal cardiopulmonary resuscitation (ECPR) compared to those with non-ECPR cardiac arrest or with no cardiac arrest preceding ECMO. Higher ECMO day 1 tau levels were significantly associated with increased hazard of unfavorable outcome in unadjusted (HR, 1.35, 95% CI 1.09–1.66) and adjusted (HR, 1.42; 95% CI 1.13–1.79) models. Higher levels of NfL or pT181 were not associated with increased hazard for unfavorable outcome in multivariable models. UCHL1 values were outside of detectable limits and thus deferred from analysis.
Conclusions
Levels of tau were significantly associated with increased hazard of death or unfavorable neurologic outcome in unadjusted and adjusted models. Biomarkers of brain injury, particularly tau, may aid in detection of neurologic injury and neuroprognostication in patients on ECMO with and without preceding cardiac arrest.
Keywords: Extracorporeal membrane oxygenation, Cardiac arrest, Extracorporeal cardiopulmonary resuscitation, Child, Brain injury, Biomarker
Introduction
Around 15,000 children suffer in-hospital cardiac arrest yearly in the United States.1 Despite increased focus on resuscitation, survival from pediatric in-hospital cardiac arrest remains low. A 2019 analysis of the American Heart Association Get With The Guidelines – Resuscitation (AHA GWTG-R) registry of 350 hospitals reporting 7,433 pediatric pulseless cardiac arrest events from 2000-2018 found that survival to discharge increased in the early years of study but has been relatively stable from 2013 to 2018 at 32–38%.2
In the last five years, nearly 4,000 neonates and children each year were supported with extracorporeal life support (ECMO) worldwide; approximately 700 (18%) of those underwent extracorporeal cardiopulmonary resuscitation (ECPR).3 ECPR is defined as the application of rapid-deployment venoarterial ECMO to provide circulatory support in patients in whom conventional CPR is unsuccessful in achieving sustained return of spontaneous circulation (ROSC), where sustained ROSC is deemed to have occurred when chest compressions are not required for 20 consecutive minutes, and signs of circulation persisted.4, 5 The Extracorporeal Life Support Organization distinguishes between the use of ECMO for ECPR, which implies the application of extracorporeal life support during conventional CPR, vs use of venoarterial ECMO for low cardiac output in patients who had suffered a cardiac arrest followed by sustained ROSC.4 It is estimated that cardiac arrest, with or without ECPR, precedes ECMO cannulation in 42% of pediatric ECMO cases.6 In a 2019 AHA GWTG-R registry review, ECPR use for patients with pulseless cardiac arrest rose steadily during the study period to 13% in 2018.2 Survival outcomes of children who undergo ECPR are worse (∼42%) than those of children supported on ECMO without having required ECPR (∼63%).3.
Neurological injury and impairment are substantial morbidities associated with cardiac arrest, ECMO and the intersection of the two represented by ECPR. The incidence of acute neurological injury for critically ill children supported on ECMO is reported as high as 25%.7, 8, 9, 10 Neurologic outcomes after pediatric cardiac arrest are typically reported as favorable in approximately 70% of survivors using broad functional assessment tools.11 However, more subtle but significant neurological deficits may be missed using these measures. The Therapeutic Hypothermia After In-hospital Pediatric Cardiac Arrest (THAPCA-IH) multicenter trial enrolled in-hospital pediatric cardiac arrest patients who were comatose after return of spontaneous circulation. While 77% of THAPCA-IH survivors had adaptive behavior scores above –2 standard deviations from the population mean at 12 months, the majority demonstrated significant declines in multiple domains of neurobehavioral functioning, with similar functioning at 3 and 12 months.12 In the subgroup of children who underwent ECPR, 71% of survivors had adaptive behavior scores above –2 standard deviations from the population mean at 12 months.13
Monitoring for neurologic injury on ECMO and after cardiac arrest is challenging due to degree of illness that may preclude neurologic exam, difficulty in transporting patients for imaging and limitations of bedside monitoring capability.14, 15, 16, 17 Brain injury blood-based biomarkers are a potential modality to monitor and direct neuroprotective therapy for patients on ECMO and following cardiac arrest.18, 19, 20
In this study we evaluated four brain injury biomarkers: neurofilament light (NfL), phosphorylated tau 181 (pT181), tau, and ubiquitin C-terminal hydrolase L1 (UCHL1), using prospectively collected plasma from a previously published cohort study of critically-ill children supported on ECMO at two institutions.10 The aim of this study was to determine if elevated plasma concentrations of NfL, pT181, tau and UCHL1 measured daily during the first three days of ECMO support were associated with in-hospital mortality or with discharge with unfavorable neurological outcome among survivors.
