Abstract
To accurately, efficiently, and environmentally prepare carrageenan oligosaccharides, we have developed a method that uses H2O2 and TiO2 as catalysts for the photodegradation of κ-carrageenan (KC). The photodegradation of KC was monitored using various amounts of TiO2 and H2O2 and different concentrations of KC via HPLC and it could decrease the average molecular weight of KC into 1.6 kDa within 2 h. Further research under optimal conditions. As a control, the effects of UV, UV/H2O2, UV/TiO2, and H2O2/TiO2 treatments were studied. In contrast, UV/H2O2/TiO2 treatments showed a coordinated effect. The effect of degradation on the structure of KC was investigated by FT-IR, XRD, and there was no obvious remotion of sulfate groups. Furthermore, oral administration of KCO prolonged the healthy lifespan of nematodes induced by ultraviolet stress and significantly regulated oxidative stress. This study suggests that the precise preparation and application of KCO may be beneficial.
Keywords: κ-Carrageenan, Photodegradation, Oligosaccharide, Structural characterization, Antioxidant activities
Highlights
-
•
Photocatalytic degradation yields narrow molecular weight carrageenan.
-
•
The sulfuric acid group of the degradation product remains unchanged.
-
•
The degradation products have strong antioxidant activity.
-
•
The degradation products can improve the healthy lifespan of C. elegans.
1. Introduction
Carrageenan, a polysaccharide found in marine plants of the Rhodophyceae class(Shafie et al., 2022), is a mixture of water-soluble linear sulfated galactoglycans (Black, Blakemore, Colquhoun, & Dewar, 1965). Consists of alternating β-(1–3)-d-galactose (G-unit) and α-(1–4)-d-galactose (D-unit) or α-(1–4)-3,6-dehydration-d-galactose (DA-unit) repeat units. The three most common forms of carrageenan are called κ-, ι-, and λ-carrageenan. It is currently attracting increasing interest due to its various biological functions (Di Rosa, 1972), including but not limited to anti-inflammatory (Ai et al., 2018), anticancer (El-Deeb et al., 2022; Khotimchenko et al., 2020), antioxidant (Ana et al., 2021), antiviral (Cosenza, Navarro, Pujol, Damonte, & Stortz, 2015), antibacterial (Júnior et al., 2021) and anticoagulant (Yermak et al., 2012) properties. Carrageenan finds wide applications in pharmaceutical preparations, cosmetics, and food industries (Prajapati, Maheriya, Jani, & Solanki, 2014). However, the bioavailability and bioactivity of carrageenan are significantly reduced and its application is limited due to its high molecular weight (Mw), water insolubility and poor tissue penetration(Jiang, Secundo, & Mao, 2023).
Carrageenan oligosaccharides are degradation products of carrageenan, which have high potential in biomedical and therapeutic applications due to their low molecular weight, unique physical and chemical properties, and improved biological propertie (Ghanbarzadeh, Golmoradizadeh, & Homaei, 2018). Therefore, it is meaningful to develop an efficient and controllable degradation method to prepare ultra-low molecular weight carrageenan with excellent biological activity so as to promotes the resource conservation and efficient use of carrageenan. Carrageenan is susceptible to various degradation techniques such as acid hydrolysis(Russo Spena et al., 2023), ultrasonic degradation (Tecson, Abad, Ebajo Jr., & Camacho, 2021), oxidative degradation (T. Sun, Tao, Xie, Zhang, & Xu, 2010), and enzymatic degradation (Riyaz et al., 2021), all of which can achieve degradation targets. Chemical hydrolysis is cost-effective and easy to operate. However, the reaction conditions may be harmful to the environment, and can damage the carbohydrate ring structure, which makes it unsuitable for the efficient preparation of carrageenan oligosaccharides with an intact structure (Y. Zhang et al., 2019; Zhao et al., 2018). Physical degradation has the advantages of low cost, easy operation and low environmental pollution. However, oligosaccharides have a low yield, making it difficult to realize the possibility of commercial production (Yu et al., 2002). The operation process of enzymatic degradation of polysaccharides is relatively simple, but the shortcomings of limited enzyme activity, strict reaction conditions and high cost hinder its application in the large-scale production of carrageenan oligosaccharides (Bouanati et al., 2020). Therefore, it is imperative to develop a degradation method that can preserve the intact structure and produce carrageenan oligosaccharides efficiently and economically.
The combination of UV/TiO2/H2O2 technologies has resulted in a new advanced oxidation process that exhibits better biological applications for highly efficient, clean, and more degradable (lower molecular weight) polysaccharides than traditional degradation methods. When irradiated with enough energy, an electron (e−) from the valence band (VB) of SMP transfers to its conduction band (CB), leaving behind a positive hole in the CB (hVB+).The e−-hVB+ pair initiates a number of redox reactions, especially the ones with the surface adsorbed water molecules which finally lead to the formation of -OH on the surface of TiO2 (Fernandes, Gągol, Makoś, Khan, & Boczkaj, 2019). The presence of free radicals promotes bond cleavage and chain cleavage in polysaccharides, which initiates the degradation and depolymerization process. Regarding the photocatalytic degradation of carrageenan, only the study by Song C et al. (Song et al., 2023) used a method that used UV/TiO2 to depolymerize κ-carrageenan, resulting in reducing its average molecular weight to 6 kDa in 4 h. However, most of the current research on carrageenan degradation is limited to acid degradation and enzymatic hydrolysis. Systematic research on the preparation of carrageenan oligosaccharides by photocatalytic degradation remains insufficient.
Therefore, the aim of this study was to develop an efficient, stable and precise degradation of κ-carrageenan by UV/H2O2/TiO2 photocatalytic reaction, and to prepare carrageenan with different molecular weights, compare the carrageenan before and after degradation through a series of chemical composition analysis, structural characterization and antioxidant activity, and reveal the influence of photodegradation technology on the structure of polysaccharides and the relationship between biological activity and structural properties.
2. Materials and methods
2.1. Materials
κ-Carrageenan was obtained from Qingdao Haida Ocean Oligosaccharide Technology Co., Ltd. (Qingdao, China); Titanium dioxide (25 nm) was purchased from Aladdin Reagent Co., Ltd. (Shanghai, China); Chromatographic grade acetonitrile and methanol were purchased from Merck SIGMA Sigma.
2.2. Optimization of UV/H2O2/TiO2 degradation conditions for κ-carrageenan
κ-Carrageenan was prepared with distilled water to form a polysaccharide solution with a concentration of 0.2%–1.2%, heated at 80 °C for 30 min and stirred to dissolve completely, and a homogeneous carrageenan polysaccharide solution was obtained. TiO2 (0.1%-1.0%) was added to the carrageenan solution. The magnetic stirrer was located at the bottom of the reactor. Its uniform TiO2 was able to maintain the suspension throughout the reaction. Immediately afterwards, H2O2 (0.3%-1.5%) was added to the reactor and irradiated with a 300 W UV lamp. The irradiation time (2–6 h) was set. After the reaction, an appropriate amount of manganese dioxide was added and stirred to remove H2O2. After centrifugation at 12,000 rpm for 20 min, the supernatant was freeze-dried separately to obtain KCO, and the degradation effect was monitored by determining the molecular weight.
2.3. Comparison of various influencing factors in photodegradation methods on carrageenan degradation
Prepare 0.8% κ-carrageenan solution and heat and stir at 80 °C to dissolve completely. UV, UV/H2O2, UV/TiO2, H2O2/TiO2 in degradation of κ-carrageenan alone, UV alone, refers to UV illumination for 2 h immediately after carrageenan is dissolved. UV/H2O2 alone refers to the addition of 0.3% H2O2 for UV illumination for 2 h after carrageenan dissolution. UV/TiO2 alone refers to the addition of 0.8% TiO2 for UV illumination for 2 h after carrageenan dissolution. H2O2/TiO2 alone refers to the addition of 0.8% TiO2 and 0.3% H2O2 for 2 h in the dark after dissolving carrageenan. Add an appropriate amount of MnO2 to the degraded polysaccharide solution with H2O2 and stir overnight to remove H2O2. The obtained polysaccharide solution was centrifuged at 12,000 rpm for 20 min to separate TiO2 and MnO2, and freeze-dried in vacuo to obtain differentially degraded carrageenan powder, and the degradation effect was monitored by determining the molecular weight.
