Abstract
OBJECTIVE
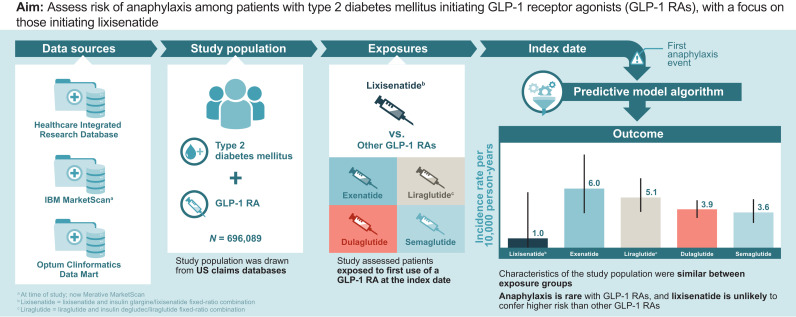
To assess risk of anaphylaxis among patients with type 2 diabetes mellitus who are initiating therapy with a glucagon-like peptide 1 receptor agonist (GLP-1 RA), with a focus on those starting lixisenatide therapy.
RESEARCH DESIGN AND METHODS
A cohort study was conducted in three large, U.S. claims databases (2017–2021). Adult (aged ≥18 years) new users of a GLP-1 RA who had type 2 diabetes mellitus and ≥6 months enrollment in the database before GLP-1 RA initiation (start of follow-up) were included. GLP-1 RAs evaluated were lixisenatide, an insulin glargine/lixisenatide fixed-ratio combination (FRC), exenatide, liraglutide or insulin degludec/liraglutide FRC, dulaglutide, and semaglutide (injectable and oral). The first anaphylaxis event during follow-up was identified using a validated algorithm. Incidence rates (IRs) and 95% CIs were calculated within each medication cohort. The unadjusted IR ratio (IRR) comparing anaphylaxis rates in the lixisenatide cohort with all other GLP-1 RAs combined was analyzed post hoc.
RESULTS
There were 696,089 new users with 456,612 person-years of exposure to GLP-1 RAs. Baseline demographics, comorbidities, and use of other prescription medications in the 6 months before the index date were similar across medication cohorts. IRs (95% CIs) per 10,000 person-years were 1.0 (0.0–5.6) for lixisenatide, 6.0 (3.6–9.4) for exenatide, 5.1 (3.7–7.0) for liraglutide, 3.9 (3.1–4.8) for dulaglutide, and 3.6 (2.6–4.9) for semaglutide. The IRR (95% CI) for the anaphylaxis rate for the lixisenatide cohort compared with the pooled other GLP-1 RA cohort was 0.24 (0.01–1.35).
CONCLUSIONS
Anaphylaxis is rare with GLP-1 RAs. Lixisenatide is unlikely to confer higher risk of anaphylaxis than other GLP-1 RAs.
Graphical Abstract
Introduction
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) are an increasingly important class of medications for the treatment of type 2 diabetes mellitus and obesity. GLP-1 RAs are used as monotherapy or in combination with other diabetes therapies. GLP-1 RAs that have been or are currently approved for use in the U.S. include dulaglutide, exenatide, liraglutide (as monotherapy or in a fixed-ratio combination [FRC] with insulin degludec), lixisenatide (as monotherapy or in an FRC with insulin glargine), semaglutide, and albiglutide (no longer marketed in the U.S.). GLP-1 RAs can be categorized as exendin-based GLP-1 RAs (e.g., exenatide, lixisenatide) or as human-analog GLP-1 RAs (e.g., albiglutide, dulaglutide, liraglutide, semaglutide) (1). The effectiveness of GLP-1 RAs for improving glycemic control, as well as for weight benefit with less hypoglycemia, is well established. However, because GLP-1 RAs are peptides, there is a potential for the therapeutic agent to trigger an immune response in which patients produce antibodies against the agent. This immunogenicity can result in injection-site reactions, neutralization of the therapeutic effects, and anaphylactic reactions (2).
Previous studies have evaluated rates of anaphylaxis with GLP-1 RA medications (1,3). Pradhan et al. (1) used a case-noncase approach using the World Health Organization’s anonymized, global, individual-case safety report database (VigiBase) to estimate the reporting odds of anaphylactic reactions and observed a modestly elevated risk of anaphylaxis with exendin-based GLP-1 RAs relative to human-analog GLP-1 RAs (adjusted odds ratio, 2.08; 95% CI, 1.37–3.19). Pradhan et al. (3) also conducted a large active-comparator cohort study set within health care databases in the U.K. and the U.S. To identify anaphylactic reactions, the authors used the definition validated by Peng and Jick (4), with a positive predictive value (PPV) of 72.5%, in the U.K.; in the U.S. databases, they adapted the algorithm validated by Walsh et al. (5) that had a PPV of 63%. The authors concluded that the GLP-1 RAs were associated with a modestly higher risk of anaphylactic reactions compared with the dipeptidyl peptidase 4 inhibitors (weighted hazard ratio, 1.15; 95% CI, 0.94–1.42) and compared with sodium-glucose cotransporter 2 inhibitors (weighted hazard ratio, 1.38; 95% CI, 1.02–1.87), but they did not evaluate the risk of anaphylaxis among specific GLP-1 RAs (3).
In the lixisenatide development program, more cases of anaphylaxis were reported for lixisenatide than placebo (incidence rate [IR] of anaphylaxis per 1,000 person-years, 1.6 cases for the lixisenatide group, and 0.7 cases for the placebo group), and the attributable risk of anaphylaxis related to lixisenatide was 1 case per 1,000 patient-years (6,7). Enhanced reporting of suspected allergic events through prospective, standardized, and blinded adjudication of all potential cases by a committee of three independent allergy experts was conducted in this clinical development program. Anaphylaxis was defined as a skin or mucosal lesion of acute onset associated with involvement of at least one other organ system. Symptoms such as hypotension, laryngeal edema, or severe bronchospasm could be present but were not required for the case definition. As a result, the adjudicated events, although clinically important, frequently did not meet a threshold of clinical severity that is typically associated with anaphylaxis.
