Abstract
Background:
People living with diabetes need constant glucose monitoring to avoid health complications. However, they do not monitor their glucose levels as often as recommended, probably because glucose measurement devices can be painful, costly, need testing strips or sensors, require lancing the finger or inserting a sensor with risk of infection, and can be inaccurate or have failures. Therefore, developing new alternatives for noninvasive glucose measurements that overcome these disadvantages is necessary, being Raman spectroscopy (RS) a solution.
Objective:
This review aims to provide an overview of the current glucose-monitoring technologies and the uses and advantages of RS to improve noninvasive transcutaneously glucose-monitoring devices.
Results:
The skin has been used to assess glucose levels noninvasively because it is an accessible tissue where glucose can be measured in the interstitial fluid (ISF) in the epidermis (especially in the stratum corneum). The most selected skin sites to apply RS for noninvasive glucose measurements were the nailfold, finger, and forearm because, in these sites, the penetration depth of the excitation light can reach the stratum corneum (10-20 µm) and the ISF. Studies found that RS is a good optical technique to measure glucose noninvasively by comparing glucose levels obtained by RS with those from invasive methods such as glucose meters with testing strips during an oral glucose tolerance test (OGTT).
Conclusions:
New alternatives for noninvasive glucose measurements that overcome the disadvantages of current devices is necessary, and RS is a possible solution. However, more research is needed to evaluate the stability, accuracy, costs, and acceptance.
Keywords: transcutaneous measurements, noninvasive, glucose monitoring, Raman spectroscopy
Introduction
Glucose monitoring (GM) is important in people living with diabetes because it provides data to make decisions related to food intake, insulin dose, physical exercise, 1 and maintain levels in the normal range to avoid health complications, such as renal failure, peripheral neuropathy, and cardiovascular diseases. 2 Current, GM devices are invasive or minimally invasive, which need a significant number of daily punctures that are uncomfortable, painful, and pose an infection risk.3,4 Also, they can be costly and uncomfortable to wear. 5 The above limitations explain why people with diabetes do not monitor their glucose levels as often as recommended. 4
However, technology is improving and focused on creating reliable, fast, painless, and cost-effective new devices for noninvasive GM that eliminates the discomfort of taking blood samples and help populations to improve GM and enhance life quality. In this case, Raman spectroscopy (RS) could be one of the most promising optical techniques. Raman spectroscopy has many advantages like no sample destruction (it can be used on skin), the ability to obtain molecular structure information (estimation of glucose concentration), no necessary reagents 4 (test strips or sensors), great specificity, and low cost.
This review aims to provide an overview of the current GM technologies and the uses and advantages of RS to improve noninvasive GM devices.
RS as a Tool for GM on Skin
Raman Spectroscopy
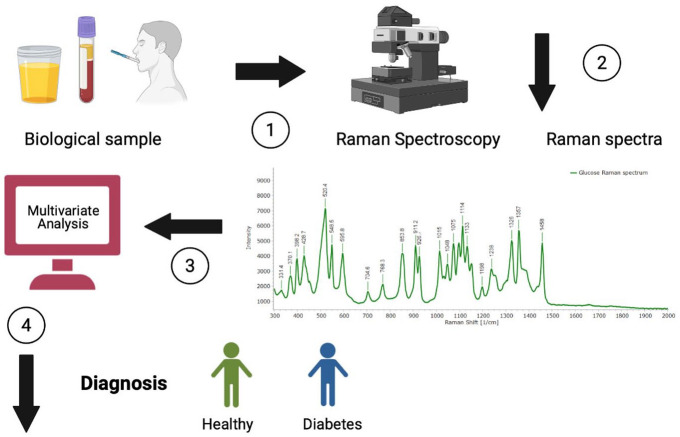
Raman spectroscopy is a good optical technique for clinical applications. It has been used for type 2 diabetes mellitus (T2DM) diagnosis by assessing skin and biofluids. Several studies differentiated healthy people from those with T2DM and predicted future complications using this technique.6-8 Figure 1 describes how RS works to provide a possible diagnosis.
Figure 1.
Description of how Raman spectroscopy works to provide a possible diagnosis: (1) Biofluids are obtained and put on a substrate (aluminum). (2) The samples are analyzed with Raman spectroscopy to obtain Raman spectra. (3) The spectra are analyzed by multivariate analysis. (4) If there are differences in the spectra and groups are separated, then the study sample can be differentiated into healthy and people with diabetes. Colored version is available online.