2.4. Determination of molecular weight
The molecular weights (Mw) of the samples were analyzed using a high-performance liquid chromatograph (Agilent1100, USA). Waters Ultrahydrogel ColumnTM500 7.8 × 300 mm (Waters UltrahydrogelTM500, USA) was selected as the column, sodium sulfate 0.2 mo1/L was used as the mobile phase, and the elution rate was 0.6 mL/min. All samples were filtered through a 0.22 μm filter membrane for analysis. Detection was performed at 35 °C using a refractive index detector (Agilent1200, USA). 1, 5, 25, 50, 80, 270 and 410 kDa dextran were used as standards.
2.5. Structural characteristics analysis
2.5.1. Chemical composition of degradation products
The reducing sugar content was determined by 3,5-dinitrosalicylic acid (DNS) assay using galactose as a standard(Goncalves, Rodriguez-Jasso, Gomes, Teixeira, & Belo, 2010). Galactose was used as a standard for the determination of total sugar content using the phenol‑sulfuric acid method(Saha & Brewer, 1994). Using D-galacturonic acid as a standard, the content of uronic acid was determined by the m-hydroxy biphenyl method(van den Hoogen et al., 1998). The content of 3,6-anhydro galactose was determined by the resorcinol method(Navarro & Stortz, 2003). Using anhydrous potassium sulfate as a standard, the total sulfate group conten was determined by the barium chloride-gelatin method(Dodgson & Price, 1962).
2.5.2. Monosaccharide composition
The monosaccharide composition of the degraded polysaccharides was analyzed using the HPLC system (Agilent 1260, Santa Clara, California, USA)(J. Zhang, Zhang, Wang, Shi, & Zhang, 2009). Carrageenan before and after degradation was hydrolyzed with 2 M trifluoroacetic acid (TFA) at 110 °C for 4 h. After hydrolysis, 2 mL of anhydrous methanol was added to completely volatilize the trifluoroacetic acid. 1 mL of distilled water was then added and the resulting solution was labeled with 1-phenyl-3-methyl-5-pyrazolone (PMP). The ZORBAX Eclipse XDB-C18 column (250 × 4.6 mm, 5 μm) was then analyzed at 30 °C. The mobile phase consists of 0.02 M phosphate buffer (pH 6.74)-acetonitrile (83:17, v/v) at a flow rate of 1.0 mL/min.
2.5.3. Ultraviolet (UV) and Fourier transform infrared (FT-IR) spectroscopy
UV spectroscopic analysis was performed using a UV spectrophotometer (model UV2550, Shimadzu, Japan). KC and four different KCOs were added to distilled water (1 mg/mL), and the spectrum was recorded in the range of 200–400 nm. Fourier transform infrared spectroscopy (IR Affinity-1 model, Shimadzu, Japan) was used to investigate the changes in functional groups before and after degradation of the polysaccharides. KC and KCOs were dried and pressed into granules using KBr powder (spectral purity). The spectra were recorded in the wavelength range of 4000–400 cm−1 (Relleve, Lopez, Cruz, & Abad, 2022).
2.5.4. Congo red test
Some modifications have been made according to the described method(J. Chen et al., 2021). In order to study the conformational structure of KC and KCO, the Congo red test was carried out. Mix 2 mg/mL of the degradation products with 80 μmol/L Congo red. Then, 1 M NaOH was added to the mixture, and the final NaOH concentration varies from 0 mol/L to 0.5 mol/L. Allow the samples to react at room temperature for 10 min and perform a UV scan (400–800 nm) to determine the maximum absorption wavelength(Semedo, Karmali, & Fonseca, 2015).
2.5.5. X-ray diffraction
Japan-Rigaku-Rigaku SmartLab X-ray diffractometer for X-ray diffraction analysis. Scanning angle of 5° to 90° at a speed of 5°/min for XRD spectra.
2.5.6. Rheological properties
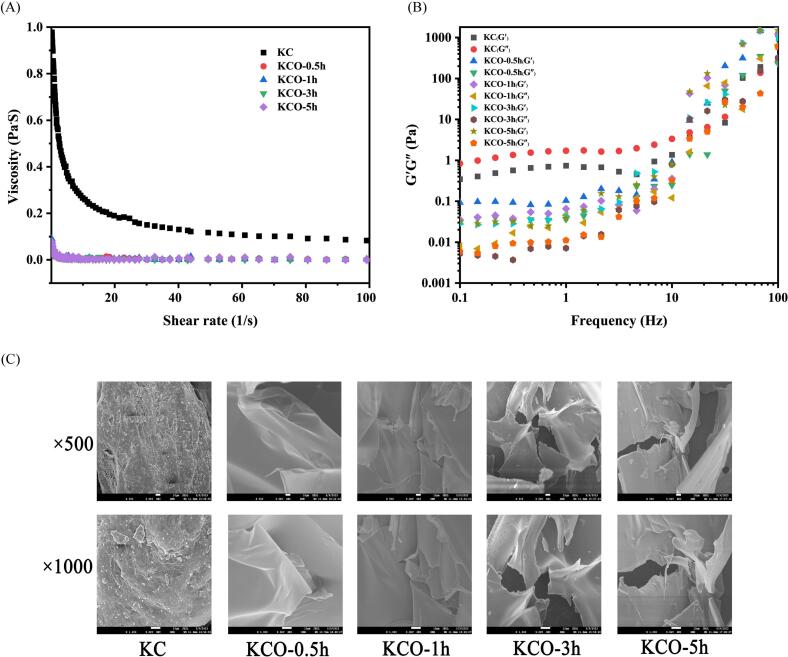
The rheological properties of carrageenan before and after degradation were determined using the HAAKE Mars60 advanced rotational rheometer and the cone plate (diameter 60 mm, gap 1 mm). KC and KCO (8 mg/mL, 1 mL) were added to the plates and equilibrated for 3 min before measurement. Apparent viscosity was measured at shear rate sweeps from 1 to 100 s−1. Evaluate the storage modulus (G′) and loss modulus (G") at 1% strain in the linear viscoelastic region at angular frequency (0.1–100 Hz)(Yang et al., 2020).
2.5.7. Scanning electron microscopy (SEM)
Observation of the microstructure of carrageenan before and after degradation by means of the SEM system. Before testing, a small sample of dry powder was sputtered with a thin gold layer and analyzed at 9.0 kV.
2.5.8. Mass spectrometry analysis
The degradation of polysaccharides was analyzed by HPLC-MSn. The HPLC-MSn experiments were performed using the Thermo Fisher Scientific U3000 high-performance liquid chromatograph. The TSKgel-amide-80 column (4.6 mm × 150 mm, 3 μm) was used for the separation of PMP-labeled sugars. When performed in negative mode, the spray voltage was set to 4.5 kV and the scan range was set to 20 to 2000 m/z. The capillary temperature was 275 °C and the mobile phase acetonitrile-water (40:60, v/v) eluted at a rate of 0.2 mL/min.
2.6. In vitro antioxidant activity
2.6.1. DPPH radical scavenging activity
The DPPH radical scavenging activity was measured by the reference method (J. Li, Chi, Yu, Jiang, & Liu, 2017). An ethanol solution of 0.1 mmo1/L DPPH was prepared and ultrasonic-treated so that it could be completely dissolved. First, KC and KCO were dissolved in distilled water at different concentrations (5–20 mg/mL, 2 mL), and freshly prepared DPPH (0.1 mM in 95% ethanol, 2 mL) was added. The absorbance was measured at 517 nm after 30 min of water bath at 33 °C. Ascorbic acid was used to replace the sample as a positive control. The removal capacity was calculated as follows: Clearance rate = [1-(A1-A2)/A0] *100%. Where A1 is the absorbance of the experimental group, A2 is the absorbance without DPPH, and A0 is the absorbance without sample.