Evidence suggests that the occurrence of anaphylaxis seems to be infrequent for GLP-1 RA medications, but no previous studies have evaluated the rates of anaphylaxis for the different GLP-1 RA medications, to our knowledge. This cohort study was voluntarily undertaken by the manufacturer of lixisenatide to assess the risk of anaphylaxis among new users of GLP-1 RAs currently marketed in the U.S. The primary objective was to assess risk of anaphylaxis among patients with type 2 diabetes mellitus initiating GLP-1 RAs, with a focus on those initiating lixisenatide.
Research Design and Methods
Study Design
This cohort study included real-world data from three U.S. administrative claims databases. Patients with type 2 diabetes mellitus who were new users of GLP-1 RA medications were followed from the index date (i.e., the date on which the patient received their first prescription for a GLP-1 RA medication within the study period) until the first occurrence of anaphylaxis after starting the GLP-1 RA medication or a censoring event. Censoring events included disenrollment from the health care system, discontinuation of the index drug (no prescription refill for >30 days after the end of the last prescription’s supply), switch to another GLP-1 RA (lixisenatide and insulin glargine/lixisenatide FRC were considered to be the same treatment, as were liraglutide and insulin degludec/liraglutide FRC), death, or end of the study period. This study was also purposed to develop and validate an algorithm to identify anaphylaxis cases in the setting of an administrative claims database (8).
This study was conducted in compliance with all international guidelines and U.S. laws and regulations, as well as any applicable guidelines. All necessary regulatory approvals (e.g., institutional review board, independent ethics committee) were obtained in accordance with local regulations, including local data protection regulations.
Setting and Data Sources
The setting for the cohort study was three U.S. administrative claims databases: the Healthcare Integrated Research Database (HIRD), IBM MarketScan, and Optum Clinformatics Data Mart (CDM). The study period began 1 January 2017 (after lixisenatide and insulin glargine/lixisenatide FRC were approved and available in the U.S.) and was extended through the most recent data available at the time of the analysis in the three data sources (MarketScan: 30 June 2021; HIRD and Optum CDM: 31 December 2021).
Participants
The study population included adults with type 2 diabetes mellitus who received a first prescription of a study drug within the study observation period (i.e., the index date). Patients had to meet all of the following inclusion criteria at their index date: diagnosis of type 2 diabetes mellitus, continuous enrollment in the health care system with medical and pharmacy coverage for ≥6 months before their index date, prescription for a GLP-1 RA study drug within the study observation period, and age ≥18 years on the index date. Patients with a prior prescription for any GLP-1 RA during all available look-back time before the index date or with a prescription for two different study drugs on their potential index date were excluded.
Exposures
The GLP-1 RAs evaluated in this study were those marketed in the U.S. for treatment of patients with type 2 diabetes mellitus between 1 January 2017 through 30 June 2021. The study medications included GLP-1 RAs alone (namely, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide [injectable and oral]) and insulin/GLP-1 RA FRCs (namely, insulin degludec/liraglutide FRC and insulin glargine/lixisenatide FRC). Albiglutide was not included as a study medication because U.S. sales for this medication ended in July 2018.
Baseline Characteristics
Baseline characteristics included demographic variables and comorbidities, identified at any time before the index date, and prescription medications used in the 6 months before the index date. These variables were identified using ICD-9-CM and ICD-10-CM diagnosis codes, Current Procedural Terminology and Healthcare Common Procedure Coding System procedure codes, and National Drug Code and General Product Identifier medication codes.
Outcome
The study outcome was the first anaphylaxis event identified using a predictive model algorithm developed as the initial stage of this study (8). The algorithm was based on ICD-9-CM and ICD-10-CM diagnosis codes, Current Procedural Terminology and Healthcare Common Procedure Coding System procedure codes, and National Drug Code and General Product Identifier medication codes. To develop the prediction model, we first developed a screening algorithm with high sensitivity and then refined the algorithm to exclude false-positive cases. Specifically, a screening algorithm with an estimated PPV of 65% (95% CI, 60–71%) in the HIRD was used to identify potential cases. Then, a subsequently developed predictive model algorithm was used to estimate case probability of each of the potential cases identified by the screening algorithm; a threshold of 0.6 was used to define case status. During the model development process, we found that using a probability threshold of 0.6 excluded 89% (n = 84 of 94) of false-positive cases identified by the screening algorithm and still had a PPV of 94% (95% CI, 91–98%). The model excluded very few true-positive cases (n = 15 of 178) and identified 92% (95% CI, 87–96%) of the cases selected by the screening algorithm (8).
Statistical Methods
Baseline characteristics were summarized by GLP-1 RA medication cohort and by outcome (anaphylaxis case and noncase). For categorical variables, frequencies and percentages were calculated for each category; continuous variables were summarized by mean, minimum, and maximum.
Unadjusted IRs and exact 95% CIs of anaphylaxis were estimated for each medication cohort (9) as the number of outcomes occurring during the person-time at risk divided by the total person-time at risk. Unadjusted IRs are reported as point estimates (number of cases per 10,000 person-years) and 95% CIs. Person-time at risk was measured as the time between the medication index date and the earlier of the end of continuous use or the date of first occurrence of either a censoring event or anaphylaxis outcome. An overall unadjusted IR of anaphylaxis for each medication exposure cohort was also calculated by pooling events and person-years across data sources.
