Abstract
Orychophragmus violaceus (L.) O. E. Schulz (Brassicaceae) is widely distributed and plentiful in China and has been widely used for its application in ornamental, oil, ecology, foraging, and food. Recent studies have revealed that the main components of Orychophragmus violaceus include flavonoids, alkaloids, phenylpropanoids, phenolic acids, terpenoids, etc., which have pharmacological activities such as antioxidation, antiradiation, antitumor, hepatic protection, antiferroptosis, anti-inflammatory, and antibacterial. In this paper, the nutritional value, chemical compositions, pharmacological activity, and application value of Orychophragmus violaceus are summarized by referring to the relevant domestic and international literature to provide a reference for further research, development, and utilization of Orychophragmus violaceus in the future.
Keywords: Orychophragmus violaceus, Brassicaceae, chemical compositions, alkaloids, ferroptosis, pharmacological activity
1. Introduction
Orychophragmus violaceus, belonging to the Orychophragmus Genus, is a plant that falls within the Brassicaceae family. It is recognized by various common names such as “Er-yue-lan” and “Zi-jin-cao”. This species is native to China and can be found as both an annual and perennial plant. Its distribution is widespread, with occurrences scattered throughout the southwest and northeast regions of China. It mostly grows in plains, grasslands, mountain slopes, roadsides, or woodland edges [1]. Legend has it that Zhuge Liang picked a roadside plant to feed his troops on their way to war because of a lack of grain. Chen Zhenhui et al. [2] suggested that the Orychophragmus Genus has only one species, namely O. violaceus, while subspecies include O. hupehensis+ O. taibaiensis, and O. diffusus.
The predominant height of Orychophragmus violaceus plants ranges from 30 to 50 cm, exhibiting erect stems and a singular central stem configuration. Flowering from April to May, the initial flowers are mostly blue-purple or light red and eventually turn white in lax racemes. The pollen polar views of O. violaceus are trilobed round and the pollen equatorial views are prolate, which have three colpi. The longitudinal growth of the pod is faster than the transverse broadening and the pod length is about 28 days after anthesis stereotypes. The fruiting periods are May–June, the fruit is long, silique cylindrical, and the seeds are ovate and rounded. O. violaceus exhibits high cold resistance and shade tolerance, remaining evergreen even during the winter months [3,4,5,6]. O. violaceus can be found everywhere along the roadsides in China and is commonly used as an ornamental plant to both prevent soil erosion and beautify the environment.
It was first recorded in “Hou Han Shu: Ji: Xiao Huan Di Ji”: “It’s warm-natured, pungent, bitter and sweet in taste, and is effective in treating jaundice in children, deep-rooted breast carbuncles, and common fever and other diseases”.
The tender leaves and stems of O. violaceus are edible, containing various nutrients such as carotene, protein, and vitamins. The whole herb of O. violaceus is employed as a medicinal resource, known for its ability to harmonize the intestine-stomach, promote diuresis, and facilitate detoxification [7]. Furthermore, the seeds of O. violaceus possess a high oil content, primarily consisting of beneficial unsaturated fatty acids. This quality makes them highly valuable as oil plants, offering potential health benefits and disease prevention [8]. Moreover, the seeds of O. violaceus are considered to have significant edible value.
It has been established in modern pharmacological studies that the natural active ingredients in O. violaceus have a wide range of pharmacological activities, including antibacterial, antitumor, antiradiation, hepatic protection, and other physiological activities [9,10,11]. Based on domestic and foreign research, flavonoids [10], alkaloids [12], phenylpropanoids [13], phenolic acids [14], and terpenoids [15] have been identified as the main components of O. violaceus. Despite being present in relatively small amounts, O. violaceus’s steroid, fatty acid, protein, and vitamin content are crucial to the plant’s overall value.
Recently, the scope of utilization of the plant has been expanding from an ornamental to an edible plant. O. violaceus seeds have high levels of unsaturated fatty acids and low levels of erucic acid, making them an outstanding quality oil resource in terms of economic value. The amino acid content of O. violaceus is well known and it can be used as feed in conjunction with maize and sorghum [16]. On the other hand, O. violaceus, as a wild vegetable in folk, also has a long-term edible history. People in Jiangsu and Anhui have been harvesting and eating the tender stems and leaves of O. violaceus for a long time. O. violaceus is rich in vitamin C, carotene, and so on, which has potential edible and medicinal value for the human body to prevent cold, cancer, and cardiovascular and cerebrovascular diseases.
Based on a review and summary of the literature, this paper comprehensively summarized and analyzed the research progress in recent years in terms of nutritional composition, chemical composition, and biological activity, intending to provide guidance and reference for the subsequent research and application of O. violaceus.
2. Research Methodology
We looked up information on the nutrients, chemicals, and health effects of O. violaceus by searching through databases like Elsevier, Journal of the American Chemical Society (ACS), PubMed, Web of Science, and China National Knowledge Infrastructure (CNKI). We searched for information in both Chinese and English. The keywords used included related words such as O. violaceus, Er-yue-lan, alkaloid, flavone, chemical composition, pharmacology, nutritional composition, application, and so on.
3. Nutritional Value
3.1. Vitamins
Vitamins are a type of small organic compounds that are essential for keeping our bodies and animals functioning properly. They are only needed in small amounts but play a crucial role in human growth, metabolism, and overall development. As the body cannot synthesize vitamins or does so in small quantities, people can only consume them from food.
Li Xinhua et al. [17] compared the vitamin content of wild O. violaceus with that of the cultivar Brassica chinensis at the seedling and moss stages. The levels of VC (vitamin C), VE (vitamin E), VB1 (vitamin B1), and VB2 (vitamin B2) were measured using the fluorometric method. On the other hand, the levels of VK (vitamin K), VB6 (vitamin B6), VB12 (vitamin B12), VB3 (vitamin B3), and β-carotene were determined using HPLC (high-performance liquid chromatography). The results are depicted in Table 1. Luo Peng et al. [18] also compared the major vitamins of O. violaceus with other plants of the genus Brassica, where β-carotene was determined by paper chromatography, which is shown in Table 2. Rich in vitamin C and carotenoids, the major vitamin content of O. violaceus is equal to that of common greens and even higher than greens in some cases. The homeostasis of vitamins in the human body is particularly important for health. Some studies have illustrated the importance of vitamin C in early infant development, especially in the brain and cognition [19]. In addition, vitamin C deficiency can have a negative impact on infectious diseases, cancer, diabetes, sepsis, and cardiovascular disease, among others [20]. Furthermore, β-carotene has been shown to significantly reduce the risk of oral, laryngeal, and breast cancers [21]. O. violaceus has a higher overall nutritional value compared to other vegetables in the Brassica genus, so it has great potential application prospects.
Table 1.
Comparison of the vitamin content of O. violaceus and Brassica chinensis.
| Stage | Sample | VC * | VB1 * | VB2 * | VE * | VK # | VB6 # | β-Carotene # |
|---|---|---|---|---|---|---|---|---|
| Seedling | O. violaceus | 132.38 | 0.06 | 0.13 | 0.08 | 0.06 | 1.14 | 1.09 |
| B.chinensis | 29.70 | 0.04 | 0.11 | 0.05 | 0.08 | 0.78 | 0.57 | |
| Moss | O. violaceus | 101.62 | 0.05 | 0.11 | 0.11 | 0.06 | 0.84 | 1.31 |
| B. chinensis | 44.57 | 0.04 | 0.09 | 0.10 | 0.07 | 0.67 | 1.01 |
*: fluorometric method; #: HPLC.
Table 2.
Comparison of the main vitamin content of O. violaceus with other plants of Brassica.
| Sample | β-Carotene @ | VB1 * | VB2 * | VC * |
|---|---|---|---|---|
| O. violaceus | 1620 | 0.10 | 0.05 | 27 |
| Brassica oleracea var. albiflora Kuntze | 3450 | 0.20 | 0.00 | 78 |
| Brassica campestris var. purpuraria | 1255 | 0.10 | 0.06 | 21 |
| Brassica caulorapa Pasq. | 20 | 0.04 | 0.82 | 41 |
| Baby Bok Choy | 1680 | 0.02 | 0.00 | 26 |
| Brassica oleracea var. botrytis Linnaeus | 20 | 0.08 | 0.08 | 81 |
| Brassica rapa var. glabra Regel | 40 | 0.08 | 0.04 | 28 |
| Brassica juncea (L.) Czern. et Coss. Var. foliosa L. H. Bailey | 1700 | 0.02 | 0.10 | 72 |
| Brassicajuncea (L.) Czern. et Coss. Var. multiceps Tsen et Lee | 310 | 0.03 | 0.11 | 31 |
@: paper chromatography; *: fluorometric method.
3.2. Amino Acid
Amino acids are the fundamental components of life. They serve as the building blocks for proteins and enzymes. They belong to a group of organic compounds that contain both amino and carboxyl groups within their molecules. The regulation of substance metabolism and information transmission in the body are two critical functions of amino acids, which are crucial for the nutrition, survival, and development of all life forms. As macromolecules, amino acids have a crucial role to play in the activity and physiological functions of biomacromolecules; as ligands, they coordinate with a variety of metal ions and offer information for the study of antitumor and anticancer drugs.
Amino acids can be divided into two categories: essential amino acids and nonessential amino acids. About 60% of amino acids can be synthesized in the liver, while the remaining 40% cannot be synthesized in the body and need to be obtained from food, which are called essential amino acids.
Weng Debao [22] determined the content of common amino acids in wild O. violaceus, as shown in Table 3. By using the method of fuzzy discernment and ratio coefficient of amino acids, using egg protein as standard protein, comparing with legume vegetables and wild vegetables, it was found that the protein content of fresh O. violaceus at the seedling stage was 3.01% and the amino acid content of the stem and leaf accounted for 89.98% of the crude protein. The results showed that the protein quality of O. violaceus was far superior to the contrast.
Table 3.
Amino acid content (mg/g).
| Amino Acid | Content | |
|---|---|---|
| Seedling | Moss | |
| * Ile | 42.30 | 43.99 |
| * Leu | 79.27 | 84.03 |
| * Phe | 38.66 | 40.21 |
| * Lys | 58.49 | 61.99 |
| * Thr | 41.92 | 44.85 |
| * Val | 63.16 | 63.79 |
| * Met | 6.66 | 6.56 |
| * Trp | 13.54 | 12.32 |
| Ser | 42.18 | 43.02 |
| Tyr | 27.51 | 28.89 |
| Ala | 60.62 | 62.44 |
| Glu | 112.33 | 117.95 |
| His | 20.09 | 20.09 |
| Arg | 41.71 | 44.21 |
| Pro | 114.88 | 92.05 |
| Cys | 6.74 | 6.94 |
| Asp | 78.42 | 83.13 |
| Gly | 51.29 | 52.83 |
*: Essential amino-acid.
The variety of amino acids in O. violaceus is very rich. In addition to the eight essential amino acids, two uncommon amino acids, ornithine, and γ-aminobutyric acid, were also detected by Li Xinhua et al. [17].
Weng Debao et al. [23] used the Kjeldner method to determine the total protein content of O. violaceus and obtained the optimal extraction process conditions of the total protein of O. violaceus through single-factor experiments and response surface designs. Under the optimal extraction conditions, the protein extraction efficiency of O. violaceus’s leaf was 24.40 g/100 g.
3.3. Mineral Element
A variety of mineral elements are present in O. violaceus. Cao Weidong et al. [24] conducted measurements of macroelements, secondary elements, heavy metal elements, trace beneficial elements, and rare earth elements in different organs of O. violaceus, and the results are shown in Table 4.
Table 4.
Contents of elements in different organs of O. violaceus (μg/g).
| Elements | Flower | Root | Leaf | |
|---|---|---|---|---|
| Macroelement | B | 19.69 | 12.01 | 22.05 |
| Na | 210.53 | 199.25 | 240.61 | |
| Mg | 1204.99 | 380.79 | 1271.30 | |
| Si | 322.58 | 239.72 | 207.08 | |
| P | 3864.03 | 1747.57 | 2613.93 | |
| K | 12,911.21 | 8075.82 | 10,043.72 | |
| Ca | 4750.79 | 3121.87 | 12,834.32 | |
| Secondary element | Mn | 32.64 | 16.31 | 86.86 |
| Fe | 234.52 | 123.06 | 1776.91 | |
| Cu | 8.56 | 3.34 | 4.98 | |
| Zn | 44.10 | 16.65 | 28.75 | |
| Br | 2.69 | 2.02 | 7.86 | |
| Sr | 23.13 | 35.13 | 88.83 | |
| I | 0.95 | 1.90 | 1.30 | |
| Heavy metal element | Al | 170.03 | 131.51 | 95.07 |
| Ti | 15.38 | 12.30 | 43.33 | |
| Cr | 2.14 | 2.22 | 221.68 | |
| Ni | 1.66 | 0.90 | 18.70 | |
| Pb | 1.357 | 0.184 | 0.679 | |
| Trace beneficial element | Li | 0.301 | 0.198 | 0.414 |
| Co | 0.374 | 0.174 | 0.578 | |
| Mo | 0.885 | 0.576 | 1.476 | |
| Rare earth element | La | 0.248 | 0.135 | 0.114 |
| Ce | 0.717 | 0.420 | 0.343 | |
| Nd | 0.185 | 0.102 | 0.087 | |
| Sc | 0..233 | 0.158 | 0.179 | |
3.4. Fatty Acids
As a new type of oil plant resource with outstanding quality, the seed of O. violaceus has a high oil content that can reach 46.91%, which is suitable for oil extraction. O. violaceus seeds have a fatty acid profile that is extremely low in erucic acid and high in nutrient-dense unsaturated fatty acids like linoleic and linolenic acids [25,26].
