Abstract
OBJECTIVE
Glucagon-like peptide 1 receptor agonists (GLP-1RA) are widely used for the management of diabetes mellitus (DM), but their efficacy in familial partial lipodystrophy (FPLD) is unknown. In this retrospective study, we evaluated the effect of GLP-1RA in patients with FPLD.
RESEARCH DESIGN AND METHODS
We analyzed data, reported with SDs, from 14 patients with FPLD (aged 58 ± 12 years; 76.47% female) and 14 patients with type 2 DM (aged 58 ± 13 years; 71% female) before and 6 months after starting GLP-1RA.
RESULTS
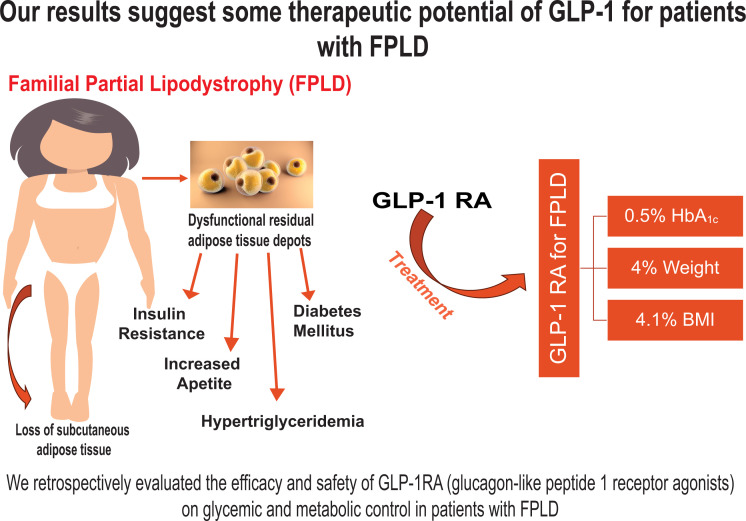
We observed reduction in weight (95 ± 23 to 91 ± 22 kg; P = 0.002), BMI (33 ± 6 to 31 ± 6 kg/m2; P = 0.001), HbA1c (8.2% ± 1.4% to 7.7% ± 1.4%; P = 0.02), and fasting glucose (186 ± 64 to 166 ± 53 mg/dL; P = 0.04) in patients with FPLD. The change in triglycerides after treatment was greater in the FPLD group compared with the DM group (P = 0.02). We noted acute pancreatitis in two case subjects with FPLD with longer therapy.
CONCLUSIONS
Our study demonstrates the relative safety and effectiveness of GLP-1RA in patients with FPLD.
Graphical Abstract
Introduction
Lipodystrophy (LD) is a group of heterogeneous and rare disorders characterized by either generalized or partial lack of adipose tissue, depending on the type of LD (1,2). Familial partial lipodystrophy (FPLD) presents with partial loss of adipose tissue, insulin resistance, hypertriglyceridemia, and hepatic steatosis that result in metabolic comorbidities that are refractory to treatment.
Glucagon-like peptide 1 receptor agonists (GLP-1RA) are an effective treatment for type 2 diabetes mellitus (T2DM) and obesity; they decrease weight and glucose levels, improve the lipid profile, and reduce the risk of major adverse cardiac events (3–6). There have been reports of GLP-1RA significantly lowering glucose levels in FPLD (7) and, in two case reports, there was a HbA1c decrease of 2.7% and 4% and a weight loss of up to 30 kg (8). Although GLP-1RA are widely used for the management of more common forms of diabetes and obesity, there is currently no large-scale study investigating the efficacy and safety (including side effects such as pancreatitis) in FLPD, to our knowledge.
In this retrospective study, we used our LD registry and electronic medical record system to systematically evaluate efficacy and safety of GLP-1RA on glycemic and metabolic control in patients with FPLD.
