Abstract
Background:
The increased use of continuous glucose monitoring (CGM) and automated insulin delivery systems raises the question about therapeutic targets for glucose profiles in people with diabetes. This study aimed to assess averaged pre- and postprandial glucose profiles in people without diabetes to provide guidance for normal glucose patterns in clinical practice. For that, number and timing of meal intake were predefined.
Material and Methods:
To assess glucose traces in 36 participants without diabetes (mean age = 23.7 ± 5.7 years), CGM was performed for up to 14 days, starting with a run-in phase (first 3 days, excluded from analysis) followed by 4 days with fixed meal times at 8:00 am, 1:00 pm, and 6:00 pm and the remaining 7 days spent under everyday life conditions. Data from two simultaneously worn CGM sensors were averaged and adjusted to capillary plasma-equivalent glucose values. Glucose data were evaluated through descriptive statistics.
Results:
Median glucose concentration on days with fixed meal times and under everyday life conditions was 95.0 mg/dL (91.6-99.1 mg/dL, interquartile range) and 98.1 mg/dL (93.7-100.8 mg/dL), respectively. On days with fixed meal times, mean premeal glucose was 92.8 ± 9.4 mg/dL, and mean peak postmeal glucose was 143.3 ± 23.5 mg/dL.
Conclusions:
By defining the time of meal intake, a clear pattern of distinct postprandial glucose excursions in participants without diabetes could be demonstrated and analyzed. The presented glucose profiles might be helpful as an estimate for adequate clinical targets in people with diabetes.
Keywords: type 1 diabetes, continuous glucose monitoring, postprandial glucose, glucose profiles
Introduction
For more than 25 years, self-monitoring of blood glucose (SMBG) has been an adequate tool for the treatment of people with diabetes, especially for those on intensified insulin therapy. 1 However, these measurements are usually performed preprandially, whereas postprandial measurements are often missing. The use of continuous glucose monitoring (CGM) systems provides comprehensive glucose profiles with the opportunity to improve diabetes therapy outcomes. 2
The goal of diabetes therapy is to avoid pronounced glucose fluctuations and to achieve a near-normal glucose profile to prevent acute and long-term complications. 3 However, the selection of appropriate goals, including postprandial targets, in diabetes therapy is of importance because in particular elevated postprandial glucose levels have been shown to be associated with an increased cardiovascular risk.4,5 Nevertheless, the definition of postprandial glucose targets in current guidelines varies. Whereas the International Diabetes Federation (IDF) guideline 6 makes the recommendation of a postmeal glucose target of <160 mg/dL (9 mmol/L), the American Diabetes Association (ADA) guideline 3 recommends that patients with diabetes should aim for postprandial plasma glucose values of <180mg/dL (10 mmol/L). Some data on continuous glucose curves in people without diabetes, especially with a focus on the postprandial glucose course, which could serve as guidance on what constitutes a normal glucose profile, are available.7 -16 However, some of these studies7 -9 used earlier-generation CGM systems for adjunctive use, which are known to be less accurate than current devices. 17 In addition, some of the studies8,9 used capillary whole blood values for calibration in contrast to the capillary plasma-equivalent values that are used today, further limiting the comparability of results. A constant factor of 1.11 is recommended to convert concentration in whole blood to the equivalent concentration in the pertinent plasma. 18
Typical distinct glucose peaks after meal intake can only be observed and analyzed if meals are taken at defined time points.8,14 -16 Otherwise, if the meals are consumed at any time during the day, the glucose values will be averaged over several days or persons and the resulting mean glucose profile will not reflect any postprandial peak.10,13
The present study therefore investigated glucose profiles in people without diabetes after fixed meal times and under everyday life conditions with the goal to provide relevant postprandial glucose profiles in healthy individuals, which can support the definition of clinical targets in people with diabetes.
Methods
The presented open, mono-center study was performed between January and March 2018 at the Institut für Diabetes-Technologie, Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Germany. The Declaration of Helsinki, the Guideline for Good Clinical Practice and the national regulations and provisions have been considered. The study protocol was approved by the responsible Ethics Committee, and the study was registered at clinicaltrials.gov (NCT03405415).