Figure 2a shows how RS works using a monochromatic light source (visible to mid-infrared light) to detect the glucose concentration based on the Raman effect. First, monochromatic light hits the tissue sample (in this case, skin), producing scattered rays traveling in all directions. Most of these rays come out with the same wavelength as incident light (elastic or Rayleigh scattering). However, some photons are inelastically scattered (Raman scattering) with different wavelengths from the incident light due to the interaction with the skin sample causing rotation and vibration of the molecules. The difference between the incidence frequency and the scattered frequency is the Raman shift (1/cm) which provides specific information about the rotational and vibrational states of the molecules from the body fluids. 9 Therefore, the shift in the frequency of the scattered light depends on the type of molecules or the sample’s chemical structure and is independent of the wavelength of the light source. 10 Finally, the intensity of the vibrations between molecules (intensity of the Raman spectrum) depends on the molecular concentration of the human fluids, allowing to create numerical models to predict molecular concentrations from Raman spectra measurements. 11
Figure 2.
Summary of Raman spectroscopy as a tool for glucose monitoring on skin: (a) Raman spectroscopy functions as a monochromatic light to detect glucose on skin, (b) the skin layers, (c) skin having scattering and absorbing compounds, and (d) skin sites used to apply Raman spectroscopy to assess glucose in the studies included in the review. Colored version is available online.
Raman spectroscopy has many advantages like no sample destruction, the ability to obtain molecular structure information with high spatial resolution, no necessary reagents, 4 and as a technique for noninvasive GM includes low sensitivity to water and temperature changes, great specificity, and low cost. However, no technique is perfect, so RS has disadvantages, such as lack of laser wavelength and intensity stability, and spectra acquisition might take time depending on the amount of obtained spectrum (each spectrum can take from 30 s to 1 min). 9
Table 1 shows information regarding skin, blood, and glucose Raman spectra.
Table 1.
Relevant Peaks Positions of Raman Signals (cm−1) From Skin, Blood, and Glucose.
| Authors | Sample | Bands (cm−1) | Assignments | Biomolecules |
|---|---|---|---|---|
| Feng et al 12 | Skin | 937 | C–C stretching of prolines and valine and protein backbone | Keratin |
| Feng et al 12 | Skin | 940 | C–C stretching of protein backbone | Collagen, elastin |
| Feng et al 12 | Skin | 1003 | C–C vibration of phenyl ring | Collagen, elastin, and keratin |
| Feng et al 12 | Skin | 1063 | C–C asymmetric skeletal stretching of lipids (trans-conformation) | |
| Feng et al 12 | Skin | 1080 | C–C skeletal stretching in lipids | Triolein |
| Feng et al 12 | Skin | 1093 | O–P–O symmetric stretching vibration of the DNA backbone | Nucleus |
| Feng et al 12 | Skin | 1128 | C–C symmetric skeletal stretching | Ceramide |
| Feng et al 12 | Skin | 1248 | Amide III (ß-sheet and random coil conformations) | Collagen and elastin |
| Feng et al 12 | Skin | 1254 | ß sheet/thymine/cytosine (DNA base/DNA and RNA base) | Nucleus |
| Feng et al 12 | Skin | 1269 | Amide III (å helix conformation), C–N stretching, N–H in plane bending | Collagen, elastin, and keratin |
| Feng et al 12 | Skin | 1301 | C–H modes (CH2 twisting and wagging of lipids; CH2/CH3 bands) | Triolein |
| Feng et al 12 | Skin | 1336 | Amide III, C–N stretching, N–H in plane bending | Elastin |
| Feng et al 12 | Skin | 1337 | Adenine, guanine (DNA and RNA base) | Nucleus |
| Feng et al 12 | Skin | 1378 | Linear stretching of the C–C bonds within the rings | Melanin |
| Feng et al 12 | Skin | 1440 | CH2/CH3 bands | Triolein and ceramide |
| Feng et al 12 | Skin | 1450 | C–H bending of proteins | Keratin |
| Feng et al 12 | Skin | 1454 | C–H stretching, C–H asymmetric deformation | Collagen and elastin |
| Feng et al 12 | Skin | 1573 | In-plane stretching of the aromatic rings | Melanin |
| Feng et al 12 | Skin | 1645 | O–H bending mode of liquid water | Water |
| Feng et al 12 | Skin | 1653 | C–O stretching model of amide I | Keratin |
| Feng et al 12 | Skin | 1656 | C–C lipids | Triolein |
| Feng et al 12 | Skin | 1454 | C–H stretching, C–H asymmetric deformation | Collagen and elastin |
| Li et al 4 | Blood | 643 | Protein C–S stretching | Ascorbic acid |
| Li et al 4 | Blood | 752 | In-plane stretching of Lactams | Tryptophane |
| Li et al 4 | Blood | 827 | In-plane stretching of Heme | Fructose |
| Li et al 4 | Blood | 855 | In-plane bending C–C | Tyrosine and lactose |
| Li et al 4 | Blood | 898 | Protein C–C skeletal | Tyrosine |
| Li et al 4 | Blood | 940 | In-plane stretching C–C | Citric acid |
| Li et al 4 | Blood | 971 | Protein skeletal vibration | Fibrin |
| Li et al 4 | Blood | 1004 | In-plane stretching mode ring | Phenylalanine |
| Li et al 4 | Blood | 1026 | In-plane bending mode (=CbH2) asymmetric | Lactose |
| Li et al 4 | Blood | 1129 | In plane stretching of Lactams | Lactose |
| Li et al 4 | Blood | 1157 | In plane stretching of Heme | Heme |
| Li et al 4 | Blood | 1212 | In plane stretching of Heme | Heme |
| Li et al 4 | Blood | 1321 | Protein CH2 twist | Tyrosine |
| Li et al 4 | Blood | 1341 | In plane stretching mode | Tryptophane |
| Li et al 4 | Blood | 1423 | In plane stretching mode | Acetates |
| Li et al 4 | Blood | 1450 | In-plane bending mode (CH2/CH3) | Tryptophane |
| Li et al 4 | Blood | 1546 | In plane stretching mode | Heme |
| Li et al 4 | Blood | 1603 | In plane stretching mode of C=C | Heme |
| Li et al 4 | Blood | 1653 | Amide I | Heme |
| Shao et al 13 | Glucose a | 759 | ||
| Shokrekhodaei and Stella 10 | Glucose | 911 | ||
| Shokrekhodaei and Stella 10 | Glucose | 1060 | ||
| Shokrekhodaei and Stella 10 | Glucose a | 1125 | ||
| Shao et al 13 | Glucose a | 1549 | Hemoglobin |
Raman spectra of blood glucose obtained from a mouse.