Research Design and Methods
The University of Michigan Institutional Review Board approved this retrospective study in which we selected patients with FPLD (LD group) who had a GLP-1RA added to their clinical management. To be included, patients had to have a confirmed clinical and laboratory diagnosis of partial LD (9), with no restriction for sex and age, and have started treatment with GLP-1RA and continued for 6 months without any additional change in medical treatment (except expected dose titration of background diabetes medications). Data on demographic characteristics, such as age, sex, BMI, and weight and laboratory measurements (hemoglobin A1c [HbA1c], and triglyceride, total cholesterol, HDL cholesterol, LDL cholesterol, alanine transaminase, aspartate aminotransferase, and total bilirubin levels) were collected before the start of GLP-1RA and 6 months later. Another group, comprising patients with T2DM (DM group) were matched for age and sex with the LD group. Only those with a stable medication regimen for 6 months outside of the addition of the GLP-1RA were included. The effect of the treatment was calculated by the change in value in continuous variables (after treatment minus before treatment). We also interrogated records for occurrence of gallstones and pancreatitis during the observation period and within 12 months after.
For summarizing data distributions, means and SDs for continuous variables, and frequencies and percentages for categorical variables, were used. A paired t test was used to evaluate the differences in continuous variables in the LD group, and an unpaired t test was used when comparing the effect of treatment between the LD and DM groups. Python programming language (version 3.9.2) was used for data analysis (10). Data preprocessing was performed using the Pandas library, and Python statistical analysis was conducted using the scipy.stats module in Python. Graphs were created with GraphPad Prism (version 10.0.1.218; GraphPad Software Inc., San Diego, CA). Differences were considered significant if P < 0.05.
Statement of Ethics
This study was reviewed and approved by the ethical committee of the University of Michigan (IRB Med approval no. LD-LYNC HUM#00127427) and performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from the patients for publication of further research.
Data and Resource Availability
Deidentified data are available upon request with proper justification to the corresponding author.
Results
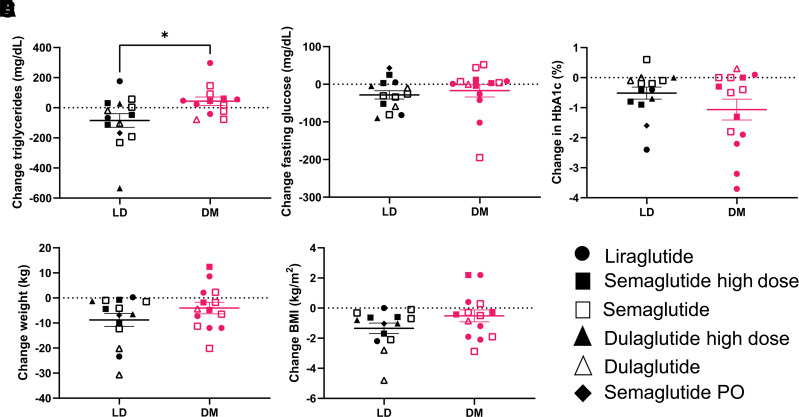
There were 14 patients with FPLD (mean age, 59 ± 11 years; 79% women) and 14 patients with T2DM (mean age, 60 ± 12 years; 71% women). All patients with FPLD had been previously diagnosed with DM associated with LD. The overall effects noted in the two groups in the parameters investigated are presented in Table 1. Treatment with GLP-1RA resulted in significant reductions in weight (P = 0.002), BMI (P = 0.001), HbA1c (P = 0.023), and fasting glucose levels (P = 0.049) in patients with FPLD. Furthermore, triglyceride levels numerically decreased from 334 ± 170 mg/dL before GLP-1RA treatment to 256 ± 82 mg/dL after 6 months of treatment (Table 1). There were comparable improvements in HbA1c in the DM group, whereas the change in fasting glucose levels, weight, BMI, and triglyceride levels did not reach statistical significance within this group. Comparing the two groups, only the change in triglyceride levels after 6 months of treatment was significantly greater in patients with FPLD compared with patients with T2DM (P = 0.026) (Fig. 1A). There were no other significant differences (Fig. 1B–E).
Table 1.
