Abstract
Background:
Fluoride exposure may increase the risk of hypothyroidism, but results from previous studies are inconsistent at low-level fluoride exposure (i.e., ≤ 0.7 mg/L). Human studies of fluoride and thyroid hormone levels in pregnancy are scarce.
Objectives:
We examined associations between fluoride exposure and maternal thyroid hormone levels in a Canadian pregnancy cohort, with consideration for fetal sex-specific effects.
Methods:
We measured fluoride concentrations in drinking water and spot urine samples collected during each trimester from 1876 pregnant women enrolled in the Maternal-Infant Research on Environmental Chemicals (MIREC) study. We also measured maternal thyroid stimulating hormone (TSH), free thyroxine (FT4), and total thyroxine (TT4) levels during the first trimester of pregnancy. We used linear and non-linear regression models to estimate associations between fluoride exposure and levels of TSH, FT4, and TT4. We explored effect modification by fetal sex and considered maternal iodine status as a potential confounder.
Results:
A 1 mg/L increase in urinary fluoride was associated with a 0.30 (95%CI: 0.08, 0.51) logarithmic unit (i.e., 35.0%) increase in TSH among women pregnant with females, but not males (B=0.02; 95%CI: −0.16, 0.19). Relative to women with urinary fluoride concentrations in the first quartile (0.05-0.32 mg/L), those with levels in the third quartile (0.49-0.75 mg/L) had higher FT4 and TT4 (i.e., inverted J-shaped associations), but the association was not statistically significant after adjustment for covariates (p= 0.06). Water fluoride concentration showed a U-shaped association with maternal FT4, whereby women with water fluoride concentrations in the second (0.13-0.52 mg/L) and third (0.52-0.62 mg/L) quartiles had significantly lower FT4 compared to those with levels in the first quartile (0.04-0.13 mg/L). Adjustment for maternal iodine status did not change the results.
Discussion:
Fluoride exposure was associated with alterations in maternal thyroid hormone levels, the magnitude of which appeared to vary by fetal sex. Given the importance of maternal thyroid hormones for fetal neurodevelopment, replication of findings is warranted.
Keywords: Fluoride, Thyroid hormone levels, Pregnancy, Sex-specific effects
1. Introduction
Fluoride is a naturally occurring element that is known for its ability to prevent tooth decay.1 In some parts of the world, fluoride is added to drinking water, constituting the largest source of exposure for children and adults living in fluoridated communities.1,2 In Canada, 0.7 mg fluoride per liter of water is the recommended concentration for dental health while minimizing the occurrence of fluorosis.3 Other sources of exposure include dental products, such as fluoridated toothpaste, and black tea.1,4
Fluoride has been shown to disrupt the thyroid system, but few studies have examined thyroid functioning in people living in areas with community water fluoridation. Some studies of children and non-pregnant adults in Asia have reported associations between higher drinking water- and urine-fluoride concentrations and elevated serum thyroid stimulating hormone (TSH), lower serum free and total thyroxine (T4) and triiodothyronine (T3) concentrations, and increased thyroid gland volume, all of which have been observed in those with hypothyroidism.5–8 Yet, other studies have reported opposite results, linking higher water-fluoride levels to elevated serum total T4 and T3 levels.9 Variability in findings may be attributed to differences in study design, methodological rigor, level and duration of fluoride exposure, as well as age at exposure. An ecologic study conducted in England found a higher prevalence of hypothyroidism in areas with higher levels of fluoride in drinking water.10 In experimental studies, lower free T4 (FT4) and T3 (FT3) were observed in Wistar rat offspring whose mothers were exposed to higher doses of fluoride (i.e., 20 mg/kg11 of body weight and >100 ppm12) in gestation. Similar findings were reported in adult Wistar rats at lower, prolonged fluoride exposure levels.13
Thyroid disruption is of particular concern in pregnancy because the developing fetus relies exclusively on maternal thyroid hormones during the first 10-12 weeks of gestation, and to a lesser extent throughout the second and third trimesters.14,15 As such, maternal hypothyroidism during pregnancy has been associated with adverse effects on offspring development, including preterm birth, increased risk of neurodevelopmental disorders, and lower intelligence quotient (IQ).16–18 A prospective birth cohort study conducted in Denmark (i.e., Danish National Birth Cohort) found that elevated maternal TSH (≥ 10 mIU/L) and low FT4 (<10 pmol/L) were associated with an 8 to 13-point reduction in child verbal IQ.17 Similar findings were reported in a recent meta-analysis.19 Importantly, even mild reductions in maternal thyroid hormone levels during gestation have been associated with lower child IQ.20
Until recently, little was known about the potential impact of fluoride exposure on maternal thyroid function in pregnancy, especially in areas with optimally fluoridated water.21,22 In a previous study of this same cohort, we found a significant association between maternal fluoride exposure and hypothyroidism in pregnancy; a 0.5 mg/L increase in drinking water fluoride concentration was associated with a 65% increase in the odds of having a diagnosis or meeting criteria for primary hypothyroidism.23 We also found that boys born to women with hypothyroidism had significantly lower Full-Scale IQ scores.23 These results suggest that maternal thyroid disruption may play a role in fluoride-induced developmental neurotoxicity observed in previous studies.24–26
Some25,26 but not all24,27 studies examining the developmental neurotoxicity of fluoride have reported that boys were adversely impacted by prenatal, but not postnatal, exposure. More broadly, a growing body of evidence suggests that the male brain may be more vulnerable to neurotoxicants than the female brain.28–30 Women’s thyroid hormone levels in pregnancy have also been observed to differ by child sex. More specifically, pregnant women who have a male fetus were found to be more likely to exhibit elevated TSH.31,32 Taken together, these findings suggest that sex differences may exist in the association between fluoride exposure and maternal thyroid hormone levels in pregnancy.
We evaluated the association between fluoride exposure and maternal TSH, FT4, and TT4 levels of pregnant women living in areas with optimally fluoridated water. Considering findings from our previous study linking fluoride exposure to hypothyroidism, we hypothesized that greater fluoride exposure during pregnancy would be associated with higher maternal TSH and lower maternal FT4 levels. We also investigated whether sex-specific differences exist in the association between fluoride and thyroid dysfunction. Finally, we considered the potential for confounding by maternal iodine status given that iodine is required for thyroid hormone production33 and iodine deficiency may exacerbate the impact of thyroid-disrupting chemicals.34
2. Methods
2.1. Participants
Pregnant women were enrolled in the Maternal-Infant Research on Environmental Chemicals (MIREC) Study35 between 2008 and 2011 from ten cities across Canada, seven of which add fluoride to drinking water (Toronto, Hamilton, Ottawa, Sudbury, Halifax, Edmonton, Winnipeg) and three of which do not (Vancouver, Montreal, Kingston). Women were eligible to participate if they were ≥18 years of age, able to communicate in English or French, and <14 weeks’ gestation. Participants were considered ineligible if they had known fetal abnormalities, medical complications, or reported drug use. Of 2001 women recruited, 1983 consented to participate. Of these, 1885 (95.1%) provided plasma samples in trimester one. We excluded those missing data on fetal sex (n= 9), for a final study sample of 1876 pregnant women.
The current study received approval from the research ethics boards at Health Canada and York University. All participants provided written informed consent at time of enrollment in MIREC.