Glucose
Plasma glucose is carried in blood arteries and transported by the circulatory system through arterioles to capillaries, and then it diffuses into the interstitial fluid (ISF) which surrounds the tissue cells. 14 Blood Raman spectrum has various peaks such as 650, 758, 837, 945, 978, 1004, 1130, 1163, 1217, 1332, 1551, and 1660 cm−1. 4
The glucose level in arterial blood and capillary blood is almost identical, but the interstitial glucose level is not the same (there is a delay of approximately 5-15 min). Therefore, any glucose changes measured within the tissue (interstitial fluid) will not represent a concurrent glucose level change in the blood. Furthermore, this delay is not constant, and it may differ between individuals, depending on the blood flow, the permeability of the capillary, glucose concentration gradients (higher lag time during a rapid change in blood glucose concentration), and the rate of glucose uptake (depends on insulin level). 14
Glucose represents a small percentage of the total volume of blood. Thus, it provides a small contribution to Raman peak amplitudes. 4 Glucose molecules (C6H12O6) vibrational modes are associated with C–O, C–C, and C–H stretching and are seen between 800 and 1200 cm−1 for C–O, C–C, and 2900 cm−1 for C–H. The typical glucose Raman fingerprints are observed at 911, 1060, and 1125 cm−1, with the highest intensity at 1125 cm−1. 10
Skin
The skin contains arterioles, venules, capillaries, and ISF, but this last component occupies significantly more volume than blood plasma. Figure 2b describes the skin layers, which are the stratum corneum (SC; 10-20 µm), the epidermis (30-100 µm), the dermis (900-1500 µm), and the subcutaneous tissue (1000-5000 µm) consisting of fat and muscle.15,16
The epidermis is an epithelial membrane in a constant state of turnover. The major substrate for metabolic activity is glucose (found in the interstitial space and intracellularly), as well it has enzymes that metabolize glucose. This layer includes 15% to 35% ISF and no blood vessels and receives all nutrients via diffusion from the underlying dermis. 17 Therefore, a careful interpretation is needed when GM is used in the epidermis since the correlation with blood may be indirect. The epidermis is divided into several layers: SC, stratum granolosum, stratum spinosum, and stratum basale. 15
The SC is the outer layer (thickness of 15-20 µm), where cells are constantly being shed from the body. Its lipophilic and dense nature is the main obstacle to overcome when attempting to sample glucose from the underlying tissue noninvasively. 18 In the stratum granusolum-SC, glucose is formed via the enzymatic breakdown of ceramides. 18
The epidermis and the dermis are interconnected by a succession of finger-like projections called papillae. The dermis comprises arterioles (that supply glucose), venules, capillaries, ISF (40%), collagen, elastic fibers, nerves, hair follicles, and sweat glands. Therefore, most home-monitoring techniques take a capillary blood sample to sense glucose. New techniques attempt to sample glucose in the dermal ISF. 19
RS and Skin
Creating new techniques for a noninvasive approach to monitor glucose levels aims to eliminate the discomfort of taking blood samples and help the populations improve GM. For this, RS could be one of the most promising optical techniques. Nevertheless, it is important to know the skin optics properties to ensure instrument functions.