Participants
Adult people without diabetes mellitus were eligible for the study. All participants signed informed consent prior to any study procedures and were included after a screening visit if they fulfilled the eligibility criteria. Inclusion criteria were age ≥18 years, and the willingness to abstain from medications containing salicylic acid or ascorbic acid during the study period. Exclusion criteria were diabetes; acute or severe chronic illness (at the physician’s discretion); pregnancy or lactation period; known severe allergy to medical grade adhesive; language or other barriers that might preclude sufficient understanding of the study procedures; and blood donation in the previous two months. After screening, 41 participants were included in the study.
Study Devices and Comparison Measurements
The factory-calibrated FreeStyle Libre (Abbott Diabetes Care, Alameda, CA) intermittent-scanning CGM system was used. It measured glucose levels every minute in the interstitial fluid and stored one value every 15 minutes for up to 14 days. To obtain continuous glucose data over this period, the CGM system needed to be actively scanned using a handheld reader device capable of displaying the results at least once every eight hours. Participants were asked to perform regular scans to retain the whole daily glycemic data. 19 Each participant wore two CGM sensors in parallel.
Capillary whole blood glucose (BG) measurements were performed using the CONTOUR NEXT ONE (Ascensia Diabetes Care Holdings AG, Basel, Switzerland) SMBG system, which yields capillary plasma-equivalent values.
Assessment of Glucose Trace Data
To minimize the effect of measurement errors and increase the reliability of the CGM data, a re-calibration and subsequent combination of sensor signals obtained from the same participant was carried out generating a single glucose curve. For that, individual sensor data were linearly interpolated on a 1-minute sampling grid thereby filling gaps in the data, if present, for up to three hours. Furthermore, duplicate SMBG measurements were averaged if the difference between the two values did not exceed 10 mg/dL (for values <100 mg/dL) or 10% (for values ≥100 mg/dL) and otherwise excluded. After removal of the first 12 hours of data, a Passing-Bablok regression 20 between CGM and SMBG data was performed and the resulting linear equation was used to re-calibrate the CGM data. Subsequently, the CGM data from simultaneously worn sensors were averaged based on the regression residuals. This procedure yielded a single SMBG-adjusted CGM glucose concentration trace per participant which is hereinafter referred to as glucose trace and resulted in improved accuracy: The mean absolute relative differences (MARDs) of the unprocessed CGM data and calculated glucose traces were 9.9 ± 4.2% and 6.5 ± 1.2%, respectively. The ±20% agreement rates were 89.2 ± 9.9% for the unprocessed data and 96.3 ± 0.5% for the calculated glucose trace data.
Further information on data preparation is provided in the Supplemental Material.
Study Design and Procedures
Study duration for each participant was 14 days. During the study, the participants completed a daily log indicating physical activity and carbohydrate or meal consumption, as well as sleep times (going to bed until wake-up) and additional information to provide context to the recorded glucose concentration data.
On day 1, potential study participants arrived for screening at the study site and were instructed on the use of CGM and SMBG devices. Two CGM sensors were then placed on the participants’ upper arms (one sensor per arm). The participants returned on the morning of day 3 for a 75-g oral glucose tolerance test (oGTT; Accu-Chek Dextrose O.G-T., Roche Diabetes Care Deutschland GmbH, Mannheim, Germany). Venous plasma glucose concentrations were determined using a hexokinase-based laboratory analyzer (Cobas Integra 400 plus; Roche Instrument Center, Rotkreuz, Switzerland) to check for diabetes, impaired fasting glucose (IFG), or impaired glucose tolerance (IGT) based on diagnostic criteria of the German Diabetes Society. 21 One additional sample was obtained to assess glycated hemoglobin A1c (HbA1c).
During study site visits on days 4 and 5 (between 7:30 am and 7:30 pm), participants received standardized meals (50 g carbohydrates) for breakfast (8:00 am) and lunch (1:00 pm), and a free-choice dinner (6:00 pm) from a buffet.
On days 6 and 7, participants did not return to the study site but were asked to consume three free-choice meals per day at the same times as the days before (8:00 am, 1:00 pm, and 6:00 pm), and to avoid carbohydrate-containing drinks and physical strain. From day 8 to the end of the study on day 14, participants were allowed to follow their usual daily-life routine.
Capillary blood glucose measurements were performed in duplicate at least 4 times per day outside of the study site, and at least once per hour during study site visits.
Statistical Analysis and Visual Report of Glucose Data
Data from participants with oGTT results indicating diabetes, IFG, or IGT were excluded from the analysis. Based on international consensus, 22 data of participants from whom <70% of glucose data could be recovered were also removed. Concerning the remaining participants, data from days 1 and 2 (≈36 hours) were excluded from the analysis due to the increased likelihood of sensor errors. 23 Data from day 3 were excluded due to an uncommon glycemic load of the oGTT.