2.6.2. ABTS radical scavenging activity
The ABTS assay was performed to evaluate the antioxidant activity of KC and KCO (Chen et al., 2021). The same volume of 7 mmol/L ABTS and 1.4 mmol/L potassium persulfate were fully mixed, stored at room temperature protected from light for 12–16 h, and an ABTS radical solution was prepared. Before use, dilute the ABTS radical solution with absolute ethanol to 734 nm with an absorbance of 0.70 ± 0.02. To 0.8 mL ABTS radical solution, add 0.2 mL sample solutions at different concentrations (5, 10, 20 mg/mL) and mix vigorously. After 5 min of reaction at room temperature, the absorbance of the mixture was determined at 734 nm. ABTS radical scavenging activity (%) = (1-A1/A0) * 100%. Where A0 is the absorbance of the blank group of distilled water and A1 is the absorbance of the sample with ABTS radical solution added.
2.6.3. Reducing power assay
The reducing power was determined as described by (Sun et al., 2015), samples from before and after degradation (0.13 mL, 5–20 mg/mL) were mixed with potassium ferricyanide (0.125 mL, 1%) in phosphate-buffered saline (1 mL, 0.2 M, pH 6.6) for 20 min at 50 °C. The reaction was terminated with a trichloroacetic acid solution (0.125 mL, 10%) and the mixture was centrifuged at 3000 rpm for 10 min. The supernatant was then mixed with ferric chloride (1.5 mL, 0.1%) and absorbance was measured at 700 nm. A higher absorbance of reaction mixture indicated a higher reducing power.
2.7. Caenorhabditis elegans experiments
2.7.1. Paraquat survival assay
Paraquat survival assays were performed using Caenorhabditis elegans as described above with slight modifications(Li et al., 2023). Synchronous L4 C. elegans were cultured for 5 days with different concentrations of carrageenan oligosaccharides (0, 0.25, 0.50 and 1.00 mg/mL). After 5 days, 30 nematodes were randomly selected from each group and placed on NGM agar plates containing 200 μM 5′-fluorodeoxyuridine (FUDR) and 100 mM paraquat in a 20 °C incubator. The number of dead nematodes was recorded regularly every day until all nematodes had died. Survival curves were plotted based on the survival and death times of the nematodes.
2.7.2. Ultraviolet injury
Synchronous L4 C. elegans were cultured for 5 days with different concentrations of carrageenan oligosaccharides (0, 0.25, 0.50 and 1.00 mg/mL). After 5 days, 30 nematodes from each group were randomly selected and placed on NGM plates containing 200 μM 5′-fluorodeoxyuridine (FUDR) and irradiated with a 188 mW/cm2 UV lamp for 2 h before being placed in a 20 °C incubator. The number of dead nematodes was recorded regularly every day until all nematodes had died. Survival curves were plotted based on the survival and death times of the nematodes.
2.8. Statistical analysis
All results were expressed as means ± standard deviation (SD), and the significant differences between the groups were determined by one-way analysis of variance (ANOVA) with Tukey's test using SPSS 26.0 software (SPSS, Chicago, IL, USA). All figures were created using Origin 2022 (OriginLab Corp., MA, USA) and GraphPad 8.
3. Results and discussion
3.1. Optimization of photocatalytic degradation conditions
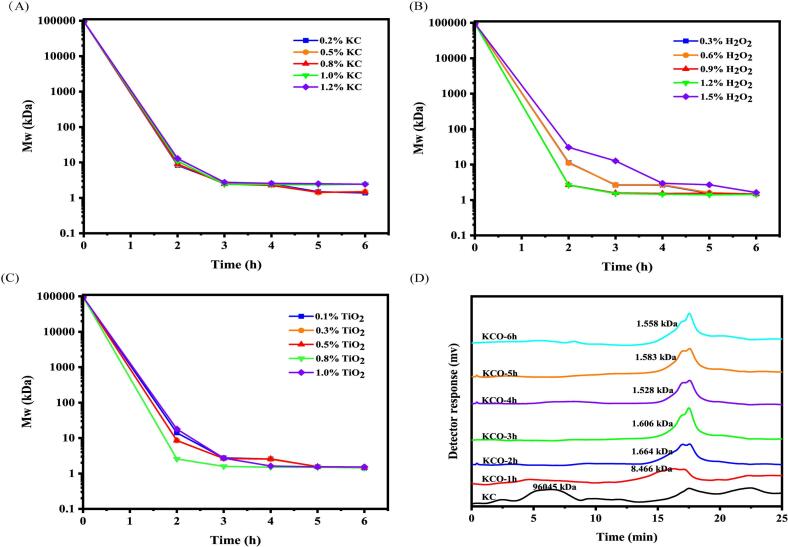
To investigate the molecular weight changes of κ-carrageenan in the ultraviolet photocatalytic reaction of H2O2 and TiO2 with different additives, the hydrolysates were monitored by HPLC during the photocatalytic reaction to optimize these reaction conditions. As shown in Fig. 1 A, the change in KC concentration during photocatalytic degradation had no significant effect on the change in Mw, while the Mw of carrageenan decreased more rapidly with increasing H2O2 concentration (Fig. 1 B). After the ideal conditions for KC concentration and H2O2 addition were established (0.8% KC concentration and 0.3% H2O2 addition), the optimal amount of TiO2 was investigated. KC degradation was best when TiO2 was added at a concentration of 0.8%, and there was no significant difference in the degradation rate of Mw after 3 h (Fig. 1 C). However, the addition of H2O2 and TiO2, which reached 1.2% and 1.0%, respectively, inhibited the degradation of Mw. The degradation efficiency was indeed limited, as the excess of TiO2 and H2O2 could lead to the reduction of hydroxyl radicals in the system, decreasing the degradation efficiency of polysaccharides(X. Chen, Sun-Waterhouse, et al., 2021). Under optimized photocatalytic degradation conditions (0.8% KC, 0.3% H2O2 and 0.8% TiO2), the average molecular weight of κ-carrageenan to 1.6 kDa within 2 h (Fig. 1 D). The degradation efficiency was significantly improved by these reaction conditions, which could generate KCO of 1.6 kDa within 2 h, compared to the previously reported conditions(Song et al., 2023).
Fig. 1.
Effect of different KC concentrations (A), H2O2 addition (B) and TiO2 addition (C) on the molecular weight of depolymerized KC. Effect of molecular weight distribution of 0.8% KC, 0.3% H2O2 and 0.8% TiO2 degradation products at different reaction times(D).
3.2. Comparison between different degradation methods
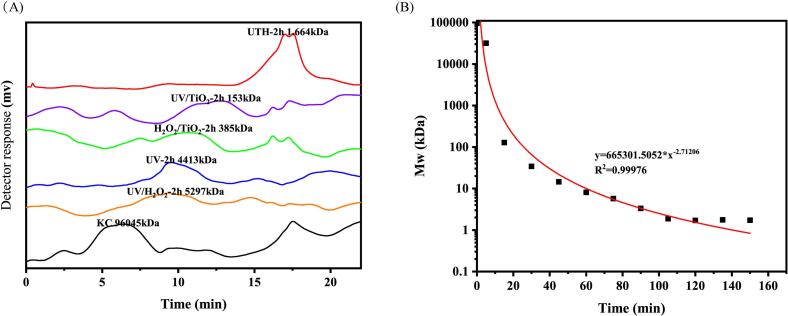
The optimal condition (UV combined with 0.8% KC, 0.3% H2O2 and 0.8% TiO2) was used for the further studies. The effects of UV, UV/ H2O2, UV/TiO2 and H2O2/TiO2 treatments were examined as controls. There were significant differences compared to the combined UV/H2O2/TiO2 treatment, suggesting that the treatment had a synergistic effect. As a strong oxidizing agent, dissociation of H2O2 could generate hydroxyl radicals and superoxide anion radicals(Semedo et al., 2015). TiO2 as a photocatalyst could produce -OH under the light (Wang et al., 2012).