Skin optics refers to how skin reflects and transmits light of different colors or wavelengths determined by the skin layers’ inherent optical properties. All skin layers have different inherent optical properties, primarily due to differences in the concentration of melanin, blood, and keratin between them. Another characteristic to consider is the optical depth of a layer, that is, the integrated attenuation coefficient of a beam going perpendicularly through the layer. In addition, light can be attenuated by absorption or scattering in another direction. 15
Also, the skin has scattering and absorbing compounds (Figure 2c). Scattering describes a change in the direction, polarization, phase of light, surface effect (reflection or refraction), or the interaction with a small region whose optical properties differ from its surroundings (particulate scatter). This scattering significantly contributes to the appearance of skin; it is estimated that 4% to 7% of visible light is reflected from the skin’s surface independently of wavelength and skin color. The rest of the light is refracted when passing from air into the skin. 20 The main scattering skin particles are lipids or proteins embedded in the fluids (mostly water) within and between skin cells. Lipids are found in the SC, cell membranes, and intracellular particles. At the same time, the main protein scatterers are keratin and melanin in the epidermis and collagen and elastin fibers in the dermis. 21 Those cells with dimensions close to the wavelength of the incoming light are the most efficient scatterers (scattering probability and scattering angle). For example, a cell with dimensions of the order of 10 µm scatters ultraviolet (UV) radiation much less efficiently than mitochondria (order of 1 µm) because UV radiation has wavelengths that are shorter than 0.4 µm (400 nm). 21
Absorption describes a reduction in light energy. The absorbing compounds considering the spectral regions UVB (280-320 nm), UVA (320-400 nm), and visible light (400-700 nm) are mainly blood, hemoglobin, and keratin. It is important to know that although blood is found beneath the epidermal layers, the amount of blood does affect the amount of UV and visible light that reaches these layers. The main blood absorption bands are found between the wavelengths 400 and 425 nm (violet light) and 500 and 600 nm (green light). The blood absorption is very low at wavelengths longer than 600 nm (red and NIR light). 15
The SC and epidermis are light-absorbing layers where specific pigments are present for UV photoprotection. However, when wavelengths in the NIR pass through the SC and epidermis, the transmittance of light is 90% to 95%, independently of skin pigmentation. However, in the dermis, light scattering is more important, and the scattering structures are collagen and elastic fibers (one-half of the volume fraction), ISF (90% of the rest), and a small fraction of cells and blood vessels. The latter are the main components for light absorption at wavelengths <600 nm. 22
With RS is possible to derive an estimation of glucose concentration due to the Raman spectrum usually considers a region between 200 to 1800 cm−1, where glucose can be differentiated from other compounds. An advantage of this technique is that it uses visible light and is very accessible. Besides, the optical components can be easily miniaturized. 23
However, this technique has limitations, such as it is sensitive to the scattering properties of the measured tissue due to scattering depolarizing the light, and skin cannot be investigated by polarimetry. Also, the specificity can be partially improved by using multiple light wavelengths. Other general sources of errors are variations in temperature and pH of the solution. 23
Aspects to Consider When Using RS for Noninvasive Glucose Measurement
Currently, several types of GM devices obtain accurate and quick glucose levels. Some are invasive or minimally invasive. However, all of them have advantages and disadvantages, such as RS.
For example, self-monitoring of blood glucose (SMBG) is performed by obtaining a capillary blood sample (<1 µL) by a lancing device. It is then measured by a glucose meter by chemical reactions (colorimetry, photometry, and electrochemistry). 1 Self-monitoring of blood glucose limitations are infection risk, and it implies a significant number of daily punctures that are uncomfortable and painful. 3 Another method to obtain glucose measurements is continuous glucose monitoring (CGM). This technology uses a sensor, a glucose oxidase platinum electrode that generates an electrical current generating an average glucose value every 5 minutes in the presence of glucose. The sensor (once every 14 days) can be placed under the skin in the buttocks, thighs, abdomen, or upper arm. 24 However, only 5% to 23% (depending on the age group) use the device; this could be due to the device costs, wear discomfort, and social factors. 5
Raman spectroscopy advantage as a noninvasive method is that no consumables are needed (testing strips, lancets, or sensors), and it is not painful (no need for lancing the finger or introducing a sensor in the skin). Therefore, the person can obtain multiple measurements (glucose oscillations) during the day without discomfort, which helps them maintain levels in the normal range and avoid health complications such as renal failure, peripheral neuropathy, and cardiovascular diseases.
Other RS advantages are less sensitive to temperature changes, minimally sensitive to water, suitable on any surface because it measures scattered light, including opaque substrates, and high specificity. 25 Water Raman spectrum has a minimal effect on glucose RS assessment. The OH stretching bond in 3400 cm−1 produces a large Raman shift, which can be used to estimate the blood water content. 4
However, RS has some disadvantages regarding accuracy and precision, especially due to physiological factors such as water and blood components. These are prone to interference from other molecules such as hemoglobin, unstable laser wavelength and intensity, long collection time, susceptible to noise interference (low signal to noise ratio), fluorescence, and turbidity. 25 The glucose Raman intensity peak can be normalized using a more stable reference within the body, such as hemoglobin. Hemoglobin concentration does not vary significantly between individuals. Thus, the relative Raman intensity of glucose can be normalized using the Raman fingerprint of hemoglobin at 1549 cm−1. 13
Regarding selecting the body site to obtain the glucose measurements, a nearly transparent epidermis, and a site with a high density of blood vessels are preferable. The fingertip’s nailfold and volar side are good sites that minimize signals from tissue components and maximize Raman spectra from blood components. Also, selecting a site with a high density of blood vessels minimizes the time lag between actual blood glucose measurements versus glucose within the tissue. 4
Skin Sites for Monitoring Glucose With RS
Strategic glucose sensor locations on the human body include the finger, ears, lip, forearm, anterior chamber of the eye, and across the tongue. Light enters the body, interacts with atoms within the tissue, and is absorbed, transmitted, or scattered. The interaction type depends on the incident light’s wavelength, tissue structure, and tissue optical properties. 10
Table 2 describes the data from studies that used RS on the skin for glucose measurements. Figure 2d shows the sites used in previous research where RS was applied to measure glucose.