Zhou Min [25] compared three approaches to extracting O. violaceus seed oil in order of oil yield: ultrasonic-assisted method > hydroenzymatic method > physical pressing method.
Zhao Ru et al. [27] microwave-mediated immiscible binary solvent extraction (MIBSE) to extract O. violaceus seed oil and epigoitrin simultaneously and optimized by response surface method. Under the optimal extraction conditions, the yield of O. violaceus seed oil by MIBSE was 34.08% ± 1.38% and that by conventional Soxhlet extraction (SE) method was 25.50% ± 2.63%. The oil yield of MIBSE was 1.34 times higher than that of the SE method. There were 11 kinds of fatty acids in O. violaceus seed oil, including 5 saturated fatty acids (SAFAs, Cn:0, 17.73%), 3 kinds of monounsaturated fatty acids (MUFAs, Cn:1, 22.12%), and 3 polyunsaturated fatty acids (PUFAs, Cn:2,3, 60.15%). The content of unsaturated fatty acids was much greater than that of saturated fatty acids and the fatty acid composition is as follows: Table 5.
Table 5.
Fatty acid composition of O. violaceus seed oil.
| Compounds | Molecular Formula | Relative Area Percentage (%) | |
|---|---|---|---|
| MIBSE | SE | ||
| Palmitic acid | C16H32O2(C16:0) | 8.84 | 9.11 |
| Linoleic acid | C18H32O2(C18:2) | 55.62 | 54.93 |
| Oleic acid | C18H34O2(C18:1) | 15.85 | 15.58 |
| Stearic acid | C18H36O2(C18:0) | 3.67 | 4.19 |
| Linolenic acid | C18H30O2(C18:3) | 4.02 | 3.78 |
| cis-11,14-Eicosenoic acid | C20H36O2(C20:2) | 0.51 | 0.56 |
| 11-Eicosenoic acid | C20H38O2(C20:1) | 3.87 | 4.11 |
| Arachidic acid | C20H40O2(C20:0) | 2.94 | 3.11 |
| Behenic acid | C22H44O2(C22:0) | 1.18 | 1.31 |
| Nervonic acid | C24H46O2(C24:1) | 2.40 | 2.32 |
| Tetracosenoic acid | C24H48O2(C24:0) | 1.10 | 1.01 |
Arachidic acid, arachidonic acid, and a small amount of erucic acid were found in O. violaceus by Sun Xiaoqin et al. [28]. Xue Yinghong et al. [29] also found that the total fatty acid content of O. violaceus seed increased gradually with increasing seed development, with the highest total fatty acid content in its late stage. Lv Zhongjin [30] compared O. violaceus with two types of oilseed rape and found that the linoleic acid content of O. violaceus seed oil was higher than that of both kale-type oilseed rape (Ning-You-7-Hao) and Canadian oilseed rape (Westar) and the erucic acid content was lower than either of these.
Liu Sifan et al. [31] isolated β-D-glucopyranosyl-12-hydroxyjasmonate, a derivative of fatty acid, from O. violaceus seed with a structure as shown in Figure 1.
Figure 1.

12-O-β-d-Glucopyranosyl-12-hydroxyjasmonic acid.
Due to its high oil content, low acid value, and favorable fatty acid ratio, the seed oil of O. violaceus holds great potential as an edible oil. It shows promising prospects for development in the food industry. High-quality oil plants with low erucic acid have been cultivated by cross-breeding of O. violaceus and kale-type oilseed rape [32].
In addition, O. violaceus is also rich in 24-carbon dihydroxy fatty acids (diOH-FAs) composed of triacylglycerol (tAG) estolides, such as Nebraska acids and Wuhan acids, which have better high-temperature resistance and lubrication properties than castor oil and are considered as a potential industrial oil crop [33,34,35].
3.5. Others
The utilization value of the seed cake of Brassicaceae depends mainly on its contents of crude proteins and toxic glucosinolates. In a study conducted by Lv Zhong jin [30], O. violaceus seed cake was compared to two types of rape (rapeseed). According to the data in Table 6, it was observed that the protein content in O. violaceus seed cake was remarkably high, reaching 52.32%. This surpassed the protein content found in typical rapeseed cake. Its glucosinolate content was similar to that of normal rapeseed cake.
Table 6.
Comparison of quality of O. violaceus and rape.
| Plant | Oil Content (%) | Crude Protein Content (%) | Glucosinolate Content (μg/g) |
|---|---|---|---|
| O. violaceus | 50.29 | 52.32 | 35.24 |
| Ning-You-7-Hao | 41.71 | 34.20 | 69.67 |
| Westar | 42.90 | 37.58 | 6.77 |
4. Chemical Compositions
4.1. Flavonoids
Flavonoids are a huge class of naturally occurring substances that are found throughout nature. They are mostly found in plants’ stems, leaves, flowers, and fruit parts and are coupled with sugars to make glycosides [36]. In the available reports, flavonoids account for the majority of the compounds in O. violaceus. Based on a consolidation of the literature, it is clear that flavonoids are also the main active site in O. violaceus. Flavonoids have a variety of biological activities such as antibacterial, antiviral, antioxidant, anti-inflammatory, and antitumor.
The ethanol extract of the stem and leaves of O. violaceus in a study by Weng Debao et al. [37] reacted positively with various color development reagents, indicating that the stem and leaves of O. violaceus contained a variety of flavonoids. The results showed that the ethanol extract contained quercetin, kaempferol, and isorhamnetin, and the total flavonoid glycoside was 0.568%. Wang Xin et al. [38,39] investigated the flavonoid content of different extraction methods of O. violaceus as follows: microwave-assisted 70% ethanol extraction > 70% cold ethanol extraction > water extraction; the flavonoids of O. violaceus were specifically tested for using the ADS-17 resin as an adsorbent. After purification, the purity of flavonoids reached 58%, which was increased by about 6.5 times. Sai Chunmei et al. [40] found that the total flavonoid content of different parts of O. violaceus: leaf > flower > stem > root > fruit.
In the existing research on O. violaceus chemical composition, it is found that there are many kinds of flavonoids. Based on reading a large number of pieces of the literature, after sorting and classifying a variety of flavonoids, all the reported flavonoids are now divided into flavanols (1~12), flavanones (13~16), isoflavones (17), flavones (18~22), and flavan-3-ols (23). Flavonoids have been reported in O. violaceus as shown in Figure 2 and Table 7.
Figure 2.
Structures of flavonoids in O. violaceus.
Table 7.
Flavonoids of O. violaceus.
| No. | Compound | Type | Formula | Mol. wt. | References |
|---|---|---|---|---|---|
| 1 | Quercetin | flavonols | C15H10O7 | 302.02 | [37] |
| 2 | Kaempferol | flavonols | C15H10O6 | 286.05 | [37] |
| 3 | Isorhamnetin | flavonols | C16H12O7 | 316.06 | [37] |
| 4 | Isorhamnetin-3-O-β-d-glucopyranoside | flavonols | C22H22O12 | 478.11 | [41] |
| 5 | 3,4′,6,8-tetyahydroxy-flavone-7-C-glucoside | flavonols | C21H20O11 | 448.10 | [42] |
| 6 | kaeMpferol 3-O-β-d-galactosyl-7-O-α-L-rhamnoside | flavonols | C27H30O15 | 594.16 | [42] |
| 7 | kaeMpferol 3-O-β-d-arabinosyl-7-O-α-L-rhamnoside | flavonols | C26H28O14 | 564.15 | [42] |
| 8 | kaeMpferol 3-O-β-d-glucopyranosyl-7-O-α-L-rhamnoside | flavonols | C27H30O15 | 594.16 | [42] |
| 9 | Quercetin 3-O-β-d-glucopyranosyl-7-O-α-L-rhamnoside | flavonols | C27H30O16 | 610.15 | [42] |
| 10 | Kaempferol-7-O-α-L-rhamnoside | flavonols | C21H20O10 | 432.11 | [42] |
| 11 | kaempferol-3-O-β-d-glucoside | flavonols | C21H20O11 | 448.10 | [42] |
| 12 | Quercetin-7-O-α-L-rhamnoside | flavonols | C21H20O11 | 448.10 | [42] |
| 13 | 5,7,3′,5′-tetyahydroxy-flavone-7-O-β-d-glucoside | flavanones | C21H22O11 | 450.12 | [41] |
| 14 | aromadendrin-7-O-β-d-glucopyranoside | flavanones | C21H22O10 | 434.12 | [41] |
| 15 | hesperetin-7-O-β-d-glucopyranoside | flavanones | C22H24O11 | 464.12 | [41] |
| 16 | 3′,5′,5,7-tetyahydroxy-dihydroflavone | flavanones | C15H12O6 | 288.06 | [9] |
| 17 | Geristein | isoflavones | C15H10O5 | 270.05 | [11] |
| 18 | acactin-7-O-β-d-glucoside | flavones | C22H22O10 | 446.12 | [11] |
| 19 | Genkwanin | flavones | C16H12O5 | 284.07 | [41] |
| 20 | acacetin-7-O-neohesperidoside | flavones | C28H32O14 | 592.18 | [41] |
| 21 | apigenin-7-O-β-d-glucoside | flavones | C21H20O10 | 432.11 | [41] |
| 22 | chrysoeriol-7-O-β-d-glucoside | flavones | C22H22O11 | 462.12 | [41] |
| 23 | Catechin | flavan-3-ols | C15H12O6 | 288.06 | [43] |
4.2. Alkaloids
O. violaceus also has a high concentration of alkaloids, which are alkaline chemicals with nitrogen heterocyclic rings and optical activity. Most alkaloids have biological activities such as antibacterial, antiviral, and anti-inflammatory, and are often the effective ingredients in many Chinese herbs and medicinal plants, which have great potential in healthcare [44,45].
Zhu Nai Liang et al. [12] obtained eight new alkaloids: Orychophragmuspine A–H, from the aqueous extract of the seeds of O. violaceus through the 10% ethanol part of D101 resin; Liu Chenqi et al. [46] also obtained a new alkaloid compound Orychophragmuspine I from the 10% ethanol part of the seed D101 resin; Zhang Guangjie et al. [47,48] isolated four new alkaloids: Orchophragine A–D from the n-butanol extract fraction of the ethanolic extract of O. violaceus seeds via the 50% ethanolic aqueous part of AB-8 resin. Xia Xinyi et al. [49] derived epigoitrin from aqueous decoction of O. violaceus seeds in the range of 0.64~0.92%.
The alkaloid compounds reported in O. violaceus are shown in Figure 3 and Table 8.
Figure 3.
Structures of alkaloids in O. violaceus.
Table 8.
Alkaloids of O. violaceus.
| No. | Compound | Formula | Mol. wt. | References |
|---|---|---|---|---|
| 24 | orychophragmuspine A | C26H33N3O5 | 467.27 | [12] |
| 25 | orychophragmuspine B | C26H33N3O5 | 467.24 | [12] |
| 26 | orychophragmuspine C | C26H33N3O5 | 467.24 | [12] |
| 27 | orychophragmuspine D | C28H35N3O6 | 509.25 | [12] |
| 28 | orychophragmuspine E | C28H35N3O6 | 509.25 | [12] |
| 29 | orychophragmuspine F | C27H35N3O5 | 481.26 | [12] |
| 30 | orychophragmuspine G | C27H35N3O5 | 481.26 | [12] |
| 31 | orychophragmuspine H | C25H33N3O4 | 439.25 | [12] |
| 32 | orychophragmuspine I | C26H35N3O5 | 469.26 | [46] |
| 33 | orychophragine A | C14H17N3O4 | 291.12 | [47] |
| 34 | orychophragine B | C16H19N5O5 | 361.14 | [47] |
| 35 | orychophragine C | C16H19N5O5 | 361.14 | [47] |
| 36 | orychophragine D | C16H18N6O4 | 358.14 | [48] |
| 37 | (+)-orychoviolines A | C25H33O4N3 | 439.25 | [50] |
| 38 | (−)-orychoviolines A | C25H33O4N3 | 439.25 | [50] |
| 39 | (+)-orychoviolines B | C27H39O5N3 | 485.29 | [50] |
| 40 | (−)-orychoviolines B | C27H39O5N3 | 485.29 | [50] |
| 41 | demethylorychophragine A | C13H15N3O4 | 277.11 | [51] |
| 42 | 4-hydroxy-5-methoxy-3-quinolinecarbonitrile | C11H8N2O2 | 200.06 | [42] |
| 43 | Cappariloside A | C16H18N2O6 | 334.12 | [42] |
| 44 | 2-Phenylacetamide | C8H9NO | 135.07 | [42] |
| 45 | Uracil | C4H4N2O2 | 112.03 | [42] |
| 46 | Uridine | C9H12N2O6 | 244.07 | [42] |
| 47 | isolumichrome | C12H10N4O2 | 242.08 | [42] |
| 48 | 1-ribosyl-2-3-diketo-1,2,3,4-tetrahydro-6,7-dimethyl-quinoxaline | C15H20N2O6 | 324.13 | [42] |
| 49 | 3,6-dibenzyl-2,5-dioxopiperazine | C16H14N2O2 | 266.11 | [42] |
| 50 | perlolyrine | C16H12N2O2 | 264.09 | [42] |
| 51 | DL-isoleucyl-leucyl anhydride | C12H22N2O2 | 226.17 | [42] |
| 52 | 4-oxo-1,4-dihydroquinoline-3-carbonitrile | C10H6N2O | 170.05 | [42] |
| 53 | epigoitrin | C5H7NOS | 129.02 | [50] |
| 54 | Adenosine | C10H13N5O4 | 267.10 | [9] |
| 55 | 2′-Dideoxyadenosine | C10H13N5O3 | 251.10 | [9] |
| 56 | 7,8-Dimethylalloxazine | C12H10N4O2 | 242.08 | [52] |
| 57 | N-2-hydroxy-3-butenyl-Benzamide | C11H13NO2 | 191.09 | [53] |
| 58 | 2-Amino-5-hydroxybenzoic acid | C7H7NO3 | 153.04 | [53] |
| 59 | N-benzoyl-epigoitri | C12H11NO2S | 233.05 | [53] |
| 60 | α-[(2-carboxyacetyl)amino]-Benzeneacetic acid | C11H11NO5 | 237.06 | [53] |
| 61 | 3-phenyl-6-vinylmorpholin-2-one | C12H13NO2 | 203.09 | [53] |
4.3. Phenylpropanoids
Phenylpropanoids are typically classified into three constituent groups: phenyl propionic acids, coumarin, and lignans.
