Abstract
The activation of cytotoxic T lymphocytes (CTLs) to cells infected with adenovirus vectors contributes to problems of inflammation and transient gene expression that attend their use in gene therapy. The goal of this study was to identify in a murine model of liver gene therapy the proteins that provide targets to CTLs and to characterize the major histocompatibility complex (MHC) class I restricting elements. Mice of different MHC haplotypes were infected with an E1-deleted adenovirus expressing human alkaline phosphatase (ALP) or β-galactosidase as a reporter protein, and splenocytes were harvested for in vitro CTL assays to aid in the characterization of CTL epitopes. A library of vaccinia viruses was created to express individual viral open reading frames, as well as the ALP and lacZ transgenes. The MHC haplotype had a dramatic impact on the distribution of CTL targets: in C57BL/6 mice, the hexon protein presented by both H-2Kb and H2Db was dominant, and in C3H mice, H-2Dk-restricted presentation of ALP was dominant. Adoptive transfer of CTLs specific for various adenovirus proteins or transgene products into either Rag-I or C3H-scid mice infected previously with an E1-deleted adenovirus verified the in vivo relevance of the adenovirus-specific CTL targets identified in vitro. The results of these experiments illustrate the impact of lr gene control on the response to gene therapy with adenovirus vectors and suggest that the efficacy of therapy with adenovirus vectors may exhibit considerable heterogeneity when applied in human populations.
A prerequisite for successful human gene therapy is the development of efficient and safe transfer technologies. Recombinant adenovirus vectors have several features which make them attractive vehicles for gene delivery. They transduce a wide variety of cell types, do not require host cell proliferation for gene transfer, and are able to transduce cells in vivo (6, 17, 19, 20, 25). The adenovirus genome is comprised of early and late genes; expression of the former leads to the activation of a cascade which culminates in the formation of new virions (9). First-generation recombinant adenoviruses used in gene therapy have been rendered replication defective by deletion of the E1A and E1B genes.
Enthusiasm for the use of recombinant adenoviruses with deletions of the E1 genes in gene therapy has been diminished by the observation that deletion of E1 sequences is insufficient to completely ablate expression of other early and late viral genes. Previous studies revealed that the block in replication achieved by deleting E1 can be overcome in vitro with high multiplicities of infection (MOIs) or through cellular factors with E1-like function (10, 24). Expression of viral genes in infected-host cells leads to direct toxicity or to the stimulation of adenovirus-specific, major histocompatibility complex (MHC) class I-restricted cytotoxic T lymphocytes (CTLs) which can contribute to the loss of transgene expression (31, 33, 34). The antigenic potential of reporter proteins as well as therapeutic proteins in models of gene replacement therapy is another potential problem. A few recent reports have highlighted the role of the transgene product in inducing destructive cellular immune responses (28, 32).
The capsid proteins of adenovirus vectors stimulate CD4+ T helper cells which recognize antigenic peptides in association with MHC class II determinants. Both TH1 and TH2 subsets are activated, the former contributing to the CTL response by augmenting the stimulation of CD8+ T cells as well as by increasing expression of MHC class I on the target cell via gamma interferon (36).
The antigenic targets recognized by MHC class I-restricted CTLs have been extensively studied for a variety of viruses including influenza virus, vesicular stomatitis virus, and lymphocytic choriomeningitis virus. Epitopes within internal regulatory proteins, as well as integral membrane proteins, have been identified as targets for CTL-mediated destruction of virus-infected cells (1, 2, 29, 37, 38). For adenovirus, however, little is known about the antigen specificity of the CTL response. Studies with replication-competent, wild-type adenovirus revealed E1A encoded within the E1 locus as a strong immunodominant antigen (15, 18). These data, however, are not relevant to the use of adenoviruses in gene therapy when E1A and E1B had been deleted.
In this study, a library of recombinant vaccinia viruses expressing viral regulatory and structural genes as well as two reporter genes was used to precisely identify the targets within E1-deleted recombinant adenoviruses which elicit CD8+ T-cell responses. Experiments were performed in four strains of inbred mice, three of which had different mouse H-2 haplotypes. This study revealed that the level of CTL response to adenovirus antigens or the transgene product is dependent on the MHC haplotype of the host.
MATERIALS AND METHODS
Animals.
C57BL/6 (H-2b), BALB/c (H-2d), C3H (H-2k), and BALB/k (H-2k) mice were purchased from Taconic Laboratory Animals and Services, Germantown, N.Y. Rag-I and C3H-scid animals were obtained from Jackson Laboratory, Bar Harbor, Maine.
Cell lines.
The following murine cell lines provided by Barbara Knowles (7, 13) served as target cells: KD2SV (H-2KdDd), K2RSV (H-2KkDb), C3H.OHSV (H-2KdDk), KHTGSV (H-2KdDb), and K5RSV (H-2KbDd). The following additional cell lines were used in this study: C57SV (H-2KbDb) and L929 (H-2KkDk) which are both from the American Type Culture Collection.
Generation of recombinant vaccinia virus.
CV-1 cells (4 × 105) were plated into six-well plates and a day later infected with 2.5 × 104 PFU of the nonrecombinant Copenhagen strain of vaccinia virus in a volume of 0.6 ml of Dulbecco modified Eagle medium containing 2.5% fetal bovine serum (DMEM/2.5% FBS). After 90 min of infection, plasmid DNA used for homologous recombination into vaccinia virus (3.4 μg) was prepared for calcium phosphate precipitation by using a CellPhect kit (Pharmacia). After 2 h of infection, the medium containing the virus was replaced by 1.35 ml of DMEM/5% FBS and the DNA was added and left on the cells for 8 h before they were washed extensively. Two days later, the cells were harvested, the cell pellet was resuspended in 0.5 ml of DMEM/5% FBS, and a lysate was generated by three cycles of freeze-thawing.
Selection of recombinant vaccinia virus.
TK− cells (4 × 105) were plated in six-well plates. The next day, the medium was replaced by 0.6 ml of DMEM/2.5% FBS, and the lysate from the recombination procedure was added at final dilutions of 10−1, 10−2, and 10−3. After a 2-h infection period, the cells were selected for 2 days in DMEM/5% FBS containing 1/200 volume of bromodeoxyuridine (5 mg/ml). To stain for recombinant vaccinia virus, the cells were overlaid with 1.5 ml of selective agarose medium (0.75 ml of 2% low melting point agarose, 0.75 ml of 2× DMEM, 12.5 μl of 40× X-Gal [5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside] solution). After 1 day, recombinant vaccinia virus appeared as blue plaques which were picked up with Pasteur pipettes and transferred into Eppendorf tubes containing DMEM/5% FBS (500 μl); after three freeze-thaw cycles, the supernatants were transferred to new Eppendorf tubes. The supernatant was retitrated on TK− cells plated in 24-well plates to eliminate contaminating the wild-type virus. Serial dilutions of the virus were prepared starting from 10−1 to 10−10. After 2 days, the supernatants of the wells were transferred individually into Eppendorf tubes and the cells were overlaid with 500 μl of selective agarose medium. For amplification of recombinant vaccinia virus, wells which showed one blue plaque and no white plaques were recovered. The blue plaques were harvested and transferred into the Eppendorf tube containing the supernatant, and after 3 freeze-thaw cycles, were used for amplification.
Amplification of recombinant vaccinia virus.