Under ultraviolet light, H2O2 and TiO2 photosensitizers exert a synergistic effect to decompose during the degradation of KC and promote the formation of hydroxyl radicals. As shown in Fig. 2 A, the Mw of UV/ H2O2-treated carrageenan was 5297 kDa, the Mw of UV-treated carrageenan was 4413 kDa, the Mw of H2O2/ TiO2-treated carrageenan was 385 kDa, and the Mw of UV/ TiO2 -treated carrageenan was 385 kDa within 2 h, respectively. In contrast, UV/ H2O2/ TiO2 showed a synergistic effect of the combined treatment, and the molecular weight of degradation could reach about 1.6 kDa and be uniform.
Fig. 2.
Molecular weight distribution of five different degradation modes (UV/H2O2, UV, H2O2/TiO2, UV/TiO2 UV/H2O2/TiO2) (A); The variation of carrageenan with photocatalytic degradation time(B).
3.3. Degradation curves
In order to explore the degradation trend of carrageenan, carrageenan was photodegraded in different time periods under optimal degradation conditions. As shown in Fig. 2 B, the molecular weight of carrageenan gradually decreases as the degradation time increases. When the degradation time reached 2 h, the degradation degree of carrageenan tended to be flat, and the molecular weight degradation reached the lowest value. The formula of the degradation curve was obtained by fitting the curve by function: y = 665,301.5052*x-2.71206, R2 = 0.99976. Photodegradation of carrageenan was performed by calculating the time required to prepare 5 kDa and 1.5 kDa carrageenan by formula. The molecular weights of the degradation products were 5.43 kDa and 1.54 kDa, which were not much different from the initial custom backup values, indicating that the specific molecular weight carrageenan could be accurately prepared. This means that photocatalytic degradation is an effective method for the degradation of polysaccharides, which can stably control the molecular weight of the degraded polysaccharides.
3.4. Chemical composition of degradation products
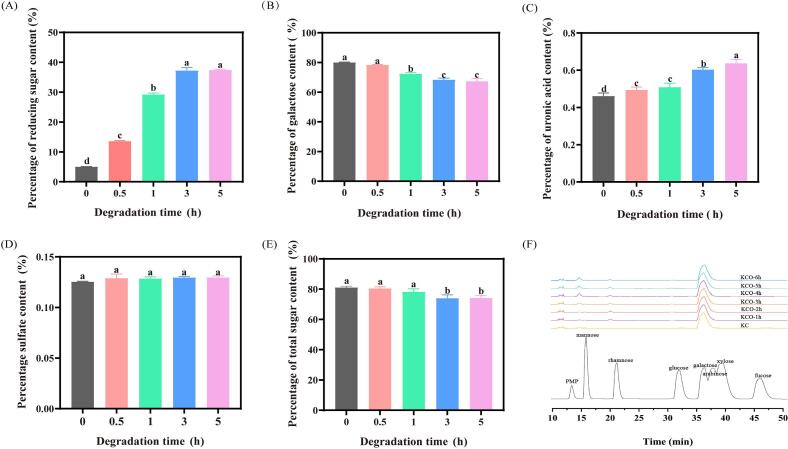
Fig. 3 visually represents the chemical composition of carrageenan before and after degradation for durations of 0, 0.5, 1, 3, and 5 h. With the increase of photodegradation time, the reducing sugar and uronic acid content of carrageenan increased, from 4.970% to 37.65% (Fig. 3 A) and from 0.45% to 0.65% (Fig. 3 C). There was no significant change in sulfate group content, probably because the sulfate groups were the least affected by free radicals (Fig. 3 D). However, the galactose and total sugar levels of carrageenan decreased after degradation (Fig. 3 B, E). The results showed that the photocatalytic degradation was effective, which was consistent with the change in molecular weight. The monosaccharide composition of KC and KCO remained consistent before and after degradation as shown in Fig. 3 F, consisting primarily of galactose. This suggests that UV/H2O2/TiO2 coordinated photodegradation did not alter the monosaccharide type. However, with extended light exposure, the proportion of galactose decreased from 97.17% to 95.23%. This outcome implies that galactose is the predominant site for the degradation of KC.
Fig. 3.
Changes in the chemical composition of κ-carrageenan. (A) reducing sugar content, (B) 3,6-dehydrated galactose content, (C) Uronic acid content (D) sulfated group content, (E) total sugar content, (F) monosaccharide composition. Data are expressed as SD ± mean (n = 3). Bars marked with different letters at the top represent statistically significant results (p < 0.05) based on Duncan's range test of one-way ANOVA, while bars marked with the same letters correspond to results showing no statistically significant differences.
3.5. FT-IR and UV analysis
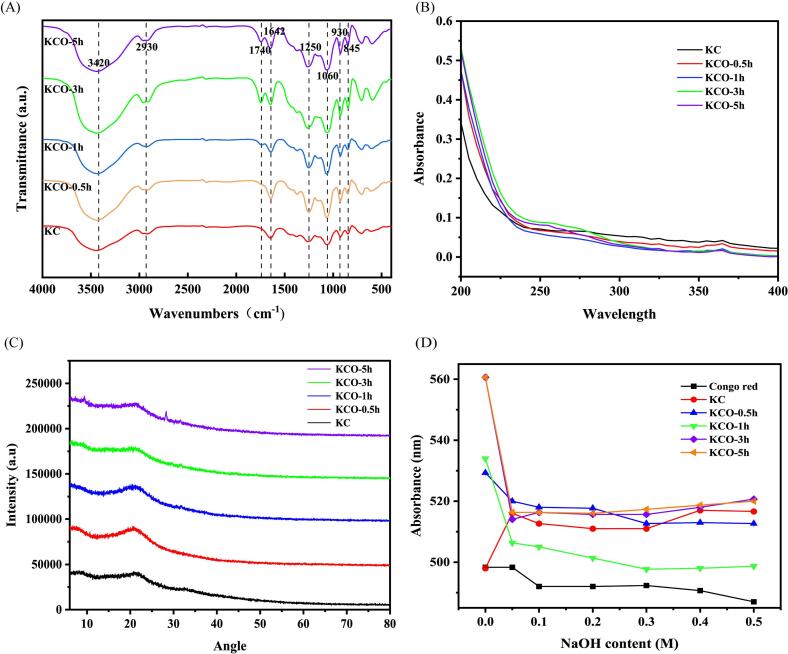
The FT-IR spectra of κ-carrageenan after 0, 0.5, 1, 3 and 5 h of photocatalytic reaction were shown in Fig. 4 A. The absorption bands at 845, 930, and 1250 cm−1 were the characteristic absorption peaks of 4-sulphate-β-D-galactose, α-D-3,6-dehydrated galactose and sulfate, respectively, indicating that the sulfate groups were preserved during the photocatalytic reaction. The absorption band at 1054 cm−1 was the characteristic absorption peak of the pyranose ring, which was attributed to the tensile vibration of C-O-C on the sugar ring (Wang et al., 2012). It implied that the chain structure of the polysaccharide was not destroyed after degradation. In addition, although the infrared spectra of carrageenan before and after degradation were similar, the tensile vibration of the absorption band C O at 1740 cm−1 increased with the extension of photocatalytic degradation, indicating the formation of carboxyl or aldehyde groups (Song et al., 2023).
Fig. 4.
FT − IR (A), ultraviolet spectra (B), X-ray diffraction spectra (C), Congo red assay (D) of κ-carrageenan after 0, 0.5, 1, 3 and 5 h of photocatalytic reaction. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The UV–Vis scanning of 1 mg/mL KC solution that underwent degradation for 0, 0.5, 1, 3, and 5 h was conducted at 200–400 nm. Fig. 4 B graphically presents the obtained outcomes, which indicated that there are no discernible peaks at 260 and 280 nm, thereby signifying the absence of proteins or nucleic acids both before and after carrageenan degradation.