Methods
Study design and population
We enrolled 99 pediatric patients (age 0 to <18 years) supported on ECMO for any indication at two academic ECMO centers between July 2010 and June 2015 in the prospective observational cohort parent study. This study was approved by the Institutional Review Boards at both participating sites (IRB00210733; Pro00003406). Inclusion and exclusion criteria, as well as detailed cohort characteristics were previously published.10 Informed consent was obtained within 24 hours of ECMO cannulation, with subsequent blood samples obtained daily during the first three days of ECMO support. Patients eligible for the current study had the first blood sample collected at 24 ± 3 hours from ECMO initiation, with plasma aliquots that had never been thawed and of sufficient volume to assay the biomarkers of interest.
Pre-ECMO cardiac arrest and study outcomes
The primary stratification was presence of pre-ECMO cardiac arrest. Specifically, this was classified as no cardiac arrest within 7 days pre-ECMO initiation (reference group), cardiac arrest within 7 days pre-ECMO initiation that did not meet ECPR definition criteria, and ECPR. Both in-hospital and out-of-hospital cardiac arrest events were recorded, along with total duration of CPR.
The primary outcome was time to in-hospital mortality or to discharge with unfavorable neurological outcome among survivors. Neurological outcome at hospital discharge was assessed with the Pediatric Cerebral Performance Category (PCPC) scale,21, 22 in which a score of 1 indicates no neurological deficit; 2, mild cerebral disability; 3, moderate cerebral disability; 4, severe cerebral disability; 5, coma or vegetative state; and 6, brain death. A discharge PCPC score >2 with decline of at least one point from baseline PCPC was considered unfavorable neurological outcome.
Biomarker sampling and analysis
Blood samples were collected daily from indwelling catheters or from the ECMO circuit, at the same time as routine clinical lab draws. Blood samples were collected in 3.2% sodium citrate tubes, with platelet poor plasma separated by centrifugation, aliquoted and stored at –80 °C in continuously-monitored, temperature-controlled freezers without freeze–thaw cycles. Plasma concentrations for NfL, pT181, tau, and UCHL1 were measured for ECMO days 1, 2 and 3 using electrochemiluminescent assays (Meso Scale Discovery, Rockville, MD). Laboratory personnel were blinded to clinical data.
Statistical analysis
Continuous variables are presented as median with 25th to 75th percentile [P25, P75] and categorical variables as counts (frequencies). We first compared plasma biomarkers by pre-ECMO cardiac arrest status. Plasma biomarker levels tended to be right skewed which was ameliorated by log transformation. To characterize levels on ECMO days 1 to 3, we calculated geometric means for each ECMO day. To characterize initial levels and highest levels observed, we separately analyzed ECMO day 1 levels (i.e., first available sample within 24 ± 3 h from ECMO initiation) and peak levels across serially drawn samples.
We used conventional lognormal models to summarize the distribution of ECMO day 1 and of peak biomarker levels by pre-ECMO cardiac arrest status. In addition to the mean central tendency being interpreted as the geometric mean, these models allowed interval censoring to account for data to describe the distribution below and above the lower and upper limits of detection and quantification, respectively. To validly describe the full distribution of the biomarkers, including those beyond the lower and upper limits of detection, we plotted lognormal cumulative incidence curves with these censoring methods.
We then examined the association between biomarker levels and time to in-hospital death or discharge with unfavorable neurological outcome. We used Cox proportional hazards models to estimate the effect of two main predictors: (1) 3 times higher ECMO day 1 biomarker levels between patients, and (2) a ≥3 times increase from ECMO day 1 in biomarker levels within patients in serially drawn samples. Cox proportional hazards models were both unadjusted and adjusted for age group (neonate [<1 month], infant [1 month to <1 year], child [1 to <12 years], or adolescent [≥12 years]), sex, and pre-ECMO cardiac arrest status (ECPR, non-ECPR cardiac arrest, or no cardiac arrest). Indicators for censored observations above the upper limit of quantification were also included for adjustment in models (9 measurements for pT181 and 13 measurements for tau). The tripling of levels generally corresponded to a one standard deviation increase for most biomarkers, and this allowed for a comparison on the same scale across biomarkers. This scaling factor was primarily for interpretability and comparisons and did not change the statistical significance of the association with outcomes.
As a supplementary analysis, we estimated the predictive discrimination of ECMO day 1 NfL, pT181 and tau levels on unfavorable outcomes using area under the receiver operating characteristic (AUROC) curves.
Statistical significance was defined when 95% confidence intervals did not cross 0 (difference in location of lognormal models) or 1 (geometric mean ratios and hazard ratios [HR]). All analyses were conducted in R 4.0.2 (R Core Team, Vienna, Austria).