The person-year accrual, and thus the number of anaphylaxis events, in the lixisenatide cohort was lower than expected and deemed to be too low to adequately support planned covariate-adjusted comparative analyses. Instead, we took the unadjusted incidence rate ratios (IRRs) to compare anaphylaxis rates in the lixisenatide cohort with all other GLP-1 RAs combined. We also conducted a quantitative bias analysis to evaluate how strong the association between a confounder and the outcome (anaphylaxis) would need to be to produce the observed IRR if the true IRR was 1.5 (10).
Data and Resource Availability
Study data are confidential due to the privacy policies of each of the data sources.
Results
Study Population
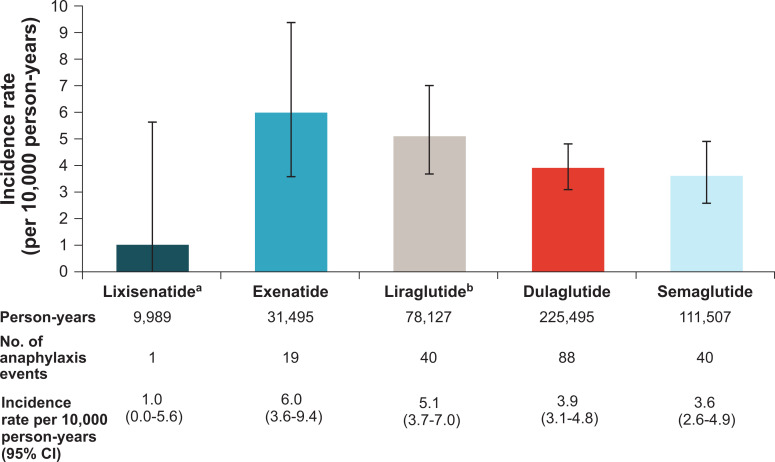
There were 696,089 new users of GLP-1 RAs with 456,612 person-years of exposure to the GLP-1 RA: 15,995 individuals in the lixisenatide cohort, 99% of whom were insulin glargine/lixisenatide FRC users (9,989 person-years); 47,425 exenatide users (31,495 person-years); 112,932 liraglutide users (78,127 person-years); 296,988 dulaglutide users (225,495 person-years); and 222,749 semaglutide users (111,507 person-years). The average duration of follow-up was shortest for semaglutide (6.0 months) and longest for dulaglutide (9.1 months) (Table 1).
Table 1.
Baseline characteristics of all GLP-1 RA medication cohorts, pooled across data sources
| Variable | Lixisenatide (n = 15,995) | Pooled other GLP-1 RA* (n = 680,094) | Exenatide (n = 47,425) | Liraglutide (n = 112,932) | Dulaglutide (n = 296,988) | Semaglutide (n = 222,749) |
|---|---|---|---|---|---|---|
| Age, mean (min, max), years | 58.6 (18, ≥89) | 56.7 (18, ≥89) | 57.4 (18, ≥89) | 56.1 (18, ≥89) | 57.4 (18, ≥89) | 56.2 (18, ≥89) |
| Age by group category, n (%), years | ||||||
| 18–29 | 180 (1.1) | 9,530 (1.4) | 550 (1.2) | 1,815 (1.6) | 3,655 (1.2) | 3,510 (1.6) |
| 30–39 | 903 (5.6) | 43,035 (6.3) | 2,818 (5.9) | 7,960 (7.0) | 17,186 (5.8) | 15,071 (6.8) |
| 40–49 | 2,607 (16.3) | 125,897 (18.5) | 8,389 (17.7) | 22,061 (19.5) | 52,638 (17.7) | 42,809 (19.2) |
| 50–59 | 4,642 (29.0) | 218,556 (32.1) | 14,828 (31.3) | 36,135 (32.0) | 93,857 (31.6) | 73,736 (33.1) |
| 60–69 | 4,458 (27.9) | 186,089 (27.4) | 13,721 (28.9) | 30,204 (26.7) | 82,773 (27.9) | 59,391 (26.7) |
| 70–79 | 2,504 (15.7) | 79,875 (11.7) | 5,959 (12.6) | 12,448 (11.0) | 37,783 (12.7) | 23,685 (10.6) |
| ≥80 | 701 (4.4) | 17,112 (2.5) | 1,160 (2.4) | 2,309 (2.0) | 9,096 (3.1) | 4,547 (2.0) |
| Sex, n (%) | ||||||
| Male | 8,270 (51.7) | 332,555 (48.9) | 23,704 (50.0) | 50,042 (44.3) | 150,358 (50.6) | 108,451 (48.7) |
| Female | 7,723 (48.3) | 347,532 (51.1) | 23,721 (50.0) | 62,889 (55.7) | 146,625 (49.4) | 114,297 (51.3) |
| Missing data | 2 (0.0) | 7 (0.0) | 0 (0.0) | 1 (0.0) | 5 (0.0) | 1 (0.0) |
| Region, n (%) | ||||||
| Midwest | 2,831 (17.7) | 148,663 (21.9) | 11,069 (23.3) | 26,334 (23.3) | 65,382 (22.0) | 45,878 (20.6) |
| Northeast | 1,412 (8.8) | 80,586 (11.8) | 3,889 (8.2) | 13,163 (11.7) | 38,164 (12.9) | 25,370 (11.4) |
| South | 9,346 (58.4) | 349,409 (51.4) | 24,305 (51.2) | 54,581 (48.3) | 147,872 (49.8) | 122,651 (55.1) |
| West | 2,271 (14.2) | 96,112 (14.1) | 7,733 (16.3) | 17,770 (15.7) | 42,861 (14.4) | 27,748 (12.5) |
| Other | 3 (0.0) | 202 (0.0) | 15 (0.0) | 38 (0.0) | 91 (0.0) | 58 (0.0) |
| Missing data | 132 (0.8) | 5,122 (0.8) | 414 (0.9) | 1,046 (0.9) | 2,618 (0.9) | 1,044 (0.5) |
| Calendar year, n (%) | ||||||
| 2017 | 2,987 (18.7) | 103,997 (15.3) | 12,698 (26.8) | 39,121 (34.6) | 52,178 (17.6) | 0 (0.0) |
| 2018 | 3,813 (23.8) | 117,599 (17.3) | 16,027 (33.8) | 34,203 (30.3) | 56,999 (19.2) | 10,370 (4.7) |