Demographic characteristics for the LD and DM groups, metabolic parameters before and after GLP-1RA treatment, and percentage of changes from baseline observed for each group
| Patients with FPLD | Patients with common type 2 DM | |||||
|---|---|---|---|---|---|---|
| Age (years) | 59 ± 11 | 60 ± 12 | ||||
| Female sex (%) | 79 | 71 | ||||
| Before GLP-1RA | After GLP-1RA | Change (%) | Before GLP-1RA | After GLP-1RA | Change (%) | |
| HbA1c (%) | 8.2 ± 1.4 | 7.7 ± 1.4* | −0.5 ± 0.7 | 7.6 ± 2.0 | 6.4 ± 0.9* | −1.1 ± 1.4 |
| Total cholesterol (mg/dL) | 181 ± 52 | 178 ± 63 | −7.1 ± 43.2 | 161 ± 39 | 173 ± 35 | 13.4 ± 30.1 |
| Triglycerides (mg/dL) | 334.1 ± 170 | 256 ± 81 | −16.7 ± 53.3 | 218 ± 116 | 259 ± 213 | 7.2 ± 35.6† |
| HDL cholesterol (mg/dL) | 39 ± 9 | 36 ± 8 | −2.0 ± 14.6 | 47 ± 17 | 48 ± 15 | 4.0 ± 14.6 |
| LDL cholesterol (mg/dL) | 98 ± 39 | 87 ± 46 | 3.8 ± 63.0 | 80 ± 22 | 81 ± 21 | 12 ± 32.4 |
| Fasting glucose (mg/dL) | 186 ± 64 | 166 ± 53* | −9.6 ± 18.1 | 155 ± 91 | 138 ± 51 | −2.0 ± 25.8 |
| AST (units/L) | 29 ± 14 | 29 ± 13 | 4.1 ± 24.4 | 29 ± 6 | 33 ± 22 | 14.4 ± 67 |
| ALT (units/L) | 32 ± 12 | 29 ± 10 | −2.8 ± 25.7 | 34 ± 7 | 36 ± 22 | 9.6 ± 76.0 |
| ALP (units/L) | 78 ± 25 | 69 ± 27 | −7.3 ± 13.4 | 74 ± 24 | 70 ± 22 | −1.1 ± 18.5 |
| Bilirubin (mg/dL) | 0.5 ± 0.3 | 0.5 ± 0.2 | 6.5 ± 29.3 | 0.4 ± 0.1 | 0.5 ± 0.2 | 25.6 ± 60.0 |
| BMI (kg/m2) | 33 ± 6 | 31 ± 6* | −4.1 ± 4.0 | 34 ± 5 | 34 ± 5 | −1.4 ± 4.5 |
| Weight (kg) | 95 ± 23 | 91 ± 22* | −4.0 ± 4.3 | 98 ± 20 | 96 ± 20 | −1.8 ± 4.4 |
Data are presented as mean ± SD. ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
*P < 0.05 between before and after treatment.
†P < 0.05 comparing the LD and DM groups.
Figure 1.
Comparison between changes in triglycerides (A) after 6 months of treatment with GLP-1RA agonist in patients with LD (n = 14) and common diabetes mellitus (DM) (n = 14). *P = 0.02. Differences in changes for fasting glucose (B), HbA1c (C), weight (D), and BMI (E) were not significant. PO, by mouth.
While evaluating metabolic parameters in patients treated with different dosages and alternative GLP-1RA agents, we examined the reduction of other medications (including insulin). In the group with FPLD, though only two patients had access to 2.0 or 2.4 mg of semaglutide weekly, seven patients reduced their total daily dose of insulin, and three patients discontinued insulin treatment altogether (Table 2). Among patients with T2DM, although three patients had access to a high dose of semaglutide, no significant reduction in background medication was observed (Table 2). There were no significant changes in liver function parameters, and no cases of gallstones or pancreatitis were reported in records of either group during the observation period of 6 months. However, two patients with FPLD developed acute pancreatitis during continued longer therapy within the next 12 months. Of note, these two case subjects had prior history of acute pancreatitis as well.