2.2. Maternal Fluoride Exposure
2.2.1. Maternal Urinary Fluoride (MUF; mg/L)
We analyzed MUF concentration in spot urine samples collected in each trimester of pregnancy (at mean [SD] = 11.6 [1.6], 19.1 [2.3], and 33.1[1.5] weeks’ gestation, respectively), using a modification of the hexamethyldisiloxane (HMDS; Sigma Chemical Co., USA) microdiffusion method with ion-selective electrode by the Indiana University School of Dentistry.36 The limit of detection (LoD) was 0.02 mg/L; trimester-specific concentrations below the LoD (n= 23 or <0.005% of all urine spot samples) were replaced with the value of 0.02 mg/L. Each MUF concentration was standardized for urine specific gravity (SG) to account for variability due to urinary dilution using the following equation: , where MUFSG is the SG-adjusted fluoride concentration (mg/L), SGi is the observed SG concentration for the individual urine sample, and SGM is the median SG for the cohort.37 We derived the average dilution-adjusted MUFSG concentration by taking the average across all three trimesters for each woman. We removed one averaged MUFSG value (> 5 mg/L) because of uncertainty that it reflected an individual’s true exposure. As described previously,23 this high concentration was driven by one trimester-specific value of 16 mg/L, which was inconsistent with the other trimester values that were close to zero.
2.2.2. Water Fluoride (mg/L)
We solicited municipal drinking water reports for the ten cities in the MIREC cohort study. These reports listed water fluoride concentrations that were measured daily for cities that add fluoride to public water supplies, and weekly or monthly among for cities that do not add fluoride to public water.38 Using the first three letters of their postal code, participants’ residences were matched with boundary regions serviced by each Water Treatment Plant. Average water fluoride concentration (i.e., geometric mean; mg/L) was estimated for each woman who reported drinking tap water in pregnancy by averaging water fluoride concentrations across each woman’s pregnancy; thus, each woman has a water fluoride concentration that is matched in time to the levels of fluoride found in tap water for the duration of her pregnancy. Further details can be found in Till et al.38
2.3. Maternal Thyroid Hormones
We analyzed thyroid hormones (i.e., TSH, FT4, and TT4) and antibodies (i.e., anti-thyroglobulin [Tg] and anti-thyroid peroxidase [TPO]) in first trimester maternal plasma collected at mean [SD] = 11.6 [1.6] weeks’ gestation. Plasma FT4 and TT4 were measured using gold standard equilibrium dialysis isotope dilution mass spectrometry (ED-ID-MS) and isotope dilution high performance liquid chromatography mass spectrometry (ID-HPLC-MS), respectively, by the accredited Toxicology Laboratory at the Institut National de Santé Publique du Québec (INSPQ). Plasma TSH, anti-Tg, and anti-TPO were quantified using commercial immunoassays by an accredited biochemistry laboratory at the Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ). TSH concentrations (n=7) below the LoD (0.0025 μIU/mL) were given a value of LoD/√2, which is a validated method for estimation of the average concentration from data containing nondetectable values.39
2.4. Measures of Maternal Iodine Status
We measured thyroglobulin (Tg) concentration in plasma collected in trimester one as an indicator of long-term iodine status. We also measured urinary iodine concentration (UIC) in two spot samples collected during the first and second trimesters and adjusted for urine dilution. We standardized UIC for SG (i.e., UICSG) and also with creatinine (UIC/Cr; μg/g) to account for variability in urinary dilution, and then averaged across both trimesters.
2.5. Statistical Analysis
We examined the distribution and descriptive statistics for all demographics, maternal fluoride exposure and thyroid hormone variables. We used Spearman’s correlation coefficients to examine associations between MUFSG and water fluoride concentrations, and thyroid hormone levels. We used multiple linear regression to test the association between maternal fluoride exposure (MUFSG concentration and water fluoride concentration) and thyroid hormone (TSH, TT4, and FT4) levels. Given the right-skewed distribution of TSH and FT4, natural log transformation was used to approximate a normal distribution for both variables. Change in log TSH associated with MUFSG concentration was interpreted as a percent increase or decrease (i.e., [(ê (B coefficient log TSH) – 1) * 100]) for every 1 mg/L increase in MUFSG, which corresponds to the approximate difference in MUFSG concentrations between women at the 10th and 95th percentiles. We also explored quadratic models to test for non-linearity (or departure from log-linearity) in these associations. We observed some non-linear relationships, which we probed by rerunning models with MUFSG and water fluoride concentrations divided into quartiles. Women in the first quartile (i.e., lowest exposure levels) served as the reference group. Where there was evidence of a non-linear association (i.e., significant differences in thyroid hormone levels between quartiles of fluoride exposure), results from the linear regression model were not reported; instead, we estimated regression coefficients and 95% confidence intervals for MUFSG and water fluoride levels modeled in quartiles.
We adjusted all models for potential confounding variables: maternal age, level of education (dichotomized as bachelor’s degree or higher), pre-pregnancy body mass index (BMI), and race (White or Other), and precision covariates associated with thyroid hormone measurement: parity, anti-Tg and anti-TPO levels, gestational age at time of blood sampling, second-hand smoke exposure, and fetal sex (assigned at birth). All covariates were included in models based on a directed acyclic graph (Supplemental Figure 1). We also adjusted for study site when MUFSG was used as the independent variable because thyroid health outcomes could vary across the study sites. We did not control for study site in our water fluoride models because site is collinear with fluoride levels in municipal drinking water.
We only included women who reported drinking tap water during pregnancy in models involving water fluoride concentration. Women who reported taking medication to treat a thyroid disorder at the time of study enrolment were excluded from all analyses. Additionally, we tested for effect modification by fetal sex in all models by including a sex*exposure interaction term. If the interaction term was significant (p <.05), we estimated the sex-specific slopes. As a sensitivity analysis, we reran models with a significant main effect with Tg, a biomarker of long-term iodine nutrition,40 UICSG, and UIC/Cr added as covariates to evaluate potential confounding by maternal iodine status.
We used STATA version 18.0 (STATA corporation) for all statistical analyses. Two-sided p values ≤.05 were considered to indicate statistical significance.
3. Results
3.1. Model Diagnostics
Regression diagnostics (i.e., Cook-Weisberg test) indicated that the models of MUFSG and water fluoride concentrations with maternal TSH (log transformed) violated the assumption of variance homogeneity (i.e., variance of the residuals was not equal across the levels of fluoride exposure), but the deviations were small (Cooks’ d values < 0.1). There were no other assumption violations, issues with model fit, collinearity, influential cases, or outliers in any of the models.
3.2. Participant Characteristics
We studied a total of 1876 women with blood plasma samples collected in trimester one; 1707 (91.0%), 1791 (95.5%), and 1766 (94.1%) of the women had data on TSH, TT4, and FT4, respectively, and were not taking thyroid medication. Figure 1 shows the sample flow chart for thyroid hormone subgroups with water fluoride, MUFSG, and covariate data.
Figure 1. Study sample flow chart.
