Abstract
Dendritic cell (DC)-based vaccines are recognized as a promising immunotherapeutic strategy against cancer; however, the efficacy of immunotherapy with DCs is controlled via immune checkpoints, such as programmed death-ligand 1 (PD-L1). PD-L1 expressed on DC and tumor cells binds to programmed death-1 (PD-1) receptors on the activated T cells, which leads to the inhibition of cytotoxic T cells. Blocking of PD-L1 on DC may lead to improve the efficacy of DC therapy for cancer. Here we demonstrated that DC vaccination in combination with pomalidomide and programmed death-ligand 1 (PD-L1) blockade inhibited tumor growth of a multiple myeloma (MM) mouse model. DCs + pomalidomide with dexamethasone + PD-L1 blockade significantly inhibited immune immunosuppressive factors and promoted proportions of immune effector cells in the spleen and tumor microenvironment. Additionally, functional activities of cytotoxic T lymphocytes and NK cells in spleen were enhanced by DCs + pomalidomide with dexamethasone + PD-L1 blockade. Taken together, this study identifies a potential new therapeutic approach for the treatment of MM. These results also provide a foundation for the future development of immunotherapeutic modalities to inhibit tumor growth and restore immune function in MM.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02654-0) contains supplementary material, which is available to authorized users.
Keywords: Myeloma, Dendritic cells, Pomalidomide, Anti-PD-L1, Combination therapy, Cancer immunotherapy
Introduction
Multiple myeloma (MM) is a malignancy of plasma cells marked by abnormal proliferation, expansion, and production of immunoglobulin (antibody) [1]. The multiple-step development of MM involves increasing abnormal stages during MM progression and immunosuppression in the bone marrow (BM) milieu that lead to MM growth [2].
Although novel agents have been developed which include immunomodulatory drugs (IMiDs) as well as protease inhibitory agents which have shown some effect on disease progression in MM patients, most patients eventually relapse which is caused by drug resistance [3–5].
Dendritic cell (DC)-based vaccines present a promising immunotherapeutic strategy against cancer [1, 6–11]. DCs are the most effective antigen-presenting cells expressing both major histocompatibility (MHC) and costimulatory molecules, particularly in priming CD8+ T cell-mediated immune responses [1, 6, 12, 13]. Therefore, DC-based systems are expected to serve as an immunotherapeutic tool for treating patients with MM. Our previous preclinical studies showed that vaccination with tumor antigen-loaded DCs elicited potent antigen-specific anti-myeloma responses [6, 14–16]. However, the clinical efficacy of the current DC vaccination is not satisfactory [17, 18]. The effect of DC immunotherapies can be disturbed by immune-suppressive functions, such as programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1) molecules, and regulatory T cells (Tregs) [19]. Therefore, combined therapies may improve the efficacy of DC-based immunotherapy and break down the tumor microenvironment. Recently, strategies to inhibit these immune-suppressive functions have become a major focus of cancer immunotherapy [20].
Pomalidomide is a distinct oral IMiD with direct anti-myeloma effects under immunosuppressive microenvironments. It stimulates effector cytotoxic T lymphocytes (CTLs), inhibits Tregs, and alters a broad range of cytokines [21–23]. Importantly, pomalidomide plus low-dose dexamethasone is commonly used for salvage treatment of patients with relapsed or refractory MM [24]. Our previous study demonstrated that the combination of pomalidomide and low-dose dexamethasone synergistically enhanced the effects of DC vaccination in a murine MM model [6]. Pomalidomide, possessing many potentially synergistic properties, could enhance the efficacy of PD-L1 blockade. Recent studies have defined immune checkpoint receptor PD-1/PD-L1 signaling as a key pathway regulating the critical balance between immune activation and tolerance [25–27]. Binding of PD-1 on effector cells to PD-L1 or PD-L2 on non-hematopoietic cells triggers inhibitory signaling in effector cells, leading to induction and maintenance of tolerance [28]. Accordingly, clinical progression is observed in patients whose myeloma cells express high levels of PD-L1 [29]. Anti-PD-L1 antibody therapy displays very modest activity for MM, and combination approaches seem to be crucial for successful PD-1/PD-L1 blockade in MM when a single agent of PD-1 or PD-L1 blockades has been unsatisfactory [30]. The logical synergistic partners of checkpoint inhibitors appear to be IMiDs, which effect the co-stimulation of T and NK cells, reduction of Tregs, and myeloid-derived suppressor cells (MDSCs), and direct downregulation of the PD-1/PD-L1 axis [30–33]. Moreover, our previous preclinical study demonstrated that lenalidomide and PD-1 blockade synergistically enhance the effects of DC vaccination in a murine MM model [14].
In the present study, we investigated whether a DC-based vaccine combined with pomalidomide and PD-L1 blockade has a synergistic effect in a murine MM model. The study was designed to closely mimic the clinical MM treatment protocol. To this end, we used flow cytometry, enzyme-linked immunosorbent (ELISA) assay, and interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) to determine antitumor immune responses in myeloma-bearing mice. We clearly demonstrated that combination treatment with DCs + pomalidomide with dexamethasone + PD-L1 blockade exerts potent anti-myeloma immunity by suppressing immunosuppressive cells, inhibitory cytokines and angiogenic factors, as well as activating and recovering effector cells with superior polarization towards the Th1 immune response in the spleen and tumor microenvironment of treated mice. Results from this study provide evidence that DC vaccination combined with pomalidomide and PD-L1 blockade synergistically enhances antitumor immunity in myeloma-bearing mice. In addition, this study provides a foundation for the future development of new treatments to inhibit tumor growth and restore immune function in MM.
Materials and methods
Ethics declarations
All animal care, experiments, and euthanasia were performed in accordance with protocols approved by the Chonnam National University Animal Research Committee.
Mice and tumor cell lines
Six- to eight-week-old female BALB/c (H-2d) mice were purchased from Orient Bio (Iksan, Republic of Korea) and maintained under specific pathogen-free conditions. The MOPC-315 murine plasmacytoma cell line (induced with mineral oil in a BALB/c mouse) and YAC-1 cell line were purchased from the American Type Culture Collection (Rockville, MD, USA). The cell lines were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco-BRL) and 1% (w/v) penicillin/streptomycin (PS).
Pomalidomide and PD-L1 blockade
Pomalidomide was donated by Celgene Corporation (Summit, NJ, USA) and was dissolved in dimethylsulfoxide (DMSO) to 1 mg/mL immediately before use. For injection into mice, pomalidomide stock solutions were diluted in sterile 0.9% (v/v) normal saline. The final concentration of DMSO in all experiments was < 0.01% (v/v). The PD-L1 blockade agent (Clone 10F.9G2) was purchased from BioXcell (West Lebanon, NH, USA). For injection into mice, PD-L1 blockade stock solutions were diluted in sterile 0.9% (v/v) normal saline.
Generation of BM-derived DCs
BALB/c BM-derived immature DCs (imDCs) were generated as described previously [6, 14, 15, 34]. Briefly, BM was harvested from the femurs and tibiae of mice and cultured in RPMI-1640 medium (Gibco-BRL) supplemented with 10% (v/v) FBS (Gibco-BRL) and 1% (w/v) PS in the presence of 20 ng/mL recombinant murine (rm) granulocyte–macrophage-colony stimulating factor (R&D Systems, Minneapolis, MN, USA) and 10 ng/mL recombinant mouse interleukin (IL)-4 (rmIL-4; R&D Systems). On culture days 2–4, half of the medium was removed and replaced with fresh medium containing cytokines. On day 6, imDCs were purified by positive selection with CD11c+-magnetic beads (Miltenyi Biotec Inc., Auburn, CA, USA). Mature DCs (mDCs) were generated by further cultivation, for 48 h, of CD11c+ DCs with 10 ng/mL rm tumor necrosis factor-alpha (rmTNF-α, R&D Systems), 10 ng/mL rmIL-1β (R&D Systems), and 10 ng/mL rmGM-CSF (R&D Systems).