| 2019 | 3,782 (23.6) | 139,552 (20.5) | 9,646 (20.3) | 20,582 (18.2) | 63,987 (21.5) | 45,337 (20.4) |
| 2020 | 2,891 (18.1) | 148,958 (21.9) | 6,260 (13.2) | 11,502 (10.2) | 59,606 (20.1) | 71,590 (32.1) |
| 2021 | 2,522 (15.8) | 169,988 (25.0) | 2,794 (5.9) | 7,524 (6.7) | 64,218 (21.6) | 95,452 (42.9) |
| Season, n (%) | ||||||
| Winter: December–February | 3,673 (23.0) | 160,281 (23.6) | 11,366 (24.0) | 27,646 (24.5) | 70,937 (23.9) | 50,332 (22.6) |
| Spring: March–May | 3,883 (24.3) | 169,299 (24.9) | 12,626 (26.6) | 29,245 (25.9) | 75,445 (25.4) | 51,983 (23.3) |
| Summer: June–August | 4,182 (26.1) | 175,150 (25.8) | 12,143 (25.6) | 28,592 (25.3) | 76,090 (25.6) | 58,325 (26.2) |
| Fall: September–November | 4,257 (26.6) | 175,364 (25.8) | 11,290 (23.8) | 27,449 (24.3) | 74,516 (25.1) | 62,109 (27.9) |
| Medical history, n (%)† | ||||||
| Allergic rhinitis | 3,369 (21.1) | 166,246 (24.4) | 11,167 (23.5) | 26,690 (23.6) | 69,262 (23.3) | 59,127 (26.5) |
| Drug allergy | 1,588 (9.9) | 61,819 (9.1) | 3,962 (8.4) | 10,085 (8.9) | 26,121 (8.8) | 21,651 (9.7) |
| Food, insect, or latex allergy | 2,098 (13.1) | 105,201 (15.5) | 7,292 (15.4) | 18,751 (16.6) | 45,209 (15.2) | 33,949 (15.2) |
| Other allergy | 5,110 (31.9) | 246,711 (36.3) | 16,468 (34.7) | 41,111 (36.4) | 104,225 (35.1) | 84,907 (38.1) |
| Anaphylaxis | 58 (0.4) | 2,854 (0.4) | 158 (0.3) | 487 (0.4) | 1,213 (0.4) | 996 (0.4) |
| Asthma | 2,215 (13.8) | 109,669 (16.1) | 7,364 (15.5) | 19,420 (17.2) | 45,709 (15.4) | 37,176 (16.7) |
| Atopic dermatitis | 382 (2.4) | 19,615 (2.9) | 1,149 (2.4) | 2,891 (2.6) | 8,081 (2.7) | 7,494 (3.4) |
| Autoimmune disease | 2,039 (12.7) | 87,859 (12.9) | 6,125 (12.9) | 15,165 (13.4) | 37,425 (12.6) | 29,144 (13.1) |
| Eczema | 2,099 (13.1) | 108,097 (15.9) | 7,034 (14.8) | 17,458 (15.5) | 46,187 (15.6) | 37,418 (16.8) |
| HIV infection | 130 (0.8) | 6,848 (1.0) | 410 (0.9) | 1,114 (1.0) | 3,013 (1.0) | 2,311 (1.0) |
| Prescription medication use, n (%)† | ||||||
| Anabolic steroids (androgens) | 223 (1.4) | 11,231 (1.7) | 813 (1.7) | 1,928 (1.7) | 4,729 (1.6) | 3,761 (1.7) |
| ACE inhibitors | 5,346 (33.4) | 217,139 (31.9) | 16,244 (34.3) | 36,724 (32.5) | 98,258 (33.1) | 65,913 (29.6) |
| ARBs | 3,242 (20.3) | 138,506 (20.4) | 9,411 (19.8) | 21,953 (19.4) | 59,880 (20.2) | 47,262 (21.2) |
| Antibiotics | 4,323 (27.0) | 185,267 (27.2) | 13,552 (28.6) | 33,880 (30.0) | 79,670 (26.8) | 58,165 (26.1) |
| Glucose-lowering drug classes | 14,456 (90.4) | 618,321 (90.9) | 43,871 (92.5) | 100,629 (89.1) | 274,838 (92.5) | 198,983 (89.3) |
| Metformin | 10,024 (62.7) | 480,001 (70.6) | 34,100 (71.9) | 76,594 (67.8) | 212,351 (71.5) | 156,956 (70.5) |
| α-Glucosidase inhibitors | 71 (0.4) | 1,991 (0.3) | 206 (0.4) | 325 (0.3) | 910 (0.3) | 550 (0.2) |
| DPP-4 inhibitors | 3,218 (20.1) | 145,058 (21.3) | 11,657 (24.6) | 21,773 (19.3) | 69,531 (23.4) | 42,097 (18.9) |
| Meglitinides | 212 (1.3) | 5,282 (0.8) | 398 (0.8) | 891 (0.8) | 2,509 (0.8) | 1,484 (0.7) |
| SGLT2 inhibitors | 3,480 (21.8) | 147,223 (21.6) | 10,844 (22.9) | 17,417 (15.4) | 65,154 (21.9) | 53,808 (24.2) |
| Sulfonylureas | 4,734 (29.6) | 201,873 (29.7) | 16,177 (34.1) | 31,801 (28.2) | 97,232 (32.7) | 56,663 (25.4) |
| Thiazolidinediones | 1,261 (7.9) | 45,006 (6.6) | 4,041 (8.5) | 6,779 (6.0) | 20,487 (6.9) | 13,699 (6.1) |
| Insulin | 8,392 (52.5) | 204,923 (30.1) | 14,259 (30.1) | 39,668 (35.1) | 92,849 (31.3) | 58,147 (26.1) |
| Antihistamine | 627 (3.9) | 28,116 (4.1) | 1,988 (4.2) | 5,312 (4.7) | 11,483 (3.9) | 9,333 (4.2) |
| β-Blockers | 5,126 (32.0) | 206,505 (30.4) | 14,902 (31.4) | 37,211 (32.9) | 90,861 (30.6) | 63,531 (28.5) |
| Epinephrine | 117 (0.7) | 6,655 (1.0) | 426 (0.9) | 1,189 (1.1) | 2,725 (0.9) | 2,315 (1.0) |