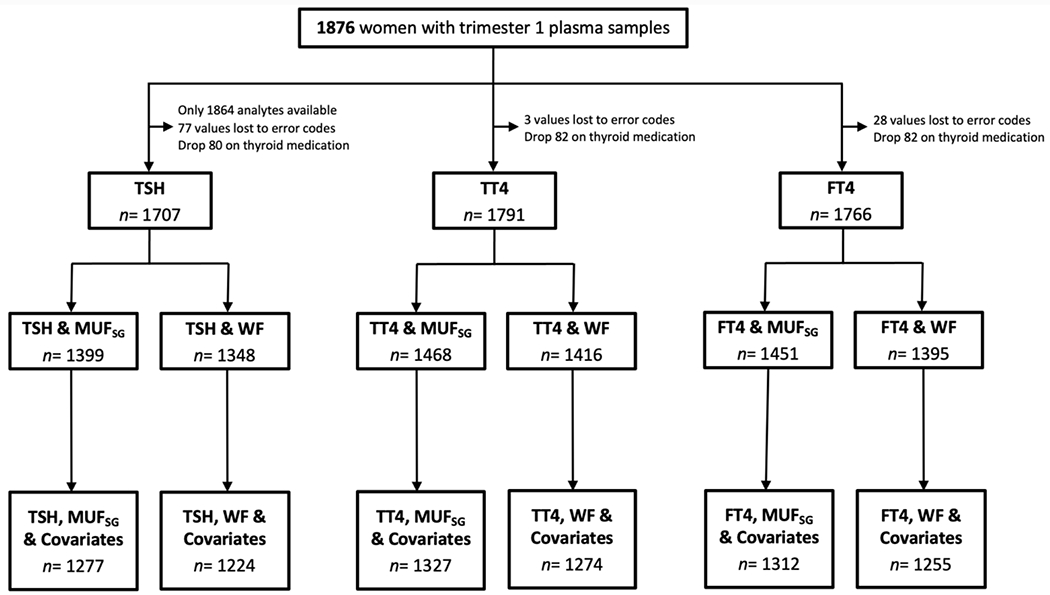
Note. One woman with a MUFSG concentrations > 5 mg/L was excluded from all models.
MUFSG= maternal urinary fluoride, standardized for specific gravity; WF= water fluoride; TSH= thyroid stimulating hormone; TT4= total thyroxine; FT4= free thyroxine.
On average, women were 32.2 years (SD= 5.0) old at time of first visit; 86% were White, 95% were married, 62% had a university education or higher, and 54% had male infants (Table 1). Maternal FT4 and TT4 were normally distributed, while maternal TSH remained skewed following log transformation. Means (Table 1) and interquartile ranges for TSH (0.73-1.74 μIU/mL), TT4 (93-120 ng/mL), and FT4 (12-15 pg/mL) were consistent with reference values for pregnant women. Mean MUFSG concentration was 0.59 mg/L (SD= 0.39; range: 0.05 to 3.33 mg/L) and concentrations at the 25th, 50th, and 75th percentiles were 0.32, 0.49, and 0.75 mg/L, respectively. Mean water fluoride concentration was 0.42 mg/L (SD= 0.25; range: 0.04 to 0.87 mg/L) and concentrations at the 25th, 50th, and 75th percentiles were 0.13, 0.52, and 0.62 mg/L, respectively; 61% of the sample lived in an area with community water fluoridation. As predicted, water fluoride concentration was moderately associated with MUFSG concentration (r= .49, p<.01). Maternal FT4 was moderately associated with TT4 (r= .33, p<.01), while FT4 (r= −.18, p<.01) and TT4 (r= −.10, p<.01) were weakly correlated with TSH.
Table 1.
Demographic characteristics of women with TSH, TT4, and FT4 data.
| TSH | TT4 | FT4 | |
|---|---|---|---|
| N | 1707 | 1791 | 1766 |
| Maternal age (years; mean; SD) | 32.1 (5.1) | 32.1 (5.0) | 32.1 (5.1) |
| Race (n; %) | |||
| White | 1465 (85.8) | 1536 (85.8) | 1517 (85.9) |
| Other | 242 (14.2) | 255 (14.2) | 249 (14.1) |
| Marital status (n; %) | |||
| Married or common law | 1624 (95.1) | 1702 (95.0) | 1677 (95.0) |
| Single | 83 (4.9) | 89 (5.0) | 89 (5.0) |
| Level of education (n; %) | |||
| College diploma or less | 644 (37.7) | 679 (37.9) | 673 (38.1) |
| University degree | 1063 (62.3) | 1112 (62.1) | 1093 (61.9) |
| Household income (n; %) | |||
| <100,000 | 985 (60.4) | 1034 (60.4) | 1020 (60.4) |
| ≥100,000 | 646 (39.6) | 678 (39.6) | 668 (39.6) |
| City (n; %) | |||
| Fluoridateda | 1041 (61.0) | 1091 (60.9) | 1074 (60.8) |
| Non-fluoridatedb | 666 (39.0) | 700 (39.1) | 692 (39.2) |
| Second-hand smoke in trimester 1 (n; %) | |||
| Yes | 107 (6.3) | 110 (6.2) | 109 (6.2) |
| No | 1599 (93.7) | 1680 (93.8) | 1656 (93.8) |
| Pre-pregnancy BMI (kg/m2; mean; SD) | 24.8 (5.4) | 24.8 (5.4) | 24.8 (5.4) |
| Parity (n; %) | |||
| 0 | 760 (44.5) | 799 (44.6) | 790 (44.7) |
| 1 | 686 (40.2) | 719 (40.2) | 707 (40.0) |
| 2+ | 261 (15.3) | 273 (15.2) | 269 (15.3) |
| Gestational age (weeks; mean; SD)c | 11.6 (1.5) | 11.6 (1.5) | 11.6 (1.5) |
| Maternal fluoride exposure | |||
| MUFSG (mg/L; mean; SD) | 0.59 (0.41) | 0.59 (0.41) | 0.59 (0.41) |
| Water fluoride (mg/L; mean; SD) | 0.42 (0.25) | 0.42 (0.25) | 0.42 (0.25) |
| Maternal thyroid hormones | |||
| TSH (log; mean; SD) | −0.01 (0.96) | −0.01 (0.96) | −0.00 (0.95) |
| TSH (μIU/mL; mean; SD) | 1.3 (0.98) | 1.3 (0.98) | 1.3 (0.98) |
| FT4 (log; mean; SD) | 2.6 (0.20) | 2.6 (0.20) | 2.6 (0.20) |
| FT4 (pg/mL; mean; SD) | 13.6 (4.5) | 13.6 (4.4) | 13.6 (4.4) |
| TT4 (ng/mL; mean; SD) | 106.2 (21.3) | 106.3 (21.4) | 106.2 (21.2) |
| Maternal thyroid antibodies | |||
| Anti-Tg (IU/mL; mean; SD) | 10.4 (50.4) | 10.2 (49.5) | 10.1 (49.8) |
| Anti-TPO (IU/mL; mean; SD) | 20.6 (86.9) | 20.8 (88.6) | 20.0 (86.7) |
| Anti-TPO + (≥5.61 IU/mL; n; %) | 216 (12.8) | 222 (12.7) | 214 (12.4) |
| Maternal iodine status | |||
| Plasma Tg (ng/mL; median; IQR) | 13.7 (13.1) | 13.8 (13.2) | 13.7 (13.0) |
| UIC unadjusted (μg/L; median; IQR) | 182.3 (179.4) | 182.3 (177.8) | 183.5 (177.2) |
| UICSG (μg/L; median; IQR) | 191.7 (128.5) | 191.9 (129.8) | 191.1 (128.3) |
| UIC/Cr (μg/g; median; IQR) | 299.0 (216.6) | 300.2 (219.5) | 299.4 (219.3) |
| Child sex (n; %) | |||
| Male | 899 (54.0) | 948 (54.2) | 931 (54.0) |
| Female | 765 (46.0) | 800 (45.8) | 792 (46.0) |
Due to missing data, percentage totals for subgroups may not sum to the total sample population; percentages are reported based on total sample in each subgroup with available data.
Edmonton, Winnipeg, Toronto, Hamilton, Sudbury, Ottawa, Halifax.
