Abstract
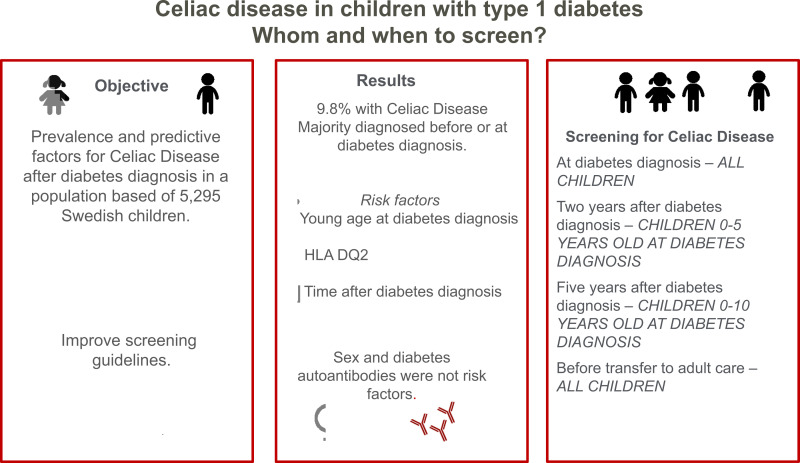
OBJECTIVE
To examine the prevalence and predictive factors for celiac disease (CD) after a diagnosis of type 1 diabetes (T1D) in children and adolescents, to improve the current screening guidelines.
RESEARCH DESIGN AND METHODS
The association between sex, age at T1D diagnosis, HLA, and diabetes autoantibodies, and a diagnosis of CD was examined in 5,295 children with T1D from the Better Diabetes Diagnosis study in Sweden.
RESULTS
The prevalence of biopsy-proven CD was 9.8%, of which 58.2% already had a CD diagnosis before or at T1D onset. Almost all, 95.9%, were diagnosed with CD within 5 years after the T1D diagnosis. Younger age at the T1D diagnosis and being homozygote for DQ2 increased the risk of CD after T1D, but neither sex nor diabetes-related autoantibodies were associated with the risk.
CONCLUSIONS
Age at and time after diabetes diagnosis should be considered in screening guidelines for CD in children with T1D.
Graphical Abstract
Introduction
Routine screening for celiac disease (CD) in children with type 1 diabetes (T1D) is common (1), but the recommendations/guidelines for screening are not well established (2,3). The aims of this study were to 1) investigate the prevalence of confirmed CD before, at, and up to 10 years after the diagnosis of T1D, 2) study potential predictive factors for CD after T1D diagnosis in children and adolescents, and 3) improve current screening guidelines for CD in children with T1D.
Research Design and Methods
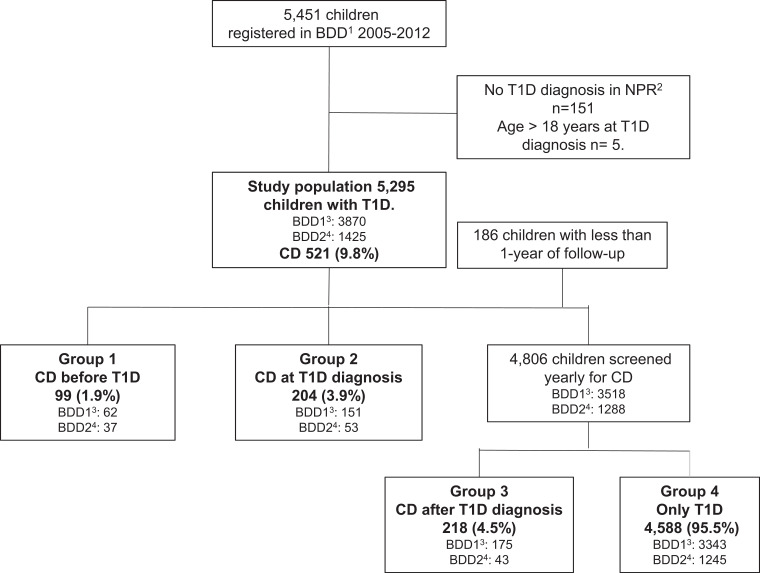
The study group was from the nationwide cohort study, the Better Diabetes Diagnosis (BDD) study, ongoing since 2005 and including >90% of all children and adolescents aged ≤18 years with a diabetes diagnosis in Sweden (4). Between May 2005 and December 2012, 5,451 children were included in BDD, of whom 5,295 were diagnosed with T1D (Fig. 1). Diagnosis of T1D was based on criteria by the American Diabetes Association (5) and validated using inpatient and outpatient registries from the Swedish National Patient Register (NPR) (6), administered by the Swedish National Board of Health and Welfare. T1D was defined using the International Classification of Diseases (ICD) 9th and 10th editions: 250A-X (ICD-9) or E10.0–9 (ICD-10).