| Immunosuppressive agents | 121 (0.8) | 5,359 (0.8) | 241 (0.5) | 982 (0.9) | 2,331 (0.8) | 1,805 (0.8) |
| Immunotherapy | 65 (0.4) | 5,308 (0.8) | 353 (0.7) | 963 (0.9) | 2,169 (0.7) | 1,823 (0.8) |
| Loop diuretics | 1,797 (11.2) | 69,891 (10.3) | 4,678 (9.9) | 13,770 (12.2) | 30,402 (10.2) | 21,041 (9.4) |
| Metaraminol | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| NSAIDs | 2,825 (17.7) | 132,092 (19.4) | 9,434 (19.9) | 22,661 (20.1) | 55,595 (18.7) | 44,402 (19.9) |
| Noradrenaline | 0 (0.0) | 10 (0.0) | 0 (0.0) | 2 (0.0) | 1 (0.0) | 7 (0.0) |
| Proton pump inhibitors | 3,135 (19.6) | 145,702 (21.4) | 10,028 (21.1) | 25,134 (22.3) | 62,768 (21.1) | 47,772 (21.4) |
| Steroids (glucocorticoids) | 3,090 (19.3) | 147,790 (21.7) | 9,931 (20.9) | 26,347 (23.3) | 61,710 (20.8) | 49,802 (22.4) |
| Injectable | 1,864 (11.7) | 89,631 (13.2) | 6,187 (13.0) | 16,253 (14.4) | 37,327 (12.6) | 29,864 (13.4) |
| Oral | 1,737 (10.9) | 84,563 (12.4) | 5,436 (11.5) | 15,091 (13.4) | 35,175 (11.8) | 28,861 (13.0) |
| Thiazides and thiazide-like diuretics | 1,745 (10.9) | 86,953 (12.8) | 5,799 (12.2) | 14,474 (12.8) | 37,297 (12.6) | 29,383 (13.2) |
| Vasopressin | 0 (0.0) | 20 (0.0) | 0 (0.0) | 2 (0.0) | 7 (0.0) | 11 (0.0) |
| Antivirus vaccines | 1,718 (10.7) | 93,493 (13.7) | 4,458 (9.4) | 11,470 (10.2) | 39,248 (13.2) | 38,317 (17.2) |
| Total no. of prescription medications taken | ||||||
| 0–3 | 8,709 (54.4) | 375,860 (55.3) | 25,698 (54.2) | 61,017 (54.0) | 162,680 (54.8) | 126,465 (56.8) |
| 4–6 | 6,736 (42.1) | 279,982 (41.2) | 20,041 (42.3) | 47,522 (42.1) | 123,966 (41.7) | 88,453 (39.7) |
| ≥7 | 550 (3.4) | 24,252 (3.6) | 1,686 (3.6) | 4,393 (3.9) | 10,342 (3.5) | 7,831 (3.5) |
| Procedures, n (%)‡ | ||||||
| Bronchodilator therapy | 940 (5.9) | 39,026 (5.7) | 2,758 (5.8) | 6,966 (6.2) | 16,613 (5.6) | 12,689 (5.7) |
| Administration of intravenous fluids | 2,366 (14.8) | 84,710 (12.5) | 5,883 (12.4) | 14,236 (12.6) | 36,865 (12.4) | 27,726 (12.4) |
| Duration of follow-up | ||||||
| Total length of follow-up, person-years | 9,989 | 446,624 | 31,495 | 78,127 | 225,495 | 111,507 |
| Average length of follow-up, months | 7.5 | 7.9 | 8.0 | 8.3 | 9.1 | 6.0 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; DPP-4, dipeptidyl peptidase 4 inhibitor; max, maximum; min, minimum; NSAID, nonsteroidal anti-inflammatory drug; SGLT2, sodium–glucose cotransporter 2.
*The pooled other GLP-1 RA cohort included exenatide, liraglutide, dulaglutide, and semaglutide.
†Presence of claims for dispensing during the 183-day look-back period before and including the index date.
‡Presence of outpatient or inpatient code during the all-available-claims look-back period.
Supplementary Tables 1–3 present baseline characteristics of the HIRD population (n = 193,353), the Optum CDM population (n = 269,571), and the MarketScan population (n = 233,165), respectively. The study population in the Optum CDM data source was older (the mean age for the lixisenatide cohort was 63 years, and for the pooled other GLP-1 RA cohort, it was 61 years) than that reflected in the HIRD (the mean age for both the lixisenatide and the pooled other GLP-1 RA cohorts was 54 years) and MarketScan (the mean age for both the lixisenatide and the pooled other GLP-1 RA cohorts was 54 years). Consequently, the Optum population had some characteristics that were somewhat different (e.g., a greater proportion of individuals with a history of coronary heart disease, hypertension, serious infection, chronic obstructive pulmonary disease, autoimmune disease, and drug allergies). Other characteristics were comparable across the data sources.