Generation of dying myeloma cell-loaded DCs
The generation of dying myeloma cell-loaded DCs was performed as described previously [6, 14, 15, 34]. Briefly, dying MOPC-315 tumor cells were induced by gamma irradiation (100 Gy, Gammacell-1000 Elite; MDS Nordion, Ottawa, Ontario, Canada) followed by overnight culture in FBS-free RPMI-1640. The cells were mixed with imDCs 2 h after maturation in a 2:1 ratio (DCs: dying tumor cells).
Animal treatment
The following four treatment groups (five mice per group) were established: (1) PBS control, (2) DC vaccination + pomalidomide with dexamethasone, (3) pomalidomide with dexamethasone + PD-L1 blockade, and (4) DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade. On day 0, mice were injected subcutaneously with 5 × 105 MOPC-315 cells in a volume of 0.1 mL into the right flank. After tumor growth, pomalidomide (0.06 mg/kg/day) was orally administered once per day for 18 days with a 3-day break after the first 8-day dosing period, and dexamethasone (0.6 mg/kg/day) was injected intravenously in a volume of 0.1 mL on days 7, 11, 18 and 21. The PD-L1 blockade agent (200 µg/mouse) was injected intraperitoneally in a 0.1-mL volume on days 9, 13, 19, and 23. Each dose of DCs (1 × 106 per mouse) was injected subcutaneously into the left flanks of BALB/c mice in a volume of 0.1 mL PBS on days 8, 12, 18, and 22. To assess the tumor therapeutic effects, we established a mouse model for monitoring tumor growth inhibition and survival prolongation. The length, width and height of each tumor were measured every 3–4 days using a Vernier caliper, and the tumor volume was calculated using the standard formula for the volume of an ellipsoid: V = 4/3π(length × width × height/8), the mice were euthanized when the tumor reached 2000 mm3, which was considered equivalent to death because of the size of the tumor.
Phenotypic analysis of splenocytes and tumors from the treated mice
To assess the immune responses in treated mice, we established a mouse model for performing immune responses. At the indicated time points after treatment (days 31–34), two or three mice were killed at each time point, their spleens and tumors were collected, and single-cell suspensions were prepared to characterize the expression of cell markers using fluorescently labeled monoclonal antibodies (mAbs) in a flow cytometry assay. The cells were stained with the following mAbs (all from eBioscience, San Diego, CA, USA): CD11b-FITC, CD11b-PE, Gr-1-PE, CD4-APC, CD4-PE, CD8-FITC, CD49b-PE, CD44-PE, CD62L-FITC, CD69-FITC, CD25-FITC, Foxp3-APC, CD86-FITC, F4/80-PE, CD206-APC, CD45-Pacific Blue, PD-1-FITC, PD-L1-PE and CTLA4-PE. Isotype-matched controls were run in parallel. Cell debris was eliminated by forward- and side-scatter gating. The samples were acquired on a BD FACS CantoII (Becton Dickinson, Mountain View, CA, USA) and the data were analyzed using Flow Jo software (TreeStar, San Carlos, CA, USA).
Tumor antigen-specific CTL activity of the treated mice
Tumor antigen-specific CTL activity was investigated as described previously [6, 14, 15, 34]. Briefly, at the indicated time points after treatment (days 31–34), two or three mice were killed at each time point and splenocytes (1 × 106) were added to 24-well plates and restimulated with irradiated MOPC-315 cells (5 × 105 cells) for 5 days in RPMI-1640 (Gibco-BRL) containing 10% FBS (Gibco-BRL) and 1% PS supplemented with 20 ng/mL rmIL-2 (R&D Systems). After restimulation, the splenocytes were assessed for tumor antigen-specific CTLs using a mouse interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay (BD Bioscience, Franklin Lakes, NJ, USA). The MOPC-315 cell line and NK-sensitive YAC-1 cell line were used as target cells, and the spots were enumerated using an ImmunoSpot Image Analyzer system (Cellular Technology Ltd., Shaker Heights, Cleveland, OH, USA) for automated plate scanning, imaging, and spot counting.
In vitro analysis of cytokine production from spleen and tumor of treated mice
Using the BD OptEIA™ enzyme-linked immunosorbent (ELISA) assay (BD Bioscience) we determined cytokine [IFN-γ, IL-10, transforming growth factor-beta (TGF-β) and vascular endothelial growth factor (VEGF)] production from spleens and tumors of treated mice at days 31–34 post-treatment, two or three mice were killed at each time point. Supernatants from cultures of restimulated splenocytes, and from tumors of all treated mice, were assayed to measure the production of Th1- and Th2-polarizing cytokines. Each sample was analyzed in triplicate, and the mean absorbance for each set of standards and samples was calculated.
Intracellular staining assay of Tregs and macrophages generated in the spleens and tumors of treated mice
To evaluate the proportion of Tregs and macrophages, mice were killed at the indicated time points after treatment (days 31–34), two or three mice were killed at each time point, their spleens and tumors were collected, and single-cell suspensions were prepared to characterize the expression of cell markers using fluorescently labeled mAbs. First, 1 × 106 splenocytes and tumor cells from treated mice were harvested, washed, and stained with surface-staining antibodies for Tregs (CD45-Pacific Blue, CD4-PE, and CD25-FITC) and M2 macrophages (CD45-Pacific Blue, F4/80-PE, and CD11b-FITC or CD86-FITC) for 30 min at 4 °C. An Fc blocker was added before incubation with surface antibodies. The cells were washed and permeabilized with FACSTM Permeabilizing Solution 2 (BD Bioscience) for 30 min at room temperature. After washing twice, the cells were stained with an intracellular staining antibody for Tregs (AlexaFluor-conjugated Foxp3 antibody; Miltenyi Biotec, Bergisch Gladbach, Germany) and M2 macrophages (CD206-APC) for 30 min at room temperature. The samples were acquired on a BD FACS CantoII (Becton Dickinson, Mountain View, CA, USA) and the data were analyzed using Flow Jo software (TreeStar, San Carlos, CA, USA).
Statistical analyses
GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA) was used to analyze tumor growth and survival in treated mice, and to determine the statistical significance of differences between groups using Ordinary One-way ANOVA analysis, and multiple comparisons using Tukey test. P values < 0.05 were considered significant. Means ± standard deviation or standard error of the mean are shown.