3.6. XRD analysis and spiral coil transition changes
Polysaccharides are either completely amorphous or semi-crystalline, and some can form crystalline fiber structures (Pérez, Mazeau, & Hervé du Penhoat, 2000). XRD analysis is often used to investigate the crystal structure of a sample. The results of XRD analysis of carrageenan before and after photocatalytic degradation were shown in Fig. 4 C, and the peak shape trends of KC and KCO were similar, indicating that the structure is the same. The wide hump was in the range of 10°-25°, indicating that it is a semi-crystalline polysaccharide (Abu Bakar, Azeman, Mobarak, Mokhtar, & Bakar, 2020). However, in the diffraction pattern of κ-carrageenan degraded for 5 h, the intensity peaks increase at 2θ = 9.36° and 28.3° to form new diffraction peaks, indicating that the crystallization performance of κ-carrageenan was slightly improved after 5 h of photocatalytic degradation.
Denaturation of the triple helix can occur at relatively high concentrations of alkaline solutions (Guo et al., 2021). Whether the polysaccharide has a triple helix conformation can be assessed by the formation of Congo red polysaccharide complexes and the resulting shift in the maximum adsorption wavelength. If a polysaccharide has a triple helix conformation, its maximum absorption wavelength initially increases and then decreases as the concentration of NaOH increases. Polysaccharides without this tendency do not have a triple helix conformation (Fariña, Siñeriz, Molina, & Perotti, 2001). Fig. 4 D showed the variation of the maximum absorption wavelength of KC and KCO at different concentrations of NaOH. The results implied that KC shows a significant red shift (from 497 nm to 516 nm), indicating the presence of a typical ordered triple helix conformation. However, the maximum absorption wavelength of KCO gradually decreased with increasing NaOH without a significant redshift, indicating that the triple helix in the polysaccharide was disrupted, which may be due to photocatalytic degradation that destroyed the intermolecular and intramolecular hydrogen bonds that maintain the triple helix structure. Therefore, carrageenan has no triple helix conformation after photocatalysis.
3.7. Rheological properties
Mw is an obvious measure of the polymer hydrolysis because it is a direct reflection of the result of macromolecular chain breakage. Gel strength and viscosity are indirect but equally related properties as they are connected to the gel network formed by the association of the double helix. Due to the shortened helix length, shorter polymer chains produce weaker gels with reduced rheological properties (Russo Spena et al., 2023).
The viscosity decreased with increasing shear rate from 0.1 to 5 s−1, and at a high shear rate (5–100 s−1) the behavior close to Newtonian fluid was observed, and the apparent viscosity of the photodegradation product was <0.1 Pa·S, indicating that the degraded solution was close to the properties of Newtonian fluid (Fig. 5 A). The G“ and G′ values of carrageenan before and after degradation increased with increasing frequency, and the G" and G′ of carrageenan after photodegradation were lower than carrageenan at the frequency of 0.1–10 Hz. The carrageenan before degradation was a viscoelastic fluid in the low-frequency range (G“ > G′), in the high-frequency range (G"< G′), the product after degradation was in the high and low-frequency range G" < G′, indicating elastic deformation (Fig. 5 B).
Fig. 5.
Apparent viscosity (A), storage modulus (G′) and loss modulus (G″) (B) and Scanning electron microscope image (C) of κ-carrageenan after 0, 0.5, 1, 3 and 5 h of photocatalytic reaction.
3.8. SEM measurements
The surface topography of carrageenan sample particles before and after degradation (×500 and ×1000) was analyzed by scanning electron microscopy. As shown in Fig. 5 C, undegraded KC has an irregular shape with a dense and rough surface structure and some debris. After UV/H2O2/TiO2 treatment for 0.5, 1, 3, and 5 h, the surface of the KCO sample became thin, smooth, dense, and gradually fragmented, especially after 3 h of degradation, the carrageenan appearance was broken and torn. With the photodegradation time, carrageenan degradation to different degrees, changes in intermolecular distance and cross-linking, and significant differences in surface appearance.
3.9. Characterization of carrageenan oligosaccharides
The repeating disaccharide units of 3-linked-4-sulfate-β-d-galactopyranose (G4S) and 3,6-anhydro-α-d-galactose (AnG) make up κ-carrageenan with different DPs. Free radical depolymerization leads to the non-selective degradation of polymers and changes in chemical structure. In order to understand the oligosaccharide fragments of carrageenan after photocatalytic degradation, the oligosaccharides in KCO were characterized by HPLC-MSn anion mode. Since κ-carrageenan oligosaccharides are rich in galactose, the oligosaccharide mixture may be represented by partially desulphated fragments of kappa-carrageenan and its precursors with no more than three degrees of sulphation (Kravchenko et al., 2020).
The κ-carrageenan oligosaccharides obtained by photodegradation for 5 h were different in terms of polymerization degree (Dp) and structural sequence. As shown in Table 1, KCO mainly contained a series of odd-numbered oligosaccharides-G4S-(An-G4S) n-, including monosaccharides (m/z 259. 00), trisaccharide (m/z 322.03, 563.09, 645.12 and 669.99), pentaccharide (m/z 474.07), heptasaccharide (m/z 630.27, 678.65, 1175.48 and 1480.42), and nona-saccharides (m/z 1584.97, 1686.36, 1889.42). Thus, the DP of the photocatalytically degraded carrageenan oligosaccharide fragment is in the range of 1–9, and the degree of sulfation of oligosaccharides is 1–3.
Table 1.
ESI-MS analysis of photocatalytic depolymerization of κ-carrageenan oligosaccharides.
| Retention time (min) | Observed m/z | Dp | Molecular ionsa |
|---|---|---|---|
| 2.27 | 259.00 | 1 | [(G4S)] − |
| 2.29 | 385 | 2 | [(G4S - An)] − -H2O |
| 2.29 | 403.05 | 2 | [(G4S-An)] − |
| 2.29 | 322.03 | 3 | [(G4S-An-G4S)] − |
| 2.57 | 563.09 | 3 | [(G4S-An-G)] − |
| 2.59 | 669.99 | 3 | [(G4S-An-G4S) Na] − |
| 2.61 | 645.12 | 3 | [(G4S-An-G4S) H] − |
| 2.29 | 394.05 | 4 | [(G4S - An)2]2− |
| 2.29 | 793.28 | 4 | [(G4S-An) (G4S-Anol) H− |
| 2.29 | 914.34 | 4 | [(G4S-An2S) (G4S-Anol)2Na] − |
| 3.88 | 708.76 | 4 | [(G4S-An) (G-An)] − |
| 2.33 | 474.07 | 5 | [(G4S-An) (G4S-An-G)]2− |
| 3.41 | 391.05 | 6 | [(An-G4S)3]3− |
| 2.27 | 545.08 | 6 | [(An-G4S)2(An-G)]2− |
| 3.85 | 547.1 | 6 | [(An-G4S)2(An-G)]2− |
| 3.84 | 1116.16 | 6 | [(An-G4S)2(An-G) Na] − |
| 6.69 | 1175.48 | 7 | [(G-An)2(G4S-An-G)] − |
| 3.84 | 630.27 | 7 | [(G4S-An)2(G-An-G)]2− |
| 2.23 | 678.65 | 7 | [(G4S-An)2(G4S-An-G) Na]2− |
| 2.23 | 1480.42 | 7 | [(G4S-An)2(G4S-An-G4S)3 Na] − |
| 2.25 | 1584.97 | 9 | [(G4S-An)2(G-An) (G-An-G) Na] − |
| 2.25 | 1686.36 | 9 | [(G4S-An)3(G-An-G)2 Na] − |
| 6.69 | 1889.42 | 9 | [(G-An)2(G4S-An-G)] − |
An is 3,6-anhydro-α-d-galactose; G4S is β-d-galactose-4-sulfate.