The demographic characteristics of the individuals initiating lixisenatide-containing GLP-1 RAs were generally similar to those of individuals initiating other GLP-1 RAs, although more women than men took liraglutide (Table 1). Comorbidities, measured over all time before the index date, were comparable across medication cohorts. Prescription medication use during the 6 months before the index date were generally comparable across medication cohorts with one exception: a greater proportion of the lixisenatide cohort used insulin before the index date compared with individuals initiating other GLP-1 RAs (lixisenatide cohort, 52.5%; pooled other GLP-1 RA cohort, 30.1%).
Incidence of Anaphylaxis
A total of 188 anaphylactic events, according to the study algorithm (8), were identified for the pooled population of 696,089 new users of GLP-1 RAs with 456,612 person-years of exposure. Supplementary Table 4 presents the number of anaphylaxis events and IRs of anaphylaxis by data source. IRs of anaphylaxis per 10,000 person-years were 1.0 for lixisenatide, 6.0 for exenatide, 5.1 for liraglutide, 3.9 for dulaglutide, and 3.6 for semaglutide, with wide 95% CIs (Fig. 1). The IR of anaphylaxis in the pooled other GLP-1 RA cohort (including exenatide, liraglutide, dulaglutide, and semaglutide) was 4.2 per 10,000 person-years (95% CI, 3.6–4.8) versus 1.0 (95% CI, 0.0–5.6) in the lixisenatide cohort. The unadjusted IRR for the anaphylaxis rate for the lixisenatide cohort compared with the anaphylaxis rate for the other GLP-1 RA cohort was 0.24 (95% CI, 0.01–1.35).
Figure 1.
Crude anaphylaxis incidence rates and 95% CIs pooled across data sources. aLixisenatide refers to lixisenatide and insulin glargine/lixisenatide fixed-ratio combination. bLiraglutide refers to liraglutide and insulin degludec/liraglutide fixed-ratio combination.
We evaluated IRs of anaphylactic events in discrete time intervals after the index date (Supplementary Table 5 and Supplementary Fig. 1). No obvious time trend was present in the incidence of anaphylactic events for up to 2 years of follow-up after the initiation of the study medication.
Quantitative Bias Analysis
The quantitative bias analysis revealed that if the true IRR is 1.5 and the prevalence of the confounder is 10% in the exposed cohort and 100% in the comparator cohort, then the relative risk of the association between the confounder and disease (anaphylaxis) would have to be 15 to produce the observed IRR (IRROBS) of 0.24 (Table 2). As the prevalence of the confounder gets closer together in the two exposure groups, then the relative risk of the association between the confounder and disease must be markedly stronger to see an IRROBS of 0.24; in fact, with a difference of 83% or less (e.g., prevalence of 100% and 17%), it is impossible to reach an IRROBS of 0.24 regardless of the strength of the association between the confounder and the outcome. The largest difference seen in prevalence of a potential confounder was 22.4% for use of insulin prior to the index date (52.5% of the lixisenatide cohort and 30.1% of the pooled other GLP-1 RA cohort).
Table 2.
Quantitative bias analysis
| IRRT | PC1 (%) | PC0 (%) | RRCD | IRROBS |
|---|---|---|---|---|
| 1.5 | 0 | 100 | 6.2 | 0.24 |
| 1.5 | 10 | 100 | 15 | 0.24 |
| 1.5 | 15 | 100 | 85 | 0.24 |
| 1.5 | 16 | 100 | 3,000 | 0.24 |
| 1.5 | 17 | 100 | 5,000 | 0.26* |
IRROBS, observed incidence rate ratio; IRRT, true incidence rate ratio; PC0, prevalence of the confounder in the unexposed group; PC1, prevalence of the confounder in the exposed group; RRCD, relative risk of the association between the confounder and disease (outcome).
*Using this methodology, an IRROBS of 0.24 could not be achieved, given the other parameters.
Conclusions
This cohort study evaluated the risk of anaphylaxis among new users of lixisenatide-containing GLP-1 RAs (lixisenatide and insulin glargine/lixisenatide FRC) and other GLP-1 RAs. A total of 188 anaphylactic events among 696,089 new users of GLP-1 RAs were identified. Only one anaphylaxis event was identified in the lixisenatide cohort (including lixisenatide and insulin glargine/lixisenatide FRC) among 15,995 new users with 9,989 person-years of exposure to lixisenatide, resulting in an IR of 1.0 events per 10,000 person-years (95% CI, 0.0–5.6). The IR estimates for all other GLP-1 RAs ranged from 3.9 to 6.0, and when pooled, the IR for the other GLP-1 RAs was 4.2 per 10,000 person-years (95% CI, 3.6–4.8). Because of the limited size of the lixisenatide cohort, adjusted comparative analyses were not conducted. As a post hoc analysis, the unadjusted IRR for anaphylaxis comparing lixisenatide users with the other GLP-1 RA users was calculated as 0.24, with a 95% CI around the unadjusted IRR of 0.01 to 1.35, suggesting little or no increased risk associated with lixisenatide use compared with other GLP-1 RAs. Because this is a crude estimate, unadjusted for possible confounding, it is subject to error from confounding bias. However, as determined from the quantitative bias analysis, the prevalence of a confounder would have to be highly unbalanced in the two cohorts, and the association between the confounder and outcome would have to be strong and specifically in the direction that biases the IRR downward to be masking even a modestly higher risk of anaphylaxis with lixisenatide versus other GLP-1 RAs. We believe this combination of circumstances is unlikely and that the results, despite the imprecision, provide reassurance against a higher risk of anaphylaxis among users of lixisenatide or insulin glargine/lixisenatide FRC than in users of the other GLP-1 RA medications.