Results
Dying myeloma cell-loaded DC vaccination in combination with pomalidomide and PD-L1 blockade induces a synergistic anti-myeloma immunity effect
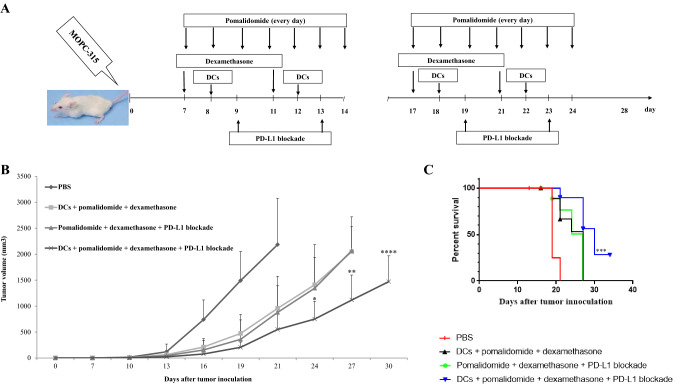
Our previous study demonstrated that murine DCs maturated with GM-CSF, TNF-α and IL-1β expressed higher levels of several molecules related to DC maturation, and produced higher levels of IL-12p70 and lower levels of IL-10, compared to imDCs [35]. We established myeloma-bearing mice to evaluate the antitumor efficacy of DC-based immunotherapy. After tumor growth, the established myeloma-bearing mice were initially treated with (1) PD-L1 blockade alone, (2) pomalidomide plus dexamethasone, and (3) DCs alone (Fig. 2a). Treatment with PD-L1 blockade did not show significant difference in tumor growth compared to the PBS control. In contrast, the tumor-bearing mice treated with DC vaccination or pomalidomide with dexamethasone exhibited significant inhibition of tumor growth compared to the PBS control (Supplemental Figure 1A and 1B; *, P < 0.05 on day 19). Therefore, combination therapy of DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade was applied to more potently inhibit tumor growth in the murine MM model (Figs. 1, 2a). All tumor-bearing mice treated with PBS experienced rapid tumor growth that led to death within 3 weeks. In contrast, tumor-bearing mice treated with DCs + pomalidomide with dexamethasone, pomalidomide with dexamethasone + PD-L1 blockade or DCs + pomalidomide with dexamethasone + PD-L1 blockade exhibited significant inhibition of tumor growth compared to the PBS control group. Treatment with a combination of DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade more strongly inhibited tumor growth compared to pomalidomide with dexamethasone + PD-L1 blockade or DC vaccination + pomalidomide with dexamethasone (Fig. 2b, Supplemental Figure 2A and 2B; *, P < 0.05 on day 24). Survival was prolonged in mice that received a combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade compared to mice that received the pomalidomide with dexamethasone + PD-L1 blockade or DCs + pomalidomide with dexamethasone (Fig. 2c; ***, P < 0.001). These results indicated that DCs + pomalidomide with dexamethasone + PD-L1 blockade induced long-term systemic anti-myeloma effects in the mouse MM model.
Fig. 2.
In vivo animal vaccination. Four treatment groups were established: (1) PBS control, (2) tumor antigen-loaded dendritic cell (DC) vaccination + pomalidomide with dexamethasone, (3) pomalidomide with dexamethasone + programmed death-ligand 1 (PD-L1) blockade, and (4) DCs + pomalidomide with dexamethasone + PD-L1 blockade. a Schematic representation of the combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade. On day 0, mice were injected subcutaneously with 5 × 105 MOPC-315 cells in a volume of 0.1 mL into the right flank. After tumor growth, pomalidomide (0.06 mg/kg/day) was orally administered once a day for 18 days with a 3-day break after the first 8-day dosing period, and dexamethasone (0.6 mg/kg/day) was injected intravenously in a volume of 0.1 mL on days 7, 11, 18, and 21. PD-L1 blockade (200 µg/mouse) was injected intraperitoneally in a 0.1-mL volume on days 9, 13, 19, and 23. Each dose of DCs (1 × 106 per mouse) was injected subcutaneously into the left flank of BALB/c mice in a volume of 0.1 mL PBS on days 8, 12, 18, and 22. b Data are presented as mean ± standard error of the mean (SEM) and are representative of two independent experiments. c The survival of the tumor-bearing mice is shown. The combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade significantly inhibited tumor growth (*, P < 0.05 on day 24) and induced a long-term systemic anti-myeloma immune response (34 days)
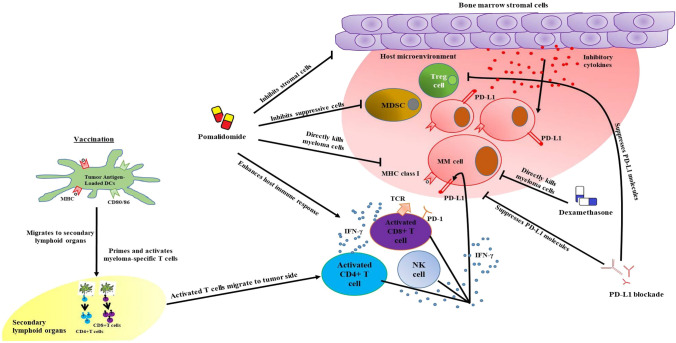
Fig. 1.
Graphical abstract. Pomalidomide with dexamethasone direct anti-myeloma effects under immunosuppressive microenvironments. Pomalidomide stimulates effector cytotoxic T lymphocytes (CTLs), inhibits Tregs, and alters a broad range of cytokines, while programmed death-ligand 1 (PD-L1) blockade suppresses PD-L1 molecules expressed on antigen-presenting cells and myeloma cells which is highly correlated to the infiltration of effector cells into the tumor bed. On the other hand, the host immune effector cells are specifically induced by the dying myeloma-loaded DC vaccine. Therefore, DC vaccination combined with pomalidomide and PD-L1 blockade exerts potent anti-myeloma immunity by suppressing immunosuppressive cells, inhibitory cytokines and angiogenic factors, as well as activating and recovering effector cells with superior polarization towards the Th1 immune response in the spleen and tumor microenvironment of treated mice
Decreased expression of checkpoint molecules in the tumor microenvironment of mice treated with pomalidomide/dexamethasone and PD-L1 blockade
Our previous study [14] revealed high levels of PD-L1 expression on MOPC-315 cell lines. In the present study, at the indicated time points after treatment (days 31–34), two or three mice were killed at each time point, their tumors were collected, and single-cell suspensions were prepared to characterize the expression of PD-1, PD-L1, and CTLA-4 using flow cytometry. We found that there was a significant reduction in the level of PD-1 expression on CD3+ T cells in the tumor microenvironment in all treatment groups compared to the PBS control group (Supplemental Figure 3A and 3B). Treatment with pomalidomide with dexamethasone + PD-L1 blockade led to significantly reduced levels of PD-L1 expression in the tumor microenvironment (Supplemental Figure 3C and 3D) and CTLA4 expression on CD3+ T cells in the tumor microenvironment (Supplemental Figure 3E and 3F) compared to the DCs + pomalidomide with dexamethasone group or the PBS control group. These results demonstrated that pomalidomide with dexamethasone + PD-L1 blockade treatment led to decreased expression of PD-1, PD-L1 and CTLA-4 in the tumor microenvironment of treated mice, which further induced effector cell infiltration of the tumor microenvironment.