Results
The parent study population comprised 99 children supported on ECMO at the two participating centers; of these, 9 participants had no samples obtained on ECMO day 1, and 2 participants did not have complete biomarker data on ECMO day 1. These 11 subjects were excluded from this current analysis. Table 1 presents the clinical characteristics of the children included for analysis (n = 88). In summary, 40 (46%) were neonates and 46 (52%) were male. About one third had a primary respiratory indication for ECMO. Fifty children (57%) had no cardiac arrest within 7 days pre-ECMO initiation, 21 (24%) had non-ECPR cardiac arrest within 7 days pre-ECMO, and 17 (19%) underwent ECPR. Forty-nine patients (56%) had unfavorable outcome, including 39 (44%) who died prior to hospital discharge.
Table 1.
Clinical characteristics and distribution of plasma samples among 88 children on ECMO supporta.
| Variable | All (n = 88) |
|---|---|
| Patient and ECMO Characteristics | |
| Age group | |
| Neonate, <1 mo | 40 (46) |
| Infant, 1 mo-<1 y | 24 (27) |
| Child, 1 y-<12 y | 17 (19) |
| Adolescent, ≥12 y | 7 (8) |
| Male | 46 (52) |
| Weight, kg | 4.0 [3.0, 11.7] |
| Primary indication for ECMO | |
| Respiratory failure | 28 (32) |
| Non-respiratory (cardiac failure, sepsis) | 60 (68) |
| Cardiac arrest within 7 days pre-ECMO | |
| No cardiac arrest | 50 (57) |
| Non-ECPR cardiac arrest | 21 (24) |
| Total duration of CPR, minutes | 8 [3,15] |
| ECPR | 17 (19) |
| Total duration of CPR, minutes | 49 [31, 60] |
| Duration of ECMO support, days | 4.9 [ 3.3, 10.4] |
| Outcomes | |
| ICU length of stay, days | 28 [13, 54] |
| Hospital length of stay, days | 49 [23, 81] |
| Abnormal neuroimaging (n = 80)b | 38 (48) |
| Unfavorable outcomec | 49 (56) |
| In-hospital mortality | 39 (44) |
| Number of plasma samples in the first 3 ECMO days | |
| 1 | 5 (6) |
| 2 | 7 (8) |
| 3 | 52 (59) |
| ≥4 | 24 (27) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; ECPR, extracorporeal cardiopulmonary resuscitation; CPR, cardiopulmonary resuscitation.
Continuous variables are presented as medians [25th to 75th percentile] and categorical variables as counts (frequencies).
Neuroimaging during or within 6 weeks post-ECMO decannulation: daily head ultrasound (HUS) during and/or post-ECMO, 33 (38%); daily HUS during ECMO, brain computed tomography (CT) during or post-ECMO, and/or brain magnetic resonance imaging (MRI) post-ECMO, 29 (33%); at least one brain CT during or post-ECMO and/or brain MRI post-ECMO, without HUS, 16 (18%); no neuroimaging, 10 (11%).
Unfavorable outcome was defined as in-hospital mortality or discharge Pediatric Cerebral Performance Category (PCPC) > 2 with decline of at least one point from baseline PCPC among survivors to hospital discharge.
Assay performance
The estimated lower limits of detection for NfL, pT181, tau, and UCHL1 were 3.6 pg/mL, 2.5 pg/mL, 1.8 pg/mL, and 570 pg/mL, respectively and the estimated upper limits of quantification were 100 ng/mL, 0.5 ng/mL, 25 ng/mL, and 2,500 ng/mL, respectively. Supplemental Tables 1 and 2 summarize the availability of measured biomarkers and censored values on ECMO day 1 and for peak measurements, respectively. The number of measurements below the lower limit of detection was zero for all biomarkers except for UCHL1, which had 49% of samples below the lower limit of detection. No measurements were above the upper limit of quantification for NfL and UCHL1, while pT181 and tau had 2% and 5% of values above the upper limit of quantification, respectively.
ECMO day 1 and longitudinal plasma brain injury biomarker levels stratified by pre-ECMO cardiac arrest status
Cumulative incidence functions displaying distribution of ECMO day 1 biomarker levels by pre-ECMO cardiac arrest status are presented in Fig. 1. Tau concentrations were significantly higher on ECMO day 1 in children who underwent ECPR (geometric mean 2,395 pg/mL) compared to those with no cardiac arrest (460 pg/mL, p < 0.001) or with non-ECPR cardiac arrest within 7 days pre-ECMO (577 pg/mL, p = 0.003). NfL and pT181 concentrations were higher on ECMO day 1 in children who underwent ECPR vs those with no cardiac arrest or with non-ECPR cardiac arrest within 7 days pre-ECMO, however differences were not significant. Lastly, there were no between patient significant differences in ECMO day 1 levels of UCHL1 by pre-ECMO cardiac arrest status. The same patterns were noted for peak biomarker levels across pre-ECMO cardiac arrest strata (Supplemental Figure 1).
