Abstract
Background:
Automated insulin delivery is an efficient treatment for patients with type 1 diabetes. Little is known on its impact on patients with excessive time in hypoglycaemia.
Methods:
We performed a post hoc analysis of three randomized control trials that used the DBLG1 (Diabeloop Generation 1) hybrid closed-loop solution. Patients whose time below 70 mg/dL during baseline, open-loop phase exceeded 5% were selected. The outcomes were the differences between the closed-loop and the open-loop phases in time in various ranges and Glycemia Risk Index (GRI).
Results:
We identified 45 patients exhibiting ≥5% of time below 70 mg/dL during the open-loop phase. Under closed-loop, the time in hypoglycaemia (54 to <70 mg/dL) dropped from 7.9% (SD 2.4) to 3.2% (SD 1.6) (difference −4.7% [−5.3; −4.1], P < 10−4). The time below 54 mg/dL decreased from 1.9% (SD 1.3) to 0.8% (SD 0.7) (difference −0.9% [−1.4; –0.8], P < 10−4). The time in range (TIR 70-180 mg/dL) improved from 63.3 (SD 9.5) to 68.2% (SD 8.2) (difference 5.1% [2.9; 7.0], P < 10−4). The GRI improved from 51.2 (SD 12.4) to 38.0 (SD 10.9) (difference 13.2 [10.4; 16.0], P < 10−4).
Conclusion:
DBLG1 decreased time in hypoglycaemia by more than 50% even in patients with excessive time in hypoglycaemia at baseline, while also improving both TIR and GRI, under real-life conditions. The improvement in GRI (13.2%) exceeded that of the improvement in TIR (5.1%) indicating that in this data set, GRI was more sensitive than TIR to the improvement in glycaemia achieved with closed-loop. These results support the safety and efficacy of this treatment.
Keywords: artificial pancreas, closed-loop, hypoglycaemia, type 1 diabetes
Introduction
Automated insulin delivery has recently emerged as the most-efficient treatment for patients with type 1 diabetes (T1D). Several pivotal trials have allowed various hybrid closed-loop solutions to be cleared by regulatory authorities.1-5 These studies aimed at describing the time in range (TIR; 70-180 mg/dL) as the main efficacy outcome, and the incidence of acute metabolic events as the safety criterium. In most if all of these studies, patients with a past history of severe hypoglycaemia or with hypoglycaemia unawareness have been excluded and, if not, the average baseline of time below range (0 to <70 mg/dL) was below the threshold of 5% that was proposed in the international consensus on continuous glucose monitoring (CGM) data.6,7 So far, very few trials targeted patients at risk for hypoglycaemia. 8 Whether closed-loop insulin delivery is also efficient and safe among patients with excessive time in hypoglycaemia is the question that we addressed in the following study, that contributes to the knowledge in this field. Time in a target range of 70 to 180 mg/dl has been proposed as a CGM metric that is linked to the quality of glycemic control. 9 The Glycemia Risk Index (GRI) is a new composite metric that has been demonstrated to correlate with opinions of clinicians as to the quality of glycaemia. Compared to TIR, the GRI has been shown to correlate more closely with the opinions of clinicians as to the quality of CGM control. 10 It is not known whether either metric is linked to improved time in the hypoglycemic range (below 70 mg/dl) with the use of closed-loop control compared to open-loop control in patients with T1D whose baseline frequency of time in hypoglycaemia range exceeds 5%. In this post hoc study, we assessed the impact of using the DBLG1 closed-loop solution on the time in hypoglycemia among patients with excessive time in hypoglycemia at baseline, and we looked at the evolution of TIR and GRI in this population.
Methods
Objective
The main objective of this study was to assess the impact of using the DBLG1 (Diabeloop Generation 1) hybrid closed-loop system on the time in hypoglycemic range (0 to <70 mg/dL) among T1D patients selected with more than 5% (≥) in hypoglycemic range during baseline, open-loop evaluation. The secondary objectives were to describe the evolution of the different glucose metrics, including TIR and GRI, under closed loop.