Table 2.
Important clinical details about patients included in this retrospective study
| Patient no. | Group | Sex | Type of LD, if applicable, and treatment | Genetic test details if applicable for LD group | Summary of background therapy changes during study period | Other details on oral hypoglycemic use |
|---|---|---|---|---|---|---|
| 1 | LD | Male | FPLD type 1, DM, liraglutide | WES performed, negative for known LD genes, interesting variant being studied, brother of patient 11 | Basal insulin decreased from 30 units b.i.d. to just once a day | Taking empagliflozin 25 mg. Never had exposure to TZDs |
| 2 | LD | Female | FPLD type 1, DM, semaglutide* | WES at UM negative, in RADIANT study | TDD insulin decreased from 2,000 to 1,000 units | Taking metformin 1,000 mg b.i.d. Was treated with TZDs in the distant past |
| 3# | LD | Female | FPLD type 1, DM, high-dose dulaglutide | LD panel by Blueprint negative, referred to RADIANT study | TDD insulin decreased from 300 units in a pump to only basal (Tresiba; Novo Nordisk) 60 units | Was treated with TZDs in the distant past |
| 4 | LD | Female | FPLD type 1, DM, semaglutide | LD panel by Blueprint negative; considering RADIANT study | Background therapy stayed stable (metformin and 36 units of basal insulin) | No TZD exposure |
| 5 | LD | Female | FPLD type 1, DM, liraglutide | WES in-house is negative for LD genes | Stopped all insulin (25 units Levemir) | No TZD exposure. Taking stable doses of topiramate and metformin chronically |
| 6 | LD | Female | FPLD type 1, DM, semaglutide | Genetic testing not covered by insurance; in RADIANT study | Stopped all insulin (TDD 40 basal + bolus) | Used TZDs in the distant past; metformin 1,000 mg b.i.d. |
| 7 | LD | Female | FPLD type 1, DM, semaglutide | Genetic testing by WES negative so far, referral to RADIANT study | On insulin pump, TDD insulin decreased from 85 to 22 units | Metformin 1,000 mg b.i.d. Was in a metreleptin study in the distant past (FHA101). No TZD exposure in the past |
| 8 | LD | Female | FPLD type 1, DM, high-dose dulaglutide | Genetic testing by Blueprint panel negative, referral to RADIANT study | TDD insulin decreased from 1,200 to 250 units in a pump. | Uses metformin 1,000 mg b.i.d. Took pioglitazone in the distant past |
| 9 | LD | Female | FPLD type 1, DM, high-dose dulaglutide | Genetic testing by Blueprint panel negative, referral to RADIANT study | Stopped all insulin (∼100 units in basal + bolus) | Takes acarbose 50 mg t.i.d. and dapagliflozin 10 mg daily. No TZD exposure |
| 10 | LD | Female | FPLD type 2, DM, semaglutide* | LMNA R482 W variant, part of a large pedigree | Insulin in pump decreased from 277 units to <80 units daily | Took pioglitazone for years, stopped because of fractures. Treated with metreleptin in a study and discontinued because of cirrhosis. Takes empagliflozin 25 mg daily (sees a bone specialist for IV bisphosphonate therapy and vitamin D) |
| 11 | LD | Male | FPLD type 1, DM, semaglutide PO only | WES performed, negative for known LD genes, interesting variant being studied, brother of patient 1 | Insulin decreased from 60 units basal to 24 units | Takes empagliflozin 25 mg. Metformin 1,000 mg b.i.d. TZD exposure in the distant past |
| 12 | LD | Female | FPLD type 1, DM, semaglutide* | WES performed, negative for known LD genes, RADIANT study referral made | Insulin dose decreased from 1,200 units to 400 units (uses U500) | Metformin 1,000 mg b.i.d. Empagliflozin 10 mg only (25 mg resulted in UTIs). TZD exposure in the distant past |