Fig. 1.
Cumulative incidence functions displaying distribution of ECMO day 1 biomarker levels, stratified by pre-ECMO cardiac arrest status. Solid lines present step functions of the data, while parametric cumulative incidence functions are depicted as dashed smoothed curves. Dashed vertical lines correspond to limits of detection (upper limit of quantification for pT181 and tau, and lower limit of detection for UCHL1). Gray, no cardiac arrest within 7 days pre-ECMO; orange, cardiac arrest within 7 days pre-ECMO (not ECPR); blue, ECPR.
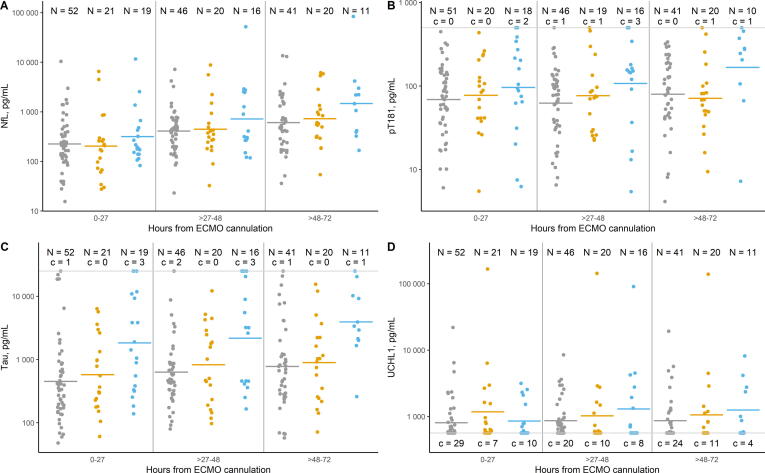
Comparisons of geometric means stratified by ECMO day and by pre-ECMO cardiac arrest status are presented in Table 2 and Fig. 2. There were no significant differences between pre-ECMO cardiac arrest strata in mean daily NfL levels on ECMO days 1 and 2, but on ECMO day 3, mean NfL levels were 2.87 times higher (95% CI, 1.17–7.02) and 2.41 times higher (95% CI, 0.90–6.42) in children who underwent ECPR vs those who had no cardiac arrest or who had non-ECPR cardiac arrest within 7 days pre-ECMO. Similarly, on ECMO day 3, mean pT181 levels were 2.23 times higher (95% CI, 1.03–4.82) and 2.44 times higher (95% CI, 1.05–5.65) in the ECPR group compared to no cardiac arrest and non-ECPR cardiac arrest within 7 days pre-ECMO groups. Mean tau levels were significantly higher in the ECPR group compared to both the no pre-ECMO cardiac arrest and the non-ECPR cardiac arrest groups on each of the first three ECMO days: day 1, 5.20 times higher (95% CI, 2.18–12.42) and 4.15 times higher (95% CI, 1.52–11.38), respectively; day 2, 3.79 times higher (95% CI, 1.58–9.05) and 2.97 times higher (95% CI, 1.09–8.09), respectively; and day 3, 5.29 times higher (95% CI, 1.85–15.15) and 4.65 times higher (95% CI, 1.47–14.73), respectively. No significant differences by pre-ECMO cardiac arrest status were detected for UCHL1 on any of the first three days of ECMO support.
Table 2.
Geometric means for plasma brain injury biomarker concentrations on ECMO days 1 to 3, stratified by pre-ECMO cardiac arrest status (n = 88)a.
| Biomarkerb |
ECMO Day |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
1 |
2 |
3 |
|||||||
| No cardiac arrest | Cardiac arrest pre-ECMO | ECPR | No cardiac arrest | Cardiac arrest pre-ECMO | ECPR | No cardiac arrest | Cardiac arrest pre-ECMO | ECPR | |
| NfL | 225 (157, 316) |
204 (121, 343) |
341 (191, 632) |
411 (289, 583) |
447 (268, 736) |
718 (406, 1286) |
608 (387, 897) |
726 (426, 1288) |
1747 (831, 3739) |
| pT181 | 70 (52, 95) |
77 (49, 124) |
109 (68, 184) |
65 (47, 87) |
78 (49, 129) |
118 (69, 205) |
80 (58, 112) |
73 (47, 116) |
177 (90, 357) |
| Tau | 460 (293, 696) |
577 (292, 1182) |
2395 (1110, 5057) |
648 (429, 991) |
826 (435, 1594) |
2456 (1142, 4903) |
784 (485, 1261) |
893 (458, 1804) |
4153 (1649, 10766) |
| UCHL1 | 1288 (817, 2012) |
1692 (959, 2853) |
1358 (653, 2646) |
1207 (754, 1848) |
1859 (929, 3667) |
2997 (1373, 6369) |
1577 (923, 2623) |
2273 (1192, 4436) |
2406 (987, 5909) |
Abbreviations: ECMO, extracorporeal membrane oxygenation; GM, geometric mean; NfL, neurofilament light; pT181, phosphorylated tau 181; UCHL1, ubiquitin C-terminal hydrolase L1.