Coumarin is a general term for a class of compounds with a benzo-α-pyranone parent core, which is widely found in various plants in nature and also in a few fungi and bacteria. Studies have shown that coumarins have a wide range of pharmacological activities, such as anti-inflammatory, antioxidation, antivirus, antitumor, anticoagulation, and antibacterial effects [54,55]. The oxidative polymerization of phenylpropanoids, which are frequently found in plant resins or xylem, results in the formation of lignans, a class of natural products.
In published studies [13], three new iso-coumarins and one new dihydroisocoumarin were isolated from the seeds of O. violaceus: orychophramarin A–D.
The reported phenylpropanoids from O. violaceus are shown in Figure 4 and Table 9.
Figure 4.
Structures of phenylpropanoids in O. violaceus.
Table 9.
Phenylpropanoids of O. Violaceus.
| No. | Compound | Type | Formula | Mol. wt. | References |
|---|---|---|---|---|---|
| 62 | orychophramarin A | coumarin | C15H10O5 | 270.05 | [13] |
| 63 | orychophramarin B | coumarin | C15H10O5 | 270.05 | [13] |
| 64 | orychophramarin C | coumarin | C16H12O6 | 300.06 | [13] |
| 65 | orychophramarin D | coumarin | C21H22O10 | 434.12 | [13] |
| 66 | 7-hydroxy-6-methoxy coumarin | coumarin | C10H8O4 | 192.04 | [13] |
| 67 | lariciresinol | lignans | C20H24O6 | 360.16 | [13] |
| 68 | 11-deoxykompasinol A | lignans | C25H24O7 | 436.15 | [31] |
| 69 | (+)-lariciresinol-9-O-β-d-glucopyranoside | lignans | C26H34O11 | 522 | [31] |
| 70 | (+)-isolariciresinol-9′-O-β-d-glucopyranoside | lignans | C26H34O11 | 522 | [31] |
| 71 | (+)-isolariciresinol-9-O-β-d-glucopyranoside | lignans | C26H34O11 | 522 | [31] |
| 72 | (−)-isolariciresinol-9′-O-β-d-glucopyranoside | lignans | C26H34O11 | 522 | [31] |
| 73 | (−)-5′-methoxyisolariciresinol-9′-O-β-d-glucopyranoside | lignans | C27H36O11 | 434.16 | [31] |
| 74 | p-hudroxycoumaric | phenylpropanoic acids | C9H8O3 | 164.05 | [42] |
| 75 | caffeic acid | phenylpropanoic acids | C9H8O4 | 180.04 | [14] |
| 76 | trans-Ferulic acid | phenylpropanoic acids | C10H10O4 | 194.06 | [42] |
| 77 | 3,4-dimethoxy cinnamic acid | phenylpropanoic acids | C11H12O4 | 208.07 | [42] |
4.4. Phenolic Acid
Phenolic acids are natural compounds with a polyphenolic structure and are also important active ingredients for medicinal plants to function, with pharmacological activities such as antitumor, antioxidant, anti-inflammatory, anticoagulant, antiatherosclerosis, and lowering blood lipids, etc. Chinese herbs such as Angelica Sinensis and Salvia miltiorrhiza are rich in phenolic acids [56,57,58].
The O. violaceus water extract contains a significant number of phenolic acids. Xia Xinyi [14] isolated 15 compounds, including salicylic acid and p-hydroxycinnamic acid, from the aqueous extracts of O. violaceus using a macroporous resin column.
The phenolic acids reported in O. violaceus are shown in Figure 5 and Table 10.
Figure 5.
Structures of phenolic acids in O. violaceus.
Table 10.
Phenolic acids of O. Violaceus.
| No. | Compound | Type | Formula | Mol. wt. | References |
|---|---|---|---|---|---|
| 78 | salicylic acid | benzoic acid | C7H6O3 | 138.03 | [14] |
| 79 | vanillic acid | benzoic acid | C8H8O4 | 168.04 | [14] |
| 80 | protocatechuic acid | benzoic acid | C7H6O4 | 154.03 | [14] |
| 81 | syringate | benzoic acid | C9H10O5 | 198.05 | [14] |
| 82 | benzonic acid | benzoic acid | C7H6O2 | 122.04 | [41] |
| 83 | 2-hydroxybenzoic acid | benzoic acid | C7H6O3 | 138.03 | [41] |
| 84 | 4-hydroxybenzoic acid | benzoic acid | C7H6O3 | 138.03 | [41] |
| 85 | 4-hydroxy-3-methoxy-benzoic acid | benzoic acid | C8H8O4 | 168.04 | [41] |
| 86 | Eudesmic acid | benzoic acid | C10H12O5 | 212.07 | [41] |
| 87 | Isovanillic acid | benzoic acid | C8H8O4 | 168.04 | [42] |
| 88 | 2,3-dihydroxybenzoic acid | benzoic acid | C7H6O4 | 154.03 | [42] |
4.5. Terpenoids
Terpenoids are the most abundant group of natural products with complex skeletons and diverse biological activities, including antibacterial, antiallergic, antitumor, anti-inflammatory, vasodilating, and blood-glucose- and lipid-regulating properties [59,60,61].
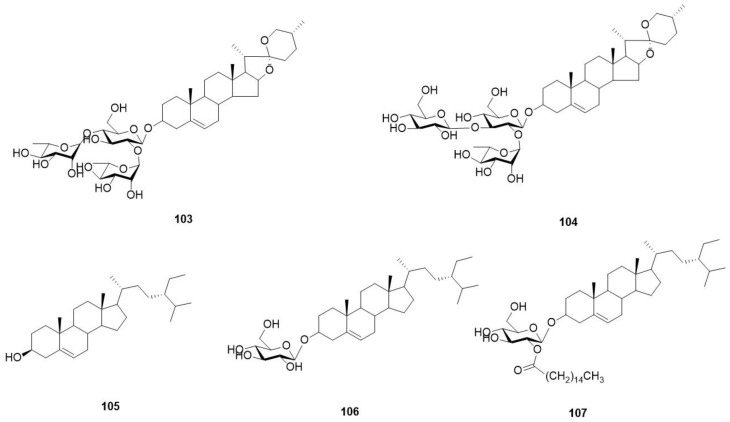
In addition to its content of triterpenes and their saponins, O. violaceus is known to possess various terpenoids. Studies have revealed the presence of a novel triterpene saponin, along with ten previously identified triterpene saponins, isolated from the seeds of O. violaceus [15,41]. The reported terpenoids of O. violaceus are shown in Figure 6 and Table 11.
Figure 6.
Structures of terpenoids in O. violaceus.
Table 11.
Terpenoids of O. Violaceus.
| No. | Compound | Type | Formula | Mol. wt. | References |
|---|---|---|---|---|---|
| 89 | orychovioside A | monoterpene glycoside | C22H38O10 | 462.25 | [46] |
| 90 | orychoside A | triterpene saponin | C54H88O22 | 1088.58 | [15] |
| 91 | orychoside B | Diterpene glycosides | C26H40O10 | 512.26 | [15] |
| 92 | rubusoside | Diterpene glycosides | C32H50O13 | 642.33 | [15] |
| 93 | mimengoside C | triterpene saponin | C54H88O22 | 1088.58 | [15] |
| 94 | mimengoside D | triterpene saponin | C47H76O17 | 912.51 | [15] |
| 95 | mimengoside E | triterpene saponin | C54H88O22 | 1088.58 | [15] |
| 96 | mimengoside F | triterpene saponin | C47H76O17 | 912.51 | [15] |
| 97 | buddlejasaponin I | triterpene saponin | C54H88O22 | 1088.58 | [15] |
| 98 | buddlejasaponin III | triterpene saponin | C47H76O17 | 912.51 | [15] |
| 99 | buddlejasaponin Ia | triterpene saponin | C55H92O23 | 1120.60 | [15] |
| 100 | buddlejasaponin IIIa | triterpene saponin | C48H80O18 | 944.53 | [15] |
| 101 | 3-O-α-L-rhamnose-(1→4)-β-d-glucose(1→3)-[β-d-glucose-(1→2)]-β-d-fucose-23,28-dihydroxysaikosaponin-11,13(18)-diene | triterpene saponin | C54H88O21 | 1072.58 | [15] |
| 102 | 3β-O-(β-d-glucose-(1→4)-β-d-glucose(1→3)-[β-d-glucose-(1→2)]-β-d-fucose-23,28-dihydroxysaikosaponin-11,13(18)-diene | triterpene saponin | C53H86O21 | 1058.57 | [15] |
4.6. Steroids
Steroid compounds are widely available as a natural chemical constituent and all contain a cyclopentane and polyhydrophenanthrene steroid nucleus in their structure. Steroids are capable of performing important physiological functions with antitumor, anti-inflammatory, cerebral-blood-flow-improving, and cerebrovascular-resistance-reducing effects [62,63,64]. Common steroidal drugs, such as steroid hormones and cardenolide, play an important role in medical applications.
Steroids have been reported for O. violaceus, see Figure 7 and Table 12.
Figure 7.
Structures of steroids in O. violaceus.
Table 12.
Steroids of O. Violaceus.
4.7. Anthocyanidin
Anthocyanidins are a kind of compound based on the flavonoid nucleus, which is widely found in the cytosol of flowers, fruits, stems, leaves, and other organs of plants and has activities such as eliminating free radicals in the body, anti-inflammatory, and antitumor. Anthocyanins are a class of compounds that combine anthocyanidins with sugars.
Toshio Honda et al. [65] extracted three acylated cyanidin 3-sambubioside-5-glucosides (Orychophragonus violet-blue anthocyanins, OVAs) from the petals of O. violaceus, cyanidin3-[2-(2-(4-(6-(4-glucosylcaffeoyl)-glucosyl)-caffeoyl)-xylosyl)-6-(4-glucosyl-p-coumaroyl)gIucoside]-5-gjucoside,cyanidin3-[2-(2-(4-(6-(4-glucosylcaffeoyl)-glucosyl)-caffeoyl)-xylosyl)-6-(4-glucosyl-feruloyl)glucoside]-5-glucoside and cyanidin3-[2-(2-(4-(6-(4-glucosylcaffeoyl)-glucosyI)-caffeoyl)-xylosyl)-6-(4-glucosyl-sinapoyl)glucoside]-5-(6-malonylglucoside). A study [66] demonstrated that anthocyanins derived from the petals of O. violaceus have the potential to serve as an alternative to phenolphthalein in monitoring the carbonation process in cement paste and evaluating the long-term strength and resilience of concrete.
Based on a study [67], it was found that both pink and blue-purple petals of C. chinensis contained a total of seven anthocyanin components. Among these, six were identified with chemical structures presented in Figure 8 and Table 13. Interestingly, white petals of the same species did not have any anthocyanins and none of the colors (pink, blue purple, and white) contained carotenoids.
Figure 8.
Structures of anthocyanins in O. violaceus.
Table 13.
Anthocyanins of O. Violaceus.
| No. | Compound | Formula | Mol. wt. | References |
|---|---|---|---|---|
| 108 | Centaureidin 3-rutinoside | C28H33O14+ | 593.19 | [67] |
| 109 | Malvidin 3,5-diglucoside | C29H35O17+ | 655.19 | [67] |
| 110 | Centaureidin 3-coumaroylglucoside-5-glucoside | C38H41O20+ | 817.22 | [67] |
| 111 | Centaureidin 3-p-coumaroyl diglucoside-5-glucoside | C43H49O25+ | 965.26 | [67] |
| 112 | Centaureidin 3-feruloylsophoroside-5-malonyl glucoside | C47H53O26+ | 1033.28 | [67] |
| 113 | Centaureidin 3-coumaroyl locust glycoside-5-malonyl glucoside | C48H55O27+ | 1063.29 | [67] |
5. Pharmacological Activity
5.1. Antioxidant Properties
Many human diseases, such as tumors, inflammation, cancer, coronary heart disease, aging, and atherosclerosis, are caused by free radicals that are not removed in time and continue to react to generate reactive oxygen species (ROS). ROS can react with almost all biological macromolecules in various types, causing a series of harmful effects [68].
Flavonoids exhibit excellent free radical scavenging and antioxidant capacity, blocking the production of free radicals in the body. The mechanism of action includes a reaction with O2-to block the free radical chain reaction, chelation with metal ions to block free radical production, and reaction with lipid peroxides (ROO) to block the lipid peroxidation process [69,70].
Quercetin and catechins, two well-known flavonoids, are extensively found in various plant species. These compounds exhibit a synergistic effect in protecting HepG2 cells from oxidative stress damage induced by H2O2. Quercetin and catechins concertedly enhanced HepG2 cell survival and catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) activities and cooperatively inhibited cellular ROS production, MDA accumulation, and apoptosis [71].