Table 2.
Description of Studies That Used Raman Spectroscopy on Skin for Noninvasive Glucose Monitoring.
| Author | Study group | Evaluated skin site | Glucose standard | Raman spectroscopy instrument | Laser wavelength | Region | Applied statistics |
|---|---|---|---|---|---|---|---|
| Li et al 4 | 12 volunteers (6 women and 6 men) Average age 25 years, healthy, not taking medications |
The nailfold of the volunteer’s fourth finger. The fingertip was placed under the probe head, and the objective lens was adjusted to focus on the microvessels of each volunteer’s nailfold | OGTT. 12 h fasting, then asked to drink 250 mL water with 75 g glucose in 5 min. Every 5 min, a blood sample was obtained from the fingers of the unengaged hand with a glucose meter OneTouch | Renishaw inVia confocal Raman spectrometer (Renishaw, Inc., New Mills, UK) | 785 nm | 552-1675 cm−1 | PCA, BP-ANN |
| Lundsgaard-Nielsen et al 26 | 35 px with T2DM between 20 and 60 years. 14 males and 21 females. 32 with T1DM and 3 with T2DM |
Thenar (base of the thumb). They assessed the stratum corneum of participants (average 166 µm) | Capillary blood with HemoCue 201 RT (HemoCue AB, Ängelholm, Sweden) | Custom-built confocal Raman setup of external dimensions 475 mm (l) × 212 mm (h) × 361 mm (w) | 830 nm | 300-1800 cm−1 | PLS |
| Enejder et al 27 | 17 healthy Caucasian and Asian human volunteers | Forearm | OGTT. 220 ml of a beverage (SUN-DEX) containing 75 g of glucose. Reference capillary blood samples were collected from finger sticks every 10 min (277 total) and analyzed by means of a Hemocue glucose analyzer | Custom-built Raman system | 830 nm | 355-1545 cm–1 | Means of PLS regression. Spectra were smoothed with a 13-point Savitsky-Golay algorithm to increase the effective SNR and the mean centered |
Abbreviations: OGTT, oral glucose tolerance test; PCA, principal component analysis; BP-ANN, back propagation artificial neural network; T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes; PLS, partial least squares; SNR, signal to noise ratio.
Nailfold
Nailfold refers to the small area of the skin beneath the nail. The microvessels structure parallel to the skin surface of the nailfold can be seen clearly, indicating that the nailfold SC and epidermis are very thin and have good light transmittance. In addition, the nailfold contains a thinner epidermis that is only about 100 µm, so it can gather more information from the dermis where microvessels are found. 4
It has been analyzed glucose measurements in the nailfold (fourth finger) by RS of 12 healthy volunteers. They performed an oral glucose tolerance test (OGTT) and evaluated capillary glucose measurements every 5 minutes with a glucose meter (OneTouch, Johnson & Johnson, New Brunswick, NJ, USA) to compare RS glucose measurements. The authors reported no time lag between the predicted blood glucose and the actual blood glucose in the OGTT. Also, the mean prediction performance of 12 volunteers was obtained as the root mean square error of prediction (RMSEP) of 0.45 mmol/L and R2 of 0.95. From day 1 to 9, 12 volunteers were used as the calibration set; day 10 was used as the prediction set, obtaining the total predicted performance of all volunteers with RMSEP of 0.27 mmol/L and R2 of 0.98. Suggesting that anatomical differences between volunteer fingernails do not reduce the prediction accuracy, and 100% of the predicted glucose concentrations fell within Region A and B of the Clarke error grid, allowing acceptable predictions in a clinically relevant range. 4
Finger
The depth of penetration of the excitation light might be shallow depending on the laser wavelength and skin thickness. For example, a wavelength of 532 nm can reach the epidermis, 785 nm from the SC to the dermis, and a wavelength of 830 nm might assess from the SC to the dermal vessels. 28
Glucose in the microvascular of the dermis diffuses to the epidermis. Glucose concentration in the ISF is lower than blood glucose. The excitation efficiency of Raman scattering is very low, and its intensity is only one-thousandth of Rayleigh scattering. To obtain a low concentration of ISF glucose percutaneously, much of the existing work has focused on improving the detection instrument to increase the Raman spectral intensity of blood glucose. Fortunately, the skin at the special anatomical site of the human body has an ultra-thin SC and a nearly transparent epidermis while also having a high density of blood vessels such as nailfold. 4
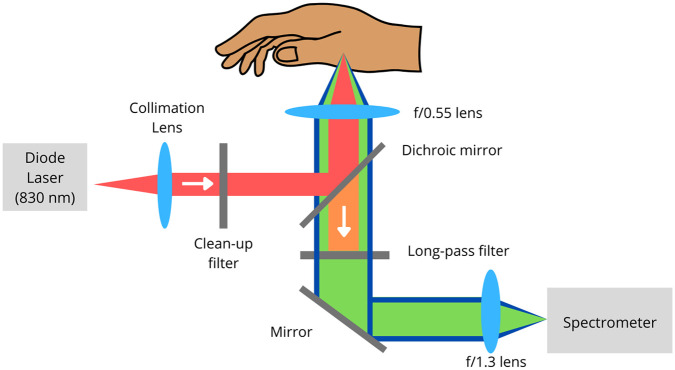
Critical-depth RS was applied on the base of the thumb (the thenar) to measure glucose levels (ISF located below the SC) in 35 patients with diabetes for 60 days using a table-top confocal Raman spectrometer that was used at the patients’ home. Figure 3 describes the custom-built Raman spectrometer researchers used to analyze glucose in the thenar.