Results were calculated separately for days of fixed meal times (days 4-7) and days under everyday life conditions (days 8-14).
All analyses were performed using the aforementioned glucose traces as these were the most reliable continuous glucose signals. Population results were obtained by first calculating the mean values of the glucose trace in the specified period per participant and then calculating the mean and median of all participants. Coefficient of variation (CV) was calculated and the glucose management indicator (GMI) was determined. 24 Time spent within different glucose ranges was calculated as the number of values that fell within the corresponding range divided by the total number of values per participant (represented as percentage).
Glucose data for days with fixed (days 4-7) and free meal times (days 8-14) were visualized in a glucose profile based on Bergenstal et al 25 and the recommendations of the international consensus statement. 22
Results are given as either mean ± standard deviation (SD), median with interquartile range (IQR), or range (min-max).
Results
Population Characteristics
Abnormal oGTT results (3 cases) or insufficient glucose data (2 cases) led to exclusion of five participants’ data from analysis. The remaining 36 participants, 15 men and 21 women, had a mean age of 23.7 ± 5.7 years and a mean body mass index (BMI) of 23.8 ± 3.5 kg/m². Mean HbA1c was 34 ± 2 mmol/mol (30-38 mmol/mol) (5.2 ± 0.2% [4.9%-5.6%]), indicating the absence of diabetes. 21 In total, 10 adverse events at the site of sensor application were documented (2× redness, 5× itching, 1× pain, 2× hematoma) all of which were mild.
Glucose Trace Data on Days With Fixed Meal Times (Days 4-7)
The mean duration for glucose trace data on days with fixed meal times was 93.6 ± 3.5 hours (Table 1). The 10th and 90th percentiles (containing 80% of glucose trace data) were found at 81.8 mg/dL (71.5–108.3 mg/dL) and at 112.5 mg/dL (93.9–175.5 mg/dL), respectively (Figure 1). Median glucose concentration was 95.0 mg/dL (91.6-99.1 mg/dL). Relative times spent between 70 and 140 mg/dL, below 70 mg/dL, and above 140 mg/dL were 93.5±3.5%, 1.7±2.8%, and 4.8±2.8%, respectively.
Table 1.
Glucose Traces and CGM-Derived Daily Population Metrics of n = 36 Participants Without Diabetes for Days of Fixed Meal Times (4 Days per Participant) and Under Everyday Life Conditions (7 Days per Participant). Data are Presented as Either Mean ± SD (With Range [Min-Max]), or Median With 25%-75% Range.
| Fixed meal times (days 4-7) |
Everyday life conditions (days 8-14) |
|
|---|---|---|
| Mean CGM use, hours | 93.6 ± 3.5 [84.5-96.0] | 155.4 ± 15.3 [102.9-168.0] |
| GMI, % | 5.7 ± 0.1 | 5.8 ± 0.1 |
| Median Glc, mg/dL | 95.0 (91.6-99.1) | 98.1 (93.7-100.8) |
| Mean Glc, mg/dL | 100.2 ± 5.6 | 102.0 ± 5.6 |
| SD, mg/dL | 19.1 ± 3.8 | 17.5 ± 3.6 |
| CV, % | 19.1 ± 3.9 | 17.2 ± 3.6 |
| Times in ranges, % | ||
| <70 mg/dL | 1.7 ± 2.8 | 0.9 ± 1.7 |
| 70-140 mg/dL | 93.5 ± 3.5 | 95.2 ± 3.0 |
| >140 mg/dL | 4.8 ± 2.8 | 4.0 ± 2.8 |
Abbreviations: CGM, continuous glucose monitoring; CV, coefficient of variation; Glc, glucose concentration; GMI, glucose management indicator; SD, standard deviation.
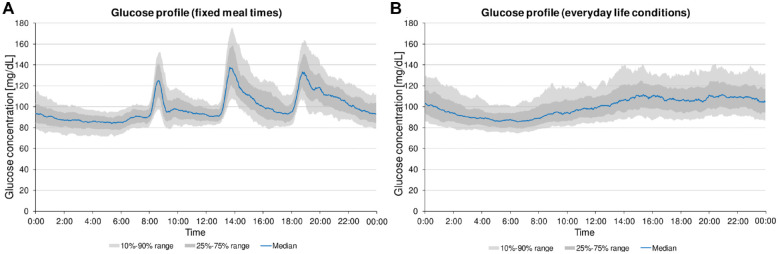
Figure 1.