It has been reported [42] that 3,4′,6,8-tetyahydroxy-flavone-7-C-glucoside, 2,3-dihydroxybenzoic acid, quercetin 3-O-β-d-glucopyranosyl-7-O-α-L-rhamnoside, Kaempferol-7-O-α-L-rhamnoside, and quercetin isolated from the whole herb of O. violaceus have antioxidant capacity. Among them, 3,4′,6,8-tetyahydroxy-flavone-7-C-glucoside, 2,3-dihydroxybenzoic acid, and quercetin had stronger antioxidant capacity than the positive control drug Trolox.
The autooxidation of lard can be significantly delayed by using the ethanol extract of O. violaceus and its antioxidant activity increases with concentration. Flavonoids can provide active hydrogen protons, thus blocking lipid peroxidation [72]. The total flavonoid extract of O. violaceus has a scavenging effect on OH produced by the Fenton system. It is known that flavonoids in O. violaceus have antioxidant activity and the higher the flavonoid content, the stronger the antioxidant capacity [38,39].
Using the oxidative stress injury model of HepG2 cells induced by H2O2, it was found that orychophragmuspine I pretreatment increased the cell survival rate, suggesting that orychophragmuspine I has a significant protective effect on cell damage caused by H2O2. In this study, antioxidant biochemical parameters were also measured to verify that orychophragmuspine I could inhibit the leakage of LDH, inhibit the increase in MDA level, and enhance the activity of SOD and GSH-Px. LDH is leaked from cells during cell membrane damage and can be used as a marker of cell damage; MDA, as a degradation product of cell membrane lipid peroxidation, is an essential marker of oxidative damage; SOD and GSH-Px both have functions of resisting oxidative damage. Western blot analysis showed that orychophragmuspine I enhanced Nrf2, HO-1, and GCL expression, revealing that orychophragmuspine I may mediate antioxidant function by activating the Nrf2-ARE pathway to reduce cell damage caused by H2O2 [46].
Zhu Nai-Liang et al. [12] demonstrated that orychophragmuspine A could inhibit H2O2-induced oxidative stress and LDH leakage indirectly or directly by reducing ROS levels and enhancing SOD activity, validating the antioxidant effect of orychophragmuspine A.
5.2. Antiradiation Properties
Ionizing radiation causes cell damage and apoptosis, leading to various related diseases. It acts directly on biological macromolecules and indirectly affects water molecules in living organisms [73]. Natural active ingredients in plants and animals can provide protection or repair against radiation damage through various effects such as antiemetic, anti-inflammatory, antioxidant, cell proliferation, and wound healing [74]. At present, it has become a research hotspot to explore the active ingredients of traditional Chinese medicine. Among them, the common alkaloids with antiradiation damage effects mainly include tetramethylpyrazine and matrine [75].
Zhang Guangjie et al. [41,48] used an 8 Gy dose of 60Coγ radiation to induce a radiation model of HUVEC cells and found that orychophragine C and orychophragine D significantly increased the survival rate of HUVECs after irradiation. Treatment with orychophragine D markedly enhanced the survival rate of male C57/BL mice exposed to 8Gy(lethal dose) 60Coγ radiation in a dose-dependent manner; from 10 to 14 days after irradiation, the leukocyte, erythrocyte, hemoglobin, and platelet counts of mice in the orychophrine-D-treated group were significantly higher than those of the control group, indicating that orychophrine D can reduce radiation-induced cell damage, accelerate the progress of cell repair, and alleviate radiation-induced hematopoietic toxicity. It has a good radiation protective effect.
Xu Zanxin et al. [51] induced HUVEC by irradiation with 60Coγ ray at a dose of 8Gy and determined by the CCK-8 method, showing that (−)-orychoviolines A and (±)-orychoviolines A at a concentration of 25 μM respectively increased the viability of irradiated HUVEC cells from (52.4% ± 2.3%) to (66.4% ± 2.8%) and (62.2% ± 2.6%). Based on the findings from the comet assay, it was proposed that (−)-orychoviolines A may exert a radioprotective effect by safeguarding the DNA of HUVEC cells.
Phenylacetamide and uridine, both isolated from the whole herb of O. Violaceus, exerted antiradiation effects, which raised the survival of irradiated HUVEC cells from (38.7% ± 1.44%) to (56.2% ± 2.66%) and (54.4% ± 2.89%) at a concentration of 25 μM [42].
5.3. Antineoplastic Properties
Malignant tumors have gradually become one of the major diseases threatening human health due to their biological characteristics, such as cell differentiation, abnormal proliferation, and metastasis, with increasing morbidity and mortality. Currently, surgery, radiotherapy, and chemotherapy are the standard methods for treating cancer but these treatments frequently have serious side effects. Contemporary research has found that the active ingredients of traditional Chinese medicine can effectively inhibit tumor growth, reduce tumor recurrence rates, and improve patients’ quality of life and survival time, and have few toxic side effects [76]. Chinese medicine has two main types of antitumor effects: one is to exert direct inhibitory effects on the growth of tumor cells, such as cytotoxic effects and induction of apoptosis; the other is to exert indirect antitumor effects by improving the pathological state of the body and thus activating the immune response of tumor cells, alleviating complications and inhibiting the migration of tumor cells [77,78]
Zhang Guangjie et al. [13] used Hela and HCT-116 cells as in vitro tumor models and found that orychophramarin A, orychophramarin B, and orychophramarin C all had the activity of inhibiting the growth and proliferation of tumor cells in vitro. In particular, orychophramarin A showed a significant inhibitory effect on the above two cell lines, whose IC50 values, respectively, were 8.91 and 5.10 μM, which surpassed the efficacy of the positive drug cisplatin. The coumarins orychophramarin A–C showed better activity than the dihydroisocoumarins orychophramarin D in inhibiting the growth of tumor cells. The activities of orychophramarin A and C were higher than those of orychophramarin B, presuming that the 8-OH in the structure was the synergist structure by the structural-activity relationship analysis while orychophramarin A was more active because of the 7,8-hydroxyl substitution in the ring.
It was found that orychophramarin A blocked the HCT-116 cell cycle at the G2 phase in a dose-dependent manner. The DNA content of the G2 phase was 14.51% in the blank control group and was 20.77%, 27.86%, and 36.36% when the drug concentrations were 3.13, 6.25, and 12.5 μM, respectively (Table 14).
Table 14.
Antitumor effect of O. Violaceus.
| Compounds | IC50 (μM) | References | ||||
|---|---|---|---|---|---|---|
| Hela | HepG2 | A549 | HCT-116 | SH-SY5Y | ||
| 11-deoxykompasinol A | 9.43 ± 0.62 | 18.26 ± 1.31 | / | / | / | [31] |
| (+)-lariciresinol-9-O-β-d-glucopyranoside | 17.82 ± 1.17 | 36.78 ± 1.21 | / | / | / | [31] |
| β-d-glucopyranosyl-12-hydroxyjasmonate | 22.20 ± 2.24 | 29.10 ± 2.45 | / | / | / | [31] |
| orychophragine A | 11.91 ± 0.46 | 7.73 ± 0.55 | 10.79 ± 0.86 | 9.93 ± 0.71 | / | [47] |
| orychoside A | / | 7.13 ± 0.10 | 8.77 ± 0.31 | / | / | [41] |
| mimengoside C | / | 7.82 ± 0.17 | 6.78 ± 0.21 | / | / | [41] |
| mimengoside D | / | 9.62 ± 0.68 | 11.63 ± 0.50 | / | / | [41] |
| mimengoside E | / | 16.82 ± 1.46 | 14.03 ± 0.49 | / | / | [41] |
| mimengoside F | / | 22.20 ± 1.24 | 19.10 ± 0.45 | / | / | [41] |
| buddlejasaponin I | / | 0.78 ± 0.21 | 0.48 ± 0.04 | / | / | [41] |
| buddlejasaponin III | / | 1.06 ± 0.09 | 0.96 ± 0.12 | / | / | [41] |
| orychophramarin A | 8.91 ± 0.65 | / | / | 5.10 ± 0.46 | / | [13] |
| orychophramarin B | 23.47 ± 1.37 | / | / | 28.30 ± 0.91 | / | [13] |
| orychophramarin C | 14.24 ± 0.67 | / | / | 10.12 ± 0.54 | / | [13] |
| orychophramarin D | >50 | / | / | 44.71 ± 1.93 | / | [13] |
| perlolyrine | 51.3 ± 1.32 | / | / | / | 23.4 ± 2.88 | [42] |
In early apoptosis, phosphotidylserine can flip from being primarily distributed on the inside of the cell membrane to the outside. Flow cytometry analysis showed that orychophramarin A could significantly promote the apoptosis of HCT-116 cells in a dose-dependent manner. The apoptosis rate was (8.22% ± 0.53%) in the blank control and after treatment with 3.13, 6.25, and 12.5 μM orychophramarin A, the apoptosis rates, respectively, were (13.47% ± 2.42%), (25.03% ± 3.86%), and (35.43% ± 3.39%). This finding suggests that orychophramarin A exerts its antitumor activity by preventing tumor cells from growing and multiplying by directly inducing apoptosis.
Orychoside A, mimengoside C, mimengoside D, buddlejasaponin I, and buddl-ejasaponin III demonstrated potent inhibition of tumor cell growth in vitro. These compounds exhibited significant inhibitory effects on HepG2 and A549 cells, with IC50 values ranging from 0.48 to 11.63 μM. Among them, buddlejasaponin I and III have the most significant activity. A conformational study of these compounds showed that HepG2 and A549 cells are sensitive to triterpenoid saponins whose glycosides are saikogenin. When 28-OH forms an intramolecular ether bond with C-13, its tumor cell suppressive activity is remarkably enhanced [41].
5.4. Hepaticprotective Properties
Hepatic damage can be caused by viruses, drugs, alcohol, and trauma, and liver disease has become one of the most common diseases worldwide. Alanine aminotransferase (ALT), glutamic oxalacetic transaminase (AST), and total bilirubin (TBiL) in serum can reflect the damage to hepatocytes. Elevated levels of ALT, AST, and TBiL indicate severe damage to liver cells. In addition, MDA (malondialdehyde) often plays a role in apoptotic necrosis, often in conjunction with cytokines released from hepatocytes [79,80].
Thioacetamide (TAA), a common industrial bactericide, is hepatotoxic to the liver and can be metabolized into sulphone and sulfoxide in the body, producing large amounts of free radicals and ROS, causing hepatocyte necrosis, which leads to the entry of ALT and AST from the cytoplasm into the blood, ultimately increasing the levels of ALT and AST in the serum [81]. Peng Min et al. [82] used male ICR mice to establish a TAA-induced acute liver injury model. The results showed that compared to the model group, MDA content in the liver tissue in the high-dose group of aqueous extract of O. violaceus (AEOV) decreased by 52.4%, and SOD, GSH-Px, and GSH increased by 42.01%, 38.8%, and 136.41%, respectively. In addition, compared to the model group, the activities of ALT and AST and the content of TBiL in the serum of the high-dose AEOV group, respectively, decreased by 84.57%, 87.62%, and 10.26%, confirming the protective effect of AEOV on the liver. In terms of the protective effect on TAA-induced liver injury, it has been observed that AEOV can decrease the levels of NF-κB p65 and keap-1 proteins in liver tissue while increasing the content of Nrf2 protein. This indicates that AEOV promotes the activation of the Nrf2 pathway. Additionally, AEOV inhibits the phosphorylation of p38 and ERK, effectively suppressing the activation of the NF-κB-signaling pathway and reducing the occurrence of inflammation. By inhibiting the phosphorylation of p38 and ERK, the activation of the NF-κB-signaling pathway and the occurrence of inflammation are suppressed. By controlling the phosphorylation of JNK, it can increase the ratio of Bcl-2/Bax, restrain the Caspase-3 conversion into Cleaved Caspase-3, and curb the apoptosis of hepatocytes, thus playing a role in liver protection.
Acetaminophen (APAP) is a widely used analgesic-antipyretic. Prolonged or large doses of APAP produce a large accumulation of the toxic metabolite NAPQI, which causes oxidative stress and mitochondrial damage by depleting glutathione and producing ROS, eventually leading to hepatocyte apoptosis. Mitochondrial oxidative stress has been implicated as the key to APAP-induced liver injury and the caspase pathway by which APAP mediates apoptosis is essential for its contribution to acute liver injury [83]. In a study conducted by Pang Min et al. [84], a model of APAP-induced acute liver injury was established using male C57BL/6J mice. The researchers discovered that the high-dose group of AEOV exhibited a significant decrease of 29.69% in MDA content in the liver homogenate compared to the model group. Additionally, they observed a substantial increase of 48.68% in SOD levels and a remarkable increase of 232.80% in GSH levels. These findings indicate that the aqueous extract of O. violaceus effectively enhances the antioxidant capacity in the liver. Compared to the model group, the ALT and AST activities of the AEOV high-dose group, respectively, decreased by 71.38% and 59.33%, indicating that the aqueous extract of O. violaceus had a significant effect on hepatic protection; the AEOV high-dose group could significantly improve the liver swelling caused by APAP in mice and the morphology of mouse liver cells improved obviously. Based on the mechanism of its protective effect on APAP-induced liver injury, it was shown that AEOV could promote the phosphorylation of AMPK, AKT, and GSK-3β protein expression, activate the AMPK/AKT/GSK-3β signaling pathway and inhibit Caspase-3 expression; it could also inhibit JNK phosphorylation, up-regulate Bcl-2 protein expression and down-regulate Bax and Caspase-9 protein expression, thus inhibiting apoptosis and exerting a protective effect on APAP-induced liver injury in mice.