TK− cells (106) were plated into a 25-cm2 flask. One day later, the cells were overlaid with 1 ml of DMEM/2.5% FBS and infected with recombinant virus in the supernatant (400 μl). After 2 h of infection, DMEM/5% FBS (4 ml) containing bromodeoxyuridine (25 μl, 5 mg/ml) was added. Cells were harvested 2 or 3 days after infection with a cell scraper and resuspended in DMEM/5% FBS (2 ml), and the virus was released from the cells in three freeze-thaw cycles. The supernatant (1.5 ml) was used to infect HeLa cells (5 × 107 cells plated the day before into a 150-cm2 flask) as described above except that the infection volume was 3 ml. The pellet was resuspended in 3 ml of DMEM/5% FBS, the virus was released from the cells in three freeze-thaw cycles, and the supernatant was used to infect three 175-cm2 flasks of HeLa cells (6 × 106 cells per flask plated the day before). The cells were pooled and resuspended in 3 ml of DMEM/2.5% FBS, the virus was released from the cells in three freeze-thaw cycles, and the supernatant was stored at −70°C in 100-μl aliquots.
Titration of recombinant vaccinia virus.
To determine the titer of the recombinant vaccinia virus, serial dilutions of the virus starting at 104 to 109 were prepared in 1 ml of DMEM/2.5% FBS. The virus was titrated on TK− cells which had been plated the day before at a density of 9 × 104 cells/well in 24-well plates. After 2 days, the titer was evaluated by counting the plaques.
Identification of recombinant proteins (data not shown).
Protein expression of the individual open reading frames (ORFs) expressed from recombinant vaccinia viruses was assayed by Western blot analysis of lysates for hexon, penton, fiber, DBP, pTP, and DNA-Pol. The E2A gene product was detected with a monoclonal antibody directed against its protein product, a 72-kDa DNA binding protein (DBP). The products of E2B encoding terminal protein (pTP) and DNA polymerase (DNA-Pol) were detected with polyclonal rabbit serum (21). Expression of the adenovirus genes encoding capsid proteins from recombinant vaccinia viruses was confirmed by using a polyclonal rabbit serum isolated following intravenous (i.v.) infusion of purified adenovirus. Hexon, penton, and fiber gave signals at the predicted positions of 108, 86, and 65 kDa, respectively.
Expression of the ALP gene from recombinant vaccinia virus was determined by staining infected C57SV cells with 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium (33). Uninfected C57SV cells did not show any staining, whereas cells infected with recombinant, ALP-expressing virus at an MOI of 2 for 6 h resulted in expression of an enzymatically active recombinant protein in 100% of cells (data not shown).
Recombinant vaccinia virus constructs. (i) ALP-pSCII.
The human placental alkaline phosphatase (ALP) cDNA was cloned into the vaccinia virus expression vector pSCII by using restriction enzyme SalI (22).
(ii) DBP-pSCII.
The DBP cDNA was isolated from the plasmid pMSG-DBP-EN by using restriction enzymes HindIII and KpnI and subcloned into vector pSP72 by using restriction enzymes XhoI and KpnI. This fragment was ligated into the SalI and KpnI sites of the vaccinia virus expression vector pSCII (3).
(iii) pTP-pSCII.
The adenovirus type 5 (Ad5) DNA was cut with the unique restriction enzyme PmeI and a 13-kb fragment isolated which was further digested with enzymes XbaI and KpnI and cloned into the same sites of the pBluescript cloning vector KS+. From the pBluescript KS+ vector, the pTP fragment was transferred into the pSV1180 cloning vector by using restriction enzymes NotI and KpnI and cloned into the vaccinia virus expression vector pSCII by using enzymes AflII and KpnI.
(iv) DNA-Pol-pSCII.
The Ad5 DNA was cut with restriction enzyme PmeI, and a 13-kb fragment was isolated. This fragment was further digested by using enzymes SphI and PstI and transferred into cloning vector pSV1180. DNA-Pol pSV1180 was digested with the enzymes BamHI and PstI and ligated into pBluescript KS+. The DNA-Pol KS+ plasmid was digested with SalI and SpeI and cloned into the SalI and NheI sites of the vaccinia virus expression vector pSCII.
(v) Penton-pSCII.
A 3-kb fragment encoding the penton protein was isolated after digesting the Ad5 DNA with restriction enzymes SfiI and PmeI. This fragment was redigested with enzymes NcoI and NotI and cloned into the NcoI and NotI sites of the cloning vector pSV1180. The plasmid Penton-pSV1180 was cut with NotI and EcoRI, and the isolated fragment was cloned into the NotI and EcoRI sites of the pBluescript cloning vector KS+. From this subcloning construct, the penton ORF was transferred into the vaccinia virus expression vector pSCII with restriction enzymes SalI and NotI (16).
(vi) Hexon-pSCII.
A 7-kb fragment was isolated after restricting Ad5 DNA with the unique restriction enzyme SfiI. This fragment was digested with enzymes BssHII and BglII and cloned into the BssHII and BamHI sites of the cloning vector pSV1180. From the pSV1180 plasmid, the hexon ORF was transferred into pBluescript cloning vector KS+ with restriction enzymes EcoRI and HindIII. The Hexon KS+ plasmid was cut with XhoI and SpeI, and the fragment was cloned into the SalI and NheI sites of the vaccinia virus expression vector pSCII (12).
(vii) Fiber-pSCII.
Fiber-pSCII was constructed using a plasmid called fiber-trunc-pSV1180 which contains a truncated form of the fiber gene. Fiber-trunc-pSV1180 was generated as follows. Ad5 DNA was cut with the SpeI enzyme, and a fragment of ∼6.5 kb was isolated. This fragment was redigested with restriction enzymes SacI and ApaI, and a fragment of 1.4 kb was cloned into the SacI and ApaI sites of cloning vector pSV1180. To reduce the size of the 5′ untranslated region, the construct was cut with SacI and BssHII and an oligonucleotide was cloned into these sites. To obtain the full-length fiber ORF, the 3′ region (from the ApaI site to the stop codon) of the gene was amplified via PCR. The newly created full-length fiber gene in the pSV1180 plasmid was cut with AflII and KpnI, and the isolated fragment was cloned into the same cloning sites of the vaccinia virus expression vector pSCII (5).
Cytotoxicity assay.
The CTL assay was performed as described previously (30). In brief, mice were injected i.v. with recombinant adenovirus H5.010CBALP (109 PFU) on day 0 and spleens were harvested on day 10. A single-cell suspension of splenocytes from groups of three mice was cultured for 5 days at 5 × 106/well in a 24-well plate in the presence of H5.010CBALP at an MOI of 0.8. After secondary in vitro stimulation, nonadherent spleen cells were harvested and assayed on MHC-compatible cells infected with recombinant vaccinia virus, using different effector/target cell ratios. Target cells (106) were infected at the day of the assay for 1 h with recombinant vaccinia virus at an MOI of 1 in 300 μl of DMEM/5% FBS. DMEM/10% FBS (8 ml) was added, and the cells were incubated for one additional hour, prior to 1 h of labeling with 100 μCi of 51Cr (Na251CrO4; New England Nuclear). Cells were washed three times with DMEM (10 ml) and resuspended in assay medium at 5 × 104/ml. Aliquots of target cells (100 μl) were plated with splenocytes (100 μl) at various effector/target cell ratios in V-bottom-shaped microtiter plates. The plates were incubated for 6 h at 37°C in 10% CO2, and the supernatant (100 μl) was removed from each well and counted in a Packard Cobra II gamma counter. A vaccinia virus expressing the glycoprotein of rabies virus (VRG) was used as a negative control (14). The percentage of specific 51Cr release was calculated as follows: [(cpm of sample − cpm of spontaneous release)/(cpm of maximal release − cpm of spontaneous release)] × 100, where cpm is counts per minute. All sample values represent the averages of four wells; maximum (i.e., target cells incubated with 5% sodium dodecyl sulfate) and spontaneous (i.e., target cells incubated with medium only) releases were averaged from eight wells.
Adoptive transfer.