Figure 1.
Flowchart of inclusion and exclusion criteria in the study population and the prevalence of CD in the different groups based on when CD is diagnosed in relation to T1D diagnosis.1BDD, Better Diabetes Diagnosis study. 2NPR, Swedish National Patient Register. 3BDD1, children included in BDD between May 2005 and December 2010. 4BDD2, children included in BDD from January 2011 until December 2012.
Information on patients with a CD recorded in the NPR (6) was collected retrospectively (1987–2016). CD was defined using ICD-9 and ICD-10 as 579A (ICD-9) or K90.0, K90.0A, K90.0B, or K90.0x (ICD-10). CD was diagnosed by intestinal biopsy according to national guidelines in Sweden and those of the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) (7).
The date of T1D diagnosis was collected from the BDD study. The first date with an ICD code defining CD in the NPR was the date of CD diagnosis.
In Sweden, all children are screened by transglutaminase autoantibodies at diagnosis of T1D and generally yearly thereafter; therefore, the study population was divided into four groups according to the timing of the CD diagnosis in relation to the T1D diagnosis.
Group 1: Children with a known CD before T1D diagnosis.
Group 2: Children diagnosed with CD at T1D. These children were detected with transglutaminase autoantibodies at T1D diagnosis and received a CD diagnosis within 11.9 months after T1D diagnosis. This group probably had an undiagnosed CD at T1D diagnosis.
Group 3: Children who developed CD after T1D diagnosis; that is, CD diagnosis ≥12 months after the T1D diagnosis.
Group 4: Children with no diagnosis of CD after up to 10 years’ follow-up.
Follow-up time started at T1D diagnosis and ended at CD diagnosis, 18 years of age, or at the end of follow-up on 31 December 2016. Information on age, sex, HLA-DQ type, and diabetes-related autoantibodies at diagnosis of T1D was obtained from BDD (4). The cohort was stratified by age at T1D diagnosis (i.e., 0–4.9, 5–9.9, 10–14.9, and 15–18 years).
Extended HLA-DQ types were determined using information on DQA1 and DQB1 alleles available from the BDD study (4). The alleles were combined into haplotypes and encoded as DQ types (Supplementary Appendix 1). Other DQ-alleles besides DQ2 and DQ8 are named DQX.
Diabetes-related autoantibodies at T1D diagnosis, GAD antibody (GADA), insulin autoantibody (IAA), islet antigen 2 antibodies (IA-2A), and zinc transporter 8 autoantibodies (ZnT8A), were analyzed in all children diagnosed with diabetes between May 2005 and December 2010 (named BDD1). As part of a national clinical routine, children diagnosed with diabetes after December 2010 were only tested for GADA and IA-2A, and if negative, analyses of IAA or/and ZnT8A were also performed (named BDD2) (4). Therefore, only children from BDD1 were included to study associations between any of the four autoantibodies at diagnosis of T1D and CD. The methods used for analyses of HLA and autoantibodies have been described previously (4).
This study was approved by the Ethics Committee of the Medical Faculty, Lund University (Dnr 2014/476) and by the Regional Ethics Board at Karolinska Institutet, Stockholm (Dnr 04-826/1, 2006/1082-32, 2007/1383-32).
Statistical analyses were performed with IBM SPSS Statistics 25 and 28 software. Descriptive data are presented as frequencies and percentages or means and SDs. To compare means, the Student t test and one-way ANOVA were used. For categorical variables, the Pearson χ2 test was used. Statistical significance was determined at α = 0.05.