Figure 3.
Custom-built confocal Raman spectrometer. It explains how the Raman spectrometer works to assess the glucose onto the thenar (base of the thumb). Colored version is available online.
Source: The image was adapted from Lundsgaard-Nielsen et al. 26
The obtained glucose measurements were correlated with capillary blood glucose values using a standard finger-stick and test strip product. However, it is not indicated how many capillary blood glucose values were taken or if they were obtained after an OGTT. 26
Lundsgaard-Nielsen et al 26 found that the critical depth was greater than 240 µm (necessary to probe beyond the SC to measure dynamic glucose levels) and Inter-Subject Unified Performance (indicator developed by the authors) larger than 0.5 gave an independent validation of 93% for readings in zone A and B of the consensus error grid and an overall mean average relative difference (MARD) of 25.8%.
Mean average relative difference is based on comparing paired measurements of a given CGM system and a reference method. With 100 points, the true MARD value is expected to be between 8.2% and 12%. After 5000 points, the confidence interval would be reduced to 9.8% and 10.4%. 29
Forearm
Performance of RS applied in the forearm (transcutaneous) monitoring of glucose concentrations was evaluated in 17 healthy volunteers whose glucose levels were elevated for 2 to 3 h using OGTT. A total of 461 Raman spectra were collected transcutaneously during the test, with glucose reference values provided by standard capillary blood analysis. They observed multiple peaks corresponding to the Raman glucose spectrum distinct from human skin tissue, ensuring they assessed glucose. In addition, calibrations appear good for almost all volunteers having an R2 of more than 0.8 and mean absolute errors of 9% or less. Only 2 participants had an R2 < 0.7. 27
The advantages of analyzing the skin for noninvasive glucose measurement are that it is an accessible tissue where glucose can be measured in the ISF in the epidermis (especially in the SC). The most selected skin sites for using RS for glucose noninvasive measurements were the nailfold, finger, and forearm due to the penetration depth of the excitation light in these sites can reach the SC (10-20 µm) and the ISF. The nailfold was the site with a greater R2 compared to the forearm. Studies found that RS is a good optical technique to measure noninvasively glucose on the skin by comparing the glucose levels obtained by RS with glucose levels from invasive methods such as glucose meters with testing strips, mostly during an OGTT.
However, it is difficult to compare the studies since they had different study samples (healthy participants or people living with diabetes), laser wavelengths (785 or 830 nm), regions (from 300 to 1800 cm−1), analyzed skin sites, and multivariate analysis. Besides, 2 of the studies used custom-built Raman systems.
On the contrary, more research is needed, especially clinical studies to assess the stability, accuracy, and reproducibility in short (<60 days) and long periods in populations of different group ages. As well, it will be interesting to compare RS glucose measurements between healthy and people living with diabetes. People living with diabetes or older people might have differences in the skin that can interfere with RS glucose measurements. It has been reported that changes in the skin hydration state, degradation of type I collagen, and greater glycation were associated with diabetes and chronological aging. 8
Another important issue in clinical research is the sample size, ethnical background, and demographic traits. It will be important to assess glucose through RS in healthy populations of different ages to establish a calibration (gold standard) according to age, sex, and ethnical background.
In addition, it is essential to assess the costs of these new devices because if they are expensive, people will not use them. Therefore, people will not beneficiate from RS advantages. As is the case of CGM, which is expensive, and not many people with diabetes can afford it.