The new-user design, selection of active comparators, and use of a validated anaphylaxis case-identification algorithm (8) enhance comparability of treatment groups and accurate ascertainment of anaphylaxis events in this study. The characteristics of individuals initiating lixisenatide-containing GLP-1 RAs were similar to those of individuals initiating other GLP-1 RAs. The only notable difference was that a greater proportion of the lixisenatide cohort used insulin before the index date compared with those initiating other GLP-1 RAs. Because the insulin glargine/lixisenatide FRC was used much more frequently than lixisenatide alone (99% insulin glargine/lixisenatide FRC vs. 1% lixisenatide), the more common use of insulin in the 6 months before the index date in this cohort is not unexpected.
For context, the frequency of anaphylactic reactions from phase 3 clinical trials, long-term extensions, and postmarketing spontaneous reports for the GLP-1 RAs is uncommon (n ≥ 10 per 10,000 to <100 per 10,000) for lixisenatide and rare (n < 10 per 10,000) for exenatide, liraglutide, insulin degludec/liraglutide FRC, dulaglutide, and semaglutide (11–19). In the present study, the IRs of anaphylaxis for all the GLP-1 RA medications fall into the “rare” classification. The IRs of anaphylaxis observed in this study are also consistent with prior observational evidence, including the cohort study by Pradhan et al. (3) in which they estimated the IR of anaphylactic reactions for GLP-1 RAs to be 4.1 per 10,000 person-years. Pradhan et al. (3) used the algorithm developed in the U.S. Food and Drug Administration’s Mini-Sentinel study using ICD-9-CM coding (5), which had a PPV of 63% but no estimate of sensitivity, and adapted this algorithm to accommodate the change to ICD-10-CM coding. In comparison, the algorithm used in the present study was based on the algorithm developed in the Food and Drug Administration’s Mini-Sentinel study but included two steps: a screening algorithm to enable estimates of sensitivity and a predictive model algorithm. The screening algorithm had a PPV of 65% (95% CI, 60–71%) and a presumed sensitivity close to 100% (20). The performance characteristics for the predictive model algorithm (at the selected probability threshold) were a PPV of 94% (95% CI, 91–98%), sensitivity of 92%, and specificity of 89% (8).
A key strength of this study is more accurate ascertainment of anaphylaxis cases than in the study by Pradhan et al. (3), which did not estimate sensitivity and, therefore, the number of true anaphylaxis cases that might have been missed is unknown. Because the outcome of anaphylaxis is rare, a large population was required to evaluate the risk; a strength of this real-world study is its size, with 696,089 new users of GLP-1 RAs contributing 456,612 person-years of exposure. Because this study of the occurrence of a rare outcome used data from large health care databases, a more timely result could be achieved than in a prospective study. The study also included three databases to boost the number of new users of lixisenatide and insulin glargine/lixisenatide FRC. Collectively, these three data sources represent a large portion of the commercially insured U.S. population, supporting generalizability to the insured U.S. population. In addition, the study included patients prescribed these medications in a usual clinical care setting and followed per usual clinical practice. Therefore, the results are likely to be relevant to clinical practice, particularly in the U.S.
Nonetheless, limitations are noted. First, the data used for this study were collected for billing purposes, not for research purposes. Issues related to coding of conditions to allow billing for procedures to diagnostically exclude a condition are well known (21); thus, there was the potential for misclassification of the anaphylaxis outcome. However, the validation study of patients with type 2 diabetes in the HIRD suggested the outcome algorithm, at the selected probability threshold, was largely accurate (8). Because GLP-1 RA medications are self-administered, it is difficult to know whether patients used the GLP-1 RA medications as prescribed and the exact timing of medication administration; therefore, errors may exist in determining the timing of anaphylaxis in relation to medication use. Although we did not observe any notable time trend in the incidence of anaphylactic events for up to 2 years of follow-up after initiation of a GLP-1 RA medication, we did not assess the risk of anaphylaxis associated with use of a GLP-1 RA after a gap in treatment.
Because the study population was pooled across the three data sources, a small percentage of overlap between the populations in the different data sources was possible. Because of low accrual in the lixisenatide cohort, adjusted comparative analyses were not done. Finally, the algorithm used to identify anaphylaxis, despite having excellent performance characteristics, did not differentiate between anaphylaxis related to GLP-1 RA use and anaphylaxis from other exposures.
Conclusion
Results of this real-world cohort study indicate that anaphylaxis is a rare adverse event with GLP-1 RA treatments. The results of this study indicate little meaningful difference in risk of anaphylaxis between drugs of this class.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25054862.
Article Information
Acknowledgments. The authors thank Claire Bocage for providing project management support for the Carelon Research group during this study. Kate Lothman, of RTI Health Solutions, Research Triangle Park, NC, provided medical writing services during the development of the manuscript. These services were funded by Sanofi. Ms. Lothman received no additional financial support for her participation.
Funding. This study was funded by Sanofi. This study was conducted under research contracts between Sanofi and RTI Health Solutions and between Sanofi and Elevance Health. The research contract between Sanofi and RTI Health Solutions included independent publication agreements.
Duality of Interest. M.S.A., C.B.J., K.J.R., C.W.S., and B.C. are employees of RTI Health Solutions. C.L.C., D.C.B., L.E.P., and S.L. are employees of Elevance Health. S.B., L.D., J.J., and C.P. are employees of Sanofi. V.R.A. has served as a consultant for Applied Therapeutics, Fractyl, Novo Nordisk, Pfizer, and Sanofi, conducting this work as a paid consultant to Sanofi, and is publishing in that capacity. V.R.A. has received research contracts (payable to institution) from Applied Therapeutics, Eli Lilly, Fractyl, Novo Nordisk, and Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.S.A., V.R.A., L.E.P., S.B., B.C., D.C.B., C.L.C., L.D., C.B.J., J.J., S.L., C.P., K.J.R., C.W.S., and K.E.W. declare that they have made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, data analysis and interpretation, or in all these areas; have substantially revised or critically reviewed the article; have reviewed and approved the final article to be published; and agree to take responsibility and be accountable for the contents of the article. M.S.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
V.R.A. is an editor of Diabetes Care but was not involved in any of the decisions regarding review of the manuscript or its acceptance.