Results
Figure 1 shows the prevalence of biopsy-proven CD. Mean follow-up time after T1D diagnosis was 5.8 (SD 2.5) years, where 317 children (6.0%) were followed for ≥10 years. More girls, 11.7%, had a double diagnosis compared with 8.3% of boys (P < 0.001), but in those diagnosed with CD after T1D, there was no significant difference in the proportion of girls versus boys (see demographic characteristics in Table 1).
Table 1.
Demographic characteristics at the time of T1D diagnosis by subgroups and comparisons between groups 3 and 4
| Group 1: CD before T1D | Group 2: CD at T1D diagnosis | Group 3: CD after T1D | Group 4: only T1D | Group 3 vs. group 4 P value | |
|---|---|---|---|---|---|
| Age at T1D diagnosis (years), mean (SD) | 10.7 (4.06) | 9.9 (4.12) | 6.6 (4.26) | 9.7 (4.29) | <0.001 |
| Sex | |||||
| Female | 52 (52.5) | 121 (59.3) | 109 (50.0) | 2,042 (44.5) | 0.11 |
| Male | 47 (47.5) | 83 (40.7) | 109 (50.0) | 2,546 (55.5) | |
| HLA | |||||
| DQ2/DQ8 | 39 (39.4) | 83 (40.7) | 80 (36.7) | 1,339 (29.2) | 0.036 |
| DQ2/DQ2 | 21 (21.2) | 36 (17.6) | 25 (11.5) | 212 (4.6) | <0.001 |
| DQ2/DQX | 22 (22.2) | 24 (11.8) | 37 (17.0) | 551 (12.0) | 0.044 |
| DQ8/DQ8 | 8 (8.1) | 28 (13.7) | 24 (11.0) | 476 (10.4) | 0.874 |
| DQ8/DQX | 7 (7.1) | 29 (14.2) | 50 (22.9) | 1,364 (29.7) | 0.016 |
| DQX/DQX | 0 (0) | 1 (0.5) | 0 (0) | 478 (10.4) | <0.001 |
| Missing | 2 (2.0) | 3 (1.5) | 2 (0.9) | 168 (3.7) | |
| Autoantibodies* | |||||
| 0 | 5 (8.1) | 5 (3.3) | 7 (4.0) | 249 (7.4) | 0.095 |
| 1 | 5 (8.1) | 27 (17.9) | 25 (14.3) | 388 (11.6) | 0.245 |
| 2 | 20 (32.3) | 35 (23.2) | 51 (29.1) | 833 (24.9) | 0.161 |
| 3 | 23 (37.1) | 44 (29.1) | 57 (32.6) | 1,106 (33.1) | 0.995 |
| 4 | 6 (9.7) | 34 (22.5) | 23 (13.1) | 589 (17.6) | 0.148 |
| Missing | 3 (4.8) | 6 (4.0) | 12 (6.9) | 178 (5.3) | |
| GADA* | |||||
| Positive | 43 (69.4) | 99 (65.6) | 106 (60.6) | 2,052 (61.4) | 0.80 |
| Negative | 19 (30.6) | 52 (34.4) | 69 (39.4) | 1,283 (38.4) | |
| Missing | 0 (0) | 0 (0) | 0 (0) | 8 (0.2) | |
| IA-2A* | |||||
| Positive | 41 (66.1) | 109 (72.2) | 121 (69.1) | 2,449 (73.3) | 0.21 |
| Negative | 21 (33.9) | 42 (27.8) | 54 (30.9) | 886 (26.5) | |
| Missing | 0 (0) | 0 (0) | 0 (0) | 8 (0.2) | |
| IAA* | |||||
| Positive | 16 (25.8) | 66 (43.7) | 86 (49.1) | 1,375 (41.1) | 0.026 |
| Negative | 43 (69.4) | 84 (55.6) | 82 (46.9) | 1,862 (55.7) | |
| Missing | 3 (4.8) | 1 (0.7) | 7 (4.0) | 106 (3.2) | |
| ZnT8A* | |||||
| Positive | 39 (62.9) | 99 (65.6) | 93 (53.1) | 2,075 (62.1) | 0.033 |
| Negative | 21 (33.9) | 46 (30.5) | 71 (40.6) | 1,123 (33.6) | |
| Missing | 2 (3.2) | 6 (4.0) | 11 (6.3) | 145 (4.3) |
Data are presented as n (%) unless indicated otherwise as mean (SD).