(a) Glucose profile (based on Bergenstal et al 25 ) of 36 participants without diabetes for days with fixed meal times (4 days, n = 144). (b) Glucose profile of these participants for days under everyday life conditions (7 days, n = 252).
A potential dawn effect of glucose concentrations between 01:00 and 05:00 in the morning could not be observed (Figure 1).
Figure 2 illustrates postprandial glucose profiles of 36 participants without diabetes for all consumed meals on days with fixed meal times. Median premeal glucose concentration was 91.7 mg/dL (86.4-98.3 mg/dL, IQR). In less than one hour (50 ± 19min), after meal intake median postprandial glucose traces reached a peak at 139.8 mg/dL (127.0-157.4 mg/dL, IQR), with the 10th percentile at 116.0 mg/dL and the 90th percentile at 174.2 mg/dL (Table 2). The postprandial glucose profile of all consumed meals (n = 432) showed a marked median increase of glucose concentrations by 47.3 mg/dL. Detailed results are provided in Tables 1 and 2.
Figure 2.
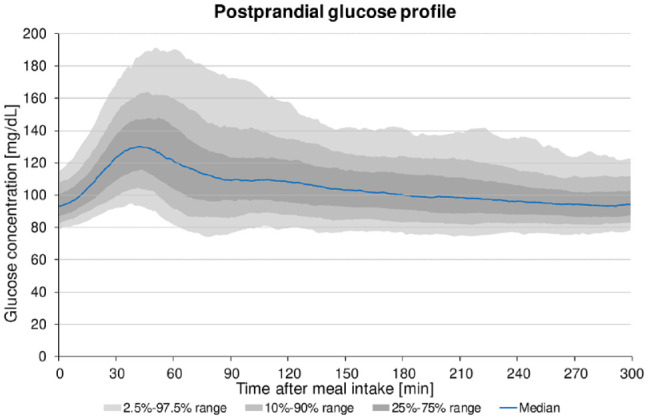
Postprandial glucose profile (based on Bergenstal et al 25 ) of 36 participants without diabetes summarized for all consumed meals on days with fixed meal times (12 meals, n = 432).
Table 2.
Pre- and Postprandial Glucose Traces and Glucose Characteristics of 36 Participants Without Diabetes Summarized for All Consumed Meals on Days With Fixed Meal Times (12 Meals per Participant, n = 432).
| Fixed meal times | Mean ± SD | 2.5% Qu | 10% Qu | 25% Qu | Median | 75% Qu | 90% Qu | 97.5% Qu |
|---|---|---|---|---|---|---|---|---|
| Premeal glucose, mg/dL | 92.8 ± 9.4 | 77.0 | 81.7 | 86.4 | 91.7 | 98.3 | 104.7 | 113.6 |
| Time to peak, min | 50 ± 19 | 26 | 32 | 38 | 47 | 56 | 78 | 105 |
| Peak glucose, mg/dL | 143.3 ± 23.5 | 107.1 | 116.0 | 127.0 | 139.8 | 157.4 | 174.2 | 198.9 |
| Glucose increase, mg/dL | 50.9 ± 23.6 | 18.6 | 23.4 | 32.6 | 47.3 | 65.2 | 80.3 | 113.7 |
Abbreviations: CGM, continuous glucose monitoring; Qu, quantile; SD, standard deviation.
Glucose Trace Data on Days Under Everyday Life Conditions (Days 8-14)
On days under everyday life conditions (days 8-14), glucose trace data were available for 155.4 ± 15.3 hours (Table 1). A corresponding median 24-hour glucose profile is shown in Figure 1, in which no glucose peaks are apparent throughout the day. Individual glucose peaks can occur at any given time of day or night depending on the time of food intake but are averaged over the whole population. The 10th to 90th percentiles (containing 80% of glucose trace data) were found at 85.3 mg/dL (74.8-93.9 mg/dL) and 125.2 mg/dL (99.3-140.9 mg/dL), respectively (Figure 1). Median glucose concentration was 98.1 mg/dL (93.7-100.8 mg/dL). Time between 70 and 140 mg/dL, time below 70 mg/dL, and time above 140 mg/dL were 95.2±3.0%, 0.9±1.7%, and 4.0±2.8%, respectively. Detailed results are provided in Table 1.