Activation of CTLs by DC vaccination combined with pomalidomide and PD-L1 blockade
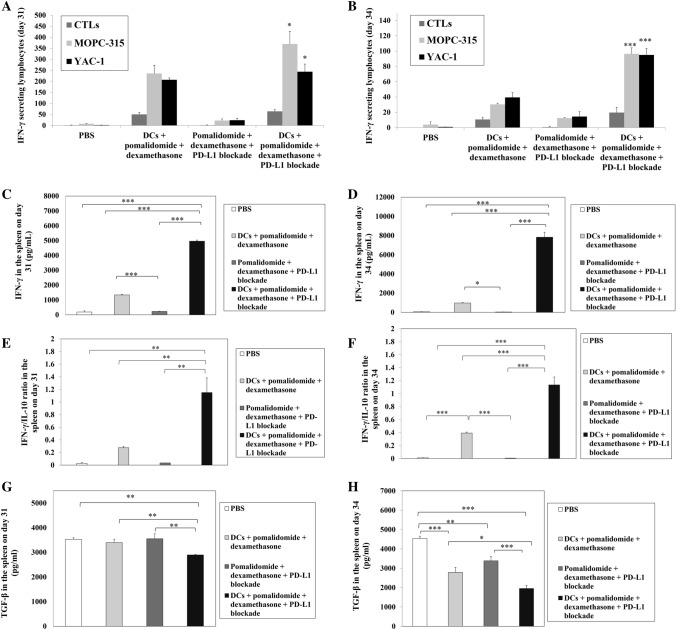
DC vaccination is a strategy for boosting immune responses by increasing the levels of specific CTLs. CTLs are known to produce the Th1 cytokine IFN-γ, which is an important mediator of effector immune responses [36, 37]. To assess whether DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade potentiates the activation of CTL-mediated immunity against myeloma in the in vivo mouse model at 31–34 days after treatment, two or three mice were killed at each time point, and splenocytes from each group of treated mice were prepared for IFN-γ ELISPOT assays. MOPC-315 and YAC-1 cells were used as the target cells. In comparison to the PBS control, pomalidomide with dexamethasone + PD-L1 blockade treatment partially increased the number of IFN-γ-secreting splenocytes against MOPC-315 and YAC-1 cells. The number of IFN-γ-secreting splenocytes against MOPC-315 and YAC-1 cells significantly increased after DCs + pomalidomide with dexamethasone treatment, as well as with combination treatment of DCs + pomalidomide with dexamethasone + PD-L1 blockade, compared to the pomalidomide with dexamethasone + PD-L1 blockade and PBS control groups. Treatment with a combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade achieved the highest levels of IFN-γ-secreting splenocytes against MOPC-315 and YAC-1 cells (Fig. 3a, b; *, P < 0.05; ***, P < 0.001). These results indicated that the tumor-inhibiting effects of DCs + pomalidomide with dexamethasone + PD-L1 blockade resulted from CTL-mediated cytotoxicity response (represented by the number of IFN-γ-secreting splenocytes against MOPC-315 cells) and NK cell-mediated cytotoxicity response (represented by the number of IFN-γ-secreting splenocytes against YAC-1 cells). To investigate the antitumor effect of DCs + pomalidomide with dexamethasone + PD-L1 blockade on Th1 cytokine production, the supernatants from CTL cultures for each group of treated mice were collected for ELISA assays. DCs + pomalidomide with dexamethasone + PD-L1 blockade treatment led to the production of higher levels of IFN-γ compared to the PBS control, DCs + pomalidomide with dexamethasone, and pomalidomide with dexamethasone + PD-L1 blockade groups (Fig. 3c, d; *, P < 0.05; ***, P < 0.001). Moreover, the IFN-γ/IL-10 ratio was significantly higher in the group treated with DCs + pomalidomide with dexamethasone + PD-L1 blockade compared to the other groups (Fig. 3e, f; **, P < 0.01; ***, P < 0.001). In contrast, TGF-β production was lower in the DCs + pomalidomide with dexamethasone + PD-L1 blockade treatment group compared to the PBS control, DCs + pomalidomide with dexamethasone, and pomalidomide with dexamethasone + PD-L1 blockade groups (Fig. 3g, h; *, P < 0.05; **, P < 0.01; ***, P < 0.001). These results suggested that the combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade enhanced Th1 immune responses in addition to CTL responses.
Fig. 3.
Activation of cytotoxic T lymphocytes (CTLs) and cytokine production induced by treatment with DC vaccination combined with pomalidomide/dexamethasone and PD-L1 blockade. The number of interferon (IFN)-γ-secreting lymphocytes in the spleens of mice treated with PBS, DCs + pomalidomide with dexamethasone, pomalidomide with dexamethasone + PD-L1 blockade, and DCs + pomalidomide with dexamethasone + PD-L1 blockade was counted using IFN-γ enzyme-linked immunospot (ELISPOT) assays. Treatment with pomalidomide with dexamethasone + PD-L1 blockade partially increased the number of IFN-γ-secreting splenocytes against MOPC-315 and YAC-1 cells compared to the PBS control, whereas treatment with a combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade increased the number of IFN-γ-secreting lymphocytes targeting MOPC-315 and YAC-1 cells compared to all other groups at days 31 a and 34 b after treatment (*, P < 0.05; (***, P < 0.001). These results indicated that the tumor-inhibiting effects of DCs + pomalidomide with dexamethasone + PD-L1 blockade resulted from the CTL-mediated cytotoxicity response (represented by the number of IFN-γ-secreting splenocytes against MOPC-315 cells) and natural killer (NK) cell-mediated cytotoxicity response (represented by the number of IFN-γ-secreting splenocytes against YAC-1 cells). The supernatants from CTL cultures from each group of treated mice were collected for enzyme-linked immunosorbent (ELISA) assays. The combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade led to the production of higher levels of IFN-γ compared to the PBS control, DCs + pomalidomide with dexamethasone, and pomalidomide with dexamethasone + PD-L1 blockade groups at days 31 c and 34 d after treatment (*, P < 0.05; ***, P < 0.001). Moreover, the IFN-γ/interleukin (IL)-10 ratio was significantly higher in the group treated with DCs + pomalidomide with dexamethasone + PD-L1 blockade compared to the other groups (e, f; **, P < 0.05; ***, P < 0.001). In contrast, transforming growth factor-beta (TGF-β) production under DCs + pomalidomide with dexamethasone + PD-L1 blockade treatment was lower compared to all other groups at days 31 g and 34 h after treatment (*, P < 0.05; **, P < 0.01; ***, P < 0.001). These results suggested that the combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade enhanced Th1 immune responses in addition to CTL responses. Data are shown as mean (pg/mL) ± standard deviation (SD) of triplicate cultures from three independent experiments
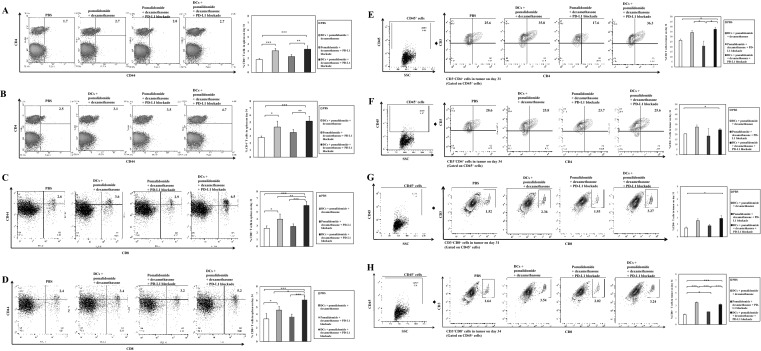
Efficient induction of infiltrating immune effector cells in the spleen and tumor microenvironment of mice treated with DC vaccination plus pomalidomide and PD-L1 blockade
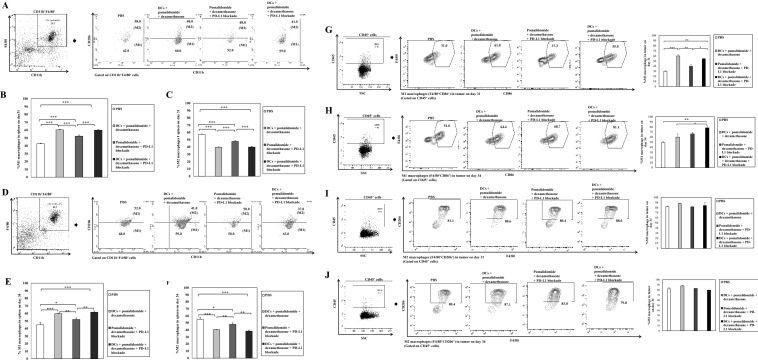
To explore the immunological mechanisms underlying the enhanced tumor-specific immune response described above, we evaluated the effects of combination therapy on infiltrating effector cells in the spleen and tumor microenvironment at days 31–34 post-treatment, two or three mice were killed at each time point. The percentage of splenic M1 macrophages was significantly decreased in the PBS control, while the DC vaccination groups exhibited significantly higher proportions of splenic M1 macrophages compared to the PBS control and the pomalidomide with dexamethasone + PD-L1 blockade group (Fig. 4a, b, 4d, e; **, P < 0.01; ***, P < 0.001).Vaccination with DCs + pomalidomide with dexamethasone + PD-L1 blockade yielded significantly increased percentages of splenic effector CD4+ T cells (Fig. 5a, b), effector CD8+ T cells (Fig. 5c, d), effector memory T cells (Supplemental Figure 4A and 4B) and effector NK cells (Supplemental Figure 4C and 4D) compared to the other groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Moreover, the DC vaccination groups exhibited significantly higher proportions of tumor-infiltrating immune cells, including CD4+ T cells (Fig. 5e, f), CD8+ T cells (Fig. 5g, h) and M1 macrophages (Fig. 4g, h) compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade group. Importantly, at day 34 after treatment, combination treatment with DCs + pomalidomide with dexamethasone + PD-L1 blockade produced a significant increase in the proportion of tumor-infiltrating M1 macrophages (Fig. 4h) compared to the other groups. These results suggested that the combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade stimulated robust infiltrating immune effector cells in the spleen and tumor microenvironment of treated mice.