*Only BDD1, that is, those diagnosed with type 1 diabetes between May 2005 and December 2010, was analyzed.
Of those with a double diagnosis, 58.2% (n = 303) were diagnosed before or at T1D diagnosis (Fig. 2). Mean time to CD diagnosis for those diagnosed after T1D was 2.69 (SD 1.63) years, and 95.9% (n = 209) were found within 5 years after T1D diagnosis. Of 2,137 children who were screened for CD >5 years after T1D diagnosis, 9 (0.4%) had a CD diagnosis. Children diagnosed with CD after T1D were younger at T1D diagnosis than those with T1D only, 6.6 years compared with 9.7 years (95% CI 2.5–3.66, P < 0.001) (Table 1). Children <5 years of age at T1D diagnosis had the highest prevalence, 15.0%, and the highest risk of a CD diagnosis after T1D diagnosis (Table 2).
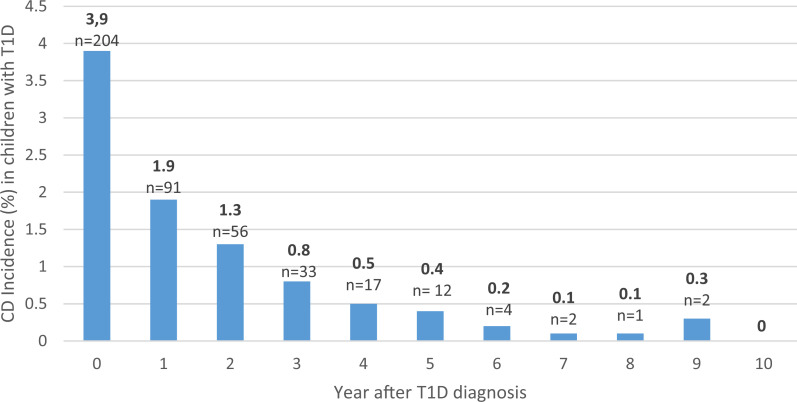
Figure 2.
Yearly incidence of CD after T1D diagnosis. “Year after T1D diagnosis” represents the year screening took place (e.g., year 0 = children screened at T1D diagnosis and CD diagnosed within the first year after T1D diagnosis, and year 1 = children screened 1 year after T1D diagnosis and CD diagnosed between the first and second year after T1D diagnosis etc.). Follow-up began at T1D diagnosis and ended at CD diagnosis, 18 years of age, or study period completion, meaning that the oldest age-groups did not have a 10-year follow-up time.
Table 2.
Prevalence of CD in children with T1D divided into groups based on age at T1D diagnosis
| No. | Total | Group 1: CD before T1D | Group 2: CD at T1D | Group 3: CD after T1D | Group 4: only T1D | |
|---|---|---|---|---|---|---|
| 0–4.9 years | 946 | 15.0 (142) | 1.5 (14) | 3.2 (30) | 10.4 (98) | 85.0 (804) |
| 5–9.9 years | 1,621 | 10.5 (170) | 1.6 (26) | 4.9 (80) | 3.9 (64) | 89.5 (1,451) |
| 10–14.9 years | 1,934 | 8.2 (159) | 2.2 (42) | 3.5 (67) | 2.6 (50) | 91.8 (1,775) |
| 15–18 years | 794 | 6.4 (51) | 2.1 (17) | 3.5 (28) | 0.8 (6) | 93.6 (743) |
Data are presented as % (n).
All HLA combinations with at least one copy of DQ2 were significantly more common in those diagnosed with CD after T1D than in those with T1D only, while DQ8/X was less common (Table 1). The child with DQX/DQX and CD also had Down syndrome.
The prevalence of CD after T1D diagnosis was highest (10.5%) among individuals who were homozygous for DQ2 (Supplementary Appendix 2). Individuals positive for DQ8/X and DQ8/8 were older than those who were DQ2/8 positive, (P = 0.002 resp. P < 0.001), while no significant differences were found between other groups (Supplementary Appendix 2).