ConA, which is a T cell mitogen, can activate T lymphocytes and induce excessive immune responses. Simultaneously, it stimulates the body to secrete significant amounts of inflammatory factors such as INF-γ, TNF-α, IL-1, IL-2, and IL-6. Under the mediation of these inflammatory factors, it causes an autoimmune inflammatory response, which leads to liver damage. ALT and AST will enter the blood in large amounts from hepatocytes and their concentrations increase with increasing doses of ConA [85,86]. Using male BALB/C mice to compose a model of acute liver injury induced by ConA, Pang Min [87] showed that the high-dose group of AEOV could significantly reduce ALT and AST activity levels, enhance SOD and CAT activities and increase GSH content, and reduce MDA content by 36.70% compared to the model group, indicating that AEOV could increase the antioxidant capacity and improve the damage of hepatocytes in mice caused by ConA.
The liver serves as the primary metabolic organ for alcohol and oxidative stress triggered by alcoholism in the liver is considered a crucial mechanism behind alcoholic liver injury. Ethanol is metabolized into acetaldehyde in liver cells, during which a large number of ROS will be produced, thus promoting oxidative stress of liver cells, leading to liver damage and inflammatory response, resulting in a series of changes such as liver degeneration [88,89]. Liu Chenqi et al. [90] established a model of acute alcoholic liver injury in ICR mice by giving 56° liquor. The results of the experiment showed that AEOV was able to antagonize the increased hepatic metabolic enzymes ALT, AST, and triglycerides (TG) caused by excessive alcohol and had a protective effect on the liver, whereas the positive drug bifendate did not have a significant effect on TG. AEOV significantly boosted the activity of SOD, ADH, and the levels of GSH-Px and GSH in the liver of mice with alcoholic liver injury and its intensity was comparable to that of the positive drug bifendate. The conclusion drawn suggests that AEOV may exhibit hepatoprotective effects by acting through the antioxidant damage pathway. Additionally, it is believed to inhibit lymphocyte infiltration as well as prevent hepatocyte apoptosis or necrosis.
Studies have shown that cortex dictamni can induce liver damage in animals. Furthermore, its main component, dictamin, has been found to have a toxic effect on the HepG2 cell line. In the case of prolonged consumption of aqueous decoction of the cortex, dictamni alone can cause significant liver damage [91]. By using cortex dictamni aqueous decoction and ICR mice to establish a mouse model of acute liver injury, Yewei Zhan et al. [92] measured the biochemical indexes of each group of mice and found that the levels of GPT, GOT, and LDH decreased by 99%, 98%, and 96%, respectively, in the AEOV high-dose group compared to the model group, indicating that AEOV could significantly alleviate the acute liver injury caused by cortex dictamni. The elevated MDA content in liver tissue and the decreased reduced GSH/GSSG ratio observed in the model group, when compared to the blank control group, indicate that the liver injury caused by cortex dictamni may be attributed to oxidative stress damage. The high-dose group of AEOV showed a 22% decrease in MDA and a 54% increase in GSH/GSSG ratio compared to the cortex dictamni model group, confirming the protective effect of AEOV against acute hepatic injury induced by cortex dictamni in mice through antioxidative stress effect.
Cocaine, which is highly addictive, can cause psychological dependence within a very short period after use, and long-term use can lead to languor. Other than the psychoactive effects mentioned above, cocaine causes other damage to the body, such as cardiovascular, gastrointestinal, muscular, and skeletal [93]. Cocaine is hepatotoxic to mice, rats, and humans. The oxidative metabolism of cocaine in the liver, facilitated by P-450 enzymes, plays a significant role in its hepatotoxicity. This metabolic process converts cocaine into highly toxic intermediates and generates a substantial amount of reactive oxygen species (ROS). As a result, lipid peroxidation occurs, particularly in cell membranes, leading to hepatocyte necrosis [94]. Xu Ziqian et al. [95] used cocaine hydrochloride to establish a model of acute liver injury in ICR mice. Compared to the model group, the GPT, GOT, and LDH activities of mice in the AEOV group decreased in a dose-dependent manner, indicating a reduction in the degree of liver injury after prophylactic administration of AEOV. This suggests that AEOV exhibits hepatoprotective effects against toxic chemicals, safeguarding the liver from their harmful impacts. The content of MDA in the liver tissue of the AEOV group was noticeably decreased compared to that of the model group, while the activity of CAT was substantially increased, which indicated that AEOV protected the liver cell membrane by antilipid peroxidation to achieve a protective effect against liver injury.
CCl4 activates hepatic Nrf2 protein and downstream gene expression, causing hepatocyte necrosis and fatty degeneration of the liver and long-term exposure can also induce liver fibrosis, resulting in hepatorenal toxicity [96,97]. Huo Xiaowei et al. [43] established a CCl4-induced liver injury model of BALB/C mice. When compared to the model group, mice in the AEOV group had lower serum levels of ALT and AST, higher levels of T-SOD, CAT, Gsh-PX, and GSH, and improved histopathological changes to the liver. AEOV ameliorates CCl4-induced liver injury by mediating Nrf2 to attenuate oxidative stress in the liver, reducing IKKα expression, and inhibiting p-IκBα protein phosphorylation to mediate the NF-κB pathway to inhibit inflammatory responses and TNF-α production.
Against H2O2-induced HepG2 cells, the epigoitrin isolated from the AEOV is protective [43]. Pretreatment of HepG2 cells with epigoitrin improved HepG2 cell viability, reduced LDH activity and MDA content, and elevated SOD and GSH-Px activity, while Nrf2, GCL, p-IκBα, and NF-κB expression were down-regulated, revealing that epigoitrin may have anti-inflammatory and antioxidant effects through regulating Nrf2 and NF-κB pathways, thereby reducing liver injury. Figure 9 illustrates the primary mechanism underlying the hepatoprotective effect of O. violaceus extracts.
Figure 9.
Mechanism of hepatic-protective action of O. Violaceus.
5.5. Antiferroptosis Properties
Ferroptosis is a pattern of iron-dependent regulated cell death, closely related to dysregulation of iron metabolism, accumulation of ROS, lipid peroxidation, and inactivation of GPX4. Radiation produces large amounts of ROS, indirectly causing cell damage and inducing ferroptosis in cells [98].
Xiao Fengjun et al. [99] used Erastin to induce ferroptosis in IEC6 cells and found that orychophragine D had a ferroptosis inhibitory effect and was more potent than the positive drug Fer-1 at a concentration of 1 μM. In a radiation-induced ferroptosis cell model, the ferroptosis inhibitory activity of orychophragine D was also better than that of the positive drug Fer-1. Orychophragine D ameliorates pathological structural changes in the small intestinal crypts of mice after radiation exposure.
5.6. Anti-Inflammatory Properties
Inflammation is a defensive response of the body to stimulation by inflammatory mediators, usually manifested as redness, heat, swelling, and pain. Anti-inflammatory alkaloids generally have a ring structure with the nitrogen atom located within the ring, which can form salts with acids [100].
(−)-Orychoviolines A exhibits anti-inflammatory activity and displays a notable inhibitory effect on the release of NO, with an IC50 value of 20.3 ± 1.58 μM, which is comparable to that of the positive drug dexamethasone. Perlolyrine also demonstrates anti-inflammatory activity by inhibiting the release of NO, with an IC50 value of 27.3 ± 1.29 μM [50]
5.7. Antibacterial Properties
A study [101] isolated a protein, Zp, from the seeds of O. violaceus. Zp protein has an inhibitory effect on a variety of plant pathogenic fungi and maybe a kind of plant defensin protein. The semi-inhibitory concentration (IC50) for Fusarium oxysporum growth and spore germination was 54.36 μg/mL and 15.97 μg/mL, respectively.
6. Toxicity
ICR mice were subjected to acute oral toxicity and subacute toxicity tests using the limited dose method. The mice in each group grew and developed well, without poisoning or death, and their behavior and urine and feces were normal. The pathological histological examination of the organs of the mice in all groups had no obvious pathological changes, which proved that O. violaceus did not have any obvious acute toxicity and short-term toxic side effects on the test animals and was a practically nontoxic substance [102].
7. Application Value
O. violaceus is abundantly distributed in our country, with ample resources. It is known for its resilience to adverse conditions, including cold temperatures, and it is rich in diverse nutrients. It is an excellent ground cover plant that integrates ornamental and ecological benefits, feeding, edible, and medicinal plants, whose seeds can be used as excellent oil materials.
7.1. Ornamental Value
It blooms in early spring and has a long flowering period, generally lasting from March to June. The inflorescences are from bottom to top and the petals change gradually from white to purple in a transitional gradient. A few of them are pure white or pure purple. When cultivated on a large scale, O. violaceus exhibits a captivating sight during the full flowering stage, with a vast expanse of pale-blue-purple flowers creating a stunning and picturesque scenery [103].
O. violaceus is a rare ground cover plant that flowers in early spring and is green in winter in northern China, which has strong environmental adaptability and tolerance to cold, shade, and drought and can grow in grasslands, plains, hillsides, roadsides, and shady mountains [104].
7.2. Ecological Value
O. violaceus benefits from early greening, a large number of branches, a large area of single leaves, and a developed root system that can quickly cover the ground. When planted at a large scale, O. violaceus can fix ground dust and effectively hinder surface dust; at the same time, its roots promote the formation of soil agglomerate structure, reducing the surface runoff to the storage water source and effectively preventing soil erosion. Using the photosynthesis of plants to absorb carbon dioxide and release oxygen, they also absorb and decompose harmful substances in the air and purify it [105].
Studies [106,107] have shown that, as a clean and sustainable maize cultivation mode, the O. violaceus-maize rotation can adjust the pH of calcareous soil, reduce the soil C/N ratio, improve maize yield, reduce the amount of fertilizer, and improve the utilization rate of fertilizer. At the same time, it can reduce greenhouse gas emissions and reactive nitrogen loss, ameliorate soil composition, lessen environmental damage, and achieve environmental and economic benefits.
7.3. Feeding Value
O. violaceus is rich in vitamins and proteins and has a high yield, with a higher variety and content of amino acids than ordinary fodder. It can be used as green fodder and can also be dried and made into compound fodder with corn and wheat bran as overwintering fodder grazing areas. Furthermore, the seed cake remaining after oil extraction from O. violaceus is rich in protein content. This seed cake can be processed into a high-protein concentrate feed, thus offering a novel and valuable source of high-quality vegetable protein [108].
7.4. Edible Value
O. violaceus can be eaten as a vegetable; its stems and leaves contain high levels of protein, calcium, iron, carotene, vitamin C, and other nutrients. Each 100 g of O. violaceus stems and leaves contains 3.32 mg of carotene, VB20.16 mg, and 59 mg of VC, which is beneficial for both the treatment and prevention of illnesses like scurvy and night blindness. To get rid of the bitterness, the stems and leaves can be cooked in a stir-fry or added to soups [109].
Early spring sees consumption of the young leaves, late spring consumption of the moss, and autumn harvesting of the seeds. The seed of O. violaceus is a high-quality oil resource with an oil content of over 50%, which with high linoleic acid and low erucic acid and can be extracted into premium edible oil, and an important natural genetic resource for improving the quality of rape. Linoleic acid is not only easily digested and absorbed by the body but also has the function of lowering cholesterol and triglyceride and will soften blood vessels and prevent the formation of blood clots, which has therapeutic effects. O. violaceus can also be developed to make food products such as vegetable biscuits and drinks [108].
7.5. Medicinal Value
According to a description, “O. violaceus is warm-natured, pungent, bitter, and sweet in taste” and it is effective in treating jaundice in children and deep-rooted breast carbuncles. The whole herb is used as a medicine for regulating pneuma, harmonizing the stomach, and promoting diuresis and detoxification.
Modern research has confirmed the pharmacological effects of O. violaceus, including antioxidant, antiradiation, antitumor, hepatoprotective, antiferroptosis, anti-inflammatory, and antibacterial effects, and its health and medicinal values are increasingly being studied by scientists.
8. Conclusions and Future Perspectives
O. violaceus, which is abundant in resources and widely distributed, exhibits a range of pharmacological activities and has numerous potential uses in agriculture, the production of oil, food, and medicine. The seeds of O. violaceus have a high oil content with high linoleic acid and low erucic acid. In addition, as an edible vegetable, it is rich in many essential nutrients such as vitamins, proteins, and minerals. Among other things, it contains a variety of bioactive compounds such as flavonoids, alkaloids, phenylpropanoids, phenolic acids, terpenoids, and steroids that have been isolated and identified from O. violaceus. The extracts and the isolated compounds have been reported to have antioxidant, antiradiation, antitumor, hepatoprotective, antiferroptosis, anti-inflammatory, and antibacterial activities. Hence, it can be concluded that the development and utilization of O. violaceus in the fields of medicine and food are still considerably lacking and have yet to reach their full potential.
This paper provides an overview of the nutritional composition, chemical composition, bioactivity, and application value of O. violaceus and analyzes the correlation between its chemical composition and bioactivity (see Figure 10), providing a theoretical basis for in-depth research and product development of O. violaceus. To date, over 110 compounds have been identified in O. violaceus, most of which are flavonoids and alkaloids, as well as being the main bioactive compounds responsible for most of its pharmacological activity in several of the identified chemical constituents. The extensive literature has supported these ideas but more in-depth research is still required. Based on reports in the literature, a summary of their biological activities is provided in this paper (see Figure 11).
Figure 10.