Splenocytes were isolated from C57BL/6 mice (adoptive transfer into Rag-I mice) or C3H mice (adoptive transfer into C3H-scid mice) which had been infected with recombinant vaccinia virus (107 PFU in 100 μl of phosphate-buffered saline, given i.v.) 7 days before. Cells (5 × 107 cells in 200 μl of serum-free DMEM) were infused i.v. into Rag-I or C3H-scid recipient animals inoculated i.v. with H5.010CMVlacZ (Rag-I) or H5.010CBALP (C3H-scid mice) 7 days prior to the adoptive transfer. Animals were given recombinant mouse interleukin 2 (IL-2) on the day of cell transfer (100 ng of recombinant IL-2, given i.v.) and 1 day after (500 ng of recombinant IL-2, given intraperitoneally). Liver tissues were analyzed for β-galactosidase expression (Rag-I) by X-Gal histochemistry or ALP expression (C3H-scid) by ALP histochemistry 12 days later.
Detection of CD8+ and CD4+ cells by double immunofluorescence.
Frozen sections were fixed in methanol as described previously (33). After blocking with 10% goat serum in phosphate-buffered saline, liver sections were incubated with 10 mg of rat anti-mouse CD4 (anti-L3T4; Boehringer Mannheim) per ml for 60 min followed with 5 mg of rhodamine-labeled goat anti-rat immunoglobulin G (IgG) per ml for 30 min. Sections were treated with 10 mg of anti-L3T4 per ml to block the unbound paratopes to CD4 on anti-rat IgG molecules prior to incubation of samples with 50 mg of rat anti-mouse CD8a–fluorescein isothiocyanate (anti-Ly-2; Boehringer Mannheim) per ml for 60 min. Liver sections were washed and mounted with the antifading agent Citifluor (Citifluor, Canterbury, United Kingdom).
RESULTS
The ORFs from adenovirus structural and regulatory genes (Table 1), as well as the DNAs for the commonly used reporter genes, ALP and lacZ, were cloned into the vaccinia virus expression vector pSCII, and recombinants were generated (4). Protein expression of the individual ORFs cloned into recombinant vaccinia viruses was assayed by Western blot analysis or cytochemical analysis (data not shown).
TABLE 1.
Ad5 sequences used for cloning regulatory and structural genesa
| Viral DNA | Start codon (bp) | Stop codon (bp) | Map units (kbp) | Transcription unit |
|---|---|---|---|---|
| pTP | 10544 | 8585 | 28.8–23.4 | l-strand |
| DNA-Pol | 8367 | 5200 | 22.8–14.1 | l-strand |
| DBP | 24032 | 22446 | 61.6–66.5 | l-strand |
| Hexon | 18842 | 21700 | 51.6–59.7 | r-strand |
| Penton | 14156 | 15870 | 39.3–44.2 | r-strand |
| Fiber | 31043 | 32790 | 86.4–91.3 | r-strand |
Ad5 ORFs used for cloning into the vaccinia virus expression vector pSCII. The precise positions of start and stop codons from Ad5 regulatory genes (pTP, DNA-Pol, and DBP) and structural genes (hexon, penton, and fiber) as well as the map units within the 35,935-bp Ad5 genome and the transcription unit used for transcribing the gene are indicated.
Viral capsid proteins are the predominant CTL targets in C57BL/6 mice.
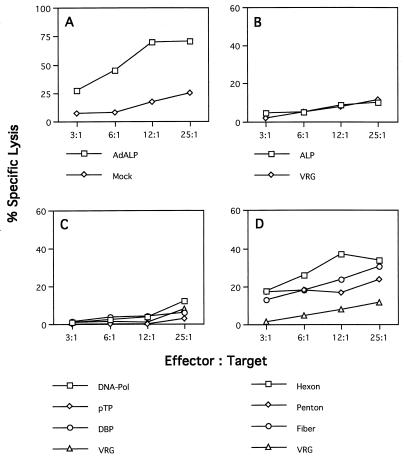
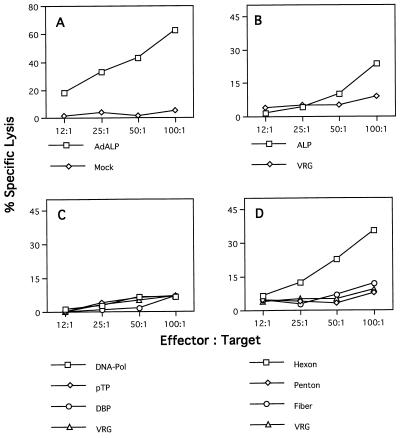
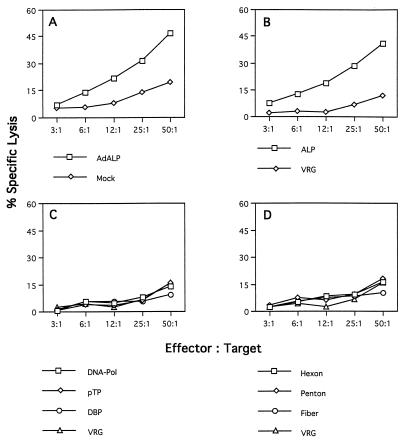
Splenocytes isolated from C57BL/6 mice (H-2b) infected with an ALP-expressing adenovirus efficiently lysed syngeneic target cells (C57SV [H-2KbDb]) infected with the ALP-expressing adenovirus (Fig. 1A), as well as recombinant vaccinia virus expressing the structural genes hexon and fiber and to a lesser extent penton (Fig. 1D). Target cells expressing the adenovirus regulatory genes DNA-Pol, pTP, and DBP were not specifically recognized by the same effector cell population (Fig. 1C). This recognition proved to be MHC restricted because allogeneic target cells (KD2SV [H-2KdDd) expressing the adenovirus structural genes were not lysed, although these cells are productively infected and can be lysed by splenocytes isolated from immunized BALB/c (H-2d) mice (data not shown). Lymphocytes from immunized C57BL/6 mice did not lyse syngeneic targets infected with the ALP-expressing vaccinia virus (Fig. 1B), suggesting that the product of this reporter gene is not a target for CTLs in this strain of mice; this differs from a previous study that demonstrated in vitro cytolytic activity to β-galactosidase in the same animal model (32). In each experiment, specificity of CTL activity was assessed by comparison to cytolysis observed with targets infected with recombinant vaccinia virus expressing the rabies glycoprotein (i.e., VRG). These experiments show that the adenovirus coat proteins hexon and fiber and to a lesser extent penton can provide antigenic determinants for presentation by the H-2b class I MHC molecules which elicit a cellular immune response in C57BL/6 mice infected with E1-deleted adenovirus.
FIG. 1.
Identification of target proteins for CTLs in C57BL/6 mice in response to adenovirus vectors. Spleens from three C57BL/6 mice injected with ALP-expressing adenovirus were restimulated in vitro for 5 days with ALP-expressing adenovirus. Effector cells were tested on MHC-compatible target cells (C57SV [H-2b]), which had been infected with either ALP-expressing adenovirus or recombinant vaccinia virus and loaded with 51Cr. Each graph shows the percent specific lysis assayed at four different effector/target cell ratios. (A) Target cells were infected with ALP-expressing adenovirus (AdALP) or mock infected. (B) Target cells were infected with recombinant vaccinia virus expressing the ALP transgene or a control vaccinia virus expressing rabies glycoprotein (VRG). (C and D) Target cells were infected with ORF-specific vaccinia viruses expressing the early adenovirus proteins DNA-Pol, pTP, and DBP (C) or the late adenovirus proteins hexon, penton, and fiber (D). The VRG virus was used as a negative control for target cells expressing various recombinant vaccinia viruses in panels C and D. These data are from one of three experiments done. The standard deviations were less than 10%. Spontaneous release ranged from 10 to 15%.
To determine if the CTLs were formed against the incoming virus or against de novo synthesized adenovirus proteins, C57BL/6 animals were injected with UV-inactivated adenovirus (i.v.) and the CTL assays were performed as described above. Effector cells isolated from these animals were not able to lyse either adenovirus-infected target cells or target cells infected with a recombinant vaccinia virus expressing hexon, penton, or fiber (data not shown). These experiments clearly demonstrate that de novo synthesis of proteins is essential for presentation of epitopes in the context of MHC class I molecules.