There was no statistically significant difference in the distribution of the number of autoantibodies between those diagnosed with CD after T1D and those with T1D only (Table 1). When the autoantibodies alone were analyzed, there was only a statistically significance for IAA positivity when comparing, those diagnosed with CD after T1D (more common) with those with T1D only. However, when the proportion of IAA positivity within the age-groups was analyzed, the only statistically significant difference within groups was for 10–14.9 years old, where IAA was more common in those with only T1D than those with CD diagnosed after T1D (Supplementary Appendix 3).
Conclusions
In this national study of 5,295 children with T1D, the prevalence of confirmed CD was 9.8%, which is in line with earlier smaller studies in Sweden (8,9) but higher than reported in many other populations (10–12).
Most of the children (58%) were diagnosed with CD before or at the time of T1D diagnosis. For those diagnosed after T1D, most received a CD diagnosis within 2–3 years. Screening up to 5 years after diagnosis of T1D captured 95.9% of individuals who developed CD, which is more than the previously reported 79% (10). One study concluded that CD is rarely found after 10 years of diabetes (13); however, we showed that CD is uncommon already after 5 years.
Younger age at T1D diagnosis increases the risk of developing CD, which has been demonstrated previously (13–15). This difference in prevalence of CD between age-groups may support age-related endotypes (16).
As in the general population (17), the HLA-genotype DQ2/DQ2 conveys the highest risk for CD, regardless of age at T1D diagnosis. Individuals lacking DQ2 or DQ8 have no risk of CD, meaning that 10% of patients with T1D could be excluded from further screening.
There are conflicting results regarding female sex as a risk factor (10,12,13,18) for CD. We found no difference between the sexes in risk of CD after T1D diagnosis. Boys with T1D seem to have the same risk of CD as girls with T1D.
Diabetes-related autoantibodies were not correlated with the risk of CD and do not need to be considered in screening guidelines.
Based on these results, we propose the following screening for CD:
Children <5 years of age, at diagnosis, after 2 and 5 years;
Children aged 5 to 10 years, at diagnosis and after 5 years;
Children aged >10 years of age, at diagnosis;
Final screening considered before referral to adult care;
If HLA typing is a clinical routine, and the child does not have HLA DQ2 or DQ8, no CD screening is needed.
With this approach, some children will experience undiagnosed CD for a longer period than in today’s screening. However, studies have not shown that late confirmation of CD has a negative impact on metabolic control or CD-related complications (19,20).
We believe that our results are applicable to other similar populations, but further research on ethnic differences may further refine the screening guidelines.
Conclusion
Less than 50% of all children with double diagnoses developed CD after T1D diagnosis. Age at and time after T1D diagnosis should be considered in screening guidelines for CD in children with T1D.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25105946.
Article Information
Acknowledgments. The authors thank Qefsere Brahimi, Lund University, for help with the data from the BDD study, and thank Prof. Ludvig Sollid, Oslo University, for the help with reanalyses of some of the HLA blood samples.
Funding. The study was financed by grants from the Swedish state under the agreement between the Swedish government and the country councils, the ALF agreement 2018/2022, and by a grant from the Swedish Diabetes Foundation (Svenska Diabetesstiftelsen) and from Barndiabetesfonden.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.L. was involved in the study concept and design as well as the acquisition, analysis, and interpretation of data, performed statistical analyses, and wrote the manuscript. F.N. was involved in data analyses and interpretation, performed statistical analyses, and critically reviewed and edited the manuscript. M.P., H.E.L., G.F., K.Å., U.S., and J.L. critically reviewed and edited the manuscript. A.C. was involved in the study concept and design as well as the acquisition, analysis, and interpretation of data and critically reviewed and edited the manuscript. A.C. is the guarantor of this work and, as such, had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented as a poster at the 45th Annual Conference of the International Society for Pediatric and Adolescent Diabetes, Boston, MA, 30 October–2 November 2019.