3.10. Antioxidant activity
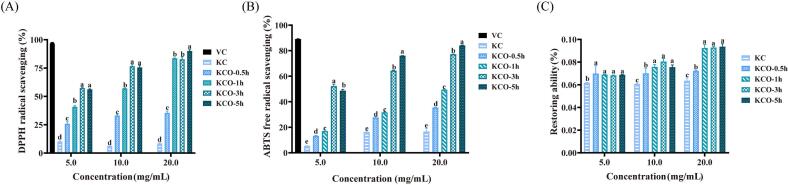
Previous research has shown that the antioxidant activity of polysaccharides is closely related to their molecular weight, configuration, degree of branching of polysaccharide chains, monosaccharide composition and chain arrangement (Zhou, Yu, Zhang, He, & Ma, 2012) (Lahrsen, Schoenfeld, & Alban, 2019). The antioxidant activity of carrageenan before and after photodegradation was investigated by measuring the scavenging activity and reducing capacity of DPPH and ABTS free radicals (Fig. 6 A-C).
Fig. 6.
Antioxidant assay of photocatalytic degradation of carrageenan. Values with different letters in each figure are significantly different (p < 0.05).
As a polyhydroxy compound with strong reducing properties, polysaccharides are easily oxidized with DPPH to form quinones. The effect of antioxidants on DPPH radical scavenging is thought to be due to its hydrogen supply capacity (Yuan et al., 2005). As shown in Fig. 6, the reducing ability of the sample and its scavenging effect on DPPH and ABTS free radicals increased with the increase of photodegradation time and concentration dose. The antioxidant scavenging activity of KCO obtained after photodegradation was higher than that of KC. At concentrations of 5–20 mg/mL, the DPPH clearance of KC was 5.93–10.88%, while the DPPH clearance of KCO obtained after 5 h of degradation was 55.60–90.85% (Fig. 6 A), which may be due to the strong hydrogen supply capacity of the degraded sample. The scavenging effect of the sample on ABTS radicals before and after degradation was determined (Fig. 6 B). The scavenging capacity of undegraded carrageenan on ABTS radicals was 5.30% at 5 mg/mL and increased to 16.98% in a concentration-dependent manner at 20 mg/mL, while the ABTS scavenging effect of carrageenan after 5 h of degradation was 49.38–84.32%. The results showed that the photocatalytically degraded samples were good at scavenging ABTS radicals.
The reducing ability of polysaccharides is one of the important indicators of their potential antioxidant activity. Fig. 6 C shows the reduction capacity of the sample at concentrations ranging from 5 to 20 mg/mL before and after degradation. The reducing power after degradation was stronger than that of undegraded carrageenan, and the reducing power was closely related to the increase in concentration, and the reducing power of the product degraded for 1, 3, and 5 h could reach 0.09% when the concentration reached 20 mg/mL. The lower the molecular weight of the polysaccharide, the more abundant free hydroxyl and reducing sugars are present at the same concentration, resulting in significant free radical scavenging capacity (H. Chen et al., 2021). The results showed that KCO showed a stronger ability to scavenge free radicals and could be used as a promising natural antioxidant.
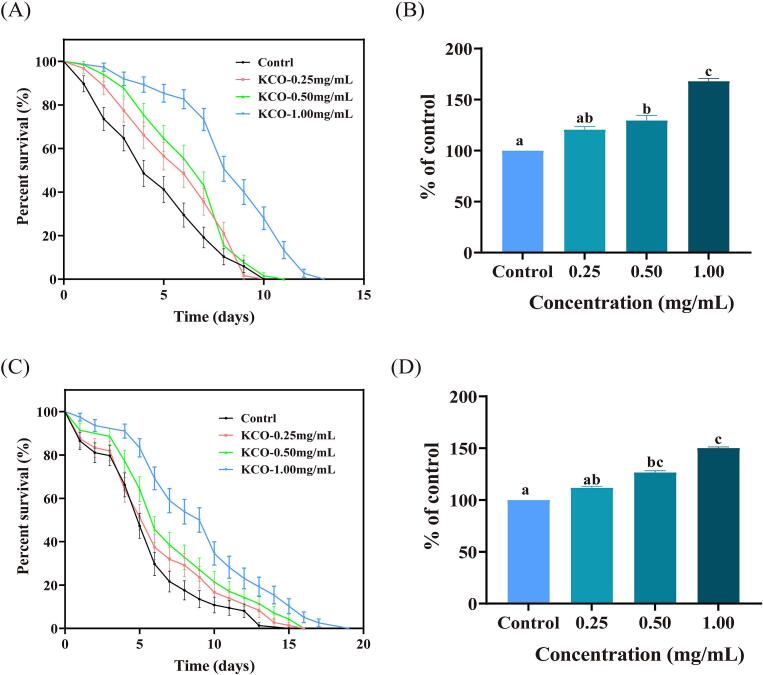
3.11. KCO ameliorates paraquat (PQ)-induced oxidative stress
To further evaluate the antioxidant effect of KCO in vivo, the Caenorhabditis elegans model of oxidative stress was used. Paraquat is often used in ROS (Reactive Oxygen Species) generators to study ROS signals due to its convenience. Paraquat is a generic name for the herbicide methyl viologen (N, -N′-dimethyl-4, −4′-dichlorobipyridine), which is involved in ROS generation through a light-dependent mechanism. Methyl viologen can induce the production of ROS in C. elegans. (Yao et al., 2023). This experiment induced oxidative stress in Caenorhabditis elegans using 100 mM paraquat. As shown in Fig. 7A and B, KCO (0.25–1.00 mg/mL) significantly increased the lifespan of nematodes by 22.57–69.98% (p < 0.05) compared to the control, suggesting a protective effect of carrageenan oligosaccharides against oxidative stress induced by paraquat.
Fig. 7.
Effect of carrageenan oligosaccharides on the lifespan of nematodes under oxidative stress and ultraviolet injury. Oxidative stress nematode survival rate (A), survival percentage (B); Ultraviolet-damaged nematode survival rate (C) and survival percentage (D); The results represent three independent experiments, and the data are presented as Kaplan-Meier curves and compare significance by log-rank test(p < 0.05).
3.12. Effect of carrageenan oligosaccharides on ultraviolet damage in nematodes
Nematodes have varying degrees of sensitivity to sunlight and their life cycle is adapted to exposure to high levels of ultraviolet light (Dijk, Louw, Kalis, & Morgan, 2009). UV damage is one of the indicators used to assess stress resistance in nematodes. The effect of KCO on ultraviolet damage in Caenorhabditis elegans was studied, and the results were shown in Fig. 7 C and D. The lifespan of nematodes fed KCO was significantly longer compared to the control, increasing by 12.72–50.87%. In addition, nematodes fed 1 mg/mL KCO showed the highest resistance, with a maximum lifespan of 19 d. The results showed that nematodes showed extremely high stress resistance after eating KCO. KCO may serve as a promising anti-aging ingredient for food, pharmaceutical and cosmetic uses.
4. Conclusion
The present study developed a photocatalytic method for the degradation of k-carrageenan using UV/H2O2/TiO2, which could reduce the average molecular weight of k-carrageenan to 1.6 kDa within 2 h. Notably, no significant difference in sulfate group content was observed between κ-carrageenan and its degradation products, and the gel performance decreased with photocatalytic degradation. Then, the identification of the degraded oligosaccharide fragments showed that the degraded carrageenan oligosaccharides were mainly odd oligosaccharides in the range of 1–9 Dp. After degradation, the antioxidant activity of κ-carrageenan was significantly enhanced, which played a beneficial role in the healthy life expectancy index of nematodes. In conclusion, UV/H2O2/TiO2 photocatalytic degradation is an efficient, green, and convenient method for depolymerizing carrageenan without stripping its sulfuric acid group, which provides certain theoretical and technical support for the accurate preparation of κ-carrageenan with different molecular weights in the production process of pharmaceutical, chemical, food and other industries.