Discussion
In the present study, we have shown that defined timing of meal intake resulted in CGM profiles with pronounced postprandial peaks in healthy individuals that remained visible even after averaging over the full population. In contrast, under everyday life conditions with meal intake at any time of the day, the average CGM glucose profile summarized over the entire population was absent of obvious postprandial glucose peaks, which is often presented as the “typical” average glucose profile of people without diabetes in several studies.10,13
Importantly, the comparison of days with defined meal intake to days under everyday life conditions showed no clinically relevant changes in the calculated CGM-based metrics. The scheduling of meal intake has thus only changed the form of the median glucose profiles, with the possibility of making postprandial courses in healthy individuals visible and enabling more detailed assessment.
Analyzing the postprandial glucose traces in this study (Figure 2), the median peak was found at 140 mg/dL, with the 90th percentile almost reaching 180 mg/dL. In fact, postprandial glucose traces above 160 mg/dL or even up to 180 mg/dL occurred regularly in healthy individuals in our study. This contrasts with the statement in the IDF guideline which says that postprandial blood glucose levels rarely exceed 140 mg/dL 6 in people with normal glucose tolerance. Even the postmeal glucose targets for people with diabetes of 160 mg/dL in the IDF and of 180 mg/dL in the ADA guideline were not achieved by all participants in our study. Nevertheless, all participants reached >90% time in the target range of 70-180 mg/dL which clearly exceeds the target recommended by Battelino et al 22 for diabetes patients (>70% time in the target range 70-180 mg/dL).
In comparison to our study, DuBose and colleagues 10 recently presented smoother postprandial glucose courses with a lower mean peak of about 130 ± 13 mg/dL. In addition to a different age distribution of the included participants (age 7-80 years), the main difference between this study and the study by DuBose et al 10 was that meal intake was not defined and there may have been differences in meal timing. Our study, however, was partially inpatient and had strict mealtime requirements for days with fixed meal times even at home. Still, unreported snacks throughout the day cannot be ruled out in both studies.
In 2007, we performed a similar study investigating CGM profiles in people without diabetes with fixed meal times and after ingestion of different meals. 8 It is noticeable that the highest postprandial peak was observed after breakfast in our previous study, whereas the postprandial increase after breakfast showed a less pronounced peak in this study. This is likely due to the fact that the study participants in the previous study stayed the night at the study site without substantial activity before breakfast. In contrast, the study participants in the current study came to the study site in the morning and were therefore already active, possibly resulting in a less pronounced glucose increase (Figure 1a).26 -28
The median and mean 24-hour glucose concentrations under everyday life conditions of approximately 100 mg/dL found in our study are similar to other CGM studies in people without diabetes with current CGM systems.10,11,13 In earlier studies,7 -9 generally lower mean concentrations were found, for example, approximately 90 mg/dL in our previous study. 8 This systematic difference is most likely due to the calibration of CGM systems on whole blood glucose values in earlier studies, because currently used plasma-equivalent values are expected to be approximately 11% higher. 18 In addition, early generation CGM systems were often less accurate.
The long observation period of 14 days and the resulting amount of glucose traces contribute to the reliability of the calculated profiles. In particular, the scheduling of food intake maximized the impact of meals on the average population curves, whereas the detailed specification of percentiles attempted not to overestimate the individual glucose traces and to exclude potential residual artifacts.
Furthermore, the proposed CGM data adjustment procedure (integrating capillary plasma-equivalent glucose values resulting in SMBG-adjusted CGM values) led to a marked improvement in the reliability and ability to interpret glucose data and associated results, thus endorsing its application in future studies.
A limitation of the study is the use of a first generation intermittent-scanning CGM. However, this limitation was to a large extent compensated by the described interpolation procedure. Other limitations of the present study are the small sample size and the homogenous group composition that prohibit adequate stratification regarding, for example, age, gender, BMI, or ethnicity. Another limiting factor is the low mean age (≈24 years) of the participants in this study which is not representative for an older population, since glucose concentrations reportedly increase with age. 11 In general, it should not be neglected that glucose excursions always depend on the composition of the meal (eg carbohydrate/fat/protein content, glycemic index) and may differ between studies according to eating patterns in the respective country. The limited range of meals in the study does not cover different eating habits.