| 13 | LD | Male | FPLD type 1, DM, semaglutide | WES performed by GenDx identified an interesting variant on WNT10A gene; functional follow-up in progress | No other DM treatment was used | Intolerant of many other diabetes and lipid-lowering medications |
| 14# | LD | Female | FPLD type 1, DM, dulaglutide | University of Chicago Genetics Laboratory LD panel negative, WES done in-house identified an interesting variant; functional follow-up in progress | Lantus 42 units daily switched to 15 units b.i.d.; meal insulin stopped | Took Jardiance in the past, which resulted in AKI. Limited exposure to TZDs in the distant past |
| 15 | DM | Male | T2DM, semaglutide | Not applicable | No significant change on background medication | Metformin 1,000 mg b.i.d.; empagliflozin 25 mg daily |
| 16 | DM | Female | T2DM, liraglutide | No significant change on background medication | Metformin 1,000 mg b.i.d. | |
| 17 | DM | Male | T2DM, semaglutide | No significant change on background medication | Basal + bolus insulin; metformin 1,000 mg b.i.d.; empagliflozin 25 mg b.i.d. | |
| 18 | DM | Female | T2DM, semaglutide* | No significant change on background medication | Metformin 1,000 mg b.i.d. | |
| 19 | DM | Female | T2DM, semaglutide PO | No significant change on background medication | Metformin 1,000 mg b.i.d.; empagliflozin 25 mg daily | |
| 20 | DM | Female | T2DM, semaglutide | No significant change on background medication | Metformin 1,000 mg b.i.d. | |
| 21 | DM | Male | T2DM, semaglutide* | No significant change on background medication | Metformin 1,000 mg b.i.d. | |
| 22 | DM | Female | T2DM, dulaglutide | No significant change on background medication | Metformin 1,000 mg b.i.d. | |
| 23 | DM | Female | T2DM, liraglutide | No significant change on background medication | Metformin 1,000 mg b.i.d.; dapagliflozin 10 mg daily | |
| 24 | DM | Male | T2DM, semaglutide | No significant change on background medication | Basal + bolus insulin; metformin 1,000 mg b.i.d.; Jardiance 25 mg daily; glipizide 10 mg b.i.d. | |
| 25 | DM | Female | T2DM, liraglutide | No significant change on background medication | Basal + bolus insulin; empagliflozin 25 mg daily; glipizide 10 mg b.i.d. | |
| 26 | DM | Female | T2DM, liraglutide | No significant change on background medication | Basal + bolus insulin; empagliflozin 25 mg daily; metformin 1,000 b.i.d. | |
| 27 | DM | Female | T2DM, liraglutide | No significant change on background medication | Basal + bolus insulin; empagliflozin 25 mg daily; metformin 1,000 b.i.d. | |
| 28 | DM | Female | T2DM, semaglutide* | No significant change on background medication | Metformin 1,000 mg b.i.d.; dapagliflozin 10 mg |
AKI, acute kidney injury; IV, intravenous; PO, by mouth; RADIANT, Rare and Atypical Diabetes Network; TDD, total daily dose; TZD, thiazolidinedione; UM, University of Michigan; UTI, urinary tract infection; WES, whole-exome sequencing.
#Has previous history of acute pancreatitis and also developed pancreatitis during the next 12 months after this study period.
*Has been able to receive semaglutide 2.0 or 2.4 mg every week.
Conclusions
We retrospectively evaluated the safety and efficacy of GLP-1RA use in 14 patients with FPLD (which leads to atypical diabetes, a well-recognized complication of FPLD). To our knowledge, this is the largest study to date with this class of drugs in FPLD, and it offers a comparison with 14 age- and sex-matched individuals with common T2DM. Our data suggest GLP-1RA is effective and safe for use in patients with FPLD for reducing weight and glucose and triglyceride levels.