CRediT authorship contribution statement
Ziyu Li: Methodology, Formal analysis, Validation, Writing – original draft. Kit-Leong Cheong: Methodology, Data curation, Formal analysis. Bingbing Song: Writing – review & editing, Supervision, Project administration. Huan Yin: Methodology, Data curation, Formal analysis. Qian Li: Methodology, Visualization. Jing Chen: Methodology, Visualization. Zhuo Wang: Investigation. Baojun Xu: Resources, Software. Saiyi Zhong: Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research project is supported by the Guangdong Basic and Applied Basic Research Foundation (2023A1515010005), Special Funds for Universities in Guangdong Province in the Key Areas of Biomedicine and Health (2023ZDZX2025), Guangdong Ocean University College Student Innovation Team Project (CXTD2023005), and the Innovative Team Program of High Education of Guangdong Province (2021KCXTD021).
Data availability
Data will be made available on request.
References
- Abu Bakar M.H., Azeman N.H., Mobarak N.N., Mokhtar M.H.H., Bakar A.A.A. Effect of active site modification towards performance enhancement in biopolymer κ-carrageenan derivatives. Polymers. 2020;12(9) doi: 10.3390/polym12092040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai L., Chung Y.-C., Lin S.-Y., Jeng K.-C.G., Lai P.F.H., Xiong Z.-Q., Wang G. Carrageenan polysaccharides and oligosaccharides with distinct immunomodulatory activities in murine microglia BV-2 cells. International Journal of Biological Macromolecules. 2018;120:633–640. doi: 10.1016/j.ijbiomac.2018.08.151. [DOI] [PubMed] [Google Scholar]
- Ana P., Nathalie B., Gilles B., Daniel R., Tomás M.-S., Yolanda F.-P. Anti-herpes simplex virus (HSV-1) activity and antioxidant capacity of carrageenan-rich enzymatic extracts from Solieria filiformis (Gigartinales, Rhodophyta) International Journal of Biological Macromolecules. 2021;168:322–330. doi: 10.1016/j.ijbiomac.2020.12.064. [DOI] [PubMed] [Google Scholar]
- Black W.A., Blakemore W.R., Colquhoun J.A., Dewar E.T. The evaluation of some red marine algae as a source of carrageenan and of its k- and lambda-components. Journal of the Science of Food and Agriculture. 1965;16(10):573–585. doi: 10.1002/jsfa.2740161001. [DOI] [PubMed] [Google Scholar]
- Bouanati T., Colson E., Moins S., Cabrera J.-C., Eeckhaut I., Raquez J.-M., Gerbaux P. Microwave-assisted depolymerization of carrageenans from Kappaphycus alvarezii and Eucheuma spinosum: Controlled and green production of oligosaccharides from the algae biomass. Algal Research. 2020;51 [Google Scholar]
- Chen H., Zeng J., Wang B., Cheng Z., Xu J., Gao W., Chen K. Structural characterization and antioxidant activities of Bletilla striata polysaccharide extracted by different methods. Carbohydrate Polymers. 2021;266 doi: 10.1016/j.carbpol.2021.118149. [DOI] [PubMed] [Google Scholar]
- Chen J., Li L., Zhang X., Wan L., Zheng Q., Xu D., Li Y., Liang Y., Chen M., Li B., Chen Z. Structural characterization of polysaccharide from Centipeda minima and its hypoglycemic activity through alleviating insulin resistance of hepatic HepG2 cells. Journal of Functional Foods. 2021;82 [Google Scholar]
- Chen X., Sun-Waterhouse D., Yao W., Li X., Zhao M., You L. Free radical-mediated degradation of polysaccharides: Mechanism of free radical formation and degradation, influence factors and product properties. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.130524. [DOI] [PubMed] [Google Scholar]
- Cosenza V.A., Navarro D.A., Pujol C.A., Damonte E.B., Stortz C.A. Partial and total C-6 oxidation of gelling carrageenans. Modulation of the antiviral activity with the anionic character. Carbohydrate Polymers. 2015;128:199–206. doi: 10.1016/j.carbpol.2015.04.030. [DOI] [PubMed] [Google Scholar]
- Di Rosa M. Biological properties of carrageenan. The Journal of Pharmacy and Pharmacology. 1972;24(2):89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- Dijk J.V., Louw M.D.E.D., Kalis L.P.A., Morgan E.R. Ultraviolet light increases mortality of nematode larvae and can explain patterns of larval availability at pasture. International Journal for Parasitology. 2009;39(10):1151–1156. doi: 10.1016/j.ijpara.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Dodgson K.S., Price R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. The Biochemical Journal. 1962;84:106–110. doi: 10.1042/bj0840106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Deeb N.M., Ibrahim O.M., Mohamed M.A., Farag M.M.S., Farrag A.A., El-Aassar M.R. Alginate/κ-carrageenan oral microcapsules loaded with Agaricus bisporus polysaccharides MH751906 for natural killer cells mediated colon cancer immunotherapy. International Journal of Biological Macromolecules. 2022;205:385–395. doi: 10.1016/j.ijbiomac.2022.02.058. [DOI] [PubMed] [Google Scholar]
- Fariña J.I., Siñeriz F., Molina O.E., Perotti N.I. Isolation and physicochemical characterization of soluble scleroglucan from Sclerotium rolfsii. Rheological properties, molecular weight and conformational characteristics. Carbohydrate Polymers. 2001;44(1):41–50. [Google Scholar]
- Fernandes A., Gągol M., Makoś P., Khan J.A., Boczkaj G. Integrated photocatalytic advanced oxidation system (TiO2/UV/O3/H2O2) for degradation of volatile organic compounds. Separation and Purification Technology. 2019;224:1–14. [Google Scholar]
- Ghanbarzadeh M., Golmoradizadeh A., Homaei A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochemistry Reviews. 2018;17(3):535–571. [Google Scholar]
- Goncalves C., Rodriguez-Jasso R.M., Gomes N., Teixeira J.A., Belo I. Adaptation of dinitrosalicylic acid method to microtiter plates. Analytical Methods. 2010;2(12):2046–2048. [Google Scholar]
- Guo X., Kang J., Xu Z., Guo Q., Zhang L., Ning H., Cui S.W. Triple-helix polysaccharides: Formation mechanisms and analytical methods. Carbohydrate Polymers. 2021;262 doi: 10.1016/j.carbpol.2021.117962. [DOI] [PubMed] [Google Scholar]
- van den Hoogen B.M., van Weeren P.R., Lopes-Cardozo M., van Golde L.M., Barneveld A., van de Lest C.H. A microtiter plate assay for the determination of uronic acids. Analytical Biochemistry. 1998;257(2):107–111. doi: 10.1006/abio.1997.2538. [DOI] [PubMed] [Google Scholar]
- Jiang C., Secundo F., Mao X. Expanding the application range of the ?-carrageenase OUC-FaKC16A when preparing oligosaccharides from ?-carrageenan and furcellaran. Marine Life Science & Technology. 2023;5(3):387–399. doi: 10.1007/s42995-023-00181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Júnior E.H., Gonçalves A.G., Noseda M.D., Duarte M.E.R., Murakami F.S., Ducatti D.R.B. Semi-synthesis of N-alkyl-kappa-carrageenan derivatives and evaluation of their antibacterial activity. Carbohydrate Research. 2021;499 doi: 10.1016/j.carres.2021.108234. [DOI] [PubMed] [Google Scholar]
- Khotimchenko M., Tiasto V., Kalitnik A., Begun M., Khotimchenko R., Leonteva E., Bryukhovetskiy I., Khotimchenko Y. Antitumor potential of carrageenans from marine red algae. Carbohydrate Polymers. 2020;246 doi: 10.1016/j.carbpol.2020.116568. [DOI] [PubMed] [Google Scholar]