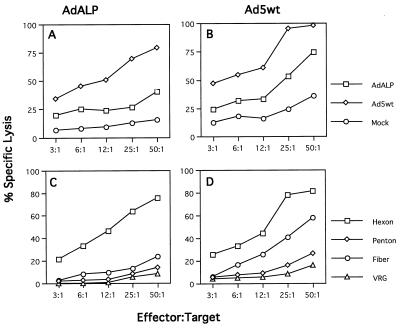
The distribution of CTL targets that emerge following injection of a replication-defective vector was compared to the results obtained with i.v. administration of a similar dose of wild-type Ad5. Figure 2 presents results of C57BL/6 mice injected with either E1-deleted adenovirus expressing ALP (Fig. 2A and C) or wild-type Ad5 (Fig. 2B and D) in which splenocytes were assayed for cytolysis against MHC identical targets infected with a series of adenoviruses or ORF-specific vaccinia viruses. Adenovirus-specific CTLs were generated in animals infected with either adenovirus vector or wild-type adenovirus, although the efficiency of cytolysis was slightly higher when the targets were infected with wild-type adenovirus (Fig. 2B and D). Hexon was a dominant CTL target upon immunization with both vector and wild-type virus (Fig. 2C and D), with detectable activity present to fiber that was higher in animals receiving wild-type adenovirus than in vector-treated mice.
FIG. 2.
Comparison of CTL targets in C57BL/6 mice infected with wild-type or E1-deleted adenovirus. Splenocytes isolated on day 7 after infection of C57BL/6 mice with wild-type adenovirus (Ad5wt) (109 particles per injection) (B and D) or E1-deleted adenovirus expressing ALP (AdALP) (1011 particles per injection) were analyzed for specific lysis on target cells infected with recombinant vaccinia virus expressing hexon, penton, fiber, or a rabies glycoprotein (C and D) or on target cells infected with AdALP or Ad5wt (A and B) in a 6-h 51Cr release assay. Specific lysis was assayed at five different effector/target cell ratios. These data are from one of two experiments done. The standard deviations were less than 10%. Spontaneous release ranged from 10 to 15%.
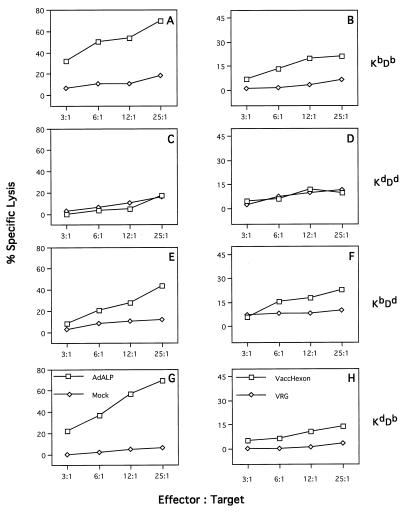
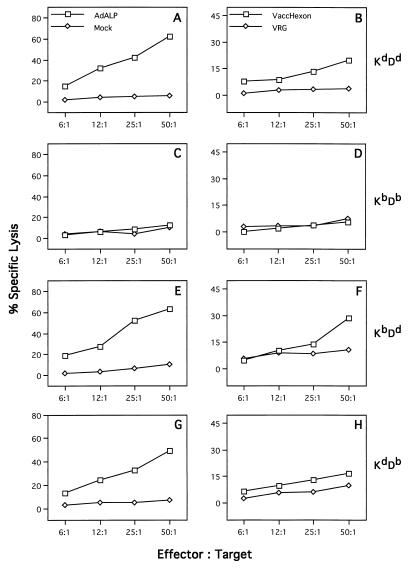
To determine the restricting element for the recognition of hexon, in vitro-restimulated, Ad5-specific CTLs were prepared from C57BL/6 mice and assayed on target cells matched at either the K or D end of the H-2 complex (Fig. 3). C57SV cells (H-2KbDb) infected with either H5.010CBALP (Fig. 3A) or the recombinant vaccinia virus expressing hexon protein (Fig. 3B) were specifically lysed, whereas target cells infected with the same viruses but not matched at either end of the H-2 complex (KD2SV [H-2KdDd]) did not show specific lysis (Fig. 3C and D). The relative contributions of H-2Kb and H-2Db to the presentation of hexon epitopes to CTL were determined with target cells of mixed H-2 genotypes. CTL assays were performed with target cells isogenic with H-2b at either the K locus (K5RSV [H-2KbDd]) or the D locus (KHTGSV [H-2KdDb]). High levels of cytolysis were observed when anti-adenovirus CTLs from C57BL/6 mice were incubated with either K5RSV (Fig. 3E) or KHTGSV (Fig. 3G) infected with ALP-expressing adenovirus, whereas intermediate but significant levels of cytolysis was detected with both target cells infected with hexon-expressing vaccinia virus (Fig. 3F and H). These data suggest that both Kb and Db MHC molecules present hexon epitopes and that proteins besides hexon harbor antigenic epitopes within E1-deleted adenoviruses because target cells infected with the E1-deleted adenovirus show stronger specific cytolysis than the target cells infected with the recombinant vaccinia virus (Fig. 3E to H).
FIG. 3.
Identification of the MHC restricting element responsible for the recognition of hexon within adenovirus-infected C57BL/6 mice. Spleens from three adenovirus-infected C57BL/6 mice were harvested on day 7, and isolated splenocytes were used as effector cells after 5 days of in vitro restimulation. MHC congenic cells differentiating the K and D alleles within the H-2b locus were used as target cells: C57SV cells (H-2KbDb) (A and B), KD2SV cells (H-2KdDd) (C and D), K5RSV (H-2KbDd) (E and F), and HTGSV (H-2KdDb) (G and H). For the determination of the MHC restricting element for E1-deleted adenovirus, the target cells were infected with ALP-expressing adenovirus (AdALP) or mock infected (negative control) (left panels). Target cells were also infected with recombinant vaccinia virus expressing hexon (VaccHexon) or recombinant vaccinia virus VRG expressing the rabies glycoprotein (negative control) (right panels). These data are from one of the two experiments done. The standard deviations were less than 10%. Spontaneous release values were 10% for C57SV and KD2SV cells and less than 18% for HTGSV and K5RSV cells.