References
- 1. Cohn A, Sofia AM, Kupfer SS.. Type 1 diabetes and celiac disease: clinical overlap and new insights into disease pathogenesis. Curr Diab Rep 2014;14:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fröhlich-Reiterer E, Elbarbary NS, Simmons K, et al. ISPAD Clinical Practice Consensus Guidelines 2022: other complications and associated conditions in children and adolescents with type 1 diabetes. Pediatr Diabetes 2022;23:1451–1467 [DOI] [PubMed] [Google Scholar]
- 3. ElSayed NA, Aleppo G, Aroda VR, et al. ; on behalf of the American Diabetes Association . 14. Children and adolescents: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S230–S253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Persson M, Becker C, Elding Larsson H, et al. The Better Diabetes Diagnosis (BDD) study—a review of a nationwide prospective cohort study in Sweden. Diabetes Res Clin Pract 2018;140:236–244 [DOI] [PubMed] [Google Scholar]
- 5. American Diabetes Association Professional Practice Committee . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45(Suppl. 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 6. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Husby S, Koletzko S, Korponay-Szabó IR, et al. ; ESPGHAN Working Group on Coeliac Disease Diagnosis ; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–160 [DOI] [PubMed] [Google Scholar]
- 8. Bybrant MC, Örtqvist E, Lantz S, Grahnquist L.. High prevalence of celiac disease in Swedish children and adolescents with type 1 diabetes and the relation to the Swedish epidemic of celiac disease: a cohort study. Scand J Gastroenterol 2014;49:52–58 [DOI] [PubMed] [Google Scholar]
- 9. Larsson K, Carlsson A, Cederwall E, et al. ; Skåne Study Group . Annual screening detects celiac disease in children with type 1 diabetes. Pediatr Diabetes 2008;9:354–359 [DOI] [PubMed] [Google Scholar]
- 10. Pham-Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME.. Screening for celiac disease in type 1 diabetes: a systematic review. Pediatrics 2015;136:e170–e176 [DOI] [PubMed] [Google Scholar]
- 11. Elfström P, Sundström J, Ludvigsson JF.. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther 2014;40:1123–1132 [DOI] [PubMed] [Google Scholar]
- 12. Craig ME, Prinz N, Boyle CT, et al. ; Australasian Diabetes Data Network (ADDN) ; T1D Exchange Clinic Network (T1DX); National Paediatric Diabetes Audit (NPDA) and the Royal College of Paediatrics and Child Health; Prospective Diabetes Follow-up Registry (DPV) initiative. Prevalence of celiac disease in 52,721 youth with type 1 diabetes: international comparison across three continents. Diabetes Care 2017;40:1034–104028546222 [Google Scholar]
- 13. Cerutti F, Bruno G, Chiarelli F, Lorini R, Meschi F; Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetology . Younger age at onset and sex predict celiac disease in children and adolescents with type 1 diabetes: an Italian multicenter study. Diabetes Care 2004;27:1294–1298 [DOI] [PubMed] [Google Scholar]
- 14. Pham-Short A, Donaghue KC, Ambler G, Chan AK, Craig ME.. Coeliac disease in Type 1 diabetes from 1990 to 2009: higher incidence in young children after longer diabetes duration. Diabet Med 2012;29:e286–e289 [DOI] [PubMed] [Google Scholar]
- 15. Vajravelu ME, Keren R, Weber DR, Verma R, De León DD, Denburg MR.. Incidence and risk of celiac disease after type 1 diabetes: a population-based cohort study using the health improvement network database. Pediatr Diabetes 2018;19:1422–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parviainen A, Härkönen T, Ilonen J, But A; Finnish Pediatric Diabetes Register . Heterogeneity of type 1 diabetes at diagnosis supports existence of age-related endotypes. Diabetes Care 2022;45:871–879 [DOI] [PubMed] [Google Scholar]
- 17. Sollid LM.. The roles of MHC class II genes and post-translational modification in celiac disease. Immunogenetics 2017;69:605–616 [DOI] [PubMed] [Google Scholar]
- 18. Slae M, Romem A, Edri S, Toker O, Wilschanski M, Strich D.. Celiac disease and celiac antibodies in DM1 patients: when are screening and biopsy recommended? Dig Dis Sci 2019;64:487–492 [DOI] [PubMed] [Google Scholar]
- 19. Simmons JH, Klingensmith GJ, McFann K, et al. Celiac autoimmunity in children with type 1 diabetes: a two-year follow-up. J Pediatr 2011;158:276–81.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamrath C, Tittel SR, Dunstheimer D, et al. Early vs late histological confirmation of coeliac disease in children with new-onset type 1 diabetes. Diabetologia 2022;65:1108–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]