Vancouver, Kingston, Montreal.
Gestational age at time of maternal blood collection in trimester one (T1).
Abbreviations: SD= standard deviation; MUFSG = maternal urinary fluoride, adjusted for specific gravity; TSH= thyroid stimulating hormone; FT4= free thyroxine; TT4= total thyroxine; Tg= thyroglobulin; TPO = thyroid peroxidase; UICSG = urinary iodine concentration, adjusted for specific gravity.
Means represent geometric means.
3.3. Maternal Fluoride Exposure and Thyroid Hormone Levels
In a covariate-adjusted multiple linear regression analysis, we found a non-significant (p= .08) positive association between MUFSG and TSH; a 1 mg/L increase in MUFSG was associated with a 0.13 logarithmic unit (i.e., 13.9%) increase in maternal TSH (Figure 2A; Table 2). There was an inverted J-shaped association between MUFSG and maternal FT4 (Figure 2B). Compared with women with MUFSG concentrations in the first quartile (0.05-0.32 mg/L), those with levels in the third quartile (0.49-0.75 mg/L) had non-significantly (p = 0.055) higher FT4 (Table 3). A similar trend was observed between MUFSG concentration and maternal TT4 (Figure 2C; Table 3).
Figure 2. Associations between MUFSG concentration and (A) maternal TSH, (B) FT4, and (C) TT4 levels.
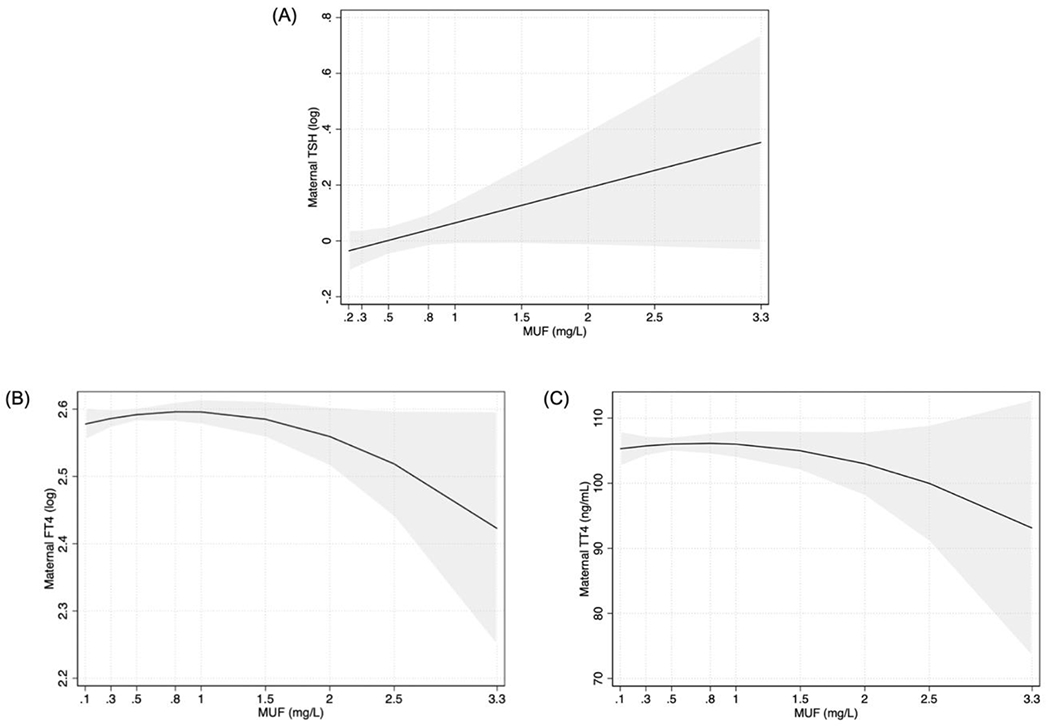
Note. The depicted associations were adjusted for covariates.
MUFSG= maternal urinary fluoride, standardized for specific gravity; TSH= thyroid stimulating hormone; FT4= free thyroxine; TT4= total thyroxine.
Table 2.
Linear associations between MUFSG and water fluoride concentrations, and TSH, FT4, and TT4 levels in pregnant women participating in the MIREC study.
| Unadjusteda | Adjustedb | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | B | 95% CI | p | n | B | 95% CI | p | |
| TSH (log) | ||||||||
| MUFSG (mg/L) | 1399 | 0.09 | −0.04,0.21 | 0.17 | 1277 | 0.13 | −0.02,0.27 | 0.08 |
| Water fluoride (mg/L) | 1348 | −0.08 | −0.28,0.12 | 0.43 | 1224 | −0.17 | −0.38,0.04 | 0.10 |
| FT4 (log) | ||||||||
| MUFSG (mg/L) | 1451 | −0.02 | −0.04,0.01 | 0.18 | 1312 | −0.01 | −0.04,0.02 | 0.50 |
| Water fluoride (mg/L) | 1395 | −0.04 | −0.08,0.00 | 0.05 | 1255 | −0.04 | −0.08,−0.00 | 0.04 |
| TT4 (ng/mL) | ||||||||
| MUFSG (mg/L) | 1468 | −0.72 | −3.49,2.06 | 0.61 | 1327 | −1.16 | −4.26,1.95 | 0.47 |
| Water fluoride (mg/L) | 1416 | 4.92 | 0.55,9.28 | 0.03 | 1274 | 3.88 | −0.53,8.29 | 0.09 |
Multiple linear regression models of associations between MUFSG and water fluoride concentration, and maternal TSH, FT4, and TT4 levels, not adjusted for covariates.
Multiple linear regression models of associations between MUFSG and water fluoride concentration, and maternal TSH, FT4, and TT4 levels, adjusted for maternal age, level of education, pre-pregnancy BMI, race, parity, anti-Tg and anti-TPO levels, gestational age at time of blood sampling, second-hand smoke exposure, and fetal sex; study site was also adjusted in models involving MUFSG.
Abbreviations: CI= confidence interval; TSH= thyroid stimulating hormone; MUFSG= maternal urinary fluoride, standardized for specific gravity; FT4= free thyroxine; TT4= total thyroxine.
Table 3.
Quartile regression effect estimates (95% CI) for the association between urinary and water fluoride concentration, and TSH, FT4, and TT4 levels in pregnant women participating in the MIREC study.