Cardiac arrest status is classified as: no cardiac arrest within 7 days pre-ECMO, cardiac arrest within 7 days pre-ECMO (not ECPR), and ECPR.
All plasma biomarker concentrations are presented in pg/mL, with geometric means (95% confidence intervals).
Fig. 2.
Distribution of plasma brain injury biomarker concentrations on ECMO days 1 to 3, stratified by pre-ECMO cardiac arrest status. Solid horizontal lines indicate geometric mean biomarker concentrations. Gray, no cardiac arrest within 7 days pre-ECMO; orange, cardiac arrest within 7 days pre-ECMO (not ECPR); blue, ECPR. c denotes censored values above the upper limit of quantification (for pT181 and tau) or below the lower limit of detection (for UCHL1).
Association of plasma brain injury biomarker levels and outcomes
Results from unadjusted and adjusted Cox proportional hazards models of time to unfavorable outcome for NfL, pT181, and tau are presented in Supplemental Table 3 and Fig. 3. We did not model UCHL1 due to the high percentage of censored observations. In a Cox proportional hazards model estimating the effect of 3 times higher ECMO day 1 biomarker levels (between-patient comparison) and a 3-fold increase in biomarker levels from ECMO day 1 in serially drawn samples (within-patient comparison), higher levels of NfL or pT181 were not significantly associated with increased hazard of unfavorable outcome. Three times higher ECMO day 1 tau levels were significantly associated with increased hazard of unfavorable outcome in unadjusted models and after adjusting for age group, sex, and pre-ECMO cardiac arrest status (tau: unadjusted HR, 1.35, 95% CI 1.09–1.66; adjusted HR: 1.42; 95% CI 1.13–1.79). A 3-fold increase in tau levels from ECMO day 1 in serially drawn samples (within-patient comparison) was not associated with unfavorable outcome after adjusting for baseline (ECMO day 1) tau levels, age group, sex, and pre-ECMO cardiac arrest status.
Fig. 3.
Risk of time to in-hospital death or unfavorable neurological outcome associated with a 3 times higher biomarker level on ECMO day 1 (●) and a 3-fold increase from day 1 longitudinally (▲), both expressed as hazard ratios from a multivariate Cox proportional hazards model. Model in Panel A reports the association with outcomes for ECMO day 1 biomarker levels, with adjustment for age category (neonate, infant, child and adolescent), sex and pre-ECMO cardiac arrest status (ECPR, non-ECPR cardiac arrest, and no cardiac arrest within 7 days pre-ECMO). Model in Panel B reports the associations with outcomes for longitudinal biomarker level changes and for ECMO day 1 biomarker levels, with additional adjustment for age category, sex and pre-ECMO cardiac arrest status.
The unadjusted model to estimate unfavorable outcome from tau values on ECMO day 1 achieved an AUROC of 0.77 (95%CI: 0.67–0.86). The AUROC curve is presented in Supplemental Figure 2 with the Youden index point being attained for the cutoff for tau concentration on ECMO day 1 of 415 pg/mL, at a sensitivity of 71% and specificity of 79%. AUROC for pT181 (0.55) and NfL (0.58) were nondiscriminatory for unfavorable outcome.
Discussion
In this prospective observational study of critically ill children receiving ECMO support, tau levels were significantly higher on ECMO days 1–3 in children who underwent ECPR compared to children who did not experience cardiac arrest pre-ECMO, or those with non-ECPR cardiac arrest preceding ECMO cannulation. Additionally, three times higher ECMO day 1 tau levels were associated with increased hazard of in-hospital death or unfavorable neurofunctional outcome among survivors after adjusting for potential confounders including pre-ECMO cardiac arrest status.