- Kravchenko A.O., Anastyuk S.D., Glazunov V.P., Sokolova E.V., Isakov V.V., Yermak I.M. Structural characteristics of carrageenans of red alga Mastocarpus pacificus from sea of Japan. Carbohydrate Polymers. 2020;229 doi: 10.1016/j.carbpol.2019.115518. [DOI] [PubMed] [Google Scholar]
- Lahrsen E., Schoenfeld A.-K., Alban S. Degradation of eight sulfated polysaccharides extracted from red and Brown algae and its impact on structure and pharmacological activities. ACS Biomaterials Science & Engineering. 2019;5(3):1200–1214. doi: 10.1021/acsbiomaterials.8b01113. [DOI] [PubMed] [Google Scholar]
- Li C., Huang W., Zheng H., Shi H., Bi S., Song L.…Wang Y. Structural elucidation of a novel heteropolysaccharide from Arca inflata reeve and its immunomodulatory and antioxidant activities. Journal of Functional Foods. 2023;107 [Google Scholar]
- Li J., Chi Z., Yu L., Jiang F., Liu C. Sulfated modification, characterization, and antioxidant and moisture absorption/retention activities of a soluble neutral polysaccharide from Enteromorpha prolifera. International Journal of Biological Macromolecules. 2017;105:1544–1553. doi: 10.1016/j.ijbiomac.2017.03.157. [DOI] [PubMed] [Google Scholar]
- Navarro D.A., Stortz C.A. Determination of the configuration of 3,6-anhydrogalactose and cyclizable alpha-galactose 6-sulfate units in red seaweed galactans. Carbohydrate Research. 2003;338(20):2111–2118. doi: 10.1016/s0008-6215(03)00345-8. [DOI] [PubMed] [Google Scholar]
- Pérez S., Mazeau K., Hervé du Penhoat C. The three-dimensional structures of the pectic polysaccharides. Plant Physiology and Biochemistry. 2000;38(1):37–55. [Google Scholar]
- Prajapati V.D., Maheriya P.M., Jani G.K., Solanki H.K. Carrageenan: A natural seaweed polysaccharide and its applications. Carbohydrate Polymers. 2014;105:97–112. doi: 10.1016/j.carbpol.2014.01.067. [DOI] [PubMed] [Google Scholar]
- Relleve L.S., Lopez G.E.P., Cruz R.M.M.D., Abad L.V. High radiation dose studies of kappa-carrageenan in dilute aqueous solution. Radiation Physics and Chemistry. 2022;197 [Google Scholar]
- Riyaz S.U.M., Inbakandan D., Manikandan D., Bhavadharani P., Elson J., Prabhu N.S.…Simal-Gandara J. Microbiome in the ice-ice disease of the farmed red algae Kappaphycus alvarezii and degradation of extracted food carrageenan. Food Bioscience. 2021;42 [Google Scholar]
- Russo Spena S., Pasquino R., Sarrica A., Delmonte M., Yang C., Grizzuti N. Kinetics of acid hydrolysis of k-carrageenan by in situ rheological follow-up. Food Hydrocolloids. 2023;144 [Google Scholar]
- Saha A.K., Brewer C.F. Determination of the concentrations of oligosaccharides, complex type carbohydrates, and glycoproteins using the phenol-sulfuric acid method. Carbohydrate Research. 1994;254:157–167. doi: 10.1016/0008-6215(94)84249-3. [DOI] [PubMed] [Google Scholar]
- Semedo M.C., Karmali A., Fonseca L. A high throughput colorimetric assay of β-1,3-d-glucans by Congo red dye. Journal of Microbiological Methods. 2015;109:140–148. doi: 10.1016/j.mimet.2014.12.020. [DOI] [PubMed] [Google Scholar]
- Shafie M.H., Kamal M.L., Zulkiflee F.F., Hasan S., Uyup N.H., Abdullah S.…Zafarina Z. Application of carrageenan extract from red seaweed (Rhodophyta) in cosmetic products: A review. Journal of the Indian Chemical Society. 2022;99(9) [Google Scholar]
- Song C., You Y., Wen C., Fu Y., Yang J., Zhao J., Song S. Characterization and gel properties of low-molecular-weight Carrageenans prepared by photocatalytic degradation. POLYMERS. 2023;15(3) doi: 10.3390/polym15030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Tao H., Xie J., Zhang S., Xu X. Degradation and antioxidant activity of kappa-Carrageenans. Journal of Applied Polymer Science. 2010;117(1):194–199. [Google Scholar]
- Sun Y., Yang B., Wu Y., Liu Y., Gu X., Zhang H., Wang C., Cao H., Huang L., Wang Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chemistry. 2015;178:311–318. doi: 10.1016/j.foodchem.2015.01.105. [DOI] [PubMed] [Google Scholar]
- Tecson M.G., Abad L.V., Ebajo V.D., Jr., Camacho D.H. Ultrasound-assisted depolymerization of kappa-carrageenan and characterization of degradation product. Ultrasonics Sonochemistry. 2021;73 doi: 10.1016/j.ultsonch.2021.105540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhang P., Yu G.-L., Li C.-X., Hao C., Qi X., Zhang L.-J., Guan H.-S. Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphated derivatives. Food Chemistry. 2012;133(3):880–888. [Google Scholar]
- Yang H., Bai J., Ma C., Wang L., Li X., Zhang Y., Xu Y., Yang Y. Degradation models, structure, rheological properties and protective effects on erythrocyte hemolysis of the polysaccharides from Ribes nigrum L. International Journal of Biological Macromolecules. 2020;165:738–746. doi: 10.1016/j.ijbiomac.2020.09.093. [DOI] [PubMed] [Google Scholar]
- Yao J., Zeng J., Tang H., Shi Q., Li X., Tan J., Cheng Y., Li T., He J., Zhang Y. Preparation of Auricularia auricula polysaccharides and their protective effect on acute oxidative stress injury of Caenorhabditis elegans. International Journal of Biological Macromolecules. 2023;253 doi: 10.1016/j.ijbiomac.2023.127427. [DOI] [PubMed] [Google Scholar]
- Yermak I.M., Barabanova A.O., Aminin D.L., Davydova V.N., Sokolova E.V., Solov’eva T.F.…Shin K.S. Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities. Carbohydrate Polymers. 2012;87(1):713–720. doi: 10.1016/j.carbpol.2011.08.053. [DOI] [PubMed] [Google Scholar]
- Yu G., Guan H., Ioanoviciu A.S., Sikkander S.A., Thanawiroon C., Tobacman J.K.…Linhardt R.J. Structural studies on κ-carrageenan derived oligosaccharides. Carbohydrate Research. 2002;337(5):433–440. doi: 10.1016/s0008-6215(02)00009-5. [DOI] [PubMed] [Google Scholar]
- Yuan H., Zhang W., Li X., Lü X., Li N., Gao X., Song J. Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives. Carbohydrate Research. 2005;340(4):685–692. doi: 10.1016/j.carres.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Zhang J., Zhang Q., Wang J., Shi X., Zhang Z. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chinese Journal of Oceanology and Limnology. 2009;27(3):578–582. [Google Scholar]
- Zhang Y., Lang B., Zeng D., Li Z., Yang J., Yan R., Xu X., Lin J. Truncation of κ-carrageenase for higher κ-carrageenan oligosaccharides yield with improved enzymatic characteristics. International Journal of Biological Macromolecules. 2019;130:958–968. doi: 10.1016/j.ijbiomac.2019.02.109. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chi Z., Xu Y., Shi N., Chi Z., Liu G. High-level extracellular expression of κ-carrageenase in Brevibacillus choshinensis for the production of a series of κ-carrageenan oligosaccharides. Process Biochemistry. 2018;64:83–92. [Google Scholar]
- Zhou C., Yu X., Zhang Y., He R., Ma H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydrate Polymers. 2012;87(3):2046–2051. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.