| Unadjusteda | Adjustedb | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| n | B | 95% CI | p | n | B | 95% CI | p | |
| TSH (log) | ||||||||
| MUF (mg/L) | 1399 | 1277 | ||||||
| Q2 | −0.04 | −0.17,0.10 | 0.62 | −0.06 | −0.21,0.09 | 0.44 | ||
| Q3 | −0.11 | −0.25,0.02 | 0.10 | −0.10 | −0.26,0.06 | 0.22 | ||
| Q4 | 0.04 | −0.10,0.18 | 0.58 | 0.05 | −0.11,0.22 | 0.52 | ||
| Water fluoride (mg/L) | 1348 | 1224 | ||||||
| Q2 | 0.08 | −0.06,0.22 | 0.28 | 0.07 | −0.08,0.22 | 0.38 | ||
| Q3 | 0.01 | −0.13,0.16 | 0.88 | −0.08 | −0.23,0.08 | 0.31 | ||
| Q4 | −0.03 | −0.17,0.12 | 0.71 | −0.08 | −0.23,0.07 | 0.31 | ||
| FT4 (log) | ||||||||
| MUF (mg/L) | 1451 | 1312 | ||||||
| Q2 | 0.02 | −0.01,0.04 | 0.27 | 0.02 | −0.01,0.05 | 0.19 | ||
| Q3 | 0.01 | −0.02,0.04 | 0.55 | 0.03 | −0.00,0.06 | 0.06 | ||
| Q4 | −0.01 | −0.04,0.02 | 0.55 | 0.01 | −0.02,0.04 | 0.59 | ||
| Water fluoride (mg/L) | 1395 | 1255 | ||||||
| Q2 | −0.03 | −0.06,−0.01 | 0.01 | −0.03 | −0.06,0.00 | 0.06 | ||
| Q3 | −0.04 | −0.06,−0.01 | 0.01 | −0.04 | −0.07,−0.01 | 0.01 | ||
| Q4 | −0.03 | −0.05,0.00 | 0.06 | −0.02 | −0.05,0.00 | 0.10 | ||
| TT4 (ng/mL) | ||||||||
| MUF (mg/L) | 1468 | 1327 | ||||||
| Q2 | 1.43 | −1.62,4.47 | 0.36 | 1.30 | −1.92,4.52 | 0.43 | ||
| Q3 | 4.39 | 1.34,7.44 | 0.01 | 3.33 | −0.13,6.79 | 0.06 | ||
| Q4 | 0.50 | −2.55,3.55 | 0.75 | 0.59 | −2.97,4.14 | 0.75 | ||
| Water fluoride (mg/L) | 1416 | 1274 | ||||||
| Q2 | 3.76 | 0.69,6.83 | 0.02 | 1.66 | −1.51,4.83 | 0.30 | ||
| Q3 | 2.20 | −0.97,5.38 | 0.17 | 2.09 | −1.17,5.36 | 0.21 | ||
| Q4 | 2.96 | −0.18,6.10 | 0.07 | 2.00 | −1.21,5.22 | 0.22 | ||
Multiple linear regression models of associations between MUFSG and water fluoride concentration, divided into quartiles, and maternal TSH, FT4, and TT4 levels, not adjusted for covariates.
Multiple linear regression models of associations between MUFSG and water fluoride concentration, divided into quartiles, and maternal TSH, FT4, and TT4 levels, adjusted for maternal age, level of education, pre-pregnancy BMI, race, parity, anti-Tg and anti-TPO levels, gestational age at time of blood sampling, second-hand smoke exposure, and fetal sex; study site was also adjusted in models involving MUFSG.
Q2: corresponds to results for women with MUFSG and water fluoride concentrations in the second quartile (i.e., between the 25th and 50th percentiles), relative to those with levels in the first quartile (i.e., below the 25th percentile).
Q3: corresponds to results for women with MUFSG and water fluoride concentrations in the third quartile (i.e., between the 50th and 75th percentiles), relative to those with levels in the first quartile (i.e., below the 25th percentile).
Q4: corresponds to results for women with MUFSG and water fluoride concentrations in the fourth quartile (i.e., above the 75th percentile), relative to those with levels in the first quartile (i.e., below the 25th percentile).
Abbreviations: CI= confidence interval; TSH= thyroid stimulating hormone; MUFSG= maternal urinary fluoride, standardized for specific gravity; FT4= free thyroxine; TT4= total thyroxine.
Water fluoride concentration showed a U-shaped association with maternal FT4 (Figure 3). Relative to women with water fluoride concentrations in the first quartile (0.04-0.13 mg/L), those with levels in the third (0.52-0.62 mg/L) quartile had significantly lower FT4 (Table 3). No statistically significant association was observed between water fluoride concentration and maternal TSH or TT4 (Table 2; Table 3).
Figure 3. U-shaped association between water fluoride concentration and maternal FT4 levels.
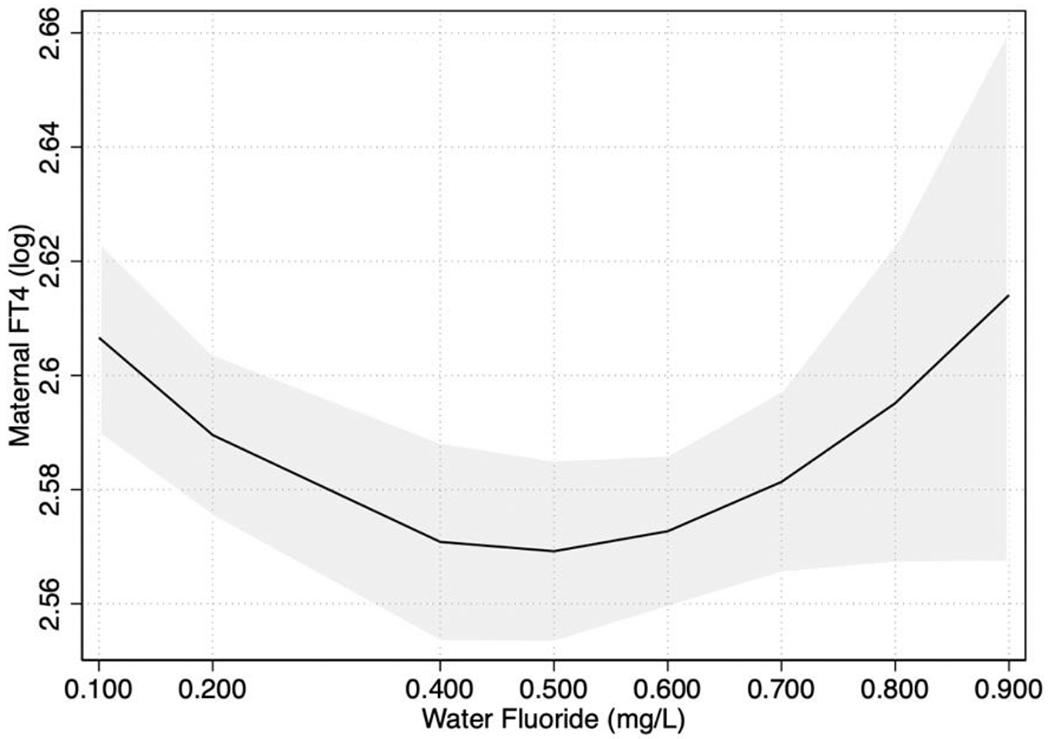
Note. The depicted association was adjusted for covariates. FT4= free thyroxine.
3.4. Effect Modification by Fetal Sex
The interaction between MUFSG concentration and fetal sex in predicting maternal TSH levels was statistically significant (p interaction term= .04). Among pregnant women who had a female fetus, a 1 mg/L increase in MUFSG was associated with a 0.30 (SE= 0.11; 95% CI: 0.08, 0.51; p= .01) logarithmic unit (i.e., 35.0%) increase in TSH (Figure 4). In contrast, MUFSG was not significantly associated with TSH among pregnant women with males (B= 0.02; SE= 0.09; 95% CI: −0.16, 0.19; p= .84; Figure 4). No evidence of effect modification by fetal sex was observed for the associations between MUFSG concentration and maternal FT4 or TT4, or between water fluoride concentration and maternal TSH, FT4, or TT4.
Figure 4. Association between MUFSG concentration and maternal TSH levels by fetal sex.