Fig. 4.
Enhanced M1 and impaired M2 macrophage polarization in the spleen and tumor microenvironment of mice treated with DC vaccination plus pomalidomide with dexamethasone and PD-L1 blockade. Using flow cytometry, we measured the proportions of M1 and M2 macrophages in the spleen and tumor microenvironment of mice at days 31–34 after treatment. DC vaccination groups displayed a significantly increased proportion of splenic M1 macrophages (a, b, d, e) and decreased proportion of splenic M2 macrophages (a, c, d, f) compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Moreover, DC vaccination groups exhibited significant increases in the proportion of tumor-infiltrating M1 macrophages compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade groups (g, h). Importantly, at day 34 after treatment, treatment with DCs + pomalidomide with dexamethasone + PD-L1 blockade produced a significant increase in the proportion of tumor-infiltrating M1 macrophages compared to all other groups (h). There was no significant difference in the proportion of M2 macrophages in the tumor microenvironment among groups (I, j). These results suggested that the combination of DCs + pomalidomide with dexamethasone + PD-L1 blockade enhanced M1 and impaired M2 macrophage polarization in the spleen and tumor microenvironment of treated mice. The data are representative of three independent experiments
Fig. 5.
Induction of infiltrating immune effector cells in the spleen and tumor microenvironment of mice treated with DC vaccination plus pomalidomide with dexamethasone and PD-L1 blockade. Using flow cytometry, we measured the proportions of infiltrating effector cells in the spleen and tumor microenvironment at days 31–34 after treatment. The combination of DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade resulted in significantly increased percentages of splenic effector CD4+ T cells (a, b) and effector CD8+ T cells (c, d) compared to the other groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Moreover, the DC vaccination groups exhibited significantly increased proportions of tumor-infiltrating CD4+ T cells (e, f) and CD8+ T cells (g, h) compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade groups. These results suggested that the combination of DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade stimulated robust infiltration of immune effector cells in the spleen and tumor microenvironment of treated mice. Data are representative of three independent experiments
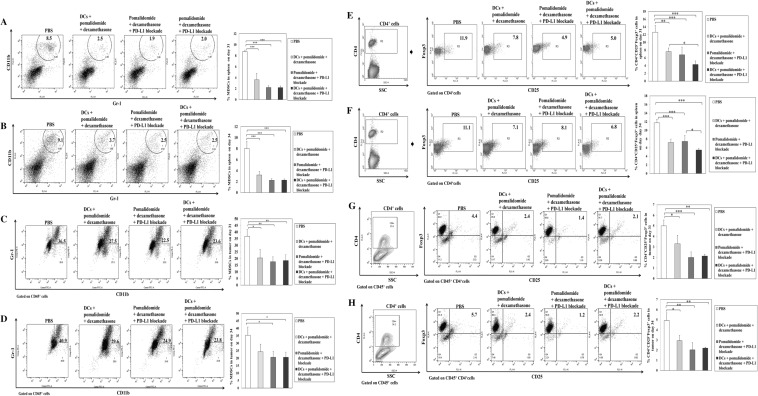
We next investigated the effects of combination therapy on the proportions of MDSCs, M2 macrophages and Tregs in the spleen and tumor microenvironment of treated mice at days 31–34 after treatment, two or three mice were killed at each time point. In comparison to the treatment groups, the PBS control group had significantly higher percentages of MDSCs and Tregs in the spleen and tumor microenvironment of treated mice. Conversely, pomalidomide with dexamethasone + PD-L1 blockade treatment decreased the generation of MDSCs in the spleen (Fig. 6a, b) and tumor microenvironment (Fig. 6c, d) compared to the PBS control and DCs with pomalidomide + dexamethasone group (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The proportion of splenic Tregs was significantly decreased in the DCs + pomalidomide with dexamethasone + PD-L1 blockade treatment group compared to the other groups (Fig. 6e, f; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Moreover, treatment with pomalidomide with dexamethasone + PD-L1 blockade led to a significantly decreased proportion of Tregs in the tumor microenvironment compared to the PBS control and DCs + pomalidomide with dexamethasone group (Fig. 6g, h; *, P < 0.05; **, P < 0.01; ***, P < 0.001). DC vaccination groups exhibited significantly decreased proportions of splenic M2 macrophages compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade group (Fig. 4a, c, 4d, f; *, P < 0.05; **, P < 0.01; ***, P < 0.001). However, there were no significant differences among groups in the percentage of M2 macrophages in the tumor microenvironment (Fig. 4i, j). These findings suggested that DCs + pomalidomide with dexamethasone + PD-L1 blockade enhanced therapeutic anti-myeloma immunity by inhibiting immunosuppressive cells in the spleen and tumor microenvironment of treated mice during the treatment phases.
Fig. 6.
Inhibition of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in the spleen and tumor microenvironment of mice treated with DCs plus pomalidomide with dexamethasone and PD-L1 blockade. Using flow cytometry, we measured the proportions of MDSCs (CD11b+Gr-1+) and CD4+CD25+Foxp3+ Tregs in the spleen and tumor microenvironment of mice at days 31–34 after treatment. The proportions of MDSCs and Tregs were significantly increased in the PBS control and DCs + pomalidomide with dexamethasone groups compared to the groups treated with pomalidomide/dexamethasone + PD-L1 blockade. Pomalidomide with dexamethasone + PD-L1 blockade treatment decreased the generation of MDSCs in the spleen (a, b) and tumor microenvironment (c, d) compared to the PBS control and DCs + pomalidomide with dexamethasone groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (e, f) The proportion of splenic Tregs was significantly decreased in the DCs + pomalidomide with dexamethasone + PD-L1 blockade treatment group compared to all other groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). (g, h) The groups treated with pomalidomide/dexamethasone + PD-L1 blockade exhibited significantly lower proportions of Tregs in the tumor microenvironment compared to the PBS control and DCs + pomalidomide with dexamethasone groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). These findings suggested that DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade enhanced therapeutic anti-myeloma immunity by inhibiting immunosuppressive cells in the spleen and tumor microenvironment of treated mice during the vaccination phases. Data are representative of at least three experiments
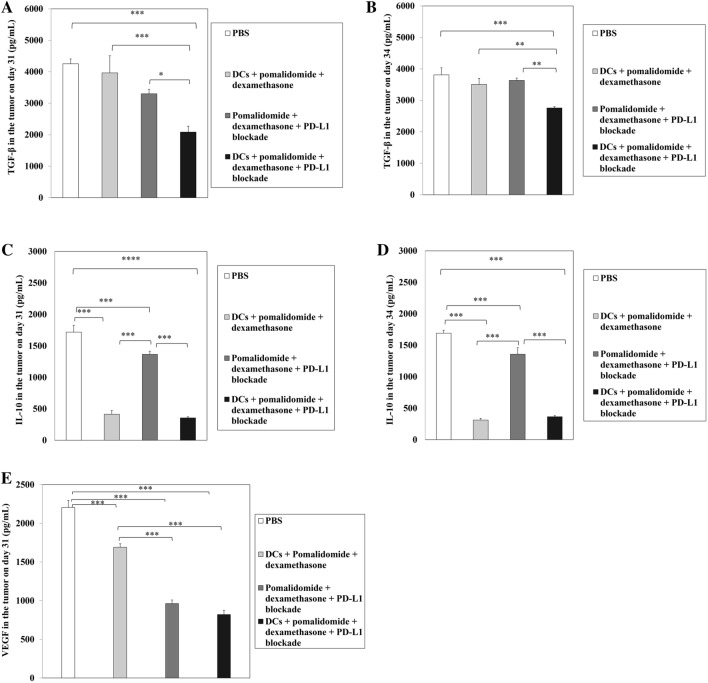
Efficient suppression of angiogenesis and inhibitory cytokines production by DC vaccination plus pomalidomide and PD-L1 blockade in the tumor microenvironment of myeloma-bearing mice
To determine the impact of combination therapy with DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade on the production of angiogenesis and inhibitory cytokines in the tumor microenvironment of myeloma-bearing mice at days 31–34 after treatment, two or three mice were killed at each time point, tumors from each group of treated mice were collected and single-cell suspensions were prepared for ELISA assays. Compared to the treatment groups, the PBS control displayed the highest levels of VEGF, TGF-β, and IL-10. In contrast, combination treatment with DCs + pomalidomide with dexamethasone + PD-L1 significantly decreased the production of TGF-β compared to the other groups (Fig. 7a, b; *, P < 0.05; **, P < 0.01; ***, P < 0.001). Groups treated with DCs had significantly decreased production of IL-10 compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade group (Fig. 7c, d; ***, P < 0.01). Moreover, in comparison to the PBS control and DCs + pomalidomide with dexamethasone groups, treatment with pomalidomide with dexamethasone + PD-L1 blockade significantly decreased VEGF production in the tumor microenvironment (Fig. 7e; ***, P < 0.01). These results suggested that combination therapy with DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade suppressed angiogenesis and inhibitory cytokines by inhibiting TGF-β, IL-10 and VEGF production in the tumor microenvironment of myeloma-bearing mice.