Participants
This study compiled data obtained from three randomized controlled trials and declared on ClinicalTrials.gov as NCT02987556 (WP7 trial), 3 NCT04190277 (WP8 DBLUS trial), and NCT03671915 (WP9 DBL4K trial). 11 Briefly, WP7 was a crossover trial performed among adult patients during two periods of 12 weeks each, only patients of second arm who used the Kaleido pump were selected (n = 30); WP9 crossover trial had involved children aged 6 to 12 year old who used the Kaleido pump for 6 weeks (n = 17); in these crossover trials, the open-loop data came from the crossover period in open-loop; WP8 was a parallel group trial including adolescents and adult patients who used the Dana-i pump (n = 142), where we used the 14-day run-in period performed with the trial pump and CGM as the baseline, open-loop control period. Of note, regarding the risk for hypoglycaemia, all three trials had excluded patients with hypoglycaemia unawareness (Gold score >4) and with a recent history of severe hypoglycaemia.3,11 Among all these patients, we selected those whose time below optimal range (0 to <70 mg/dL) during baseline, open-loop phase exceeded 5% (≥). We based this criterion upon the international consensus that recommends a time <4% below 70 mg/dL (≥54 to <70, low glucose) and a time <1% below 54 mg/dL (<54, very low glucose) in non-high-risk patients. 7
Insulin Delivery DBLG1 System
DBLG1 is a hybrid, closed-loop insulin delivery system combining a Dexcom G6® continuous glucose monitoring device, an insulin pump (Kaleido® or Dana-i®) and the DBLG1 software into a dedicated controller handset. Briefly, algorithm is a machine learning system, allowing customization through 10 different settings in order to respond to the diversity of existing T1D metabolic profiles [details in reference # 3].
Study design
In a post hoc analysis, we looked at the glucose metrics among all patients who had gone through the WP7 trial (arm 2), the WP8 trial, and the WP9 trial, and we selected the population of patients whose baseline, open-loop data showed an average time below optimal range (0 to <70 mg/dL) exceeding 5% (≥). Among this population, we then looked at the time in optimal range (70-180 mg/dL) and the time below range (0 to <70 mg/dL) during the whole period using the DBLG1 closed-loop system. The main outcome was the difference in time below range between the closed-loop and the open-loop phases. We also looked at the GRI, which is a recently introduced CGM metric that was validated by clinician ratings. 10 Briefly, GRI was built with a data set of 14-day CGM tracings from 225 insulin-treated adults with diabetes. These tracings were ranked by 330 highly experienced clinicians worldwide from best to worst glycemic control. A model was built to predict the clinician ranking based on seven standard metrics in an Ambulatory Glucose Profile (AGP): very low-glucose and low-glucose hypoglycaemia; very high-glucose and high-glucose hyperglycaemia; TIR; mean glucose; and coefficient of variation. 10 The GRI can present as a single score expressing both the effects of hypoglycaemia and hyperglycaemia on a 0 to 100 scale of percentiles, with 0 the best and 100 the worst. The GRI can be expressed either as a population mean (similar to how the TIR can be expressed) or else as a distribution of the quality of a population’s individual patients’ glycaemia in five quintiles from A (the best) to E (the worst).
Statistical analysis
A paired t-test was performed to compare the time below range, the TIR and the GRI in our selected population between the closed-loop and the open-loop phases. In order to take into account any size effect in the observed differences between closed-loop and open-loop outcomes, we performed the Cohen’s d test with Hedges and Olkin’s correction (a d test lower than 0.2, lower than 0.5 and higher than 0.8 suggests a minor, moderate and high effect, respectively). All results were reported in intention to treat (i.e., whether the closed-loop function was on, off, or dysfunctioning). In this article, we defined time in low glucose as (54 to <70 mg/dL), and time in very low glucose as (<54 mg/dL).