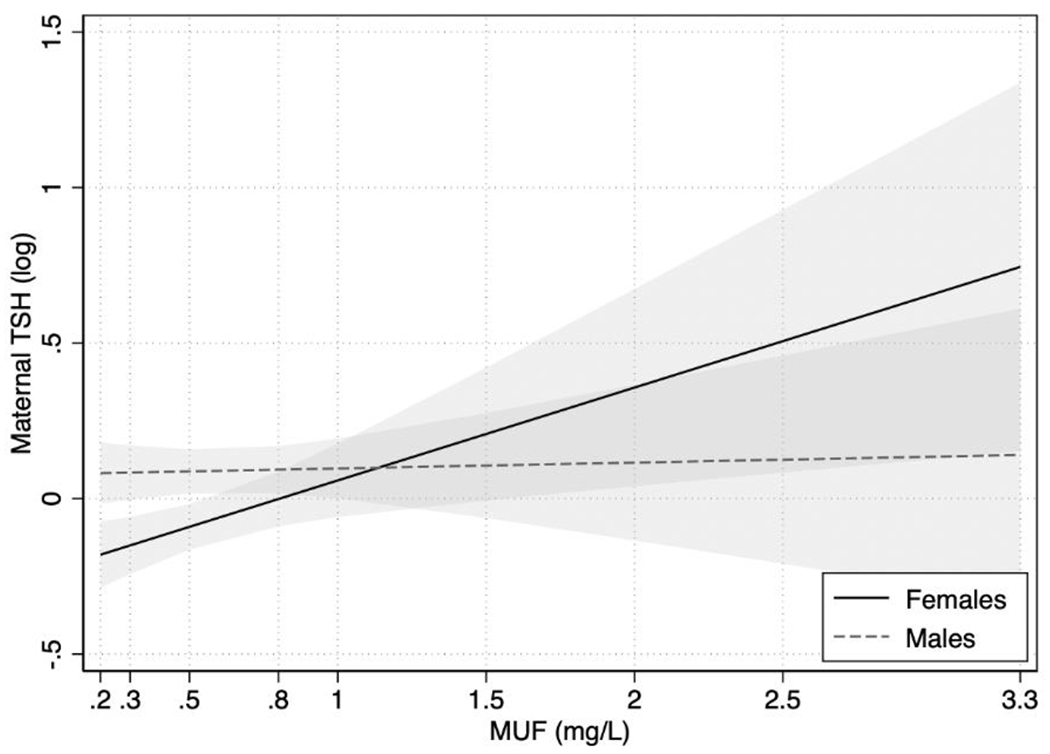
Note. Depicted associations were adjusted for covariates. Females: women pregnant with female fetuses; Males: women pregnant with male fetuses.
MUFSG= maternal urinary fluoride, standardized for specific gravity; TSH= thyroid stimulating hormone.
3.5. Sensitivity Analysis
When maternal Tg, UICSG, and UIC/Cr were added as covariates, we did not observe any substantial differences in the observed associations between maternal MUFSG concentration and water fluoride concentration and thyroid hormones (TSH and FT4) (data not shown). Notably, the interaction between MUFSG concentration and fetal sex in predicting maternal TSH levels remained statistically significant in all models (p interaction term < .05).
4. Discussion
In this Canadian pregnancy cohort, we found a significant and positive log-linear association between MUFSG concentration and maternal TSH for women pregnant with female fetuses. We found that a 1 mg/L increase in MUFSG was associated with a 35% increase in maternal TSH. In our sample, a 1 mg/L increase in MUFSG represents the difference in MUFSG concentrations between women at the 10th and 95th percentiles. We also found a non-linear trend between MUFSG and maternal FT4 and TT4 (i.e., inverted J-shaped associations), such that women with MUFSG concentrations in the third quartile had higher FT4 and TT4 relative to those with concentrations in the first quartile.
In comparison, water fluoride concentration showed a U-shaped association with maternal FT4 (i.e., women with levels in the third quartile had significantly lower FT4 than those with levels in the first quartile) and was not significantly associated with TT4 or TSH levels. These findings should be interpreted with caution, however, given the bi-modal distribution of water fluoride concentration resulting from a gap in water fluoride data (from 0.20 to 0.40 mg/L) between those with lower levels of fluoride living in non-fluoridated cities (values: ≤ 0.20 mg/L), and those with higher fluoride levels living in cities with community water fluoridation (values: 0.41 – 0.87 mg/L). As such, there was a greater range of water fluoride concentration in the second quartile (n=337; range: 0.13 – 0.52 mg/L) relative to the other three quartiles. Accordingly, the observed non-linear association between water fluoride concentration and maternal FT4 may not be interpolated within the range of missing water fluoride data (i.e., between 0.20 and 0.40 mg/L).
Urinary fluoride concentration is an objective biomarker of short-term fluoride exposure that allows for more precise estimates of individuals’ fluoride intake from multiple sources (e.g., fluoridated water, high-fluoride foods, black tea, dental products, etc.). Further, MUFSG concentrations were measured during pregnancy, coinciding with measurement of thyroid hormones. As such, MUFSG is likely a more accurate measure of women’s contemporaneous exposure than water fluoride, and it would be reasonable to expect a short-term urinary biomarker of fluoride exposure to be more strongly associated with maternal thyroid hormone levels. In contrast, water fluoride concentration is a better measure of cumulative or chronic exposure, which may explain the lack of association observed between water fluoride and maternal thyroid hormone levels in the current study.
In a previous study, we found that water fluoride concentration, and not MUFSG, was significantly associated with increased risk of hypothyroidism in pregnancy.23 As mentioned, water fluoride concentration is more likely to be indicative of cumulative or chronic fluoride exposure. If hypothyroidism develops over a longer period, it is plausible that our long-term measure of fluoride exposure (i.e., water fluoride concentration) would be more strongly associated with risk of hypothyroidism.
Our finding that MUFSG concentration was not significantly associated with maternal FT4 or TSH levels (in the total sample) is consistent with results from two studies of pregnant women.21,22 One cross-sectional study of 583 pregnant women from Sweden22 also did not observe associations between maternal urinary fluoride levels and thyroid biomarkers, except for a weak positive association with plasma FT3:FT4 ratio. Likewise, another study of 966 pregnant women from the United States21 did not observe an association between maternal urinary fluoride levels and TSH. Neither of these studies reported whether fetal sex may modify the association between maternal fluoride exposure and thyroid hormone levels.
In our study, MUFSG concentration was only significantly associated with higher TSH levels among pregnant women carrying females. While little is known about potential sex-specific effects in the association between maternal fluoride exposure and thyroid function in pregnancy, a recent study from China reported sex differences in school-aged children’s thyroid gland volumes in response to fluoride exposure.8 Specifically, thyroid gland volumes of males were found to be more vulnerable (i.e., larger) in response to fluoride exposure compared to females. Sex-specific effects have also been found in pregnant women and children’s thyroid hormone levels in response to other neurotoxicants,42 such as perfluorooctanoic acid.43 Furthermore, sex differences have been established in children’s vulnerability to neurotoxicants, with the developing male brain being disproportionally affected.24,25 Considering this and the importance of the maternal thyroid for supporting optimal fetal neurodevelopment, it is possible that the increase in TSH observed among women pregnant with females might indicate a potential protective effect, whereby maternal TSH levels increase in response to fluoride exposure to ensure adequate supply of thyroid hormone to the developing fetus. The biological mechanism to explain why this effect would only be observed among women pregnant with females is unknown, but some evidence suggests that environmental chemicals may induce sex-specific changes in the expression of thyroid related genes in the placenta.44,45 More specifically, prenatal exposure to persistent organic pollutants was found to influence the methylation of thyroid transporter genes in the placenta (e.g., deiodinase type 3 [DIO3] and monocarboxylate transporter 8 [MCT8]), in a sexually dimorphic manner.44 Thus, it is also possible that sex-specific changes in the quantity and frequency of thyroid hormone transport from mother to fetus may explain the fluoride-associated increase in TSH observed among women pregnant with females. Given the complexity of the hypothalamic-pituitary-thyroid axis, we cannot dismiss this elevation in TSH as a potential indicator of maternal thyroid dysfunction; accordingly, it is also possible that the developing female may be more resilient to disturbances in maternal thyroid function in pregnancy. Considering this is the first study to investigate sex differences in the association between fluoride exposure and maternal thyroid hormone levels in pregnancy, and that the mechanism remains unknown, future research in this area will be important for replicating these findings.