Fig. 7.
Reduced angiogenesis and inhibitory cytokine production by DC vaccination plus pomalidomide with dexamethasone and PD-L1 blockade in the tumor microenvironment of myeloma-bearing mice. Using flow ELISA, we measured the production of TGF-β, IL-10, and vascular endothelial growth factor (VEGF) in the tumor microenvironment of treated mice at days 31–34 after treatment. Compared to all treatment groups, the PBS control group had higher levels of TGF-β, IL-10, and VEGF production (***, P < 0.001). In contrast, the DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade group exhibited significantly decreased production of TGF-β (a, b) compared to the other groups (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Conversely, the groups treated with DCs had significantly decreased production of IL-10 (c, d) compared to the PBS control and pomalidomide with dexamethasone + PD-L1 blockade groups (***, P < 0.001). In comparison to the PBS control and DCs + pomalidomide with dexamethasone groups, treatment with pomalidomide/dexamethasone + PD-L1 blockade significantly decreased VEGF production (e) in the tumor microenvironment of treated mice (***, P < 0.001). These results suggested that combination therapy with DC vaccination + pomalidomide/dexamethasone + PD-L1 blockade suppressed angiogenesis and inhibitory cytokines by inhibiting TGF-β, IL-10 and VEGF production in the tumor microenvironment of myeloma-bearing mice. Data are representative of at least three experiments
Discussion
MM is known to be associated with an inflammatory microenvironment, leading to a state of effector cell exhaustion, and thereby allowing tumor escape from immune surveillance and enhancing tumor growth [38]. Therefore, targeting the tumor microenvironment may be a promising therapeutic option for the future development of anticancer treatments [39]. In previous studies, we established several strategies to enhance anti-myeloma immunity in murine tumor models using DC vaccination combined with immunomodulating drugs, such as lenalidomide [14, 40] and pomalidomide [6]. All of these studies focused on modulating the MM microenvironment to augment the efficacy of DC vaccination through the recovery and induction of effector cells, while suppressing immune-suppressive cells.
Many studies have shown that PD-1 and its ligands, PD-L1 and PD-L2, play key roles in regulating the critical balance of immune activation, tolerance, and autoimmunity [41]. Binding of PD-1 on effector cells to PD-L1 or PD-L2 on non-hematopoietic cells triggers inhibitory signaling in effector cells, inducing apoptosis and exhaustion of activated immune cells, thus leading to induction and maintenance of tolerance [42]. Specifically, PD-L1 is highly expressed on tumor cells and infiltrating immune cells in the tumor microenvironment, resulting in inhibition of effector cell activation and contributing to tumor development and growth [43, 44]. PD-L1 is reported to be expressed at high levels in plasma cells from MM patients, but not in normal plasma cells [45]. Our previous study also confirmed that PD-L1 is overexpressed on MOPC-315 cell lines (99%) [14]. Inhibition of the PD-L1 signaling pathway by the addition of anti-PD-L1-blocking antibodies induces an anti-MM immune response and represents a promising approach for anti-myeloma therapy, but there is still limited information on long-term outcomes [34].
In this study, we investigated whether the combination of DCs plus pomalidomide and PD-L1 blockade has a synergistic therapeutic effect on a MM mouse model. Our expectation was that the efficacy of DC vaccine will be more effective when combined with pomalidomide and PD-L1 blockade. Pomalidomide and PD-L1 blockade suppresses very well immunosuppressive factors while the host immune effector cells specifically induced by the DC vaccine. As expected, this study showed that DCs + pomalidomide with dexamethasone + PD-L1 blockade strongly inhibited tumor growth, and prolonged the survival of vaccinated mice. This combination strongly increased proportions of effector cells while effectively reduced the proportions of suppressor cells in the systemic immune compartment. Furthermore, treatment with pomalidomide with dexamethasone + PD-L1 blockade effectively reduced levels of PD-L1 and CTLA-4 expression in treated mice, which is highly correlated to the infiltration of effector cells into the tumor bed. Moreover, in this study, we chose two end points, days 31–34, to look at the kinetics of in vivo immune responses in treated mice over time. Our data showed an enduring increase in immune responses in the treatment with DCs + pomalidomide with dexamethasone + PD-L1 blockade compared to other groups.
Similarly, in our previous study, we observed that combination therapy of tumor antigen-loaded DC vaccination plus lenalidomide and PD-1 blockade synergistically established potent anti-myeloma immunity in a murine model through the superior polarization of Th1/Th2 balance in favor of the tumor immune response [14]. In this study, DCs + pomalidomide with dexamethasone + PD-L1 blockade induced activation of cell-mediated immunity by increasing the production of IFN-γ and decreasing the production of TGF-β, IL-10, and VEGF in the spleen and tumor microenvironment.
Mice models of MM provide a critical tool for studying myeloma disease progression, resistance, and pathogenesis, and enable the development of new therapeutic approaches [6, 14, 46, 47]. However, this study was limited in that it used only a subcutaneous model based on the MOPC-315 cell line. To overcome this limitation, our group will develop an intravenous myeloma mouse model to mimic human MM, which occurs in BM. This study demonstrated that pomalidomide plus dexamethasone and PD-L1 blockade was more powerful in enhancing the capability of DC vaccination to target specific lymphoid organs, inducing the activity of effector cells targeting MM.