Results
Population
The total number of patients exhibiting ≥5% of time in low glucose during the open-loop phase of their respective trials was 45, including 9 among 30 adult patients in the WP7 trial, 28 among 142 patients in the WP8 trial (among which 7 were between 14 and 17 year old) and 8 among 17 children in the WP9 trial. Table 1 summarizes demographic data (age, gender, duration of diabetes, baseline HbA1c, and mean total daily insulin dosage) of both categories of patients (those exhibiting ≥5% or <5% of time in low glucose). There was a trend to a lower HbA1c and a lower insulin requirement in the population with excessive time in low glucose. The median duration under closed-loop therapy for the whole population was 37 days [36.4, 51.2], as opposed to median time under open-loop treatment which was 17 days [24.3, 38.9].
Table 1.
Baseline Characteristics of Participants.
| Characteristics | WP7 (n = 30) | WP8 (n = 142) | WP9 (n = 17) | |||
|---|---|---|---|---|---|---|
| % Time in Low Glucose (0 to <70 mg/dL) | ≥5% LG |
<5% LG |
≥5% LG |
<5% LG |
≥5% LG |
<5% LG |
| Number of patients | 9 | 21 | 28 | 114 | 8 | 9 |
| Gender: female (n) | 3 (33.33) | 9 (42.86) | 18 (64.29) | 73 (64.04) | 4 (50.0) | 5 (55.56) |
| Age (years) | 46.78 (10.15) | 48.19 (12.23) | 35.71 (16.7) | 36.23 (16.45) | 7.88 (0.99) | 8.44 (2.07) |
| Duration of diabetes (years) | 25.56 (13.57) | 26.05 (12.75) | 23.07 (11.94) | 20.66 (13.43) | 5.25 (1.58) | 5.89 (2.37) |
| HbA1c (%) | 6.88 (0.75) | 7.76 (0.75) | 7.53 (0.89) | 8.07 (1.08) | 6.92 (0.47) | 7.42 (0.38) |
| Total daily insulin (U/d) | 36.25 (12.22) | 36.84 (9.16) | 40.57 (14.18) | 44.12 (16.05) | 21.31 (3.51) | 24.08 (6.8) |
Data are given as n (%) or mean (SD). LG: low glucose
Main outcome
Among patients in WP7 trial, the time in low glucose dropped from 8.3% in open-loop phase to 3.3% in closed-loop period (difference −5.9% [−6.8; −3.1]). In patients from WP8 trial, the time in low glucose dropped from 7.9 to 3.1% (difference −4.9% [−5.6; −4.0]). In children from WP9 trial, the time in low glucose was decreased from 7.4% to 3.5% (difference −4.0% [−5.3; −2.7]). Thus overall, while using the closed-loop device, the whole population experienced a reduction in time in low glucose that dropped from 7.9% (SD 2.4) to 3.2% (SD 1.6) (difference −4.7% [−5.3; −4.1], P < 10−4). As summarized in Table 2, the time in very low glucose (<54 mg/dL) was also reduced from 1.9% (SD 1.3) in open-loop to 0.8% (SD 0.7) in closed-loop (difference −0.9% [−1.4; −0.8], P < 10−4). Individual data showing that time in low glucose ranged from 5.1 to 15.6% are provided in appendix (Supplemental Table S1). The glucose metrics for the population with baseline time in low glucose under 5% (<) are provided in Supplemental Table S2.
Table 2.
24-h Glucose Control During OL and CL Periods in Patients Exhibiting ≥ 5% of Time in Low Glucose.