Besides, it is necessary to evaluate the acceptance by people living with diabetes and health professionals of this kind of device that works with RS. For example, a study reported that parents of children with diabetes using CGM perceived barriers in the use of this kind of device were: it provides much information that can be difficult to interpret, feeling disturbed or disrupted by the alerts for low and high glucose values, concerns about the device inaccuracy or failure, frustrations related to gaps in data due to lost signals between the transmitter and display device. 30
Finally, a critical issue to consider for future technology innovation for glucose-monitoring devices that uses RS is the portability, size, weight, and software.
Conclusion
Currently, invasive methods are the most employed GM devices as the gold standard. Although these give high-accuracy measurements, they have disadvantages in their use, such as SMBG sensors require drawing a drop of blood to be tested through finger-pricking, and CGM sensors are based on a needle implanted subcutaneously, which is painful, can promote infections, be costly, and it can be seen a deterioration of accuracy over time.
Therefore, developing new alternatives for noninvasive glucose measurements that overcome these disadvantages is necessary to improve the life quality of people living with diabetes being RS a possible solution. Some of RS advantages are that it is not painful (no need for lancing the finger or introducing a sensor in the skin) and no consumables are needed (testing strips, lancets, or sensors), therefore, no waste production (ecological benefits).
The few studies that have assessed RS for transcutaneous noninvasive glucose measurements found that it is a good optical technique (compared with OGTT data), and the preferred skin sites to analyze glucose were nailfold, finger (base of the thumb), and forearm. However, more research is needed to assess accuracy (other study designs), stability, costs, and acceptance.
Acknowledgments
This work was supported in part by CONACYT through “Estancias Posdoctorales por México” and by the National Laboratory program from CONACYT, through Terahertz Science and Technology National Lab (Laboratorio Nacional de Ciencia y Tecnología de Terahertz, LANCYTT).
Footnotes
Abbreviations: CGM, continuous glucose monitoring; GDM, gestational diabetes; GM, glucose monitoring; HbA1c, glycated hemoglobin; ISF, interstitial fluid; LDL, low-density lipoprotein; MARD, mean average relative difference; NIR, near-infrared; OGTT, oral glucose tolerance test; RMSEP, root mean square error of prediction; RS, Raman spectroscopy; SC, stratum corneum; SMBG, self-monitoring of blood glucose; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alejandra Loyola-Leyva  https://orcid.org/0000-0002-0355-803X
https://orcid.org/0000-0002-0355-803X
Karen Hernández-Vidales  https://orcid.org/0000-0002-3355-4785
https://orcid.org/0000-0002-3355-4785
Juan Pablo Loyola-Rodríguez  https://orcid.org/0000-0003-0892-0082
https://orcid.org/0000-0003-0892-0082
Francisco Javier González  https://orcid.org/0000-0002-1346-9073
https://orcid.org/0000-0002-1346-9073
References
- 1. Patton SR, Clements MA. Continuous glucose monitoring versus self-monitoring of blood glucose in children with type 1 diabetes-the pros and cons. US Endocrinol. 2012;8(1):27-29. [PMC free article] [PubMed] [Google Scholar]
- 2. Tourkmani AM, Alharbi TJ, Rsheed AMB, et al. Hypoglycemia in type 2 diabetes mellitus patients: a review article. Diabetes Metab Syndr. 2018;12(5):791-794. doi: 10.1016/j.dsx.2018.04.004. [DOI] [PubMed] [Google Scholar]
- 3. Frontino G, Meschi F, Bonfanti R, et al. Future perspectives in glucose monitoring sensors. Eur Endocrinol. 2010;9(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li N, Zang H, Sun H, et al. A noninvasive accurate measurement of blood glucose levels with Raman spectroscopy of blood in microvessels. Molecules. 2019;24(8):1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lanning MS, Tanenbaum ML, Wong JJ, Hood KK. Barriers to continuous glucose monitoring in people with type 1 diabetes: clinician perspectives. Diabetes Spectr. 2020;33(4):324-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loyola-Leyva A, Hernández-Vidales K, Loyola-Rodríguez JP, González FJ. Raman spectroscopy applications for the diagnosis and follow-up of type 2 diabetes mellitus. A brief review. Biomed Spectrosc Imaging. 2020;9:119-140. [Google Scholar]
- 7. Guevara E, Torres-Galván JC, Ramírez-Elías MG, Luevano-Contreras C, González FJ. Use of Raman spectroscopy to screen diabetes mellitus with machine learning tools. Biomed Opt Express. 2018;9:4998-5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paolillo FR, Mattos VS, de Oliveira AO, Guimarães FEG, Bagnato VS, de Castro Neto JC. Noninvasive assessments of skin glycated proteins by fluorescence and Raman techniques in diabetics and nondiabetics. J Biophotonics. 2019;12(1):e201800162. [DOI] [PubMed] [Google Scholar]