It can, however, be argued that the glucose traces found in this study represent a scenario of optimal physiologic glucose control, since participants were young, had a BMI in the normal range, and HbA1c and oGTT results indicating normal glucose tolerance. 21 Thus, the presented glucose profiles can support clinical targets in people with diabetes and, potentially, the detection of prediabetes using CGM. 29
Likewise, with regard to interoperable CGM systems and insulin pumps, as well as the further development of (hybrid) closed-loop systems30,31 it is essential to take into account the physiologic level of glycemic control in healthy individuals.
Conclusions
In this study, fixed meal times resulted in a clear pattern of distinct postprandial glucose excursions in participants without diabetes, thus enabling statements about the course of glucose in healthy subjects throughout the day and allowing for the establishment of a standardized normative glucose profile. These results, therefore, not only add to the clinical evidence of the glucose values that are occurring in healthy subjects, but also show CGM-derived metrics, which were not observed in other studies likely due to their study design. Based on these data, people with diabetes or their healthcare professionals might get a clearer picture of what “normal” pre- and postprandial glucose levels, and thus realistic therapeutic goals, are.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968221113341 for Continuous Glucose Profiles in Healthy People With Fixed Meal Times and Under Everyday Life Conditions by Guido Freckmann, Sebastian Schauer, Anne Beltzer, Delia Waldenmaier, Sina Buck, Annette Baumstark, Nina Jendrike, Manuela Link, Eva Zschornack, Cornelia Haug and Stefan Pleus in Journal of Diabetes Science and Technology
Acknowledgments
The authors thank the study personnel and all volunteers who contributed to the study, as well as Dr. Manuel Eichenlaub (IfDT) for his help and feedback in creating the manuscript.
Footnotes
Abbreviations: ADA, American Diabetes Association; BG, blood glucose; CGM, continuous glucose monitoring; CV, coefficient of variation; Glc, glucose concentration; GMI, glucose management indicator; HbA1c, glycated hemoglobin A1c; IDF, International Diabetes Federation; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; IQR, interquartile range; MARD, mean absolute relative difference; oGTT, oral glucose tolerance test; SD, standard deviation; SMBG, self-monitoring of blood glucose.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.F. is general manager of the Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm (IfDT, Ulm, Germany), which carries out clinical studies on the evaluation of BG meters and medical devices for diabetes therapy on its own initiative and on behalf of various companies. G.F./IfDT have received grants, speakers’ honoraria or consulting fees from Abbott, Agamatrix, Ascensia, Berlin Chemie, Beurer, Boydsens, CRF Health, Dexcom, i-SENS, LifeScan, Lilly, Metronom Health, Medtronic, Menarini, MySugr, Novo Nordisk, PharmaSens, Roche, Sanofi, Sensile, and Ypsomed. All other authors are employees of IfDT.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
Sebastian Schauer, PhD  https://orcid.org/0000-0002-9873-0989
https://orcid.org/0000-0002-9873-0989
Anne Beltzer  https://orcid.org/0000-0002-8308-8540
https://orcid.org/0000-0002-8308-8540
Delia Waldenmaier  https://orcid.org/0000-0003-3280-2369
https://orcid.org/0000-0003-3280-2369
Sina Buck  https://orcid.org/0000-0001-8428-1038
https://orcid.org/0000-0001-8428-1038
Annette Baumstark  https://orcid.org/0000-0002-3439-7400
https://orcid.org/0000-0002-3439-7400
Stefan Pleus  https://orcid.org/0000-0003-4629-7754
https://orcid.org/0000-0003-4629-7754
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2. Rodbard D. Continuous glucose monitoring: a review of recent studies demonstrating improved glycemic outcomes. Diabetes Technol Ther. 2017;19(S3):S25-S37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Standards of Medical Care in Diabetes—2022. Diabetes Care. 2022;45(suppl 1):S1-S264. [DOI] [PubMed] [Google Scholar]
- 4. DECODE Study Group, The European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med. 2001;161(3):397-405. [DOI] [PubMed] [Google Scholar]
- 5. Hanefeld M, Fischer S, Julius U, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39(12):1577-1583. [DOI] [PubMed] [Google Scholar]
- 6. International Diabetes Federation. Guideline for management of postmeal glucose in diabetes [Internet]. 2011. https://www.idf.org/e-library/guidelines/82-management-of-postmeal-glucose.html. Accessed May 5, 2022. [DOI] [PMC free article] [PubMed]