| WP7 (n = 9) | WP8 (n = 28) | WP9 (n = 8) | Total (n = 45) | ||||
|---|---|---|---|---|---|---|---|
| OL | CL | OL | CL | OL | CL | OL | CL |
| Diff CL-OL P value size effect |
Diff CL-OL P value size effect |
Diff CL-OL P value size effect |
Diff CL-OL P value size effect |
||||
| Time in low glucose (54 to <70 mg/dL) (%) | |||||||
| 8.3 ± 2.3 | 3.3 ± 1.6 | 7.9 ± 2.5 | 3.1 ± 1.7 | 7.4 ± 2.1 | 3.5 ± 1.7 | 7.9 ± 2.4 | 3.2 ± 1.6 |
| −5.9 [−6.8, −3.1] 0.0003 2.1 |
−4.9 [−5.6, −4.0] <10−4 2.1 |
−4.0 [−5.3, −2.7] 0.0002 1.7 |
−4.7 [−5.3, −4.1] <10−4 2.2 |
||||
| Time in very low glucose (<54 mg/dL) (%) | |||||||
| 2.1 ± 1.1 | 0.8 ± 0.5 | 1.9 ± 1.5 | 0.7 ± 0.8 | 1.6 ± 0.9 | 0.9 ± 0.6 | 1.9 ± 1.3 | 0.8 ± 0.7 |
| −1.3 [−2.1, −0.5] 0.0044 1.3 |
−0.9 [−1.6, −0.8] <10-4 0.9 |
−0.6 [−1.1, −0.3] 0.0047 0.8 |
−0.9 [−1.4, −0.8] <10−4 1.0 |
||||
| Time in range (70-180 mg/dL) (%) | |||||||
| 65.4 ± 9.4 | 72.0 ± 10.6 | 62.5 ± 10.3 | 67.4 ± 8.2 | 63.6 ± 6.8 | 66.8 ± 3.8 | 63.3 ± 9.5 | 68.2 ± 8.2 |
| 6 [2.4, 10.7] 0.0066 0.5 |
4.7 [1.8, 8.0] 0.0028 0.5 |
0.2 [−0.7, 7.1] 0.0944 0.5 |
5.1 [2.9, 7.0] <10−4 0.5 |
||||
| Time in high glucose (>180 mg/dL) (%) | |||||||
| 26.3 ± 10.1 | 24.7 ± 10.8 | 29.6 ± 10.6 | 29.5 ± 7.9 | 29 ± 7.7 | 29.8 ± 3.6 | 28.8 ± 9.9 | 28.6 ± 8.1 |
| 0.4 [−5.8, 2.7] 0.417 0.1 |
−0.3 [−3.2, 3] 0.955 0.0 |
0.5 [−4.2, 5.7] 0.773 0.1 |
−0.2 [−2.4, 1.9] 0.827 0.0 |
||||
| Time in very high glucose (>250 mg/dL) (%) | |||||||
| 7.7 ± 5 | 6.3 ± 4.8 | 9.7 ± 5.9 | 8.5 ± 4.6 | 10.5 ± 5.2 | 8.4 ± 3.1 | 9.4 ± 5.6 | 8 ± 4.4 |
| −1.2 [−3.6, 0.9] 0.196 0.2 |
−0.7 [−3, 0.7] 0.204 0.2 |
−2.7 [−5.2, 1.1] 0.161 0.4 |
−1.1[−2.6, −0.1] 0.035 0.3 |
||||
| Glycemia Risk Index | |||||||
| 48.8 ±10.9 | 33.7 ± 12.5 | 52.0 ± 13.9 | 38.8 ± 11.4 | 50.8 ± 9.0 | 39.9 ± 6.7 | 51.2 ± 12.4 | 38.0 ± 12.9 |
| −15.3 [−21.0, −9.1] 0.0003 1.1 |
−12.6 [−17.3, −9.2] <10−4 1.0 |
−11.0 [−15.4, −6.5] 0.0007 1.1 |
−13.1 [−16.0, −10.4] <10−4 1.1 |
||||
Data are given as mean (SD). Differences are shown as median [95% CI]. Paired t-test and Cohen’s d test are provided.
Abbreviation: OL, open loop; CL, closed loop; CI, confidence interval.