These results are consistent with previous studies of neonatal encephalopathy and cardiac arrest evaluating tau. Tau is a microtubule-associated protein that functions to stabilize cellular structure in axons.23 In a multicenter trial of adults after cardiac arrest, 24-, 48- and 72-hour serum tau levels improved prediction of poor outcome compared to clinical information alone.24 Tau levels were associated with poor neurofunctional outcome at 6 months and, in long term follow-up, up to 956 days, were significantly associated with survival.24 In newborns with hypoxic-ischemic encephalopathy, elevated tau levels were associated with death or severe brain injury on MRI, and higher baseline tau levels were associated with worse 1-year outcomes.25, 26 In a multicenter study of biomarker concentrations following pediatric in-hospital or out-of-hospital cardiac arrest, tau levels measured 1–3 days post-arrest were associated with death or poor neurobehavioral outcome at one year in multivariable analyses.20
While we also evaluated other biomarkers that previously have been shown to be associated with outcomes following cardiac arrest in adults as well as children, namely NfL, pT181 and UCHL1, we found no association between these biomarker levels on ECMO days 1–3 and outcome at hospital discharge. NfL levels measured in 717 adults at 24, 48, and 72 hours after out-of-hospital cardiac arrest were predictive of long term poor neurologic outcome.27, 28 In the same population, higher 24-hour pT181 levels were associated with poor 6-month neurofunctional outcome. This association was not present for 48- or 72-hour levels, implying that pT181 may be more useful early in injury timeframe.29 In a single center prospective study of 32 pediatric in hospital cardiac arrest survivors, NfL levels were significantly higher in nonsurvivors vs survivors and higher in survivors compared with healthy controls. Similar to our findings, NfL levels were not associated with unfavorable vs favorable outcomes, where unfavorable outcome had the same definition as in this study.30 In a multicenter study of biomarker concentrations following pediatric cardiac arrest, NfL levels measured 1–3 days following cardiac arrest were significantly associated with death or poor neurologic outcome in multivariable analyses.20 Our analysis found that NfL and pT181 levels trended higher in the ECPR patients on days 1 and 2 and were significantly higher on day 3 compared to the no cardiac arrest and non-ECPR cardiac arrest within 7 days pre-ECMO groups. However, in this cohort, pT181 and NfL levels were not associated with unfavorable outcome.
UCHL1 is a neuronal protein with minimal release by other cells. Higher plasma UCHL1 was associated with worse cognitive outcomes after neonatal hypoxic encephalopathy25, 31 and worse neurofunctional outcomes at 6 months after pediatric in-hospital or out-of-hospital cardiac arrest. 20, 32 UCHL1 in combination with GFAP highly predicted poor outcomes in a study of 717 adult patients after cardiac arrest.33 In a post hoc analysis of 249 adults who suffered out of hospital cardiac arrest, UCHL1 had significantly higher levels in patients with poor vs good neurofunctional outcomes at one year, however its predictive value was only moderate.34 While UCHL1 has been associated with degree of brain injury and outcomes in other studies, values for UCHL1 were outside of detectable limits in our patient samples and this biomarker was deferred from further analysis. Further studies in children on ECMO should be undertaken with UCHL1 assays of higher sensitivity. Prior research in other patient populations with cardiac arrest or traumatic brain injury conducted using assays with a lower value for the lower limit of detection (e.g., 160 pg/mL,34 30 pg/mL,35 or 5.45 pg/mL20) had positive findings, and thus it is possible that the negative findings in our study are in fact false negative and driven by the performance characteristics of the assay used.
This study has several limitations. While PCPC is as a measure of neurofunctional outcome recommended for cardiac arrest reporting guidelines,5 it can miss subtle but meaningful deficits. Hospital discharge as an endpoint provides limited information about longer term outcomes, which were collected10 but would have severely limited the sample size. We used banked plasma, and findings will need to be validated in contemporary patient cohorts, which the authors are actively acquiring. Also, we were careful in selecting only aliquots that had not been previously thawed and all samples were maintained continuously in temperature-controlled ultra low temperature freezers, with electronic tracking and no freeze–thaw cycles.
Conclusions
Brain-specific biomarker tau levels were significantly higher on ECMO day 1 and across the first 3 days of ECMO in children who underwent ECPR compared with children with no cardiac arrest within 7 days pre-ECMO, and significantly associated with increased hazard of death or unfavorable neurologic outcome in unadjusted and adjusted models. Brain-specific biomarkers, particularly tau, may aid in detection of neurologic injury and neuroprognostication in patients on ECMO with and without preceding cardiac arrest.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (R21HD096389, MMB) and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health (K23NS076674, MMB).