Although there are no U.S. Food and Drug Administration (FDA)-approved therapies for FPLD in the U.S., metreleptin is an option for patients in Europe and Japan (11). Metreleptin supplementation helps restore leptin levels and can lead to improvements in metabolic control and triglyceride levels, increases in insulin sensitivity, reduction of appetite (11,12). According to the data reviewed by the European Medicines Agency (11), metreleptin caused a 20.6% statistically significant reduction in triglyceride levels and a 0.6% reduction in HbA1c in patients with partial LD. Our data demonstrate that GLP-1RA reduced weight by 4.0%, BMI by 4.1%, fasting glucose levels by 9.6%, and HbA1c by 0.5%. Furthermore, triglyceride levels decreased by 16.7%. Both metreleptin and GLP-1RA have notable impacts on metabolic parameters. Whereas metreleptin has the potential to address leptin deficiency (13) in LD, GLP-1RA can influence glucose control and appetite regulation. Although our findings reveal that the effect of GLP-1RA trails slightly behind the reported effects of metreleptin in reducing HbA1c and fasting triglyceride levels, there is currently no information on either concurrent use or comparative efficacies of these two agents.
Although the exact mechanism of action remains an area of interest, there are hypotheses as to why incretin analogs and GLP-1RA are effective in FPLD. Previously, a patient with FPLD type 2 had a combined intravenous glucose tolerance–euglycemic clamp test that demonstrated severe insulin resistance (as anticipated in LD), but also an insulin secretory defect with strongly diminished first-phase insulin secretion, which normalized after GLP-1RA administration (8). Additionally, patients with FPLD type 2 have elevated dipeptidyl peptidase-4 (DPP-4) levels; DPP-4 is known to inactivate GLP-1 when compared with healthy control study participants (14), which is believed to have led to higher levels of central fat distribution. Therefore, it is foreseeable that the effect of GLP-1RA improves first-phase insulin secretion and offsets zthe high DPP-4 levels, which lead to reduced glucose level, reduced weight, a lower body adiposity index, and an improved lipid profile (14). Finally, the possibility of GLP-1 to work as a leptin sensitizer centrally may be an important contributor (15,16).
The strengths of this study were that all patients were followed longitudinally with standardized variables in a large, single institution where a single provider oversaw therapeutic decisions. Therefore, the prescribing practices and patterns were homogeneous. An additional strength of this study is the relatively large number of patients within this cohort for a rare disease who were matched to those with T2DM. This gave us the benefit of comparing a standard FDA-approved GLP-1RA treatment for T2DM with novel use in FPLD.
There are several limitations to our study. One limitation is that FPLD represents a heterogeneous population that may have variable degrees of severity and response to treatment. An attempt was made to control for this aspect by including FPLD with similar phenotypes, in addition to matching for age and sex in patients with T2DM. A second limitation is that this was a retrospective study and, as a result, there were variables of interest that were not controlled for that could be better controlled for in a prospective study. Last, not all patients had the opportunity to receive the highest dose of a given agent or the most potent agent in the GLP-1RA class, because this was a retrospective evaluation and not an interventional study. There is hesitancy when prescribing GLP-1RA, given their previously reported association with pancreatitis (which occurred in two case subjects in this study on long-term follow-up), which limited the number of patients receiving a GLP-1RA.
In conclusion, to our knowledge, this is the first and largest data set that demonstrates GLP-1RA is a safe and effective treatment for patients with FPLD, offering benefits in lowering HbA1c, weight, and BMI.
Article Information
Acknowledgments. We are grateful to our patients who participate in our registry and translational studies and give permission to us to use their data in future research studies.
Funding. E.A.O., M.C.F.-F., and D.T.B. receive grant support from the Caswell Diabetes Institute and from the National Institutes of Health (NIH; grant U54DK118612-04). E.A.O. and M.C.F.-F. were also partially funded by NIH grant R01DK125513.