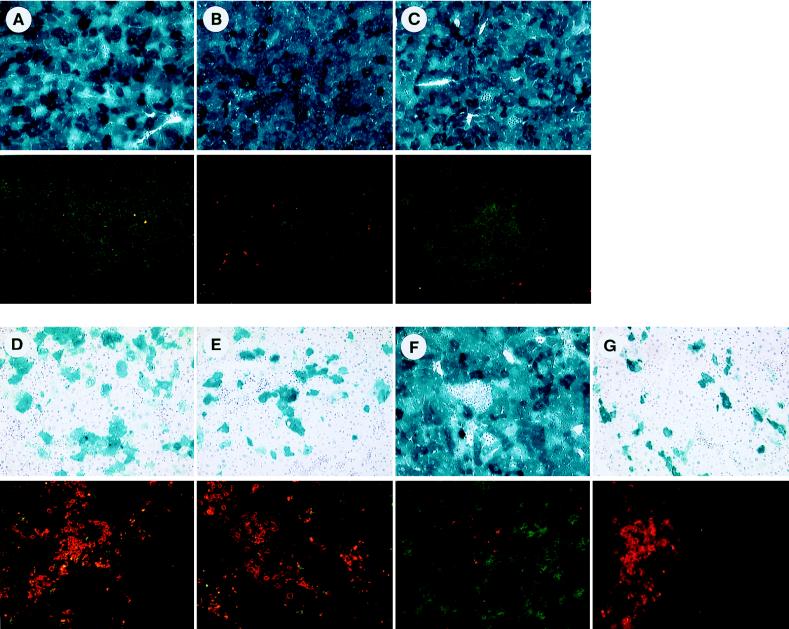
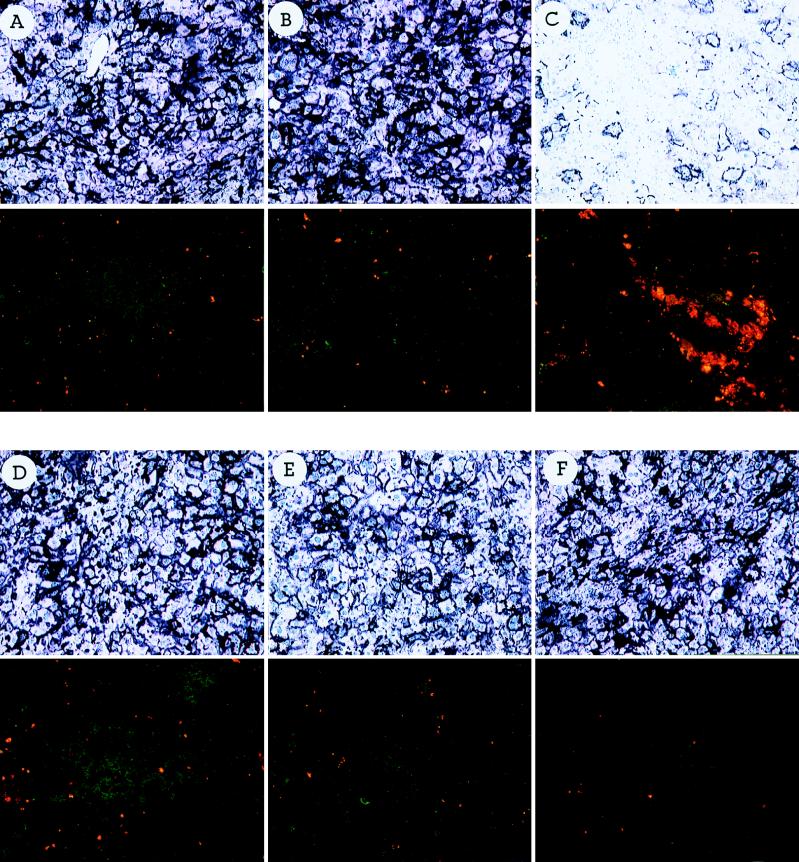
To demonstrate that CTLs specific for the adenovirus targets identified in an in vitro 51Cr release assay are indeed responsible for target cell destruction in vivo, CTLs specific for various adenovirus proteins were adoptively transferred into Rag-I animals previously infected with an E1-deleted adenovirus expressing the lacZ transgene. For generating CTLs specific for different adenovirus proteins, C57BL/6 animals were injected with a recombinant vaccinia virus expressing one of several adenovirus genes (hexon, penton, and fiber) or the lacZ transgene. Splenocytes were isolated 7 days later and adoptively transferred into adenovirus-infected Rag-I animals. A portion of splenocytes was restimulated in vitro with UV-inactivated adenovirus for 5 days and tested for lytic activity in a 51Cr release assay. Target cells were either mock infected or infected with an E1-deleted adenovirus. Specific lysis was detected for effector populations to all three late gene products, whereas naive splenocytes restimulated with an adenovirus did not result in specific lysis (data not shown). Liver tissue isolated and stained for lacZ expression 12 days after adoptive transfer showed stable transgene expression in Rag-I animals which either did not receive CTLs (Fig. 4A) or received splenocytes from naive C57BL/6 animals (Fig. 4B) or received CTLs isolated from C57BL/6 animals infected with a recombinant vaccinia virus expressing the rabies virus glycoprotein (Fig. 4C). In contrast, liver tissue isolated from animals that had received either CTLs specific for the hexon protein (Fig. 4D) or the fiber protein (Fig. 4E) showed severe infiltration of T lymphocytes into the area of lacZ-expressing hepatocytes and diminution of transgene expression. Penton-specific CTLs, however, induced only marginal hepatic infiltration (Fig. 4F), whereas adoptive transfer of CTLs specific for the lacZ transgene resulted in efficient target cell killing of virus-infected hepatocytes (Fig. 4G). These data clearly demonstrate that the CTLs specific for the target proteins identified in the in vitro CTL assays are able to recognize adenovirus-infected hepatocytes in vivo, resulting in transient gene expression.
FIG. 4.
Adoptive transfer of primed splenocytes to congenic Rag-I mice infected i.v. with H5.010CMVlacZ. Splenocytes from C57BL/6 mice infected with recombinant vaccinia virus expressing the adenovirus hexon protein (D panels), fiber protein (E panels), or penton protein (F panels) or the lacZ transgene product (G panels) were isolated 7 days after infection, and cells (5 × 107) were infused into the tail veins of Rag-I mice previously infected with H5.010CMVlacZ (109 PFU). Liver tissues were analyzed for lacZ expression 12 days after the adoptive transfer by X-Gal histochemistry (upper panel of each pair of panels) or for the presence of CD8+ (FITC) and CD4+ (rhodamine) lymphocytes by double immunofluorescence (lower panel of each pair of panels). Control animals did not receive any splenocytes (A) or received splenocytes from naive C57BL/6 mice (B) or splenocytes from mice infected with recombinant vaccinia virus expressing the rabies virus glycoprotein (C).
BALB/c mice activate CTLs to both viral capsid proteins and the ALP transgene.
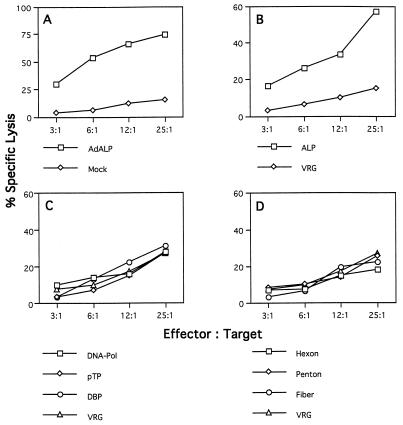
Similar experiments were performed with lymphocytes recovered from BALB/c mice immunized with ALP-expressing adenovirus to evaluate CTL responses in the context of the MHC class I H-2d haplotype (Fig. 5). H-2d-restricted target cells (KD2SV [H-2KdDd]) infected with the E1-deleted adenovirus showed strong specific lysis compared to uninfected cells (Fig. 5A). Syngeneic target cells expressing the ALP transgene exhibited lysis when cocultured with lymphocytes from BALB/c mice infected with adenovirus expressing ALP (Fig. 5B); however, recognition of ALP epitopes is weak because specific lysis could be detected only at high effector/target cell ratios. Lysis of cells expressing adenovirus regulatory proteins was below the detection level of the assay (Fig. 5C). Specific lysis was again consistently obtained with syngeneic target cells infected with recombinant vaccinia virus expressing hexon, while the other two adenovirus coat proteins, penton and fiber, did not serve as targets for CTLs isolated from BALB/c mice (Fig. 5D). The results presented here confirm previous findings showing that, in addition to the viral genes, the transgene product can indeed activate CD8+ cytotoxic T lymphocytes (28, 32).
FIG. 5.
Identification of target proteins for CTLs in H-2d mice. Splenocytes from three adenovirus-infected BALB/c mice were assayed on H-2d-restricted target cells (KD2SV cells [H-2KdDd]). See the legend to Fig. 1 for the target cells used. These data are from one of three experiments done. The standard deviations were less than 13%. Spontaneous release ranged from 10 to 15%.