Controlling for maternal urinary iodine status in all models of fluoride exposure and maternal thyroid hormone levels did not change the results. Iodine is an essential nutrient for thyroid hormone synthesis and plays an important role in determining the magnitude of fluoride’s effect on the thyroid. Specifically, iodine has been found to modify the association between urinary fluoride concentration and TSH levels among pregnant women21 and non-pregnant adults,46 such that this association was only significant among those who were classified as iodine insufficient. Effect modification was not tested directly in the current study due to limited statistical power given nearly all women in the MIREC cohort were classified as iodine sufficient in a prior study41 based on our estimate of daily iodine intake. Given reports that fluoride may interact with iodine to exert adverse effects on the thyroid47,48 and increase risk of poorer neurodevelopmental outcome49 in offspring, further research in this area is warranted.
4.1. Strengths and limitations
Strengths of this study include the use of a large Canadian pregnancy cohort with individual assessments of fluoride exposure and thyroid hormones analysed using gold-standard methods. We were also able to adjust for several potential confounding variables in our statistical analyses, including maternal urinary iodine, an essential nutrient for thyroid hormone production. However, we only had urine iodine measurements from two spot samples, which is not optimal given that urinary iodine can vary considerably. Further, women in the MIREC cohort tend to be older, more educated, more likely to be married or common law, primarily White, and more likely to report prenatal vitamin use,35 which may not be representative of the broader Canadian population of pregnant women. In this study, we excluded women who reported having a thyroid disorder or taking medication to treat a thyroid disorder; however, it is possible that some women may have failed to self-report having a thyroid disorder, which would be an additional limitation. Moreover, by excluding women who were being treated with medication for hypothyroidism, we may have reduced the sensitivity of our analyses to detect a significant association between both fluoride exposure measures and maternal thyroid hormone levels. Importantly, however, we have already explored the association between fluoride exposure and maternal hypothyroidism in a previous study.23 Another limitation is that we did not control for thyroid-binding globulins (i.e., proteins that bind T4 to form TT4), which may increase measurement error in TT4 given that these levels have been shown to fluctuate over the course of pregnancy; this may have impacted the level of precision with which the associations between fluoride exposure and TT4 were estimated. Similarly, we were also unable to control for biomarkers of other nutrients like selenium, which have been shown to play an important role in T4 to T3 activation by iodothyronine deiodinases.50 Future studies in this area may also want to obtain measures of maternal T3 as it may be more sensitive to fluoride. An additional limitation pertaining to our measure of MUFSG concentration, is that fluoride was measured in spot samples instead of 24-hour urine samples or first morning voids, preventing us from being able to control for behaviours that could contribute to fluctuations in urinary fluoride concentration given the short half-life of fluoride (approximately 5 hours). We attempted to mitigate the effects of this limitation by averaging urinary fluoride across all three trimesters.
4.2. Conclusions
In this Canadian pregnancy cohort, higher levels of fluoride exposure were associated with alterations in maternal thyroid hormone levels, the magnitude of which varied by fetal sex. Our findings make an important contribution to the growing body of evidence suggesting that higher levels of fluoride exposure in pregnancy may have adverse effects on maternal thyroid function. The implications of this work are of public health significance when considering the vital role of the maternal thyroid in supporting optimal fetal growth and neurodevelopment. Future studies in this area are warranted to replicate the current findings.
Supplementary Material
Acknowledgments
The authors would like to extend a sincere thank you to Nicole Lupien, Sarah Garrett, Stéphanie Bastien, and Romy-Leigh McMaster (Centre de Recherche, CHU Sainte-Justine), and the Maternal Infant Research on Environmental Chemicals (MIREC) Study Coordinating Staff for their administrative support, the MIREC site investigators, as well as the MIREC Biobank; Jillian Ashely-Martin for her review of our manuscript as the Knowledge Translation representative for the MIREC study; Alain LeBlanc from the Institut National de Santé Publique Québec (INSPQ) for free and total thyroxine measurement; Nathalie Ouellet at INSPQ and the team at the Institut Universitaire de Cardiologie et de Pneumologie de Québec (IUCPQ) for measuring thyroglobulin, thyroid stimulating hormone, and thyroid antibody levels; Christine Buckley, Frank Lippert, and Prithvi Chandrappa at the Indiana University School of Dentistry for their analysis of urinary fluoride; and Carly Goodman, Rivka Green, John Krzeczkowski, Taylor McGuckin, Maddy Blazer, and Raichel Neufeld at York University for their valuable contributions to, and support of this work.
This work was supported by the National Institute of Environmental Health Science (NIEHS) [grant number R01ES030365, 2020–2025], and the Maternal-Infant Research on Environmental Chemicals Study was funded by the Chemicals Management Plan at Health Canada, the Ontario Ministry of the Environment, and the Canadian Institute for Health Research (CIHR) [grant number MOP-81285, 2006]. This work was also supported by a CIHR scholarship awarded to M.H. None of the funding sources had direct involvement in the conduct of the study and preparation of the manuscript.
References
- 1.Centers for Disease Control and Prevention. Community Water Fluoridation: About Fluoride. https://www.cdc.gov/fluoridation/faqs/about-fluoride.html (2019). [Google Scholar]
- 2.United States Environmental Protection Agency. Fluoride: Exposure and relative source contribution analysis. https://www.epa.gov/sites/default/files/2019-03/documents/fluoride-exposure-relative-report.pdf (2010).
- 3.Health Canada. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document Fluoride. https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/publications/healthy-living-vie-saine/water-fluoride-fluorure-eau/alt/water-fluoride-fluorure-eau-eng.pdf (2010).