Correlation diagram of “chemical constituents-pharmacological activity” of O. Violaceus.
Figure 11.
Overview of bioactivity and potential mechanism of action of O. violaceus. “↑”: up-regulation; “↓”: down-regulation.
Most of the research on the pharmacological action of O. violaceus focuses on its aqueous and alcoholic extracts but there is a lack of studies on the biological activity of monomer compounds, leading to the uncertainty of the material basis of the pharmacological action of O. violaceus and the mechanism of pharmacological activity is even less. Currently, the O. violaceus liver protection effect serves as the main focus of its pharmacological mechanism. As well as reducing inflammation and apoptosis, it safeguards the liver from damage caused by antioxidative stress.
One of the most important factors in controlling the body’s antioxidative stress mechanism is the nuclear factor erythroid-2 related factor (Nrf2). Under normal conditions, Nrf2 exists in cells as a complex with the epoxy chloropropane Kelch sample-related protein-1 (Keap1), so Keap1 exerts a negative regulatory effect on regulating Nrf2 function [110,111]. When stimulated by external ROS or when Nrf2 is phosphorylated, Nrf2 is uncoupled from Keap1, and the activated Nrf2 translocates into the nucleus and binds to the ARE promoter, regulating downstream proteins such as phase II metabolic enzymes, antioxidant enzymes, and anti-inflammatory factors, thereby exerting an antioxidant effect [112] (see Figure 12). The antioxidant effect of O. violaceus is mainly mediated by the Keap1-Nrf2/ARE signaling pathway that regulates antioxidant proteins/enzymes including HO-1, GCL, SOD, GSH, GSH-Px, etc.
Figure 12.
Nrf2 signaling pathway diagram.
The NF-κB signaling pathway, an important regulator in the immune system, is a typical downstream inflammatory pathway, of which IKK/IκBα/NF-κB is one of the most classical pathways and is a common pathway for the production of various inflammatory factors [113]. In the resting state, NF-κB p65 is bound to IκBα and located in the cytoplasm. Upon stimulation by exogenous agents like LPS, the IκB kinase (IKK) complex phosphorylates IκBα. NF-κB p65 dissociates from phosphorylated IκBα and rapidly enters the nucleus and then binds to the κB site to promote the release of relevant inflammatory factors (IL-6, TNF-α, etc.), while cytokines further activate NF-κB, creating positive feedback regulation [114,115]. In addition, MDA can combine with proteins to form malondialdehyde-acetaldehyde adducts (MAA) that upregulate inflammatory factors and induce inflammation [116]. O. violaceus can decrease MDA content, reduce the phosphorylation of p38 and ERK protein, significantly down-regulate the expression of phosphorylated protein of IκBα and NF-κB p65, inhibit the activation of NF-κB signaling pathway, and thus play a role in inhibiting the occurrence of inflammation and preventing the spread of inflammatory reaction.
Recent studies have demonstrated that JNK is a crucial signaling pathway that mediates the cellular stress response, taking part in both cell proliferation and apoptosis. After JNK phosphorylation, it can regulate Bcl-2 family proteins, upregulate antiapoptotic protein Bcl-2, downregulate proapoptotic protein Bax, activate Caspase-8 and Caspase-9, and subsequently activate Caspase-3 [117,118]. Caspase-3 is the predominant shearing enzyme in the process of apoptosis, which cleaves cell proteins, destroys the cytoskeleton, and cleaves nuclear DNA [119]. O. violaceus can inhibit JNK phosphorylation, activate the AMPK/AKT/GSK-3β pathway, and inhibit Caspase-3 activation to form Cleaved Caspase-3, thus inhibiting apoptosis and protecting the liver from damage.
Research has indicated that radiation can indirectly impact water molecules within living organisms, resulting in their ionization and generating a significant quantity of free radicals. These free radicals interact with other substances in the body, leading to apoptosis and potentially contributing to the development of various harmful diseases [73]. Ferroptosis, as one of the programmed cell death modes, is mainly related to intracellular iron accumulation, glutathione (GSH) depletion, and lipid peroxidation [120], and studies [121] have shown that ferroptosis may become a potential target for inflammation-related diseases. Radiation generates a large amount of ROS to promote lipid peroxidation while inducing ACSL4 expression to increase the biosynthesis of PUFA-PL, thereby, in turn, inducing cell ferroptosis [122] (see Figure 13). It is speculated that the antiradiation and antiferroptosis effects of O. violaceus are also closely related to its ability to resist oxidative stress and inhibit inflammation. The Nrf2 signaling pathway not only plays a crucial role in maintaining redox homeostasis within the body but also serves as a key mediator in various essential metabolic pathways. These include lipid metabolism, iron/hemoglobin metabolism, and apoptosis [123]. Nrf2 also exerts a wide range of cytoprotective functions in anti-inflammatory, antitumor, antiapoptotic, and organ-protective activities [124,125]. It has been reported that Nrf2, a key mitigator of lipid peroxidation and ferroptosis, plays a protective role during ferroptosis in hepatocellular carcinoma cells by mediating the p62-Keap1-Nrf2 pathway and its elevated expression level can effectively protect against ferroptosis in hepatoma cells [126]. It has also been found that the NF-κB pathway is also involved in Erastin-induced ferroptosis in colon cancer cells and SPINK4 mediates ferroptosis by regulating the NF-κB pathway [127]. O. violaceus may resist oxidative and inflammatory damage and then exert antiradiation, antiferroptosis, and other pharmacological activities by mediating the Keap1-Nrf2/ARE signaling pathway as well as the NF-κB pathway, elevating Nrf2 expression, up-regulating HO-1 and GSH levels, and down-regulating TNF-α.
Figure 13.
Diagram of radiation-induced ferroptosis.
O. violaceus is a kind of medicinal resource plant with a high development value because of its rich nutritive value and health care function. It contains quercetin, catechin, and other antioxidant active ingredients. The alkaloids of O. violaceus have great benefits to human health such as anti-inflammatory, antioxidant, and antitumor, and have a protective effect on the liver and can be used to treat diseases. In particular, the new skeleton alkaloid orychophragine D shows significant antiradiation activity. Herbs are rarely used alone and are often combined with other herbs in Chinese medicine theory. Over recent years, there has been a growing trend of integrating herbs with conventional chemical drugs in clinical practice, resulting in significant therapeutic benefits. Thus, O. violaceus may have a superimposed effect when used in conjunction with conventional antiradiation drugs. Through the above findings, we can infer the mechanism of antiradiation and antiferroptosis, reveal the internal mechanism of its traditional curative effect by using clinical key indicators and data changes, and provide a scientific basis for disease treatment, which will help position O. violaceus as a promising candidate for future pharmaceutical development.
O. violaceus is known to be dense in fatty acids but has very little current application and potentially very high economic value. O. violaceus oil appears to have special lubricating properties due to the dihydroxylation of its major fatty acids and the presence of estolides in the oil. O. violaceus has the potential to be an industrial crop with oil properties distinct from those of castor oil as a chemical feedstock [128]. In addition, it is also rich in vitamins, amino acids, trace elements, etc., which can be used as a special dietary supplement to provide elements that cannot be synthesized by the human body.
Despite the research progress in chemical composition and pharmacological effects, there is also insufficient research information and a distinct lack of in-depth and systematic studies on O. violaceus. Focusing on the isolation and purification of its compounds as well as their pharmacological activity requires additional study. Further isolation and identification of chemical components can lay the foundation for their pharmacological activities and development applications. The extraction, preparation, and purification processes should adopt green chemistry methods to minimize the use of organic reagents and mitigate environmental pollution. Highly active compounds can be prepared by chemical total synthesis, laying the foundation for future industrial production of drugs. In addition, most of the identified compounds have not yet been validated in vivo, despite in vitro activity assessments to indicate that they have pharmacological effects. The in vivo pharmacodynamic evaluation and molecular biological mechanism research are essential to promote traditional and modern drug development. The specific action mechanism, signaling pathways, and targets of these compounds is also a pressing issue to be further explored by scholars nationwide and internationally. Alternatively, it is necessary to determine whether there are synergistic or antagonistic effects between these bioactive compounds [129]. The sources of O. violaceus vary slightly in different regions, but there is no uniform quality standard system yet. Therefore, a comprehensive quality standard system should be established to provide a basis for the clinical application and resource development of O. violaceus. There are still numerous promising opportunities and challenges in the research filed related to O. violaceus in future. Moderate techniques such as the high-efficiency separation technique, metabolomics, spectrum-effect relationship, network pharmacology, and molecular docking can be combined in the future to further clarify the pharmacological mechanism of action of O. violaceus, screen its active compounds, and identify its quality markers, thus providing a basis for the development of O. violaceus [130].
Author Contributions
X.C.: conceptualization, writing—original draft, methodology, investigation. G.Z.: conceptualization, writing—review and editing. W.C.: conceptualization, investigation. C.G.: methodology, investigation. B.L.: data curation, validation. M.L.: validation, methodology. S.L.: conceptualization, project administration, data curation. L.W.: conceptualization, Data curation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 81903497.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hu H., Wang Q., Liu J.-Q. Recent Advances and Future Prospects of the Important Resource Plant Orychophragmus. Plant Sci. J. 2014;32:189–198. [Google Scholar]
- 2.Chen Z., Lu Y., Hu J., Zhang Y., Jin X. Taxonomic study Orychophragmus Bunge (Brassicaceae), agenus endemic to China. J. Zhejiang Univ. Sci. Ed. 2017;44:201–205. [Google Scholar]
- 3.Chen B., Ren Q. Characteristics and application value of Orychophragmus violaceus. Mod. Agric. Sci. Technol. 2015;9:169. [Google Scholar]
- 4.Chen X., Mao L., Pan Y., Wang Y. Comparison on pollen morphological characteristics of some species of Brassicaceae. J. Plant Resour. Environ. 2022;31:13–20. [Google Scholar]
- 5.Li M., Zhao H. Pod Development Regularity of Orychophragmus violaceus. J. Anhui Sci. Technol. 2018;32:18–23. [Google Scholar]
- 6.Zhang L.Q. Study on the shade tolerance of Orychophragmus violaceus. Contemp. Hortic. 2006;8:42. [Google Scholar]
- 7.Ni S., Cheng Z., Gong J., Luo R., Li Z., Cui Y., Zhao G. Research Overview on Orychophragmus spp. Plant. J. Anhui Agric. Sci. 2009;37:6435–6460. [Google Scholar]
- 8.Luo P., Li Y., Huang B. A Desirable Genrtic Resource of Oil Plant-Orychophragmus violaceus. Chin. J. Oil Crop Sci. 1993;4:75–77. [Google Scholar]
- 9.Zhang T.-T., Ma G.-X., Xu F.-Q., Wu J.-N., Zhang X., Yang J.-S., Zhang B.-X., Xu X.-D. Water Soluble Constituents from of Seeds Orychophragmus violaceus. Chin. Pharm. J. 2014;49:2165–2167. [Google Scholar]
- 10.Wang X., Li X., Wang H. Study on the Extraction Method of Flavonoid Compounds in Orychophragmus violaceus and its Antioxidation. J. Anhui Agric. Sci. 2007;35:8667–8669. [Google Scholar]
- 11.Liu S., Zhang G., Cui H., Qin H., Dong J. Chemical constituents from the seeds of Orychophragmus violaceus. J. Int. Pharm. Res. 2018;45:238–243. [Google Scholar]
- 12.Zhu N., Wu H., Xu Z., Liu C., Tian Y., Hu M., Sun Z.H., Li P., Ma G., Xu X. New alkaloids with unusual spermidine moieties from the seeds of Orychophragmus violaceus and their cytoprotective properties. RSC Adv. 2017;7:41495–41498. doi: 10.1039/C7RA02951A. [DOI] [Google Scholar]