Secondary Outcomes
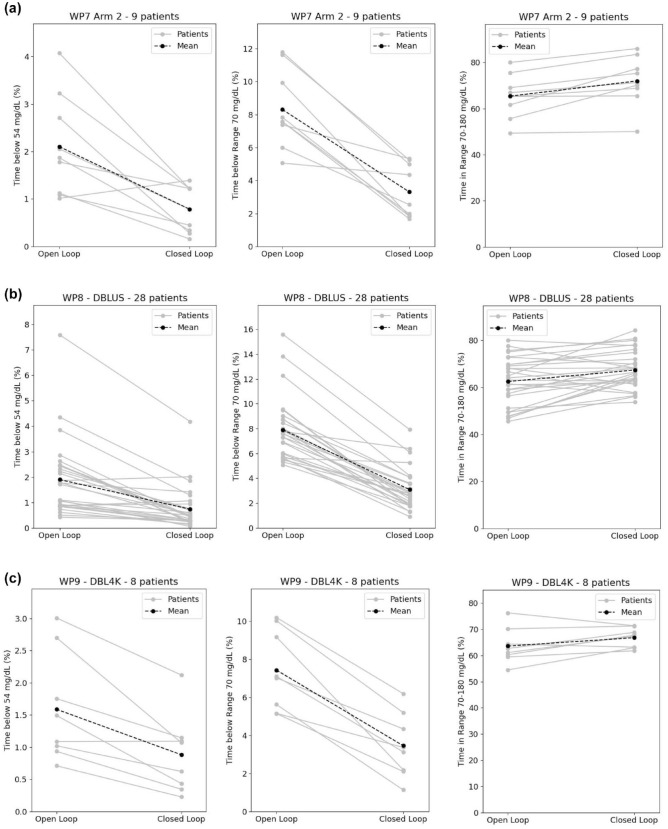
Meanwhile, the time in optimal range (TIR 70-180 mg/dL) in the selected population has been raised from 65.4% to 72.0% in WP7 trial, from 62.5% to 67.4% in WP8 trial, and from 63.6% to 66.8% in WP9 trial. The overall population increased its TIR from 63.3% (SD 9.5) to 68.2% (SD 8.2) (difference 5.1% [2.9; 7.0], P < 10−4). All these results are summarized in Table 2 and individual results are illustrated in Figure 1.
Figure 1.
Individual trajectories from open-loop to closed-loop for time below 54 mg/dL, time below 70 mg/dL and time in range 70 to 180 mg/dL for (a) WP7 trial (9 patients), (b) WP8 trial (28 patients), and (c) WP9 trial (8 patients). The black line represents the mean.
We looked at the number of patients who reached the targets from the International CGM consensus, 7 that is, a TIR 70 to 180 mg/dL above 70% (>) combined with a time below 70 mg/dL under 4% (<) and a time below 54 mg/dL under 1% (<). Overall this number increased from 0 in open-loop to 13 patients in closed-loop. The number of patients that reached a TIR 70 to 180 mg/dL above 70% (>) combined with a time below 70 mg/dL under 4% (<), a time below 54 mg/dL under 1% (<) and a Glucose Management Indicator under 7% (<) was 0 in open-loop and 11 in closed-loop.
We also looked at the GRI, which is a recently introduced composite metric integrating the hypoglycaemia and hyperglycaemia components of ambulatory glucose profile into a single index that was validated by clinician rankings. 10 Data provided in Table 2 and Supplemental Figure S1 showed an improvement of this index when patients moved from to open-loop to closed-loop therapy.
Thus, the mean GRI for our combined population from three studies improved from 51.2 (±12.4) on open-loop control to 38.0 (±10.9) on closed-loop control, for a difference of 13.2. The distribution of GRI scores (A, B, C, D, E) for the whole population (n = 45) was 0, 10, 26, 8, and 1 for open-loop control and 3, 22, 19, 1, and 0 for closed-loop control. Figure 2 illustrates that this improvement was mostly linked with the hypoglycaemia component.
Figure 2.