Immunotherapy is emerging as a promising treatment for various cancers, and investigators have focused on developing new tools to elicit myeloma-specific immune responses using daratumumab, targeting CD38, and elotuzumab, targeting signaling lymphocyte activation molecule F7 (SLAMF7), that have shown clinical activity immunotherapy or combination therapy with other agents in clinical studies. In addition, immune checkpoint inhibitors, IMiDs, cellular immunotherapy using DC vaccination and adoptive immunotherapy with CAR T cells or T cell receptor (TCR)-engineered T cells are emerging as promising treatment strategies for MM [20, 48–50]. However, not all patients are responsive to current immunotherapies, and among those patients who do respond, the effects are not always long-lasting. Thus, combination approaches are a cornerstone of cancer therapy for improving the clinical benefit to patient outcomes.
Conclusion
Our study demonstrated that the combination of DC vaccination + pomalidomide with dexamethasone + PD-L1 blockade is potent in MM treatment by restoring and enhancing myeloma immune responses, as well as reducing the generation of immune suppressor cells. The framework developed in this study provides a foundation for the future development of immunotherapeutic modalities to inhibit tumor growth and restore immune function in MM.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Je-jung Lee has received honoraria for speaking at the 17th international myeloma workshop september 2019 [51], and the 62nd American Society of Hematology Annual Meeting and Exposition 2019 [52].
Abbreviations
- BM
Bone marrow
- CTLA4
Cytotoxic T-lymphocyte-associated protein 4
- CTLs
Cytotoxic T lymphocytes
- DC
Dendritic cell
- DMEM
Dulbecco’s modified Eagle’s medium
- DMSO
Dimethylsulfoxide
- ELISA
Enzyme-linked immunosorbent
- ELISPOT
Interferon (IFN)-γ enzyme-linked immunospot
- FBS
Fetal bovine serum
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- imDC
Immature DC
- IMiDs
Immunomodulatory drugs
- MM
Multiple myeloma
- MOPC
Mineral-oil-induced plasmacytomas
- MHC
Major histocompatibility
- MDSCs
Myeloid-derived suppressor cells
- mAbs
Monoclonal antibodies
- NK
Natural killer
- PD-1
Programmed death-1
- PD-L1
Programmed death-ligand 1
- PC
Plasma cell
- PBS
Phosphate-buffered saline
- PIs
Proteasome inhibitors
- PS
Penicillin/streptomycin
- rm
Recombinant murine
- rmIL
Recombinant mouse interleukin
- TNF-α
Tumor necrosis factor-alpha
- Th1
T helper type 1
- Tregs
Regulatory T cells
- TGF-β
Transforming growth factor-beta
- VEGF
Vascular endothelial growth factor
Author contributions
MCV, THC, SHJ, and JJL designed the study. MCV, THC, HSP, and TJL performed the research and analyzed the data. MCV and JJL wrote the article. JJL, SHJ, and HJK contributed intellectually to the research.
Funding
This research was supported by grants (2018R1A5A2024181, NRF-2018R1C1B5041536, NRF-2020R1A2C2010098) from the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (MEST), Republic of Korea.
Data availability
The data that support the findings of this study are openly available in 17th International Myeloma Workshop September 2019 at https://doi.org/10.1016/j.clml.2019.09.272 [51], and the 62nd American Society of Hematology Annual Meeting & Exposition 2019 at https://doi.org/10.1182/blood-2019-127964 [52].
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tan-Huy Chu and Manh-Cuong Vo contributed equally to this work.
Contributor Information
Sung-Hoon Jung, Email: shglory@hanmail.net.
Je-Jung Lee, Email: drjejung@chonnam.ac.kr.
References
- 1.Jung SH, Lee HJ, Vo MC, Kim HJ, Lee JJ. Immunotherapy for the treatment of multiple myeloma. Crit Rev Oncol Hematol. 2017;111:87–93. doi: 10.1016/j.critrevonc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Görgün GT, Whitehill G, Anderson JL, Hideshima T, Maguire C, Laubach J, et al. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood. 2013;121(15):2975–2987. doi: 10.1182/blood-2012-08-448548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kane RC, Farrell AT, Sridhara R, Pazdur R. United States food and drug administration approval summary: bortezomib for the treatment of progressive multiple myeloma after one prior therapy. Clin Cancer Res. 2006;12(10):2955–2960. doi: 10.1158/1078-0432.CCR-06-0170. [DOI] [PubMed] [Google Scholar]
- 4.Kumar SK, Gertz MA. Risk adapted therapy for multiple myeloma: back to basics. Leuk Lymphoma. 2014;55(10):2219–2220. doi: 10.3109/10428194.2014.905775. [DOI] [PubMed] [Google Scholar]
- 5.Rosenblatt J, Avigan D. Cellular immunotherapy for multiple myeloma. Best Pract Res Clin Haematol. 2008;21(3):559–577. doi: 10.1016/j.beha.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 6.Vo MC, Yang S, Jung SH, Chu TH, Lee HJ, Lakshmi TJ, et al. Synergistic antimyeloma activity of dendritic cells and pomalidomide in a murine myeloma model. Front Immunol. 2018;9:1798. doi: 10.3389/fimmu.2018.01798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vo MC, Lakshmi TJ, Jung SH, Cho D, Park HS, Chu TH, Lee HJ, Kim HJ, Kim SK, Lee JJ. Cellular immunotherapy in multiple myeloma. Korean J Intern Med. 2019;34(5):954–965. doi: 10.3904/kjim.2018.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong CY, Lee HJ, Choi NR, Jung SH, Vo MC, Hoang MD, Kim HJ, Lee JJ. Sarcoplasmic reticulum Ca(2+) ATPase 2 (SERCA2) reduces the migratory capacity of CCL21-treated monocyte-derived dendritic cells. Exp Mol Med. 2016;48(8):e253. doi: 10.1038/emm.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim YS, P HJ, Park JH, Hong EJ, Jang GY, Jung ID, Han HD, Lee SH, Vo MC, et al. A novel function of API5 (apoptosis inhibitor 5), TLR4-dependent activation of antigen presenting cells. Oncoimmunology. 2018;7(10):e1472187. doi: 10.1080/2162402X.2018.1472187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park HJ, Jand GY, Kim YS, Park JH, Lee SE, Vo MC, Lee JJ, et al. A novel TLR4 binding protein, 40S ribosomal protein S3, has potential utility as an adjuvant in a dendritic cell-based vaccine. J Immunother Cancer. 2019;7(1):60. doi: 10.1186/s40425-019-0539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo MC, Nguyen-Pham TN, Lee HJ, Jung SH, Choi NR, Hoang MD, Kim HJ, Lee JJ. Chaetocin enhances dendritic cell function via the induction of heat shock protein and cancer testis antigens in myeloma cells. Oncotarget. 2017;8(28):46047–46056. doi: 10.18632/oncotarget.17517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi NR, Lee HJ, Jung SH, Hong CY, Vo MC, Hoang MD, Kim HJ, Lee JJ. Generation of potent dendritic cells with improved migration ability through p-cofilin and sarco/endoplasmic reticulum Ca(2+) transport ATPase 2 regulation. Cytotherapy. 2015;17(10):1421–1433. doi: 10.1016/j.jcyt.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Jung SH, Lee YK, Lee HJ, Choi NR, Vo MC, Hoang MD, Lim MS, Nguyen-Pham TN, Kim HJ, Lee JJ. Dendritic cells loaded with myeloma cells pretreated with a combination of JSI-124 and bortezomib generate potent myeloma-specific cytotoxic T lymphocytes in vitro. Exp Hematol. 2014;42(4):274–281. doi: 10.1016/j.exphem.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Vo MC, Jung SH, Chu TH, Lee HJ, Lakshmi TJ, Park HS, et al. Lenalidomide and programmed death-1 blockade synergistically enhances the effects of dendritic cell vaccination in a model of murine myeloma. Front Immunol. 2018;9:1370. doi: 10.3389/fimmu.2018.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vo MC, Nguyen-Pham TN, Lee HJ, Jaya Lakshmi T, Yang S, Jung SH, et al. Combination therapy with dendritic cells and lenalidomide is an effective approach to enhance antitumor immunity in a mouse colon cancer model. Oncotarget. 2017;18(16):27252–27262. doi: 10.18632/oncotarget.15917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen-Pham TN, Jung SH, Vo MC, Thanh-Tran HT, Lee YK, Lee HJ, Choi NR, Hoang MD, Kim HJ, Lee JJ. Lenalidomide synergistically enhances the effect of dendritic cell vaccination in a model of murine multiple myeloma. J Immunother. 2015;38(8):330–3399. doi: 10.1097/CJI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen-Pham TN, Lee YK, Kim HJ, Lee JJ. Immunotherapy using dendritic cells against multiple myeloma: how to improve? Clin Dev Immunol. 2012;2012:397648. doi: 10.1155/2012/397648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoang MD, Jung SH, Lee HJ, Lee YK, Nguyen-Pham TN, Choi NR, et al. Dendritic cell-based cancer immunotherapy against multiple myeloma: from bench to clinic. Chonnam Med J. 2015;51(1):1–7. doi: 10.4068/cmj.2015.51.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Köhler M, Greil C, Hudecek M, Lonial S, Raje N, Wäsch R, et al. Current developments in immunotherapy in the treatment of multiple myeloma. Cancer. 2018;124(10):2075–2085. doi: 10.1002/cncr.31243. [DOI] [PubMed] [Google Scholar]