The restricting element within the H-2d locus responsible for the recognition of hexon in BALB/c mice was studied with target cells that match in only one of the MHC class I determinants. No cytolysis above the background level was observed on target cells infected with either ALP adenovirus or vaccinia virus hexon that was mismatched at both the K and D loci (C57SV cells [H-2KbDb] [Fig. 6C and D]). Activity was still present with targets matched at only the K locus (KHTGSV [H-2KdDb] [Fig. 6G and H]) or the D locus (K5RSV [H-2KbDd] [Fig. 6E and F]); higher cytolysis was observed when target cells were infected with ALP adenovirus than with the vaccinia virus hexon. These data provide evidence that the Dd allele presents the hexon epitope in BALB/c animals, but the stronger cytolysis observed in both target cells infected with an adenovirus expressing the ALP transgene indicates that the adenovirus most likely harbors additional antigenic proteins which are presented in the context of an H-2d haplotype.
FIG. 6.
Determination of the MHC restricting element for the presentation of hexon to CTLs in H-2d mice infected with ALP-expressing adenovirus. Splenocytes from three adenovirus-infected BALB/c mice were assayed on various target cells differentiating the K and D determinants within the H-2d haplotype. The following target cells were used in this study: KD2SV (H-2KdDd) (A and B), C57SV (H-2KbDb) (C and D), K5RSV (H-2KbDd) (E and F), and HTGSV (H-2KdDb) (G and H). The left panels show target cells infected with the ALP-expressing adenovirus (AdALP) or mock-infected cells. The right panels show results of target cells infected with recombinant vaccinia virus expressing the hexon transgene (VaccHexon) or with the recombinant vaccinia virus VRG used in all of the experiments as a negative control for recombinant vaccinia virus-infected cells. These data are from one of two experiments done; the standard deviations varied between 10 and 12%. In all of the target cells, spontaneous release was under 20%.
The ALP transgene product dominates the activation of CTLs in C3H mice.
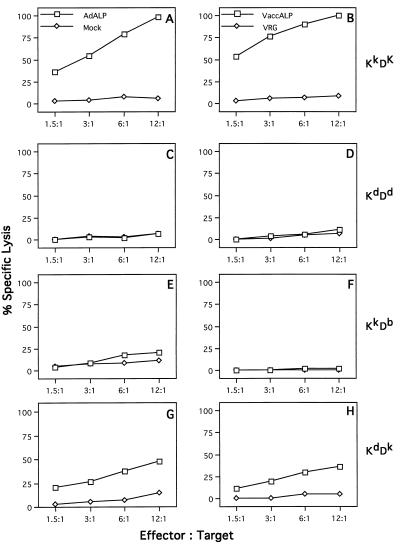
Experiments in C3H mice demonstrated a substantially different array of CTL responses in the context of the H-2k haplotype. CTLs induced by in vivo priming and in vitro stimulation of C3H splenocytes did not recognize any of the viral proteins tested (Fig. 7C and D); however, very significant specific lysis could be obtained with L929 target cells (H-2KkDk) expressing the ALP transgene (Fig. 7B).
FIG. 7.
Targets for CTLs in C3H mice infected with E1-deleted adenovirus expressing the ALP transgene. C3H mice (H-2k) were injected with E1-deleted adenovirus expressing the ALP transgene. In each experiment, splenocytes from three spleens were pooled and used as effector cells. L929 cells (H-2k) were used as target cells. See the legend to Fig. 1 for the target cells used. The data are from one of four experiments done; the standard deviations were less than 15%. Spontaneous release ranged from 8 to 12%.
To define the determinant within the H-2k MHC complex that is able to present the antigenic epitope of ALP to antiadenovirus CTLs from C3H mice, effector cells were assayed on K2RSV cells (H-2KkDb) (Fig. 8E and F) and LC3H.OHSV cells (H-2KdDk) (Fig. 8G and H) which had been infected with recombinant, ALP-expressing vaccinia virus. Specific lysis was restricted to target cells expressing Dk (LC3H.OHSV). The Kk allele appears not to be involved in the presentation of any antigenic determinants to transgene- or adenovirus-specific CTLs, as shown in Fig. 8E where no cytolysis was observed on K2RSV targets (H-2KkDb) infected with ALP-expressing adenovirus.
FIG. 8.
Identification of the MHC restricting element for ALP-derived epitopes in adenovirus-infected C3H mice. Splenocytes from three C3H mice infected with E1-deleted adenovirus expressing the ALP protein were isolated on day 7 after virus infection and restimulated in vitro for 5 days with ALP adenovirus. These effector cells were tested on target cells that differentiate H-2Kk and H-2Dk haplotypes. The MHC congenic target cell lines used were L929 cells (H-2KkDk) (A and B), KD2SV cells (H-2KdDd) (C and D), K2RSV cells (H-2KkDb) (E and F), and LC3H.OHSV (H-2KdDk) (G and H). Specific lysis was determined on target cells infected with adenovirus expressing the ALP protein (AdALP) (left panels) or recombinant vaccinia virus expressing the ALP protein (VaccALP) (right panels). Mock-infected cells were used as a negative control for adenovirus-infected target cells, whereas target cells infected with the recombinant vaccinia virus VRG expressing the rabies virus glycoprotein were used as a negative control for target cells infected with recombinant vaccinia virus. These data are from one of three experiments done; the standard deviations ranged from 8 to 12%, depending on the target cells. Spontaneous release was less than 10% for L929 and KD2SV cells, whereas it was 15 to 20% for K2RSV and LC3H.OHSV cells.
Additional experiments were performed to confirm that the recognition of the ALP protein in C3H animals was actually due to the MHC class I complex restriction and not to other genetic loci. Similar experiments were performed with lymphocytes harvested from BALB/k mice who have the same MHC haplotype as C3H mice (i.e., H-2k) but are otherwise genetically distinct. Figure 9 shows that the pattern of antigen recognition was identical to the one obtained with C3H effector cells. ALP-expressing target cells are efficiently lysed by the BALB/k CTLs (Fig. 9A and B), whereas lysis of target cells expressing viral proteins was not above the background level (Fig. 9C and D). The data obtained with C3H and BALB/k mice demonstrate an example in which the transgene product dominates as a CTL epitope to the apparent exclusion of viral protein epitopes.
FIG. 9.
Identification of proteins which activate CTLs in BALB/k mice (H-2k) infected with E1-deleted adenovirus. Splenocytes were isolated from three BALB/k mice which had been previously infected with E1-deleted adenovirus, and CTL assays were performed with L929 cells as targets. See the legend to Fig. 1 for the target cells used. Spontaneous release of L929 cells was less than 10%. These data are from one of two experiments done; the standard deviations ranged from 10 to 14%.
To assess the in vivo relevance of the various CTLs identified through in vitro CTL assays in C3H animals, adoptive transfer experiments similar to the ones described earlier for C57BL/6 animals were performed. CTLs specific for different adenovirus proteins or the ALP protein were generated in C3H animals by immunization against specific vaccinia virus constructs and subsequently adoptively transferred into C3H-scid animals previously infected with an E1-deleted adenovirus expressing the ALP transgene. No infiltration of T lymphocytes could be detected in livers from animals that received splenocytes specific for hexon, penton, or fiber 12 days after adoptive transfer resulting in stable gene expression (Fig. 10D to F). In contrast, ALP-specific CTLs recognized virus-infected hepatocytes, leading to target cell destruction and transient gene expression (Fig. 10C). Control animals that received no splenocytes or splenocytes from naive C3H animals showed stable transgene expression and no signs of infiltrating lymphocytes (Fig. 10A and B).
FIG. 10.