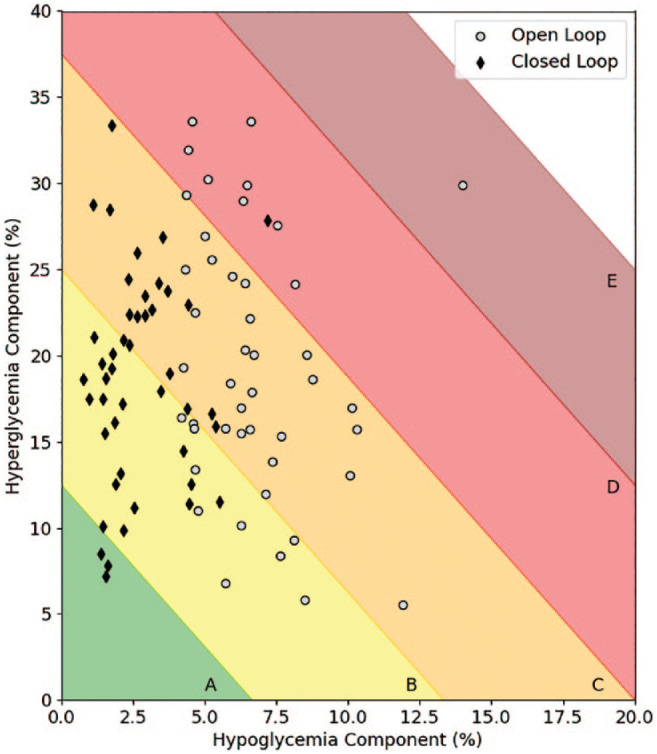
Evolution of individual Glycemia Risk Index from open-loop to closed-loop. See reference 10 for a description of this index.
Discussion
This post hoc analysis of three randomized trials using the DBLG1 hybrid closed-loop solution focused on patients that presented with excessive time in hypoglycaemia when treated with their baseline, usual open-loop treatment. We selected all patients staying more than 5% in hypoglycaemia, this threshold being proposed by the International Consensus on CGM data6,7 and meaning more than 72 minutes per day below the 70 mg/dL level. Our study showed that the time spent in low glucose (54 to <70 mg/dL) was reduced by 59% (from 114 to 46 minutes per day, i.e., 68 minutes reduction). Similarly, the time spent in very low glucose (<54 mg/dL) was also reduced by 59% (from 27 to 11 minutes, i.e., 16 minutes reduction). On the contrary, the time in optimal range (70-180 mg/dL) increased by 7.8% (15.2-16.4 hours per day, 71 minutes increase).
The GRI also improved after switching from open-loop control to closed-loop control. The difference in improvement in the two metrics, which are both on a 100-point scale, was 5.1% for the TIR and 13.2 percentile points for the GRI. The GRI thus showed greater sensitivity to improvement in control for this intervention. The reason for greater sensitivity of the GRI is related to the derivation of the GRI which accounts for time spent in both hypoglycaemia and hyperglycaemia with increased weighting to hypoglycaemia over hyperglycaemia and greater weighting to extreme hypoglycaemia over less-extreme hypoglycaemia. The TIR, on the contrary, treats all times outside of target range as equally significant, be they times in hypoglycaemia or hyperglycaemia or be they severe or not severe outliers. In this population transitioning to closed-loop intervention, where there was a goal of minimizing hypoglycaemia, the GRI, compared to TIR, showed a greater dynamic range to differences in the amount of time spent in hypoglycaemia. The original article presenting GRI featured a population of insulin users equally using different types of regimens (multiple daily injections in T1D, open-loop in T1D, closed-loop in T1D, and multiple daily injections in T2D). 10 The improvement in both metrics of control in our study argues both for the safety and efficacy of the closed-loop system in this particular population.