CRediT authorship contribution statement
Jamie McElrath Schwartz: Writing – original draft, Investigation. Derek K. Ng: Writing – review & editing, Visualization, Project administration, Methodology, Formal analysis, Data curation, Conceptualization. Jennifer Roem: Writing – review & editing, Visualization, Methodology, Formal analysis, Data curation. Nikhil Padmanabhan: Writing – review & editing, Methodology. Daniel Romero: Writing – review & editing, Methodology. Jessica Joe: Writing – review & editing, Methodology. Christopher Campbell: Writing – review & editing, Methodology. George B. Sigal: Writing – review & editing, Methodology. Jacob N. Wohlstadter: Writing – review & editing, Methodology. Allen D. Everett: Writing – review & editing, Conceptualization. Melania M. Bembea: Writing – review & editing, Supervision, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the contributing centers as well as the patients described herein and the medical staff who made their complex medical care possible.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resplu.2024.100609.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Holmberg M.J., Ross C.E., Fitzmaurice G.M., et al. Annual incidence of adult and pediatric in-hospital cardiac arrest in the united states. Circ Cardiovasc Qual Outcomes. 2019;12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg M.J., Wiberg S., Ross C.E., et al. Trends in survival after pediatric in-hospital cardiac arrest in the united states. Circulation. 2019;140:1398–1408. doi: 10.1161/CIRCULATIONAHA.119.041667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Extracorporeal Life Support Organization (ECLS registry report. https://www.elso.org/registry/internationalsummaryandreports/internationalsummary.aspx. Updated 202Accessed October 27, 2023.
- 4.Conrad S.A., Broman L.M., Taccone F.S., et al. The extracorporeal life support organization maastricht treaty for nomenclature in extracorporeal life support. A position paper of the extracorporeal life support organization. Am J Respir Crit Care Med. 2018 doi: 10.1164/rccm.201710-2130CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nolan J.P., Berg R.A., Andersen L.W., et al. Cardiac arrest and cardiopulmonary resuscitation outcome reports: Update of the utstein resuscitation registry template for in-hospital cardiac arrest: a consensus report from a task force of the international liaison committee on resuscitation (american heart association, european resuscitation council, australind new zealand council on resuscitation, heart and stroke foundation of canada, InterAmerican heart foundation, resuscitation council of southern africa, resuscitation council of asia) Circulation. 2019;140:e746–e757. doi: 10.1161/CIR.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 6.Dante S.A., Carroll M.K., Ng D.K., et al. Extracorporeal membrane oxygenation outcomes in children with preexisting neurologic disorders or neurofunctional disability. Pediatr Crit Care Med. 2022;23:881–892. doi: 10.1097/PCC.0000000000003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polito A., Barrett C.S., Wypij D., et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. an analysis of ELSO registry data. Intensive Care Med. 2013;39:1594–1601. doi: 10.1007/s00134-013-2985-x. [DOI] [PubMed] [Google Scholar]
- 8.Polito A., Barrett C.S., Rycus P.T., Favia I., Cogo P.E., Thiagarajan R.R. Neurologic injury in neonates with congenital heart disease during extracorporeal membrane oxygenation: An analysis of extracorporeal life support organization registry data. ASAIO J. 2015;61:43–48. doi: 10.1097/MAT.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 9.Barrett C.S., Bratton S.L., Salvin J.W., Laussen P.C., Rycus P.T., Thiagarajan R.R. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10:445–451. doi: 10.1097/PCC.0b013e318198bd85. [DOI] [PubMed] [Google Scholar]
- 10.Bembea M.M., Felling R.J., Caprarola S.D., et al. Neurologic outcomes in a two-center cohort of neonatal and pediatric patients supported on extracorporeal membrane oxygenation. ASAIO J. 2020;66:79–88. doi: 10.1097/MAT.0000000000000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg R.A., Nadkarni V.M., Clark A.E., et al. Incidence and outcomes of cardiopulmonary resuscitation in PICUs. Crit Care Med. 2016;44:798–808. doi: 10.1097/CCM.0000000000001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slomine B.S., Silverstein F.S., Christensen J.R., et al. Neurobehavioural outcomes in children after in-hospital cardiac arrest. Resuscitation. 2018;124:80–89. doi: 10.1016/j.resuscitation.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meert K., Slomine B.S., Silverstein F.S., et al. One-year cognitive and neurologic outcomes in survivors of paediatric extracorporeal cardiopulmonary resuscitation. Resuscitation. 2019;139:299–307. doi: 10.1016/j.resuscitation.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slovis J.C., Bach A., Beaulieu F., Zuckerberg G., Topjian A., Kirschen M.P. Neuromonitoring after pediatric cardiac arrest: cerebral physiology and injury stratification. Neurocrit Care. 2023 doi: 10.1007/s12028-023-01685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felling R.J., Kamerkar A., Friedman M.L., et al. Neuromonitoring during ECMO support in children. Neurocrit Care. 2023 doi: 10.1007/s12028-023-01675-8. [DOI] [PubMed] [Google Scholar]