- 7. Borg R, Kuenen JC, Carstensen B, et al. ; ADAG Study Group. Real-life glycaemic profiles in non-diabetic individuals with low fasting glucose and normal HbA1c: the A1c-Derived Average Glucose (ADAG) study. Diabetologia. 2010;53(8):1608-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freckmann G, Hagenlocher S, Baumstark A, et al. Continuous glucose profiles in healthy subjects under everyday life conditions and after different meals. J Diabetes Sci Technol. 2007;1(5):695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Fox LA, Beck RW, Xing D. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33(6):1297-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DuBose SN, Li Z, Sherr JL, Beck RW, Tamborlane WV, Shah VN. Effect of exercise and meals on continuous glucose monitor data in healthy individuals without diabetes. J Diabetes Sci Technol. 2021;15(3):593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Foreman YD, Brouwers MCGJ, van der Kallen CJH, et al. Glucose variability assessed with continuous glucose monitoring: reliability, reference values, and correlations with established glycemic indices-The Maastricht Study. Diabetes Technol Ther. 2020;22(5):395-403. [DOI] [PubMed] [Google Scholar]
- 13. Sofizadeh S, Pehrsson A, Ólafsdóttir AF, Lind M. Evaluation of reference metrics for continuous glucose monitoring in persons without diabetes and prediabetes. J Diabetes Sci Technol. 2020;16(2):373-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2020;32(4):690. [DOI] [PubMed] [Google Scholar]
- 15. Fechner E, Op ’t, Eyndt C, Mulder T, Mensink RP. Diet-induced differences in estimated plasma glucose concentrations in healthy, non-diabetic adults are detected by continuous glucose monitoring—a randomized crossover trial. Nutr Res. 2020;80:36-43. [DOI] [PubMed] [Google Scholar]
- 16. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079-1094. [DOI] [PubMed] [Google Scholar]
- 17. Freckmann G. Basics and use of continuous glucose monitoring (CGM) in diabetes therapy. J Lab Med. 2020;44(2):71-79. [Google Scholar]
- 18. D’Orazio P, Burnett RW, Fogh-Andersen N, et al. Approved IFCC recommendation on reporting results for blood glucose: International Federation of Clinical Chemistry and Laboratory Medicine Scientific Division, Working Group on Selective Electrodes and Point-of-Care Testing (IFCC-SD-WG-SEPOCT). Clin Chem Lab Med. 2006;44(12):1486-1490. [DOI] [PubMed] [Google Scholar]
- 19. Rodbard D. Metrics to evaluate quality of glycemic control: comparison of time in target, hypoglycemic, and hyperglycemic ranges with “risk indices.” Diabetes Technol Ther. 2018;20(5):325-334. [DOI] [PubMed] [Google Scholar]
- 20. Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, part I. J Clin Chem Clin Biochem. 1983;21(11):709-720. [DOI] [PubMed] [Google Scholar]
- 21. Petersmann A, Nauck M, Müller-Wieland D, et al. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes. 2018;126(07):406-410. [DOI] [PubMed] [Google Scholar]
- 22. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boscari F, Galasso S, Acciaroli G, et al. Head-to-head comparison of the accuracy of Abbott FreeStyle Libre and Dexcom G5 mobile. Nutr Metab Cardiovasc Dis. 2018;28(4):425-427. [DOI] [PubMed] [Google Scholar]
- 24. Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7(2):562-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Figueira FR, Umpierre D, Bock PM, et al. Effect of exercise on glucose variability in healthy subjects: randomized crossover trial. Biol Sport. 2019;36(2):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Busing F, Hagele FA, Nas A, Hasler M, Muller MJ, Bosy-Westphal A. Impact of energy turnover on the regulation of glucose homeostasis in healthy subjects. Nutr Diabetes. 2019;9(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med. 2016;2(1):e000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bergman M, Abdul-Ghani M, DeFronzo RA, et al. Review of methods for detecting glycemic disorders. Diabetes Res Clin Pract. 2020;165:108233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5(7):501-512. [DOI] [PubMed] [Google Scholar]
- 31. Bergenstal RM, Klonoff DC, Garg SK, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224-232. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968221113341 for Continuous Glucose Profiles in Healthy People With Fixed Meal Times and Under Everyday Life Conditions by Guido Freckmann, Sebastian Schauer, Anne Beltzer, Delia Waldenmaier, Sina Buck, Annette Baumstark, Nina Jendrike, Manuela Link, Eva Zschornack, Cornelia Haug and Stefan Pleus in Journal of Diabetes Science and Technology