Hypoglycaemia has been associated with an increased risk of cardiovascular events and all-cause mortality in insulin-treated patients with T1D or T2D. 12 Hypoglycaemia is also responsible for substantial healthcare costs and can have profound negative effects on quality of life.13,14 Reimbursement criteria for new technological devices in diabetes should not only put emphasis on HbA1c and TIR but also consider patients with optimal A1c level but excessive time in hypoglycaemia. It is possible that GRI will become a widely used metric for expressing the quality of glycaemia if future studies, like this one demonstrate a high dynamic range associated with a favorable outcome, and especially if future outcomes studies link better glycaemia, as represented by the GRI, with a decreased frequency of adverse diabetic vascular outcomes. Indeed, our study population had fair baseline A1c, ranging from 6.9% to 7.5%, and one can speculate that the expectations of these patients from a closed-loop solution were more related with a relief from the burden of hypoglycaemia. It is established that hypoglycaemia is the main barrier to tight glucose control. 15 Improvements in fear of hypoglycaemia and diabetes distress have been documented with the use of closed-loop. 16 A recent trial reported that patients with low baseline A1c improved mostly by reducing hypoglycaemia and concluded that there were no reason to exclude individuals with T1D from automated insulin delivery based on their HbA1c. 17
To the best of our knowledge, most controlled studies using closed-loop insulin delivery have not involved patients with excessive time in hypoglycaemia so far. In the pivotal trials of all five commercially available closed-loop solutions, the median cumulated time below 70 mg/dL and below 54 mg/dL never exceeded 5%.1-5 One study had focused on 29 patients with HbA1c <7.5%, excluding those with hypoglycaemia unawareness and reported that time below 70 mg/dL was reduced from 5.3% to 2.9% and time below 54 mg/dl fell from 1.0% to 0.3%, while time in optimal range increased from 65.6% to 76.2%. 8 The strength and originality of our study were to focus only on patients exceeding 5% time in hypoglycaemia. Its weaknesses are its post hoc design, the small sample, and possible selection bias. Actually, very positive outcomes have been recently reported with a specifically designed and customizable closed-loop solution in patients presenting with hypoglycaemia unawareness and a history of severe hypoglycemic episodes.18,19
In conclusion, our study supports the use of closed-loop treatment also in T1D patients experiencing excessive time in hypoglycaemia and brings arguments for the efficacy and safety of this treatment in this population.
Supplemental Material
Supplemental material, sj-docx-1-dst-10.1177_19322968221128565 for Efficacy of a Hybrid Closed-Loop Solution in Patients With Excessive Time in Hypoglycaemia: A Post Hoc Analysis of Trials With DBLG1 System by Pierre-Yves Benhamou, Alice Adenis, Yousra Tourki, Sylvie Pou, Stéphanie Madrolle, Sylvia Franc, Dulanjalee Kariyawasam, Jacques Beltrand, David C. Klonoff and Guillaume Charpentier in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: DBLG1, diabeloop generation 1; GRI, Glycemia Risk Index; TIR, time in range; CGM, continuous glucose monitoring; T1D, type 1 diabetes; AGP, ambulatory glucose profile.
Author Contributions: PYB: study design, patient enrolment and follow-up, data interpretation, and manuscript writing. AA, YT, SP, and SM: study design, data interpretation, and statistical analysis. SF, DK, and JB: patient enrolment and follow-up, data interpretation, and manuscript editing. GC: study design, protocol writing, relation with regulation authorities, patient follow-up, data interpretation, and manuscript editing. PYB is the guarantor of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: This study was funded by the French Innovation Fund (Banque Publique d’Investissement, Maisons-Alfort; France) and by Diabeloop SA (Grenoble, France). PYB, SF, and GC are consultants for Diabeloop SA. AA, YT, SP, and SM are employees from Diabeloop SA. DK is a consultant to EOFlow, Fractyl, Integrity, Lifecare, Roche Diagnostics, Rockley Photonics, and Thirdwayv. No author has been paid to write this article, and the findings and conclusions in this study are those of the authors and do not necessarily represent the views of the sponsors. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the French Innovation Fund (Banque Publique d’Investissement, Maisons-Alfort; France) and by Diabeloop SA (Grenoble, France).
Ethics Approval and Informed Consent: The study was conducted in accordance with the Helsinki Declaration and in accordance with the French law concerning personal healthcare.
Trial Registration: This study was registered under ClinicalTrials.gov NCT02987556, NCT04190277, and NCT03671915.