- 9. Alsunaidi B, Althobaiti M, Tamal M, Albaker W, Al-Naib I. A review of non-invasive optical systems for continuous blood glucose monitoring. Sensors (Switzerland). 2021;21:6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shokrekhodaei M, Stella Q. Review of non-invasive glucose sensing techniques: optical, electrical and breath acetone. Sensors (Switzerland). 2020;20:1251. https://sci-hub.scihubtw.tw/10.3390/s20051251. Accessed June 24, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernández-Vidales K, Guevara E, Olivares-Illana V, González FJ. Characterization of wild-type and mutant p53 protein by Raman spectroscopy and multivariate methods. J Raman Spectrosc. 2019;50:1388-1394. [Google Scholar]
- 12. Feng X, Moy AJ, Nguyen HTM, et al. Raman biophysical markers in skin cancer diagnosis. J Biomed Opt. 2018;23(5):1-10. doi: 10.1117/1.JBO.23.5.057002. [DOI] [PubMed] [Google Scholar]
- 13. Shao J, Lin M, Li Y, et al. In vivo blood glucose quantification using Raman spectroscopy. PLoS ONE. 2012;7(10):e48127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Siegmund T, Heinemann L, Kolassa R, Thomas A. Discrepancies between blood glucose and interstitial glucose—technological artifacts or physiology: implications for selection of the appropriate therapeutic target. J Diabetes Sci Technol. 2017;11(4):766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nielsen KP, Zhao L, Stamnes JJ, Stamnes K, Moan J. The optics of human skin: aspects important for human health. Sol Radiat Hum Heal. 2008;1:35-46. [Google Scholar]
- 16. Caduff A, Talary MS, Zakharov P. Cutaneous blood perfusion as a perturbing factor for noninvasive glucose monitoring. Diabetes Technol Ther. 2010;12(1):1-9. [DOI] [PubMed] [Google Scholar]
- 17. Urbach E, Lentz J. Carbohydrate metabolism and the skin. Arch Derm Syphilol. 1945;52:301-316. http://www.ncbi.nlm.nih.gov/pubmed/21009657. Accessed January 25, 2022. [DOI] [PubMed] [Google Scholar]
- 18. Sieg A, Guy RH, Delgado-Charro MB. Noninvasive and minimally invasive methods for transdermal glucose monitoring. Diabetes Technol Ther. 2005;7(1):174-197. [DOI] [PubMed] [Google Scholar]
- 19. Yum S, Il Roe J. Capillary blood sampling for self-monitoring of blood glucose. Diabetes Technol Ther. 1999;1(1):29-37. doi: 10.1089/152091599317549. [DOI] [PubMed] [Google Scholar]
- 20. Lister T, Wright PA, Chappell PH. Optical properties of human skin. J Biomed Opt. 2012;17(9):0909011. [DOI] [PubMed] [Google Scholar]
- 21. Nielsen KP, Lu Z, Juzenas P, Stamnes JJ, Stamnes K, Moan J. Reflectance spectra of pigmented and nonpigmented skin in the UV spectral region. Photochem Photobiol. 2004;80(3):450-455. http://www.ncbi.nlm.nih.gov/pubmed/15623329. Accessed January 27, 2022. [DOI] [PubMed] [Google Scholar]
- 22. Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol. 1981;77(1):13-19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 23. Tura A, Maran A, Pacini G. Non-invasive glucose monitoring: assessment of technologies and devices according to quantitative criteria. Diabetes Res Clin Pract. 2007;77(1):16-40. [DOI] [PubMed] [Google Scholar]
- 24. Nardacci EA, Bode BW, Hirsch IB. Individualizing care for the many. Diabetes Educ. 2010;36(suppl 1):4S-19S. doi: 10.1177/0145721710362798 [cited 2022 Feb 15] [DOI] [PubMed] [Google Scholar]
- 25. Villena Gonzales W, Mobashsher AT, Abbosh A. The progress of glucose monitoring—a review of invasive to minimally and non-invasive techniques, devices and sensors. Sensors (Switzerland). 2019;19:800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lundsgaard-Nielsen SM, Pors A, Banke SO, Henriksen JE, Hepp DK, Weber A. Critical-depth Raman spectroscopy enables home-use non-invasive glucose monitoring. PLoS ONE. 2018;13:e0197134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Enejder AM, Scecina TG, Oh J, et al. Raman spectroscopy for noninvasive glucose measurements. J Biomed Opt. 2005;10(3):031114. [DOI] [PubMed] [Google Scholar]
- 28. Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. Mosby; 2003. https://www.iberlibro.com/buscar-libro/titulo/dermatology-2-volume-set/autor/bolognia-jean-jorizzo-joseph-rapini/. Accessed March 24, 2022. [Google Scholar]
- 29. Reiterer F, Polterauer P, Schoemaker M, et al. Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol. 2017;11(1):59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hilliard ME, Levy W, Anderson BJ, et al. Benefits and barriers of continuous glucose monitoring in young children with type 1 diabetes. Diabetes Technol Ther. 2019;21(9):493-498. [DOI] [PMC free article] [PubMed] [Google Scholar]