- 13.Zhang G.J., Li B., Chen L., Tian Y., Liu S.J., Cui H.M., Dong J.X. Isocoumarin derivatives and monoterpene glycoside from the seeds of Orychophragmus violaceus. Fitoterapia. 2018;125:111–116. doi: 10.1016/j.fitote.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Xia X.Y. Study on Ployphenols and Quality Control of the Seeds of Orychophragmus violaceus. Harbin University of Commerce; Harbin, China: 2019. [Google Scholar]
- 15.Zhang G., Cui H., Liu S., Dong J. A new triterpenoid saponin and a diterpene glucoside from the seeds of Orychophragmus violaceus. Nat. Prod. Res. 2019;33:407–413. doi: 10.1080/14786419.2018.1455044. [DOI] [PubMed] [Google Scholar]
- 16.Luo P., Huang B., Ye D., Gao H., Chen Y., Mu Z. Study on the forage plant resources of Orychophragmus violaceus. Acta Pratacult. Sin. 1998;7:54–58. [Google Scholar]
- 17.Li X., He S., Ren B., Sheng N. Nutrient constituents of Orychophragmus violaceus (L.) O. E. Schulz and the evaluation as a wild vegetable. J. Plant Resour. Environ. 1997;6:9–13. [Google Scholar]
- 18.Luo P., Huang B., Lan Z., Yan H. A Study on the Vegetable Resource Orychophragmus violaceus. J. Sichuan Univ. Nat. Sci. Ed. 1998;35:148–151. [Google Scholar]
- 19.Schjoldager J.G., Paidi M.D., Lindblad M.M., Birck M.M., Kjærgaard A.B., Dantzer V., Lykkesfeldt J., Tveden-Nyborg P. Maternal vitamin C deficiency during pregnancy results in transient fetal and placental growth retardation in guinea pigs. Eur. J. Nutr. 2015;54:667–676. doi: 10.1007/s00394-014-0809-6. [DOI] [PubMed] [Google Scholar]
- 20.Lykkesfeldt J., Tveden-Nyborg P. The Pharmacokinetics of Vitamin C. Nutrients. 2019;11:2412. doi: 10.3390/nu11102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milani A., Basirnejad M., Shahbazi S., Bolhassani A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017;174:1290–1324. doi: 10.1111/bph.13625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weng D., Huang X. Evaluation on the protein quality of Orychophragmus violaceus. Acta Bot. Boreali-Occident. Sin. 2001;21:673–677. [Google Scholar]
- 23.Wang X., Xu G. Study on Protein Extraction Technology from Iolet Orychophragmus. J. Anhui Agric. Sci. 2009;37:9128–9129. [Google Scholar]
- 24.Cao W., Rui Y., Li X. Determination of Forty-Six Elements in Different Organs of Orychophragmus violaceus in Agricultural Farm. Asian J. Chem. 2014;26:1038–1040. doi: 10.14233/ajchem.2014.15837. [DOI] [Google Scholar]
- 25.Min Z. Study on the Extraction of Orychophragmus violaceus Seed Oil and the Preparation of Its Microencapsulation. Guizhou University; Guiyang, China: 2018. [Google Scholar]
- 26.Qi P., Li Z. Genome and Genetics of the Crucifer Orychophragmus violaceus Native to China. J. Plant Genet. Resour. 2023;24:340–348. [Google Scholar]
- 27.Zhao R., Yang X., Zhang A., Zhou T., Zhou Y., Yang L. An efficient approach for simultaneously obtaining oil and epigoitrin from Orychophragmus violaceus seeds by microwave-mediated immiscible binary solvent extraction. Food Chem. 2022;372:131258. doi: 10.1016/j.foodchem.2021.131258. [DOI] [PubMed] [Google Scholar]
- 28.Sun X., Peng H., Guo J., Peng B., Bai M., Hang Y. Fatty Acid Analysis of the Seed Oil in a Germplasm Collection of 94 Species in 58 Genera of Brassicaceae. Chem. Ind. For. Prod. 2011;31:46–54. [Google Scholar]
- 29.Xue Y., Wang Z., Hao Y., Deng M., Xue J. Analysis of Fatty Acids of Different Growth Stages in Orychophragmus violaceus Seeds. J. Shanxi Agric. Sci. 2020;48:715–718. [Google Scholar]
- 30.Lv Z. Analysis and utilization evaluation of oil and fat from seeds of Orychophragmus violaceus. Chin. Wild Plant Resour. 1992;1:1–5. [Google Scholar]
- 31.Liu S., Xu Z., Zhang G., Dong J. Lignan derivatives and a jasmonic acid derivative from the seeds of Orychophragmus violaceus. Nat. Prod. Res. 2022;36:3779–3785. doi: 10.1080/14786419.2021.1886100. [DOI] [PubMed] [Google Scholar]
- 32.Hu Q., Hansen N., Laursen J., Dixelius C., Andersen B. Intergeneric hybrids between Brassica napus and Orychophragmus violaceus containing traits of agronomic importance for oilseed rape breeding. Theor. Appl. Genet. 2002;105:834–840. doi: 10.1007/s00122-002-1017-y. [DOI] [PubMed] [Google Scholar]
- 33.Huang F., Chen P., Tang X., Zhong T., Yang T., Nwafor C.C., Yang C., Ge X., An H., Li Z., et al. Genome assembly of the Brassicaceae diploid Orychophragmus violaceus reveals complex whole-genome duplication and evolution of dihydroxy fatty acid metabolism. Plant Commun. 2023;4:100432. doi: 10.1016/j.xplc.2022.100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romsdahl T., Shirani A., Minto R.E., Zhang C., Cahoon E.B., Chapman K.D., Berman D. Nature-Guided Synthesis of Advanced Bio-Lubricants. Sci. Rep. 2019;9:11711. doi: 10.1038/s41598-019-48165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hao J. Study on the Difference of Di-hydroxy Fatty Acid Composition in the Seeds of Orychophragmus Limprichtianus and Orychophragmus violaceus. Huazhong Agricultural University; Wuhan, China: 2019. [Google Scholar]
- 36.Petrussa E., Braidot E., Zancani M., Peresson C., Bertolini A., Patui S., Vianello A. Plant Flavonoids-Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013;14:14950–14973. doi: 10.3390/ijms140714950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weng D., Wang H., Weng J. Study on flavonoids in leaves and stalks of Orychophragmus violaceus. Chin. Wild Plant Resour. 2000;19:13–15. [Google Scholar]
- 38.Wang X., Xia X., Xu G. Research on Extraction of Total Isoflavones in Oryehophragmus violaceus by Microwave-assisted. J. Anhui Agric. Sci. 2007;35:6705–6706. [Google Scholar]
- 39.Xin W. Total Flavonoids Extraction, Purification and Oxidation Resistance of Orychophragmus. Northwest Agriculture and Forestry University; Xi’an, China: 2008. [Google Scholar]
- 40.Sai C., Cui L., Yang M., Zhou M., Jin X., Wang J. Optimization of ultrasound assisted extraction technology of total flavonoids from orychophragmus violaceus by response surface method and study on the antioxidant activity. J. Jining Med. Univ. 2018;41:53–58. [Google Scholar]
- 41.Zhang G. Studies on the Chemical Constituments and Bioactivities from the Seeds of Orychophragmus violaceus. Academy of Military Sciences; Beijing, China: 2018. [Google Scholar]
- 42.Xu Z. Master’s Thesis. Guangdong Pharmaceutical University; Guangzhou, China: 2022. Study on the Chemical Compositions and Bioactivities of Orychophragmus violaceus. [Google Scholar]
- 43.Huo X., Liu C., Gao L., Xu X., Zhu N., Cao L. Hepatoprotective Effect of Aqueous Extract from the Seeds of Orychophragmus violaceus against Liver Injury in Mice and HepG2 Cells. Int. J. Mol. Sci. 2017;18:1197. doi: 10.3390/ijms18061197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L. Studies on Antiviral Effect and Antibacterial Effect of Rdix Isatidis Alkaloid In Vitro. Henan Agricultural University; Zhengzhou, China: 2010. [Google Scholar]
- 45.Zhang H. Neurotoxicity and Neuroprotection as well as Metabolic Differences of Gelsemium Alkaloids. Northwest Agriculture and Forestry University; Xi’an, China: 2022. [Google Scholar]
- 46.Liu C.-Q., Zhu N.-L., Yang S.-X., Dai Y.-H., Li L.-Y., Xu X.-D., Cao L. Protective Effect of Orychophragmuspine I Against Oxidative Damage in HepG2 Cells Induced by Hydrogen Peroxide. Mod. Food Sci. Technol. 2017;33:19–25. [Google Scholar]
- 47.Zhang G.J., Li B., Cui H.M., Chen L., Tian Y., Liu S.J., Li B.W., Li M., Xia Z.M., Chen X.X., et al. Orychophragines A-C, Three Biologically Active Alkaloids from Orychophragmus violaceus. Org. Lett. 2018;20:656–659. doi: 10.1021/acs.orglett.7b03801. [DOI] [PubMed] [Google Scholar]
- 48.Zhang G., Dong J., Liu S., Li B., Tian Y., Li M. Application of Orychophragines D in the Preparation of Drugs against Radiation Damage. CN113262229B. China Patent. 2023 February 24;
- 49.Xia X., Zhu N., Ji Y., Xu X. Determination of epigoitrin in seeds of Orychophragmus violaceus by UPLC. Sci. Technol. Innov. 2018;31:10–11. [Google Scholar]
- 50.Xu Z.X., Li B., Tian Y., Li M., Dong J.X., Zhang G.J. Pentacyclic spermidine alkaloids with radioprotective and anti-inflammatory activities from Orychophragmus violaceus. Org. Biomol. Chem. 2021;19:9844–9848. doi: 10.1039/D1OB01973B. [DOI] [PubMed] [Google Scholar]
- 51.Xu Z., Bin L., Ying T., Min L., Dong J., Zhang G. Chemical constituents from the aerial parts of Orychophragmus violaceus. Nat. Prod. Res. 2022;38:278–286. doi: 10.1080/14786419.2022.2118745. [DOI] [PubMed] [Google Scholar]
- 52.Zhang G., Sang T., Chen X., Ge C., Li B., Tian Y., Li M., Liu S., Xia Z., Li H., et al. Orychophragine D:A new 2-piperazinone fused 5-azacytosine type alkaloid with radioprotective activity from the seeds of Orychophragmus violaceus. Fitoterapia. 2023;168:105544. doi: 10.1016/j.fitote.2023.105544. [DOI] [PubMed] [Google Scholar]
- 53.Zhu N., Yang W., Xu Z., Liu C., Wu H., Ma G., Xu X. Study on the alkaloidal components of Orychophragmus violaceus; Proceedings of the 2016 First China Traditional Chinese Medicine Resources Conference; Xi’an, China. 26 October 2016; pp. 362–363. [Google Scholar]
- 54.Kong L.-L., Hu J.-F., Chen N.-H. Advances in pharmacology and toxicology of coumarins. Chin. Pharmacol. Bull. 2012;28:165–168. [Google Scholar]
- 55.Ye C., Chen J., Wu L., Liu C. Advances in the study of the anti-inflammatory activity of coumarins. J. Mod. Med. Health. 2022;38:4063–4065+4069. [Google Scholar]
- 56.Yang Q., Chen M. Research progress in the prevention and treatment of cardiovascular diseases by regulating intestinal flora with phenolic acids. Chin. Tradit. Pat. Med. 2022;44:3920–3926. [Google Scholar]
- 57.Wang J., Ding H. Research progress on bacteriostatic effects of phenolic acids. Chin. Tradit. Pat. Med. 2022;44:1906–1911. [Google Scholar]
- 58.Wan X., Wang Y., Zhou C., Guo H., Ma S. Research progress on chemical constituents and pharmacological effects of Salvia miltiorrhiza. Chin. Tradit. Herb. Drugs. 2020;51:788–798. [Google Scholar]
- 59.Wei C., Cui P., Xiao J., Luo X., Jiang S. Research progress of natural diterpenoids with antibacterial activity. Chin. Tradit. Pat. Med. 2022;44:2240–2249. [Google Scholar]
- 60.Zhou Q. Research on the Vasorelaxant Substances, Novel Terpenoids from the Aerial Parts of Pogostemon cablin and Leonurus japonicus. Chengdu University of Traditional Chinese Medicine; Chengdu, China: 2017. [Google Scholar]
- 61.Chen L., Wang L., Yang J. Research Progress on Diterpenoids and Their Biological Activities in Rubus Plants. Mod. Chin. Med. 2022;24:2040–2047. [Google Scholar]
- 62.Wang B., Gao L., Wang J., Zhang J., Sun X. Establishment of a liquid chromatographic method for determination of the content of steroidal saponin 25-epi-officinalisninⅡin Asparagi Radix. J. Int. Pharm. Res. 2018;45:799–803. [Google Scholar]
- 63.Liu L. Study on STeroidal AAlkaloids and Antitumor Effect of Solanum nigrum and Solanum lyratum. China Academy of Chinese Medical Sciences; Beijing, China: 2022. [Google Scholar]
- 64.Zhao Q., Xie B., Yan J., Zhao F., Xiao J., Yao L., Zhao B., Huang Y. In Vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohydr. Polym. 2012;87:392–396. doi: 10.1016/j.carbpol.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 65.Honda T., Tatsuzawa F., Kobayashi N., Kasai H., Nagumo S., Shigihara A., Saito N. Acylated anthocyanins from the violet-blue flowers of Orychophragonus violaceus. Phytochemistry. 2005;66:1844–1851. doi: 10.1016/j.phytochem.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 66.Cui D., Liu W., Wang J., Gu C., Wan Y., Wang J. Tracing carbonation in cementitious slurry using anthocyanin extracted from fresh or stale petals. RSC Adv. 2022;12:32557–32566. doi: 10.1039/D2RA04980E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jia X., Deng Y., Sun X., Liang L., Xiao Z. Anthocyanin Profiles in Petals of Orychophragonus violaceus. Chin. Agric. Sci. Bull. 2018;34:60–64. [Google Scholar]
- 68.Demirci-Çekiç S., Özkan G., Avan A.N., Uzunboy S., Çapanoğlu E., Apak R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022;209:114477. doi: 10.1016/j.jpba.2021.114477. [DOI] [PubMed] [Google Scholar]
- 69.Maleki S.J., Crespo J.F., Cabanillas B. Anti-inflammatory effects of flavonoids. Food Chem. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- 70.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:162750. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hui G. Molecular Mechanism of Quercetin and Catechin in Protecting Cell against Oxidative Stress by Targeting FOXO3 to Synergistically Inhibit CHUK Transcription. Shandong Agriculutural University; Taian, China: 2022. [Google Scholar]