- 21.Chanan-Khan AA, Swaika A, Paulus A, Kumar SK, Mikhael JR, Rajkumar SV, et al. Pomalidomide: the new immunomodulatory agent for the treatment of multiple myeloma. Blood Cancer J. 2013;3:e143. doi: 10.1038/bcj.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mark TM, Coleman M, Niesvizky R. Preclinical and clinical results with pomalidomide in the treatment of relapsed/refractory multiple myeloma. Leuk Res. 2014;38(5):517–524. doi: 10.1016/j.leukres.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Richardson PG, Mark TM, Lacy MQ. Pomalidomide: new immunomodulatory agent with potent antiproliferative effects. Crit Rev Oncol Hematol. 2013;88(Suppl 1):S36–44. doi: 10.1016/j.critrevonc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Bristol-myers squibb (NYSE: BMY) (2018) US. Food and drug administration accepts for priority review bristol-myers squibb’s application for empliciti (elotuzumab) plus pomalidomide and low-dose dexamethasone in patients with relapsed or refractory multiple myeloma. Link: https://news.bms.com/press-release/bristolmyers/us-food-and-drug-administration-accepts-priority-review-bristol-myers-squ. Accessed 25 Feb 2020
- 25.Dong H, Strome S, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 26.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 28.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203(4):883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura H, Ishibashi M, Yamashita T, Tanosaki S, Okuyama N, Kondo A, et al. Marrow stromal cells induce B7–H1 expression on myeloma cells, generating aggressive characteristics in multiple myeloma. Leukemia. 2013;27(2):464–472. doi: 10.1038/leu.2012.213. [DOI] [PubMed] [Google Scholar]
- 30.Jelinek T, Hajek R. PD-1/PD-L1 inhibitors in multiple myeloma: the present and the future. Oncoimmunology. 2016;5(12):e1254856. doi: 10.1080/2162402X.2016.1254856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–2294. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, et al. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62(1):39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sedlarikova L, Kubiczkova L, Sevcikova S, Hajek R. Mechanism of immunomodulatory drugs in multiple myeloma. Leuk Res. 2012;36(10):1218–1224. doi: 10.1016/j.leukres.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK. PD-1 and PD-l1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vo MC, Lee HJ, Kim JS, Hoang MD, Choi NR, Rhee JH, et al. Dendritic cell vaccination with a toll-like receptor agonist derived from mycobacteria enhances anti-tumor immunity. Oncotarget. 2015;6(32):33781–33790. doi: 10.18632/oncotarget.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 37.Nikitina EY, Gabrilovich D. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94(6):825–833. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 38.Romano A, Conticello C, Cavalli M, Vetro C, La Fauci A, Parrinello NL, et al. Immunological dysregulation in multiple myeloma microenvironment. Biomed Res Int. 2014;2014:198539. doi: 10.1155/2014/198539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sounni NE, Noel A. Targeting the tumor microenvironment for cancer therapy. Clin Chem. 2013;59(1):85–93. doi: 10.1373/clinchem.2012.185363. [DOI] [PubMed] [Google Scholar]
- 40.Vo MC, Anh-Nguyen Thi T, Lee HJ, Nguyen-Pham TN, Jaya Lakshmi T, Jung SH, et al. Lenalidomide enhances the function of dendritic cells generated from patients with multiple myeloma. Exp Hematol. 2017;46:48–55. doi: 10.1016/j.exphem.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 41.He J, Hu Y, Hu M, Li B. Development of PD-1/PD-L1 pathway in tumor immune microenvironment and treatment for non-small cell lung cancer. Sci Rep. 2015;5:13110. doi: 10.1038/srep13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 2014;20(4):265–271. doi: 10.1097/PPO.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juneja VR, McGuire KA, Manguso RT, LaFleur MW, Collins N, Haining WN, et al. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J Exp Med. 2017;214(4):895–904. doi: 10.1084/jem.20160801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1) Proc Natl Acad Sci USA. 2018;115(43):E10119–E10126. doi: 10.1073/pnas.1802166115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremblay-LeMay R, Rastgoo N, Chang H. Modulating PD-L1 expression in multiple myeloma: an alternative strategy to target the PD-1/PD-L1 pathway. J Hematol Oncol. 2018;11(1):46. doi: 10.1186/s13045-018-0589-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lwin ST, Edwards CM, Silbermann R. Preclinical animal models of multiple myeloma. Bonekey Rep. 2016;5:772. doi: 10.1038/bonekey.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tassone P, Neri P, Burger R, Di Martino MT, Leone E, Amodio N, et al. Mouse models as a translational platform for the development of new therapeutic agents in multiple myeloma. Curr Cancer Drug Targets. 2012;12(7):814–822. doi: 10.2174/156800912802429292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdallah N, Kumar SK. Daratumumab in untreated newly diagnosed multiple myeloma. Ther Adv Hematol. 2019;10:2040620719894871. doi: 10.1177/2040620719894871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fancher KM, Bun EJ. Elotuzumab: the first monoclonal antibody for the treatment of multiple myeloma. J Adv Pract Oncol. 2016;7(5):542–547. doi: 10.6004/jadpro.2016.7.5.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah UA, Mailankody S. CAR T and CAR NK cells in multiple myeloma: expanding the targets. Best Pract Res Clin Haematol. 2020;33(1):101141. doi: 10.1016/j.beha.2020.101141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J-J, Jung S-H, Chu T-H, Vo M-C, Park H-S, Kim H-J. Potent anti-myeloma efficacy of dendritic cell therapy in combination with pomalidomide and programmed death-ligand 1 blockade in a preclinical model of multiple myeloma 17th international myeloma workshop September 2019. Clin Lymp Myeloma Leuk. 2019;19(10):e163. doi: 10.1016/j.clml.2019.09.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee J-J, Chu T-H, Vo M-C, Park H-S, Lakshmi TJ, Ahn S-Y, Song G-Y, Jung S-H, Kim H-J. A combination therapy with dendritic cells, pomalidomide and programmed death-ligand 1 blockade exerts a potent antitumor immunity in a murine model of multiple myeloma. 2019 by the American society of hematology. Blood. 2019 doi: 10.1182/blood-2019-127964. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in 17th International Myeloma Workshop September 2019 at https://doi.org/10.1016/j.clml.2019.09.272 [51], and the 62nd American Society of Hematology Annual Meeting & Exposition 2019 at https://doi.org/10.1182/blood-2019-127964 [52].