- 4.Krishnankutty N et al. Public-health risks from tea drinking: Fluoride exposure. Scand. J. Public Health 50, 355–361 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khandare AL, Validandi V, Gourineni SR, Gopalan V & Nagalla B Dose-dependent effect of fluoride on clinical and subclinical indices of fluorosis in school going children and its mitigation by supply of safe drinking water for 5 years: an Indian study. Environ. Monit. Assess 190, 110 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Kheradpisheh Z et al. Impact of Drinking Water Fluoride on Human Thyroid Hormones: A Case- Control Study. Sci. Rep 8, 2674 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M et al. Thyroid function, intelligence, and low-moderate fluoride exposure among Chinese school-age children. Environ. Int 134, 105229 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Du Y et al. Iodine Modifies the Susceptibility of Thyroid to Fluoride Exposure in School-age Children: a Cross-sectional Study in Yellow River Basin, Henan, China. Biol. Trace Elem. Res 199, 3658–3666 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Yasmin S, Ranjan S, Hilaluddin & D’Souza D. Effect of excess fluoride ingestion on human thyroid function in Gaya region, Bihar, India. Toxicol. Environ. Chem 95, 1235–1243 (2013). [Google Scholar]
- 10.Peckham S, Lowery D & Spencer S Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. J. Epidemiol. Community Health 69, 619–624 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Banji D, Banji OJF, Pratusha NG & Annamalai AR Investigation on the role of Spirulina platensis in ameliorating behavioural changes, thyroid dysfunction and oxidative stress in offspring of pregnant rats exposed to fluoride. Food Chem. 140, 321–331 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Basha PM, Rai P & Begum S Fluoride toxicity and status of serum thyroid hormones, brain histopathology, and learning memory in rats: a multigenerational assessment. Biol. Trace Elem. Res 144, 1083–1094 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y, Guo X, Sun Q, Shan Z & Teng W Effects of Excess Fluoride and Iodide on Thyroid Function and Morphology. Biol. Trace Elem. Res 170, 382–389 (2016). [DOI] [PubMed] [Google Scholar]
- 14.de Escobar GM, Obregón MJ & del Rey FE Maternal thyroid hormones early in pregnancy and fetal brain development. Best Pract. Res. Clin. Endocrinol. Metab 18, 225–248 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Morreale de Escobar G, Obregón MJ & Escobar del Rey F Is neuropsychological development related to maternal hypothyroidism or to maternal hypothyroxinemia? J. Clin. Endocrinol. Metab 85, 3975–3987 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Andersen SL, Olsen J, Wu CS & Laurberg P Low Birth Weight in Children Born to Mothers with Hyperthyroidism and High Birth Weight in Hypothyroidism, whereas Preterm Birth Is Common in Both Conditions: A Danish National Hospital Register Study. Eur. Thyroid J 2, 135–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen SL, Andersen S, Liew Z, Vestergaard P & Olsen J Maternal Thyroid Function in Early Pregnancy and Neuropsychological Performance of the Child at 5 Years of Age. J. Clin. Endocrinol. Metab 103, 660–670 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chevrier J et al. Maternal Thyroid Function during the Second Half of Pregnancy and Child Neurodevelopment at 6, 12, 24, and 60 Months of Age. J. Thyroid Res 2011, 426427 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levie D et al. Thyroid Function in Early Pregnancy, Child IQ, and Autistic Traits: A Meta-Analysis of Individual Participant Data. J. Clin. Endocrinol. Metab 103, 2967–2979 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Moog NK et al. Influence of maternal thyroid hormones during gestation on fetal brain development. Neuroscience 342, 68–100 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griebel-Thompson A et al. Iodine Status, Fluoride Exposure, and Thyroid Function in Pregnant Women in the United States. Curr. Dev. Nutr 6, 652 (2022). [Google Scholar]
- 22.Kampouri M et al. Association of maternal urinary fluoride concentrations during pregnancy with size at birth and the potential mediation effect by maternal thyroid hormones: The Swedish NICE birth cohort. Environ. Res 214, 114129 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Hall M et al. Fluoride exposure and hypothyroidism in a Canadian pregnancy cohort. Sci. Total Environ 869, 161149 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashash M et al. Prenatal Fluoride Exposure and Cognitive Outcomes in Children at 4 and 6-12 Years of Age in Mexico. Environ. Health Perspect 125, 097017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green R et al. Association Between Maternal Fluoride Exposure During Pregnancy and IQ Scores in Offspring in Canada. JAMA Pediatr. 173, 940–948 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantoral A et al. Dietary fluoride intake during pregnancy and neurodevelopment in toddlers: A prospective study in the progress cohort. Neurotoxicology 87, 86–93 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ibarluzea J et al. Prenatal exposure to fluoride and neuropsychological development in early childhood: 1-to 4 years old children. Environ. Res 207, 112181 (2022). [DOI] [PubMed] [Google Scholar]
- 28.Green R, Rubenstein J, Popoli R, Capulong R & Till C Sex-specific neurotoxic effects of early-life exposure to fluoride: A review of the epidemiologic and animal literature. Curr. Epidemiol. Rep 7, 263–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kern JK et al. Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol. Exp. (Warsz.) 77, 269–296 (2017). [PubMed] [Google Scholar]
- 30.Goodman CV et al. Sex difference of pre- and post-natal exposure to six developmental neurotoxicants on intellectual abilities: a systematic review and meta-analysis of human studies. Environ. Health 22, 80 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitoris G et al. Does foetal gender influence maternal thyroid parameters in pregnancy? Eur. Thyroid J 11, e210001 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X et al. Maternal Thyroid-Stimulating Hormone level in the first trimester and sex ratio at birth. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol 25, 315–319 (2019). [DOI] [PubMed] [Google Scholar]
- 33.Krassas GE, Poppe K & Glinoer D Thyroid function and human reproductive health. Endocr. Rev 31, 702–755 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Demeneix BA Evidence for Prenatal Exposure to Thyroid Disruptors and Adverse Effects on Brain Development. Eur. Thyroid J 8, 283–292 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arbuckle TE et al. Cohort profile: the maternal-infant research on environmental chemicals research platform. Paediatr. Perinat. Epidemiol 27, 415–425 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Martínez-Mier EA et al. Development of gold standard ion-selective electrode-based methods for fluoride analysis. Caries Res. 45, 3–12 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duty SM, Ackerman RM, Calafat AM & Hauser R Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect 113, 1530–1535 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Till C et al. Community Water Fluoridation and Urinary Fluoride Concentrations in a National Sample of Pregnant Women in Canada. Environ. Health Perspect 126, 107001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornung RW & Reed LD Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg 5, 46–51 (1990). [Google Scholar]
- 40.Dineva M et al. Exploration of thyroglobulin as a biomarker of iodine status in iodine-sufficient and mildly iodine-deficient pregnant women. Eur. J. Nutr (2023) doi: 10.1007/s00394-023-03131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krzeczkowski JE et al. Iodine status in a large Canadian pregnancy cohort. Am. J. Obstet. Gynecol. MFM 5, 100784 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ballesteros V et al. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: A systematic review of epidemiologic studies. Environ. Int 99, 15–28 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Liang H et al. Prenatal exposure to perfluoroalkyl substances and thyroid hormone concentrations in cord plasma in a Chinese birth cohort. Environ. Health 19, 127 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S et al. Maternal exposures to persistent organic pollutants are associated with DNA methylation of thyroid hormone-related genes in placenta differently by infant sex. Environ. Int 130, 104956 (2019). [DOI] [PubMed] [Google Scholar]
- 45.Leonetti C et al. Brominated flame retardants in placental tissues: associations with infant sex and thyroid hormone endpoints. Environ. Health 15, 113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malin AJ, Riddell J, McCague H & Till C Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ. Int 121, 667–674 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Buckalew AR et al. Evaluation of potential sodium-iodide symporter (NIS) inhibitors using a secondary Fischer rat thyroid follicular cell (FRTL-5) radioactive iodide uptake (RAIU) assay. Arch. Toxicol 94, 873–885 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waugh DT Fluoride Exposure Induces Inhibition of Sodium/Iodide Symporter (NIS) Contributing to Impaired Iodine Absorption and Iodine Deficiency: Molecular Mechanisms of Inhibition and Implications for Public Health. Int. J. Environ. Res. Public. Health 16, 1086 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodman CV et al. Iodine Status Modifies the Association between Fluoride Exposure in Pregnancy and Preschool Boys’ Intelligence. Nutrients 14, 2920 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arthur JR, Nicol F & Beckett GJ The role of selenium in thyroid hormone metabolism and effects of selenium deficiency on thyroid hormone and iodine metabolism. Biol. Trace Elem. Res 34, 321–325 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