- 72.Li G., Yang S. Extraction of Codonopsis Pilosula Polysaccharide and Its Effect of Antiactive Oxygen Free Radicals. Chem. World. 2001;8:421–422+434. [Google Scholar]
- 73.Calvo-Asensio I., Barthlott T., von Muenchow L., Lowndes N.F., Ceredig R. Differential Response of Mouse Thymic Epithelial Cell Types to Ionizing Radiation-Induced DNA Damage. Front. Immunol. 2017;8:418. doi: 10.3389/fimmu.2017.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arora R., Gupta D., Chawla R., Sagar R., Sharma A., Kumar R., Prasad J., Singh S., Samanta N., Sharma R.K. Radioprotection by plant products: Present status and future prospects. Phytother. Res. PTR. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 75.Liu L., Chen M., Zhao H., Yao L. Recent Advances in Understanding the Radioprotective Effect of Natural Products. Food Sci. 2018;39:269–274. [Google Scholar]
- 76.Zhou A., Wang D., Yue X., Zhou C., Huang L. Anti-tumor Effect of Chinese Herbal Polysaccharides: A Review. Chin. J. Exp. Tradit. Med. Formulae. 2022;28:236–246. [Google Scholar]
- 77.Niu C., Zhang L., Huang J. Research Progress on Antitumor Mechanism of Traditional Chinese Medicine. J. Liaoning Univ. Tradit. 2023;25:132–136. [Google Scholar]
- 78.Xia X., Cole S.P.C., Cai T., Cai Y. Effect of traditional Chinese medicine components on multidrug resistance in tumors mediated by P-glycoprotein. Oncol. Lett. 2017;13:3989–3996. doi: 10.3892/ol.2017.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y., Yang L. Expert consensus on liver inflammation and its prevention and treatment. Chin. J. Pract. Intern. Med. 2014;34:152–162. [Google Scholar]
- 80.Hamdy N., El-Demerdash E. New therapeutic aspect for carvedilol: Antifibrotic effects of carvedilol in chronic carbon tetrachloride-induced liver damage. Toxicol. Appl. Pharmacol. 2012;261:292–299. doi: 10.1016/j.taap.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 81.Gu X., Manautou J.E. Molecular mechanisms underlying chemical liver injury. Expert Rev. Mol. Med. 2012;14:e4. doi: 10.1017/S1462399411002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pang M., Wang F.-f., Yang S., Li L., Cao L. Protective effect of water extracts of Orychophragmus violaceus seeds on TAA-induced acute liver injury in mice. China J. Chin. Mater. Med. 2020;45:1399–1405. doi: 10.19540/j.cnki.cjcmm.20191112.405. [DOI] [PubMed] [Google Scholar]
- 83.da Rocha B.A., Ritter A.M., Ames F.Q., Gonçalves O.H., Leimann F.V., Bracht L., Natali M.R., Cuman R.K., Bersani-Amado C.A. Acetaminophen-induced hepatotoxicity: Preventive effect of trans anethole. Biomed. Pharmacother. 2017;86:213–220. doi: 10.1016/j.biopha.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 84.Pang M., Shan Y., Wang F.-f., Yang S., Li L., Cao L. Protective Effect of Water Extracts of Orychophragmus violaceus Seeds on Acetaminophen-induced Acute Liver Injury in Mice: Roles of Activating Hepatic AMPK/AKT/GSK-3β Signaling. Mod. Food Sci. Technol. 2020;36:34–41. [Google Scholar]
- 85.Xie J., Han Z., Yun W. Protective effect of salvianolate on ConA-induced immune liver injury in mice. J. Chin. Med. Mater. 2017;40:2686–2688. [Google Scholar]
- 86.Gong F., Shen Y., Zhang C., Xu J., Wu X., Hua Z., Xu Q. Dregea volubilis ameliorates concanavalin A-induced liver injury by facilitating apoptosis of activated T cells. Exp. Biol. Med. 2008;233:1124–1132. doi: 10.3181/0801-RM-15. [DOI] [PubMed] [Google Scholar]
- 87.Min P. The Molecular Mechanism of Water Extracts from Orychophragmus violaceus Seed on Liver Injury. Harbin University of Commerce; Harbin, China: 2020. [Google Scholar]
- 88.Hagymási K., Blázovics A., Lengyel G., Kocsis I., Fehér J. Oxidative damage in alcoholic liver disease. Eur. J. Gastroenterol. Hepatol. 2001;13:49–53. doi: 10.1097/00042737-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 89.Gao B., Bataller R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu C.-Q., Gao L., Huo X.-w., Zhu N., Dai Y., Yang S., Li L., Cao L. Protective Effect of Water Extracts of Orychophragmus violaceus Seeds on Alcohol-induced Acute Liver Injury in Mice. Mod. Food Sci. Technol. 2017;33:7–11+79. [Google Scholar]
- 91.Wang L., Li Z., Li L., Li Y., Yu M., Zhou Y., Lv X., Arai H., Xu Y. Acute and sub-chronic oral toxicity profiles of the aqueous extract of Cortex Dictamni in mice and rats. J. Ethnopharmacol. 2014;158 Pt A:207–215. doi: 10.1016/j.jep.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 92.Zhan Y.-w., Xu Z.-Q., Guo X.-h., Li R., Shen J., Xu X., Zhang B. Protective effect of Orychophragmus violaceus seed against acute liver injury induced by Cortex Dictamni in mice. Chin. J. Pharmacol. Toxicol. 2016;30:101–106. [Google Scholar]
- 93.Sun W., Lu Y. Progress incocaine toxicology research. Chin. Pharmacol. Bull. 2004;11:1212–1214. [Google Scholar]
- 94.Ndikum-Moffor F.M., Roberts S.M. Cocaine-protein targets in mouse liver. Biochem. Pharmacol. 2003;66:105–113. doi: 10.1016/S0006-2952(03)00246-6. [DOI] [PubMed] [Google Scholar]
- 95.Xu Z., Li R., Shen J., Zhu N., Zhang B. Protective effect of Orychophragmus violaceus on cocaine-induced acute liver injury in mice. J. Toxicol. 2017;31:51–54+59. [Google Scholar]
- 96.Hang L. Ph.D. Thesis. China Medical University; Shenyang, China: 2021. The Role of Nrf2 in CCl4 Induced Liver Fibrosis and Related Mechanisms. [Google Scholar]
- 97.Schuppan D., Kim Y.O. Evolving therapies for liver fibrosis. J. Clin. Investig. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J., Cao F., Yin H.L., Huang Z.J., Lin Z.T., Mao N., Sun B., Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11:88. doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao F., Zhang G., Du L., Lu Y., Chen X., Dong J., Wang L. Application of Orychophragine D in the Preparation of Drugs to Inhibit Ferroptosis in IEC6 Cells. CN114028404A. China Patent. 2022 February 11;
- 100.Li J., Li Y., Lu R., Wei J., Liao G. Research Progress on Anti-Inflammatory Alkaloids. Guangzhou Chem. Ind. 2020;48:14–19. [Google Scholar]
- 101.Zhang W., Yuan S., Wang C., Ye X. Extraction of antifungal protein from Orychophragmus violaceus seeds and its antifungal activity. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2019;48:296–302. [Google Scholar]
- 102.Zhang M., Jia F., Zhang B. Studies on acute and subacute toxicity of Orychophragmus violaceus; Proceedings of the 4th Annual Conference of Chinese Society of Toxicology on Toxicology and Safety Evaluation of Traditional Chinese Medicine and Natural Medicine; Haikou, China. 15 November 2019; p. 171. [Google Scholar]
- 103.Ma M., Mei Y. Research Status and Development Prospect of Orychophragmus violaceus. J. Anhui Agric. 2012;40:5109–5111+5113. [Google Scholar]
- 104.Hongmei J. Discussion on cultivation technique and landscape application of Orychophragmus violaceus. Agric. Technol. 2018;38:218. [Google Scholar]
- 105.Shi Y., Liang F., Ji Y., Ji Y., Ma N., Ye S. The application value of Orychophragmus violaceus in garden. Sci. Technol. Tianjin Agric. 2011;2:18–20. [Google Scholar]
- 106.Zhang Z., An J., Han Y., Feng L., Li X., Xiong S., Xing F., Xin M., Li Y., Wang Z. Advantages of an Orychophragmus violaceus-maize rotation in reducing greenhouse gas emissions and reactive nitrogen losses and increasing net ecosystem economic benefits on the North China Plain. J. Clean. Prod. 2021;317:128426. doi: 10.1016/j.jclepro.2021.128426. [DOI] [Google Scholar]
- 107.Wu D. Studies on Bio-Ecology of Landscape Cover Green Plant Orychophragmus violaceus. Hunan Agricultural University; Changsha, China: 2010. [Google Scholar]
- 108.Jia L. Study on Nutrition Charracteristics and Green Manure Effect of Orychophragmus violaceus. Chinese Academy of Agricultural Sciences; Beijing, China: 2010. [Google Scholar]
- 109.Daohong Z. Application value and intensive planting management technology of Orychophragmus violaceus. Mod. Agric. Sci. Technol. 2018;24:74–75. [Google Scholar]
- 110.Cui W., Ma H., Kong L. Research progress on Nrf2/ARE pathway and mechanism of antioxidation. J. Jilin Univ. Med. Ed. 2011;37:187–190. [Google Scholar]
- 111.Lu C., Xu W., Zheng S. Nrf2 activation is required for curcumin to induce lipocyte phenotype in hepatic stellate cells. Biomed. Pharmacother. 2017;95:1–10. doi: 10.1016/j.biopha.2017.08.037. [DOI] [PubMed] [Google Scholar]
- 112.Hu L.-f., Wang Y., Ren R.-j., Huo H., Sun J. Anti-oxidative stress actions and regulation mechanisms of Keap1-Nrf2/ARE signal pathway. J. Int. Pharm. Res. 2016;43:146–152+166. [Google Scholar]
- 113.Tang L. The Research on the Mechanism of Kuijieling to the NF-κB Activation of IκBα Phosphorylation/Ubiquitin-Proteasome System. Guangzhou University of Chinese Medicine; Guangzhou, China: 2012. [Google Scholar]
- 114.Tang J., Xu L., Zeng Y., Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int. Immunopharmacol. 2021;91:107272. doi: 10.1016/j.intimp.2020.107272. [DOI] [PubMed] [Google Scholar]
- 115.Ding W., Wang W., He Z., Chen X., Liu Z., Tao X., Zhao P., Yang Z., Zhao H. Anti-inflammatory Protective Effect of Lingguizhugan Decoction via NF-κB Pathway on LPS-induced Acute Lung Injury in Mice. Chin. J. Exp. Tradit. Med. Formulae. 2023;29:14–21. [Google Scholar]
- 116.Yue S., Hu B., Wang Z., Yue Z., Wang F., Zhao Y., Yang Z., Shen M. Salvia miltiorrhiza compounds protect the liver from acute injury by regulation of p38 and NFκB signaling in Kupffer cells. Pharm. Biol. 2014;52:1278–1285. doi: 10.3109/13880209.2014.889720. [DOI] [PubMed] [Google Scholar]
- 117.Saito C., Zwingmann C., Jaeschke H. Novel mechanisms of protection against acetaminophen hepatotoxicity in mice by glutathione and N-acetylcysteine. Hepatology. 2010;51:246–254. doi: 10.1002/hep.23267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang Z., Hu J.N., Yan M.H., Xing J.J., Liu W.C., Li W. Caspase-Mediated Anti-Apoptotic Effect of Ginsenoside Rg5, a Main Rare Ginsenoside, on Acetaminophen-Induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017;65:9226–9236. doi: 10.1021/acs.jafc.7b03361. [DOI] [PubMed] [Google Scholar]
- 119.Che Y. Berberine Decreases Apoptosis by Regulating Bcl-2 Family Proteins and Activating Caspase Pathway in Hypoxia/Reoxygenation-Induced H9C2 Cardiomyocytes. Fourth Military Medical University; Xi’an, China: 2014. [Google Scholar]
- 120.Yu H., Guo P., Xie X., Wang Y., Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017;21:648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sun Y., Chen P., Zhai B., Zhang M., Xiang Y., Fang J., Xu S., Gao Y., Chen X., Sui X., et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020;127:110108. doi: 10.1016/j.biopha.2020.110108. [DOI] [PubMed] [Google Scholar]
- 122.Lei G., Zhang Y., Koppula P., Liu X., Zhang J., Lin S.H., Ajani J.A., Xiao Q., Liao Z., Wang H., et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020;30:146–162. doi: 10.1038/s41422-019-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dong T. Study on the Mechanism of Nrf2 Signaling Pathway Alleviating PM2.5 Induced Lung Injury by Regulating Ferroptosis. Jilin University; Changchun, China: 2022. [Google Scholar]
- 124.Ning J. Important Roles of Nrf2 in Protection of Transient Controlled Hypothermia for Lung Injury after Limb’s Explosion Injury in Rats. Third Military Medical University; Chongqing, China: 2008. [Google Scholar]
- 125.Min L. Nrf2, HO-1 in Cerebral Ischemia-Reperfusion in Rats and the Neuroprotective Effect of Oxymatrine. Hebei Medical University; Shijiazhuang, China: 2009. [Google Scholar]
- 126.Sun X., Ou Z., Chen R., Niu X., Chen D., Kang R., Tang D. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatol. Baltim. Md. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fu Y. Erastin Induced Ferroptosis of Colon Cancer Cells through Regulation of SPINK4/VIL1 and NF-κB Pathway. Guilin Medical University; Guilin, China: 2020. [Google Scholar]
- 128.Li X., Teitgen A.M., Shirani A., Ling J., Busta L., Cahoon R.E., Zhang W., Li Z., Chapman K.D., Berman D., et al. Discontinuous fatty acid elongation yields hydroxylated seed oil with improved function. Nat. Plants. 2018;4:711–720. doi: 10.1038/s41477-018-0225-7. [DOI] [PubMed] [Google Scholar]
- 129.Haro-González J.N., Castillo-Herrera G.A., Martínez-Velázquez M., Espinosa-Andrews H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules. 2021;26:6387. doi: 10.3390/molecules26216387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jing F., Li F., Zhang T., Lv C., Zhao T. Research Progress of Chemical Constituents and Pharmacological Activities of Forsythia. J. Chin. Med. Mater. 2023;46:242–251. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.