Duality of Interest. E.A.O. has received grant support from Aegerion Pharmaceuticals (manufacturer of metreleptin), Akcea Therapeutics, Ionis Pharmaceuticals, Regeneron Pharmaceuticals, Gemphire Therapeutics, Novo Nordisk, Rhythm Pharmaceuticals, Fractyl Health Laboratories, and GI Dynamics and has served as an advisor and/or a consultant to Aegerion Pharmaceuticals, Akcea Therapeutics, Ionis Pharmaceuticals, and Regeneron. E.A.O. has royalty rights from the use of metreleptin in lipodystrophy. D.T.B. receives grant funding from Novo Nordisk, Rhythm Pharmaceuticals, Inc., and Fractyl Health Laboratories, Inc., and serves as a consultant for Tayco, Inc. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.C.F.-F. selected the patients for this study, analyzed the data, and wrote the manuscript. S.I. performed the electronic medical record review, collected the data, and wrote the abstract. D.T.B. contributed to writing and editing the manuscript. A.N. and A.D.G. collected the data. E.A.O. oversaw the data collection and analysis and reviewed and edited the manuscript. All authors approved the final version of the manuscript. E.A.O. is the guarantor of this work, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. An abstract of this work was presented as an oral presentation at the American Diabetes Association 83rd Scientific Sessions, San Diego, CA, 23–26 June 2023.
Footnotes
M.C.F-.F. and S.I. contributed equally to this work and are joint first authors.
References
- 1. Chan JL, Oral EA.. Clinical classification and treatment of congenital and acquired lipodystrophy. Endocr Pract 2010;16:310–323 [DOI] [PubMed] [Google Scholar]
- 2. Garg A.. Clinical review: lipodystrophies: genetic and acquired body fat disorders. J Clin Endocrinol Metab 2011;96:3313–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marso SP, Daniels GH, Brown-Frandsen K, et al. ; LEADER Steering Committee ; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marso SP, Bain SC, Consoli A, et al. ; SUSTAIN-6 Investigators . Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834–1844 [DOI] [PubMed] [Google Scholar]
- 5. Hernandez AF, Green JB, Janmohamed S, et al. ; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 6. Gerstein HC, Colhoun HM, Dagenais GR, et al. ; REWIND Investigators . Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121–130 [DOI] [PubMed] [Google Scholar]
- 7. Cook K, Ali O, Akinci B, et al. Effect of leptin therapy on survival in generalized and partial lipodystrophy: a matched cohort analysis. J Clin Endocrinol Metab 2021;106:e2953–e2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Banning F, Rottenkolber M, Freibothe I, Seissler J, Lechner A.. Insulin secretory defect in familial partial lipodystrophy Type 2 and successful long-term treatment with a glucagon-like peptide 1 receptor agonist. Diabet Med 2017;34:1792–1794 [DOI] [PubMed] [Google Scholar]
- 9. Brown RJ, Araujo-Vilar D, Cheung PT, et al. The diagnosis and management of lipodystrophy syndromes: a multi-society practice guideline. J Clin Endocrinol Metab 2016;101:4500–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Python Software Foundation . Python 3.9.2. Python Software Foundation; 2022 [Google Scholar]
- 11. Oral EA, Gorden P, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with partial lipodystrophy. Endocrine 2019;64:500–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown RJ, Oral EA, Cochran E, et al. Long-term effectiveness and safety of metreleptin in the treatment of patients with generalized lipodystrophy. Endocrine 2018;60:479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ajluni N, Meral R, Neidert AH, et al. Spectrum of disease associated with partial lipodystrophy: lessons from a trial cohort. Clin Endocrinol (Oxf) 2017;86:698–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Valerio CM, de Almeida JS, Moreira RO, et al. Dipeptidyl peptidase-4 levels are increased and partially related to body fat distribution in patients with familial partial lipodystrophy type 2. Diabetol Metab Syndr 2017;9:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tong J, Sandoval DA.. Is the GLP-1 system a viable therapeutic target for weight reduction? Rev Endocr Metab Disord 2011;12:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rupp AC, Tomlinson AJ, Affinati AH, et al. Suppression of food intake by Glp1r/Lepr-coexpressing neurons prevents obesity in mouse models. J Clin Invest 2023;133:e157515. [DOI] [PMC free article] [PubMed] [Google Scholar]