Adoptive transfer of primed splenocytes into C3H-scid mice infected i.v. with H5.010CBALP. Splenocytes (5 × 107 cells) isolated from C3H mice infected with recombinant vaccinia virus expressing the ALP transgene (C panels) or the adenovirus hexon protein (D panels), penton protein (E panels), or fiber protein (F panels) were adoptively transferred into C3H-scid animals previously infected with H5.010CBALP (109 PFU). Frozen liver tissues were evaluated for ALP expression 12 days later by ALP histochemistry (upper panel of each pair of panels) or for CD8+ (FITC-labeled) and CD4+ (rhodamine-labeled) infiltration by double immunofluorescence (lower panel of each pair of panels). Control animals either did not receive any splenocytes (A panels) or received naive splenocytes (B panels).
DISCUSSION
Adenovirus vectors have realized broad application in a variety of preclinical and clinical studies. Immune responses of the recipient to the vectors and to vector-transduced cells have complicated their use for stable gene transfer to correct chronic diseases. Activation of CD8+ T cells in mice to cells transduced with E1-deleted vector appears to contribute to the loss of transgene expression and the concomitant inflammatory reaction. The goal of this study was to characterize the distribution of proteins that are targeted by CTLs following i.v. administration of E1-deleted adenovirus into mice and to evaluate the influence of the MHC haplotype on the nature of this response. We showed previously that the deletion of E1 was insufficient to block expression of early and late viral genes in vivo when administered to both mice and nonhuman primates (8, 34, 35). Furthermore, many of the reporter gene products used in previous studies by us and others are potentially antigenic (28, 32). A variety of targets, including adenovirus and transgene products, were tested in this study.
Data obtained with adenovirus-specific splenocytes isolated from C57BL/6 mice indicate that from the panel of proteins tested in this study, the viral coat proteins hexon, fiber, and to a lesser extent, penton harbor epitopes which can all be presented by the H-2b complex eliciting a T-cell-mediated immune response. It is obvious that the CTL assay in vitro permits a limited quantitation of the overall relative activity of specific CTLs. Despite these difficulties, it should be mentioned that in mice with the H-2b haplotype, CTL-mediated lysis of hexon-expressing target cells was consistently higher than target cell lysis achieved with cells expressing penton or fiber. This could be due to a high frequency of hexon-specific precursor CTLs or the presence of multiple and/or high-affinity H-2b-restricted hexon epitopes.
In addition, our data revealed that adenovirus regulatory proteins as well as the ALP reporter transgene are not major targets for CTL activation in the H-2b haplotype. The data, however, do not exclude the possibility that those proteins harbor subdominant epitopes, leading to weak target cell lysis which could not be detected in the in vitro experiments.
Characterization of the CTL response in other strains of mice demonstrated a striking impact of the MHC haplotype on the distribution of CTL targets. As noted above, hexon dominated as a target in the H-2b haplotype of C57BL/6 mice, while the ALP transgene was recognized predominantly in the H-2k haplotype of C3H animals to the exclusion of viral gene products. CTLs were activated to both ALP and hexon in the H-2d haplotype of BALB/c mice. The strong dependence between recognition of viral antigens and H-2 determinants was first shown by Townsend et al. (27) for influenza virus antigens. It is now well accepted that this reflects the binding characteristics of particular K or D MHC molecules for selected viral antigen peptides (26, 27). In order to evaluate the contribution of the K and D molecules of different MHC molecules to the presentation of hexon (in C57BL/6 and BALB/c mice) and ALP (in C3H mice), various target cell lines expressing different K and D molecules were used in this study. The finding that the hexon protein is presented by K as well as D determinants in BALB/c and C57BL/6 mice strongly emphasizes that this protein is highly immunogenic. The activation of CTL to the ALP transgene product in two MHC backgrounds and the lacZ transgene in C57BL/6 animals underscores the potential problem of cellular immunity to the therapeutic product, a problem not easily overcome through simple vector modifications.
Previous studies of CTL responses to human adenovirus infection in mice were restricted to animals that received replication-competent adenovirus. Evaluation of target cells expressing viral early genes by transfection and/or infection with early gene mutants confirmed the immunodominance of epitopes within E1A (18). The relative lack of cytolysis to cells infected with viruses with deletions of the E1A gene suggested that late viral gene products do not contain dominant epitopes. The negative result in this setting is less informative because late gene expression is indirectly diminished in the absence of E1A. The availability of vaccinia virus selectively expressing late gene ORFs, in our study, confirmed that C57BL/6 mice do indeed generate CTLs to late viral proteins following i.v. injection with wild-type adenovirus.
One issue often debated in the literature is the relevance of in vitro CTL assays to the net effect on vector performance, as defined by transgene stability and inflammation in vivo. Measurement of detectable CTL activity required in vitro restimulation of cells by the recombinant adenovirus. This could potentially skew the relative contribution of CTL populations present in vivo. Bulk cultures rather than limiting dilutions of lymphocytes were assayed, making precise quantitation difficult. The process of adenovirus vector transduction is sufficiently complex that predictions from in vitro CTL activity on in vivo effects are difficult.
Our in vivo adoptive transfer experiments, however, clearly demonstrate a direct correlation between the CTL target proteins within adenovirus identified in the in vitro CTL assays and target cell destruction by adoptively transferred CTLs in vivo. All of the adenovirus- or transgene-specific CTLs which resulted in high specific lysis in the in vitro 51Cr release assays severely infiltrated the livers of immunodeficient animals previously infected with an E1-deleted adenovirus and yielded a net decrease in transgene expression. These data reveal that hepatocytes do indeed present adenovirus- and transgene- specific epitopes identified in vitro and are recognized in vivo by antigen-specific CTLs. Interestingly, CTLs generated in C57BL/6 animals specific for penton which lead to intermediate cytolysis in the in vitro CTL assays resulted only in weak infiltration into the area of lacZ-expressing hepatocytes. This contrasts with hexon- or fiber-specific CTLs, which lysed target cells in vitro very efficiently and resulted in rapid target cell destruction and hence transient gene expression in vivo. The quantitative correlation between the in vitro and in vivo data was rather surprising. A general principle is that different epitopes compete for the MHC pocket during CTL activation. Vaccinia virus and adenovirus backbones most likely harbor specific epitopes with different affinities for the MHC peptide binding sites which are expected to influence and/or interfere with the activation of hexon-, penton-, and fiber-specific CTLs.
One important finding relevant to gene therapy is the impact of the MHC determinant on cellular immune responses to adenovirus vectors. This is highly relevant for the design and interpretation of clinical trials performed in genetically heterogeneous human populations. The stability of a vector-encoded transgene, as well as associated toxicity, may be primarily influenced by the presence of MHC alleles capable of presenting viral antigens and/or the therapeutic protein. In addition, studies performed by other investigators showed that multiple immunizations with adenovirus vectors might activate CTLs against subdominant epitopes. This phenomenon appears to be dependent on haplotype and could present a further obstacle to gene therapy in a human population with diverse MHCs (23). Although our study and the study presented by Sparer et al. (23) demonstrate the importance of the haplotype on transgene persistence, other investigators have shown that the genomic structure of the vector also modulates the persistence of transgene expression from recombinant adenovirus in mouse lung (11).
In summary, we have attempted to delineate the genetics of immune responses to adenovirus vectors in syngeneic strains of mice. MHC determinants have a dramatic impact on CTL activation to adenovirus vectors. Multiple genetic determinants to the hexon protein were detected, which suggests that it may be highly antigenic. One important lesson learned from this study is the fact that in vitro 51Cr release assays indeed seem to reflect the in vivo situation, an observation essential for the interpretation of data obtained from in vitro assays in clinical gene therapy trials.
ACKNOWLEDGMENTS
Contributions of the Vector Core and Clinical Pathology Unit of the Institute for Human Gene Therapy were greatly appreciated. We are also grateful to Radha Padmanabhan for providing the anti-pTP and anti-DNA-Pol antibody, Bernard Moss for providing the recombinant vaccinia virus expressing the lacZ transgene, and Laurence Eisenlohr for technical help.