- 16.Said A.S., Guilliams K.P., Bembea M.M. Neurological monitoring and complications of pediatric extracorporeal membrane oxygenation support. Pediatr Neurol. 2020;108:31–39. doi: 10.1016/j.pediatrneurol.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bembea M.M., Felling R., Anton B., Salorio C.F., Johnston M.V. Neuromonitoring during extracorporeal membrane oxygenation: a systematic review of the literature. Pediatr Crit Care Med. 2015;16:558–564. doi: 10.1097/PCC.0000000000000415. [DOI] [PubMed] [Google Scholar]
- 18.Bembea M.M., Rizkalla N., Freedy J., et al. Plasma biomarkers of brain injury as diagnostic tools and outcome predictors after extracorporeal membrane oxygenation. Crit Care Med. 2015;43:2202–2211. doi: 10.1097/CCM.0000000000001145. [DOI] [PubMed] [Google Scholar]
- 19.Fink E.L., Berger R.P., Clark R.S., et al. Serum biomarkers of brain injury to classify outcome after pediatric cardiac arrest*. Crit Care Med. 2014;42:664–674. doi: 10.1097/01.ccm.0000435668.53188.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink E.L., Kochanek P.M., Panigrahy A., et al. Association of blood-based brain injury biomarker concentrations with outcomes after pediatric cardiac arrest. JAMA Netw Open. 2022;5:e2230518. doi: 10.1001/jamanetworkopen.2022.30518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiser D.H. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 22.Fiser D.H., Long N., Roberson P.K., Hefley G., Zolten K., Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28:2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 23.Guo T., Noble W., Hanger D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704. doi: 10.1007/s00401-017-1707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattsson N., Zetterberg H., Nielsen N., et al. Serum tau and neurological outcome in cardiac arrest. Ann Neurol. 2017;82:665–675. doi: 10.1002/ana.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massaro A.N., Wu Y.W., Bammler T.K., et al. Plasma biomarkers of brain injury in neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2018;194:67–75.e1. doi: 10.1016/j.jpeds.2017.10.060. [DOI] [PubMed] [Google Scholar]
- 26.Li R., Lee J.K., Govindan R.B., et al. Plasma biomarkers of evolving encephalopathy and brain injury in neonates with hypoxic-ischemic encephalopathy. J Pediatr. 2023;252:146–153.e2. doi: 10.1016/j.jpeds.2022.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moseby-Knappe M., Mattsson N., Nielsen N., et al. Serum neurofilament light chain for prognosis of outcome after cardiac arrest. JAMA Neurol. 2019;76:64–71. doi: 10.1001/jamaneurol.2018.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H., Bang H.J., You Y., et al. Novel serum biomarkers for predicting neurological outcomes in postcardiac arrest patients treated with targeted temperature management. Crit Care. 2023;27:113–121. doi: 10.1186/s13054-023-04400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashton N.J., Moseby-Knappe M., Benedet A.L., et al. Alzheimer disease blood biomarkers in patients with out-of-hospital cardiac arrest. JAMA Neurol. 2023;80:388–396. doi: 10.1001/jamaneurol.2023.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirschen M.P., Yehya N., Graham K., et al. Circulating neurofilament light chain is associated with survival after pediatric cardiac arrest. Pediatr Crit Care Med. 2020;21:656–661. doi: 10.1097/PCC.0000000000002294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Douglas-Escobar M.V., Heaton S.C., Bennett J., et al. UCH-L1 and GFAP serum levels in neonates with hypoxic-ischemic encephalopathy: a single center pilot study. Front Neurol. 2014;5:273. doi: 10.3389/fneur.2014.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fink E.L., Berger R.P., Clark R.S., et al. Exploratory study of serum ubiquitin carboxyl-terminal esterase L1 and glial fibrillary acidic protein for outcome prognostication after pediatric cardiac arrest. Resuscitation. 2016;101:65–70. doi: 10.1016/j.resuscitation.2016.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebner F., Moseby-Knappe M., Mattsson-Carlgren N., et al. Serum GFAP and UCH-L1 for the prediction of neurological outcome in comatose cardiac arrest patients. Resuscitation. 2020;154:61–68. doi: 10.1016/j.resuscitation.2020.05.016. [DOI] [PubMed] [Google Scholar]
- 34.Wihersaari L., Reinikainen M., Tiainen M., et al. Ubiquitin C-terminal hydrolase L1 after out-of-hospital cardiac arrest. Acta Anaesthesiol Scand. 2023;67:964–971. doi: 10.1111/aas.14257. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Arrastia R., Wang K.K.W., Papa L., et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma. 2014;31:19–25. doi: 10.1089/neu.2013.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.