ORCID iDs: Pierre-Yves Benhamou  https://orcid.org/0000-0003-4378-0468
https://orcid.org/0000-0003-4378-0468
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Tauschmann M, Thabit H, Bally L, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392:1321-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown SA, Kovatchev BP, Raghinaru D, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019;381:1707-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benhamou PY, Franc S, Reznik Y, et al. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digit Health. 2019;1(1):e17-e25. [DOI] [PubMed] [Google Scholar]
- 4. Bergenstal RM, Nimri R, Beck RW, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021;397:208-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown SA, Forlenza GP, Bode BW, et al. Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care. 2021;44(7):1630-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bally L, Thabit H, Kojzar H, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. Lancet Diabetes Endocrinol. 2017;5(4):261-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bode B, King A, Russell-Jones D, Billings LK. Leveraging advances in diabetes technologies in primary care: a narrative review. Ann Med. 2021;53(1):805-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klonoff DC, Wang J, Rodbard D, et al. A glycemia risk index (GRI) of hypoglycemia and hyperglycemia for continuous glucose monitoring validated by clinician ratings. J Diabetes Sci Technol. 2022;29:19322968221085273. doi: 10.1177/19322968221085273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kariyawasam D, Morin C, Casteels K, et al. Hybrid closed-loop insulin delivery versus sensor-augmented pump therapy in children aged 6-12 years: a randomised, controlled, cross-over, non-inferiority trial. Lancet Digit Health. 2022;4(3):e158-e168. [DOI] [PubMed] [Google Scholar]
- 12. Khunti K, Davies M, Majeed A, Thorsted BL, Wolden ML, Paul SK. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38:316-322. [DOI] [PubMed] [Google Scholar]
- 13. Foos V, Varol N, Curtis BH, et al. Economic impact of severe and non-severe hypoglycemia in patients with Type 1 and Type 2 diabetes in the United States. J Med Econ. 2015;18(6):420-432. [DOI] [PubMed] [Google Scholar]
- 14. Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10(12):711-722. [DOI] [PubMed] [Google Scholar]
- 15. Cryer PE. Glycemic goals in diabetes: trade-off between glycemic control and iatrogenic hypoglycemia. Diabetes. 2014;63(7):2188-2195. [DOI] [PubMed] [Google Scholar]
- 16. Cobry EC, Bisio A, Wadwa RP, Breton MD. Improvements in parental sleep, fear of hypoglycemia, and diabetes distress with use of an advanced hybrid closed loop system. Diabetes Care. 2022;45:1292-1295. doi: 10.2337/dc21-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekhlaspour L, Town M, Raghinaru D, Lum JW, Brown SA, Buckingham BA. Glycemic outcomes in baseline hemoglobin A1C subgroups in the international diabetes closed-loop trial. Diabetes Technol Ther. 2022;24(8):588-591. doi: 10.1089/dia.2021.0524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benhamou PY, Lablanche S, Vambergue A, Doron M, Franc S, Charpentier G. Patients with highly unstable type 1 diabetes eligible for islet transplantation can be managed with a closed-loop insulin delivery system: a series of N-of-1 randomized controlled trials. Diabetes Obes Metab. 2021;23(1):186-194. [DOI] [PubMed] [Google Scholar]
- 19. Benhamou PY, Lablanche S, Vambergue A, et al. The beneficial effects of closed-loop insulin delivery in patients with highly unstable type 1 diabetes eligible for islet transplantation are maintained over 6 months: an extension study of the DBLHU-WP10 trial. Diabetes Obes Metab. 2022;24(5):956-961. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dst-10.1177_19322968221128565 for Efficacy of a Hybrid Closed-Loop Solution in Patients With Excessive Time in Hypoglycaemia: A Post Hoc Analysis of Trials With DBLG1 System by Pierre-Yves Benhamou, Alice Adenis, Yousra Tourki, Sylvie Pou, Stéphanie Madrolle, Sylvia Franc, Dulanjalee Kariyawasam, Jacques Beltrand, David C. Klonoff and Guillaume Charpentier in Journal of Diabetes Science and Technology