Funding was provided by the Cystic Fibrosis Foundation and the National Institute of Diabetes and Digestive and Kidney Diseases and the National Institute of Child Health and Human Development of the National Institutes of Health, and Genovo, Inc. Karin Jooss was supported by the Human Frontier Science Program. H. Ertl was supported by grants from NIAID.
REFERENCES
- 1.Bennink J R, Yewdell J W, Smith G L, Moller C, Moss B. Recombinant vaccinia virus primes and stimulates influenza haemagglutinin-specific cytotoxic T cells. Nature. 1990;311:578–579. doi: 10.1038/311578a0. [DOI] [PubMed] [Google Scholar]
- 2.Bennink J R, Yewdell J W, Smith G L, Moss B. Anti-influenza virus cytotoxic T lymphocytes recognize the three viral polymerases and a nonstructural protein: responsiveness to individual viral antigens is major histocompatibility complex controlled. J Virol. 1987;61:1098–1102. doi: 10.1128/jvi.61.4.1098-1102.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brough D E, Cleghon V, Klessig D F. Construction, characterization, and utilization of cell lines which inducibly express the adenovirus DNA-binding protein. Virology. 1992;190:624–634. doi: 10.1016/0042-6822(92)90900-a. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Brechling K, Moss B. Vaccinia virus vector: coexpression of β-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985;5:3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chroboczek J, Jacrot B. The sequence of adenovirus fiber: similarities and differences between serotypes 2 and 5. Virology. 1987;161:549–554. doi: 10.1016/0042-6822(87)90150-4. [DOI] [PubMed] [Google Scholar]
- 6.Davidson B L, Allen E D, Kozarsky K F, Wilson J M, Roessler B J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993;3:219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- 7.Gmur R, Solter D, Knowles B. Independent regulation of H-2K and H-2D gene expression in murine teratocarcinoma somatic cell hybrids. J Exp Med. 1980;151:1349–1359. doi: 10.1084/jem.151.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldman M J, Wilson J M. Transfer of the CFTR gene to the lung of nonhuman primates with E1 deleted, E2a defective recombinant adenoviruses: a preclinical toxicology study. Hum Gene Ther. 1995;6:839–851. doi: 10.1089/hum.1995.6.7-839. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz M S. Adenoviridae and their replication. In: Fields B N, Knipe D M, et al., editors. Virology. New York, N.Y: Raven Press; 1990. pp. 1679–1721. [Google Scholar]
- 10.Imperiale M J, Kao H-T, Feldman L T, Nevins J R, Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984;4:867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan J M, Armentano D, Sparer T E, Wynn S G, Peterson P A, Wadsworth S C, Couture K K, Pennington P A, George J A S, Gooding L R, Smith A E. Characterization of factors involved in modulating persistence of transgene expression from recombinant adenovirus in the mouse lung. Hum Gene Ther. 1997;8:45–56. doi: 10.1089/hum.1997.8.1-45. [DOI] [PubMed] [Google Scholar]
- 12.Kinloch R, Mackay N, Mautner V. Adenovirus hexon. J Biol Chem. 1984;259:6431–6436. [PubMed] [Google Scholar]
- 13.Knowles B B, Knocar M, Pfitzenmaier K, Solter D, Aden D P, Trinchierai G. Genetic control of the cytotoxic T cell response to SV40 tumor-associated specific antigen. J Immunol. 1979;122:1798–1806. [PubMed] [Google Scholar]
- 14.Larson J K, Wunner W H, Otvos L, Ertl H C J. Identification of an immunodominant epitope within the phosphoprotein of rabies virus that is recognized by both class I- and class II-restricted T cells. J Virol. 1991;65:5673–5679. doi: 10.1128/jvi.65.11.5673-5679.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muellbacher A, Bellet A J D, Hla R T. The murine cellular immune response to adenovirus 5. Immunol Cell Biol. 1989;67:31–39. doi: 10.1038/icb.1989.4. [DOI] [PubMed] [Google Scholar]
- 16.Neumann R, Chroboczek J, Jacrot B. Determination of the nucleotide sequence for the penton-base gene of human adenovirus type 5. Gene. 1988;69:153–157. doi: 10.1016/0378-1119(88)90389-7. [DOI] [PubMed] [Google Scholar]
- 17.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan J, Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 18.Rawle F C, Knowles B B, Ricciardi R P, Brahmacheri V, Duerksen-Hughes P, Wold W S M, Gooding L R. Specificity of the mouse cytotoxic T lymphocyte response to adenovirus 5. J Immunol. 1991;146:3977–3984. [PubMed] [Google Scholar]
- 19.Rosenfeld M A, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier L E, Paakko P K, Gilardi P, Stratford-Perricaudet L D, Perricaudet M. Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science. 1991;252:431–434. doi: 10.1126/science.2017680. [DOI] [PubMed] [Google Scholar]
- 20.Rosenfeld M A, Yoshimura K, Trapnell B C, Yoneyama K, Rosenthal E R, Dalemans W, Fukayama M, Bargon J, Stier L E, Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992;68:143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- 21.Sasaguri Y, Sanford T, Aguirre P, Padmanabhan R. Immunological analysis of 140-kDa adenovirus-encoded DNA polymerase in adenovirus type 2-infected Hela cells using antibodies raised against the protein expressed in escherichia coli. Virology. 1987;160:389–399. doi: 10.1016/0042-6822(87)90010-9. [DOI] [PubMed] [Google Scholar]
- 22.Schreiber J H, Schisa J A, Wilson J M. Recombinant retroviruses containing novel reporter genes. BioTechniques. 1993;14:818–823. [PubMed] [Google Scholar]
- 23.Sparer T E, Wynn S G, Clark D J, Kaplan J M, Cardoza L M, Wadsworth S C, Smith A E, Gooding L R. Generation of cytotoxic T lymphocytes against immunorecessive epitopes after multiple immunizations with adenovirus vectors is dependent on haplotype. J Virol. 1997;71:2277–2284. doi: 10.1128/jvi.71.3.2277-2284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spergel J M, Hsu W, Akira S, Thimmappaya B, Kishimoto T, Chen-Kiang S. NF-IL6, a member of the C/EBP family, regulates E1A-responsive promoters in the absence of E1A. J Virol. 1992;66:1021–1030. doi: 10.1128/jvi.66.2.1021-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. Widespread long-term gene transfer to mouse skeletal muscle and heart. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 27.Townsend A R M, Rothbard J, Gotch F M, Bahadur G, Wraith D, McMichael A J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- 28.Tripathy S K, Black H B, Goldwasser E, Leiden J M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996;2:545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- 29.Whitton J L, Southern P J, Oldstone M B A. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virology. 1988;162:321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Z, Spitalnik S, Tran M, Wunner W H, Cheng J, Ertl H C J. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Ertl H C J, Wilson J M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994;1:433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 32.Yang Y, Jooss K U, Su Q, Ertl H C J, Wilson J M. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 1996;3:137–144. [PubMed] [Google Scholar]
- 33.Yang Y, Li Q, Ertl H C J, Wilson J M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenovirus. J Virol. 1995;69:2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y, Nunes F A, Berencsi K, Furth E E, Gonczol E, Wilson J M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci USA. 1994;91:4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Y, Nunes F A, Berencsi K, Gonczol E, Engelhardt J F, Wilson J M. Inactivation of E2a in recombinant adenoviruses improves the prospect for gene therapy in cystic fibrosis. Nat Genet. 1994;7:362–369. doi: 10.1038/ng0794-362. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Xiang Z, Ertl H C J, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon γ is necessary for T-cell mediated elimination of recombinant adenovirus-infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yewdell J, Bennink J R, Mackett M, Lefrancois L, Lyles D S, Moss B. Recognition of cloned vesicular stromatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med. 1986;163:1529–1538. doi: 10.1084/jem.163.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yewdell J W, Bennink J R, Smith G L, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1985;82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]