Prior Presentations. This work was presented in part at the 2020 36th ICPE International Virtual Conference on Pharmacoepidemiology & Therapeutic Risk Management, 16 September 2020, and as a poster at the American Diabetes Association’s 83rd Scientific Sessions, San Diego, CA, 23–26 June 2023.
References
- 1. Pradhan R, Montastruc F, Rousseau V, Patorno E, Azoulay L.. Exendin-based glucagon-like peptide-1 receptor agonists and anaphylactic reactions: a pharmacovigilance analysis. Lancet Diabetes Endocrinol 2020;8:13–14 [DOI] [PubMed] [Google Scholar]
- 2. Madsbad S.. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab 2016;18:317–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pradhan R, Patorno E, Tesfaye H, et al. Glucagon-like peptide 1 receptor agonists and risk of anaphylactic reaction among patients with type 2 diabetes: a multisite population-based cohort study. Am J Epidemiol 2022;191:1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peng MM, Jick H.. A population-based study of the incidence, cause, and severity of anaphylaxis in the United Kingdom. Arch Intern Med 2004;164:317–319 [DOI] [PubMed] [Google Scholar]
- 5. Walsh KE, Cutrona SL, Foy S, et al. Validation of anaphylaxis in the Food and Drug Administration’s Mini-Sentinel. Pharmacoepidemiol Drug Saf 2013;22:1205–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sanofi U.S . Medication guide: Soliqua 100/33. June 2022. Accessed 3 September 2022. Available from https://products.sanofi.us/soliqua100-33/soliqua100-33.pdf
- 7. Sanofi U.S . ADLYXIN (lixisenatide) injection, for subcutaneous use. Sanofi U.S.; June 2022. Accessed 3 September 2022. Available from https://products.sanofi.us/Adlyxin/Adlyxin.pdf
- 8. Beachler DC, Taylor DH, Anthony MS, et al. Development and validation of a predictive model algorithm to identify anaphylaxis in adults with type 2 diabetes in U.S. administrative claims data. Pharmacoepidemiol Drug Saf 2021;30:918–926 [DOI] [PubMed] [Google Scholar]
- 9. Dobson AJ, Kuulasmaa K, Eberle E, Scherer J.. Confidence intervals for weighted sums of Poisson parameters. Stat Med 1991;10:457–462 [DOI] [PubMed] [Google Scholar]
- 10. Schneeweiss S.. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15:291–303 [DOI] [PubMed] [Google Scholar]
- 11. Sanofi Groupe . Lyxumia (lixisenatide)10 (or 20) micrograms solution for injection. Sanofi Groupe; 23 September 2021. Accessed 30 November 2022. Available from https://www.ema.europa.eu/en/documents/product-information/lyxumia-epar-product-information_en.pdf.
- 12. Sanofi Groupe . Suliqua 100 units/mL insulin glargine + 50 (or 33) micrograms/mL lixisenatide solution for injection in prefilled pen. Sanofi Groupe; 5 September 2022. Accessed 3 November 2022. Available from https://www.ema.europa.eu/en/documents/product-information/suliqua-epar-product-information_en.pdf
- 13. AstraZeneca AB . Byetta (exenatide) 5 micrograms solution for injection in prefilled pen. AstraZeneca AB; 25 August 2022. Accessed 3 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/byetta-epar-product-information_en.pdf
- 14. AstraZeneca AB . Bydureon (exenatide) 2 mg powder and solvent for prolonged-release suspension for injection. AstraZeneca AB; 26 August 2022. Accessed 3 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/bydureon-epar-product-information_en.pdf
- 15. Novo Nordisk A/S . Victoza (liraglutide) 6 mg/mL solution for injection in prefilled pen. Novo Nordisk A/S; 2 November 2021. Accessed 3 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/victoza-epar-product-information_en.pdf
- 16. Novo Nordisk A/S . Xultophy 100 units/mL degludec + 3.6 mg/mL liraglutide solution for injection. Novo Nordisk A/S; 13 October 2020. Accessed 3 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/xultophy-epar-product-information_en.pdf
- 17. Eli Lilly . Trulicity (dulaglutide) 0.75 mg (or 1.5 mg or 3 mg or 4.5 mg) solution for injection in prefilled pen. Eli Lilly Nederland B.V.; 5 July 2021. Accessed 3 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/trulicity-epar-product-information_en.pdf
- 18. Novo Nordisk A/S . Ozempic (semaglutide) 0.25 mg (or 0.5 mg or 1 mg or 2 mg) solution for injection in prefilled pen. Novo Nordisk A/S; 16 November 2022. Accessed 3 September 2022. Available from https://www.ema.europa.eu/en/documents/product-information/ozempic-epar-product-information_en.pdf
- 19. Novo Nordisk A/S . Rybelsus (semaglutide) 3 mg (or 7 mg or 14 mg) tablets Novo Nordisk A/S; 26 July 2022. Accessed 30 November 2022. Available from https://www.ema.europa.eu/en/documents/product-information/rybelsus-epar-product-information_en.pdf
- 20. Lanes S, Beachler DC.. Validation to correct for outcome misclassification bias. Pharmacoepidemiol Drug Saf 2023;32:700–703 [DOI] [PubMed] [Google Scholar]
- 21. Lanes S, Brown JS, Haynes K, Pollack MF, Walker AM.. Identifying health outcomes in healthcare databases. Pharmacoepidemiol Drug Saf 2015;24:1009–1016 [DOI] [PubMed] [Google Scholar]